ABSTRACT
Open cystogastrostomy is the standard treatment for the operative management of pancreatic pseudocysts. We describe our technique of minimally invasive open cystogastrostomy for giant pediatric pancreatic pseudocyst. Preoperative incision marking on the most prominent part of the pseudocyst was done by ultrasound guidance. A transverse incision of approximately 3–4 cm was made, and a minilaparotomy was performed. Stay sutures were applied on the anterior wall of the stomach. The anterior wall was exteriorized; transverse gastrotomy was performed, and superior and inferior flaps were made. Deaver’s retractor was placed inside the lumen, and cystogastrostomy was completed. We employed this technique in five male patients without any complications. All patients were allowed clear liquids on postoperative day 4 or 5; and gradually shifted to a soft diet. The mean duration of postoperative stay was 7 days. The size of the scar ranged from 3 to 5 cm. All patients were doing well on follow-up. Our technique of minimally invasive open cystogastrostomy is a viable option for pancreatic pseudocyst in pediatric patients.
KEYWORDS: Cystogastrostomy, minimally invasive, open, pediatric, pancreatic, pseudocyst, trauma
Dear Sir,
Cystogastrostomy is the surgical procedure of choice for drainage of the pancreatic pseudocyst.[1,2,3,4] Laparoscopic surgery is challenging in pediatric patients with giant pancreatic pseudocyst cysts.[5] Furthermore, the endoscopic approach is less invasive but is technically demanding in the pediatric age group and associated with recurrence.[6] We describe our technique of minimally invasive open cystogastrostomy for giant pancreatic pseudocysts.
TECHNIQUE
All pancreatic pseudocyst was included [Figure 1] except those primarily arising from the tail of the pancreas and/or in the infracolic compartment. Preoperative incision marking on the most prominent part of the pseudocyst was done by ultrasound guidance, a day before the surgery [Figure 1]. A transverse incision of approximately 3–4 cm in size was made and a mini-laparotomy was performed. Stay sutures were applied on the anterior wall of the body of the stomach [Figure 2]. The anterior wall was exteriorized; transverse gastrotomy was performed. It was followed by the creation of superior and inferior flaps of the stomach for better visualization of the tented posterior wall. We ensured the protection of both the wound edges and the peritoneal cavity. Four traction sutures (two each on the edges of superior and inferior flaps) were placed. The cyst was aspirated at its most prominent part to evaluate its contents and confirmation of the diagnosis. Deaver’s/Langenbach’s retractors were gently placed inside the lumen and the cyst was opened with the help of electrocautery [Figure 2]. The opening was widened for ≥2.5 cm and cyst fluid and debris were sucked out with a suction cannula. The septa present inside the cyst were broken down completely. Cystogastrostomy was completed by application of interrupted PGA 2-0 sutures circumferentially. The anterior wall of the stomach was closed using PGA 3-0 sutures and the procedure was completed. Postoperatively, the patients were allowed clear liquids on either postoperative day 4 or 5, depending on the bowel movements; they were gradually given a soft diet. During the follow-up visit (7 days, one and then monthly), patients were checked for the presence of complications.
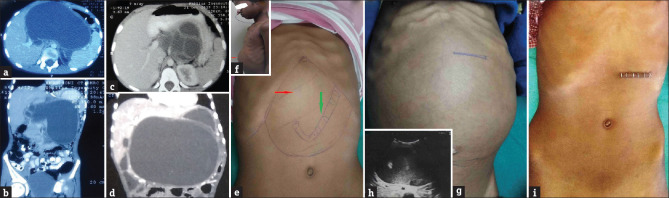
Figure 1.
Contrast-enhanced computed tomography (CECT) with both oral and intravenous contrast images of patients: (a) Axial section image (case A) showing a large 16.7 cm × 9.6 cm sized cyst in relation to the body of pancreas with 3 mm wall thickness. It is present in the lesser sac, pushing the stomach (nasogastric tube in situ) antero-laterally and causing displacement of the portal vein. It extends into the splenic hilum. (b) Coronal section image (case A) of the cyst in the supracolic compartment and extending into the paracolic compartment. The cyst is abutting the porta hepatis and pushing the stomach superolaterally. (c) Axial section images (case B) of a large (8.2 cm × 11.2 cm) sized, well-defined hypodense cystic lesion with internal echoes and septations in relation to the tail and body of the pancreas. The cyst is thick walled and abutting the celiac axis and superior mesenteric artery; encasement of the splenic artery is present. (d) Coronal section image (case C) of the cyst shows a large well-defined thin-walled (3 mm) cyst (15 cm × 10 cm × 9 cm) in close relation to the body of the pancreas) The cyst is pushing the bowel loops inferiorly, and transverse colon antero-superiorly. Clinical photographs of the patients with pseudocysts of the pancreas: (e) epigastric lump with nasogastric tube palpable per-abdominally. (f) Inset image showing a large epigastric lump causing bulging of the upper abdominal wall. (g) A 4-year-old boy with a large epigastric lump causing bulging of the anterior abdominal wall; the site marking of the incision site has been performed under ultrasound guidance. (h) Inset ultrasound image shows a large cystic lesion (11.1 cm × 8.4 cm) in the epigastrium having a cyst wall of 2.6 mm, arising in relation to the body of the pancreas. (i) Postoperative photograph shows a small scar with staples in place
Figure 2.
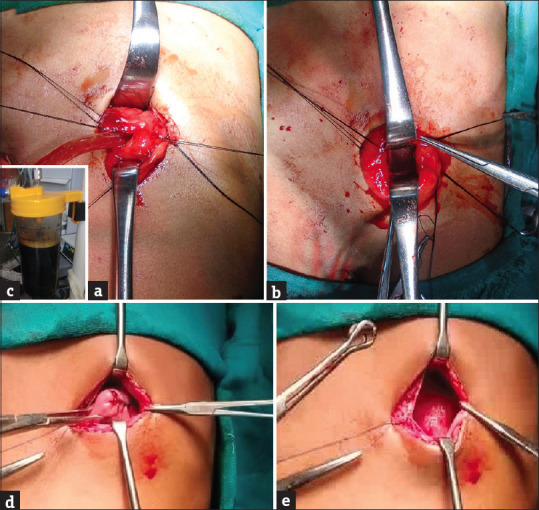
Intraoperative photographs show (a) traction sutures on the edges of superior and inferior flaps of the stomach wall and stay sutures on the posterior wall. Langenbach’s retractors were placed inside the gastric lumen and the contents of the cyst were sucked with a suction cannula; (b) Cystogastrostomy completed and hemostasis checked. (c) Contents of cyst (>1500 mL) in suction apparatus. (d) Intraoperative photographs show (d) closure of the anterior gastric wall and (e) sheath closure
We employed this technique in five male patients with giant pancreatic pseudocyst. The size of the cyst was >10 cm in all patients. The age of patients ranged from 4 to 12 years. The duration of illness ranged from 2 to 12 months. The etiology was posttraumatic in all cases. The contents of the cyst were pancreatic fluid and debris. There were two patients with septa inside the cyst; they were broken down with the help of a suction cannula. There were no intraoperative or postoperative complications. The mean duration of postoperative stay was 7 days. The duration of hospital stay ranged from 9 to 23 days (mean = 13.2 days). The size of the scar ranged from 3 to 5 cm; initially, it was 5 cm, which has gradually reduced to 3 cm in length. All patients were doing well at 1–3 years follow-up.
Pancreatic pseudocyst (lack epithelial lining) is a rare entity. The cysts with a major diameter of 10 cm or more (as seen in all patients in our series) are referred to as giant pancreatic pseudocyst.[3,7] In pediatric patients, blunt abdominal trauma is invariably its most common etiology followed rarely by acute pancreatitis.[6] Abdominal ultrasound has a sensitivity of 70%–90% in the detection of pancreatic pseudocyst. Computer tomography suggests a thick-walled and clear fluid-filled mass adjacent to the pancreas with a history of pancreatitis or pancreatic trauma (sensitivity ≈ 90%–100%). Magnetic resonance imaging is the most accurate and sensitive imaging modality for the evaluation of the pancreatic and biliary ductal anatomy and pseudocyst.[8]
Operative interventions (approximately one-third of patients) are undertaken for symptomatic cysts and with complications, for example, infection, fistula formation, gastric outlet obstruction, obstructive jaundice, spontaneous rupture, and massive gastrointestinal hemorrhage.[9] Pancreatic pseudocysts follow a “Rule of 6” for their treatment with cysts >6 cm in diameter or duration >6 weeks following the initial injury.[10]
Open cystogastrostomy (success rate – 90%, recurrence rate – 0%–6%) is a standard practice in the operative treatment of pancreatic pseudocyst (Jedlicka, 1931).[1,9] The procedure is contemplated if the cyst is in the supracolic compartment (epigastric) and retrogastric position. But it is associated with severe postoperative pain due to a relatively large incision. Our technique is similar to the method used by Sekioka (2018) et al., who performed open cystogastrostomy by a mini-laparotomy (4 cm) using a wound retractor (Smart Retractor®).[2]
Our technique of minimally invasive open cystogastrostomy is a viable option for pediatric pancreatic pseudocysts. The insertion of conventional retractors created a satisfactory operative field. It is cost-effective without the use of stapling devices and additional wound retractors. It is safe with shortened operative time.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Department of Paediatric Surgery, SMS Medical College, Jaipur, Rajasthan, India.
REFERENCES
- 1.Hassan MA. Open cystogastrostomy in the management of a large pancreatic pseudocyst in a child. J Pediatr Surg Case Rep. 2020;59:1–3. [Google Scholar]
- 2.Sekioka A, Takahashi T, Fukumoto K, Urushihara N. Intragastric cystogastrostomy in a 4-year-old child with a pancreatic pseudocyst: A novel technique. Afr J Paediatr Surg. 2018;15:148–50. doi: 10.4103/ajps.AJPS_71_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang GC, Misra S. A giant pancreatic pseudocyst treated by cystogastrostomy. BMJ Case Rep. 2015;2015:bcr2014207271. doi: 10.1136/bcr-2014-207271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frey CF. Pancreatic pseudocyst –Operative strategy. Ann Surg. 1978;188:652–62. doi: 10.1097/00000658-197811000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makris KI, St Peter SD, Tsao KJ, Ostlie DJ. Laparoscopic intragastric stapled cystgastrostomy of pancreatic pseudocyst in a child. J Laparoendosc Adv Surg Tech A. 2008;18:771–3. doi: 10.1089/lap.2007.0126. [DOI] [PubMed] [Google Scholar]
- 6.Melman L, Azar R, Beddow K, Brunt LM, Halpin VJ, Eagon JC, et al. Primary and overall success rates for clinical outcomes after laparoscopic, endoscopic, and open pancreatic cystgastrostomy for pancreatic pseudocysts. Surg Endosc. 2009;23:267–71. doi: 10.1007/s00464-008-0196-2. [DOI] [PubMed] [Google Scholar]
- 7.Tuboku-Metzger VR, Seenath MM, Tan LC. Peritonitis secondary to traumatic duodenal laceration in the presence of a large pancreatic pseudocyst: A case report. J Med Case Rep. 2011;5:528. doi: 10.1186/1752-1947-5-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agalianos C, Passas I, Sideris I, Davides D, Dervenis C. Review of management options for pancreatic pseudocysts. Transl Gastroenterol Hepatol. 2018;3:18. doi: 10.21037/tgh.2018.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalifa M, Gobran T, Shreef KS, Waly A. Pancreatic pseudocyst in children: A single institute experience. Ann Pediatr Surg. 2015;11:127–31. [Google Scholar]
- 10.Yeo CJ, Bastidas JA, Lynch-Nyhan A, Fishman EK, Zinner MJ, Cameron JL. The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gynecol Obstet. 1990;170:411–7. [PubMed] [Google Scholar]