Abstract
Cancer cachexia, characterized by muscle wasting and widespread inflammation, poses a significant challenge for patients with cancer, profoundly impacting both their quality of life and treatment management. However, existing treatment modalities remain very limited, accentuating the necessity for innovative therapeutic interventions. Many recent studies demonstrated that changes in autonomic balance is a key driver of cancer cachexia. This review consolidates research findings from investigations into autonomic dysfunction across cancer cachexia, spanning animal models and patient cohorts. Moreover, we explore therapeutic strategies involving adrenergic receptor modulation through receptor blockers and agonists. Mechanisms underlying adrenergic hyperactivity in cardiac and adipose tissues, influencing tissue remodeling, are also examined. Looking ahead, we present a perspective for future research that delves into autonomic dysregulation in cancer cachexia. This comprehensive review highlights the urgency of advancing research to unveil innovative avenues for combatting cancer cachexia and improving patient well-being.
Keywords: cancer cachexia, Autonomic dysfunction, Sympathetic signaling, Cardiac innervation, Adipose tissue innervation, Beta blockers, Skeletal muscle, Heart rate variability
1. Introduction
Throughout evolution, the autonomic nervous system (ANS) has played a pivotal role in enabling our bodies to effectively respond to and adapt to environmental fluctuations. This adaptive capacity extends to instances of illness. For instance, the sympathetic nervous system (SNS) orchestrates the well-known “fight or flight” response, gearing the body to combat disease by modulating immune responses and triggering heightened caloric expenditure (Elias et al., 1998; Bellinger et al., 2008). While these acute responses can confer advantages during acute challenge (Cannon and Nedergaard, 2004; Luan et al., 2019), the prolonged activation or inactivation of the sympathetic response can devolve into a counterproductive state, yielding adverse outcomes. This phenomenon is prominently evident in individuals grappling with cancer, culminating in debilitating conditions like cachexia that significantly curtail life quality and increase mortality.
Cancer cachexia is a debilitating and multifactorial syndrome that is often observed in patients with lung, pancreatic, and gastrointestinal malignancies. It is characterized by progressive loss of muscle mass and adipose tissue, accompanied by systemic inflammation, fatigue, and metabolic abnormalities (Fig. 1) (Murphy, 2016; Argiles et al., 2023). This condition significantly impacts the quality of life of patients with cancer, and is a major cause of morbidity and mortality. Indeed, it is estimated that cachexia accounts for up to 20 % of cancer related deaths (Tisdale, 2002; Poisson et al., 2021; Argiles et al., 2023). Despite its impact, effective treatment for cancer cachexia remains elusive, highlighting a significant gap in therapeutic options.
Fig. 1.

Schematic illustrating features of cancer cachexia. The systemic inflammation associated with cancer cachexia results in metabolic imbalance. As such, patients with cancer cachexia experience fatigue and a reduction in appetite and body weight that is accompanied by a paradoxical increase in metabolic rate and catabolism of both skeletal and cardiac muscle. Additionally, the reprograming of metabolism orchestrates adipose tissue loss and remodeling and reduces tolerance to many anti-neoplastic therapies.
In clinical categorization, this condition is stratified into three stages: precachexia, cachexia, and refractory cachexia (Fearon et al., 2011). During the precachectic phase, patients encounter anorexia and altered nutrient flux without substantial weight loss. Diagnostic benchmarks for the cachectic phase encompass either a body weight reduction exceeding 5 % over a span of 6 months, or a body mass index (BMI) below 20 kg/m2 coupled with ongoing weight loss surpassing 2 % or the presence of sarcopenia alongside ongoing weight loss beyond 2 %. In the terminal stage, refractory cachexia may manifest in terminally ill patients, characterized by active catabolism where the management of weight loss is unattainable, and the projected survival duration is approximately 3 months (Fearon et al., 2011). There have been many refinements to this consensus, but the basic principles still apply (Roeland et al., 2020; Arends, Strasser et al., 2021; Meza-Valderrama et al., 2021; Nishikawa et al., 2021).
Presently, anamorelin, a selective agonist of the ghrelin/growth hormone secretagogue receptor (HGSR), stands as the sole approved cachexia treatment in Japan (Wakabayashi et al., 2021). Given the multifaceted nature of cancer cachexia, a holistic therapeutic approach that concurrently addresses various metabolic and inflammatory pathways may yield the most favorable outcomes. Thus, understanding pathways that play a pivotal role in cancer cachexia – such as disrupted energy equilibrium, inflammation, and neural mechanisms contributing to this syndrome – are important in identifying intervention targets.
A plethora of recent studies in both human patients and pre-clinical animals demonstrate the role of adrenergic signaling in the pathogenesis of cancer cachexia. Since many of the organs affected by cancer cachexia (e.g., adipose tissue, heart, and skeletal muscle) are densely innervated by sympathetic nerves, the perturbed balance between the sympathetic and parasympathetic inputs can modulating metabolic pathways, culminating in deleterious consequences for target organs. Both white adipose tissue (WAT) and brown adipose tissue (BAT), for example, are densely innervated by sympathetic neurons (Jiang et al., 2017; Chi et al., 2018). In rodents the sympathetic fibers innervating BAT mostly originate from stellate ganglia as well as T2-T5 paravertebral ganglia (Francois et al., 2019; Barrett et al., 2022), whereas the neurons innervating inguinal WAT (iWAT) originate from T12-L1 paravertebral ganglia as well as the celiac ganglion (Jiang et al., 2017; Huesing et al., 2021). In addition to BAT, the postganglionic sympathetic nerves that innervate the heart also originate from the stellate ganglia, and they are important regulators of cardiac rhythm and homeostasis. Finally, in addition to motor neutrons, sympathetic nerves also widely innervate the neuromuscular junction of skeletal muscle (Khan et al., 2016; Straka et al., 2018). The sympathetic nerves innervating skeletal muscles originate from paravertebral sympathetic ganglia and reach muscle fibers via Remak fibers (Rodrigues et al., 2019) (Fig. 2).
Fig. 2.

Schematic illustrating post ganglionic sympathetic innervation of heart, brown adipose tissue (BAT), inguinal white adipose tissue (iWAT), and skeletal muscle in rodents. Heart receives sympathetic innervation from neurons in stellate ganglion (SG). BAT is innervated by neurons originating from stellate ganglion and T2-T5 paravertebral ganglion. The celiac ganglion (CG) and T12-L1 paravertebral ganglia innervate iWAT. Finally, skeletal muscle is innervated by paravertebral ganglia.
In this review paper, we provide an overview of the current state of knowledge pertaining to changes in sympathetic tone within the context of cancer cachexia. Since sympathetic outflow to target tissues is differentially regulated, we will discuss tissue specific impacts of adrenergic dysfunction and how it contributes to disease progression. Therapeutic implications of adrenergic receptor blockers and agonists are also discussed, alongside an exploration of future research directions in this field. By addressing these aspects, we will highlight potential novel therapeutic strategies and pave the way for future research in this critical area of cancer cachexia therapeutics.
2. Methods
We conducted an extensive search of the PubMed database, covering articles published up to June 2023. Our search strategy encompassed various keyword combinations, including variations for “Autonomic signaling and cancer cachexia”; “Adrenergic and cancer cachexia”; “Heart rate variability and cancer cachexia”. Additionally, we evaluated the bibliographies of the retrieved articles to identify any further pertinent publications.
3. Autonomic imbalance in patients with cancer cachexia
Cancer cachexia is a systemic disease that leads to dysregulation of many physiologic processes in the body. The autonomic nervous system is one of the many systems in the body affected by this debilitating condition. The evidence for autonomic dysregulation includes multiple clinical studies demonstrating changes in the heart rate variability (HRV) of patients with cancer. In one study, for example, Chauhan et al. obtained a 5-minute electrocardiogram from 9 patients with cancer cachexia and compared the different frequency domains of HRV to age-matched healthy controls. The authors demonstrated that patients with cancer cachexia had significantly lower HRV in both the low frequency and high frequency spectral bands (Chauhan et al., 2012). Furthermore, in a prospective study, body composition and different elements of heart rate variability were analyzed in 50 patients with colorectal cancer (CRC). The authors reported patients with CRC had a lower lean mass and a global reduction in HRV in the very low frequency, low frequency, and high frequency spectral bands (Cramer et al., 2014).
The decrease in HRV was also found to be associated with unfavorable outcomes in patients with cancer. A recent meta-analysis, which included 6 studies and 1286 patients, demonstrated lower survival rates in patients with lower HRV, indicating the prognostic value of HRV in the clinical practice (Zhou et al., 2016). Interestingly, this paper excluded studies with terminally ill or cachectic patients due to the known reduction in the HRV of cachectic patients. Nonetheless, the results provide support for autonomic dysfunction as an indicator of poor outcomes in patients with cancer and cancer cachexia.
The observed reduction in the high frequency band indicates a decrease in vagal tone to the heart. Conversely, the low frequency band is a mix of both sympathetic and parasympathetic signals. Therefore, a change in the low frequency band may be indicative of alterations in the activity of either sympathetic or parasympathetic nerves. As such, the available HRV data do not offer precise insights into how sympathetic signaling is modified in patients. Nevertheless, an elevation in cardiac sympathetic tone leads to a reduction in the low frequency domain of HRV. As we discuss later in this review, studies in both patients and animal models also support the notion that cardiac adrenergic tone is increased in patients with cancer cachexia.
In addition to cancer cachexia, some cancer therapeutics may also result in changes in HRV. Antineoplastic agents such as doxorubicin are well-known inducers of cardiac toxicity in patients with cancer, but their effect on the sympathovagal balance seem to be distinct from that observed in patients with cancer cachexia. For example, studies in rats reveled that doxorubicin treatment results in an increase in all elements of heart rate variability (Loncar-Turukalo et al., 2015; Vasic et al., 2019). Another study by Afonso et al. also demonstrated an increase in the high frequency band along with a decrease in LF/HF ration (Afonso et al., 2023). Therefore, these results suggest that the global reduction in HRV observed in patients with cancer cachexia is most likely driven by cancer and cancer cachexia rather than chemotherapeutic agents. Indeed, analysis of HRV in chemotherapy-naïve patients also revealed a reduction in HRV (Cramer et al., 2014).
Changes in autonomic signaling is also one of the key drivers of cachexia pathophysiology. Presently, it is not clear whether the onset of cachexia precedes autonomic imbalances and changes in HRV or if the inflammation associated with cancer orchestrates a modulation of autonomic nerves, thereby leading to cachexia in patients. Conceivably, a positive feedback loop may be at play, wherein inflammation within the central and peripheral nervous systems of patients with cancer cachexia reshapes autonomic signaling, and these autonomic alterations at the level of end organs, in turn, exacerbate symptoms of cachexia (Fig. 3). This process becomes even more convoluted when considering patients with cancer dissemination. Multiple studies have pointed to the role of autonomic nerves in cancer development and metastasis in patients (Ondicova and Mravec, 2010; Sloan et al., 2010; Magnon et al., 2013; Renz et al., 2018; Mohammadpour et al., 2019), who generally present with a greater cachexia burden (Biswas and Acharyya, 2020). Therefore, the autonomic imbalance can further increase cachexia burden by facilitating tumor growth and metastasis (Fig. 3). Gaining a more profound insight into the temporal dynamics of autonomic nerve imbalance, the onset of cachexia, and the spread of cancer can provide valuable guidance on when during the course of the disease distinct therapeutic interventions are most likely to benefit patients.
Fig. 3.

Diagram depicting the impact of autonomic imbalance on cancer progression and development of cancer cachexia. The presence of a peripheral tumor leads to the development of cancer cachexia in patients, resulting in the disruption of numerous physiological processes in the body. The disruption in the autonomic nervous system, in turn, exacerbates cachexia symptoms. Moreover, Changes in autonomic signaling can also facilitate tumor growth and dissemination, which will further exacerbate cachexia burden.
4. The impact of adrenergic receptor blockade therapies in patients and animal models of cancer cachexia
Although the HRV studies implicate changes in both sympathetic and parasympathetic inputs, most studies to date have focused on the role of sympathetic signaling in cancer cachexia, and adrenergic receptor blockade shows promise in multiple models of cancer cachexia. Bisoprolol, a selective beta-1 adrenergic receptor antagonist, for example, was able to improve food intake and reduce lean and fat mass loss in a rat model of hepatoma associated cachexia (AH-130) (Springer et al., 2014). Additionally, bisoprolol significantly reduced mortality rates compared to vehicle treated animals (Springer et al., 2014). Similarly, specific stereoisomers of beta blockers, such as S-pindolol and S-oxprenolol, reduced mortality rates and cachexia burden in preclinical animal models by improving food intake, lean mass, fat mass, and cardiac function (Lainscak and Laviano, 2016; Potsch et al., 2020; Yuan et al., 2022; Poetsch et al., 2023; Springer et al., 2023). Furthermore, in a randomized double-blind phase II multicenter trial, S-pindolol significantly improved lean mass and strength in patients with lung and colorectal cancer, but had no effect on fat mass loss (Stewart Coats, Ho et al., 2016). Collectively, these studies point to the therapeutic potential of beta-blockers in patients suffering from cancer cachexia (Table 1).
Table 1.
Summary of Beta-blocker studies in patients with cancer cachexia as well as various cancer cachexia animal models.
| Animal model/patient population | Beta-blocker | Findings | Reference |
|---|---|---|---|
| Patients with various solid tumors | Propranolol | Propranolol reduced resting energy expenditure | (Hyltander et al., 1993) |
| Patients with gastric, rectal, pancreatic, and hepatocellular carcinomas | Atenolol and propranolol | Reduced heart rate and resting energy expenditure | (Hyltander et al., 2000) |
| Rat Yoshida Hepatoma model of cachexia | Bisoprolol | Improved food intake, lean mass, fat mass, activity level, cardiac function, and survival | (Springer et al., 2014) |
| Patients with Small cell lung cancer or colorectal cancer (ACT-ONE trial) | S-pindolol | Attenuated weight loss and improved handgrip strength | (Stewart Coats, Ho et al., 2016) |
| Rat Yoshida Hepatoma model of cachexia | S-pindolol | Improved food intake, activity level, and survival and attenuated loss of lean mass and fat mass | (Potsch et al., 2020) |
| Mouse model of pancreatic and lung cancer | S-pindolol | Improved lean mass and grip strength, but had no effect on fat mass loss | (Springer et al., 2023) |
| Rat Yoshida Hepatoma model of cachexia | S-pindolol, carvedilol, metoprolol, nebivolol, and tertatolol | Only S-pindolol Improved cardiac function, food intake, activity level, and survival and attenuated loss of lean mass and fat mass | (Poetsch et al., 2023) |
| Rat Yoshida Hepatoma model of cachexia | S-oxprenolol | Improved lean mass, fat mass, food intake, and survival, but did not improve cardiac function | (Yuan et al., 2022) |
| K5-SOS model of skin cancer | Selective Beta-3 adrenergic receptor blocker | Reduced white adipose tissue browning | (Petruzzelli et al., 2014) |
Although adrenergic receptor blockade showed promising results, not all beta blockers seem to have anti-cachectic properties. In one study, Poetsch and colleagues compared the therapeutic effects of S-pindolol, carvedilol, metoprolol, nebivolol, and tertatolol on cachexia burden as well as cardiac mass and function in the Yoshida Hepatoma model of cachexia. Interestingly, only S-pindolol was able to significantly improve survival and cardiac function while attenuating loss of lean mass (Poetsch et al., 2023). These results suggest that the type of beta-blocker needs to be taken into consideration when choosing a therapeutic regimen. On the molecular level, both S-pindolol and S-oxprenolol alleviate tissue wasting by inhibiting catabolic pathways through beta-1 receptor antagonism, activate anabolic pathways through beta-2 receptor agonism and stimulate appetite and food intake though a central serotonin receptor (5HT1α) antagonism (Lainscak and Laviano, 2016; Potsch et al., 2020; Yuan et al., 2022). This non-specific receptor action of S-pindolol and S-oxprenolol is the mechanistic basis for their anti-cachectic effects.
In addition to the type of beta-blocker, the type of cancer also needs to be taken into consideration. The majority of the beta-blocker research has been conducted in the Yoshida Hepatoma model, which demonstrates a global elevation of sympathetic tone with augmented plasma norepinephrine levels. In other cachexia models, on the other hand, sympathetic transmission to distinct targets is differentially regulated with no change in plasma norepinephrine levels (Xie et al., 2022). Therefore, it is critical to assess the effects of various beta-blockers in different cancer models in order to establish the most effective type of beta-blocker for each cancer type.
Many studies have also investigated tissue specific effects of altered adrenergic tone. Although adrenergic dysfunction in cancer cachexia affects multiple organs, we will only focus on the heart, skeletal muscle, and adipose tissue in this review. The tissue specific dysfunction in the parasympathetic arm of ANS, on the other hand, is less explored and warrants further investigation. This is especially true considering patients with cancer cachexia experience a global reduction in heart rate variability. Therefore, although not within the primary scope of this review, it is imperative to underscore the significance of conducting additional research to gain a better understanding of the respective roles of sympathetic and parasympathetic dysfunction in driving cancer cachexia pathology.
5. Cardiac adrenergic stimulation and cancer cachexia-associated cardiac remodeling
Initial observations for cardiac dysfunction and atrophy in patients with cancer was made by Burch et al. (1968). In addition to cardiac atrophy, the study also noted loss of epicardial fat and a low athero-sclerotic plaque in the aorta and coronary arteries. The authors coined the term “cachectic heart” to describe this distinctive syndrome. Since then, compromised cardiac function resulting from structural and functional remodeling has been commonly observed in patients with cancer cachexia and survivors, leading to a more difficult treatment management course and reduced quality of life (Cramer et al., 2014; Springer et al., 2014; Anker et al., 2016; Barkhudaryan et al., 2017; Anker et al., 2018; Kazemi-Bajestani et al., 2019a, 2019b; Cai et al., 2020; Anker et al., 2021; Lena et al., 2023). Additionally, numerous studies in chemotherapy-naïve animal models also demonstrated signs of remodeling and cardiac insufficiency such as atrophy, reduced ejection fraction, reduced calcium cycling, and switching of myosin heavy chain isoforms (Tian et al., 2010; Cosper and Leinwand, 2011; Tian et al., 2011; Springer et al., 2014; Mishra et al., 2018; Law and Metzger, 2021; Uurasmaa et al., 2022; Wiggs et al., 2022). Although these findings indicate cachexia and metabolic reprogramming as important drivers of adverse cardiac remodeling in chemotherapy-naïve patients, the mediators of cancer cachexia-induced cardiac remodeling still remain an active area of research.
Although the exact mechanism of cardiac remodeling in cancer cachexia is not well understood, several studies point to the role of increased adrenergic transmission in the heart. For example, in a 5-year prospective study, 145 patients with colorectal, pancreatic, and non-small cell lung cancers along with 59 healthy control subjects underwent ECG recordings for evaluation of resting heart rate (RHR). Patients with cancer demonstrated a significant elevation in their RHR, which was independent of chemotherapy status (Anker et al., 2016). The authors also found that cancer patients with heart rates greater than 75 beats per minute had lower one-year, two-year and three-year survival rates, highlighting the prognostic value of RHR in patients with cancer cachexia (Anker et al., 2016). Furthermore, in a separate retrospective study of 4786 patients with stage I-III breast cancer, elevated RHR was also significantly associated with higher mortality rates (Lee et al., 2016).
It is important to acknowledge that the elevation in RHR reported in clinical studies could be the result of either dysfunctional sympathetic or parasympathetic input to the heart, and further research is required to determine the respective roles of these systems. However, clinical studies in patients with cancer cachexia highlighted the significance of adrenergic hyperactivity. In two separate studies, administration of atenolol and propranolol was able to significantly reduce resting heart rate and resting energy expenditure (REE) in patients with cancer cachexia (Hyltander et al., 1993; Hyltander et al., 2000). Interestingly, the authors attributed the decrease in REE to a decrease in heart rate and concluded that the wasting observed in patients with cancer cachexia is partially attributable to the increased adrenergic stimulation of the heart as a result of anemia and diminished contractile capacity (Hyltander et al., 2000). It is essential to note, however, that beta-blockers can exert their effects on numerous organs in the body. Therefore, additional studies with ivabradine, a funny channel pacemaker current (If) blocker, are required to determine if the decrease in REE is truly due to a decrease in heart rate or due to blockade of adrenergic signaling on other organs, such as adipose tissue and skeletal muscle.
In addition to regulating resting energy expenditure, elevated sympathetic transmission in the heart also regulates adverse cardiac remodeling. A study by Springer and colleagues reported significant functional and structural remodeling of the heart in a hepatoma associated cachexia model (AH-130) (Springer et al., 2014). Interestingly, the authors found elevated norepinephrine levels in the plasma of tumor bearing animals, and treatment with bisoprolol, a selective beta-1 antagonist, was able to reduce adverse cardiac remodeling by improving cardiac mass, left ventricular stroke volume, and left ventricular end diastolic volume. On the other hand, treatment with imidapril, an angiotensin converting enzyme inhibitor, was unable to rescue the cardiac phenotype or improve survival. These observations suggest that possibly a direct sympathetic hyperactivity to the cardiovascular system, rather than an indirect mechanism such as activation of the renin angiotensin system by stimulating beta-1 adrenergic receptors on juxtaglomerular cells of the kidney, is regulating adverse remodeling.
Despite the large body of research, the mechanism of adrenergic hyperactivity to the heart is not clear and remains an active area of research. A recent study in a model of fibrosarcoma associated cachexia demonstrated increased expression of inflammatory markers in various adrenergic centers in the brain, including locus coeruleus, hypothalamic paraventricular nucleus, and A1/C1 neurons (Cernackova et al., 2023). C1 neurons are catecholaminergic neurons located in the rostral ventrolateral medulla (RVLM) and are a critical mediator of cardiovascular homeostasis by synapsing to pre-ganglionic sympathetic neurons in the intermediolateral cell column (Guyenet and Stornetta, 2022). Therefore, the inflammatory milieu in the RVLM of tumor bearing animals can modulate the activity of these neurons, thereby increasing cardiac adrenergic transmission. Indeed, VLM catecholaminergic neurons were found to be activated in a murine colon adenocarcinoma model (MC-38) (Zhang et al., 2021). The authors reported that activation of these neurons promoted tumor growth, while ablation of the neurons slowed down tumor growth by modulating cytotoxic T cells. Although, cardiac adrenergic signaling was not investigated in this study, it is plausible that activation of VLM catecholaminergic neurons also mediate adrenergic hyperactivity in the heart (Fig. 5). Additional studies are also needed to investigate adrenergic signaling at the level of stellate ganglia, the nerve terminals projecting to the heart, and how adrenergic receptor signaling is perturbed in the cardiomyocytes of tumor bearing animals.
Fig. 5.
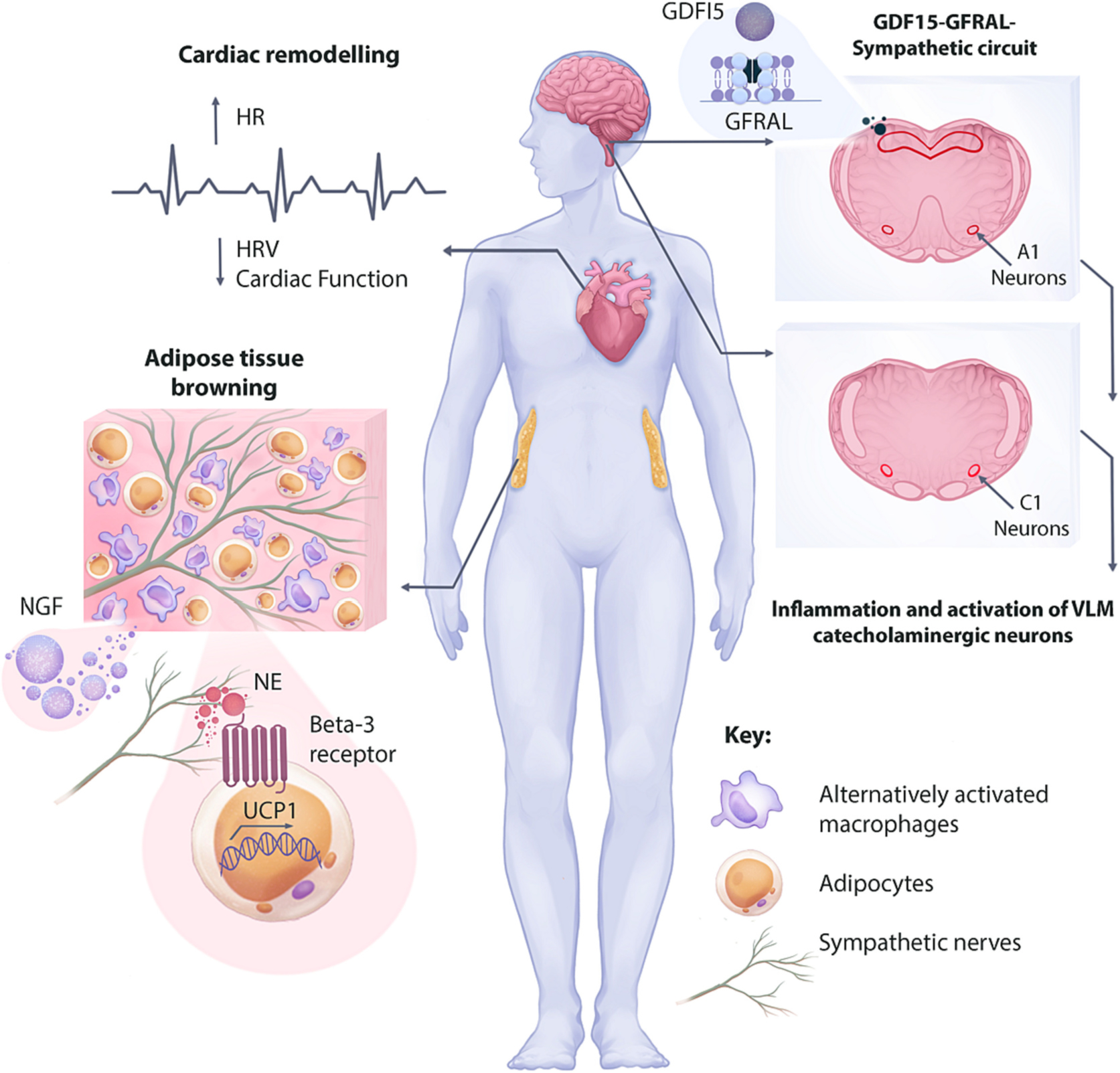
Summary of findings and potential mechanisms of increased sympathetic signaling and tissue remodeling during cancer-associated cachexia. An increased adrenergic transmission in the heart increases heart rate while decreasing heart rate variability and regulating maladaptive cardiac remodeling. Although the exact mechanism of enhanced adrenergic transmission in the heart is unknown, inflammation and activating of VLM catecholaminergic neurons are reported in animal models. Additionally, the GDF15-GFRAL-Sympathetic circuit in involved in increasing skeletal muscle adrenergic transmission, leading to increased energy expenditure. Increased nerve density in the white adipose tissue (WAT) is regulated by the nerve growth factor (NGF) secreted from alternatively activated macrophages. The increased nerve density and adrenergic signaling on beta-3 adrenergic receptor regulates WAT browning and increased energy expenditure in some animal models of cancer cachexia.
6. Adrenergic stimulation of skeletal muscle and anti-cachectic effects of beta-2 adrenoreceptor agonists
Sympathetic nerves widely innervate the neuromuscular junction of skeletal muscle, and their input is important for synaptic maintenance and function (Khan et al., 2016; Straka et al., 2018). Local sympathectomy of skeletal muscle with 6-OHDA resulted in reduced complexity and size of neuromuscular junctions (NMJ). This phenomenon was rescued in animals receiving clenbuterol, highlighting the role of Beta-2 adrenoreceptors in NMJ biology of skeletal muscle. Sympathetic innervation of skeletal muscle is also an important regulator of skeletal muscle size and proteolysis. For example, local sympathectomy of skeletal muscle in mice via lumbar ganglionectomy resulted in muscle atrophy and increased expression of the E3 Ubiquitin Ligase MuRF1 (Rodrigues et al., 2019). Collectively, these studies highlight the role of adrenergic signaling in regulating proteolysis and NMJ function in skeletal muscle and provide support for the use of selective sympathomimetics for treatment of skeletal muscle wasting disorders such as cancer cachexia.
Beta-2 adrenergic receptor is the major subtype of beta-adrenergic receptor on skeletal muscle, and its stimulation can mediate muscle hypertrophy by increasing protein synthesis and slowing down protein degradation (Sato et al., 2011). On the molecular level, beta-2 adrenoreceptor is coupled to Gαs, and stimulation of the receptor results in conversion of adenosine triphosphate to cyclic adenosine mono-phosphate (cAMP), a second messenger that activates protein kinase A (PKA). Additionally, the Gβγ dimer activates the phosphoinositol 3-kinase (PI3K)-AKT signaling pathway. Activation of these signaling pathways is the mechanistic basis of muscle hypertrophy induced by Beta-2 adrenoreceptor agonists (Lynch and Ryall, 2008; Goncalves et al., 2019) (Fig. 4).
Fig. 4.
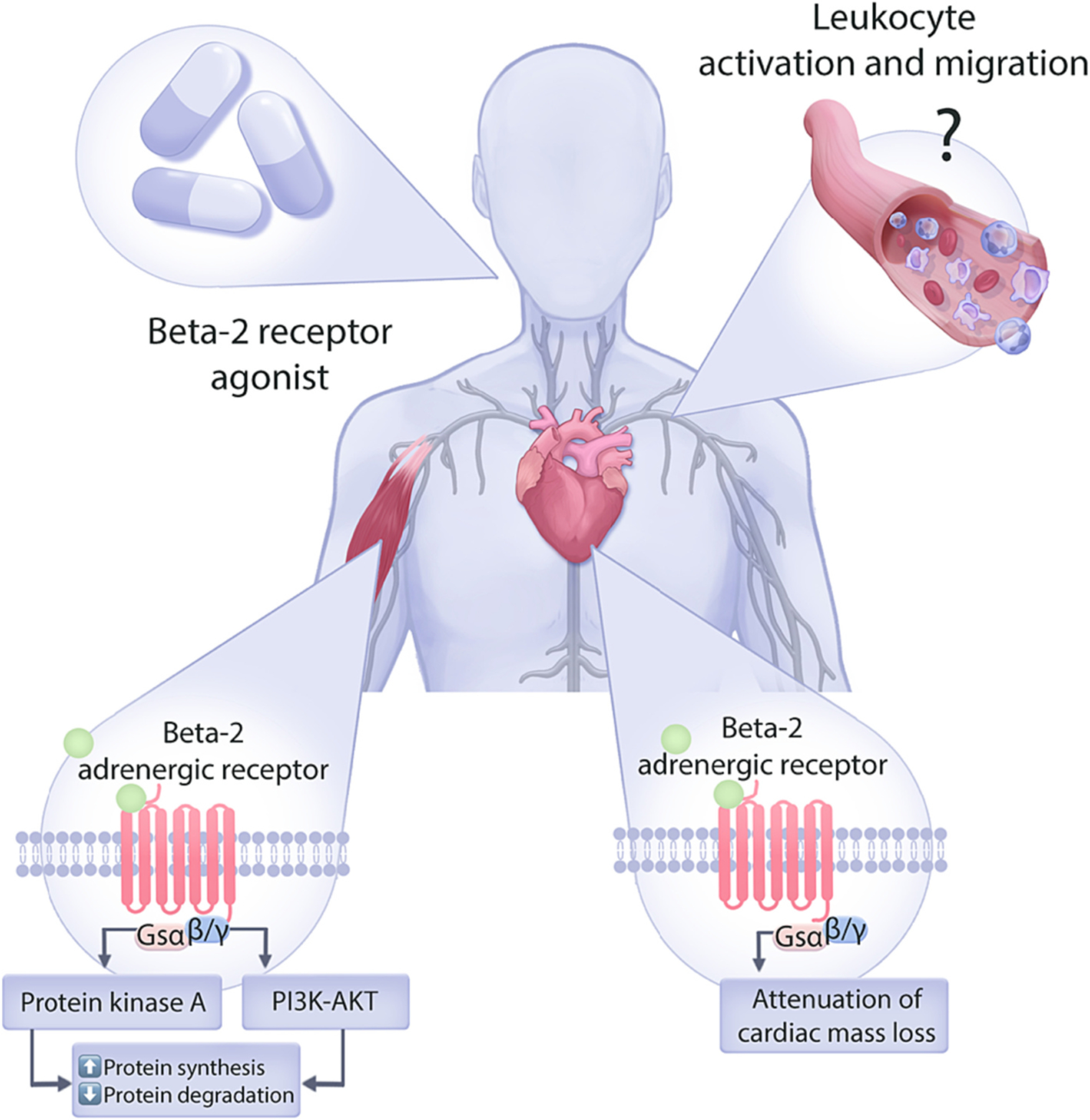
Graphical representation of the influence of beta-2 adrenergic receptor agonists on loss of skeletal and cardiac muscle during cancer cachexia. Stimulation of beta-2 adrenergic receptor on both skeletal and cardiac muscle leads to increased protein synthesis and decreased protein degradation, which attenuates loss of lean mass in patients with cancer cachexia.
Interestingly, Beta-2 receptor signaling is also implicated as a regulator of cardiomyocyte size. Sympathetic denervation studies with 6-OHDA demonstrated activation of E3 ubiquitin ligases and autophagy genes in the heart, resulting in an atrophic remodeling (Zaglia et al., 2013). Furthermore, a beta-2 receptor agonist was able to abrogate atrophic remodeling in denervated animals, suggesting that the loss of beta-2 adrenergic signal on cardiomyocytes is the key mediator of atrophy.
Our knowledge regarding how sympathetic signaling is altered within skeletal muscle in the context of cancer cachexia remains very limited. Nonetheless, due to the hypertrophic effects of beta-2 adrenergic receptor stimulation, beta-2 adrenoreceptor agonists have received significant attention in recent years as a therapeutic option for the treatment of cancer cachexia. Initial studies by Costelli et al. implemented the use of clenbuterol in a rat ascites hepatoma Yoshida AH-130 model. The authors demonstrated that Clenbuterol suppressed the rate of protein breakdown, attenuating loss of both skeletal (gastrocnemius and soleus) and cardiac muscles (Costelli et al., 1995). However, clenbuterol had no effect on food intake, suggesting that the trophic effects on skeletal and cardiac muscle are independent of food intake.
Similarly, formoterol, a long-acting beta-2 adrenoreceptor agonist, offered protective effects in the Yoshida AH-130 ascites hepatoma and the Lewis lung carcinoma models by decreasing the rate of protein degradation (Busquets et al., 2004). Additionally, formoterol suppressed apoptosis, proteasome activity, and autophagy in skeletal muscle while accelerating the rate of protein synthesis (Busquets et al., 2004; Salazar-Degracia et al., 2018). As such, tumor bearing animals receiving formoterol exhibited preservation of skeletal and cardiac muscle (Busquets et al., 2004, Salazar-Degracia et al., 2018). However, formoterol had no effect on WAT mass or food intake. Additional studies demonstrated that the trophic effects of formoterol is accompanied by improved proxy measures of quality of life as demonstrated by increased grip force and activity level of tumor bearing animals (Busquets et al., 2011). Combining formoterol with other anticachectic medications also seem to augment the trophic properties. For example, Toledo et al. demonstrated that combination of formoterol and the soluble myostatin receptor ActRIIB resulted in a more dramatic recovery of skeletal and cardiac tissue compared to animals receiving individual treatments of these drugs (Toledo et al., 2016).
Novel beta-blockers, such as S-pindolol and S-oxprenolol, also hold a beta-2 agonistic property, which in combination with antagonistic effects on beta-1 adrenergic receptor and serotonin receptor (5HT1α) provide a powerful therapeutic potential (Potsch et al., 2020; Yuan et al., 2022). However, how these medications and other beta-2 adrenoreceptor agonists modulate the immune system still needs to be investigated. Interestingly, Beta-2 is also one of the major adrenergic receptors on immune cells and can influence their activation and recruitment to different organs. For example, beta-2 adrenergic receptor regulates expression of chemokine receptor 2 (CCR2) on leukocytes and their recruitment to tissues (Grisanti et al., 2016). Other studies demonstrated formoterol treatment perturbs leukocyte migration and locomotion, resulting in an impaired immune response (Devi et al., 2021). Lastly, beta-2 adrenergic signaling also regulates egress of hematopoietic stem cells from bone marrow (Katayama et al., 2006). Therefore, additional research is needed to better understand if modulation of immune system is involved in the anti-cachectic properties observed with beta-2 receptor agonists.
Although most studies point to the beneficial effects of increased sympathetic transmission in skeletal muscle, increased activity of a recently identified sympathetic circuit involving the growth and differentiation factor-15 (GDF15) seems to have detrimental effects on skeletal muscle (Fig. 5). GDF15 is a member of the transforming growth factor-β (TGFβ) family that was initially found to be expressed by macrophages (Bootcov et al., 1997). However, various other cell types and tissues, including cardiomyocytes, adipocytes, endothelial cells, kidney, liver, lung, and placental trophoblasts were later found to also produce GDF15 (Tsai et al., 2018; Coll et al., 2020; Rochette et al., 2020). In healthy individuals, GDF15 is expressed at low levels, but the levels are significantly elevated in serum of patients with cancer cachexia (Johnen et al., 2007), thereby mediating a decrease in food intake and body mass by signaling though glial-cell-derived neurotrophic factor family receptor α-like (GFRAL) in the brainstem (Emmerson et al., 2017; Suriben et al., 2020).
Interestingly, in addition to the canonical GDF15 signaling pathway, a GDF15-GFRAL-sympathetic circuit was recently identified to regulate metabolism in cancer cachexia. In a study by Suriben et al. the authors treated control and sympathectomized mice with GDF15, and although both cohorts had the same level of drop in their food intake, the sympathectomized animals had blunted GDF15-induced weight loss (Suriben et al., 2020). Therefore, the authors concluded that the GDF15-GFRAL signaling regulates body weight through a sympathetic nervous system mechanism that is independent of the food intake effects of GDF15. In a more recent study, a GDF15-GFRAL-sympathetic axis was found to locally increase norepinephrine in skeletal muscle, thereby increasing energy expenditure by increasing calcium signaling and fatty acid oxidation in skeletal muscle (Wang et al., 2023). Although this study was not conducted in tumor bearing animals, it provides an indication that the same process may be happening in patients with cancer cachexia. However, additional studies are needed to better understand how GDF15 influences skeletal muscle adrenergic signaling in patients with cancer cachexia. In addition to skeletal muscle, it is also important to investigate the effects of GDF15-GFRAL-sympathetic circuit on cardiac sympathetic input during cancer cachexia.
7. Adrenergic signaling in adipose tissue
The dense network of sympathetic fibers in white adipose tissue (WAT) are important regulators of metabolism and thermogenesis. For example, during cold challenge, the sympathetic innervation of iWAT regulates adipose tissue browning (Jiang et al., 2017). WAT browning results in increased expression of thermogenic factors, such as uncoupling protein-1 (Ucp1), which uncouples mitochondrial respiration and increases energy expenditure. This phenomenon was abolished in animals that underwent a local sympathectomy of iWAT with 6-OHDA. Additionally, Jiang et al. demonstrated loss of sympathetic arborization in the iWAT and BAT of both diet-induced and genetic (ob/ob) models of obesity (Jiang et al., 2017), which further highlights the role of adipose sympathetic innervation in regulating energy homeostasis. Numerous other studies also demonstrated the link between sympathetic innervation of adipose tissue and metabolism in various obesity models (Zeng et al., 2015; Pirzgalska et al., 2017; Chen et al., 2022).
Similarly, enhanced sympathetic transmission to adipose tissue was demonstrated in animal models of cancer cachexia in recent years. For example, Petruzzelli and colleagues demonstrated a role for adrenergic signaling in browning of WAT in various models of cancer cachexia (Petruzzelli et al., 2014). Similar to models of cold challenge, WAT browning in cancer cachexia resulted in increased expression of thermogenic factors, such as Ucp1. The authors demonstrated that the activation of browning programs is mediated by increased adrenergic outflow to the beta-3 adrenergic receptor in WAT. Indeed, WAT browning was abolished in tumor bearing animals receiving a novel and selective beta-3 adrenergic receptor blocker.
Although cancer cachexia could result in systemic dysregulation of adrenergic signaling, the browning of adipose tissue is mediated by a local increase in sympathetic neurotransmission. Recent studies revealed elevated norepinephrine levels in both iWAT and BAT of tumor bearing animals but not in plasma, indicating an increase in adrenergic signaling at the tissue site (Xie et al., 2022). The authors further demonstrated increased neurotrophin signaling in the iWAT of tumor bearing animals as indicated by elevated expression of nerve growth factor (NGF) and enhanced sympathetic neuronal outgrowth in both iWAT and BAT. Utilizing a co-culture system of sympathetic neurons and macrophages, the authors unveiled a macrophage-sympathetic neuron crosstalk that contributes to the sympathetic hyperactivity of adipose tissue in cancer cachexia. Sympathetic neurons co-cultured with macrophages that were pre-treated with plasma of cachectic animals demonstrated reduced arborization, suggesting the presence of an anti-neurotrophic factor in plasma. However, co-cultures of sympathetic neurons with macrophages that were pre-treated with IL-4 and plasma from cachectic animals demonstrated increased arborization. In this elegant co-culture experiment, the authors demonstrated that IL-4 signaling increases NGF expression in alternatively activated macrophages, promoting neuronal arborization (Fig. 5).
Cancer cachexia-induced WAT browning and cold-induced thermogenesis share many similarities. For example, elevated macrophage recruitment and modulation of sympathetic innervation by alternatively activated macrophages are also implicated in models of cold-induced WAT browning (Qiu et al., 2014; Jiang et al., 2017). Although the invivo source of IL-4, which primes alternatively activated macrophages, is not well understood in cancer cachexia, studies in cold-induced thermogenesis suggested eosinophils as a major source of IL-4 (Qiu et al., 2014). Additionally, a recent study found that eosinophils also produce NGF in response to cold-induced thermogenesis, promoting sympathetic neuronal growth (Meng et al., 2022). Therefore, additional research is warranted to explore the role of eosinophils in WAT innervation in the setting of cancer cachexia.
In addition to eosinophils, sympathetic associated macrophages (SAMs) are another type of immune cell that modulate sympathetic transmission in adipose tissue. SAMs are responsible for taking up and degrading norepinephrine in adipose tissue and their abundance increases in models of obesity (Pirzgalska et al., 2017). Additionally, genetic ablation of SAMs results in WAT browning and activation of thermogenic programs. However, the role and abundance of SAMs in cancer cachexia is unclear and remains an active area of research.
Although a few studies in animal models show signs of adipose tissue browning and increased Ucp1 expression, it is crucial to acknowledge that browning of white adipose tissue is a controversial topic and is not observed across all animal models and patient cohorts. For example, using an orthotopic model of pancreatic cancer cachexia Michaelis et al. demonstrated loss of Ucp1 expression in all adipose tissues in tumor bearing animals (Michaelis et al., 2017). Rohm et al. also demonstrated that staining of iWAT in the Lewis Lung carcinoma and C26 colon adenocarcinoma models did not show any UCP1 specific staining (Rohm et al., 2016). Furthermore, studies in both animal models and human patients also point to the fact that UCP1 and brown adipose tissue are not key drivers of cachexia. For example, UCP1 knockout tumor bearing animals were still susceptible to cachexia, and lost the same amount of body weight and fat mass as their Wild-type counterparts (Rohm et al., 2016). Furthermore, in a retrospective study fluoro-deoxyglucose (FDG) positron-emission tomography (PET) imaging analysis of patients with cancer revealed no correlation between BAT and survival (Eljalby et al., 2023). Interestingly, the authors even found a negative correlation between BAT and cachexia in patients (Eljalby et al., 2023). In another retrospective intra-individual longitudinal study, FDG-PET/CT imaging of patients with cancer did not reveal any correlation between BAT activation and body mass index (BMI) (Becker et al., 2020). These data challenge the notion that WAT browning and activation of thermogenic pathways are drivers of cancer cachexia, and it is possible that WAT browning is a phenomenon observed only in some mouse models and other factors may be involved in loss of fat mass and cachexia development in human patients. Indeed, studies by Rohm and colleagues demonstrated inactivation and degradation of AMP-activated protein kinase (Ampk) as a mediator of WAT wasting (Rohm et al., 2016).
Apart from WAT browning, sympathetic innervation of white adipose tissue also plays an important role in governing lipolysis by phosphorylating hormone sensitive lipase (HSL) and activating other lipolytic programs (Bartness et al., 2014). Although the activation of lipolytic pathways and HSL phosphorylation is well-established in cancer cachexia (Das et al., 2011), the involvement of sympathetic innervation in this process remains controversial and an active area of research. Studies by Rohm and colleagues demonstrated that local sympathectomy of WAT using 6-OHDA had no effect on adipose tissue wasting and levels of non-esterified free fatty acid in the serum of C26 tumor-bearing animals (Rohm et al., 2016). Conversely, Xie et al. utilized a genetic approach for selective peripheral sympathectomy and observed prevention of WAT loss and HSL phosphorylation in the Lewis Lung Carcinoma model (Xie et al., 2022). These discrepancies could stem from differences in the experimental approach used to induce sympathectomy or variations in tumor models employed. Therefore, further investigation is warranted to gain a better understanding of the role of sympathetic signaling in cancer cachexia-associated lipolysis.
The beta-3 adrenergic receptor is the predominant type of adrenergic receptor found in adipose tissue, and it plays a critical role in the regulation of adipose tissue metabolism under both metabolic and cold challenges. However, less is known about the role of other beta-adrenergic subtypes in the pathophysiology of cancer cachexia and how they promote adipose wasting. In a prospective study, the expression of various adrenergic receptors and lipolytic enzymes in the abdominal adipose tissue of 34 chemotherapy-naïve patients with gastrointestinal malignancies was evaluated. The authors demonstrated increased mRNA and protein expression of beta-1 adrenergic receptor (ADRB1) and hormone sensitive lipase (HSL) in patients with cancer cachexia compared to non-cachectic patients and nonmalignant controls (Cao et al., 2010). The authors also found a positive correlation between ADRB1 and HSL. However, how much of adipose tissue wasting and increased energy expenditure is mediated through ADRB1 signaling still needs to be investigated.
8. Conclusions and considerations for future studies
This review summarizes the current knowledge of the role of the sympathetic nervous system in cachexia pathophysiology, including autonomic imbalance in patients, therapeutic effects of various adrenergic receptor blockers and adrenergic receptor agonists, and tissue specific impacts of increased adrenergic signaling. It is clear that the majority of the literature in cancer cachexia has focused on role of sympathetic innervation in various organ systems, but further investigation is needed to evaluate other nerve fibers, including parasympathetic and sensory nerves, to provide a comprehensive understanding of tissue innervation in cancer cachexia. Indeed, recent investigations highlighted alterations in parasympathetic transmission within cancer cachexia models, underscoring the importance of implementing a holistic tissue innervation approach (Gams et al., 2023).
For future research, it is also essential to consider several critical factors that can significantly influence experimental outcomes in the context of autonomic signaling studies and cancer cachexia progression. One such crucial factor is the ambient temperature of the animal housing facility. Typically, animal facilities maintain temperatures around 21 degrees Celsius, which falls below the thermoneutral range for rodents. This low temperature can activate sympathetic drive to initiate thermogenesis and directly alter immune function, potentially confounding experimental results (Karp, 2012). To address this, it is recommended to house animals in thermoneutral environments (between 28 and 30 degrees Celsius) for investigations involving autonomic signaling.
Furthermore, the location of tumor implantation also warrants careful consideration. Most studies employ intraperitoneal or subcutaneous tumor implantation, and depending on the specific site of implantation (e.g., Subcutaneous flank or interscapular space), tumors may come into closer proximity with autonomic nerves and different adipose tissue depots. Consequently, the location of tumor implantation can potentially impact experimental outcomes. To gain a deeper understanding of how cancer cells influence autonomic signaling and cachexia progression within their native environment, it is important to develop better models of cancer cachexia that employ orthotopic implantation.
While norepinephrine stands as the chief neurotransmitter discharged from sympathetic nerve terminals, exerting multifarious regulatory effects within skeletal, cardiac, and adipose tissue domains, the release of adenosine triphosphate (ATP) and neuropeptide-Y (NPY) as co-transmitters adds an intriguing layer to this dynamic interaction. However, in the context of cancer cachexia, the ramifications of sympathetic co-transmitters remain overshadowed and warrant in-depth exploration. Unveiling the distinct impacts of different co-transmitters constitutes an essential avenue for advancing our comprehension of this intricate interplay.
Finally, there is a need for the refinement of techniques for achieving potent and specific sympathectomy in animal models. Presently, numerous investigations in cancer cachexia lean on the deployment of 6-OHDA to initiate the degeneration of sympathetic nerves. It is important to note, however, that 6-OHDA disrupts adrenergic and dopaminergic axons throughout the periphery. Therefore, in addition to the loss of norepinephrine signal, there is also a loss of other neurotransmitters (e. g., neuropeptide-Y) as well as potential direct trophic signals from the sympathetic neurons. Loss of adrenergic innervation to organs like the spleen or bone marrow can also have a significant impact on the immune response. Additionally, the administration of 6-OHDA is also accompanied by the generation of substantial amounts of reactive oxygen species, which can introduce variables that potentially distort experimental outcomes. Genetic models of sympathectomy have also been utilized to induce a global sympathectomy. However, considering sympathetic signaling is differentially regulated, utilizing alternative approaches such as chemogenetic or optogenetic methods to induce local sympathectomy that afford heightened selectivity and precision holds potential to considerably enhance accuracy of research in this field.
Acknowledgements
This work was supported by R01CA264133 (DLM), AACR Mark Foundation 20-60-51-Mark (DLM), R01 HL093056 & HL146833 (BAH), the Oregon Clinical and Translational Research Institute (OCTRI), grant number TL1TR002371 from the National Center for Advancing Translational Sciences (NCATS) at the National Institute of Health (PD), and by the National Heart, Lung, and Blood Institute of the National Institute of Health under Award Number F30HL170476 (PD).
Footnotes
CRediT authorship contribution statement
Conceptualization, P.D. and D.L.M; writing – original draft preparation, P.D. and DLM; writing – review and editing, P.D., T.K., A.S., B.A. H., and D.L.M.; supervision D.L.M. and B.A.H.; funding acquisition, P.D., B.A.H., and D.L.M. All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
D.L.M. is a consultant for Pfizer, Inc. and Alkermes, Inc. D.L.M. is a consultant, has received grant funding, is the chief medical officer, and has equity in Endevica Bio Inc. The other authors declare no competing interest.
Data availability
No data was used for the research described in the article.
References
- Afonso AI, Amaro-Leal A, Machado F, Rocha I, Geraldes V, 2023. Doxorubicin dose-dependent impact on physiological balance-a holistic approach in a rat model. Biology (Basel) 12 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker MS, Ebner N, Hildebrandt B, Springer J, Sinn M, Riess H, Anker SD, Landmesser U, Haverkamp W, von Haehling S, 2016. Resting heart rate is an independent predictor of death in patients with colorectal, pancreatic, and non-small cell lung cancer: results of a prospective cardiovascular long-term study. Eur. J. Heart Fail 18 (12), 1524–1534. [DOI] [PubMed] [Google Scholar]
- Anker MS, von Haehling S, Landmesser U, Coats AJS, Anker SD, 2018. Cancer and heart failure-more than meets the eye: common risk factors and co-morbidities. Eur. J. Heart Fail 20 (10), 1382–1384. [DOI] [PubMed] [Google Scholar]
- Anker MS, von Haehling S, Coats AJS, Riess H, Eucker J, Porthun J, Butler J, Karakas M, Haverkamp W, Landmesser U, Anker SD, 2021. Ventricular tachycardia, premature ventricular contractions, and mortality in unselected patients with lung, colon, or pancreatic cancer: a prospective study. Eur. J. Heart Fail 23 (1), 145–153. [DOI] [PubMed] [Google Scholar]
- Arends J, Strasser F, Gonella S, Solheim TS, Madeddu C, Ravasco P, Buonaccorso L, de van der Schueren MAE, Baldwin C, Chasen M, Ripamonti CI, E. G. C. E. A. clinicalguidelines@esmo.org, 2021. Cancer cachexia in adult patients: ESMO clinical practice guidelines(☆). ESMO Open 6 (3), 100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiles JM, Lopez-Soriano FJ, Stemmler B, Busquets S, 2023. Cancer-associated cachexia - understanding the tumour macroenvironment and microenvironment to improve management. Nat. Rev. Clin 20, 250–264. [DOI] [PubMed] [Google Scholar]
- Barkhudaryan A, Scherbakov N, Springer J, Doehner W, 2017. Cardiac muscle wasting in individuals with cancer cachexia. ESC Heart Fail 4 (4), 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett MS, Hegarty DM, Habecker BA, Aicher SA, 2022. Distinct morphology of cardiac- and brown adipose tissue-projecting neurons in the stellate ganglia of mice. Physiol. Rep 10 (10), e15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Liu Y, Shrestha YB, Ryu V, 2014. Neural innervation of white adipose tissue and the control of lipolysis. Front. Neuroendocrinol 35 (4), 473–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AS, Zellweger C, Bacanovic S, Franckenberg S, Nagel HW, Frick L, Schawkat K, Eberhard M, Bluthgen C, Volbracht J, Moos R, Wolfrum C, Burger IA, 2020. Brown fat does not cause cachexia in cancer patients: a large retrospective longitudinal FDG-PET/CT cohort study. PloS One 15 (10), e0239990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, Molinaro C, Lubahn C, Lorton D, 2008. Sympathetic modulation of immunity: relevance to disease. Cell. Immunol 252 (1–2), 27–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas AK, Acharyya S, 2020. Understanding cachexia in the context of metastatic progression. Nat. Rev. Cancer 20 (5), 274–284. [DOI] [PubMed] [Google Scholar]
- Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN, 1997. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl. Acad. Sci. U. S. A 94 (21), 11514–11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch GE, Phillips JH, Ansari A, 1968. The cachetic heart. A clinico-pathologic, electrocardiographic and roentgenographic entity. Dis. Chest 54 (5), 403–409. [DOI] [PubMed] [Google Scholar]
- Busquets S, Figueras MT, Fuster G, Almendro V, Moore-Carrasco R, Ametller E, Argiles JM, Lopez-Soriano FJ, 2004. Anticachectic effects of formoterol: a drug for potential treatment of muscle wasting. Cancer Res. 64 (18), 6725–6731. [DOI] [PubMed] [Google Scholar]
- Busquets S, Toledo M, Sirisi S, Orpi M, Serpe R, Coutinho J, Martinez R, Argiles JM, Lopez-Soriano FJ, 2011. Formoterol and cancer muscle wasting in rats: effects on muscle force and total physical activity. Exp. Ther. Med 2 (4), 731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Mao Y, Yang Q, Wen H, Lv Y, Zhang R, 2020. Are left ventricular muscle area and radiation attenuation associated with overall survival in advanced pancreatic cancer patients treated with chemotherapy? Clin. Radiol 75 (3) (238 e231–238 e239). [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J, 2004. Brown adipose tissue: function and physiological significance. Physiol. Rev 84 (1), 277–359. [DOI] [PubMed] [Google Scholar]
- Cao DX, Wu GH, Yang ZA, Zhang B, Jiang Y, Han YS, He GD, Zhuang QL, Wang YF, Huang ZL, Xi QL, 2010. Role of beta1-adrenoceptor in increased lipolysis in cancer cachexia. Cancer Sci. 101 (7), 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernackova A, Tillinger A, Bizik J, Mravec B, Horvathova L, 2023. Dynamics of cachexia-associated inflammatory changes in the brain accompanying intra-abdominal fibrosarcoma growth in Wistar rats. J. Neuroimmunol 376, 578033. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Sequeria A, Manderson C, Maddocks M, Wasley D, Wilcock A, 2012. Exploring autonomic nervous system dysfunction in patients with cancer cachexia: a pilot study. Auton. Neurosci 166 (1–2), 93–95. [DOI] [PubMed] [Google Scholar]
- Chen D, Qi Y, Zhang J, Yang Y, 2022. Deconstruction of a hypothalamic astrocyte-white adipocyte sympathetic axis that regulates lipolysis in mice. Nat. Commun 13 (1), 7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi J, Wu Z, Choi CHJ, Nguyen L, Tegegne S, Ackerman SE, Crane A, Marchildon F, Tessier-Lavigne M, Cohen P, 2018. Three-dimensional adipose tissue imaging reveals regional variation in beige fat biogenesis and PRDM16-dependent sympathetic neurite density. Cell Metab. 27 (1), 226–236 e223. [DOI] [PubMed] [Google Scholar]
- Coll AP, Chen M, Taskar P, Rimmington D, Patel S, Tadross JA, Cimino I, Yang M, Welsh P, Virtue S, Goldspink DA, Miedzybrodzka EL, Konopka AR, Esponda RR, Huang JT, Tung YCL, Rodriguez-Cuenca S, Tomaz RA, Harding HP, Melvin A, Yeo GSH, Preiss D, Vidal-Puig A, Vallier L, Nair KS, Wareham NJ, Ron D, Gribble FM, Reimann F, Sattar N, Savage DB, Allan BB, O’Rahilly S, 2020. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 578 (7795), 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosper PF, Leinwand LA, 2011. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Res. 71 (5), 1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costelli P, Garcia-Martinez C, Llovera M, Carbo N, Lopez-Soriano FJ, Agell N, Tessitore L, Baccino FM, Argiles JM, 1995. Muscle protein waste in tumor-bearing rats is effectively antagonized by a beta 2-adrenergic agonist (clenbuterol). Role of the ATP-ubiquitin-dependent proteolytic pathway. J. Clin. Invest 95 (5), 2367–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer L, Hildebrandt B, Kung T, Wichmann K, Springer J, Doehner W, Sandek A, Valentova M, Stojakovic T, Scharnagl H, Riess H, Anker SD, von Haehling S, 2014. Cardiovascular function and predictors of exercise capacity in patients with colorectal cancer. J. Am. Coll. Cardiol 64 (13), 1310–1319. [DOI] [PubMed] [Google Scholar]
- Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner M, Zimmermann R, Vesely P, Haemmerle G, Zechner R, Hoefler G, 2011. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 333 (6039), 233–238. [DOI] [PubMed] [Google Scholar]
- Devi S, Alexandre YO, Loi JK, Gillis R, Ghazanfari N, Creed SJ, Holz LE, Shackleford D, Mackay LK, Heath WR, Sloan EK, Mueller SN, 2021. Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity 54 (6), 1219–1230 e1217. [DOI] [PubMed] [Google Scholar]
- Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK, 1998. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21 (6), 1375–1385. [DOI] [PubMed] [Google Scholar]
- Eljalby M, Huang X, Becher T, Wibmer AG, Jiang CS, Vaughan R, Schoder H, Cohen P, 2023. Brown adipose tissue is not associated with cachexia or increased mortality in a retrospective study of patients with cancer. Am. J. Physiol. Endocrinol. Metab 324 (2), E144–E153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, Coskun T, Hamang MJ, Sindelar DK, Ballman KK, Foltz LA, Muppidi A, Alsina-Fernandez J, Barnard GC, Tang JX, Liu X, Mao X, Siegel R, Sloan JH, Mitchell PJ, Zhang BB, Gimeno RE, Shan B, Wu X, 2017. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med 23 (10), 1215–1219. [DOI] [PubMed] [Google Scholar]
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE, 2011. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12 (5), 489–495. [DOI] [PubMed] [Google Scholar]
- Francois M, Torres H, Huesing C, Zhang R, Saurage C, Lee N, Qualls-Creekmore E, Yu S, Morrison CD, Burk D, Berthoud HR, Munzberg H, 2019. Sympathetic innervation of the interscapular brown adipose tissue in mouse. Ann. N. Y. Acad. Sci 1454 (1), 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gams A, Nevarez A, Perkail S, Venegas A, George SA, Efimova T, Efimov IR, 2023. Evidence of sex differences in cancer-related cardiac complications in mouse models of pancreatic and liver cancer. Physiol. Rep 11 (8), e15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves DA, Silveira WA, Manfredi LH, Graca FA, Armani A, Bertaggia E, BT ON, Lautherbach N, Machado J, Nogara L, Pereira MG, Arcidiacono D, Realdon S, Kahn CR, Sandri M, Kettelhut IC, Navegantes LCC, 2019. Insulin/IGF1 signalling mediates the effects of beta(2) -adrenergic agonist on muscle proteostasis and growth. J. Cachexia. Sarcopenia Muscle 10 (2), 455–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti LA, Traynham CJ, Repas AA, Gao E, Koch WJ, Tilley DG, 2016. beta2-adrenergic receptor-dependent chemokine receptor 2 expression regulates leukocyte recruitment to the heart following acute injury. Proc. Natl. Acad. Sci. U. S. A 113 (52), 15126–15131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, 2022. Rostral ventrolateral medulla, retropontine region and autonomic regulations. Auton. Neurosci 237, 102922. [DOI] [PubMed] [Google Scholar]
- Huesing C, Qualls-Creekmore E, Lee N, Francois M, Torres H, Zhang R, Burk DH, Yu S, Morrison CD, Berthoud HR, Neuhuber W, Munzberg H, 2021. Sympathetic innervation of inguinal white adipose tissue in the mouse. J. Comp. Neurol 529 (7), 1465–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyltander A, Korner U, Lundholm KG, 1993. Evaluation of mechanisms behind elevated energy expenditure in cancer patients with solid tumours. Eur. J. Clin. Invest 23 (1), 46–52. [DOI] [PubMed] [Google Scholar]
- Hyltander A, Daneryd P, Sandstrom R, Korner U, Lundholm K, 2000. Beta-adrenoceptor activity and resting energy metabolism in weight losing cancer patients. Eur. J. Cancer 36 (3), 330–334. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ding X, Cao Y, Wang H, Zeng W, 2017. Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab. 26 (4), 686–692 e683. [DOI] [PubMed] [Google Scholar]
- Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, Bauskin AR, Wu L, Pankhurst G, Jiang L, Junankar S, Hunter M, Fairlie WD, Lee NJ, Enriquez RF, Baldock PA, Corey E, Apple FS, Murakami MM, Lin EJ, Wang C, During MJ, Sainsbury A, Herzog H, Breit SN, 2007. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat. Med 13 (11), 1333–1340. [DOI] [PubMed] [Google Scholar]
- Karp CL, 2012. Unstressing intemperate models: how cold stress undermines mouse modeling. J. Exp. Med 209 (6), 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS, 2006. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124 (2), 407–421. [DOI] [PubMed] [Google Scholar]
- Kazemi-Bajestani SMR, Becher H, Butts C, Basappa NS, Smylie M, Joy AA, Sangha R, Gallivan A, Chu Q, Baracos VE, 2019a. Undiagnosed cardiac deficits in non-small cell carcinoma patients in the candidate population for anti-cachexia clinical trials. Support. Care Cancer 27 (4), 1551–1561. [DOI] [PubMed] [Google Scholar]
- Kazemi-Bajestani SMR, Becher H, Butts C, Basappa NS, Smylie M, Joy AA, Sangha R, Gallivan A, Kavsak P, Chu Q, Baracos VE, 2019b. Rapid atrophy of cardiac left ventricular mass in patients with non-small cell carcinoma of the lung. J. Cachexia. Sarcopenia Muscle 10 (5), 1070–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MM, Lustrino D, Silveira WA, Wild F, Straka T, Issop Y, O’Connor E, Cox D, Reischl M, Marquardt T, Labeit D, Labeit S, Benoit E, Molgo J, Lochmuller H, Witzemann V, Kettelhut IC, Navegantes LC, Pozzan T, Rudolf R, 2016. Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc. Natl. Acad. Sci. U. S. A 113 (3), 746–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainscak M, Laviano A, 2016. ACT-ONE - ACTION at last on cancer cachexia by adapting a novel action beta-blocker. J. Cachexia. Sarcopenia Muscle 7 (4), 400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law ML, Metzger JM, 2021. Cardiac myocyte intrinsic contractility and calcium handling deficits underlie heart organ dysfunction in murine cancer cachexia. Sci. Rep 11 (1), 23627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Park S, Lim SM, Lee MK, Giovannucci EL, Kim JH, Kim SI, Jeon JY, 2016. Resting heart rate as a prognostic factor for mortality in patients with breast cancer. Breast Cancer Res. Treat 159 (2), 375–384. [DOI] [PubMed] [Google Scholar]
- Lena A, Wilkenshoff U, Hadzibegovic S, Porthun J, Rosnick L, Frohlich AK, Zeller T, Karakas M, Keller U, Ahn J, Bullinger L, Riess H, Rosen SD, Lyon AR, Luscher TF, Totzeck M, Rassaf T, Burkhoff D, Mehra MR, Bax JJ, Butler J, Edelmann F, Haverkamp W, Anker SD, Packer M, Coats AJS, von Haehling S, Landmesser U, Anker MS, 2023. Clinical and prognostic relevance of cardiac wasting in patients with advanced cancer. J. Am. Coll. Cardiol 81 (16), 1569–1586. [DOI] [PubMed] [Google Scholar]
- Loncar-Turukalo T, Vasic M, Tasic T, Mijatovic G, Glumac S, Bajic D, Japunzic-Zigon N, 2015. Heart rate dynamics in doxorubicin-induced cardiomyopathy. Physiol. Meas 36 (4), 727–739. [DOI] [PubMed] [Google Scholar]
- Luan HH, Wang A, Hilliard BK, Carvalho F, Rosen CE, Ahasic AM, Herzog EL, Kang I, Pisani MA, Yu S, Zhang C, Ring AM, Young LH, Medzhitov R, 2019. GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell 178 (5), 1231–1244 e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GS, Ryall JG, 2008. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol. Rev 88 (2), 729–767. [DOI] [PubMed] [Google Scholar]
- Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS, 2013. Autonomic nerve development contributes to prostate cancer progression. Science 341 (6142), 1236361. [DOI] [PubMed] [Google Scholar]
- Meng X, Qian X, Ding X, Wang W, Yin X, Zhuang G, Zeng W, 2022. Eosinophils regulate intra-adipose axonal plasticity. Proc. Natl. Acad. Sci. U. S. A 119 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza-Valderrama D, Marco E, Davalos-Yerovi V, Muns MD, Tejero-Sanchez M, Duarte E, Sanchez-Rodriguez D, 2021. Sarcopenia, malnutrition, and cachexia: adapting definitions and terminology of nutritional disorders in older people with cancer. Nutrients 13 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis KA, Zhu X, Burfeind KG, Krasnow SM, Levasseur PR, Morgan TK, Marks DL, 2017. Establishment and characterization of a novel murine model of pancreatic cancer cachexia. J. Cachexia. Sarcopenia Muscle 8 (5), 824–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Tamta AK, Sarikhani M, Desingu PA, Kizkekra SM, Pandit AS, Kumar S, Khan D, Raghavan SC, Sundaresan NR, 2018. Subcutaneous Ehrlich ascites carcinoma mice model for studying cancer-induced cardiomyopathy. Sci. Rep 8 (1), 5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadpour H, MacDonald CR, Qiao G, Chen M, Dong B, Hylander BL, McCarthy PL, Abrams SI, Repasky EA, 2019. beta2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J. Clin. Invest 129 (12), 5537–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KT, 2016. The pathogenesis and treatment of cardiac atrophy in cancer cachexia. Am. J. Physiol. Heart Circ. Physiol 310 (4), H466–H477. [DOI] [PubMed] [Google Scholar]
- Nishikawa H, Goto M, Fukunishi S, Asai A, Nishiguchi S, Higuchi K, 2021. Cancer cachexia: its mechanism and clinical significance. Int. J. Mol. Sci 22 (16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondicova K, Mravec B, 2010. Role of nervous system in cancer aetiopathogenesis. Lancet Oncol. 11 (6), 596–601. [DOI] [PubMed] [Google Scholar]
- Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, Zechner R, Wagner EF, 2014. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20 (3), 433–447. [DOI] [PubMed] [Google Scholar]
- Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sanchez NM, Mahu I, Mendes R, Gres V, Kubasova N, Morris I, Arus BA, Larabee CM, Vasques M, Tortosa F, Sousa AL, Anandan S, Tranfield E, Hahn MK, Iannacone M, Spann NJ, Glass CK, Domingos AI, 2017. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med 23 (11), 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poetsch MS, Palus S, Van Linthout S, von Haehling S, Doehner W, Coats AJS, Anker SD, Springer J, 2023. The small molecule ACM-001 improves cardiac function in a rat model of severe cancer cachexia. Eur. J. Heart Fail 25 (5), 673–686. [DOI] [PubMed] [Google Scholar]
- Poisson J, Martinez-Tapia C, Heitz D, Geiss R, Albrand G, Falandry C, Gisselbrecht M, Couderc AL, Boulahssass R, Liuu E, Boudou-Rouquette P, Chah Wakilian A, Gaxatte C, Pamoukdjian F, de Decker L, Antoine V, Cattenoz C, Solem-Laviec H, Guillem O, Medjenah H, Natella PA, Canoui-Poitrine F, Laurent M, Paillaud E, 2021. Prevalence and prognostic impact of cachexia among older patients with cancer: a nationwide cross-sectional survey (NutriAgeCancer). J. Cachexia. Sarcopenia Muscle 12 (6), 1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potsch MS, Ishida J, Palus S, Tschirner A, von Haehling S, Doehner W, Anker SD, Springer J, 2020. MT-102 prevents tissue wasting and improves survival in a rat model of severe cancer cachexia. J. Cachexia. Sarcopenia Muscle 11 (2), 594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A, 2014. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157 (6), 1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, Maurer HC, Chen X, Jiang Z, Westphalen CB, Ilmer M, Valenti G, Mohanta SK, Habenicht AJR, Middelhoff M, Chu T, Nagar K, Tailor Y, Casadei R, Di Marco M, Kleespies A, Friedman RA, Remotti H, Reichert M, Worthley DL, Neumann J, Werner J, Iuga AC, Olive KP, Wang TC, 2018. beta2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 33 (1), 75–90 e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochette L, Zeller M, Cottin Y, Vergely C, 2020. Insights into mechanisms of GDF15 and receptor GFRAL: therapeutic targets. Trends Endocrinol. Metab 31 (12), 939–951. [DOI] [PubMed] [Google Scholar]
- Rodrigues ACZ, Messi ML, Wang ZM, Abba MC, Pereyra A, Birbrair A, Zhang T, O’Meara M, Kwan P, Lopez EIS, Willis MS, Mintz A, Files DC, Furdui C, Oppenheim RW, Delbono O, 2019. The sympathetic nervous system regulates skeletal muscle motor innervation and acetylcholine receptor stability. Acta Physiol (Oxf.) 225 (3), e13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeland EJ, Bohlke K, Baracos VE, Bruera E, Del Fabbro E, Dixon S, Fallon M, Herrstedt J, Lau H, Platek M, Rugo HS, Schnipper HH, Smith TJ, Tan W, Loprinzi CL, 2020. Management of cancer cachexia: ASCO guideline. J. Clin. Oncol 38 (21), 2438–2453. [DOI] [PubMed] [Google Scholar]
- Rohm M, Schafer M, Laurent V, Ustunel BE, Niopek K, Algire C, Hautzinger O, Sijmonsma TP, Zota A, Medrikova D, Pellegata NS, Ryden M, Kulyte A, Dahlman I, Arner P, Petrovic N, Cannon B, Amri EZ, Kemp BE, Steinberg GR, Janovska P, Kopecky J, Wolfrum C, Bluher M, Berriel Diaz M, Herzig S, 2016. An AMP-activated protein kinase-stabilizing peptide ameliorates adipose tissue wasting in cancer cachexia in mice. Nat. Med 22 (10), 1120–1130. [DOI] [PubMed] [Google Scholar]
- Salazar-Degracia A, Busquets S, Argiles JM, Bargallo-Gispert N, Lopez-Soriano FJ, Barreiro E, 2018. Effects of the beta(2) agonist formoterol on atrophy signaling, autophagy, and muscle phenotype in respiratory and limb muscles of rats with cancer-induced cachexia. Biochimie 149, 79–91. [DOI] [PubMed] [Google Scholar]
- Sato S, Shirato K, Tachiyashiki K, Imaizumi K, 2011. Muscle plasticity and beta(2)-adrenergic receptors: adaptive responses of beta(2)-adrenergic receptor expression to muscle hypertrophy and atrophy. J. Biomed. Biotechnol 2011, 729598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK, Cole SW, 2010. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 70 (18), 7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, Kaschina E, Palus S, Potsch M, von Websky K, Hocher B, Latouche C, Jaisser F, Morawietz L, Coats AJ, Beadle J, Argiles JM, Thum T, Foldes G, Doehner W, Hilfiker-Kleiner D, Force T, Anker SD, 2014. Prevention of liver cancer cachexia-induced cardiac wasting and heart failure. Eur. Heart J 35 (14), 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer J, Jove Q, de Lima Junior EA, Ladron NA, Lopez-Soriano FJ, Busquets S, Argiles JM, Marks DL, 2023. Effects of S-pindolol in mouse pancreatic and lung cancer cachexia models. J. Cachexia. Sarcopenia Muscle 14 (3), 1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart Coats AJ, Ho GF, Prabhash K, von Haehling S, Tilson J, Brown R, Beadle J, Anker SD, for and A. C. T. O. N. E. s. g. on behalf of the, 2016. Espindolol for the treatment and prevention of cachexia in patients with stage III/IV non-small cell lung cancer or colorectal cancer: a randomized, double-blind, placebo-controlled, international multicentre phase II study (the ACT-ONE trial). J. Cachexia. Sarcopenia Muscle 7 (3), 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka T, Vita V, Prokshi K, Horner SJ, Khan MM, Pirazzini M, Williams MPI, Hafner M, Zaglia T, Rudolf R, 2018. Postnatal development and distribution of sympathetic innervation in mouse skeletal muscle. Int. J. Mol. Sci 19 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriben R, Chen M, Higbee J, Oeffinger J, Ventura R, Li B, Mondal K, Gao Z, Ayupova D, Taskar P, Li D, Starck SR, Chen HH, McEntee M, Katewa SD, Phung V, Wang M, Kekatpure A, Lakshminarasimhan D, White A, Olland A, Haldankar R, Solloway MJ, Hsu JY, Wang Y, Tang J, Lindhout DA, Allan BB, 2020. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat. Med 26 (8), 1264–1270. [DOI] [PubMed] [Google Scholar]
- Tian M, Nishijima Y, Asp ML, Stout MB, Reiser PJ, Belury MA, 2010. Cardiac alterations in cancer-induced cachexia in mice. Int. J. Oncol 37 (2), 347–353. [DOI] [PubMed] [Google Scholar]
- Tian M, Asp ML, Nishijima Y, Belury MA, 2011. Evidence for cardiac atrophic remodeling in cancer-induced cachexia in mice. Int. J. Oncol 39 (5), 1321–1326. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ, 2002. Cachexia in cancer patients. Nat. Rev. Cancer 2 (11), 862–871. [DOI] [PubMed] [Google Scholar]
- Toledo M, Busquets S, Penna F, Zhou X, Marmonti E, Betancourt A, Massa D, Lopez-Soriano FJ, Han HQ, Argiles JM, 2016. Complete reversal of muscle wasting in experimental cancer cachexia: additive effects of activin type II receptor inhibition and beta-2 agonist. Int. J. Cancer 138 (8), 2021–2029. [DOI] [PubMed] [Google Scholar]
- Tsai VWW, Husaini Y, Sainsbury A, Brown DA, Breit SN, 2018. The MIC-1/GDF15-GFRAL pathway in energy homeostasis: implications for obesity, cachexia, and other associated diseases. Cell Metab. 28 (3), 353–368. [DOI] [PubMed] [Google Scholar]
- Uurasmaa TM, Streng T, Alkio M, Karikoski M, Heinonen I, Anttila K, 2022. Subcutaneous B16 melanoma impairs intrinsic pressure generation and relaxation of the heart, which are not restored by short-term voluntary exercise in mice. Am. J. Physiol. Heart Circ. Physiol 322, H1044–H1056. [DOI] [PubMed] [Google Scholar]
- Vasic M, Loncar-Turukalo T, Tasic T, Matic M, Glumac S, Bajic D, Popovic B, Japundzic-Zigon N, 2019. Cardiovascular variability and beta-ARs gene expression at two stages of doxorubicin - induced cardiomyopathy. Toxicol. Appl. Pharmacol 362, 43–51. [DOI] [PubMed] [Google Scholar]
- Wakabayashi H, Arai H, Inui A, 2021. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: facts and numbers. J. Cachexia. Sarcopenia Muscle 12 (1), 14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Townsend LK, DesOrmeaux GJ, Frangos SM, Batchuluun B, Dumont L, Kuhre RE, Ahmadi E, Hu S, Rebalka IA, Gautam J, Jabile MJT, Pileggi CA, Rehal S, Desjardins EM, Tsakiridis EE, Lally JSV, Juracic ES, Tupling AR, Gerstein HC, Pare G, Tsakiridis T, Harper ME, Hawke TJ, Speakman JR, Blondin DP, Holloway GP, Jorgensen SB, Steinberg GR, 2023. GDF15 promotes weight loss by enhancing energy expenditure in muscle. Nature 619 (7968), 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs MP, Beaudry AG, Law ML, 2022. Cardiac remodeling in cancer-induced cachexia: functional, structural, and metabolic contributors. Cells 11 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Heier C, Meng X, Bakiri L, Pototschnig I, Tang Z, Schauer S, Baumgartner VJ, Grabner GF, Schabbauer G, Wolinski H, Robertson GR, Hoefler G, Zeng W, Wagner EF, Schweiger M, Zechner R, 2022. An immune-sympathetic neuron communication axis guides adipose tissue browning in cancer-associated cachexia. Proc. Natl. Acad. Sci. U. S. A 119 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Springer J, Palus S, Busquets S, Jove Q, Alves de Lima Junior E, Anker MS, von Haehling S, Alvarez Ladron N, Millman O, Oosterlee A, Szymczyk A, Lopez-Soriano FJ, Anker SD, Coats AJS, Argiles JM, 2022. The atypical beta-blocker S-oxprenolol reduces cachexia and improves survival in a rat cancer cachexia model. J. Cachexia. Sarcopenia Muscle 14 (1), 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaglia T, Milan G, Franzoso M, Bertaggia E, Pianca N, Piasentini E, Voltarelli VA, Chiavegato D, Brum PC, Glass DJ, Schiaffino S, Sandri M, Mongillo M, 2013. Cardiac sympathetic neurons provide trophic signal to the heart via beta2-adrenoceptor-dependent regulation of proteolysis. Cardiovasc. Res 97 (2), 240–250. [DOI] [PubMed] [Google Scholar]
- Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, Lu YH, Kozlova A, Voss H, Martins GG, Friedman JM, Domingos AI, 2015. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163 (1), 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li Y, Lv X, Zhao L, Wang X, 2021. VLM catecholaminergic neurons control tumor growth by regulating CD8(+) T cells. Proc. Natl. Acad. Sci. U. S. A 118 (28). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Ma Z, Zhang L, Zhou S, Wang J, Wang B, Fu W, 2016. Heart rate variability in the prediction of survival in patients with cancer: a systematic review and meta-analysis. J. Psychosom. Res 89, 20–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


