Abstract
Introduction:
Osteomyelitis of the jaws is a common disease of the maxillofacial region. The goal of treatment is to alleviate pain, reduce infection, inhibit the progression of the disease and induce bone and mucosal healing. In addition to surgical management and antibiotic and oxygen hyperbaric therapy, new therapeutic strategies for the treatment of osteomyelitis are developed. One of the novel approaches is photobiomodulation therapy or low-level light therapy (LLLT).
Materials and Methods:
After surgical treatment, experimental group patients (n = 4) were treated with LLLT for five sessions with an extraoral pulsed 635-nm LED lamp (Repuls7, Repuls Lichtmedizintechnik GmbH, Austria), maximum output power: 140 mW/cm2, frequency: 2.5 Hz, duty cycle: 50%. Clinical achievement and patient pain perception (through Visual Analogue Scale score) were evaluated at 1-, 3- and 6-month follow-up appointments and compared with control group (n = 4) patients, treated with standard therapy.
Results:
At three and six months, clinical achievement was better in patients treated with LLLT. Pain and discomfort resolution was significantly greater in the experimental group.
Discussion:
Taking into consideration the results of this study, it can be concluded that LLLT shows potential for improving clinical outcome of surgical and medical treatment of secondary chronic osteomyelitis of the jaws. Furthermore, pain and discomfort were significantly reduced in patients treated with LLLT. Further research with a larger sample size is needed to obtain a more accurate insight into this promising field.
Keywords: Healing, infection, low-level light therapy, osteomyelitis, regeneration
INTRODUCTION
Osteomyelitis of the jaws is still a fairly common disease in maxillofacial clinics and offices.[1] The word ‘osteomyelitis’ originates from the ancient Greek words osteon (bone) and muelinos (marrow) and means infection of the medullary portion of the bone. Common medical literature extends the definition to an inflammation process of the entire bone including the cortex and the periosteum, recognising that the pathological process is rarely confined to the endosteum. The infection becomes established in the calcified portion of the bone when pus and oedema in the medullary cavity and beneath the periosteum compromise or obstruct the local blood supply. Following ischaemia, the infected bone becomes necrotic and leads to sequester formation, which is considered a classical sign of osteomyelitis.[2] Although other aetiological factors can cause inflammation and subsequently ischaemia of bone, such as trauma, radiation or chemical agents, the term ‘osteomyelitis’ is used in the medical literature to describe a true infection of the bone induced by pyogenic microorganisms.[3] Osteomyelitis of the jaws differs from osteomyelitis of long bones due to the specific oral environment. This plays a major role in the aetiology and pathogenesis of the disease and has a direct impact on the modality of treatment. To optimise diagnosis and treatment, many classification systems of osteomyelitis occurred in the literature, and amongst them, the Zurich classification system is considered the most reliable.[4]
Therapy of osteomyelitis of the jaws is currently controversial. The goal of treatment is to alleviate pain, reduce infection, inhibit the progression of the disease and induce bone and mucosal healing. One of the novel treatments is photobiomodulation (PBM) therapy or low-level light therapy (LLLT). PBM therapy is an application of light, usually with lasers or LEDs, for the therapeutic purpose of improving tissue regeneration, reducing inflammation or inducing analgesia.[5] One of the major features of LLLT is the promotion of angiogenesis and tissue perfusion, which has significant implications in the treatment of jaw osteomyelitis.[6] Its bio-stimulatory effect on bone repair was documented in a systematic review by Escudero et al., who also stated that acceleration of this process was present regardless of different light parameters or graft materials used in studies.[7] The aim of this study is to evaluate the effect of 635-nm pulsing LLLT on secondary chronic osteomyelitis post-operative healing.
MATERIALS AND METHODS
Following approval by the Ethical Committee of the Faculty of Dentistry, University of Belgrade (approval number & date: 36/7, 15 April 2021), a clinical study was conducted at the Clinic for Maxillofacial Surgery, Faculty of Dentistry, University of Belgrade, from May 2021 to February 2023. Patients were treated according to the principles established in the Helsinki Declaration. Signed informed consent was obtained from each patient before treatment. Osteomyelitis in patients was confirmed by biopsy and histopathological verification. At the same time, tissue microorganism cultures were obtained in order to determine if another antibiotic therapy is indicated besides empirical. Correlation was made with medical history, clinical presentation and radiological findings, after which patients diagnosed with secondary chronic osteomyelitis, according to the Zurich classification system,[4] were included in this study. Accordingly, all patients included in this study presented with infections of the mandible which were not resolved after one month of proper care, characterised by the presence of pus, bone sequestra and fistula formation [Figure 1]. Exclusion criteria were previous radiation therapy in the head & neck region, metastatic or recurrent malignant disease of the jaw, use of bisphosphonates, ongoing tobacco use or pregnancy. The study prospectively included eight patients, of which four patients were treated with standard therapy and assigned to the control group and four patients were treated additionally with LLLT and assigned to the test group (four women, mean age: 55 ± 8; four men, mean age: 58 ± 4). In all patients, the mandible was involved. In this study, patients were randomly allocated to groups using a randomisation procedure to ensure unbiased assignment. In addition, the experienced examiner responsible for the patient assessments remained blinded throughout the study.
Figure 1.
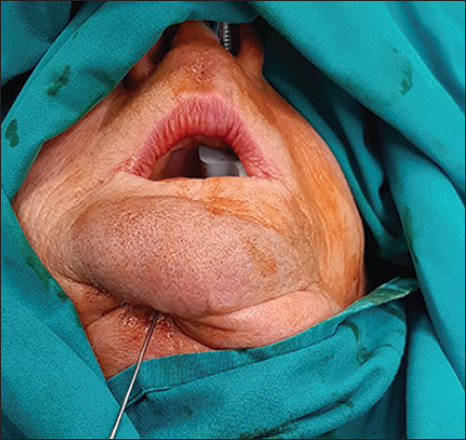
Extraoral fistula assessment
A mucoperiosteal flap of minimal disturbance to surrounding tissues was reflected. An extraoral approach was also established for patients with extraoral fistulas [Figure 2a]. Sequestra [Figure 2b], necrotic bone [Figure 2c] and overlying granulation tissue were removed. Symptomatic neighbouring teeth were extracted and sharp bony edges were removed. Specimens were sent for histopathological evaluation [Figure 2b and c]. All patients received intravenous empiric wide-spectrum antibiotic therapy, which was changed to culture-guided antibiotic therapy if indicated, based on cultures obtained at the time of biopsy. Following discharge, patients were prescribed a mouth rinse for 7 days (chlorhexidine gluconate 0.12%). Sutures were removed 7 or 10 days after surgery. Extraoral LLLT application was performed postoperatively on days 3, 5, 7, 10 and 14 [Figure 3]. In all test group patients, a pulsed 635-nm LED lamp (Repuls7, Repuls Lichtmedizintechnik GmbH, Austria) was used (maximum output power: 140 mW/cm2, frequency: 2.5 Hz, duty cycle: 50%). Patients were positioned in an upright position in a dental chair and the lamp had an extraoral position, perpendicular to the area to be illuminated at a distance of 30 cm from the skin. The treatment surface irradiance was measured at the given distance and was 27.2 mW/cm2. The most important beam parameters are summarised in Table 1.
Figure 2.
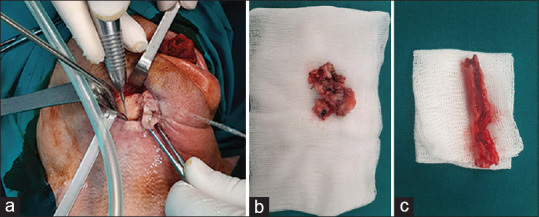
(a) Surgical procedure; (b) Sequestra; (c) Necrotic bone removal
Figure 3.
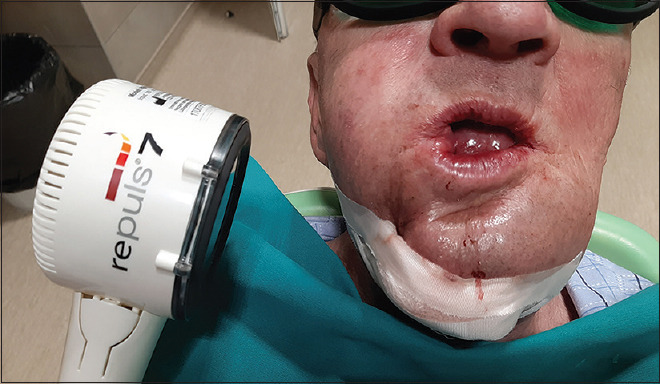
Low-level light therapy session
Table 1.
Report parameters in experimental and clinical photobiomodulation papers
| Description | Data |
|---|---|
| Manufacturer | Repuls Lichtmedizintechnik GmbH |
| Model identifier | Repuls 7 |
| Year produced | 2017 |
| Number and type of emitters (laser or LED) | LED, 7 |
| Wavelength and bandwidth (nm) | 635 |
| Pulse mode (CW or Hz, duty cycle) | 2.5 Hz, 50% |
| Beam spot size at target (cm2) | 200,96 |
| Irradiance at target (mW/cm2) | 27.2 |
| If pulsed peak irradiance (mW/cm2) | 27.2 |
| Exposure duration (min) | 6 |
| Number of treatments | 5 |
| Radiant exposure (J/cm2) | 0.2 |
| Number of points irradiated | 1 |
LED: Light-emitting diode
Patients were clinically evaluated preoperatively and postoperatively at follow-ups. Follow-up examinations were scheduled at one, three and six months after completion of treatment. The presence of oedema, pain, pus and fistulas was evaluated. The severity of patient pain perception was assessed using a Visual Analogue Scale (VAS). The VAS consisted of a 10-cm horizontal line marked from 0 (no pain) to 10 (most severe experienced). Complete resolution of the symptoms (no symptoms) was defined as the absence of any discomfort, corresponding to a zero VAS score.
Evolution of the disease was detailed as follows: Partial clinical achievement (PA) – partial covering of the previously exposed bone with re-epithelisation and no signs of oral or cutaneous draining fistulas; complete clinical achievement (CA) – complete coverage of the previously exposed bone with re-epithelisation, with no signs of oral or cutaneous draining fistulas; Suboptimal clinical achievement (SOA) – the absence of soft-tissue closure or other additional complications (e.g. pathological fracture and oral or cutaneous draining fistulas).
Statistical analysis
The results are presented as mean ± standard deviation, median (interquartile range) or count (per cent) depending on data type and distribution. Due to small sample size and lack of power, exact testing is performed, Fisher’s exact test for nominal data and Mann–Whitney U for numerical data. Graphics for plotting individual-level data were created using an interactive graph tool according to guidelines for basic science data visualisation.
RESULTS
The duration of follow-up was six months. A total of eight patients took part in the study, of which 4 (50%) were women (patients’ mean age: 56.5 ± 6). Patient healing and pain perception were examined. At one month, in all patients, complete healing was observed and maintained; at three months in two patients treated with standard therapy, complete clinical achievement was not maintained while it was maintained in all four patients of the experimental group; at six months, also in two patients, partial clinical achievement remained, while in the experimental group, all patients maintained complete clinical achievement [Table 2]. Patient pain perception at one month was significantly lower in the experimental group compared to the control group, while at three and six months in the experimental group, there was no pain perception (x = 0). The VAS scores are shown in Table 3 and Graph 1.
Table 2.
Healing during the course of the study
| Standard therapy (n=4) | LLLT (n=4) | P | |
|---|---|---|---|
| 1 month | 4 (100) | 4 (100) | 1.000 |
| 3 months | 2 (50) | 4 (100) | 0.429 |
| 6 months | 2 (50) | 4 (100) | 0.429 |
Results are presented as count (%). Testing is performed using Fisher’s exact test. LLLT: Low-level light therapy
Table 3.
Visual Analogue Scale during the course of the study
| Standard therapy (n=4) | LLLT (n=4) | P | |
|---|---|---|---|
| Pre-operative | 7 (2) | 5.5 (2) | 0.400 |
| 1 month | 2.5 (1) | 0.5 (1) | 0.029 |
| 3 months | 3 (2) | 0 | 0.029 |
| 6 months | 2 (4) | 0 | 0.429 |
| Δ 6M-pre-operative | −5 (4) | −5.5 (2) | 0.629 |
Results are presented as median (IQR). Testing is performed using Mann–Whitney U-test (exact test). IQR: Interquartile range, LLLT: Low-level light therapy
Graph 1.
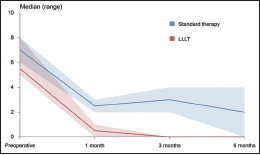
VAS scores
DISCUSSION
According to the Zurich classification system, secondary chronic osteomyelitis of the jaws is considered the same disease as acute osteomyelitis but at a different stage.[8] Acute osteomyelitis of the jaws becomes secondary chronic after 1 month of infection persistence. Both are considered true bone infections and are accompanied by more or less extensive suppuration. Complications of secondary chronic osteomyelitis predominantly include the formation as well as the development of bone sequestra.[9] The final common pathway in all treatments of acute and secondary chronic osteomyelitis of the jaws is to achieve a shift in the disturbed balance between the responsible pathogen(s) and host defences to the latter, allowing the body to overcome the infection. Improvement of local vascularisation is further accomplished by surgical decortication, exceeding conventional surgical debridement, which not only removes the poorly vascularised (infected) bone but also brings well-vascularised tissue to the affected bone, thus facilitating the healing process and allowing antibiotics to reach the target area.[10] Another modality of treatment that can be utilised in osteomyelitis therapy is hyperbaric oxygen therapy (HBO).[12,13] However, patients who undergo HBO therapy need more resources, which cannot be provided by all centres, and careful monitoring.[14] HBO therapy has an acceptable rate of complications,[14] but complications occur.[15,16,17] PBM has no reported adverse effects when used in different treatment modalities such as treatment of oral mucositis due to radiation therapy,[18,19] third molar surgery complications,[20] crown-lengthening complications[21] and COVID-19 in a patient with comorbidities.[22] Wadee et al., compared LLLT and HBO therapy in patients with chronic diabetic foot ulcers and showed more favourable results with the use of LLLT.[23] Vescovi et al., showed the most favourable results with surgical approach and laser therapy combined in terms of clinical improvement and complete healing of bisphosphonate-related osteonecrosis of the jaws.[24] In another study, the same group showed significantly higher success rates with a combined approach based on medical, surgical and LLLT therapy.[25] Pain control was also shown to be supported by the utilisation of LLLT in patients with osteonecrosis[26] as well as in other fields and treatment modalities.[27,28] A study by Scoletta et al., showed improved symptoms and decreased size of lesions in patients with bisphosphonate-related osteonecrosis of the jaws treated with only LLLT.[29] These significant results are indicative of the possible important role of LLLT in the treatment of osteomyelitis in the future. Aside from osteomyelitis research, the effect of LLLT on bone repair was thoroughly carried out and showed stimulation of bone production;[30,31,32] therefore, the positive effect of this treatment modality can be postulated. A stimulating effect was recorded in terms of blood and lymph neovascularisation,[33,34] synthesis of RNA, DNA, collagen and its precursors, as well as positive effects on the levels of prostaglandin, phagocyte cytoplasmic granules, cell proliferation and production of adenosine-5’- triphosphate.[35,36] Furthermore, an existent penetration of LED 635-nm low-level light through the soft and hard tissues of the jaws was demonstrated[37] which implies that the complete jaw bone thickness receives therapeutic doses.
In this study, patients diagnosed with secondary chronic osteomyelitis according to Zurich’s classification[4] were included. In all patients, the mandible was involved and tooth extraction performed before onset of symptoms was identified as the most significant predisposing local factor. Results showed significantly better clinical achievement after 3 and 6 months in the group treated with LLLT, as well as better pain and discomfort resolution. These results support the postulation that LLLT improves blood and lymph flow and therefore improves medical therapy in the affected area by providing antibiotics with the possibility of reaching more affected tissue. With regard to the findings of this study, it can be postulated that with the use of LLLT, it is possible to reduce the period of antibiotic therapy use in the future in patients with secondary chronic osteomyelitis.
CONCLUSIONS
It can be concluded that LLLT is a promising modality for improvement of clinical outcomes of surgical and medical treatment of secondary chronic osteomyelitis of the jaws. Furthermore, pain and discomfort were reduced in patients treated with LLLT. LLLT improves tissue perfusion, as well as bone and soft tissue healing and analgesia. This study shows preliminary results, and due to a small sample size, further inclusion and treatment of patients are indicated and will demonstrate more information on the exact benefits of adjuvant LLLT in treating patients with secondary chronic osteomyelitis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Baltensperger M, Eyrich G, editors. In: Osteomyelitis of the Jaws. Berlin Heidelberg: Springer; 2009. Osteomyelitis of the jaws: Definition and classification; pp. 5–56. [Google Scholar]
- 2.Topazian R, Goldberg M, Hupp J. Philadelphia: W. B. Saunders; 2002. Oral and Maxillofacial Infections. [Google Scholar]
- 3.Marx RE. Chronic osteomyelitis of the jaws. Oral Maxillofac Surg Clin North Am. 1991;3:367–81. [Google Scholar]
- 4.Baltensperger M, Grätz K, Bruder E, Lebeda R, Makek M, Eyrich G. Is primary chronic osteomyelitis a uniform disease?Proposal of a classification based on a retrospective analysis of patients treated in the past 30 years. J Craniomaxillofac Surg. 2004;32:43–50. doi: 10.1016/j.jcms.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Desmet KD, Paz DA, Corry JJ, Eells JT, Wong-Riley MT, Henry MM, et al. Clinical and experimental applications of NIR-LED photobiomodulation. Photomed Laser Surg. 2006;24:121–8. doi: 10.1089/pho.2006.24.121. [DOI] [PubMed] [Google Scholar]
- 6.Dungel P, Hartinger J, Chaudary S, Slezak P, Hofmann A, Hausner T, et al. Low level light therapy by LED of different wavelength induces angiogenesis and improves ischemic wound healing. Lasers Surg Med. 2014;46:773–80. doi: 10.1002/lsm.22299. [DOI] [PubMed] [Google Scholar]
- 7.Escudero JS, Perez MG, de Oliveira Rosso MP, Buchaim DV, Pomini KT, Campos LM, et al. Photobiomodulation therapy (PBMT) in bone repair: A systematic review. Injury. 2019;50:1853–67. doi: 10.1016/j.injury.2019.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Mercuri LG. Acute osteomyelitis of the jaws. Oral Maxillofac Surg Clin North Am. 1991;3:355–65. [Google Scholar]
- 9.Bruder E, Jundt G, Eyrich G. In: Osteomyelitis of the Jaws. Berlin, Heidelberg: Springer; 2009. Pathology of osteomyelitis; pp. 121–33. [Google Scholar]
- 10.Baltensperger M, Eyrich G. In: Osteomyelitis of the jaws. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009. Considerations and surgical therapy; pp. 145–78. [Google Scholar]
- 11.Savvidou OD, Kaspiris A, Bolia IK, Chloros GD, Goumenos SD, Papagelopoulos PJ, et al. Effectiveness of hyperbaric oxygen therapy for the management of chronic osteomyelitis: A systematic review of the literature. Orthopedics. 2018;41:193–9. doi: 10.3928/01477447-20180628-02. [DOI] [PubMed] [Google Scholar]
- 12.Kawashima M, Yamaguchi T, Tamura H, Kawashima M. Treatment of osteomyelitis by hyperbaric oxygen therapy. In: Hyperbaric Oxygenation Therapy, Molecular Mechanisms and Clinical Applications. 2020:67–80. [Google Scholar]
- 13.Monis PL, Bhat V, Shetty S, Salins PC. Hyperbaric oxygen therapy in the management of zygomatic bone osteomyelitis. J Maxillofac Oral Surg. 2021;20:414–7. doi: 10.1007/s12663-020-01469-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Memar MY, Yekani M, Alizadeh N, Baghi HB. Hyperbaric oxygen therapy: Antimicrobial mechanisms and clinical application for infections. Biomed Pharmacother. 2019;109:440–7. doi: 10.1016/j.biopha.2018.10.142. [DOI] [PubMed] [Google Scholar]
- 15.Aliravci ID, Vurucu S. Seizures during hyperbaric oxygen therapy: Diabetic foot patient with chronic osteomyelitis. Demiroglu Bilim Universitesi Florence Nightingale Tip Dergisi. 2022;8:74–6. [Google Scholar]
- 16.Yamamoto Y, Asai N, Furuhashi A, Ono T, Yamagishi Y, Mikamo H, et al. Metronidazole-induced encephalopathy caused by hyperbaric oxygen therapy in a patient with mandibular osteomyelitis. J Infect Chemother. 2019;25:1057–9. doi: 10.1016/j.jiac.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Costa DA, Ganilha JS, Barata PC, Guerreiro FG. Seizure frequency in more than 180,000 treatment sessions with hyperbaric oxygen therapy –A single Centre 20-year analysis. Diving Hyperb Med. 2019;49:167–74. doi: 10.28920/dhm49.3.167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genot-Klastersky MT, Paesmans M, Ameye L, Kayumba A, Beauvois S, Dragan T, et al. Retrospective evaluation of the safety of low-level laser therapy/photobiomodulation in patients with head/neck cancer. Support Care Cancer. 2020;28:3015–22. doi: 10.1007/s00520-019-05041-3. [DOI] [PubMed] [Google Scholar]
- 19.Marín-Conde F, Castellanos-Cosano L, Pachón-Ibañez J, Serrera-Figallo MA, Gutiérrez-Pérez JL, Torres-Lagares D. Photobiomodulation with low-level laser therapy reduces oral mucositis caused by head and neck radio-chemotherapy: Prospective randomized controlled trial. Int J Oral Maxillofac Surg. 2019;48:917–23. doi: 10.1016/j.ijom.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Khalighi Sigaroudi A, Maleki D, Zare H, Maleki D. Is low-level laser therapy effective for complications of mandibular third molar surgery?A literature review. Int J Sci Res Dent Med Sci. 2020;2:72–80. [Google Scholar]
- 21.Akbari N, Mohammadi Moghaddam M, Abedzade S, Osmani F. The effect of low-level laser therapy on complications after crown lengthening surgery-a double-blind clinical trial. Armaghane Danesh. 2022;27 [Google Scholar]
- 22.Sigman SA, Mokmeli S, Vetrici MA. Adjunct low level laser therapy (LLLT) in a morbidly obese patient with severe COVID-19 pneumonia: A case report. Can J Respir Ther. 2020;56:52–6. doi: 10.29390/cjrt-2020-022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadee AN, Fahmy SM, El-Deen HA. Low-level laser therapy (photobiomodulation) versus hyperbaric oxygen therapy on healing of chronic diabetic foot ulcers: A controlled randomized trial. Phys Ther Rev. 2021;26:73–80. [Google Scholar]
- 24.Vescovi P, Merigo E, Meleti M, Manfredi M, Guidotti R, Nammour S. Bisphosphonates-related osteonecrosis of the jaws: A concise review of the literature and a report of a single-Centre experience with 151 patients. J Oral Pathol Med. 2012;41:214–21. doi: 10.1111/j.1600-0714.2011.01091.x. [DOI] [PubMed] [Google Scholar]
- 25.Vescovi P, Manfredi M, Merigo E, Guidotti R, Meleti M, Pedrazzi G, et al. Early surgical laser-assisted management of bisphosphonate-related osteonecrosis of the jaws (BRONJ): A retrospective analysis of 101 treated sites with long-term follow-up. Photomed Laser Surg. 2012;30:5–13. doi: 10.1089/pho.2010.2955. [DOI] [PubMed] [Google Scholar]
- 26.Romeo U, Galanakis A, Marias C, Vecchio AD, Tenore G, Palaia G, et al. Observation of pain control in patients with bisphosphonate-induced osteonecrosis using low level laser therapy: Preliminary results. Photomed Laser Surg. 2011;29:447–52. doi: 10.1089/pho.2010.2835. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Chen XL, Zou XL, Chen SZ, Zou J, Wang Y. Efficacy of low-level laser therapy in pain management after root canal treatment or retreatment: A systematic review. Lasers Med Sci. 2019;34:1305–16. doi: 10.1007/s10103-019-02793-6. [DOI] [PubMed] [Google Scholar]
- 28.Tehrani MR, Nazary-Moghadam S, Zeinalzadeh A, Moradi A, Mehrad-Majd H, Sahebalam M. Efficacy of low-level laser therapy on pain, disability, pressure pain threshold, and range of motion in patients with myofascial neck pain syndrome: A systematic review and meta-analysis of randomized controlled trials. Lasers Med Sci. 2022;37:3333–41. doi: 10.1007/s10103-022-03626-9. [DOI] [PubMed] [Google Scholar]
- 29.Scoletta M, Arduino PG, Reggio L, Dalmasso P, Mozzati M. Effect of low-level laser irradiation on bisphosphonate-induced osteonecrosis of the jaws: Preliminary results of a prospective study. Photomed Laser Surg. 2010;28:179–84. doi: 10.1089/pho.2009.2501. [DOI] [PubMed] [Google Scholar]
- 30.Gerbi ME, Marques AM, Ramalho LM, Ponzi EA, Carvalho CM, Santos Rde C, et al. Infrared laser light further improves bone healing when associated with bone morphogenic proteins: An in vivo study in a rodent model. Photomed Laser Surg. 2008;26:55–60. doi: 10.1089/pho.2007.2026. [DOI] [PubMed] [Google Scholar]
- 31.Blaya DS, Guimarães MB, Pozza DH, Weber JB, de Oliveira MG. Histologic study of the effect of laser therapy on bone repair. J Contemp Dent Pract. 2008;9:41–8. [PubMed] [Google Scholar]
- 32.Nissan J, Assif D, Gross MD, Yaffe A, Binderman I. Effect of low intensity laser irradiation on surgically created bony defects in rats. J Oral Rehabil. 2006;33:619–924. doi: 10.1111/j.1365-2842.2006.01601.x. [DOI] [PubMed] [Google Scholar]
- 33.Kumari D, Khan H, Jiskani AR, Rafique M, Asif M, Kumar V, et al. Neovascularization: Topical effects of streptococcus thermophilus and low level laser therapy in treatment of diabetic wound in rats. Int J Res Med Sci. 2019;7:3357–61. [Google Scholar]
- 34.Sousa Albuquerque Brandão MG, Moreira Ximenes MA, de Oliveira Ramalho A, Saraiva Veras V, Moreira Barros L, de Araújo T. Effects of low-level laser therapy on the healing of foot ulcers in people with diabetes mellitus. Rev Estima. 2020;18 [Google Scholar]
- 35.Pinheiro AL, Gerbi ME. Photoengineering of bone repair processes. Photomed Laser Surg. 2006;24:169–78. doi: 10.1089/pho.2006.24.169. [DOI] [PubMed] [Google Scholar]
- 36.Vescovi P, Merigo E, Manfredi M, Meleti M, Fornaini C, Bonanini M, et al. Nd: YAG laser biostimulation in the treatment of bisphosphonate-associated osteonecrosis of the jaw: Clinical experience in 28 cases. Photomed Laser Surg. 2008;26:37–46. doi: 10.1089/pho.2007.2181. [DOI] [PubMed] [Google Scholar]
- 37.Keković V, Schicho K, Figl M, Arany P, Jezdić Z, Soldatović I, et al. Light distribution of 635 nm LED for PBM treatments in the maxillofacial region. [[Last accessed on 2022 May 03]];Oral and Maxillofacial Surgery Cases. 2021 7:100208. Available from: https://www.sciencedirect.com/science/article/pii/S221454192100002X . [Google Scholar]


