Abstract
Decreased N2 fixation in soybean (Glycine max) L. Merr. during water deficits has been associated with increases in ureides and free amino acids in plant tissues, indicating a potential feedback inhibition by these compounds in response to drought. We evaluated concentrations of ureides and amino acids in leaf and nodule tissue and the concurrent change in N2 fixation in response to exogenous ureides and soil-water treatments for the cultivars Jackson and KS4895. Exogenous ureides applied to the soil and water-deficit treatments inhibited N2 fixation by 85% to 90%. Mn fertilization increased the apparent catabolism of ureides in leaves and hastened the recovery of N2 fixation following exogenous ureide application for both cultivars. Ureides and total free amino acids in leaves and nodules increased during water deficits and coincided with a decline in N2 fixation for both cultivars. N2 fixation recovered to 74% to 90% of control levels 2 d after rewatering drought-stressed plants, but leaf ureides and total nodule amino acids remained elevated in KS4895. Asparagine accounted for 82% of the increase in nodule amino acids relative to well-watered plants at 2 d after rewatering. These results indicate that leaf ureides and nodule asparagine do not feedback inhibit N2 fixation. Compounds whose increase and decrease in concentration mirrored the decline and recovery of N2 fixation included nodule ureides, nodule aspartate, and several amino acids in leaves, indicating that these are potential candidate molecules for feedback inhibition of N2 fixation.
Water-deficit stress is often a primary constraint of soybean (Glycine max) yields (Purcell and Specht, 2004). N2 fixation in soybean is reportedly more sensitive to moderate water deficits than are many other plant processes including transpiration (Sall and Sinclair, 1991; Serraj and Sinclair, 1996) and leaf gas exchange (Durand et al., 1987; Djekoun and Planchon, 1991). It has been proposed that increased tolerance of N2 fixation to water deficits would increase overall water-deficit tolerance in soybean.
Despite much research effort in the area, the mechanisms influencing N2 fixation response to limited soil water are not well understood. Accumulating evidence indicates that the decline in N2 fixation during water deficits and genotypic differences in sensitivity to drought may be associated with levels of nitrogenous compounds, such as amino acids or ureides, in leaves or nodules of N2-fixing plants (Silsbury et al., 1986; Parsons et al., 1993; Oti-Boateng and Silsbury, 1993; Serraj et al., 1999a; Purcell et al., 2000; Vadez et al., 2000). How specific compounds regulate nodule activity is unclear, although several hypotheses have been proposed (Serraj et al., 1999b).
The ureides, allantoin and allantoate, are the final products of N2 fixation that are exported from soybean nodules to the shoot (McClure and Israel, 1979), where they are catabolized. While N2 fixation declines in response to water deficits, shoot ureides often increase (de Silva et al., 1996; Vadez et al., 2000) due to a decreased rate of ureide catabolism in leaves (Serraj et al., 1999a; Vadez and Sinclair, 2001). In support of a link between elevated shoot ureides and inhibition of N2 fixation, the drought-tolerant cv Jackson maintained lower leaf ureide concentrations than drought-sensitive KS4895 under both well-watered and water-deficit conditions (Purcell et al., 2000). Vadez and Sinclair (2001) also reported an inverse relationship between shoot ureide concentrations in well-watered plants and the drought sensitivity of N2 fixation among nine soybean genotypes.
There are conflicting reports on the pathway of ureide breakdown in soybean leaves. Winkler et al. (1987), working with the cv Williams 82, reported the breakdown of allantoate to ureidoglycine by allantoate amidohydrolase (EC 3.5.3.9), which requires Mn as a cofactor (Lukaszewski et al., 1992). Shelp et al. (1984) reported the breakdown of allantoate to urea and ureidoglycolate in the cv Maple Arrow by the enzyme allantoate imidinohydrolase (EC 3.5.3.4), which does not require Mn as a cofactor. More recently, Todd and Polacco (2004) reported evidence that both Maple Arrow and Williams 82 catabolize ureides through the same pathway, and that breakdown in both cultivars involves enzymes that are Mn dependent. Purcell et al. (2000) found that Jackson generally had higher concentrations of Mn than did KS4895 and that increased Mn in plant tissues during drought lowered leaf ureides in both Jackson and KS4895. Furthermore, supplemental Mn under moderate drought conditions increased N2 fixation in the drought-sensitive cv KS4895. Regardless of whether two ureide breakdown pathways exist, it does appear that ureide catabolism in soybean leaves responds to Mn fertility levels (Vadez et al., 2000) and that some genotypes are less sensitive to low Mn fertility than others (Purcell et al., 2000; Sinclair et al., 2003).
In both alfalfa (Schubert et al., 1995) and soybean (Serraj et al., 1998), the accumulation of free amino acids was implicated in feedback inhibition of N2 fixation in response to water deficits. Concentrations of free amino acids in the plant may also play a role in the response of nitrogenase activity to N fertility. Bacanamwo and Harper (1997) reported a decline in nitrogenase activity and a dramatic increase in shoot Asn and in nodule Asp and Glu in response to short term exposure of soybean plants to NO3−. Neo and Layzell (1997) found a 10-fold increase of Gln in soybean phloem in response to a 6-h exposure of shoots to exogenous NH3 that reduced nitrogenase activity by 54%. However, nitrogenase activity recovered when nodulated roots were exposed to elevated O2 concentrations, indicating that Gln concentrations in phloem sap may decrease nodule permeability to O2 diffusion, thereby down-regulating nitrogenase activity. Lukaszewski et al. (1992) reported increased Asn and ureides in soybean leaves in response to N fertilization. It was hypothesized that Asn chelates Mn, thereby inhibiting allantoate amidohydrolase and allantoate catabolism (Lukaszewski et al., 1992).
Evidence in the literature indicates that elevated levels of nitrogenous compounds, such as ureides and amino acids, may play a role in the decline in N2 fixation in soybean in response to environmental stimuli such as nitrogen fertilization or water-deficit stress. Despite much research in this area, no single report has evaluated the relationship between N2 fixation and changes in ureide and amino acid concentrations in leaves and nodules of soybean in response to water deficits. We hypothesized that increased concentrations of ureides or amino acids in either leaf or nodule tissues in soybean would coincide with decreased N2 fixation and that recovery of N2 fixation would be dependent upon reestablishing initial concentrations of nitrogenous compounds that might serve to regulate N2 fixation. Our objectives were to: (1) characterize ureide catabolism and N2 fixation in response to Mn treatments for two genotypes that differ in ureide concentrations and drought sensitivity of N2 fixation; and (2) evaluate the possible regulatory relationship of plant-ureide and amino acid concentrations on N2 fixation activity.
RESULTS
Experiment 1: Response to Mn Fertility and Exogenous Ureides
N2 fixation, measured as acetylene reduction activity (ARA), on day 1 of measurement, prior to application of exogenous ureides to the roots averaged 76 μmol plant−1 h−1 and was not influenced by cultivar or Mn treatment. Mn fertility and exogenous ureides did not influence plant biomass at harvest (data not shown), except that average nodule mass was reduced from 0.26 to 0.20 g plant−1 (lsd 0.05 = 0.05 g) by exogenous ureides for both cultivars. Leaf Mn concentrations ranged from 19 to 32 mg kg−1 and tended to be higher in Jackson than in KS4895 (Table I). Leaf Mn increased in Jackson but not in KS4895 in response to the +Mn treatment. Vadez and Sinclair (2002) also reported greater increases in leaf Mn in Jackson than in the cv Biloxi in response to increased Mn fertility levels.
Table I.
Leaf manganese concentration in Experiment 1 in response to cultivar and manganese fertility
| Cultivar | Manganese Treatment | Leaf Manganese Concentration |
|---|---|---|
| mg kg−1 dw | ||
| Jackson | No | 24 |
| Yes | 32 | |
| KS4895 | No | 19 |
| Yes | 20 | |
| LSD 0.05 | 6 |
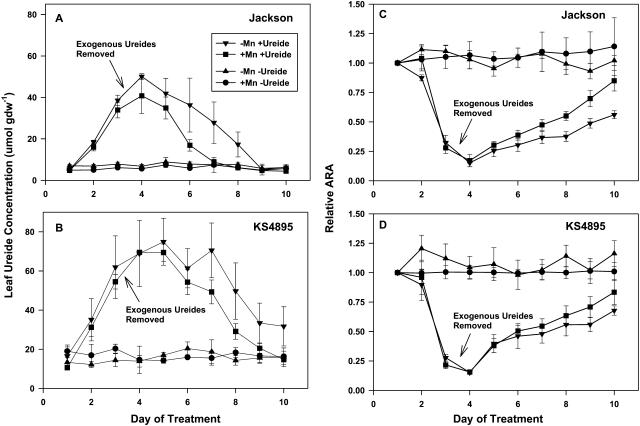
There was no difference in leaf-ureide concentration between Mn treatments on day 1 prior to application of exogenous ureides, but KS4895 had higher levels of leaf ureides (15 μmol g dry weight [dw]−1) than did Jackson (5 μmol gdw−1; Fig. 1, A and B). This is consistent with previously reported levels of ureides in Jackson and KS4895 (King and Purcell, 2001) and with other reports where drought-tolerant lines had lower ureide concentrations than drought-sensitive lines (Vadez and Sinclair, 2001) under well-watered conditions. Exogenously applied ureides increased leaf ureide concentration 5- to 8-fold for both cultivars by day 4. Concurrent with the leaf ureide increase, ARA declined to 15% of control on day 4 for all ureide-treated plants regardless of Mn fertility (Fig. 1, C and D).
Figure 1.
Ureide concentration in the uppermost expanded trifoliolate leaf and relative ARA in response to Mn fertility and exogenous ureides for Jackson and KS4895, Experiment 1. Values of ARA are expressed relative to the well-watered control for a given day. Bars represent the average se of the mean for a treatment on a given day.
The decline in leaf ureides for both cultivars from day 4 through day 10, after the exogenous ureide source was removed from the soil, was greater for plants that received Mn in the nutrient solution (Fig. 1, A and B). Leaf ureide concentration for Jackson treated with exogenous ureides declined to the same level as untreated plants by days 7 and 9 for +Mn and −Mn treatments, respectively. Leaf ureide concentrations for KS4895 treated with exogenous ureides did not reach control levels of ureides until day 10 for the +Mn treatment, and for the −Mn treatment leaf ureide concentration was approximately twice that of the control when plants were harvested on day 10.
For both cultivars, the faster decline in leaf ureides for plants that received Mn fertilizer was associated with a faster recovery of ARA relative to the −Mn treatment. Although ureides declined to control levels faster in Jackson than in KS4895, this difference was not reflected in recovery of ARA (Fig. 1, C and D). For example, by day 7, leaf-ureide concentrations in the +Mn plants given exogenous ureides were similar to control plants for Jackson but were approximately 3 times the control plants for KS4895, yet ARA had recovered to 50% of the control level for both cultivars (Fig. 1, A and C).
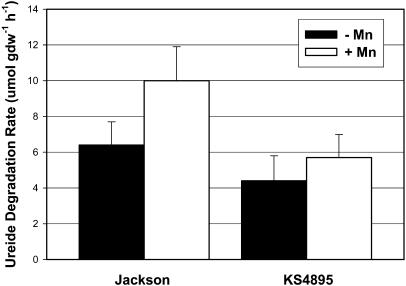
Leaves from plants that had not received ureide application via soil treatment were excised on day 10 and loaded with ureides for 5 h. Ureide concentration at the end of the 5-h loading period ranged from 111 to 133 umol gdw−1 and was not different among cultivar and Mn treatments (data not shown). Ureide degradation rates following the loading period were linear between 0 and 4 h for both cultivars, and degradation rates declined after 4 h (data not shown). Ureide degradation between 0 and 4 h for Jackson +Mn was 10 μmol gdw−1 h−1 and was greater than for other genotype-by-Mn-treatment combinations, which ranged from 4.4 to 6.4 μmol gdw−1 h−1 (Fig. 2). There was a tendency for ureide degradation to increase in response to Mn fertility in KS4895 but the difference was not significant. Higher leaf Mn concentration in the +Mn treatment of Jackson (Table I) may have enabled higher rates of ureide breakdown than in KS4895, which did not have increased leaf Mn in response to the +Mn treatment.
Figure 2.
Ureide degradation rates in excised leaves following exogenous loading with 10 mm allantoin, Experiment 1. Bars represent the slope and ses for each cultivar by Mn treatment as determined by linear regression.
The greater ureide degradation rate in leaves of Jackson may have contributed to its ability to maintain lower leaf ureides and hastened the return to control levels following removal of the exogenous ureide source, as was seen in Figure 1A. The tendency toward faster ureide degradation in the +Mn treatments for both cultivars (Fig. 2) would also explain differences between −Mn and +Mn treatments for leaf ureides in response to exogenous ureide application (Fig. 1, A and B). Purcell et al. (2000) found that vegetative growth was stimulated by Mn for both Jackson and KS4895, but that Jackson extracted more Mn from soil than did KS4895. Furthermore, Jackson had a lower ureide-to-Mn-concentration ratio in leaf tissue than KS4895, indicating that elevated Mn levels in Jackson may contribute to lower ureide levels via increased catabolic rates. In contrast, Vadez and Sinclair (2002) found that leaf ureide degradation in Jackson did not respond to increased Mn concentration in nutrient solution, but in their report, leaf Mn concentrations were greater than 50 mg kg−1 for all Mn treatments. It may be that ureide degradation rates in Jackson do respond to leaf Mn levels but that Mn may not be limiting at concentrations above 50 mg kg−1.
Experiment 2: ARA and Leaf Ureide Response to Water Deficit
As in Experiment 1, ARA of well-watered plants was not affected by Mn treatment or cultivar and averaged 58 μmol plant−1 h−1 prior to initiation of water deficits for all treatments. There was no cultivar or Mn fertility effect on leaf Mn concentrations, which were greater than those reported in Experiment 1 (>100 mg Mn kg−1; data not shown). Although the potting medium was from the same source as for Experiment 1, the potting medium used in the 2 experiments was from different lots and may have differed in Mn availability.
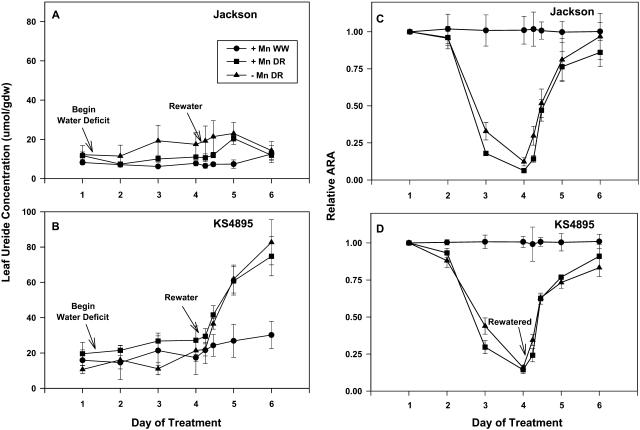
Leaf ureide concentrations on day 1 of measurement for Jackson (10 μmol gdw−1) tended to be lower than for KS4895 (15 μmol gdw−1) averaged over Mn treatments (Fig. 3, A and B). During the water-deficit period (day 2 through 4), leaf ureides increased by approximately 10 μmol gdw−1 for water-deficit treatment of Jackson −Mn and for KS4895 +Mn relative to the +Mn well-watered control. For both Mn treatments in Jackson, there was a modest increase in leaf ureides on day 4 and 5 following rewatering of the water-deficit treatment, but ureide concentrations declined to that of the control by day 6 (Fig. 3A). The most striking response of leaf ureide to water deficit and rewatering treatments was for KS4895, where there was a 4-fold increase in leaf ureide concentration from 20 to 80 μmol gdw−1 between day 4 and day 6 following rewatering (Fig. 3B). This indicates that modest increases in leaf ureides during the water deficit likely did not cause the decline in ARA.
Figure 3.
Ureide concentration in uppermost expanded trifoliolate leaf and relative ARA in response to Mn fertility and soil water treatment for Jackson and KS4895, Experiment 2. Values of ARA are expressed relative to the well-watered control for a given day. FTSW for water-deficit treatment at measurement on days 2 and 3 and for first measurement on day 4 were 0.24, 0.15, and 0.08, respectively, and was 0.6 for all other measurements. FTSW was 0.6 for all measurements in the well-watered (WW) treatment. Bars represent the average se for a treatment on a given day.
The decline in relative ARA in response to progressive water-deficit stress was similar between cultivars and Mn treatments (Fig. 3, C and D). In a previous experiment where Jackson had greater tolerance of ARA to water deficits than did KS4895, the water deficit was developed more slowly (King and Purcell, 2001). The rapid drying of potting mixture in this experiment may have masked genotypic differences in water-deficit tolerance.
By day 4, when soil for the water-deficit treatments was at a fraction of transpirable soil water (FTSW) of 0.08, ARA had declined to approximately 20% of the control for plants in all water-deficit treatments (Fig. 3, C and D). ARA recovered to 50% to 60% of control levels within 8 h of rewatering water-deficit plants on day 4. ARA was 80% to 90% of control levels by day 6, 2 d after rewatering, for all water-deficit treatments.
In this experiment, as has been previously reported (Purcell et al., 2000; Vadez et al., 2000; Vadez and Sinclair, 2001), leaf-ureide concentrations did increase in response to water-deficit stress for two of the four water-deficit treatments and there was a simultaneous decline in nitrogenase activity. However, the extreme increase in leaf-ureide concentration, especially in KS4895, following rewatering of stressed plants coincided with the recovery of ARA (Fig. 3D), indicating that feedback inhibition of N2 fixation in response to water deficits was not directly linked to elevated leaf-ureide concentrations as has been hypothesized (Serraj et al., 1999a).
Experiment 3: Ureide and Amino Acid Levels in Response to Water Deficits
The water-deficit treatments from Experiment 2 were repeated to more thoroughly evaluate the response to water deficit of nitrogenous compounds that may be involved in a signaling pathway for controlling nitrogenase activity in the plant. ARA and leaf ureides on the day of harvest responded similarly to soil-water content during the 6-d-treatment period as in the previous experiment, and these data from the days of plant harvest are presented in Table II. Ureide concentrations from the uppermost-expanded leaf reflected bulk-leaf ureide concentrations for all treatments. Leaf ureides increased slightly, though not significantly, and relative ARA was 0.11 to 0.15 for both cultivars in response to severe water deficit (FTSW = 0.08) on day 4 of treatment. Two days after rewatering (day 6) of plants that had been exposed to water deficit, ARA had recovered to 74% and 92% of well-watered levels for Jackson and KS4895, respectively (Table II). As in the previous experiment, ureide concentrations in the uppermost-expanded trifoliolate leaf declined to the same level as the control in Jackson but increased to 48 μmol gdw−1 (2.7 times that of the control) in KS4895 by 2 d after rewatering. Again, this increase in leaf ureides corresponded with almost full recovery (92%) of ARA in KS4895, indicating that nitrogenase activity was not directly inhibited by elevated leaf ureides.
Table II.
Interacting effect of cultivar and water treatment on ARA, plant ureides, and free amino acids in soybean leaf and nodule at harvest for Experiment 3
| Cultivar
|
Water Treatmenta
|
Day of Treatment at Harvest
|
Relative ARA at Harvestb
|
Leaf
|
Nodule
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bulk Leaf Ureides | Leaf-Disc Ureidesc | Total Amino Acids | Glu | Ureides | Total Amino Acids | Asn | Asp | ||||
| μmol gdw−1 | |||||||||||
| Jackson | WD | 4 | 0.11 | 13 | 12 | 91 | 13.6 | 64 | 95 | 28.8 | 16.9 |
| WD fb RW | 6 | 0.74 | 7 | 7 | 40 | 6.3 | 24 | 41 | 7.2 | 7.2 | |
| WW | 6 | 1.01 | 6 | 7 | 41 | 6.3 | 34 | 37 | 7.6 | 5.6 | |
| KS4895 | WD | 4 | 0.15 | 16 | 23 | 77 | 5.8 | 61 | 80 | 23.6 | 13.0 |
| WD fb RW | 6 | 0.92 | 27 | 48 | 43 | 3.2 | 31 | 67 | 28.7 | 7.7 | |
| WW | 6 | 1.01 | 13 | 18 | 42 | 3.9 | 24 | 35 | 2.5 | 4.6 | |
| LSD 0.05 | 0.24 | 2 | 9 | 18 | 2.4 | 11 | 17 | 11.2 | 3.2 | ||
WD, Water-deficit treatment harvested at FTSW of 0.08; WD fb RW, water deficit followed by rewatering harvested at FTSW = 0.6; WW, well watered harvested at FTSW = 0.6.
Relative ARA indicates ARA for the different soil-water treatments as a fraction of rate for the well-watered control.
Ureide concentration of uppermost fully expanded trifoliate as determined from leaf discs taken at harvest.
Leaf amino acid and nodule ureide concentrations were inversely related to ARA in Jackson and KS4895. Concentrations of total free amino acids in leaves and ureide in nodules for water-deficit plants harvested on day 4 were approximately double that of well-watered plants harvested on day 6, while concentrations for plants that were rewatered following the water deficit were not different from those of well-watered plants on day 6 (Table II). Free amino acid concentrations in nodules increased 2- to 3-fold in response to water deficit on day 4 for both cultivars. For Jackson, free amino acids in nodules declined to control levels by day 6 after plants were rewatered, but as with leaf ureides, nodule amino acids remained relatively high for KS4895 2 d after rewatering (Table II). Asn and Asp accounted for approximately 65% to 75% of the total increase in nodule amino acids during water deficit for both cultivars (Table II). Also, Asn concentration in nodules was 11.5-fold greater for KS4895 2 d after rewatering water-deficit-stressed plants than for control plants on day 6 and accounted for 82% of the increase in total nodule amino acids in this treatment relative to the well-watered control (Table II). For Jackson, nodule concentrations of Asn declined to control levels by day 6, 2 d after rewatering plants of water-deficit treatments. Although nodule Asp concentrations increased in response to water deficit, 2 d after rewatering the Asp concentrations in nodules were similar to the control for both Jackson and KS4895, indicating that nodule Asp is a possible candidate for feedback inhibition of N2 fixation. Nodule concentrations of all other amino acids evaluated were below 6 μmol gdw−1 at all harvests (data not shown).
The increase in total amino acids in leaves of Jackson and KS4895 in response to water deficit (Table III) could not be attributed to large increases for 2 amino acids (Asp and Asn) as occurred in the nodules. Rather, concentrations of 7 amino acids (Ser, Asn, Glu, Val, Ile, Leu, and Phe) increased by 3.5 to 6.7 μmol gdw−1 of leaf tissue relative to well-watered controls, accounting for approximately 70% of the amino acid increase in leaves in response to water deficit (Table III). Among these 7, the increase in Asn was the greatest at 6.7 μmol gdw−1. In leaves, concentrations of all other amino acids evaluated were below 6 μmol gdw−1 at all harvests, except for GABA, which ranged from 8 to 11 μmol gdw−1 for the 3 harvests and did not change in response to water treatment (data not shown). Interestingly, leaf Asp remained low (1.4 μmol gdw−1) for both genotypes during water deficits and was 0.3 μmol gdw−1 for plants in the well-watered and water-deficit followed by rewatering treatments (data not shown).
Table III.
Effect of soil water treatment, averaged over cultivars, on free amino acid concentrations in soybean leaf tissue for Experiment 3
There was no significant cultivar ×water treatment interaction for any leaf tissue amino acids.
| Water Treatmenta
|
Day of Treatment at Harvest
|
Amino Acid Concentration
|
||||||
|---|---|---|---|---|---|---|---|---|
| Ser | Asn | Glu | Val | Ile | Leu | Phe | ||
| μmol gdw−1 | ||||||||
| WD | 4 | 12.87 | 7.85 | 9.70 | 5.09 | 3.76 | 4.46 | 3.82 |
| WD fb RW | 6 | 9.08 | 3.19 | 4.76 | 1.22 | 0.27 | 0.30 | 0.30 |
| WW | 6 | 8.76 | 1.18 | 5.08 | 1.57 | 0.23 | 0.32 | 0.34 |
| LSD 0.05 | 2.52 | 3.02 | 1.68 | 1.12 | 0.96 | 1.22 | 0.96 | |
WD, Water-deficit treatment harvested at FTSW of 0.08; WD fb RW, water deficit followed by rewatering harvested at FTSW = 0.6; WW, well watered harvested at FTSW = 0.6.
DISCUSSION
This research evaluated nitrogenous compounds in leaves and nodules of drought-sensitive (KS4895) and drought-tolerant (Jackson) soybean cultivars in an attempt to associate a decline in nitrogenase activity with feedback inhibition by specific compounds. Following exogenous ureide treatment, ureide catabolism was increased by the Mn treatment in both cultivars. The stimulation of ureide catabolism by Mn in both Jackson and KS4895 indicates that ureide catabolism in Jackson is not strictly Mn independent as has been proposed (Vadez and Sinclair, 2003). Greater leaf Mn concentrations in Jackson than in KS4895 presented in Table I are consistent with previous conclusions (Purcell et al., 2000; Vadez and Sinclair, 2002) and indicate a greater ability by Jackson to remove Mn from the soil solution than for other genotypes. The greater Mn concentration in Jackson and increased ureide catabolic rate may explain the lower levels of shoot ureides in Jackson than other genotypes as reported previously (King and Purcell, 2001).
Although previous research has shown some association between genotypic differences in sensitivity of N2 fixation to water-deficit stress and differences in shoot-ureide levels among soybean genotypes (Vadez and Sinclair, 2001), our results clearly indicate that elevated leaf ureides do not directly inhibit nitrogenase activity. The sharp increase in leaf ureides in KS4895 following rewatering of drought-stressed plants and the concurrent recovery of nitrogenase activity establishes that, for this cultivar, elevated leaf ureides alone do not directly inhibit N2 fixation. With the possible exception of Asp, it appears that elevated levels of free-amino acids in nodules were not responsible for the inhibition of nitrogenase activity in response to water-deficit in this research. For KS4895, total nodule amino acids increased 2-fold during water deficits but at 2 d after rewatering remained 1.9 times the level in nodules of well-watered plants, while ARA had recovered to 92% of well-watered level. Asn accounted for 82% of the total increase in nodule amino acids at 2 d after rewatering of plants in the water-deficit treatment, indicating that elevated nodule Asn concentrations do not inhibit N2 fixation. This conclusion contradicts a previous report that both ureides and Asn were probably involved in the inhibition of nitrogenase activity either directly by accumulating in nodules or by feedback inhibition due to elevated levels in the shoot (Vadez et al., 2000).
In this research, increased concentrations of ureides and Asp in nodules and increases in a number of amino acids in leaves were consistently associated with reduced nitrogenase activity (Tables II and III). The increase in leaf amino acids was attributed to relatively small increases in several amino acids, with maximum contributions from individual amino acids coming from Asn and Glu. Oti-Boateng and Silsbury (1993) reported a 50% to 70% inhibition in ARA of Vicia faba within 48 h of 14C labeled Asn or Glu applied exogenously either to the root or via stem injection. At 48 h after application, radioactive Asn levels were 7 times greater in V. faba leaves than in roots. Conversely, 500 mL of 20 mm Glu supplied to soybean roots increased ARA by 25% relative to the control at 24 h after treatment (Zhu et al., 1991). Bacanamwo and Harper (1997) noted a decrease in ARA and a concomitant increase in shoot Asn and nodule Asp and Glu in soybean in response to short term exposure of roots to NO3−. Further research is required to determine the role of specific compounds and the mechanism by which these compounds may regulate N2 fixation.
A summary of our results and those of others indicate that elevated levels of nitrogenous compounds in soybean may contribute to the decline in nitrogenase activity in response to water-deficit by way of feedback inhibition. Results for KS4895 from this research indicate that elevated concentrations of leaf ureides or nodule Asn did not inhibit N2 fixation, while elevated levels of ureides or Asp in nodules and several amino acids in leaves were consistently associated with a decline in nitrogenase activity for both Jackson and KS4895.
MATERIALS AND METHODS
Experiment 1: Response to Manganese Fertility and Exogenous Ureides
Seeds were sown in 16 pots each for cv Jackson and cv KS4895 to evaluate ARA and leaf-ureide concentration in response to Mn nutrition and exogenous ureide application. Plants were grown in a growth chamber maintained at 25 C with a 16-h photoperiod (6 am–10 pm) and photosythetically active radiation of 600 umol m−2 s−1 at the top of the plants. Experimental design was completely random with a factorial arrangement of two cultivars, two Mn fertility treatments, and two soil-applied ureide treatments with four replications. Pots were constructed of 5-cm-diameter polyvinyl chloride pipe, with an approximate soil volume of 0.5 L, which allowed for nondestructive measurement of ARA as an estimate of nitrogenase activity (Purcell et al., 2000). Prior to sowing, the N-free potting mix (LB2, Sun Gro Horticulture, Bellevue, WA) was saturated with deionized (DI) water followed by the addition of 500 mL/pot of a N-free nutrient solution (de Silva et al., 1996) with (9 μm) or without (0 μm) Mn as MnCl2. After sowing, pots were inoculated with Bradyrhizobium japonicum (strain USDA 110), allowed to drain overnight, and pot capacity weights recorded. Plants were thinned to one per pot after emergence and were well watered throughout the experiment by applying DI water as needed to maintain the soil at approximately 70% of pot-capacity weights. An additional 100 mL of + or − Mn nutrients was added to each pot on 21 and 22 days after sowing (DAS) for a total of 0.37 mg Mn per pot in the +Mn treatment.
Beginning on 34 DAS (day 1), when plants were at the V5 to V6 developmental stage, ARA was measured between 9 am and 11 am daily for 10 consecutive days as an estimate of nitrogenase activity. To measure ARA, a 1:9 air to acetylene mixture was pumped through the sealed root chamber at a volumetric flow rate of 200 mL min−1/pot. After 8 min exposure to acetylene, ethylene concentration in exhaust gas was quantified by gas chromatography. This short-term exposure prevented the decline in nodule activity (data not shown) associated with long-term acetylene exposure (Minchin, et al., 1983). Following ARA measurement on day 1 and day 2, one-half of the plants for each cultivar and Mn treatment were exposed to exogenous ureides by applying 100 mL of 10 mm allantoin to the soil surface. The remaining plants received 100 mL of DI water. Following ARA measurement on day 3, 1 L of DI water was applied to the surface of each pot to flush any remaining allantoin from the soil, followed by 200 mL of the appropriate Mn nutrient solution. Allantoate concentration was determined for samples from the final volume of rinsate from each pot. Allantoin level in rinsate samples for all pots was below 3 × 10−3 mm and was not statistically different from zero (data not shown).
Daily ARA values were double normalized (Ray and Sinclair, 1997) with the first normalization correcting for differences in activity among individual plants prior to initiation of ureide treatments, and the second normalization correcting for fluctuations in ARA for control plants (no exogenous ureides, +Mn fertility) among days. As a result, relative ARA was approximately 1.0 for all plants at the beginning of the experiment and for control plants throughout the experiment.
Leaf ureide concentration was determined daily from day 1 through day 10 from 1.2-cm-diameter leaf discs. Each day at 12 pm, one disc was removed from each leaflet of the uppermost fully expanded trifoliolate leaf. To minimize ureide breakdown prior to extraction, leaf discs were frozen at −80°C and then dried at 80°C for 2 h. Dry weights were recorded for leaf-disc samples, and ureides were extracted in 1 mL of 0.2 n NaOH at 100°C for 30 min. Ureide concentrations were determined using the colorimetric procedure of Young and Conway (1942). Plants were harvested following ARA measurement on day 10 and sectioned into nodules, roots, leaves, and stem. Plant sections were dried for 96 h at 65°C and dw recorded. Leaves were ground to pass a 1-mm sieve and leaf Mn concentration was determined by inductively coupled plasma emission spectroscopy (model D, Spectro Analytical, Fitchburg, MA) by the Soil Testing and Plant Analysis Laboratory at the University of Arkansas.
In situ ureide degradation rates (Vadez and Sinclair, 2000) were determined for the uppermost fully expanded trifoliate leaves taken from the nonureide treated, +, and − Mn plants. Prior to plant harvest at 2 pm on day 10, 1 leaf/plant was removed by cutting the petiole underwater with a surgical blade. Petioles were recut underwater and petiole of each leaf was placed in DI water in a 60-mL test tube and kept in a dark growth chamber at 21°C for 14 h. After the dark equilibration period, leaves plus petioles were transferred to tubes containing 60 mL of 10 mm allantoin, pH 6.8, and placed in a growth chamber at 21°C with a light intensity of 600 μmol m−2 s−1 at the leaf surface for a 5-h ureide loading period. At the end of the loading period, leaves plus petioles were transferred to DI water and incubated in the light. A leaf disc measuring 1.2 cm was taken from each leaf blade (three per trifoliolate) at 0, 2, 4, 6, and 9 h after ureide loading. Ureides were extracted and quantified as described previously. For each cultivar by Mn treatment, rate of ureide degradation over time was determined by linear regression and including individual plants as covariates. The slopes and ses of these regressions were used to separate degradation rates among cultivar and Mn treatments.
Experiment 2: ARA and Leaf Ureide Response to Water Deficit and Manganese
Seeds were sown in 12 pots each for cv Jackson and cv KS4895 to evaluate ARA and plant ureide concentrations in response to Mn fertility and soil water availability. Soil water and Mn treatments evaluated for each cultivar included a well-watered +Mn control and water-deficit treatments with and without Mn. The experimental design was completely random with four replications. Experimental conditions, including the PVC-pot design and nutrient applications, were the same as described for Experiment 1.
All plants were maintained well watered (70% soil pot-capacity weight daily) until 31 DAS (day 1 of a 6-d measurement period), when the 2 water treatments were established. Transpirable water at pot capacity was defined as the difference between the pot capacity weight and the pot weight when daily transpiration was <10% of the well-watered plants (Ritchie, 1981). Each pot was watered to a daily target weight at 8 am and 2 pm during the 6-d measurement period. Control pots were watered to 70% of capacity weight daily. Water-deficit pots were watered to 70, 42, 35, and 30% of the capacity weight at 8 am on days 1 through 4 of measurement, respectively, which corresponded to FTSW values of 0.6, 0.24, 0.15, and 0.08 as described by King and Purcell (2001). ARA was measured between 9 am and 11 daily. At 12 pm on day 4, all pots were rewatered to 70% of capacity to evaluate recovery of ARA following water-deficit treatments. ARA was measured 2 and 8 h after rewatering on day 4. All pots were watered to 70% of capacity at 8 am on days 5 and 6 and ARA was measured at 9 am.
Leaf ureides were determined as described previously for the uppermost-expanded leaf from leaf-disc samples taken immediately after each ARA measurement. Plants were harvested after measurements on day 6 and sectioned into nodules, roots, leaves, and stem. Plant sections were dried for 96 h at 65°C, weighed, and leaf Mn concentration was determined.
Experiment 3: Ureide and Amino Acid in Leaves and Nodules in Response to Soil Water Deficit
The +Mn treatments from Experiment 2 were repeated to evaluate tissue ureides and amino acids as potential feedback inhibitors of nitrogenase activity. PVC pots were prepared and maintained as previously described except all treatments received N-free nutrients plus Mn. Seeds were sown in 12 pots each for cv Jackson and cv KS4895 and maintained well watered until 34 DAS.
At 34 DAS, two-thirds of the pots for each cultivar were taken through a soil-drying cycle similar to that described for Experiment 2. ARA was measured daily at 9 am until harvest. Leaf-disc samples were taken daily following ARA measurements for determination of leaf ureide concentration. One-half of the water-deficit plants were harvested following ARA measurement on day 4 prior to rewatering to determine tissue ureides and amino acids at the time of maximum water deficit. The remaining plants were rewatered to 70% of the saturated-soil weight and harvested on day 6, when ARA of the rewatered plants had recovered to greater than 75% of the well-watered controls. At harvest, plants were sectioned into root, stem, leaves, and nodules. Root and stem portions were immediately oven dried at 65°C. Fresh weights were recorded for leaves and nodules and one-half of the leaf and nodule samples were stored at −80°C for amino acid analysis. Fresh weights were recorded for the remaining one-half, which was then dried. Dry weights were recorded for all plant sections and dried tissue was ground for ureide extraction. Total leaf and nodule dry weights per plant were determined by multiplying the total fresh weight per plant times the ratio of dry to fresh weight for the dried subsample. Approximately 100 mg of ground tissue for each plant section was weighed into a test tube and extracted in 5 mL of 0.2 m NaOH at 100°C for 30 min and ureide concentrations were determined colorimetrically (oung and Conway, 1942).
Free amino acid content of leaf and nodule tissue was determined from the frozen samples. Samples were weighed, freeze dried and reweighed, and then extracted using 0.1 m hydrochloric acid. Amino acids were separated and quantified by the Poultry Science Laboratory at the University of Arkansas using the Pickering lithium gradient system with postcolumn ninhydrin derivatization and norLeu at 0.5 nmol μL−1 as an internal standard (Granau and Swiader, 1992). Amino acids that were analyzed included: Ser, Asn, Glu, Val, Ile, Leu, Phe, Asp, pseudoSer, Thr, Gln, Gly, Ala, citrulline, Cys, Met, Tyr, β-Ala, γ-aminobutyric acid, Trp, ethanolamine, Lys, His, and Arg. Only those amino acids followed by an abbreviation responded significantly to the imposed water regime and are discussed in results.
Acknowledgments
We thank Marilynn Davies for her assistance in the sample preparation and tissue ureide analysis.
This work was supported by the United Soybean Board (project no. 4213).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.056317.
References
- Bacanamwo M, Harper JE (1997) The feedback mechanism of nitrate inhibition of nitrogenase activity in soybean may involve asparagine and/or products of its metabolism. Physiol Plant 100: 371–377 [Google Scholar]
- de Silva M, Purcell LC, King CA (1996) Soybean petiole ureide response to water deficits and decreased transpiration. Crop Sci 36: 611–616 [Google Scholar]
- Djekoun A, Planchon D (1991) Water status effect on dinitrogen fixation and photosynthesis in soybean. Agron J 83: 316–322 [Google Scholar]
- Durand JL, Sheehy JE, Minchin FR (1987) Nitrogenase activity, photosynthesis and nodule water potential in soyabean plants experiencing water deprivation. J Exp Bot 38: 311–321 [Google Scholar]
- Granau JA, Swiader JM (1992) Chromatography of 99 amino acids and other ninhydrin-reactive compounds in the Pickering lithium gradient system. J Chromatogr 594: 165–171 [Google Scholar]
- King CA, Purcell LC (2001) Soybean nodule size and relationship to nitrogen fixation response to water deficit. Crop Sci 41: 1099–1107 [Google Scholar]
- Lukaszewski KM, Blevins DG, Randall DD (1992) Asparagine and boric acid cause allantoate accumulation in soybean leaves by inhibiting manganese-dependent allantoate amidohydrolase. Plant Physiol 99: 1670–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure PR, Israel DW (1979) Transport of nitrogen in the xylem of soybean plants. Plant Physiol 64: 411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchin FR, Witty JF, Sheehy JE, Muller M (1983) A major error in the acetylene reduction assay: decreases in nodular nitrogenase activity under assay conditions. J Exp Bot 37: 641–649 [Google Scholar]
- Neo HH, Layzell DB (1997) Phloem glutamine and the regulation of O2 diffusion in legume nodules. Plant Physiol 113: 259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oti-Boateng C, Silsbury JH (1993) The effect of exogenous amino-acid on acetylene reduction activity of Vicia faba L. cv. Fiord. Ann Bot (Lond) 71: 71–74 [Google Scholar]
- Parsons R, Stanforth A, Raven JA, Sprent JI (1993) Nodule growth and activity may be regulated by a feedback mechanism involving phloem nitrogen. Plant Cell Environ 16: 125–136 [Google Scholar]
- Purcell LC, King CA, Ball RA (2000) Soybean cultivar differences in ureides and the relationship to drought tolerant nitrogen fixation and manganese nutrition. Crop Sci 40: 1062–1070 [Google Scholar]
- Purcell LC, Specht JE (2004) Physiological traits for ameliorating drought stress. In HR Boerma, JE Specht, eds, Soybeans: Improvement, Production, and Uses, Ed 3. American Society of Agronomy, Madison, WI, pp 569–620
- Ray JD, Sinclair TR (1997) Stomatal closure of maize hybrids in response to drying soil. Crop Sci 37: 803–807 [Google Scholar]
- Ritchie JT (1981) Water dynamics in the soil-plant-atmosphere system. Plant Soil 58: 81–96 [Google Scholar]
- Sall K, Sinclair TR (1991) Soybean genotypic differences in sensitivity of symbiotic nitrogen fixation to soil dehydration. Plant Soil 133: 31–37 [Google Scholar]
- Schubert S, Serraj R, Plies-Balzer E, Mengel K (1995) Effect of drought stress on growth, sugar concentrations and amino acid accumulation in N2-fixing alfalfa. J Plant Physiol 146: 541–546 [Google Scholar]
- Serraj R, Shelp BJ, Sinclair TR (1998) Accumulation of aminobutyric acid in nodulated soybeans in response to drought stress. Physiol Plant 102: 79–86 [DOI] [PubMed] [Google Scholar]
- Serraj R, Sinclair TR (1996) Processes contributing to N2-fixation insensitivity to drought in the soybean cultivar Jackson. Crop Sci 36: 961–968 [Google Scholar]
- Serraj R, Vadez V, Denison RF, Sinclair TR (1999. a) Involvement of ureides in nitrogen fixation inhibition in soybean. Plant Physiol 119: 289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serraj R, Sinclair TR, Purcell LC (1999. b) Symbiotic N2 fixation response to drought. J Exp Bot 50: 143–155 [Google Scholar]
- Shelp BJ, Sieciechowicz K, Ireland RJ, Joy KW (1984) Determination of urea and ammonia in leaf extracts: application to ureide metabolism. Can J Bot 63: 1135–1140 [Google Scholar]
- Silsbury JH, Catchpole DW, Wallace W (1986) Effects of nitrate and ammonium on nitrogenase (C2H2 reduction) activity of swards of subterranean clover, Trifolium subterraneum L. Aust J Plant Physiol 13: 257–273 [Google Scholar]
- Sinclair TR, Vadez V, Chenu K (2003) Ureide accumulation in response to Mn nutrition by eight soybean genotypes with N2 fixation tolerance to soil drying. Crop Sci 43: 592–597 [Google Scholar]
- Todd CD, Polacco JC (2004) Soybean cultivars ‘Williams 82′ and ‘Maple Arrow’ produce both urea and ammonia during ureide degradation. J Exp Bot 55: 867–877 [DOI] [PubMed] [Google Scholar]
- Vadez V, Sinclair TR (2000) Ureide degradation pathways in intact soybean leaves. J Exp Bot 51: 1459–1465 [PubMed] [Google Scholar]
- Vadez V, Sinclair TR (2001) Leaf ureide degradation and N2 fixation tolerance to water deficit in soybean. J Exp Bot 52: 153–159 [PubMed] [Google Scholar]
- Vadez V, Sinclair TR (2002) Sensitivity of N2 fixation traits in soybean cultivar Jackson to manganese. Crop Sci 42: 791–796 [Google Scholar]
- Vadez V, Sinclair TR (2003) Ureide accumulation in response to manganese nutrition by eight soybean genotypes with N2 fixation tolerance to soil drying. Crop Sci 43: 592–597 [Google Scholar]
- Vadez V, Sinclair TR, Serraj R (2000) Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiol Plant 110: 215–223 [Google Scholar]
- Winkler RD, Blevins DG, Polacco JC, Randall DD (1987) Ureide catabolism in soybeans. II. Pathway of catabolism in intact leaf tissue. Plant Physiol 83: 585–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EG, Conway CF (1942) On the estimation of allantoin by the Rimini-Schryver reaction. J Biol Chem 142: 839–853 [Google Scholar]
- Zhu Y, Shearer G, Kohl DH (1991) Proline fed to intact soybean plants influences acetylene reducing activity and content and metabolism of proline in bacteroids. Plant Physiol 98: 1020–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]