ABSTRACT
Background
The Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial enrolled patients with estimated glomerular filtration rate 25–75 mL/min/1.73 m2 and urine albumin-to-creatinine ratio >200 mg/g. The Dapagliflozin Effect on CardiovascuLAR Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial enrolled patients with type 2 diabetes, a higher range of kidney function and no albuminuria criterion. The study objective was to estimate the cost-effectiveness of dapagliflozin in a broad chronic kidney disease population based on these two trials in the UK, Spain, Italy and Japan.
Methods
We adapted a published Markov model based on the DAPA-CKD trial but to a broader population, irrespective of urine albumin-to-creatinine ratio, using patient-level data from the DAPA-CKD and DECLARE-TIMI 58 trials. We sourced cost and utility inputs from literature and the DAPA-CKD trial. The analysis considered healthcare system perspectives over a lifetime horizon.
Results
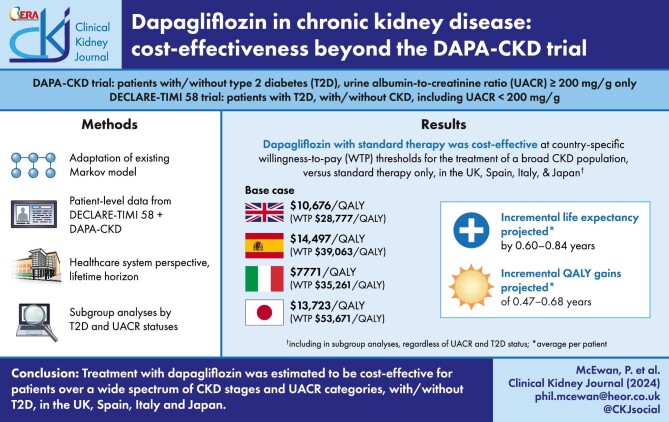
Treatment with dapagliflozin was predicted to attenuate disease progression and extend projected life expectancy by 0.64 years (12.5 versus 11.9 years, undiscounted) in the UK, with similar estimates in other settings. Clinical benefits translated to mean quality-adjusted life year (QALY; discounted) gains between 0.45 and 0.68 years across countries. Incremental cost-effectiveness ratios in the UK, Spain, Italy and Japan ($10 676/QALY, $14 479/QALY, $7771/QALY and $13 723/QALY, respectively) were cost-effective at country-specific willingness-to-pay thresholds. Subgroup analyses suggest dapagliflozin is cost-effective irrespective of urinary albumin-to-creatine ratio and type 2 diabetes status.
Conclusion
Treatment with dapagliflozin may be cost-effective for patients across a wider spectrum of estimated glomerular filtration rates and albuminuria than previously demonstrated, with or without type 2 diabetes, in the UK, Spanish, Italian and Japanese healthcare systems.
Keywords: albuminuria, chronic kidney disease, cost-effectiveness, dapagliflozin, SGLT2 inhibitor
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
Chronic kidney disease (CKD) is associated with considerable burden to healthcare systems worldwide, particularly upon progression to kidney failure.
The efficacy and safety of dapagliflozin in addition to standard therapy in patients with CKD and at least moderate degrees of albuminuria have been demonstrated in the DAPA-CKD trial, irrespective of type 2 diabetes status; the DECLARE-TIMI 58 trial demonstrated efficacy in patients with type 2 diabetes.
This study adds:
By adapting a previously published model, this study assessed the cost-effectiveness of dapagliflozin in a broader CKD population using a subset of data from the DECLARE-TIMI 58 trial comprising patients who had CKD at baseline.
Treatment with dapagliflozin was cost-effective, at country-specific willingness-to-pay thresholds; subgroup analyses suggested that dapagliflozin is expected to be cost-effective at lower levels of urinary albumin-to-creatine ratio and among patients with and without type 2 diabetes status.
Potential impact:
This analysis addresses a data gap that currently exists in patients with CKD, suggesting that there may be value in treating patients with dapagliflozin across a wider spectrum of estimated glomerular filtration rates and albuminuria than previously demonstrated, with or without type 2 diabetes.
INTRODUCTION
The prevalence of chronic kidney disease (CKD) is rising and is estimated to be 8%–16% worldwide [1], affecting an estimated 840 million people [2, 3]. CKD is usually a progressive condition, typically described by the level of estimated glomerular filtration rate (eGFR) and the presence and degree of albuminuria. Patients with reduced eGFR and/or albuminuria typically incur higher rates of hospitalization, outpatient visits, ambulance use and emergency room visits relative to patients with milder disease [4]. Accordingly, CKD has a detrimental effect on health-related quality of life (HRQoL) and yields a substantial burden to healthcare systems and society [5].
In addition to lifestyle changes, standard-of-care pharmacologic treatment options for CKD include angiotensin-converting enzyme inhibitors or angiotensin receptor blockers which aim to delay disease progression and prevent or ameliorate associated complications, yet many patients continue to progress toward advanced CKD.
Sodium-glucose co-transporter-2 (SGLT2) inhibitors are an established therapeutic option in patients with T2D. Trials designed to ensure the cardiovascular safety of SGLT2 inhibitors also demonstrated substantial benefits vis-à-vis cardiovascular outcomes distinct from their glucose lowering action. These and other studies showed protective effects on kidney function, including the potential to reduce the rate of eGFR decline and the risk of end-stage kidney disease (ESKD) in patients with type 2 diabetes (T2D) [6–12].
The efficacy and safety of dapagliflozin in addition to standard therapy in patients with CKD and albuminuria [eGFR 25–75 mL/min/1.73 m2; urinary albumin-to-creatine ratio (UACR) ≥200 mg/g] was investigated in the Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial [13]. The trial was ended prematurely due to overwhelming efficacy in patients with or without T2D. Subsequently, a cost-effectiveness analysis based on the DAPA-CKD trial demonstrated cost-effectiveness in multiple national healthcare systems [14]. While patients with CKD with lower levels of albuminuria or without albuminuria are generally at lower risk of adverse clinical outcomes, they may also gain benefit from treatment with an SGLT2 inhibitor [15, 16]. However, the potential value of treatment in these patients has not been studied.
The DAPA-CKD-like population typically have a higher per patient burden than the broad CKD population described in this analysis [17]. However, patients with lower UACR are more prevalent and therefore exert a higher burden at a population level on national healthcare systems [18]. Therefore, the largest benefit to patients and national healthcare systems could be attained by addressing the residual risk in patients outside of the DAPA-CKD population.
By adapting a previously published model driven by patient-level data from the DAPA-CKD trial, the objective of the present study is to assess the cost-effectiveness of dapagliflozin within the broader CKD population. Based on data from both the DAPA-CKD and Dapagliflozin Effect on CardiovascuLAR Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trials, the model assesses dapagliflozin from national healthcare perspectives in the UK, Spain, Italy and Japan.
MATERIALS AND METHODS
Source data
A broad data set was derived by combining individual patient-level data from two placebo-controlled randomized controlled trials of dapagliflozin plus standard therapy. The DAPA-CKD trial included patients with eGFR 25–75 mL/min/1.73 m2 and UACR 200–5000 mg/g, with or without T2D. The DECLARE-TIMI 58 study included patients with T2D and estimated creatinine clearance ≥60 mL/min/1.73 m2 and no inclusion criteria based on UACR. Study designs, patient characteristics and outcomes have been published [8, 13, 19–23].
The broad CKD population
We pooled individual patient data on patients with CKD from the two trials, including only the DECLARE-TIMI 58 subset of patients with eGFR <60 mL/min/1.73 m2 calculated by the CKD Epidemiology Collaboration equation [24, 25] and/or with albuminuria at baseline (>30 mg/g), referred to hereafter as the DECLARECKD population. Baseline patient characteristics of the pooled CKD population are provided in Table 1.
Table 1:
Summary patient characteristics of pooled CKD populations from the DAPA-CKD and DECLARE TIMI 58 trials.
| Characteristic | Pooled CKD (N = 10 273) |
|---|---|
| Demographic characteristics | |
| Age, years | 63.3 (9.6) |
| Female | 3365 (32.8) |
| Race | |
| White | 6914 (67.3) |
| Asian | 2367 (23.0) |
| Black | 411 (4.0) |
| Other | 581 (5.7) |
| Baseline body mass index, kg/m² | 31.3 (6.3) |
| Current smoker | 1482 (14.4) |
| Baseline systolic blood pressure, mmHg | 137.3 (16.7) |
| Baseline diastolic blood pressure, mmHg | 77.8 (9.8) |
| Haemoglobin, g/dL | 13.4 (1.7) |
| Baseline serum potassium, mEq/L | 4.5 (0.5) |
| Disease characteristics | |
| Baseline eGFR (mL/min per 1.73 m2) | |
| Mean (SD) | 64.4 (24.7) |
| G1 (≥90) | 2335 (22.7) |
| G2 (60–89) | 2854 (27.8) |
| G3a (45–59) | 2378 (23.1) |
| G3b (30–44) | 2062 (20.1) |
| G4 (15–29) | 640 (6.2) |
| G5 (<15) | 4 (0.0) |
| Baseline UACR (mg/g) | |
| Median (IQR) | 284 (62–932) |
| A1 (<30) | 772 (7.5) |
| A2 (30–300) | 4473 (43.5) |
| A3 (>300) | 5028 (48.9) |
| Medical history | |
| Baseline T2D | 8875 (86.4) |
| History of cardiovascular disease | 3569 (34.7) |
| History of heart failure | 1203 (11.7) |
| History of stroke | 767 (7.5) |
| History of myocardial infarction | 1760 (17.1) |
| ACE inhibitor or ARB | 9170 (89.3) |
Unless otherwise indicated, values for continuous variables are mean (SD); values for categorical variables represent counts (%).
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; IQR, interquartile range; SD, standard deviation
Patients with lower levels of albuminuria (UACR <200 mg/g) were taken primarily from the DECLARECKD data set with T2D patients only. We therefore estimated event rates and the dapagliflozin treatment effects for participants without T2D with a UACR <200 mg/g using a Poisson distribution because neither trial enrolled patients with low UACR but without T2D [26]. To do this, we applied adjustment factors for UACR and T2D status to the DECLARECKD data to estimate rates for patients without T2D with lower levels of albuminuria, relative to the treatment effect observed in patients with or without T2D in the DAPA-CKD trial (Supplementary data, Tables S1 and S2). The prevalence of T2D in the broad CKD population was assumed equivalent to the DAPA-CKD trial.
Economic model
This analysis adapts a published Markov model for the DAPA-CKD trial population [27], which modelled disease progression by discrete eGFR-defined health states and health states for patients on kidney replacement therapy (KRT).
The primary outcome was the incremental cost-effectiveness ratio (ICER), expressed as the difference in costs per quality-adjusted life year (QALY) gained. Willingness-to-pay (WTP) thresholds specified for the presented analysis were $28 777/QALY, $39 063/QALY, $35 261/QALY and $53 671/QALY (£20 000/QALY, €30 000/QALY, €25 000/QALY and ¥5 million/QALY) for the UK, Spain, Italy and Japan, respectively.
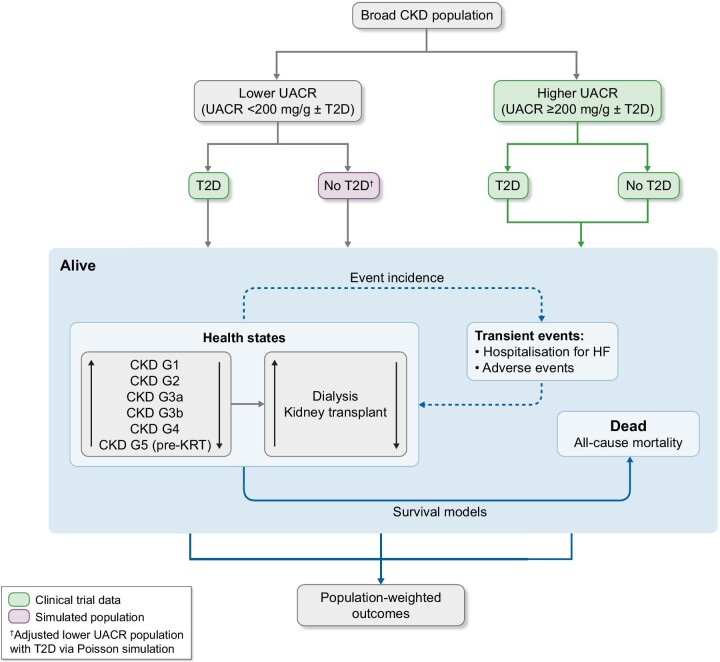
The adapted model estimated outcomes separately according to T2D status since the extrapolation estimates effects for patients without T2D and lower levels of albuminuria. The model outcomes are a weighted average according to a defined proportion of lower versus higher UACR (Fig. 1). The model assumes that 75% of the broad CKD population consists of patients with lower UACR (<200 mg/g) and 25% with higher UACR (≥200 mg/g), as observed in the DECLARECKD population, which had no albuminuria inclusion criterion.
Figure 1:
Model schematic for estimation of outcomes in the broad CKD population. HF: heart failure; UACR: urine albumin to creatinine ratio.
The model assumes a constant rate of discontinuation, which was applied to all patients receiving treatment with dapagliflozin in each modelled cycle. Upon discontinuing treatment with dapagliflozin, patients were assumed to be treated with standard therapy only and subject to the risk of outcomes associated with that treatment arm.
Disease progression
For patients with higher UACR, treatment dependent transition matrices were derived from the DAPA-CKD trial for the first 4 months of follow-up and from Month 4 thereafter, so as to replicate observed patterns of eGFR trajectory (see Supplementary data, Tables S3 and S4) [13]. For patients with lower UACR, transition matrices were derived by similarly using DECLARECKD data for CKD G1–G3 and DAPA-CKD for CKD G4 and G5, consistent with risk stratification according to guidelines (see Supplementary data, Tables S5 and S6) [28]. The model derives transitions for post-KRT outcomes from a systematic literature review of CKD modelling [29], as these events were rare in the DECLARE-TIMI 58 and DAPA-CKD trials.
To avoid double counting of mortality, we derived transition probabilities excluding the transition to death. We calculated the role of altered kidney function on the risk of death by parametric survival modelling, described below.
Mortality and event incidence
We extrapolated the incidence of all-cause mortality using adjusted parametric survival equations, in accordance with established guidelines [30]. Health state defined by eGFR were time-updated to estimate the dynamic mortality risk associated with progression. Of the distributions evaluated, we considered the Weibull distribution to yield the most consistent fits by albuminuria stratification to the pooled trial data (Supplementary data, Fig. S1).
We used adjusted generalized estimating equations to capture the incidence of hospitalization for heart failure (HHF), including first and recurrent events. As with mortality, time-updated eGFR captured the effect of disease evolution on event risk. We conducted all analyses from an intention-to-treat perspective and validated against observed data (Supplementary data, Fig. S2).
Patients could experience treatment-related adverse events of interest at a constant rate, incurring management costs and utility decrements in the incident cycle only. Considered adverse events were volume depletion, major hypoglycaemic events, fractures, diabetic ketoacidosis and amputation.
Resource use and costs
The analysis considered only direct costs from healthcare payer perspectives. We discounted costs at an annual rate of 3.5% in the UK, 3% in Spain and Italy and 2% in Japan, according to local guidelines [30–33]. Cost inputs specific to each country are presented in 2022 US dollars and native currencies in Supplementary data, Tables S7 and S8, respectively.
Health-related quality of life
As EuroQol-5 Dimension 5 Levels (EQ-5D-5L) data were not recorded in the DECLARE-TIMI 58 trial, utility estimates were solely derived from patient-level EQ-5D-5L data from the DAPA-CKD trial (Table 2). Notably, utility values for each setting were derived using country-specific life tables and tariffs; further details are available in published material [14]. The model assumes that patients with lower UACR are adequately represented by HRQoL outcomes in patients with higher albuminuria; in effect, that patient utility is driven only by eGFR-defined stage and the incidence of complications, in line with other published CKD models [29]. We also discounted benefits annually at a rate of 3.5% in the UK, 3% in Spain and Italy, and 2% in Japan [30–33].
Table 2:
Health state utility values and event-related disutility modifiers for the UK, Japan, Spain and Italy.
| Utility or utility decrement | |||||
|---|---|---|---|---|---|
| Parameter | UK | Italy | Spain | Japan | Source |
| Health-state utility | |||||
| CKD G1 | 0.77 (0.01) | 0.85 (0.01) | 0.83 (0.01) | 0.80 (0.01) | DAPA-CKD [34] |
| CKD G2 | 0.77 (0.01) | 0.85 (0.01) | 0.83 (0.01) | 0.80 (0.01) | DAPA-CKD [34] |
| CKD G3a | 0.77 (0.01) | 0.86 (0.01) | 0.83 (0.01) | 0.80 (0.01) | DAPA-CKD [34] |
| CKD G3b | 0.77 (0.01) | 0.86 (0.01) | 0.84 (0.01) | 0.80 (0.01) | DAPA-CKD [34] |
| CKD G4 | 0.76 (0.01) | 0.85 (0.01) | 0.84 (0.01) | 0.79 (0.01) | DAPA-CKD [34] |
| CKD G5, pre-KRT | 0.73 (0.01) | 0.73 (0.01) | 0.83 (0.01) | 0.76 (0.01) | DAPA-CKD [34] |
| Dialysis | 0.68 (0.01) | 0.78 (0.01) | 0.79 (0.01) | 0.73 (0.01) | DAPA-CKD [34] |
| Transplant | 0.71 (0.07) | 0.71 (0.07) | 0.77 (0.01) | 0.74 (0.07) | DAPA-CKD [34] |
| Event-related disutility modifier | |||||
| HHF | –0.09 (0.04) | –0.08 (0.04) | –0.07 (0.03) | –0.06 (0.03) | DAPA-CKD [34] |
| Volume depletion | –0.05 (0.01) | –0.05 (0.01) | –0.05 (0.01) | –0.05 (0.01) | McEwan et al. [35] |
| Major hypoglycaemic events | –0.01 (0.00) | –0.01 (0.00) | –0.01 (0.00) | –0.01 (0.00) | Beaudet et al. [36]; Currie et al. [37] |
| Diabetic ketoacidosis | –0.01 (0.01) | –0.01 (0.01) | –0.01 (0.01) | –0.01 (0.01) | Peasgood et al. [38] |
| Fracture | –0.09 (0.03) | –0.05 (0.03) | –0.07 (0.03) | –0.05 (0.02) | DAPA-CKD [34] |
| Amputation | –0.26 (0.05) | –0.32 (0.05) | –0.26 (0.05) | –0.18 (0.04) | DAPA-CKD [34] |
Subgroup analyses
Key subgroups were established for the analysis to assess model outcomes stratified by T2D status (with and without) and level of albuminuria (lower: <200 mg/g; higher: ≥200 mg/g) to yield four subgroups for evaluation.
Scenario analyses
The DAPA-CKD and DECLARE-TIMI 58 trials had median follow-up periods of 2.4 years and 4.2 years, respectively. Given that our model predicts outcomes over a lifetime horizon, we considered varying time horizons to characterize the evolution of the cost-effectiveness of dapagliflozin.
Sensitivity analyses
We conducted one-way sensitivity analyses to demonstrate the influence of varying key parameter values on modelled outcomes. We conducted probabilistic sensitivity analysis to assess the influence of uncertainty across all model parameters on health economic outcomes. For each analysis, 1000 replicates were generated in which the parameters sampled included patient baseline demographic characteristics, direct costs, utilities, adverse event risks and treatment efficacy.
Probabilistic sensitivity analyses were also conducted separately for the assessed subgroups for each of the four countries. Specific to the extrapolative analysis in the population with low UACR without T2D, the parameters estimating HHF and mortality effects were also sampled. Results were evaluated on the cost-effectiveness plane and plotted with 95% credibility ellipses.
RESULTS
Base case analysis
In the broad CKD population, dapagliflozin is predicted to slow the rate of CKD progression and lower the incidence of HHF. Patients treated with dapagliflozin were likely to spend 1.08 more years in the earlier stages of CKD (stages G1 to G4) versus those treated with standard therapy alone (dapagliflozin 14.9 years; standard therapy 13.8 years; Table 3). Patients treated with dapagliflozin had a lower rate of HHF (119 versus 140 per 1000 patient-years).
Table 3:
Base case clinical outcomes for the broad CKD population.
| Outcome | Dapagliflozin plus standard therapy | Standard therapy | Incremental |
|---|---|---|---|
| Mean time in each CKD stage, years | |||
| CKD G1 | 2.96 | 2.62 | 0.34 |
| CKD G2 | 6.16 | 5.77 | 0.39 |
| CKD G3 | 3.36 | 3.18 | 0.18 |
| CKD G4 | 2.40 | 2.23 | 0.17 |
| CKD G5 (pre-KRT) | 1.28 | 1.30 | –0.02 |
| Dialysis | 0.18 | 0.19 | –0.01 |
| Transplant | 0.71 | 0.75 | –0.03 |
| Event incidence, per 1000 patients | |||
| HHF | 119 | 140 | –21 |
Note that results are presented for the UK setting, there will be some variation in outcomes owing to the application of country-specific life tables.
These clinical benefits lead to extended life expectancy versus patients treated with standard therapy alone (Table 4), with mean predicted increase in life expectancy by 0.60 years in those treated with dapagliflozin (12.5 years) versus those treated with standard therapy alone (11.9 years) in the UK setting. Other settings demonstrated similar improvements in patient life expectancy (Spain 0.64; Italy 0.66 years; Japan 0.84).
Table 4:
Base case health economic outcomes for the broad CKD population in the UK, Japan, Spain and Italy.
| Outcome | Dapagliflozin plus standard therapy | Standard therapy | Incremental |
|---|---|---|---|
| UK | |||
| Total costs | $63 243 | $58 232 | $5011 |
| Drug acquisition | $6857 | $854 | $6002 |
| CKD management (pre-KRT) | $28 690 | $27 900 | $790 |
| KRT | $23 666 | $25 362 | –$1696 |
| HHF | $642 | $784 | –$142 |
| Adverse events | $3388 | $3331 | $57 |
| Total LYs gained | 12.51 | 11.91 | 0.60 |
| Total QALYs gained | 9.56 | 9.09 | 0.47 |
| ICER (per LY gained) | $8293/LY | ||
| ICER (per QALY gained) | $10 676/QALY | ||
| Incremental NMB | $8496 | ||
| Spain | |||
| Total costs | $156 466 | $148 659 | $7807 |
| Drug acquisition | $5679 | $839 | $4840 |
| CKD management (pre-KRT) | $105 376 | $100 171 | $5205 |
| KRT | $39 782 | $42 546 | –$2764 |
| HHF | $475 | $578 | $103 |
| Adverse events | $5154 | $4524 | $629 |
| Total LYs gained | 13.00 | 12.36 | 0.64 |
| Total QALYs gained | 10.75 | 10.21 | 0.54 |
| ICER (per LY gained) | $12 124/LY | ||
| ICER (per QALY gained) | $14 479/QALY | ||
| Incremental NMB | $13 255 | ||
| Italy | |||
| Total costs | $67 999 | $63 593 | $4406 |
| Drug acquisition | $6177 | $1297 | $4880 |
| CKD management (pre-KRT) | $30 502 | $29 175 | $1327 |
| KRT | $27 202 | $29 014 | –$1812 |
| HHF | $567 | $688 | –$122 |
| Adverse events | $3252 | $3180 | $72 |
| Total LYs gained | 13.09 | 12.43 | 0.66 |
| Total QALYs gained | 11.16 | 10.60 | 0.57 |
| ICER (per LY gained) | $6701/LY | ||
| ICER (per QALY gained) | $7771/QALY | ||
| Incremental NMB | $15 587 | ||
| Japan | |||
| Total costs | $88 386 | $79 115 | $9271 |
| Drug acquisition | $9749 | $730 | $9019 |
| CKD management (pre-KRT) | $42 230 | $40 486 | $1744 |
| KRT | $31 310 | $33 037 | –$1728 |
| HHF | $1906 | $2314 | –$408 |
| Adverse events | $3957 | $3501 | $456 |
| Total LYs gained | 14.75 | 13.91 | 0.84 |
| Total QALYs gained | 11.70 | 11.03 | 0.68 |
| ICER (per LY gained) | $10 998/LY | ||
| ICER (per QALY gained) | $13 723/QALY | ||
| Incremental NMB | $26 989 | ||
LYs, life years; NMB, net monetary benefit.
Treatment with dapagliflozin in the broad CKD population led to mean lifetime QALY gains of 0.47, 0.54, 0.57 and 0.68 in the UK, Spain, Italy and Japan, respectively (Table 4). Differences between setting are borne from country specific utility tariffs, life tables and discounting rates.
Treatment with dapagliflozin led to increased overall costs versus placebo in the UK ($5011), Spain ($7807), Italy ($4406) and Japan ($9271). The main contributors of additional cost in those treated with dapagliflozin were additional drug acquisition costs and disease management costs resulting from extended life expectancy. These costs were partially offset by reductions in costs associated with KRT through delayed time to dialysis or transplant and a reduced rate of HHF over the lifetime model horizon.
Dapagliflozin was considered cost-effective with ICERs of $10 676/QALY, $14 479/QALY, $7771/QALY and $13 723/QALY in the UK, Spain, Italy and Japan, well below WTP thresholds ($28 777/QALY, $39 063/QALY, $35 261/QALY, $53 671/QALY, respectively) and estimated net monetary benefit of $8496, $13 255, $15 587 and $26 989 respectively. Health economic outcomes are presented in their native currency in Supplementary data, Table S9.
Subgroup analysis
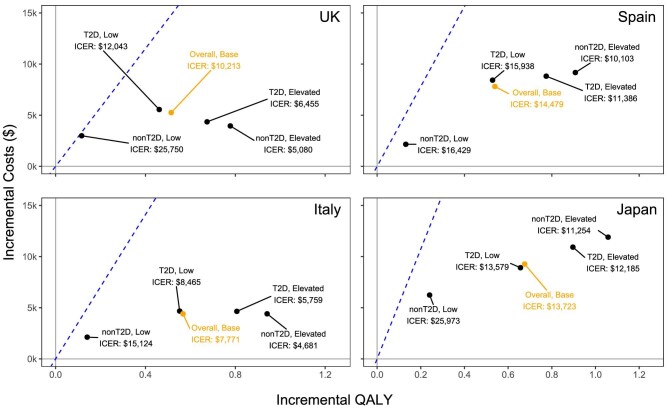
Across all subgroups analysed, dapagliflozin was considered cost effective at the corresponding WTP thresholds attributed to each country (Fig. 2). In all settings, the ICERs for patients with lower UACR and without T2D (UK $25 750; Spain $16 429; Italy $15 124; Japan $25 973) were highest, while ICERs for the subgroup with higher UACR and without T2D were lowest (UK $5080; Spain $10 103; Italy $4681; Japan $11 254). In all settings, a similar spread of incremental QALYs was observed; in terms of costs, T2D subgroups were associated with higher incremental costs than non-T2D subgroups and this differential was larger for the lower UACR than for the higher UACR groups.
Figure 2:
Subgroup analyses (top to bottom) in the UK, Spain, Italy and Japan dependent on UACR severity and T2D status. ‘Low’ and ‘elevated’ refer to albuminuria status (UACR <200 mg/g and UACR ≥200 mg/g respectively) while ‘Base’ refers to the base case analysis of 75% low and 25% elevated albuminuria. Dashed lines designate the WTP threshold of each country.
Scenario analysis
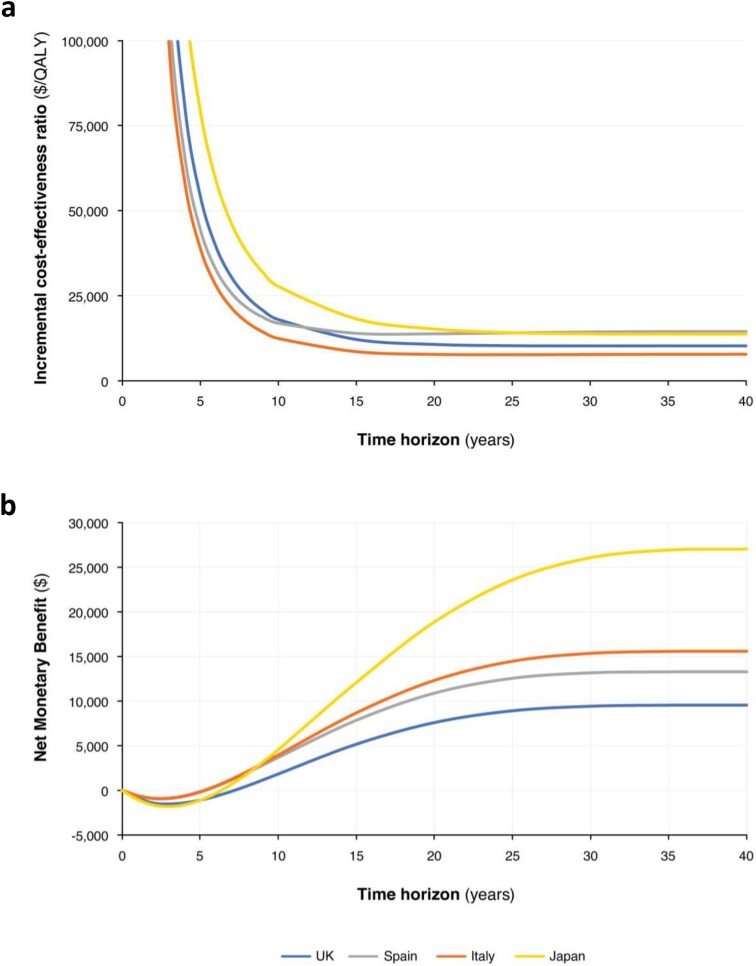
Treatment with dapagliflozin was estimated to lead to fewer patients progressing to ESKD and becoming hospitalized due to heart failure over a lifetime horizon. The largest changes in ICERs were observed during the early period, up to approximately 15 years, where increasing time horizons are associated with large decreases in the ICERs (Fig. 3). Beyond this point, the ICERs stabilize and show little change up to a lifetime time horizon. Similarly, the estimated net monetary benefit was negative over the initial years of treatment, before rising until 20 years from baseline, and the rate of increase levelled off across most settings by 25 years.
Figure 3:
(a) ICER (US dollars per QALY) and (b) net monetary benefit evolution over time in the UK, Spain, Italy and Japan.
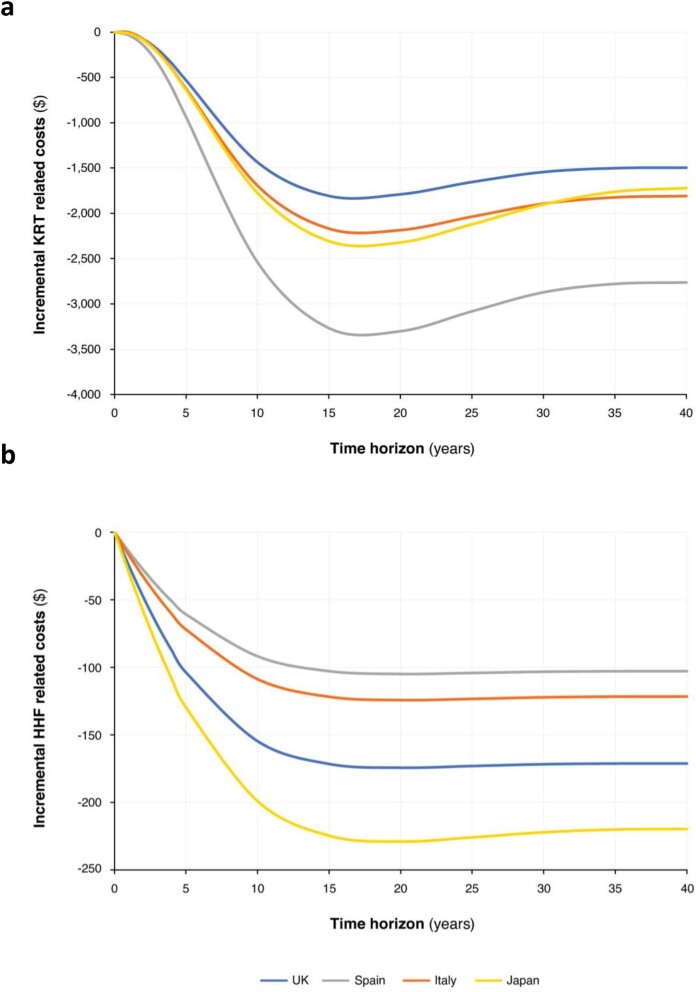
Over the patient lifetime, treatment and disease management costs accrue, and these costs are offset in the dapagliflozin arm by reductions in HHF and KRT events (Fig. 4). While average, per-patient HHF cost reductions remain constant at longer time horizons, those related to avoided KRT exhibit a local minimum at around 15 years, followed by a slight increase before levelling off, likely due to a combined effect of survival and changes in disease progression as more patients transition to later stages of CKD.
Figure 4:
Incremental costs over time associated with (a) KRT and (b) HHF avoided.
Sensitivity analysis
Deterministic sensitivity analyses revealed that, aside from discounting, subpopulation selection and time horizon had the greatest influence on outcomes. Analyses remained cost effective at the defined thresholds for each country for all parameter variations tested (Supplementary data, Table S10).
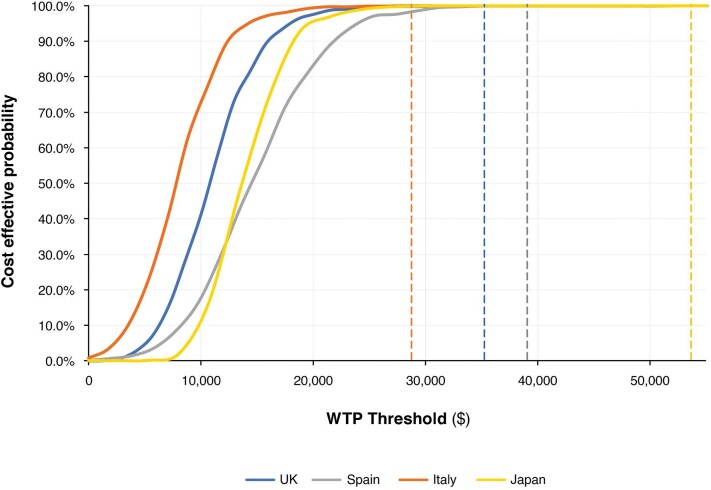
Probabilistic sensitivity analysis showed that the model was robust to joint uncertainty of all parameters. In the UK, 99.8% of simulations were found to be cost effective at a threshold of $28 777/QALY (£20 000/QALY), 99.9% were cost effective at a threshold of $39 063 (€30 000/QALY) in Spain, whilst 100% were cost effective at thresholds of $35 261 (€25 000/QALY) in Italy and $53 671/QALY (¥5 000 000/QALY) in Japan (Fig. 5). Probabilistic sensitivity analyses across subgroups showed that cost-effectiveness outcomes were robust across simulations of the elevated UACR populations and low UACR population with T2D, while simulations in the population with low UACR without T2D showed less certainty in the cost-effectiveness outcomes for this population (see Supplementary data, Fig. S3).
Figure 5:
Cost-effectiveness acceptability curves for the UK, Spain, Italy and Japan. The probability of dapagliflozin in addition to standard therapy being cost-effective at the given WTP threshold versus standard therapy only. The specified WTP threshold for each country is denoted by the corresponding-coloured dotted line parallel to the y-axis.
DISCUSSION
The present study sought to bridge a data gap to explore cost-effectiveness of dapagliflozin in patients with CKD including lower risk patients with lower levels of albuminuria with or without T2D. To model this broader population, individual patient data were pooled from randomized large outcome clinical trials of dapagliflozin plus standard therapy versus standard therapy alone that could inform overall survival and cardiorenal event risks. After adaptation and incorporation of these risk equations into a previously published cost-effectiveness model, results of the present study expand the evidence base of health economic outcomes in a population with CKD that is broader than the inclusion criteria of the DAPA-CKD trial. Analyses revealed ICERs below attributed WTP thresholds and positive net monetary benefit in all settings (UK, Spain, Italy and Japan), and outcomes were generally supportive of cost-effectiveness in patients with lower UACR (<200 mg/g) and without T2D. Outcomes were typically robust across deterministic and probabilistic sensitivity analyses.
Building on the previous cost-effectiveness analysis in the DAPA-CKD population [14], this analysis suggests that the clinical and economic value of dapagliflozin in comparison with usual care alone could be generalizable (albeit somewhat less pronounced) to patients with CKD who are not represented in the DECLARE-TIMI 58 or DAPA-CKD trials, including patients with albuminuria stage A2 without T2D. In line with the previous analysis, patients treated with dapagliflozin spent more time in CKD stages 1–4 (broad CKD population, 1.1 years; DAPA-CKD, 1.7 years) versus patients treated with standard therapy alone [14]. Moreover, dapagliflozin slowed the rate of HHF (broad CKD population, 21 events avoided; DAPA-CKD, 19 events avoided) and increased life expectancy (broad CKD population, 0.7 years; DAPA-CKD, 1.7 years) versus standard therapy alone. In comparison with the previous analysis in the higher UACR population only, potential benefits were lower as reflected by smaller incremental QALY and slightly smaller incremental costs in the context of the broader CKD population. The effect is expected since, while the rates of mortality, ESKD and cardiovascular events were lower relative to patients with more advanced eGFR and albuminuria stage of CKD, the presence of CKD will increase the risk of cardio-renal events and so the treatment effect of dapagliflozin was consistent [39]. Nevertheless, the analysis suggests that results would be considered cost effective at all country-specific thresholds for the broad CKD population, and even when restricted to the population with lower UACR. The substantial clinical benefits observed in the medium- to long-term suggest that, given management of CKD in patients within this broader population will typically lie with primary care physicians, earlier enrolment on reno-protective therapies such as dapagliflozin could be important in reducing the population level impact of CKD progression.
This study, as with any modelling analysis, is subject to several limitations. The most significant source of uncertainty relates to the requirement to extrapolate outcomes in patients without T2D and with lower UACR. Additionally, given the relative health of these patients, there are limited data available against which to externally validate these projections.
This analysis takes advantage of two trial data sets to generate a pooled population including patient-level data from 10 273 patients, including patients at lower risk of progression. Therefore, these patients would be expected to experience fewer events such as HHF or progression to advanced CKD, potentially confounding extrapolations for these individuals. The limitation is partly mitigated through the longer observation time for these patients (median trial follow-up was 4.2 years) and controlling for multiple variables including albuminuria and CKD state to exploit fully all available data to inform estimates.
The transition probabilities for post-KRT outcomes were estimated from a systematic review of published literature on modelling in CKD [29], given the small number of observed events in either trial.
Finally, the prevalence of albuminuria may vary between countries, and therefore country-specific prevalence data would best reflect outcomes specific to each setting. National prevalence data stratified by eGFR and albuminuria are limited and importantly, the DAPA-CKD trial threshold definition of higher albuminuria was 200 mg/g, a level that differs from KDIGO categories of albuminuria (A2, 30–300 mg/g; A3, >300 mg/g) [18, 24]. The relative proportions were instead approximated from the DECLARECKD trial data in which patients were not selected according to albuminuria status.
In conclusion, this analysis addresses a data gap that currently exists in patients with CKD. The country-specific results of the model suggest that dapagliflozin could be a cost-effective treatment option for the broader CKD population and irrespective of eGFR, UACR and T2D status in the UK, Spain, Italy and Japan.
Supplementary Material
Acknowledgements
Data analysis, and editorial support were provided by Andrew Gibson, PhD, of Health Economics and Outcomes Research Ltd, which was funded by AstraZeneca.
Contributor Information
Phil McEwan, Health Economics and Outcomes Research Ltd, Cardiff, UK.
Jason A Davis, Health Economics and Outcomes Research Ltd, Cardiff, UK.
Peter D Gabb, Health Economics and Outcomes Research Ltd, Cardiff, UK.
David C Wheeler, Department of Renal Medicine, University College London, London, UK.
Peter Rossing, Steno Diabetes Centre Copenhagen, Herlev, Denmark; Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Glenn M Chertow, Departments of Medicine and Epidemiology and Population Health, Stanford University School of Medicine, Stanford, CA, USA.
Ricardo Correa-Rotter, Department of Nephrology and Mineral Metabolism, National Medical Science and Nutrition Institute Salvador Zubiran, Mexico City, Mexico.
Kouichi Tamura, Department of Medical Science and Cardiorenal Medicine, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Salvatore Barone, Global Medical Affairs, AstraZeneca, Gaithersburg, MD, USA.
Juan Jose Garcia Sanchez, Health Economic and Payer Evidence, AstraZeneca, Cambridge, UK.
FUNDING
This work and its publication were funded by AstraZeneca. Author independence in study design, data interpretation, manuscript preparation for publication was ensured by the funding agreement.
AUTHORS’ CONTRIBUTIONS
Phil McEwan, Jason Davis, Peter Gabb, and Juan Garcia Sanchez conceptualised and designed the study. Jason Davis and Peter Gabb were responsible for data analysis, preparation of the manuscript outline and subsequent drafts, collating and incorporating author comments and preparing tables and figures. Phil McEwan, Jason Davis, Peter Gabb, David Wheeler, Peter Rossing, Glenn Chertow, Ricardo Correa-Rotter, Kouichi Tamura, Salvatore Barone and Juan Jose Garcia Sanchez contributed to interpretation of the results, preparation and review of the manuscript and approval of the final manuscript for publication.
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
CONFLICT OF INTEREST STATEMENT
P.M., J.A.D. and P.D.G. are employees of Health Economics and Outcomes Research Ltd, Cardiff, UK. Health Economics and Outcomes Research Ltd received fees from AstraZeneca in relation to this study. D.C.W. provided ongoing consultancy services to AstraZeneca in the last 2 years and has received honoraria and/or consultancy fees from Amgen, AstraZeneca, Boehringer Ingelheim, Bayer, Eledon, Galderma, Gilead, GlaxoSmithKline, George Clinical, Janssen, Merck Sharp and Dohme, ProKidney, Takeda, Vifor and Zydus. He also reports speaking fees from Astellas, AstraZeneca and Vifor, and support for travel/meeting attendance from Astellas, AstraZeneca and Pro. He has served on DSMBs for Eledon, Galderma, Merck, ProKidney and Pathalys. He is a member of the International Society of Nephrology and National Institute of Health Research, UK. P.R. has received honoraria to Steno Diabetes Center Copenhagen for consultancy from AstraZeneca, Astellas, Bayer, Boehringer Ingelheim, Gilead, Novo Nordisk, Merck, Mundipharma, Sanofi and Vifor, and research support from AstraZeneca, Bayer and Novo Nordisk. G.M.C. received fees from AstraZeneca for service on the DAPA-CKD trial steering committee. He serves on the Board of Directors for Satellite Healthcare, a non-profit dialysis provider. He has received research grants to his institution from NIDDK, NIAID and CSL Behring. He has served on trial-steering committees with Akebia, AstraZeneca, Gilead, Sanifit and Vertex. He has served as an advisor to Applaud, Ardelyx, CloudCath, Durect, Eliaz Therapeutics, Miromatrix, Outset, Renibus, Unicycive and Vertex. He has served on DSMBs for NIDDK, Bayer, Mineralys and ReCor. He also declares stock or stock options for Applaud, Ardelyx, CloudCath, Durect, Eliaz Therapeutics, Miromatrix, Outset and Renibus. R.C.-R. has received honoraria as consultant from AstraZeneca, Boehringer Ingelheim, Bayer, Chinook, AbbVie and Novo Nordisk, and research support from AstraZeneca, Boehringer Ingelheim, Roche and Novo Nordisk. He has received speaking fees from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Amgen. K.T. has honoraria as lecture fee from Novartis, AstraZeneca, Ono, Daiichi-Sankyo, Takeda, Otsuka, Bayer and Kyowa-Kirin. He has received research support from AstraZeneca, Ono, Bayer, Kyowa-Kirin, Otsuka, Takeda and Daiichi-Sankyo. S.B. and J.J.G.S. are employees of AstraZeneca.
REFERENCES
- 1. Jha V, Garcia-Garcia G, Iseki Ket al. Chronic kidney disease: global dimension and perspectives. Lancet North Am Ed 2013;382:260–72. 10.1016/S0140-6736(13)60687-X [DOI] [PubMed] [Google Scholar]
- 2. Jager KJ, Kovesdy C, Langham Ret al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int 2019;96:1048–50. 10.1016/j.kint.2019.07.012 [DOI] [PubMed] [Google Scholar]
- 3. Jager KJ, Fraser SDS.. The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol Dial Transplant 2017;32:ii121–8. 10.1093/ndt/gfw330 [DOI] [PubMed] [Google Scholar]
- 4. Pollock C, James G, Garcia Sanchez JJet al. Healthcare resource utilisation and related costs of patients with CKD from the UK: a report from the DISCOVER CKD retrospective cohort. Clin Kidney J 2022;15:2124–34. 10.1093/ckj/sfac168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vanholder R, Annemans L, Brown Eet al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol 2017;13:393. 10.1038/nrneph.2017.63 [DOI] [PubMed] [Google Scholar]
- 6. Wanner C, Inzucchi SE, Lachin JMet al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–34. 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 7. Neal B, Perkovic V, Mahaffey KWet al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 8. Wiviott SD, Raz I, Bonaca MPet al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–57. 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 9. McMurray JJV, Solomon SD, Inzucchi SEet al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 10. Zinman B, Wanner C, Lachin JMet al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 11. Packer M, Anker SD, Butler Jet al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 12. Mosenzon O, Wiviott SD, Cahn Aet al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019;7:606–17. 10.1016/S2213-8587(19)30180-9 [DOI] [PubMed] [Google Scholar]
- 13. Heerspink HJL, Stefánsson BV, Correa-Rotter Ret al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 14. McEwan P, Darlington O, Miller Ret al. Cost-effectiveness of dapagliflozin as a treatment for chronic kidney disease: a health-economic analysis of DAPA-CKD. Clin J Am Soc Nephrol 2022;17:1730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heerspink HJL, Chertow GM, Jongs Net al. Effects of dapagliflozin in people without diabetes and with microalbuminuria. Clin J Am Soc Nephrol 2022;17:1665–8. 10.2215/CJN.07290622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner Cet al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023;388:117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Olufade T, Lamerato L, Sánchez JJGet al. Clinical outcomes and healthcare resource utilization in a real-world population reflecting the DAPA-CKD trial participants. Adv Ther 2021;38:1352–63. 10.1007/s12325-020-01609-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murton M, Goff-Leggett D, Bobrowska Aet al. Burden of chronic kidney disease by KDIGO categories of glomerular filtration rate and albuminuria: a systematic review. Adv Ther 2021;38:180–200. 10.1007/s12325-020-01568-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wiviott SD, Raz I, Bonaca MPet al. The design and rationale for the dapagliflozin effect on cardiovascular events (DECLARE)–TIMI 58 trial. Am Heart J 2018;200:83–9. 10.1016/j.ahj.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 20. Raz I, Mosenzon O, Bonaca MPet al. DECLARE-TIMI 58: participants’ baseline characteristics. Diabetes Obes Metab 2018;20:1102–10. 10.1111/dom.13217 [DOI] [PubMed] [Google Scholar]
- 21. Mosenzon O, Wiviott SD, Cahn Aet al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019;7:606–17. [DOI] [PubMed] [Google Scholar]
- 22. Heerspink HJL, Stefansson BV, Chertow GMet al. Rationale and protocol of the Dapagliflozin and prevention of adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 2020;35:274–82. 10.1093/ndt/gfz290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wheeler DC, Stefansson BV, Batiushin Met al. The Dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: baseline characteristics. Nephrol Dial Transplant 2020;35:1700–11. 10.1093/ndt/gfaa234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eknoyan G, Lameire N, Eckardt Ket al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013;3:5–14. [DOI] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis J, Gabb PD, Garcia Sanchez JJet al. Extrapolation of DAPA-CKD trial end points in a broad urine albumin creatinine ratio population [Abstract]. J Am Soc Nephrol 2022;33:848. [Google Scholar]
- 27. McEwan P, Boyce R, Sanchez JJGet al. Extrapolated longer-term effects of the DAPA-CKD trial: a modelling analysis. Nephrol Dial Transplant 2023;38:1260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kidney Disease: Improving Global Outcomes (KDIGO) . Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2012;2013:1–150. [Google Scholar]
- 29. Sugrue DM, Ward T, Rai Set al. Economic modelling of chronic kidney disease: a systematic literature review to inform conceptual model design. Pharmacoeconomics 2019;37:1451–68. 10.1007/s40273-019-00835-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal. 2013. Available at: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (17 October 2022, date last accessed). [PubMed]
- 31. López-Bastida J, Oliva J, Antoñanzas Fet al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ 2010;11:513–20. 10.1007/s10198-010-0244-4 [DOI] [PubMed] [Google Scholar]
- 32. Agenzie Italiana del Farmaco . Linee Guida per La Compilazione Del Dossier A Supporto Della Domanda Di Rimborsabilità E Prezzo di Un Medicinale. 2019. Available at: https://www.aifa.gov.it/documents/20142/1307543/2021.01.22_estratto_linee_guida_sezione_E.pdf (21 October 2022, date last accessed).
- 33. Shiroiwa T, Fukuda T, Ikeda Set al. Development of an official guideline for the economic evaluation of drugs/medical devices in Japan. Value Health 2017;20:372–8. 10.1016/j.jval.2016.08.726 [DOI] [PubMed] [Google Scholar]
- 34. AstraZeneca. A study to evaluate the effect of Dapagliflozin on renal outcomes and cardiovascular mortality in patients with chronic kidney disease (Dapa-CKD). 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT03036150 (4 June 2020, date last accessed).
- 35. McEwan P, Darlington O, McMurray JJVet al. Cost-effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health-economic analysis of DAPA-HF. Eur J Heart Fail 2020;22:2147–56. 10.1002/ejhf.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beaudet A, Clegg J, Thuresson POet al. Review of utility values for economic modeling in type 2 diabetes. Value Health 2014;17:462–70. 10.1016/j.jval.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 37. Currie CJ, Morgan CL, Poole CDet al. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 2006;22:1523–34. 10.1185/030079906X115757 [DOI] [PubMed] [Google Scholar]
- 38. Peasgood T, Brennan A, Mansell Pet al. The impact of diabetes-related complications on preference-based measures of health-related quality of life in adults with type I diabetes. Med Decis Making 2016;36:1020–33. 10.1177/0272989X16658660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Darlington O, Dickerson C, Evans Met al. Costs and healthcare resource use associated with risk of cardiovascular morbidity in patients with chronic kidney disease: evidence from a systematic literature review. Adv Ther 2021;38:994–1010. 10.1007/s12325-020-01607-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.