Abstract
We isolated a recessive symbiotic mutant of Lotus japonicus that defines a genetic locus, LOT1 (for low nodulation and trichome distortion). The nodule number per plant of the mutant was about one-fifth of that of the wild type. The lot1 mutant showed a moderate dwarf phenotype and distorted trichomes, but its root hairs showed no apparent differences to those of the wild type. Infection thread formation after inoculation of Mesorhizobium loti was repressed in lot1 compared to that in the wild type. The nodule primordia of lot1 did not result in any aborted nodule-like structure, all nodules becoming mature and exhibiting high nitrogen fixation activity. The mutant was normally colonized by mycorrhizal fungi. lot1 also showed higher sensitivity to nitrate than the wild type. The grown-up seedlings of lot1 were insensitive to any ethylene treatments with regard to nodulation, although the mutant showed normal triple response on germination. It is conceivable that a nodulation-specific ethylene signaling pathway is constitutively activated in the mutant. Grafting experiments with lot1 and wild-type seedlings suggested that the root genotype mainly determines the low nodulation phenotype of the mutant, while the trichome distortion is regulated by the shoot genotype. Grafting of har1-4 shoots to lot1 roots resulted in an intermediate nodule number, i.e. more than that of lot1 and less than that of har1-4. Putative double mutants of lot1 and har1 also showed intermediate nodulation. Thus, it was indicated that LOT1 is involved in a distinct signal transduction pathway independent of HAR1.
Leguminous plants form nitrogen-fixing root nodules postembryonically with symbiotic bacteria called rhizobia. This cross-kingdom symbiosis is initiated by reciprocal signal exchange between the two organisms (for review, see Geurts and Bisseling, 2002; Oldroyd and Downie, 2004).
Nodulation in legumes is tightly controlled. The best characterized control mechanism is termed autoregulation of nodulation, in which the nodule formation on one part of a rhizobium-infected root systematically inhibits subsequent nodulation of nearby regions (Nutman, 1952; Kosslak and Bohlool, 1984; Caetano-Anollés and Gresshoff, 1991; van Brussel et al., 2002). A defect of autoregulation results in supernodulation or hypernodulation of soybean (Glycine max) mutants (Carroll et al., 1985a, 1985b; Gremaud and Harper, 1989; Akao and Kouchi, 1992), which are regulated by the shoot genotype (Delves et al., 1986; Sheng and Harper, 1997). Similar hypernodulation mutants have been isolated from Lotus japonicus (Schauser et al., 1998; Szczyglowski et al., 1998; Kawaguchi et al., 2002), a model legume (Handberg and Stougaard, 1992; Jiang and Gresshoff, 1997), and termed har1 for hypernodulation aberrant root formation (Wopereis et al., 2000).
The mobile signal molecules involved in autoregulation have not yet been identified. Besides autoregulation, it is generally known that leguminous plants do not form root nodules when they are exposed to high concentrations of a nitrogen source such as nitrate (Streeter, 1988; Carroll and Mathews, 1990). Ethylene is another negative factor; insensitivity to ethylene causes hypernodulation of Medicago truncatula, another model legume (Penmetsa and Cook, 1997). In addition to the above well-known signaling mechanisms, there are other mechanisms for control of the nodule number. For example, the astray mutant of L. japonicus starts nodule development early and forms approximately twice the number of nodules on a wider area of roots compared to the wild type, and shows normal sensitivity to ethylene and nitrate (Nishimura et al., 2002b, 2002c). It remains to be clarified if the recently reported sunn mutant of M. truncatula (Penmetsa et al., 2003) is orthologous to har1 of L. japonicus.
Ethyl methanesulfonate-mutagenized L. japonicus symbiotic mutants fall into four basic categories: (1) nonnodulation (Nod−); (2) hypernodulation (Nod2+); (3) defect in cooperative histogenesis (Hist−); and (4) ineffective nodulation that often accompanies early senescence (Fix−; Kawaguchi et al., 2002). Along with developing fundamentals of L. japonicus and M. truncatula genomics, an increasing number of symbiotic genes have been cloned. These include NIN (Schauser et al., 1999; Borisov et al., 2003), SYMRK/NORK (Endre et al., 2002; Stracke et al., 2002), NFR1 and NFR5 (Madsen et al., 2003; Radutoiu et al., 2003), LYK3/4 (Limpens et al., 2003), DMI1 (Ané et al., 2004), and DMI3 (Levy et al., 2004; Mitra et al., 2004) for the Nod− phenotype. On the other hand, for the Nod2+ phenotype, the HAR1/NARK (Krusell et al., 2002; Nishimura et al., 2002a; Searle et al., 2003) and ASTRAY (Nishimura et al., 2002b) genes have also been cloned.
In this article, we report the isolation and initial characterization of a L. japonicus mutant, named lot1, which shows unprecedented low nodulation, distorted trichomes, and moderate dwarfism.
RESULTS
Plant Phenotype and Growth Kinetics of the lot1 Mutant
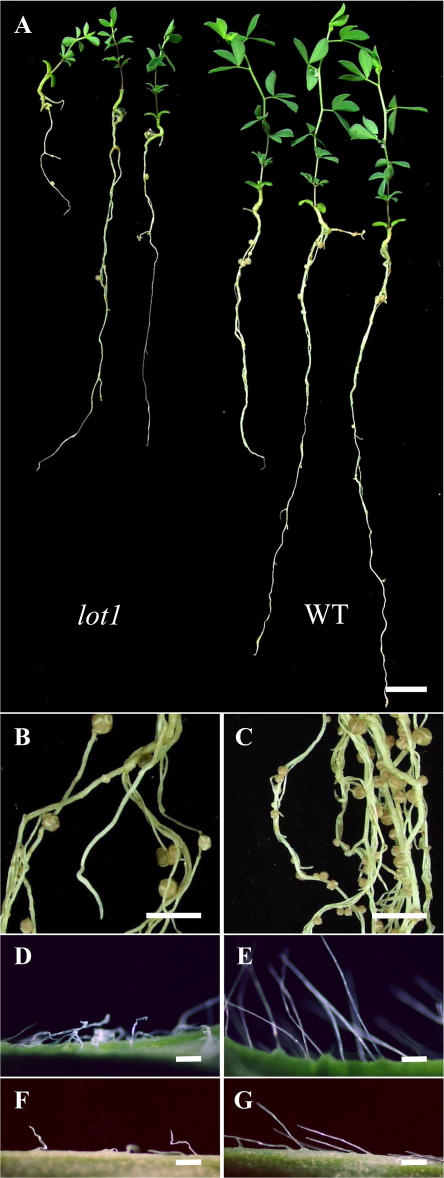
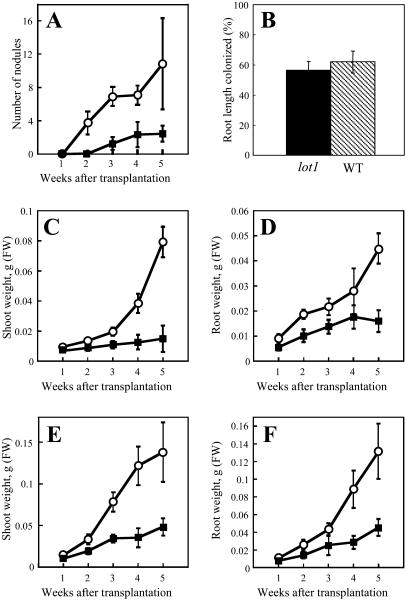
When the lot1 mutant was inoculated with Mesorhizobium loti Tono, it formed apparently healthy nodules, but the number was around 20% that of the wild type (Figs. 1A and 2A).The lot1 mutant also showed a moderate dwarf phenotype (Fig. 1A). Both the shoots and roots of lot1 were shorter than those in the wild type when the plants were grown not only on a nitrogen-free medium with M. loti (Fig. 2, C and D) but also on a nitrogen-rich medium without M. loti (Fig. 2, E and F). At 8 weeks after M. loti inoculation, nodules were formed much more sparsely than in the wild type (Fig. 1, B and C). In addition, the lot1 mutant formed wavy trichomes in calyx regions (Fig. 1D) and on the abaxial side of leaflets (Fig. 1F). According to the proposed guidelines for L. japonicus genetic nomenclature (Stougaard et al., 1999), we named the mutant lot1 for low nodulation and trichome distortion. In contrast to the unique nodulation phenotype, the lot1 mutant was colonized by mycorrhizal fungi as effectively as the wild type (Fig. 2B).
Figure 1.
The appearance of the lot1 mutant and wild type. A, Plants grown for 4 weeks after inoculation of M. loti Tono. Left, lot1; right, wild type. B and C, Mature nodules of lot1 and the wild type, respectively, at 8 weeks postinoculation (wpi). D and E, Trichomes in calyx regions of lot1 and the wild type, respectively. F and G, Trichomes on the abaxial side of leaflets of lot1 and the wild type, respectively. Bars in A to C, 1 cm; bars in D to G, 300 μm.
Figure 2.
Nodulation, mycorrhizal colonization, and growth kinetics of lot1 and the wild type. A, Numbers of nodule primordia and mature nodules as a function of time. One week after transplantation, inoculation of M. loti Tono was carried out as described in “Materials and Methods.” The means and sds are presented (n = 10). B, Comparison of mycorrhizal colonization between lot1 and the wild type. The means and sds (n = 3) are not significantly different at P < 0.01 according to the t test. C to F, Growth kinetics of shoots and roots. The means and sds are presented (n = 10). C and D, Shoot weight and root weight, respectively. Seedlings at 7 d after germination were transplanted onto nitrogen-free B & D medium on vermiculite, and, 1 week later, they were inoculated with M. loti Tono. E and F, The seedlings were transplanted onto nitrogen-rich 0.5× Gamborg B5 medium on vermiculite and then grown without bacteria. White circles and black squares in all sections except B indicate the wild type and lot1 mutant, respectively.
Monogenic and Recessive Inheritance of the lot1 Phenotype
When the lot1 mutant was backcrossed with the parental wild type L. japonicus Gifu B-129, all F1 progenies formed as many nodules as the wild type. The F1 plants were naturally self-crossed, and the resulting F2 progenies segregated at the ratio of 189:53 (3:1, χ2 = 0.228), indicating recessive and monogenic Mendelian inheritance of the low nodulation phenotype. This finding was confirmed by crossing the lot1 mutant with another wild-type line, L. japonicus Miyakojima MG-20 (Kawaguchi et al., 2001). The F2 progenies segregated at the ratio of 129:42 (3:1, χ2 = 0.002). As far as we examined the F2 population, no genetic segregation was found among low nodulation, moderate dwarfism, and crinkly trichomes.
Repressed Formation of Nodule Primordia on lot1 Roots
A series of experiments was conducted to determine why the lot1 mutant forms a smaller number of nodules than the wild type. Root hair deformation of the lot1 mutant induced by M. loti was indistinguishable from that of the wild type (data not shown). In our experimental system, the infection threads and nodule primordia were first observed at 3 and 7 d postinfection (dpi), respectively. As shown in Table I, infection thread formation with green fluorescent protein (GFP)-labeled M. loti BNO2 was significantly blocked compared to that of the wild type, although the shape of the infection threads was apparently normal in the mutant (data not shown). Similar results were obtained with lacZ-labeled M. loti ML001 (data not shown). Abortion at some step after initiation might also have occurred (Table I). It is noteworthy that once nodule primordia were formed on lot1 roots, they did not result in any aborted nodule-like structure, all nodules becoming mature. The inside structure of mature lot1 nodules was normal, bacteroid-infected cells and uninfected cells being indistinguishable from those of the wild type (data not shown). The nitrogenase activity of lot1 nodules determined as acetylene reduction was as high as that of the wild type (Table II).
Table I.
The nodulation process in lot1 mutant and the wild type
Infection threads were observed for the first time and counted microscopically at 3 dpi for M. loti ML001, which expresses GFP constitutively. At 7 dpi, nodules and primordia were observed for the first time and counted again. The successful nodulation ratios were then calculated by dividing the numbers of nodules and primordia by those of infection threads at 3 dpi. The means and sds are presented (n = 8). Each value for lot1 was significantly lower than that for the wild type at P < 0.01 according to the t test.
| Parameter | dpi | Wild Type | lot1 |
|---|---|---|---|
| Number of infection threads | 3 | 17.7 ± 6.1 | 2.8 ± 2.3 |
| 5 | 68.4 ± 20.0 | 29.2 ± 16.1 | |
| 7 | 153 ± 39.2 | 51.9 ± 21.5 | |
| Number of nodules and primordia | 7 | 3.8 ± 1.4 | 0.1 ± 0.3 |
| Successful nodulation (%) | 21 | 4 |
Table II.
Acetylene reduction activity of nodules formed by lot1 and the wild type
Acetylene reduction activity was determined with dissected root portions at 8 wpi. The means and sds are presented (n = 6). Different letter suffixes within a column indicate significant differences at P < 0.01 according to the t test.
| C2H2 Reduction Activity | ||
|---|---|---|
| μmol h−1 plant−1 | μmol h−1 g−1 (fresh nodule weight) | |
| Wild type | 1.12 ± 0.49 a | 17.4 ± 2.1 a |
| lot1 | 0.20 ± 0.09 b | 16.2 ± 4.6 a |
Sensitivity of the lot1 Mutant to Exogenous Nitrate
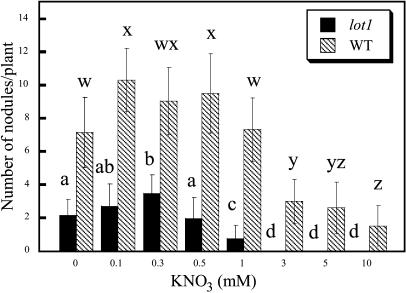
It is known that exogenous nitrate inhibits nodule formation by legumes (Streeter, 1988; Carroll and Mathews, 1990). Accordingly, we examined the effects of varying concentrations of nitrate in the medium on nodule formation by lot1 mutant and wild-type plants. As shown in Figure 3, the nodule formation by the lot1 mutant was reduced with 1 mm nitrate and completely blocked with more than 3 mm nitrate. On the other hand, wild-type plants grown with 1 mm nitrate formed a similar number of nodules to a control without nitrate. The wild type kept forming nodules even with 10 mm nitrate, although the number was reduced to some extent (Fig. 3). The latter results are similar to those of Hussain et al. (1999) and Nishimura et al. (2002c). Thus, it was suggested that lot1 shows higher sensitivity to exogenous nitrate than the wild type.
Figure 3.
Effects of varying concentrations of nitrate on nodule formation by lot1 and the wild type. Seedlings of 7 d after germination were transplanted into B & D medium on vermiculite containing the indicated concentrations of KNO3, and, after 1 week, they were inoculated with M. loti Tono. The seedlings were grown for 3 weeks, and then the number of mature nodules was determined. The means and sds (n = 6) are presented. Different letters above the bars indicate significant differences at P < 0.01 according to the t test.
Nodule Formation by the lot1 Mutant with Various Rhizobia
It is generally known that Rhizobium mutants lacking nitrogen fixation form more nodules than wild-type bacteria (Nutman, 1949; Hirsch and Smith, 1987). The low nodule number of the lot1 mutant might be caused by higher sensitivity to fixed nitrogen of bacteroids. Therefore, we checked nodule formation by a nifH-deficient mutant of M. loti. As shown in Table III, the nifH-deficient mutant formed more nodules not only on wild-type roots but also lot1 roots. Thus, lot1 is capable of perceiving fixed nitrogen. However, the inoculation of ineffective M. loti onto lot1 roots resulted in a smaller increase in nodule number than in the case of the wild type (Table III). Unlike the nifH-deficient mutant, Rhizobium etli CE3, a heterologous symbiont that forms partly effective nodules on the wild type (Banba et al., 2001), scarcely formed nodules on the lot1 mutant (Table III).
Table III.
Nodulation of lot1, har1-4, and the wild type on inoculation of various rhizobia
A nifH-defective mutant of M. loti (ΔnifH; J. Maruya and K. Saeki, unpublished data) and its parent strain, MAFF 303099, were inoculated to determine the numbers of nodules formed on lot1, har1-4, and the wild-type L. japonicus Gifu B-129. Another wild type, M. loti Tono, and R. etli CE3 (Banba et al., 2001) were also examined. The means and sds at 6 wpi are presented (n = 12–18). Different letter suffixes within a column indicate significant differences at P < 0.05 according to the t test.
| Rhizobia
|
Number of Nodules
|
||
|---|---|---|---|
| lot1 | har1-4 | Gifu B-129 | |
| M. loti MAFF 303099 | 3.3 ± 1.5 a | NDa | 8.1 ± 1.8 a |
| ΔnifH | 5.8 ± 2.9 b | ND | 26.3 ± 4.2 b |
| M. loti Tono | 2.2 ± 0.8 a | 23.4 ± 7.3 a | 6.8 ± 1.7 a |
| R. etli CE3 | 0.2b ± 0.4 c | 13.1 ± 4.3 b | 7.3 ± 2.2 a |
ND, Not determined.
About 17% of the lot1 mutant inoculated with R. etli CE3 formed only a single nodule, the others not forming any nodules.
Ethylene Sensitivity of the lot1 Mutant
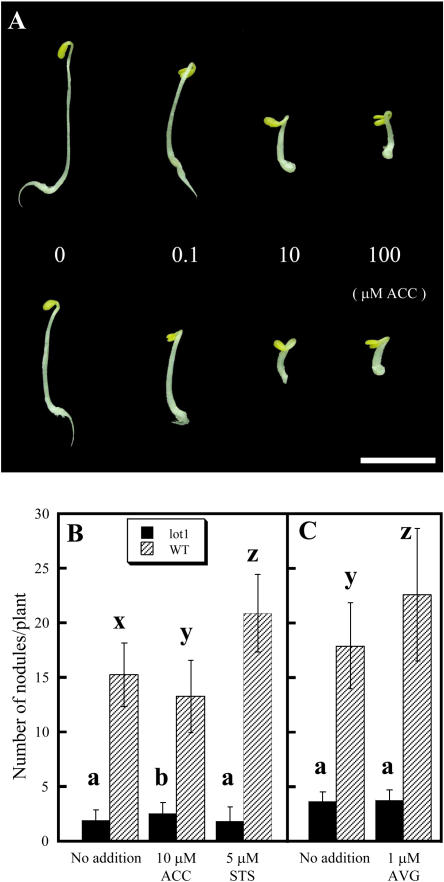
We examined the sensitivity of the lot1 mutant to ethylene, which inhibits nodule formation. As shown in Figure 4A, application of 1-aminocyclopropane-1-carboxylic acid (ACC), an ethylene precursor (Adams and Yang, 1979), on germination in the dark caused a typical triple response, i.e. inhibition of root and hypocotyl elongation, radial swelling of hypocotyls, and hypocotyl hook formation, of the lot1 mutant as well as the wild type. The effects of ACC on nodule formation by grown-up seedlings (Fearn and LaRue, 1991; Penmetsa and Cook, 1997; Wopereis et al., 2000; Penmetsa et al., 2003) were investigated next. As expected, 10 μm ACC reduced the nodule number of the wild type (Fig. 4B). On the other hand, ACC showed no effect or caused a slight increase in the nodule number of the lot1 mutant. In parallel, we applied silver thiosulfate (STS), an inhibitor of the ethylene action (Veen, 1983; Nukui et al., 2004), to the seedlings. Five micromolar STS clearly increased the nodule number of the wild type (Fig. 4B). The effect of STS on lot1 nodule formation was not significant. Application of 1 μm aminoethoxyvinylglycine (AVG), a potent inhibitor of ethylene synthesis (Yu et al., 1979), to the seedlings caused an increase in the nodule number of the wild type, but the nodule number of lot1 did not change either (Fig. 4C). Overall, lot1 showed insensitivity to ethylene with regard to nodulation, although its triple response was normal.
Figure 4.
Production and sensitivity to ethylene of lot1 mutant. A, Triple response of the wild type (top) and the lot1 mutant (bottom). Seeds were germinated in the dark in the presence of varying concentrations of ACC. Bar, 1 cm. B, Effects of ACC and STS on the nodule number of lot1 and wild-type plants. Seedlings were inoculated with M. loti Tono and grown for 5 weeks in the absence or presence of 10 μm ACC or 5 μm STS, and then the mature nodules were counted. C, Effect of 1 μm AVG on the nodule number in an experiment independent of B. The means and sds are presented (n = 16–30 for lot1, n = 24–30 for the wild type). Different letters within a section indicate significant differences at P < 0.05 according to the t test.
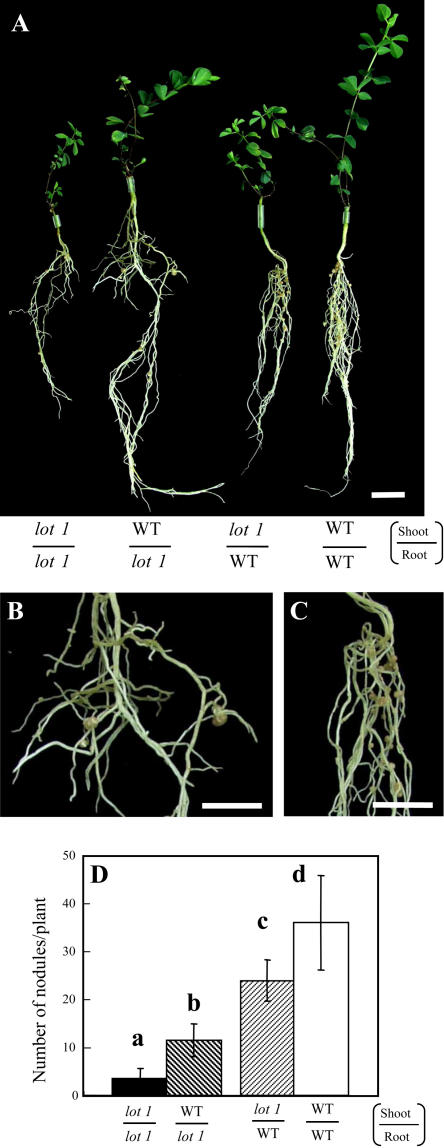
Grafting with lot1 and Wild-Type Plants
To examine what portion determines the low nodulation phenotype of lot1, we conducted grafting experiments with the lot1 mutant and wild type. As shown in Figure 5, grafting of wild-type shoots onto lot1 roots resulted in a slightly but significantly larger nodule number than that for one control, lot1/lot1. In addition, grafting of lot1 shoots to wild-type roots gave a slightly smaller nodule number than that for another control, wild type/wild type. However, the wild-type shoots were larger than lot1 shoots, and so lot1 roots with wild-type shoots are longer than those with lot1 shoots (Fig. 5A). These secondary differences in nodule number (Fig. 5D) would be attributable to different photosynthetic activity of the grafted shoots. Sparse nodules are one of the hallmarks of the lot1 mutant (Fig. 1B). Notably, lot1 roots grafted with wild-type shoots formed nodules much more sparsely than wild-type roots with lot1 shoots (Fig. 5, B and C). These results suggest that the root genotype determines the low nodulation phenotype of the lot1 mutant, although we cannot exclude a small effect of shoot genotype completely. This is in contrast to har1/nark mutants, in which the shoot genotype determines the hypernodulation phenotype in a clear manner (Delves et al., 1986; Sheng and Harper, 1997; Jiang and Gresshoff, 2002; Krusell et al., 2002; Men et al., 2002; Nishimura et al., 2002a; Searle et al., 2003). Unlike the nodule formation, trichomes on lot1 shoots grafted to wild-type roots remained wavy. In addition, trichomes on wild-type shoots grafted to lot1 roots were normal (data not shown). Therefore, trichome morphology is determined by the shoot genotype.
Figure 5.
Grafting experiments with lot1 and the wild type (WT). A, Plants grown for 6 weeks after grafting and 5 wpi of M. loti Tono. Bar, 2 cm. B, Nodulation of lot1 roots to which wild-type shoots had been grafted. Bar, 1 cm. C, Nodulation of wild-type roots to which lot1 shoots had been grafted. Bar, 1 cm. D, Number of mature nodules. The means and sds are presented (n = 10, 14, 5, and 17 for lot1/lot1, WT/ lot1, lot1/WT, and WT/WT, respectively). Different letters above the bars indicate significant differences at P < 0.05 according to the t test.
Independence of LOT1 and HAR1 Functions
The above-described high nitrate sensitivity and low nodulation are just opposite to the case of the nitrate-tolerance and hypernodulation phenotype of har1 mutants of soybean (Carroll et al., 1985a, 1985b) and L. japonicus (Wopereis et al., 2000), although some har1 mutants of L. japonicus show similar nitrate sensitivity to that of the wild type (Kawaguchi et al., 2002). As a first step to determine whether or not the LOT1 gene product is involved in the HAR1 regulation pathway, we estimated the relative expression levels of the HAR1 transcript in the lot1 mutant and wild type by real-time reverse transcription (RT)-PCR. HAR1 mRNA levels were similar in both the mutant and wild-type plants, decreasing in shoots, roots, and root nodules in that order (data not shown). This expression pattern is consistent with that reported by Nishimura et al. (2002a). As a control, the constitutive β-actin gene (Matamoros et al., 2003) was also amplified, showing a similar expression level in all tissues (data not shown). Thus, it was shown that the lot1 mutant expresses the HAR1 gene as highly as the wild type.
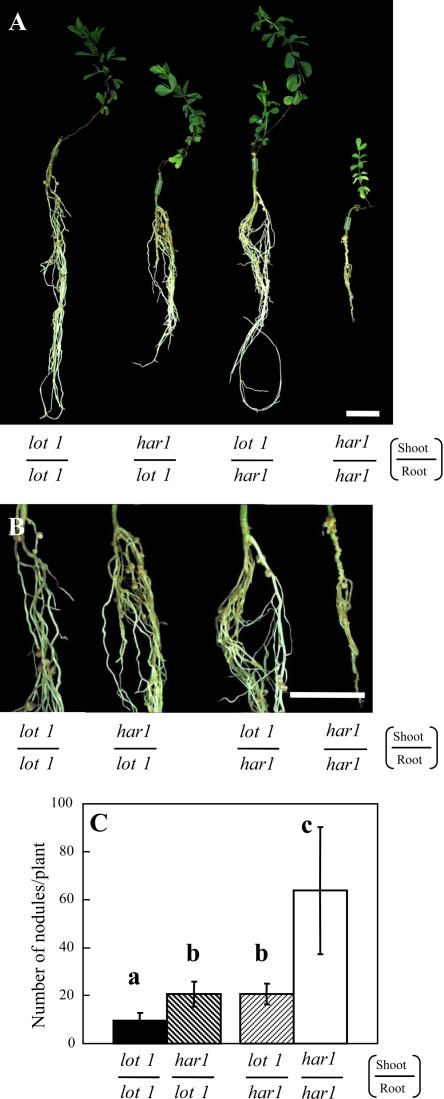
Next, we carried out grafting experiments with lot1 and har1 mutants. Since the har1 mutant shows a dwarf phenotype too, especially when inoculated with M. loti (Wopereis et al., 2000), we do not have to take into account the secondary effect caused by the difference in shoot growth in the experiments in Figure 6. The short root phenotype of the har1 mutant seems to be determined by the shoot genotype (Krusell et al., 2002) as well as super/hypernodulation (Delves et al., 1986; Sheng and Harper, 1997; Nishimura et al., 2002a; Searle et al., 2003). Interestingly, grafting of har1-4 shoots onto lot1 roots resulted in an intermediate number of nodules. The grafted plants formed more nodules than one control, lot1/lot1, and much less nodules than another control, har1-4/har1-4 (Fig. 6C). Grafting of lot1 shoots onto har1-4 roots gave similar results.
Figure 6.
Grafting experiments with the lot1 and har1-4 mutants. A, Plants grown for 6 weeks after grafting and 5 wpi of M. loti Tono. Bar, 2 cm. B, Enlarged root portions. Bar, 2 cm. C, Number of mature nodules. The means and sds are presented (n = 22, 12, 12, and 10 for lot1/lot1, har1/lot1, lot1/har1, and har1/har1, respectively). Different letters above the bars indicate significant differences at P < 0.01 according to the t test.
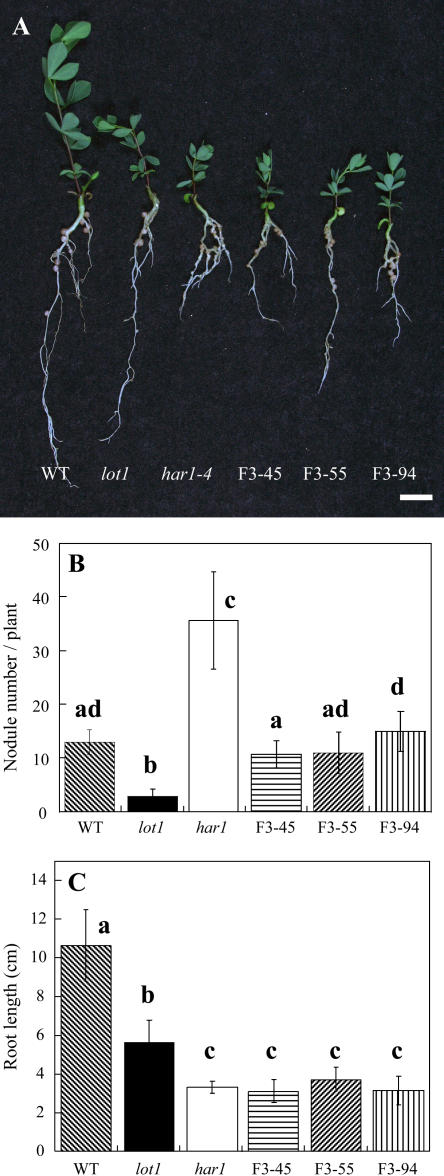
Finally, we prepared putative double mutant lines of lot1 and har1-4, named F3-45, F3-55, and F3-94. As shown in Figure 7, all putative double mutants showed intermediate nodule number between those of the single mutants. Unexpectedly, however, the putative double mutants showed straight trichomes (data not shown) and short root phenotype like har1 (Fig. 7) when inoculated with M. loti. The homozygous har1-4 allele seems to suppress the lot1 phenotypes to some extent. Taken together, these results indicate that the LOT1 gene product acts independently from the HAR1 gene product in the regulation of nodule number.
Figure 7.
Phenotype of putative double mutants of lot1 and har1-4. A, The appearance of the putative double mutants F3-45, F3-55, and F3-94. The wild type (Gifu B-129) and the single mutants are also shown for comparison. The plants were grown for 4 weeks after inoculation of M. loti Tono. Bar, 1 cm. B and C, Nodule number and root length, respectively, of the plants. The means and sds are presented (n = 10, 15, 15, 15, 14, and 12 for the wild type, lot1, har1-4, F3-45, F3-55, and F3-94, respectively). Different letters above the bars indicate significant differences at P < 0.01 according to the t test.
Chromosomal Mapping
Using the F2 plants obtained by crossing lot1 and Miyakojima MG-20, linkage analysis with a total of 24 simple sequence repeat markers was carried out. The results suggested that LOT1 is near an intraspecific translocation site between Gifu B-129 and Miyakojima MG-20 involving chromosomes 1 and 2 (data not shown). Combined with the phenotypical difference of the lot1 mutant from thus far reported L. japonicus mutants (Schauser et al., 1998; Szczyglowski et al., 1998; Kawaguchi et al., 2002; Tansengco et al., 2003) and pea (Pisum sativum) mutants (Provorov et al., 2002), this finding strongly suggests that LOT1 is a novel locus on the L. japonicus genome.
DISCUSSION
We isolated a novel L. japonicus symbiotic mutant, lot1. Although the lot1 mutant is monogenic and recessive, it shows some distinct phenotypes such as low nodulation, trichome distortion, and moderate dwarfism (Figs. 1 and 2). These findings indicate that LOT1 is involved not only in control of nodule formation but also in trichome formation and growth control. In Arabidopsis, a gene that controls both trichome and root hair formation has been reported (Rerie et al., 1994; Ohashi et al., 2003). The crinkle mutant of L. japonicus also shows alteration of both trichome and root hair formation (Tansengco et al., 2003). In the case of the lot1 mutant, however, the root hairs were normal. Other pleiotropic nodulation mutants have been described (Caetano-Anollés and Gresshoff, 1991; Penmetsa and Cook, 1997; Wopereis et al., 2000; Nishimura et al., 2002a, 2002c; Tansengco et al., 2003, 2004). It is thought that most nodule-enhanced genes, or nodulin genes, are recruited for nodule-specific functions from among preexisting common genes (Hata et al., 1998; Gualtieri and Bisseling, 2000; Nakagawa et al., 2003; Szczyglowski and Amyot, 2003), although there is an exceptional galegoid legume-specific gene family (Mergaert et al., 2003). At least some key genes involved in nodule formation seem to have also been recruited from among preexisting genes involved in coordinated plant development. These include LOT1 (this study), HAR1/NARK (Wopereis et al., 2000; Krusell et al., 2002; Nishimura et al., 2002a; Searle et al., 2003), ASTRAY (Nishimura et al., 2002b, 2002c), and CRINKLE (Tansengco et al., 2003, 2004). This view is in accord with the prediction of Szczyglowski and Amyot (2003).
Although the shape of infection threads was normal, their formation was significantly blocked (Table I). Subsequent nodule primordia formation may also be repressed compared to the wild type (Table I). Since it has long been known that a high proportion of infection results in abortion (Nutman, 1962), careful examination like that of Vasse et al. (1993) would be necessary to determine the blocked step(s). In any case, once nodule primordia were formed on lot1 roots, all nodules developed fully (Fig. 1; Table II). This is in contrast to crinkle (Tansengco et al., 2003) and alb1 (Imaizumi-Anraku et al., 1997). In these other mutants, many bumps or empty nodules are formed and infection thread development is markedly arrested. Linkage analysis also indicated that LOT1 is distinct from both CRINKLE and ALB1 (Y. Ooki, M. Hayashi, and M. Kawaguchi, unpublished data). The phenotype of lot1 is similar to that of Ljsym73, which nodulates at very low frequency and exhibits normal mycorrhizal colonization (Kawaguchi et al., 2002). However, crossing of lot1 with Ljsym73 indicated that they are not allelic (M. Banba, M. Yoshikawa, M. Hayashi, and M. Kawaguchi, unpublished data). It is worth noting that lot1 does not belong to any of the four basic categories of nodulation mutants: Nod−, Nod2+, Hist−, and Fix−.
The results of grafting experiments with the lot1 mutant and wild type suggested that the root genotype mainly determines low nodulation phenotype of the lot1 mutant (Fig. 5). This is in sharp contrast to har1 mutants, in which the shoot genotype determines the hypernodulation phenotype systemically. Besides the HAR1 autoregulation pathway, Postma et al. (1988) described a pea hypernodulation mutation that was determined by the root genotype. A root meristem-derived factor that controls nodulation of soybean has also been reported (Caetano-Anollés et al., 1991). It is possible that the LOT1 gene is involved in these root-derived local regulations of nodule formation. Unlike nodulation, the wavy trichome morphology of the lot1 mutant was determined by the shoot genotype. Therefore, we speculate that the LOT1 gene is expressed not only in roots but also in shoots, where it acts in trichome formation and growth regulation.
The effects of nitrate on plant morphogenesis are complicated. Lateral roots of Arabidopsis, for example, show very contrasting responses to high concentrations of nitrate (Casimiro et al., 2003; Lopez-Bucio et al., 2003). When plants are grown on a medium with a uniformly high nitrate supply, lateral root elongation is inhibited throughout the root system. On the other hand, when a section of a primary root grown on low nitrate is exposed to a high nitrate supply, localized stimulation of lateral root elongation occurs. In the former case, the systemic inhibition is thought to be due to sufficient nitrogen metabolites. In the latter case, the signal is thought to be nitrate itself (Casimiro et al., 2003; Lopez-Bucio et al., 2003). Since most hypernodulation mutants of legumes show nitrate tolerance, the relationship between nitrate inhibition of nodule formation and autoregulation of the nodule number has long been pointed out (Delves et al., 1986; Caetano-Anollés and Gresshoff, 1991). In most cases, the availability of nitrogen per se does not seem to repress the new nodule formation, but preexisting nodules do so systemically. However, the control mechanism may be complicated as in the case of Arabidopsis lateral roots. In this work, lot1 showed higher sensitivity to exogenous nitrate than the wild type (Fig. 3). As described above, lot1 seems to be involved in local regulation of root nodule formation. Thus, lot1 may exhibit aberrant nitrogen sensing in roots, causing the low nodulation phenotype. The inoculation of a nifH-defective strain of M. loti onto lot1 roots resulted in a smaller increase of nodule number than that in the wild type (Table III). Therefore, it is still possible that lot1 also has a defect in the recognition of unknown signals from preexisting nodules other than nitrate.
We found that the low nodulation phenotype of lot1 is not caused by overexpression of the HAR1 gene. We next designed grafting experiments with lot1 and har1-4 mutants. If LOT1 and HAR1 act in the same genetic pathway, one can expect that either the lot1 phenotype or the har1-4 phenotype is observed after grafting. For example, Delves et al. (1986) reported that the har1/nark hypernodulation phenotype of soybean was completely suppressed by a root-expressed nonnodulation mutation when hypernodulation shoots were grafted onto nonnodulation roots. In that case, it is highly likely that the causal gene for nonnodulation acts downstream of the HAR1/NARK gene in the same regulation pathway. Interestingly, however, grafting of har1-4 shoots onto lot1 roots resulted in an intermediate number of nodules, showing an additive effect of the two mutations (Fig. 6). The phenotype of the putative double mutants of lot1 and har1 supported this observation (Fig. 7). These findings strongly indicate that LOT1 and HAR1 determine distinct control pathways, LOT1 and HAR1 exhibiting augmentative and suppressive effects, respectively, on the nodule number.
What is the mechanism underlying the low nodulation by lot1? In this regard, it is noteworthy that ACC, AVG, nor STS showed any significant effect on the nodule number of grown-up lot1 seedlings, although lot1 showed the normal triple response just after germination (Fig. 4). These results are in contrast to those for some pea mutants showing a reduced nodule number that are hypersensitive to ethylene (Fearn and LaRue, 1991) or overproducers of ethylene (Lee and LaRue, 1992). The plant hormone ethylene regulates a variety of functions in plant growth and development. In Arabidopsis, ethylene modulates plant responses through common upstream pathways, including receptor-CTR1 complexes (Gao et al., 2003), EIN2 (Alonso et al., 1999), etc., and specialized downstream pathways involving the EIN3/EIL family (Solano et al., 1998) and ethylene-responsive element binding proteins (Riechmann and Meyerowitz, 1998). It is possible that a nodulation-specific downstream pathway is constitutively activated in the lot1 mutant of L. japonicus. In this sense, lot1 seems to be in symmetry with sickle of M. truncatula that is insensitive to ethylene (Penmetsa and Cook, 1997). On the other hand, the normal triple response suggests that the common upstream pathways and the other downstream pathways except the nodulation-specific one are functioning well in the lot1 mutant. This working hypothesis would explain the low nodulation phenotype of lot1, although we cannot rule out other mechanisms at present. The moderate dwarfism of the mutant may also be related to the conceivable constitutive ethylene response.
In summary, we isolated a hitherto-unknown low nodulation mutant, designated as lot1. Positional cloning of the LOT1 gene in the future will provide new insights into the homeostatic control of symbiotic root nodule formation.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Lotus japonicus Gifu B-129 was used as the parental line for mutation and as the wild-type control in other experiments. As a crossing partner, L. japonicus Miyakojima MG-20 was used (Kawaguchi et al., 2001). Unless otherwise stated, seeds were scarified, surface sterilized, and allowed to germinate on 0.8% agar plates under sterile conditions. The plants were kept dark at 25°C for 2 d and then subjected to greening for 5 d in a controlled-environment growth chamber (Sanyo, Tokyo) with a 16-h-day/8-h-night cycle at 25°C and a light intensity of 260 μE s−1 m−2 with 60% humidity. Then, up to 25 seedlings were transferred to a Magenta jar containing 150 mL of vermiculite supplied with 125 mL of Broughton and Dilworth (B & D) medium (Broughton and Dilworth, 1971). A whole (1 cm diameter) was made at the center of the lid and sealed with MilliSeal (Millipore, Billerica, MA). The modified lid was set on the jar, and the seedlings were grown under the above conditions. After 2 to 3 weeks, the lid was removed just before the shoots reached to it. When M1 plants were grown to collect M2 seeds, they were grown on Power Soil (Kreha Chemical Industry, Tokyo) in an air-conditioned greenhouse at 25°C with 60% humidity.
Microbial Strains and Inoculation
Mesorhizobium loti Tono was isolated by M. Kawaguchi (Kawaguchi et al., 2002) and used as a standard symbiont. M. loti MAFF303099 was also used as a wild-type control in some experiments. M. loti BNO2, which constitutively expresses GFP, was prepared by K. Saeki (to be published elsewhere) from M. loti JRL501 (Imaizumi-Anraku et al., 1997), which is a spontaneous nalidixic acid-tolerant derivative of MAFF303099. M. loti MAFF303099 derivative ML001 carrying pGD499 (nodB:lacZ; Ditta et al., 1985), which constitutively expresses the lacZ reporter gene, was provided by Dr. K. Minamisawa, Tohoku University, Japan. Another M. loti MAFF303099 derivative that lacks the nifH gene was established by J. Maruya and K. Saeki (to be published elsewhere). The M. loti strains and Rhizobium etli CE3 were grown at 28°C for 2 d with shaking in yeast extract-mannitol medium (Vincent, 1970) in the presence of appropriate antibiotics. One week after transplantation of the germinated seedlings onto vermiculite, 2.6 × 1010 bacterial cells per Magenta jar were inoculated to the seedlings. A soil inoculum containing spores and hyphae of the arbuscular fungus Glomus mosseae was a kind gift from Dr. K. Nagashima, Idemitsu Kosan, Tokyo. One-week-old seedlings of L. japonicus were transplanted into 38-mL glass tubes (2.2 cm diameter × 10 cm high) containing vermiculite and modified Hornum nutrient solution (Handberg and Stougaard, 1992) with a lowered NaH2PO4 concentration of 250 μm. Then, 2 g of the soil inoculant was added to each plant. The plants were watered with 5 mL of the modified Hornum solution at 5-d intervals.
Mutagenesis and Screening
Seeds of L. japonicus Gifu B-129 were scarified, shaken gently in water for 2 h and in 0.4% (w/v) ethyl methanesulfonate (Sigma, St. Louis) for 6 h at room temperature, and then rinsed more than 8 times with water. After germination, M1 plants were grown to maturity as described above, and the resulting M2 seeds were individually harvested to obtain a seed family. About 17 seeds from each M2 seed family were sterilized, germinated, inoculated with R. etli CE3, a heterologous symbiont (Banba et al., 2001), and then grown for 5 weeks on vermiculite containing nitrogen-free B & D medium in sterile Magenta jars. Vermiculite was carefully removed from the plant roots, and then gross changes in the nodule number and morphology were assessed for about 11,500 M2 seedlings. The resulting putative mutants were transplanted into Magenta jars containing vermiculite and half-strength Gamborg B5 medium (Wako, Osaka) to collect M3 seeds and to check the heritability of the M2 phenotypes. A number of mutants showing a nodulation deficiency, an increased shoot number, round-shaped leaves, etc. were isolated (Y. Ooki, unpublished data). Fortuitously, R. etli scarcely forms nodules on lot1 roots (Table III), which is probably why we could discover the mutant among them. In turn, our screening system is not suitable for isolation of hypernodulating mutants. Actually, har1-4 inoculated with R. etli CE3 formed a much lower number of nodules than in the case with M. loti (Table III).
Microscopy of the Nodule Formation Process and Arbuscular Mycorrhizal Colonization
The morphology of root hairs and their deformation by M. loti were examined by the following two methods. When the deformation was observed within 12 h after inoculation of M. loti, Fåhraeus slides were used (Fåhraeus, 1957; Heidstra et al., 1994; Niwa et al., 2001). Two-day-old seedlings were prepared in vertically positioned agar plates and then transplanted into Fåhraeus slides. The roots were left to stand in 108 cells/mL of M. loti Tono and examined at hourly intervals by bright-field microscopy. When the deformation was examined at 2 d after inoculation, the seedlings were incubated with the above density of M. loti in vertically positioned agar plates (Bonfante et al., 2000).
The numbers of infection threads and nodule primordia were determined with M. loti BNO2, which expresses GFP constitutively. One-week-old seedlings were inoculated with 6.6 × 109 cells/plant M. loti BNO2 in Magenta jars containing vermiculite and nitrogen-free B & D medium. At 1, 3, 5, or 7 dpi, infection threads and nodule primordia were visualized and counted as to green fluorescence under a Nikon ECLIPSE E600 microscope (Nikon, Tokyo). Excitation and detection were carried out at 490 nm and 520 nm, respectively.
The entire nodule-formation process was monitored by inoculation of M. loti ML001 harboring pGD499. The symbiont was inoculated onto 1-week-old seedlings as described above. After appropriate periods, whole roots were fixed and stained for β-galactosidase activity as described by Tansengco et al. (2003). Then, the infection threads and nodule primordia were counted under bright-field optics.
For assessment of G. mosseae colonization, roots were cleared with 10% KOH and then stained with 0.05% trypan blue in lactoglycerol (Phillips and Hayman, 1970). The stained roots were examined under a light microscope.
Acetylene Reduction Assay
The acetylene reduction assay was carried out as described previously (Banba et al., 2001).
Grafting Experiments
Grafting was performed as described by Nishimura et al. (2002a) using plastic tubes (diameter, 0.79 mm).
Real-Time RT-PCR
Total RNA was extracted from shoots under symbiotic conditions, shoots under nonsymbiotic conditions, noninfected roots, and mature nodules using an RNeasy plant mini kit (Qiagen, Hilden, Germany). Each RNA preparation was reverse transcribed with oligo(dT) and Superscript II (Invitrogen, Carlsbad, CA), and then subjected to real-time PCR with specific primer pairs and SYBR Green I according to the manufacturer's instructions (Real Time RT-PCR Core kit; TaKaRa BIO, Otsu, Japan) using a Smart Cycler system (Cepheid, Sunnyvale, CA). The forward and reverse primers for HAR1 and the β-actin gene were 5′-GATACCCCTTGACAAGTGTCATC-3′ and 5′-GTTGTTTCACTTCTCACAATCTAGG-3′, and 5′-GCATTGTTGGTCGTCCTCGT-3′ and 5′-TGTGCCTCATCCCCAACATA-3′, respectively. For HAR1, the reaction mixture was heated at 95°C for 30 s and then subjected to PCR cycles of 95°C for 10 s, 52°C for 20 s, and 72°C for 10 s, the resulting fluorescence being monitored. For the constitutive β-actin gene (Matamoros et al., 2003), the mixture was heated at 95°C for 30 s, and then subjected to PCR cycles of 95°C for 10 s, 62°C for 20 s, and 72°C for 10 s. Heat dissociation curves showed that a single PCR product was amplified for each gene. Melting temperatures were 86.3°C and 85.3°C for the PCR products of HAR1 and the β-actin gene, respectively.
Preparation of Putative Double Mutants of lot1 and har1
To generate double mutant lines having mutations in both LOT1 and HAR1 genes, a lot1 homozygote was crossed with a har1-4 homozygote. The F1 plants were allowed to self, and 15 plants homozygous for the har1-4 allele were selected from the resulting F2 plants making use of a CAPS marker. The F2 plants were naturally self-crossed, and the resulting F3 plants were inoculated with M. loti Tono. Three lines that show different nodulation from that of the har1 single mutant, F3-45, F3-55, and F3-94, were further characterized. Confirmation of their genotype is now under way by crossing them to the wild type for segregation of the two parental phenotypes.
Linkage Analyses
The lot1 mutant, which was derived from L. japonicus Gifu B-129, was crossed with L. japonicus Miyakojima MG-20, the resulting F1 plants were self-crossed, and 32 F2 plants with the mutant phenotype were used for subsequent analysis. To map the lot1 locus roughly, simple sequence repeat markers in the genetic linkage map of L. japonicus (Hayashi et al., 2001) were selected, and then bulked segregant analyses involving agarose gel electrophoresis using 3% NuSieve 3:1 agarose or 3% Metaphor agarose (Cambrex, East Rutherford, NJ) were performed.
Acknowledgments
We thank Prof. P.M. Gresshoff for critical reading of the manuscript, and Drs. D.R. Cook, N. Sandal, H. Kouchi, J. Stougaard, N. Nukui, M.L. Tansengco, M. Yoshikawa, R. Geurts, N. Ellis, Y. Saijo, Y. Umehara, and H. Imaizui-Anraku for the valuable discussions. Thanks are also due to K. Minamisawa, S. Isobe, and K. Nagashima for the generous gifts of the materials, H. Okamoto for the technical assistance, and Y. Deguchi for the kind instruction regarding mycorrhizal colonization.
This work was supported in part by Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.056630.
References
- Adams DO, Yang SF (1979) Ethylene biosynthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci USA 76: 170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akao S, Kouchi H (1992) A supernodulating mutant isolated from soybean cultivar Enrei. Soil Sci Plant Nutr 38: 182–187 [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Ané JM, Kiss GB, Riely BK, Penmetsa RV, Oldroyd GE, Ayax C, Levy J, Debelle F, Baek JM, Kalo P, et al (2004) Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303: 1364–1367 [DOI] [PubMed] [Google Scholar]
- Banba M, Siddique A-BM, Kouchi H, Izui K, Hata S (2001) Lotus japonicus forms early senescent root nodules with Rhizobium etli. Mol Plant Microbe Interact 14: 173–180 [DOI] [PubMed] [Google Scholar]
- Bonfante P, Genre A, Faccio A, Martini I, Schauser L, Stougaard J, Webb J, Parniske M (2000) The Lotus japonicus LjSym4 gene is required for the successful symbiotic infection of root epidermal cells. Mol Plant Microbe Interact 13: 1109–1120 [DOI] [PubMed] [Google Scholar]
- Borisov AY, Madsen LH, Tsyganov VE, Umehara Y, Voroshilova VA, Batagov AO, Sandal N, Mortensen A, Schauser L, Ellis N, et al (2003) The Sym35 gene required for root nodule development in pea is an ortholog of Nin from Lotus japonicus. Plant Physiol 131: 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton WJ, Dilworth MY (1971) Control of leghemoglobin synthesis in snake bean. Biochem J 125: 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anollés G, Gresshoff PM (1991) Plant genetic control of nodulation. Annu Rev Microbiol 45: 345–382 [DOI] [PubMed] [Google Scholar]
- Caetano-Anollés G, Paparozzi ET, Gresshoff PM (1991) Mature nodules and root tips control nodulation in soybean. J Plant Physiol 137: 389–396 [Google Scholar]
- Carroll BJ, Mathews A (1990) Nitrate inhibition of nodulation in legumes. In PM Gresshoff, ed, Molecular Biology of Symbiotic Nitrogen Fixation. CRC Press, Boca Raton, FL, pp 159–180
- Carroll BJ, McNeil DL, Gresshoff PM (1985. a) Isolation and properties of soybean [Glycine max (L.) Merr.] mutants that nodulate in the presence of high nitrate concentrations. Proc Natl Acad Sci USA 82: 4162–4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll BJ, McNeil DL, Gresshoff PM (1985. b) A supernodulation and nitrate-tolerant symbiotic (nts) soybean mutant. Plant Physiol 78: 34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Delves AC, Mathews A, Day DA, Carter AS, Carroll BJ, Gresshoff PM (1986) Regulation of the soybean-Rhizobium nodule symbiosis by shoot and root factors. Plant Physiol 82: 588–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G, Schmidhauser T, Yakobson E, Lu P, Liang XW, Finlay DR, Guiney D, Helinski DR (1985) Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid 13: 149–153 [DOI] [PubMed] [Google Scholar]
- Endre G, Kereszt A, Kevei Z, Mihacea S, Kalo P, Kiss GB (2002) A receptor kinase gene regulating symbiotic nodule development. Nature 417: 962–966 [DOI] [PubMed] [Google Scholar]
- Fåhraeus G (1957) The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol 16: 374–381 [DOI] [PubMed] [Google Scholar]
- Fearn JC, LaRue TA (1991) Ethylene inhibitors restore nodulation to sym 5 mutants of Pisum sativum L. cv Sparkle. Plant Physiol 96: 239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Chen YF, Randlett MD, Zhao XC, Findell JL, Kieber JJ, Schaller GE (2003) Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem 278: 34725–34732 [DOI] [PubMed] [Google Scholar]
- Geurts R, Bisseling T (2002) Rhizobium Nod factor perception and signaling. Plant Cell (Suppl) 14: S239–S249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremaud MF, Harper JE (1989) Selection and initial characterization of partially nitrate tolerant nodulation mutants of soybean. Plant Physiol 89: 169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri G, Bisseling T (2000) The evolution of nodulation. Plant Mol Biol 42: 181–194 [PubMed] [Google Scholar]
- Handberg K, Stougaard J (1992) Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant J 2: 487–496 [Google Scholar]
- Hata S, Izui K, Kouchi H (1998) Expression of a soybean nodule-enhanced phosphoenolpyruvate carboxylase gene that shows striking similarity to another gene for a house-keeping isoform. Plant J 13: 267–273 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Miyahara A, Sato S, Kato T, Yoshikawa M, Taketa M, Hayashi M, Pedrosa A, Onda R, Imaizumi-Anraku H, et al (2001) Construction of a genetic linkage map of the model legume Lotus japonicus using an intraspecific F2 population. DNA Res 8: 301–310 [DOI] [PubMed] [Google Scholar]
- Heidstra R, Geurts R, Franssen H, Spaink HP, van Kammen A, Bisseling T (1994) Root hair deformation activity of nodulation factors and their fate on Vicia sativa. Plant Physiol 105: 787–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AM, Smith CA (1987) Effects of Rhizobium meliloti nif and fix mutants on alfalfa root nodule development. J Bacteriol 169: 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain AKMA, Jiang Q, Broughton WJ, Gresshoff PM (1999) Lotus japonicus nodulates and fixes nitrogen with the broad host range Rhizobium sp. NGR234. Plant Cell Physiol 40: 894–899 [Google Scholar]
- Imaizumi-Anraku H, Kawaguchi M, Koiwa H, Akao S, Syono K (1997) Two ineffective-nodulating mutants of Lotus japonicus: different phenotypes caused by blockage of endocytotic bacterial release and nodule maturation. Plant Cell Physiol 38: 871–881 [Google Scholar]
- Jiang Q, Gresshoff PM (1997) Classical and molecular genetics of the model legume Lotus japonicus. Mol Plant Microbe Interact 10: 59–68 [DOI] [PubMed] [Google Scholar]
- Jiang Q, Gresshoff PM (2002) Shoot control of hypernodulation and aberrant root formation in the har1-1 mutant of Lotus japonicus. Funct Plant Biol 29: 1371–1376 [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Imaizumi-Anraku H, Koiwa H, Niwa S, Ikuta A, Syono K, Akao S (2002) Root, root hair, and symbiotic mutants of the model legume Lotus japonicus. Mol Plant Microbe Interact 15: 17–26 [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Motomura T, Imaizumi-Anraku H, Akao S, Kawasaki S (2001) Providing the basis for genomics in Lotus japonicus: the accessions Miyakojima and Gifu are appropriate crossing partners for genetic analyses. Mol Genet Genomics 266: 157–166 [DOI] [PubMed] [Google Scholar]
- Kosslak RM, Bohlool BB (1984) Suppression of nodule development of the one side of a split root system of soybeans caused by prior inoculation of the other side. Plant Physiol 75: 125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusell L, Madsen LH, Sato S, Aubert G, Genua A, Szczyglowski K, Duc G, Kaneko T, Tabata S, de Bruijn F, et al (2002) Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 420: 422–426 [DOI] [PubMed] [Google Scholar]
- Lee KH, LaRue TA (1992) Pleiotropic effects of sym-171: A mutation in Pisum sativum L. cv Sparkle causes decreased nodulation, altered root and shoot growth, and increased ethylene production. Plant Physiol 100: 1326–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J, Bres C, Geurts R, Chalhoub B, Kulikova O, Duc G, Journet EP, Ane JM, Lauber E, Bisseling T, et al (2004) A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science 303: 1361–1364 [DOI] [PubMed] [Google Scholar]
- Limpens E, Franken C, Smit P, Willemse J, Bisseling T, Geurts R (2003) LysM domain receptor kinases regulating rhizobial Nod factor-induced infection. Science 302: 630–633 [DOI] [PubMed] [Google Scholar]
- Lopez-Bucio J, Cruz-Ramirez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6: 280–287 [DOI] [PubMed] [Google Scholar]
- Madsen EB, Madsen LH, Radutoiu S, Olbryt M, Rakwalska M, Szczyglowski K, Sato S, Kaneko T, Tabata S, Sandal N, et al (2003) A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature 425: 637–640 [DOI] [PubMed] [Google Scholar]
- Matamoros MA, Clemente MR, Sato S, Asamizu E, Tabata S, Ramos J, Moran JF, Stiller J, Gresshoff PM, Becana M (2003) Molecular analysis of the pathway for the synthesis of thiol tripeptides in the model legume Lotus japonicus. Mol Plant Microbe Interact 16: 1039–1046 [DOI] [PubMed] [Google Scholar]
- Men AE, Laniya TS, Searle IR, Iturbe-Ormaetxe I, Gresshoff I, Jiang Q, Carroll BJ, Gresshoff PM (2002) Fast neutron mutagenesis of soybean (Glycine soja L.) produces a supernodulating mutant containing a large deletion in the linkage group H. Genome Lett 3: 147–155 [Google Scholar]
- Mergaert P, Nikovics K, Kelemen Z, Maunoury N, Vaubert D, Kondorosi A, Kondorosi E (2003) A novel family in Medicago truncatula consisting of more than 300 nodule-specific genes coding for small, secreted polypeptides with conserved cysteine motifs. Plant Physiol 132: 161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra RM, Gleason CA, Edwards A, Hadfield J, Downie JA, Oldroyd GE, Long SR (2004) A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: gene identification by transcript-based cloning. Proc Natl Acad Sci USA 101: 4701–4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Takane K, Sugimoto T, Izui K, Kouchi H, Hata S (2003) Regulatory regions and nuclear factors involved in nodule-enhanced expression of a soybean phosphoenolpyruvate carboxylase gene: implications for molecular evolution. Mol Genet Genomics 269: 163–172 [DOI] [PubMed] [Google Scholar]
- Nishimura R, Hayashi M, Wu G-J, Kouchi H, Imaizumi-Anraku H, Murakami Y, Kawasaki S, Akao S, Ohmori M, Nagasawa M, et al (2002. a) HAR1 mediates systemic regulation of symbiotic organ development. Nature 420: 426–429 [DOI] [PubMed] [Google Scholar]
- Nishimura R, Ohmori M, Fujita H, Kawaguchi M (2002. b) A Lotus basic leucine zipper protein with a RING-finger motif negatively regulates the developmental program of nodulation. Proc Natl Acad Sci USA 99: 15206–15210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura R, Ohmori M, Kawaguchi M (2002. c) The novel symbiotic phenotype of enhanced-nodulating mutant of Lotus japonicus: astray mutant is an early nodulating mutant with wider nodulation zone. Plant Cell Physiol 43: 853–859 [DOI] [PubMed] [Google Scholar]
- Niwa S, Kawaguchi M, Imazumi-Anraku H, Chechetka SA, Ishizaka M, Ikuta A, Kouchi H (2001) Responses of a model legume Lotus japonicus to lipochitin oligosaccharide nodulation factors purified from Mesorhizobium loti JRL501. Mol Plant Microbe Interact 14: 848–856 [DOI] [PubMed] [Google Scholar]
- Nukui N, Ezura H, Minamisawa K (2004) Transgenic Lotus japonicus with an ethylene receptor gene Cm-ERS1/H70A enhances formation of infection threads and nodule primordia. Plant Cell Physiol 45: 427–435 [DOI] [PubMed] [Google Scholar]
- Nutman PS (1949) Physiological studies on nodule formation. II. The influence of delayed inoculation on the rate of nodulation in red clover. Ann Bot (Lond) 13: 261–283 [Google Scholar]
- Nutman PS (1952) Physiological studies on nodule formation. III. Experiments on the excision of root-tips and nodules. Ann Bot (Lond) 16: 79–101 [Google Scholar]
- Nutman PS (1962) The relation between root hair infection by Rhizobium and nodulation in Trifolium and Vicia. Proc R Soc Lond B Biol Sci 156: 122–137 [Google Scholar]
- Ohashi Y, Oka A, Rodrigues-Pousada R, Possenti M, Ruberti I, Morelli G, Aoyama T (2003) Modulation of phospholipid signaling by GLABRA2 in root-hair pattern formation. Science 300: 1427–1430 [DOI] [PubMed] [Google Scholar]
- Oldroyd GED, Downie JA (2004) Calcium, kinases and nodulation signaling in legumes. Nat Rev Mol Cell Biol 5: 566–576 [DOI] [PubMed] [Google Scholar]
- Penmetsa RV, Cook DR (1997) A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275: 527–530 [DOI] [PubMed] [Google Scholar]
- Penmetsa RV, Frugoli JA, Smith LS, Long SR, Cook DR (2003) Dual genetic pathways controlling nodule number in Medicago truncatula. Plant Physiol 131: 998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JM, Hayman DS (1970) Improved procedure for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55: 158–161 [Google Scholar]
- Postma JG, Jacobsen E, Feenstra WJ (1988) Three pea mutants with an altered nodulation studied by genetic analysis and grafting. J Plant Physiol 132: 424–430 [Google Scholar]
- Provorov NA, Borisov AY, Tikhonovich IA (2002) Developmental genetics and evolution of symbiotic structures in nitrogen-fixing nodules and arbuscular mycorrhiza. J Theor Biol 214: 215–232 [DOI] [PubMed] [Google Scholar]
- Radutoiu S, Madsen LH, Madsen EB, Felle HH, Umehara Y, Gronlund M, Sato S, Nakamura Y, Tabata S, Sandal N, et al (2003) Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425: 585–592 [DOI] [PubMed] [Google Scholar]
- Rerie WG, Feldmann KA, Marks MD (1994) The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes Dev 8: 1388–1399 [DOI] [PubMed] [Google Scholar]
- Riechmann JL, Meyerowitz EM (1998) The AP2/EREBP family of plant transcription factors. Biol Chem 379: 633–646 [DOI] [PubMed] [Google Scholar]
- Schauser L, Handberg K, Sandal N, Stiller J, Thykjaer T, Pajuelo E, Nielsen A, Stougaard J (1998) Symbiotic mutants deficient in nodule establishment identified after T-DNA transformation of Lotus japonicus. Mol Gen Genet 259: 414–423 [DOI] [PubMed] [Google Scholar]
- Schauser L, Roussis A, Stiller J, Stougaard J (1999) A plant regulator controlling development of symbiotic root nodules. Nature 402: 191–195 [DOI] [PubMed] [Google Scholar]
- Searle IR, Men AE, Laniya TS, Buzas DM, Iturbe-Ormaetxe I, Carroll BJ, Gresshoff PM (2003) Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science 299: 109–112 [DOI] [PubMed] [Google Scholar]
- Sheng C, Harper JE (1997) Shoot versus root signal involvement in nodulation and vegetative growth in wild-type and hypernodulating soybean genotypes. Plant Physiol 133: 825–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stougaard J, Szczyglowski K, de Bruin FJ, Parnicke M (1999) Genetic nomenclature guidelines for the model legume Lotus japonicus. Trends Plant Sci 4: 300–301 [DOI] [PubMed] [Google Scholar]
- Stracke S, Kistner C, Yoshida S, Mulder L, Sato S, Kaneko T, Tabata S, Sandal N, Stougaard J, Szczyglowski K, et al (2002) A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 417: 959–962 [DOI] [PubMed] [Google Scholar]
- Streeter J (1988) Inhibition of legume nodule formation and N2 fixation by nitrate. Crit Rev Plant Sci 7: 1–23 [Google Scholar]
- Szczyglowski K, Amyot L (2003) Symbiosis, inventiveness by recruitment? Plant Physiol 131: 935–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczyglowski K, Shaw RS, Wopereis J, Copeland S, Hamburger D, Kasiborski B, Dazzo FB, de Bruijn FJ (1998) Nodule organogenesis and symbiotic mutants of the model legume Lotus japonicus. Mol Plant Microbe Interact 11: 684–697 [Google Scholar]
- Tansengco ML, Hayashi M, Kawaguchi M, Imaizumi-Anraku H, Murooka Y (2003) crinkle, a novel symbiotic mutant that affects the infection thread growth and alters the root hair, trichome, and seed development in Lotus japonicus. Plant Physiol 131: 1054–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansengco ML, Imaizumi-Anraku H, Yoshikawa M, Takagi S, Kawaguchi M, Hayashi M, Murooka Y (2004) Pollen development and tube growth are affected in the symbiotic mutant of Lotus japonicus, crinkle. Plant Cell Physiol 45: 511–520 [DOI] [PubMed] [Google Scholar]
- van Brussel AAN, Tak T, Boot KJM, Kijne JW (2002) Autoregulation of root nodule formation: signals of both symbiotic partners studied in a split-root system of Vicia sativa subsp. nigra. Mol Plant Microbe Interact 15: 341–349 [DOI] [PubMed] [Google Scholar]
- Vasse J, de Billy F, Truchet G (1993) Abortion of infection during the Rhizobium meliloti-alfalfa symbiotic interaction is accompanied by a hypersensitive reaction. Plant J 4: 555–566 [Google Scholar]
- Veen H (1983) Silver thiosulfate: an experimental tool in plant science. Sci Hort 20: 211–224 [Google Scholar]
- Vincent JM (1970) A Manual for the Practical Study of Root Nodule Bacteria. IBP Handbook No. 15. Blackwell Scientific Publications, Oxford
- Wopereis J, Pajuelo E, Dazzo FB, Jiang Q, Gresshoff PM, de Bruijn FJ, Stougaard J, Szczyglowski K (2000) Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J 23: 97–114 [DOI] [PubMed] [Google Scholar]
- Yu Y-B, Adams DO, Yang SF (1979) 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys 198: 280–286 [DOI] [PubMed] [Google Scholar]