Abstract
Objectives
Determine uptake of furan, a potential human carcinogen, in waterpipe tobacco (WPT) smokers in home settings.
Methods
We analysed data from a US convenience sample of 50 exclusive WPT smokers, mean age 25.3 years, and 25 non-smokers, mean age 25.5 years. For WPT smokers, data were collected at a home visit by research assistants during which participants smoked one WPT head of one brand for a mean of 33.1 minutes in their homes. Research assistants provided and prepared a WP for participants by weighing and loading 10g of WPT in the WP head. At the completion of the smoking session, research assistants measured the remaining WPT. Cotinine and six furan metabolites were quantified in first morning urine samples provided on 2 consecutive days for non-smokers, and on the morning of a WPT smoking session and on the following morning for smokers.
Results
WPT smokers consumed a mean of 2.99g WPT. In WPT smokers, urinary cotinine levels increased significantly 26.1 times the following morning; however, urinary metabolites of furan did not increase significantly. Compared to non-smokers, 2 furan metabolites, N-acetyl-S-[1-(5-acetylamino-5-carboxylpentyl)-1H-pyrrol-3-yl]-L-cysteine and N-acetyl-S-[1-(5-amino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide, were significantly higher in WPT smokers in pre and in post WPT smoking levels.
Conclusions
To enable a more rigorous assessment of furan exposure from WPT smoking, future research should determine furan concentrations in WPT smoke, quantify furan metabolites from users of various WPT brands; and extend the investigation to social settings where WPT smoking is habitually practiced.
Keywords: Furan, Waterpipe Tobacco, Hookah
INTRODUCTION
Waterpipe tobacco (WPT) use is currently considered a global health problem, particularly among youth and young adults in several eastern Mediterranean, eastern European and western countries, including the United States (US).1-11 In the US, a nationally representative sample (2013-2014) showed that 13% of youth (15-17 year olds) have ever used WPT, and 2.9% have used WPT in the past 30 days; among young adults (18-24 year olds), 44.4% have ever used WPT and 18.2% are current WPT users.11
WPT is smoked using a waterpipe (WP) device (hookah) in which smoke passes through water. The WP consists of a head (bowl) containing WPT and charcoal, a stem (body) which is a vertical tube that passes into a partially filled bowl at the base (water jar), with a flexible hose and a mouthpiece. Burning charcoal heats the WPT producing smoke that the user inhales.
The most popular WPT, worldwide, is flavored WPT in which sugar-containing ingredients are added.3,12-17 Flavored WPT (aka Moassel, Maassel) is a mixture of ~30% tobacco and natural/artificial flavorings, sweeteners (e.g., molasses, honey, sugars) and humectants (e.g., propylene glycol).13-18 WPT smokers smoke in home settings,19-24 and are at increased risk for a number of preventable harmful health effects including cancers.25-27 Furan exposure among WPT smokers in home settings has not been investigated.
Furan is hepatotoxic and carcinogenic in rats and mice.28-31 It is a pulmonary and hepatic toxicant in mice when inhaled.31 Human exposure to furan is likely significant because furan is a ubiquitous environmental volatile chemical found in processed food, air pollution, car exhaust, and cigarette smoke.32-36 The National Toxicology Program and International Agency for Research on Cancer classified furan as a possible human carcinogen.28,34
For human adults, the average dietary exposure to furan was ~0.3 μg/kg bodyweight/day.37 Cigarette smokers may be exposed to higher amounts of furan than non-smokers, as cigarette smoke contains non-trivial levels of furan (20-40 μg/cigarette).38,39 Furan exposure from tobacco use is attributed, in part, to sugar content in tobacco products.40 Heating sugar-rich consumable products results in chemical conversions, yielding toxic furans.41,42,49 Sugars are natural components of tobacco in levels up to 20% by weight, and are commonly added, in varying levels, to tobacco during manufacturing.40,43 Flavored WPT typically contains higher sugar concentrations than cigarettes40,44-46 (WPT: 350 mg/g sugar vs cigarettes: 7-180 mg/g).44 Flavored WPT is typically manufactured through the fermentation of tobacco with molasses or honey or other sugars.45 In some WPT brands, 125–250 mL of honey is added per kg of flavored WPT (~17–35 wt%).46 Sugars serve as binders, casing ingredients, flavors, or humectants.40
WPT mainstream smoke is a major source of furanic compounds, particularly 5-(hydroxymethyl)-2-furaldehyde (HMF).44,47 HMF aerosol concentration per puff in WPT is ~33 times higher than in cigarettes.47,48 Machine-smoked WPT yields a range of furanic compounds (e.g., furfuryl alcohol, 2-furoic acid, 2-furaldehyde, 2-furyl methyl ketone and HMF), and up to 62,300 μg HMF per smoking session.44,47 While furan was not measured in these studies, it is likely present since furan is produced from glucose and sucrose under Maillard-like reaction conditions.49 Such findings highlight the need to investigate furan exposure associated with WPT smoking, a practice believed to be less harmful than cigarette smoking, particularly among college/university students, a population with high WPT use.11,50
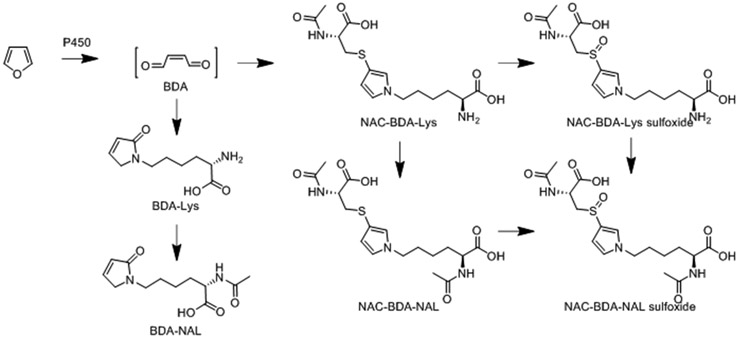
Measuring metabolites of furan exposure is important in order to quantify the associated human health risks.51 Furan is oxidized to a reactive α,β-unsaturated dialdehyde metabolite, cis-2-butene-1,4-dial (BDA), by cytochrome P450 (P450).52-58 BDA is highly toxic and mutagenic, and forms adducts with various cellular nucleophiles (e.g., DNA, protein, and polyamines).55-63 Consequently, BDA is considered responsible for furan’s toxic effects.55-64 Additional urinary metabolites of furan are derived from the reaction of BDA with a variety of cellular nucleophiles.63,65-68,36 The lysine and cysteine-BDA-lysine cross-links, and their sulfoxides (Figure 1) are major urinary furan metabolites in rodents.65,67,36 These metabolites are elevated in the urine of cigarette smokers,36 indicating exposure of the smoker to furan in tobacco product emissions and to the risk of possible harm from that exposure.
Figure 1.
Six urinary metabolites are derived from the reactive intermediate, BDA (CAS No: 3675-13-6), formed as a result of furan oxidation. Furan (CAS No: 110-00-9) is classified by IARC as a group 2b carcinogen (possibly carcinogenic to humans).
To our knowledge this is the first exploratory study in private home settings in the US to quantified urinary cotinine and six furan metabolites in WPT smokers and non-smokers. Furan metabolites measured were BDA-Lys [L-2-(amino)-6-(2,5-dihydro-2-oxo-1H-pyrrol-1-yl)-1-hexanoic acid], BDA-NAL [L-2-(acetylamino)-6-(2,5-dihydro-2-oxo-1H-pyrrol-1-yl)-1-hexanoic acid], NAC-BDA-Lys [N-acetyl-S-[1-(5-amino-5-carboxylpentyl)-1H-pyrrol-3-yl]-L-cysteine], NAC-BDA-NAL [N-acetyl-S-[1-(5-acetylamino-5-carboxylpentyl)-1H-pyrrol-3-yl]-L-cysteine] and their sulfoxides, NAC-BDA-Lys sulfoxide [N-acetyl-S-[1-(5-amino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide] and NAC-BDA-NAL sulfoxide [N-acetyl-S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide] (Figure 1). Findings will contribute to identifying possible health risks of WPT use.
METHODS
Participant recruitment, screening and consent
Between December 2017 and September 2018, we recruited a convenience sample of 50 WPT smokers (25 men, 25 women) and 25 non-smokers (12 men and 13 women) from San Diego County, California, US via bulletin board flyers (e.g., community colleges, universities, cafés), electronic flyers on social media (e.g. Instagram, Facebook, Snapchat, Reddit, Twitter), and participant enlistment of others by word of mouth. Trained research assistants (RAs) qualified participants via phone-screening based on inclusion/exclusion criteria. At an office visit we explained study activities, obtained signed informed consent forms, collected smoking history and demographics via self-administered questionnaire, took physiologic measures (height, weight, exhaled carbon monoxide (eCO)), and scheduled a home visit.
WPT smoker inclusion criteria were: exclusive WPT smoker; ≥21 years old; smoked ≥1WPT head/month; smoked ≥1WPT head/smoking session; and no WPT home smoking restrictions. Non-smoker inclusion criteria were: ≥21 years old; not living with a smoker; not using any tobacco or nicotine replacement products; not allowing tobacco smoking at home during the study.
Exclusion criteria for all participants were: major physical/psychiatric illnesses that might interfere with providing informed consent or completing an interview; history of chronic health problems (e.g. asthma); regular prescription medication use (other than vitamins or birth control); pregnancy.
We phone-screened 101 WPT smoker respondents; 54 were eligible and were enrolled; 4 withdrew before the home visit (due to travel/change of mind). We phone-screened 51 non-smoker respondents; 31 were eligible and were enrolled; 1 non-smoker dropped out before the home visit (due to school/work schedule); 5 were excluded because of potential exposure to tobacco secondhand smoke (SHS) based on eCO levels ≥3.6 ppm.69
Study design
RAs collected data during an office visit and a home visit for smokers and non-smokers. During the home visit, smokers smoked one WPT head (not shared with other smokers), with a 7-day washout period before the visit. During the wash-out period, prescheduled phone texts reminded WPT smokers about smoking abstinence, and all participants about avoiding SHS, collecting urine samples, and confirming scheduled home visits. All participants received $75 in cash at study completion. San Diego State University’s Institutional Review Board approved the study.
WPT smoking session
During the home visit, the RAs provided the smokers with a single-hose medium-sized WP with glass water bowl (height=22 inches (55.8 cm); Khalil Mamoon Safari brand, Egypt) and a disposable plastic hose (length=50 inches (125 cm); Fancy Hose, Zebra Smoke brand, Amazon.com). Following a standardized protocol, RAs placed a standardized volume (2 cups=470 mL) of room temperature commercial drinking water (Nestlé Pure Life purified bottled water brand, US) in the WP bowl, covering the metal stem with an inch (2.5 cm) of water. RAs weighed (using an analytical balance) and loaded one head of flavored WPT (10g; Exotic Double Apple flavor, Starbuzz brand, US, Supplementary Figure 1) in the ceramic head, covering it with a manufacturer pre-perforated sheet of aluminum foil (Zebra Smoke brand, Amazon.com). The WPT was purchased between December 2017 and August 2018 from two WPT supply stores in San Diego, California, and stored at room temperature. Using a portable lighter, RAs lit a single quick-light charcoal (40 mm; Three Kings brand, Holland), placing it on the foil-covered WP head (no additional charcoal was added). Smokers smoked as they usually do, with no other smokers present, during the evening hours between 5:00pm and 8:00pm. They were informed that the WP would be available for 45 minutes per smoking session, and ended smoking when desired. At the conclusion of the home visit, RAs returned the WP device and WPT use waste (in sealed Ziploc bags) to our center. RAs thoroughly cleaned WP devices with soap and water between sessions and between participants. Each participant had a new disposable hose.
Measures
Demographics and WPT smoking behaviors
During the office visit, participants completed a self-administered questionnaire that asked about demographics (age, gender, education, race/ethnicity), and WPT smoking behaviors.
Physiologic measurements
During the office visit, height (Ht.) and weight (Wt.) were taken to calculate Body Mass Index [BMI=Wt.(kg)/Ht.(m)2]. Using a CO breath analysis monitor (Micro+pro™ Smokerlyzer® Covita, Bedfont, US; sensitivity=1 parts per million (ppm)), an eCO measurement was taken. Participants were asked to inhale deeply, hold breath for 15 seconds, and then exhale slowly into the mouthpiece, aiming to empty lungs completely.
Smoking duration and amount of WPT smoked
During the home visit, RAs recorded length of smoking time, from first to last puff, and weighed the WPT remaining at smoking session conclusion.
Urinary metabolites of furan analyses
Participants provided two first-void urine samples: one sample the morning of the WPT smoking session day (pre-smoking) and one sample the following morning for smokers, and on 2 consecutive days for non-smokers. Participants stored urine samples in their refrigerator’s freezer section until pickup by RAs within 12 hours and transfer (frozen) to our research center laboratory. Urine samples were aliquoted and stored in a freezer (−20°C), then sent frozen in dry ice to two laboratories. The San Diego State University Environmental Health Laboratory analyzed urinary creatinine using isotope-dilution liquid chromatography/tandem mass spectrometry (LC-MS/MS) with the sample preparation methods and multiple-reaction-monitoring transitions,70 and an adapted chromatographic separation.71 Urinary cotinine was analyzed by isotope-dilution LC/MS/MS.72 Furan metabolites were analyzed at the University of Minnesota’s Masonic Cancer Center by isotope-dilution LC-MS/MS method with minor modifications.36 Specifically, fractions containing NAC-BAD-NAL were analyzed with the column heated to 40°C and utilized different transitions (NAC-BDA-NAL, m/z 398 → m/z 226; NAC-BDA-[2H3]NAL, m/z 401 → m/z 228; NAC-BDA-[13C615N2]NAL, m/z 406 → m/z 235; and NAC-BDA-[13C62H315N2]NAL m/z 409 → m/z 236).
Statistical Analyses
Data were double entered and analyzed using SPSS v26 and Stata v14.2. Descriptive analyses included computing measures of central tendency, standard deviations (SDs), and minimum and maximum values for BMI, eCO, and creatinine-corrected urinary cotinine and furan metabolites; Wilcoxon signed-rank tests to identify within-person differences in urinary metabolites levels from pre to post the WPT smoking session; Mann–Whitney U tests to identify differences in urinary metabolites levels between smokers and non-smokers prior to and following the WPT smoking session; independent t tests or chi-square tests, as applicable, to identify differences in demographics by smoking status and to compare urinary cotinine levels to those in our previous study.20 For urinary metabolites, limit of detection (LOD) values and instances of laboratory interference are described in Table 3; we excluded the laboratory interference values and replaced <LOD values by half of the LOD. All statistical tests were two-tailed with an alpha level of 0.05; ‘pmol/mg creatinine’ is referred to as ‘pmol/mg’.
RESULTS
Demographics and WPT use
WPT smokers (mean age=25.3 years), were 50% males, identifying as White (22%); Hispanic (32%); Black (10%); Middle Eastern/Arab, Chaldean, Persian, or Kurdish (26%); or multi-ethnic (10%) (Table 1). Most WPT smokers had a college degree (50%) or some college (36%). About two-thirds (60%) resided in houses; one-third (38%) resided in apartments. WPT smokers smoked weekly (62%) or monthly (38%), and were borderline overweight (mean BMI=27.5 kg/m2).73 WPT smokers and non-smokers did not differ significantly by age, gender, education, home setting, BMI or eCO concentrations (Table 1).
Table 1.
Characteristics of waterpipe tobacco (WPT) smokers and non-smokers.
| WPT smokers (n=50) | Non-smokers (n=25) | ||
|---|---|---|---|
| n (%) | n (%) | p † | |
| Age (years) | |||
| Mean ± SD ‡ | 25.3 ±3.1 | 25.5 ±4.6 | 0.668 |
| Median (Minimum-Maximum) | 25.0 (21-35) | 23.5 (21-37) | |
| Gender | |||
| Male | 25 (50) | 12 (48.0) | 0.870 |
| Female | 25 (50) | 13 (52.0) | |
| Race/ethnicity | |||
| White, Caucasian, European | 11 (22) | 5 (20) | 0.005 |
| Mexican, Hispanic, or Latino | 16 (32) | 8 (32) | |
| Black or African American | 5 (10) | 1 (4) | |
| Middle Eastern § | 13 (26) | 0 (0) | |
| Asian/Multi-ethnic Σ | 5 (10) | 11 (44) | |
| Highest level of education completed | |||
| High school | 7 (14) | 1 (4.2) | 0.219 |
| College, no degree | 18 (36) | 8 (33.3) | |
| College degree | 25 (50) | 15 (62.5) | |
| Type of home setting | |||
| House | 30 (60) | 12 (48) | 0.324 |
| Apartment/Townhouse Δ | 19 (40) | 13 (52) | |
| WPT smoking frequency Ω | |||
| Weekly | 31 (62) | N/A | |
| Monthly | 19 (38) | ||
| Type of WPT currently smoke | |||
| Only flavored | 47 (92) | N/A | |
| Flavored and unflavored | 3 (8) | ||
| How often do you share a WP with someone you know? | |||
| Almost always/often | 44 (88) | N/A | |
| Rarely/never | 6 (12) | ||
| Do you live with a tobacco smoker? | |||
| Yes | 17 (34) | 0 (0) | <0.001 |
| No | 33 (66) | 25 (100) | |
| Body Mass Index (BMI) ß | |||
| Mean ± SD | 27.5 ±4.4 | 26.3 ±5.7 | 0.351 |
| Median (Minimum-Maximum) | 27.1 (19.6-39.9) | 25.4 (18.0-38.5) | |
| Exhaled Carbon Monoxide (ppm) Φ | |||
| Mean ± SD | 5.8 ±21.9 | 1.6 ±1.3 | 0.519 |
| Median (Minimum-Maximum) | 2.0 (0.0-156) | 2.0 (0.0-4) | |
p WPT Smokers vs non-smokers: p values were derived from Chi-Square test, or Mann-Whitney U. tests where applicable; two-tailed alpha level p<0.05. Significant levels are bolded.
SD = Standard Deviation.
Participants considered themselves as Middle Eastern/Arab, Chaldean, Persian, or Kurdish.
Asian/Multi-ethnic: WPT Smokers considered themselves German/Mexican (n=1), Lebanese/Black/White (n=1), and Mexican/Caucasian (n=3); Non-smokers considered themselves Native Hawaiian or Pacific Islander (n=8), Filipino (n=2), and Mexican/Filipino (n=1).
Townhouse (n=1).
Weekly: at least once each week but less than daily; Monthly: at least once a month but less than weekly
BMI calculation = Weight in (kg) / Height in (m)2; kg = Kilogram; m = Meter.
ppm = Parts Per Million.
WPT smoking duration and consumption
Mean WPT smoking session duration was 33.1 minutes (Table 2). Of the 10g WPT initially placed in the WP head, smokers consumed a mean of 2.99 g WPT (Table 2).
Table 2.
Waterpipe Tobacco (WPT) smoking for one smoking session (N=50)
| WPT consumed (g) † ‡ § Σ | |
| Mean (±SD) Δ | 2.99 (±0.93) |
| Median (25-75 percentile) | 3.00 (2.4-3.7) |
| (Minimum-Maximum) | (0.5-5.2) |
| Length of time smoked WPT per session (minutes) | |
| Mean (±SD) | 33.1 (±9.35) |
| Median (25-75 percentile) | 30.0 (26-37) |
| (Minimum-Maximum) | (21-64) Ω |
WPT = Waterpipe Tobacco.
Research Assistants weighted 10 g WPT using an analytical balance and loaded it in the WP head.
Research Assistants weighted the remaining WPT using an analytical balance at end of smoking session.
g = Gram.
SD = Standard Deviation.
5 WPT smokers smoked above the allotted 45 minutes/ smoking session as follows: 52,53,54,55 & 64 minutes.
Urinary metabolites of WPT smoking
Urinary Cotinine.
Geometric mean (GM) urinary cotinine levels increased significantly 26.1 times post a WPT smoking session (35 vs 1.3 ng/mg) (Table 3). Compared to non-smokers, GM urinary cotinine levels in smokers were 44.7 times higher pre smoking session (1.3 vs 0.03 ng/ml) and 1702 times higher post smoking session (35 vs 0.02 ng/mg) (Table 3).
Table 3.
Creatinine-Corrected urinary furan metabolites in waterpipe tobacco (WPT) smokers (n=50) compared to non-smokers (n=25).
| WPT Smokers pmol/mg Creatinine |
Non-Smokers pmol/mg Creatinine |
Pre-Smoking WPT vs Non- Smokers |
Post- Smoking WPT vs Non- Smokers |
|||||
|---|---|---|---|---|---|---|---|---|
| Pre-Smoking | Post-Smoking | Day 1 † | Day 2 † | Ratio ♪ | p ♥ | Ratio ♫ | p ₼ | |
| BDA-NAL ‡ | ||||||||
| M ± SD § | 147 ± 89 | 161 ± 138 | 120 ± 80 | 127 ± 66 | 1.3 | 0.084 | 1.2 | 0.431 |
| GM (95% CI) Σ | 128 (111-148) | 131 (111-155) | 99 (76-129) | 112 (91-139) | ||||
| Median (25-75 percentile) | 121 (94-167) | 122 (92-180) | 103 (59-151) | 113 (99-141) | ||||
| (Minimum-Maximum) | (52-544) | (36-860) | (37-346) | (38-311) | ||||
| % above LOD Δ (Freq/n) Ω | 100% (50/50) | 100% (50/50) | 100% (25/25) | 100% (25/25) | ||||
| Ratio ß p-value Φ | 1.03 0.896 | |||||||
| BDA-Lys ψ | ||||||||
| M ± SD | 57 ± 45 | 63 ± 53 | 51 ± 37 | 53 ± 34 | 1.0 | 0.537 | 1.1 | 0.515 |
| GM (95% CI) | 42 (32-55) | 47 (36-60) | 41 (31-54) | 44 (34-57) | ||||
| Median (25-75 percentile) | 42 (27-71) | 50 (33-73) | 42 (24-56) | 42 (31-66) | ||||
| (Minimum-Maximum) | (0.3-260) | (1-318) | (14-149) | (13-140) | ||||
| % above LOD (Freq/n) | 98% (49/50) | 96% (48/50) | 100% (25/25) | 100% (25/25) | ||||
| Ratio ß p-value Φ | 1.1 1.000 | |||||||
| NAC-BDA-NAL Ж | ||||||||
| M ± SD | 0.10 ± 0.11 | 0.08 ± 0.07 | 0.05 ± 0.07 | 0.04 ± 0.04 | 6.0 | 0.006 | 5.0 | 0.017 |
| GM (95% CI) | 0.06 (0.04-0.09) | 0.05 (0.04-0.07) | 0.01 (0.00-0.03) | 0.01 (0.00-0.03) | ||||
| Median (25-75 percentile) | 0.08 (0.04-0.11) | 0.07 (0.03-0.11) | 0.04 (0.00-0.08) | 0.04 (0.00-0.08) | ||||
| (Minimum-Maximum) | (0.00-0.64) | (0.00-0.37) | (0.00-0.25) | (0.00-0.16) | ||||
| % above LOD (Freq/n) | 94% (47/50) | 92% (45/49) x | 56% (14/25) | 64% (16/25) | ||||
| Ratio ß p-value Φ | 0.8 0.085 | |||||||
| NAC-BDA-Lys Я | ||||||||
| M ± SD | 4.5 ± 2.1 | 4.5 ± 2.3 | 3.5 ± 1.6 | 3.7 ± 1.9 | 1.3 | 0.078 | 1.7 | 0.199 |
| GM (95% CI) | 4.0 (3.5-4.6) | 4.0 (3.5-4.6) | 3.1 (2.6-3.9) | 2.4 (1.1-5.3) | ||||
| Median (25-75 percentile) | 4.2 (2.9-5.6) | 3.8 (3.0-5.2) | 4.0 (2.1-4.4) | 3.7 (2.7-4.7) | ||||
| (Minimum-Maximum) | (1.6-11) | (1.7-13) | (1.2-7.7) | (0.0-8.6) | ||||
| % above LOD (Freq/n) | 100% (50/50) | 100% (49/49) x | 100% (25/25) | 96% (24/25) | ||||
| Ratio ß p-value Φ | 1.0 1.000 | |||||||
| NAC-BDA-Lys sulfoxide Ъ | ||||||||
| M ± SD | 4.2 ± 3.4 | 3.6 ± 2.1 | 2.3 ± 0.9 | 2.3 ± 1.1 | 1.6 | 0.001 | 1.5 | 0.002 |
| GM (95% CI) | 3.4 (2.9-4.1) | 3.1 (2.6-3.6) | 2.1 (1.8-2.5) | 2.1 (1.8-2.5) | ||||
| Median (25-75 percentile) | 3.6 (2.1-4.6) | 3.1 (2.1-4.5) | 2.2 (1.6-2.9) | 1.8 (1.4-3.1) | ||||
| (Minimum-Maximum) | (0.98-20) | (0.7-10) | (0.9-4.5) | (1.3-5) | ||||
| % above LOD (Freq/n) | 100% (50/50) | 100% (50/50) | 100% (25/25) | 100% (25/25) | ||||
| Ratio ß p-value Φ | 0.9 0.119 | |||||||
| Cotinine | ||||||||
| M ± SD | 46 ± 135 | 84 ± 148 | 0.07 ± 0.08 | 0.06 ± 0.10 | 44.7 | <0.001 | 1702 | <0.001 |
| GM (95% CI) | 1.34 (0.59-3.04) | 35 (24-51) | 0.03 (0.02-0.05) | 0.02 (0.01-0.04) | ||||
| Median (25-75 percentile) | 0.45 (0.13-12) | 31 (17-61) | 0.04 (0.01-0.08) | 0.01 (0.01-0.08) | ||||
| (Minimum-Maximum) | (0.01-752) | (1-797) | (0.01-0.27) | (0.01-0.41) | ||||
| % above LOD (Freq/n) | 98% (49/50) | 100% (50/50) | 60% (15/25) | 40% (10/25) | ||||
| Ratio ß p-value Φ | 26.10 <.0001 | |||||||
Two first morning urine samples were provided on 2 consecutive days. All values and percentages are rounded up.
BDA-NAL (L-2-(acetylamino)-6-(2,5-dihydro-2-oxo-1H-pyrrol-1-yl)-1-hexanoic acid), a metabolite of furan; Limit of Detection (LOD) = 0.65 pmol/mL.
M (± SD) = Arithmetic mean (± Standard Deviation); the number of decimal places displayed is 2 for values<0, 1 for 0<values<10, and 0 for values>10.
GM (95% CI) = Geometric mean (95% Confidence Intervals).
% above LOD = Percentage of urine samples above the LOD.
Freq/n = Frequency of urine samples with levels above the LOD / n size of urine samples minus missing values due to laboratory interference, as denoted by (x).
Ratio = Ratio of GM metabolites levels post to pre WPT smoking.
p = Post vs pre smoking; p values were derived from Wilcoxon signed-rank tests; two-tailed alpha level p < 0.05. Significant levels are bolded.
BDA-Lys = L-2-(amino)-6-(2,5-dihydro-2-oxo-1H-pyrrol-1-yl)-1-hexanoic acid, a metabolite of furan; LOD = 1.95 pmol/mL.
NAC-BDA-NAL = N-acetyl-S-[1-(5-acetylamino-5-carboxylpentyl)-1H-pyrrol-3-yl]-L-cysteine, a metabolite of furan; LOD = 0.0022 pmol/mL. x Laboratory interference: n=1.
NAC-BDA-Lys = N-acetyl-S-[1-(5-amino-5-carboxylpentyl)-1H-pyrrol-3-yl]-L-cysteine, a metabolite of furan; LOD = 0.0019 pmol/ML. x Laboratory interference: n=1.
NAC-BDA-Lys sulfoxide = N-acetyl-S-[1-(5-amino-5-carboxypentyl)-1H-pyrrol-3-yl]-L-cysteine sulfoxide, a metabolite of furan; LOD = 0.0010 pmol/mL.
Ratio = Ratio of GM metabolites levels pre-smoking WPT vs non-smokers.
p = Pre-smoking WPT vs non-smokers; p values derived from Mann-Whitney U tests; two-tailed alpha level p < 0.05. Significant levels are bolded.
Ratio = Ratio of GM metabolites levels post-smoking WPT vs non-smokers.
p = post-smoking WPT vs non-smokers; p values derived from Mann-Whitney U tests; two-tailed alpha level p < 0.05. Significant levels are bolded.
LOD values of metabolites were replaced with (LOD/2).
Urinary Furan Metabolites
Five of the six metabolites were detected in urine of WPT smokers and non-smokers. The 5 detected urinary metabolites of furan—BDA-NAL, BDA-Lys, NAC-BDA-NAL, NAC-BDA-Lys sulfoxide, and NAC-BDA-Lys—did not increase significantly post smoking WPT. However, for smokers, 2 furan metabolites, NAC-BDA-NAL and NAC-BDA-Lys sulfoxide, were significantly higher than non-smokers in pre and post smoking levels; NAC-BDA-NAL (pre-smoking GM: smoker=0.06 vs non-smoker=0.01 pmol/mg; and post-smoking GM: smoker=0.05 vs non-smoker=0.01 pmol/mg), and NAC-BDA-Lys sulfoxide (pre-smoking GM: smoker=3.4 vs non-smoker=2.1 pmol/mg; and post-smoking GM: smoker=3.1 vs non-smoker=2.1 pmol/mg). Urinary levels of furan metabolites were not statistically correlated with cotinine, length of time smoked, amount of WPT smoked, age, or BMI. (See supplementary Table 1. Correlations of urinary cotinine and furan metabolites).
DISCUSSION
Available evidence of furan-induced toxicity and hepatocarcinogenicity in animals warrants investigating furan’s role in human disease. The first step would be to investigate exposure levels of furan in various human populations. One emphasis should be on tobacco smokers.36 In this study, we focused on WPT smokers in home settings.
We detected 5 out of 6 furan metabolites in urine of WPT smokers and non-smokers. Levels of two urinary furan metabolites, NAC-BDA-NAL and NAC-BDA-Lys sulfoxide, were significantly higher in WPT smokers compared to non-smokers. These findings are similar to those reported for cigarette smokers in a small sample size study where levels of NAC-BDA-Lys sulfoxide were 10 times higher in cigarette smokers (average ±SD=69 ±33 pmol/mg, N=16) compared to non-smokers (6.5 ±2.9 pmol/mg, N=15).36 In this study, average ±SD levels of NAC-BDA-Lys sulfoxide in cigarette smokers and non-smokers were significantly higher than in our WPT smokers (3.6 ±2.1 pmol/mg, p<0.001) and in our non-smokers (2.3 ±1.1 pmol/mg, p<0.001).36 The difference in furan exposure between the two studies could be due, in part, to factors such as frequency of smoking, amount of tobacco smoked and study design; for example, while cigarette smokers reported smoking daily an average ±SD of 21.8 ±6.7 cigarettes per day,36,74 our WPT smoker participants were asked to abstain from smoking WPT for one week before smoking, and they were asked to smoke only one WPT head on the day they smoked (mean WPT consumed = 2.99 g). Despite the differences in study designs, findings from these two human studies are important because they set the stage for future studies on furan exposure in tobacco smokers, especially because furan was found to induce liver fibrosis in rats.28 Tobacco smoking has been found to be an independent risk factor for non-alcoholic fatty liver disease.75,76 FibroScan tests for patients with non-alcoholic fatty liver disease showed that liver stiffness values, as an indicator for liver fibrosis, were significantly higher in smokers than in non-smokers.76 More studies are needed to assess exposure to furan in smokers of various tobacco products, taking into consideration smoking behaviors.
It is likely that our findings on levels of urinary furan metabolites post WPT smoking underestimate levels that typically occur in uncontrolled conditions; we asked WPT smokers to smoke alone, smoke only 1 WPT head, and we limited the smoking session to a maximum of 45 minutes. Normally, WPT smoking is habitually practiced in social settings in homes and in hookah lounges where smokers spend lengthy hours smoking WPT and sharing with other smokers.1,3,12,19,20,77 Furthermore, WPT smokers in social settings are not only exposed to mainstream smoke, they are also exposed to SHS from other smokers.78-83 SHS levels in home settings and more so in WPT smoking commercial venues (e.g., hookah lounges/bars/cafés) were found to contain hazardous levels of indoor air pollutants.77-82 Hookah lounges operate globally, and are opening throughout the US, providing the opportunity to smoke WPT while exposing their patrons to unhealthy air quality.78-84 Furan has not been measured in WPT SHS. To estimate, in part, furan exposure levels, we suggest including furan quantification in studies aimed to investigate SHS constituents and indoor air quality in homes where WPT smokers and non-smoking family members reside, and in hookah lounges where WPT smokers and non-smokers socialize.
It is likely that post smoking furan metabolites provided underestimates of typical levels, considering that urinary cotinine levels in our WPT smoker participants, although significantly elevated post smoking, were lower than previously reported.20 For example, we previously found that in WPT smokers who also resided in San Diego County and had similar characteristics [age, mean ±SD=26.9 ±10.5 years, and 50% males] as our 50 WPT smoker participants [age, mean ±SD=25.3 ±3.1 years, and 50% males], had about 4 times higher urinary cotinine levels in post smoking urine samples after attending a ~3 hour-exclusive WPT smoking social event in homes (N=50) [mean ±SD=333 ±339 ng/mg vs our participants 84 ±148 ng/mg, p<0.001], and in hookah lounges (N=54) [mean ±SD=305 ±461 ng/mg vs our participants 84 ±148 ng/mg, p=0.002].20 In WPT smoking social gatherings, smokers are likely to smoke more and share with others; for example, we previously found that WPT smokers in a social gathering smoked an average of 2 WPT heads and almost all shared with others20 compared to the 1 WPT head allowed for our participants to smoke without sharing. Future studies are encouraged to quantify furan uptake in WPT smokers in homes and commercial smoking venues without placing restrictions on habitual WPT smoking behaviors.
We did not find a significant difference in levels of urinary furan metabolites between pre-smoking and post-smoking samples in WPT smokers, or in uptake of furan between WPT smokers and non-smokers in 3 urinary furan metabolites. These findings are likely due to different kinetics of metabolite excretion for furan metabolites relative to cotinine. Furan metabolites are likely derived from protein adducts and have a half-life of days in smokers following cessation.36 Furthermore, potential exposures from other sources may also boost values of furan uptake in pre-smoking samples of WPT smokers and in urine samples of non-smokers. For example, one source could be air pollution from car exhaust because all smoker and non-smoker participants resided in a metropolitan area with major multilane highways. Another source could be occupational exposure as participants opted to provide their urine samples during week days after work hours. Another source could be food, such as consuming heat-processed foods sold in jars and cans;37 we did not ask about food intake during the study period. None of the measured furan metabolites were correlated with the amount of WPT smoked, length of time smoked, BMI, or age (21-35 years). This could be the result of inadequate variance in these variables, in part because of standardization of study activities, such as providing a standardized amount of WPT (10 g) to participants, the use of one WPT brand, and setting a maximum duration for smoking (45 minutes). Studies are needed in natural settings without standardization of smoking behaviors, and among a wider range of age and BMI levels.
In the interim, calls for regulating flavored WPT should target the highly honeyed/sweetened tobacco, particularly since almost all WPT smokers (92%) reported that they smoke only flavored WPT. While the added sweeteners/sugars attract WPT smokers,1,12 the nicotine in WPT will get them addicted,85 thereby exposing them to many toxicants, including furan.
In an effort to regulate WPT products in the US, the FDA proposed rules to require the manufacturers of WPT to report a listing of all ingredients by quantity, including additives in their WPT products.86-88 Furan is a byproduct of tobacco as well as sugars.28,36,40,44,47 The FDA and regulatory agencies outside the US are urged to require reporting of sugar ingredients in WPT products, along with methods of curing, which largely determine the sugar level of tobacco products.40 Furthermore, the FDA is urged to support future research to inform regulating flavored WPT products standards, such as setting maximum allowable concentrations of sugar additives to WPT.
Limitations and Recommendations
Our findings and recommendations were based on standardized smoking sessions in which participants in our small San Diego US sample, ages 21–35 years, smoked alone in their home. However, WPT smokers typically smoke in social settings with other smokers; the majority (88%) of participants reported that they almost always smoked with someone else. Therefore, data on furan metabolites in WPT smokers could be underestimated. Furthermore, data were generated using one brand of commercial WPT product and WP device; thus, findings may not generalize to other WPT products and WP devices available to consumers. The ethnic distribution of WPT users and non-smokers was different, possibly influencing differential metabolism of furan and confounding the comparison of furan urinary metabolites. We did not measure extraneous sources of furan in the environment where smokers smoked. We also collected spot urine samples, which may have underestimated peak levels of furan metabolites excretion. It is possible that furan and its metabolites have different toxicokinetics. In rats, the metabolism of furan is quite rapid, with the majority of the parent compound removed within a few hours,89,90 whereas the metabolites take much longer to show up in urine and feces.90 More research is required to better understand the toxicokinetic properties of furan and its metabolites.
For rigorous assessment of furan exposure from WPT smoking, investigators should determine the impact of the following strategies on furan levels in smoke and on levels of urinary furan metabolites: collecting data from various WPT brands and WP device styles; using larger sample sizes and group smoking sessions; sampling different populations including older adults with various socio-demographic backgrounds; matching smoker and non-smoker comparison groups by demographics, including ethnic background; conducting experimental studies in laboratory smoking chambers where potential ambient sources of furan are controlled; conducting laboratory analyses of the chemical composition of WPT; determining the unknown time course of furan metabolites elimination in humans; collecting and analyzing 24-hour urine samples; and extending the investigation to social settings where WPT smoking is habitually practiced, such as home WPT smoking parties and commercial WPT smoking venues. Furthermore, identifying furan retained in the water of the WP and in WPT mainstream smoke are warranted.
WPT smoking inside homes is hazardous to the health of children and adult non-smokers.19,20,78,80 Therefore, it is warranted to assess metabolites of furan exposure in non-smokers who socialize or live with WPT smokers, and to determine furan concentrations in WPT SHS when assessing the quality of indoor air in homes of WPT smokers.
Because furan is a byproduct of sugars as well as tobacco,28,36,40,44,47 reduction of furan exposure requires research to identify furan sources in WPT products.
CONCLUSION
We investigated uptake of furan in WPT smokers vs non-smokers in home settings. Levels of two urinary furan metabolites, NAC-BDA-NAL and NAC-BDA-Lys sulfoxide, were significantly higher in WPT smokers compared to non-smokers. In WPT smokers, urinary cotinine levels the following morning were significantly 26.1 times higher; however, urinary metabolites of furan did not increase significantly. Furthermore, levels of three urinary furan metabolites, BDA-NAL, BDA-Lys, and NAC-BDA-Lys did not differ significantly between WPT smokers and non-smokers, possibly due to exposures from other sources that may have boosted values of furan uptake in urine samples of non-smokers and pre-smoking WPT smokers, such as air pollution from car exhaust, occupational exposure, and consuming heat-processed foods sold in jars and cans.
Although exposure to furan may result from multiple, interacting endogenous and exogenous factors,28,34,37 elevated levels of furan metabolites in WPT smokers compared to non-smokers is a concern. Further investigation into levels of furan in WPT smoke, and factors that may influence those levels and potential harm to human health are warranted.
Supplementary Material
Acknowledgments
We thank our participants, our research assistants, and our CBEACH support staff. We thank Linda Chu, Jade Wong, Kaylen Wilson, and Pamela Olguin of the School of Public Health, San Diego State University, for assistance with the urine sample preparation for cotinine and creatinine laboratory analyses. We also thank Dr. Yingchun Zhao, Dr. Sharon Murphy and Mr. Steve Carmella for their assistance with various aspects of the furan metabolite assay.
Funding
Research reported in this publication was supported by the United States Food and Drug Administration (FDA) Center for Tobacco Products (CTP) and the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (NIH) under Award Number R01DA042471 to Nada O.F. Kassem. The furan analyses were supported in part by the National Institute of Environmental Health Sciences of NIH under Award Number 1U2C ES026533 to Lisa A. Peterson and the Masonic Cancer Center, University of Minnesota. The University of Minnesota’s Masonic Cancer Center Analytical Biochemistry and Flow Cytometry Shared Resources are supported in part by NIH P30 CA77598.
Footnotes
Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the FDA or NIH.
Ethics approval The study was approved by San Diego State University’s Institutional Review Board (IRB# 2445100).
Competing interests None declared.
ID# NCT03253653 with a release date of 8.18.2017
Data sharing statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Kassem NOF, Jackson SR, Boman-Davis M, et al. Hookah Smoking and Facilitators/Barriers to Lounge Use among Students at a US University. Am J Health Behav 2015;39(6):832–8. doi: 10.5993/AJHB.39.6.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jawad M, Charide R, Waziry R, et al. The prevalence and trends of waterpipe tobacco smoking: a systematic review. PLoS One 2018;13:e0192191. doi: 10.1371/journal.pone.0192191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maziak W, Taleb ZB, Bahelah R, et al. The global epidemiology of waterpipe smoking. Tob Control 2015;24 Suppl 1:i3–12. doi: 10.1136/tobaccocontrol-2014-051903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morton J, Song Y, Fouad H, et al. Cross-country comparison of waterpipe use: nationally representative data from 13 low and middle-income countries from the global adult tobacco Survey (GATS). Tob Control 2014;23(5):419–27. doi: 10.1136/tobaccocontrol-2012-050841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agaku IT, Filippidis FT, Vardavas CI, et al. Poly-tobacco use among adults in 44 countries during 2008-2012: evidence for an integrative and comprehensive approach in tobacco control. Drug Alcohol Depend 2014;139:60–70. doi: 10.1016/j.drugalcdep.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Jawad M, Lee JT, Millett C. Waterpipe tobacco smoking prevalence and correlates in 25 Eastern Mediterranean and Eastern European countries: cross-sectional analysis of the global youth tobacco survey. NICTOB 2016;18(4):395–402. doi: 10.1093/ntr/ntv101. [DOI] [PubMed] [Google Scholar]
- 7.Singh T, Arrazola RA, Corey CG, et al. Tobacco Use Among Middle and High School Students-United States, 2011-2015. MMWR Morb Mortal Wkly Rep 2016;65(14):361–367. doi: 10.15585/mmwr.mm6514a1. [DOI] [PubMed] [Google Scholar]
- 8.Grekin ER, Ayna D. Waterpipe smoking among college students in the United States: a review of the literature. J Am Coll Health 2012;60(3):244–49. doi: 10.1080/07448481.2011.589419. [DOI] [PubMed] [Google Scholar]
- 9.Johnson AL, Collins LK, Villanti AC, et al. Patterns of nicotine and tobacco product use in youth and young adults in the United States, 2011-2015. Nicotine Tob Res 2018;20(suppl_1):S48–S54. doi: 10.1093/ntr/nty018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odani S, Armour BS, Graffunder CM, et al. State-Specific Prevalence of Tobacco Product Use Among Adults - United States, 2014-2015. MMWR Morb Mortal Wkly Rep 2018;67(3):97–102. doi: 10.15585/mmwr.mm6703a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasza KA, Ambrose BK, Conway KP, et al. Tobacco-Product use by adults and youths in the United States in 2013 and 2014. N Engl J Med 2017;376(4):342–53. doi: 10.1056/NEJMsa1607538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kassem NOF, Kassem NO, Jackson SR, et al. Arab-American hookah smokers: initiation, and pros and cons of hookah use. Am J Health Behav 2015;39(5):680–97. doi: 10.5993/AJHB.39.5.10. [DOI] [PubMed] [Google Scholar]
- 13.Khater AEM, Abd El-Aziz NS, Al-Sewaidan HA, Chaouachi K. Radiological hazards of narghile (hookah, shisha, goza) smoking: activity concentrations and dose assessment. J Environ Radioact 2008;99(12):1808–14. doi: 10.1016/j.jenvrad.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Schubert J, Luch A, Schulz TG. Waterpipe smoking: analysis of the aroma profile of flavored waterpipe tobaccos. Talanta 2013;115:665–674. doi: 10.1016/j.talanta.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Schubert J, Heinke V, Bewersdorff J, et al. Waterpipe smoking: the role of humectants in the release of toxic carbonyls. Arch Toxicol 2012;86(8):1309–16. doi: 10.1007/s00204-012-0884-5. [DOI] [PubMed] [Google Scholar]
- 16.Sepetdjian E, Abdul Halim R, Salman R, et al. Phenolic compounds in particles of mainstream waterpipe smoke. Nicotine Tob Res 2013;15(6):1107–12. doi: 10.1093/ntr/nts255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kassem NOF, Kassem NO, Liles S, et al. Acrolein exposure in hookah smokers and nonsmokers exposed to hookah tobacco secondhand smoke: implications for regulating hookah tobacco products. Nicotine Tob Res 2018;20(4):492–501. doi: 10.1093/ntr/ntx133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akl EA, Ward KD, Bteddini D, et al. The allure of the waterpipe: a narrative review of factors affecting the epidemic rise in waterpipe smoking among young persons globally. Tob Control 2015;24(Suppl 1):i13–21. doi: 10.1136/tobaccocontrol-2014-051906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kassem NOF, Daffa RM, Liles S, et al. Children’s Exposure to Secondhand and Thirdhand Smoke Carcinogens and Toxicants in Homes of Hookah Smokers. Nicotine Tob Res 2014;16(7):961–75. doi: 10.1093/ntr/ntu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassem NOF, Kassem NO, Liles S, et al. Levels of urine cotinine from hookah smoking and exposure to hookah tobacco secondhand smoke in hookah lounges and homes. Int J High Risk Behav Addict 2018;7(1):e67601. doi: 10.5812/ijhrba.67601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agaku I, Odani S, Armour B, Glover-Kudon R. Social aspects of hookah smoking among US youth. Pediatrics 2018;142(2):pii:e20180341. doi: 10.1542/peds.2018-0341. [DOI] [PubMed] [Google Scholar]
- 22.Heinz AJ, Giedgowd GE, Crane NA, et al. A comprehensive examination of hookah smoking in college students: use patterns and contexts, social norms and attitudes, harm perception, psychological correlates and co-occurring substance use. Addict Behav 2013;38(11):2751–60. doi: 10.1016/j.addbeh.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Lipkus IM, Eissenberg T, Schwartz-Bloom RD, et al. Affecting perceptions of harm and addiction among college waterpipe tobacco smokers. Nicotine Tob Res 2011;13(7):599–610. doi: 10.1093/ntr/ntr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maziak W, Ward KD, Eissenberg T. Factors related to frequency of narghile (waterpipe) use: the First insights on tobacco dependence in narghile users. Drug Alcohol Depend 2004;76(1):101–6. doi: 10.1016/j.drugalcdep.2004.04.007 [DOI] [PubMed] [Google Scholar]
- 25.Montazeri Z, Nyiraneza C, El-Katerji H, Little J. Waterpipe smoking and cancer: systematic review and meta-analysis. Tob Control 2017;26(1):92–7. doi: 10.1136/tobaccocontrol-2015-052758. [DOI] [PubMed] [Google Scholar]
- 26.Waziry R, Jawad M, Ballout RA, et al. The effects of waterpipe tobacco smoking on health outcomes: an updated systematic review and meta-analysis. Int J Epidemiol 2017;46(1):32–43. doi: 10.1093/ije/dyw021. [DOI] [PubMed] [Google Scholar]
- 27.Rezk-Hanna M, Benowitz NL. Cardiovascular Effects of Hookah Smoking: Potential Implications for Cardiovascular Risk. Nicotine Tob Res 2019;21(9):1151–61. doi: 10.1093/ntr/nty065. [DOI] [PubMed] [Google Scholar]
- 28.National Toxicology Program. Toxicology and Carcinogenesis Studies of Furan in F344/N Rats and B6C3F1 Mice. Vol NTP Technical Report No. 402. Research Triangle Park, NC: US Department of Health and Human Services, Public Health Service, National Institutes of Health Research, Triangle Park, NC; 1993. Accessed April 10, 2020. https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr402.pdf [Google Scholar]
- 29.Elmore LW, Sirica AE. "Intestinal-type" of adenocarcinoma preferentially induced in right/caudate liver lobes of rats treated with furan. Cancer Res 1993;53(2):254–9. https://cancerres.aacrjournals.org/content/canres/53/2/254.full.pdf [PubMed] [Google Scholar]
- 30.Terrell AN, Huynh M, Grill AE, et al. Mutagenicity of furan in female Big Blue B6C3F1 mice. Mutat Res Genet Toxicol Environ Mutagen 2014;770:46–54. doi: 10.1016/j.mrgentox.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tǎbǎran AF, O'Sullivan MG, Seabloom DE, et al. Inhaled Furan Selectively Damages Club Cells in Lungs of A/J Mice. Toxicol Pathol 2019;47(7):842–50. doi: 10.1177/0192623319869306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakhiya N, Appel KE. Toxicity and carcinogenicity of furan in human diet. Arch Toxicol 2010;84(7):563–78. doi: 10.1007/s00204-010-0531-y. [DOI] [PubMed] [Google Scholar]
- 33.Moro S, Chipman JK, Wegener JW, et al. Furan in heat-treated foods: Formation, exposure, toxicity, and aspects of risk assessment. Mol Nutr Food Res 2012;56(8):1197–1211. doi: 10.1002/mnfr.201200093. [DOI] [PubMed] [Google Scholar]
- 34.International Agency for Research on Cancer. Furan. In: Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals. 63rd ed. Lyon, France: International Agency for Research on Cancer; 1995:393. Accessed April 10, 2020. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono63.pdf [Google Scholar]
- 35.Gertler AW, Sagebiel JC, Dippel WA, Farina RJ. Measurements of Dioxin and Furan Emission Factors from Heavy-Duty Diesel Vehicles. J Air Waste Manag Assoc 1998;48(3):276–78. doi: 10.1080/10473289.1998.10463677. [DOI] [PubMed] [Google Scholar]
- 36.Grill AE, Schmitt T, Gates LA, et al. Abundant rodent furan-derived urinary metabolites are associated with tobacco smoke exposure in humans. Chem Res Toxicol 2015;28(7):1508–16. doi: 10.1021/acs.chemrestox.5b00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.European Food Safety Authority Panel on Contaminants in the Food Chain (EFSA CONTAM Panel), Knutsen HK, Alexander J, Barregard L, et al. Risks for public health related to the presence of furan and methylfurans in food. EFSA Journal 2017;15(10):5005,142 pp. Accessed April 10, 2020. 10.2903/j.efsa.2017.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pouli AE, Hatzinikolaou DG, Piperi C, et al. The cytotoxic effect of volatile organic compounds of the gas phase of cigarette smoke on lung epithelial cells. Free Radical Biol Med 2003;34(3):345–55. doi: 10.1016/s0891-5849(02)01289-3. [DOI] [PubMed] [Google Scholar]
- 39.Baek SO, and Jenkins RA. Characterization of trace organic compounds associated with aged and diluted sidestream tobacco smoke in a controlled atmosphere-volatile organic compounds and polycyclic aromatic hydrocarbons. Atmos Environ 2004;38(38):6583–99. doi: 10.1016/j.atmosenv.2004.08.016. [DOI] [Google Scholar]
- 40.Talhout R, Opperhuizen A, van Amsterdam JG. Sugars as tobacco ingredient: Effects on mainstream smoke composition. Food Chem Toxicol 2006;44(11):1789–98. doi: 10.1016/j.fct.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 41.Onishi M, Inoue M, Araki T, et al. Characteristic coloring curve for white bread during baking. Biosci Biotechnol Biochem 2011;75(2):255–60. doi: 10.1271/bbb.100558. [DOI] [PubMed] [Google Scholar]
- 42.Tomasik P. The thermal decomposition of carbohydrates. Part I. The decomposition of mono-, di-, and oligo-saccharides. Adv Carbohydr Chem Biochem 1989; 47:203–78. doi: 10.1016/S0065-2318(08)60415-1 [DOI] [Google Scholar]
- 43.Elson LA, Betts TE, Passey RD. The sugar content and the pH of the smoke of cigarette, cigar and pipe tobaccos in relation to lung cancer. Int J Cancer 1972;9(3):666–75. doi: 10.1002/ijc.2910090325. [DOI] [PubMed] [Google Scholar]
- 44.Brinkman MC, Teferra AA, Kassem NO, Kassem NOF. Effect of electric heating and ice added to the bowl on mainstream waterpipe semivolatile furan and other toxicant yields. Tob Control 2019. Sep 21. pii: tobaccocontrol-2019-054961. doi: 10.1136/tobaccocontrol-2019-054961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khater AE, Abd El-Aziz NS, Al-Sewaidan HA, Chaouachi K. Radiological hazards of Narghile (hookah, shisha, goza) smoking: activity concentrations and dose assessment. J Environ Radioact 2008;99(12):1808–14. doi: 10.1016/j.jenvrad.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Hadidi KA, Mohammed FI. Nicotine content in tobacco used in hubblebubble smoking. Saudi Med J 2004;25(7):912–17. https://pdfs.semanticscholar.org/d3d2/ea961d123d24b5ef760e2e0616206142c3bf.pdf?_ga=2.147521719.1889952986.1578688206-1668895357.1573868715 [PubMed] [Google Scholar]
- 47.Schubert J, Bewersdorff J, Luch A, Schulz TG. Waterpipe smoke: a considerable source of human exposure against furanic compounds. Anal Chim Acta 2012;709:105–12. doi: 10.1016/j.aca.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Matsushima S, Ishiguro S, Sugawara S. Composition studies on some varieties of tobacco and their smoke. I. Major components in smoke condensate. Beitr Tabakforsch Int 1979;10:31–8. doi: 10.2478/cttr-2013-0466 [DOI] [Google Scholar]
- 49.Limacher A, Kerler J, Davidek T, et al. Formation of furan and methylfuran by maillard-type reactions in model systems and food. J Agric Food Chem 2008;56(10):3639–47. doi: 10.1021/jf800268t. [DOI] [PubMed] [Google Scholar]
- 50.Arshad A, Matharoo J, Arshad E, et al. Knowledge, attitudes, and perceptions towards waterpipe tobacco smoking amongst college or university students: a systematic review. BMC Public Health 2019;19(1):439. doi: 10.1186/s12889-019-6680-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albertini R, Bird M, Doerrer N, et al. The use of biomonitoring data in exposure and human health risk assessments. Environ Health Perspect 2006;114(11):1755–62. doi: 10.1289/ehp.9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Von Tungeln LS, Walker NJ, Olson GR, et al. Low dose assessment of the carcinogenicity of furan in male F344/N Nctr rats in a 2-year gavage study. Food Chem Toxicol 2017;99:170–81. doi: 10.1016/j.fct.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cordelli E, Leopardi P, Villani P, et al. Toxic and genotoxic effects of oral administration of furan in mouse liver. Mutagenesis. 2010;25(3):305–314. doi: 10.1093/mutage/geq007. [DOI] [PubMed] [Google Scholar]
- 54.Gill S, Kavanagh M, Barker M, et al. Subchronic oral toxicity study of furan in B6C3F1 Mice. Toxicol Pathol. 2011;39(5):787–794. doi: 10.1177/0192623311412980. [DOI] [PubMed] [Google Scholar]
- 55.Chen LJ, Hecht SS, Peterson LA. Identification of cis-2-butene-1,4- dial as a microsomal metabolite of furan. Chem Res Toxicol 1995;8(7):903–906. doi: 10.1021/tx00049a001. [DOI] [PubMed] [Google Scholar]
- 56.Peterson LA, Naruko KC, Predecki DP. A reactive metabolite of furan, cis-2-butene-1,4-dial, is mutagenic in the Ames assay. Chem Res Toxicol 2000;13(7):531–4. doi: 10.1021/tx000065f. [DOI] [PubMed] [Google Scholar]
- 57.Gingipalli L, Dedon PC. Reaction of cis- and trans-2-Butene-1,4-dial with 2'-deoxycytidine to form stable oxadiazabicyclooctaimine adducts. J Am Chem Soc 2001;123(11):2664–5. doi: 10.1021/ja0056421. [DOI] [PubMed] [Google Scholar]
- 58.Gates LA, Lu D, Peterson LA. Trapping of cis-2-butene-1,4-dial to measure furan metabolism in human liver microsomes by cytochrome P450 enzymes. Drug Metab Dispos 2012;40(3):596–01. doi: 10.1124/dmd.111.043679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Byrns MC, Predecki DP, Peterson LA. Characterization of nucleoside adducts of cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol 2002;15(3):373–39. doi: 10.1021/tx0101402. [DOI] [PubMed] [Google Scholar]
- 60.Byrns MC, Vu CC, Peterson LA. The formation of substituted 1,N6-etheno-2'-deoxyadenosine and 1,N2-etheno-2'-deoxyguanosine adducts by cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol 2004;17(12):1607–13. doi: 10.1021/tx049866z. [DOI] [PubMed] [Google Scholar]
- 61.Peterson LA, Cummings ME, Vu CC, Matter BA. Glutathione trapping to measure microsomal oxidation of furan torcis-2-butene-1,4-dial. Drug Metab Dispos 2005;33:1453–58. doi: 10.1124/dmd.105.004432. [DOI] [PubMed] [Google Scholar]
- 62.Byrns MC, Vu CC, Neidigh JW, et al. Detection of DNA adducts derived from the reactive metabolite of furan, cis-2-butene-1,4-dial. Chem Res Toxicol 2006;19(3):414–20. doi: 10.1021/tx050302k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peterson LA, Phillips MB, Lu D, Sullivan MM. Polyamines are traps for reactive intermediates in furan metabolism. Chem Res Toxicol 2011;24(11):1924–36. doi: 10.1021/tx200273z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peterson LA. Reactive metabolites in the biotransformation of molecules containing a furan ring. Chem Res Toxicol 2013;26(1):6–25. doi: 10.1021/tx3003824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peterson LA, Cummings ME, Chan JY, et al. Identification of a cis-2-butene-1,4-dial-derived glutathione conjugate in the urine of furan-treated rats. Chem Res Toxicol 2006;19(9):1138–41. doi: 10.1021/tx060111x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kellert M, Wagner S, Lutz U, Lutz WK. Biomarkers of furan exposure by metabolic profiling of rat urine with liquid chromatography-tandem mass spectrometry and principal component analysis. Chem Res Toxicol 2008;21(3):761–68. doi: 10.1021/tx7004212. [DOI] [PubMed] [Google Scholar]
- 67.Lu D, Sullivan MM, Phillips MB, Peterson LA. Degraded protein adducts of cis-2-butene-1,4-dial are urinary and hepatocyte metabolites of furan. Chem Res Toxicol 2009;22(6):997–1007. doi: 10.1021/tx800377v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu D, Peterson LA. Identification of furan metabolites derived from cysteine-cis-2-butene-1,4-dial-lysine cross-links. Chem Res Toxicol 2010;23(1):142–51. doi: 10.1021/tx9003215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deveci SE, Deveci F, Açik Y, et al. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respir Med 2004;98(6):551–56. doi: 10.1016/j.rmed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 70.Fraselle S, De Cremer K, Coucke W, et al. Development and validation of an ultra-high performance liquid chromatography–tandem mass spectrometry method to measure creatinine in human urine. Journal of Chromatography B. 2015;988:88–97. doi: 10.1016/j.jchromb.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 71.Ou M, Song Y, Li S, et al. LC-MS/MS Method for Serum Creatinine: Comparison with Enzymatic Method and Jaffe Method. Bueno V, editor. PLoS One 2015;10:e0133912. doi: 10.1371/journal.pone.0133912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quintana PJE, Hoh E, Dodder NG, et al. Nicotine levels in silicone wristband samplers worn by children exposed to secondhand smoke and electronic cigarette vapor are highly correlated with child’s urinary cotinine. J Expo Sci Environ Epidemiol 2019;29(6):733–41. doi: 10.1038/s41370-019-0116-7. [DOI] [PubMed] [Google Scholar]
- 73.Centers for Disease Control and Prevention (CDC). Defining Adult Overweight and Obesity. Adult Body Mass Index (BMI). 2020. Accessed April 10, 2020. https://www.cdc.gov/obesity/adult/defining.html [Google Scholar]
- 74.Carmella SG, Chen M, Han S, et al. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol 2009. Apr;22(4):734–41. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamabe A, Uto H, Imamura Y, et al. Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period. J Gastroenterol 2011;46(6):769–78. doi: 10.1007/s00535-011-0376-z. [DOI] [PubMed] [Google Scholar]
- 76.Ou H, Fu Y, Liao W, Zheng C, Wu X. Association between smoking and liver fibrosis among patients with nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol 2019. Oct 15;2019:6028952. doi: 10.1155/2019/6028952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kassem NOF, Jackson SR, Kassem NO, et al. College Student Beliefs and Behavior Regarding Sharing When Smoking Hookahs. Am J Health Behav 2019;43(1):133–44. doi: 10.5993/AJHB.43.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar SR, Davies S, Weitzman M, Sherman S. A review of air quality, biological indicators and health effects of second-hand waterpipe smoke exposure. Tob Control 2015;24 Suppl 1:i54–i59. doi: 10.1136/tobaccocontrol-2014-052038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kassem NOF, Kassem NO, Jackson SR, et al. Benzene uptake in Hookah smokers and non-smokers attending Hookah social events: regulatory implications. Cancer Epidemiol Biomarkers Prev 2014;23(12):2793–809. doi: 10.1158/1055-9965.EPI-14-0576. [DOI] [PubMed] [Google Scholar]
- 80.Weitzman M, Yusufali AH, Bali F, et al. Effects of hookah smoking on indoor air quality in homes. Tob Control 2016;26(5):586–91. doi: 10.1136/tobaccocontrol-2016-053165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moon KA, Rule AM, Magid HS, et al. Biomarkers of Secondhand Smoke Exposure in Waterpipe Tobacco Venue Employees in Istanbul, Moscow, and Cairo. Nicotine Tob Res 2018;20(4):482–91. doi: 10.1093/ntr/ntx125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang B, Haji F, Kaufman P, et al. 'Enter at your own risk': a multimethod study of air quality and biological measures in Canadian waterpipe cafes. Tob Control 2015;24(2):175–81. doi: 10.1136/tobaccocontrol-2013-051180. [DOI] [PubMed] [Google Scholar]
- 83.Gurung G, Bradley J, Delgado-Saborit JM. Effects of shisha smoking on carbon monoxide and PM2.5 concentrations in the indoor and outdoor microenvironment of shisha premises. Sci Total Environ 2016;548-549:340–6. doi: 10.1016/j.scitotenv.2015.12.093. [DOI] [PubMed] [Google Scholar]
- 84.Colditz JB, Chu KH, Switzer GE, et al. Online data to contextualize waterpipe tobacco smoking establishments surrounding large US universities. Health Informatics J 2019;25(4):1314–24. doi: 10.1177/1460458217754242. [DOI] [PubMed] [Google Scholar]
- 85.Maziak W, Ben Taleb Z , Jawad M , et al. Consensus statement on assessment of waterpipe smoking in epidemiological studies. Tob Control 2017;26(3):338–343. doi: 10.1136/tobaccocontrol-2016-052958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.U.S. Food and Drug Administration. Harmful and Potentially Harmful Constituents (HPHCs). 2019. Accessed January 10, 2020. https://www.fda.gov/tobacco-products/products-ingredients-components/harmful-and-potentially-harmful-constituents-hphcs [Google Scholar]
- 87.U.S. Food and Drug Administration. Deeming Tobacco Products to Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Regulations on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. Federal Register. 2014;79:23142–23207. Accessed January 10, 2020. https://www.fda.gov/media/88754/download [PubMed] [Google Scholar]
- 88.McCarthy M FDA moves to regulate e-cigarettes and pipe and hookah tobacco. BMJ. 2014;348:g2952. doi: 10.1136/bmj.g2952. [DOI] [PubMed] [Google Scholar]
- 89.Kedderis G, Carfagna M, Held S, et al. Kinetic analysis of furan biotransformation by F-344 rats in vivo and in vitro. Toxicol Appl Pharmacol 1993;123(2):274–282. doi: 10.1006/taap.1993.1246. [DOI] [PubMed] [Google Scholar]
- 90.Burka L, Washburn K, Irwin R. Disposition of [14C]furan in the male F344 rat. J Toxicol Environ Health 1991;34(2):245–257. doi: 10.1080/15287399109531564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.