Abstract
The fluxes of (1) exogenous nitrogen (N) assimilation and (2) remobilization of endogenous N from vegetative plant compartments were measured by 15N labeling during the seed-filling period in pea (Pisum sativum L. cv Caméor), to better understand the mechanism of N remobilization. While the majority (86%) of exogenous N was allocated to the vegetative organs before the beginning of seed filling, this fraction decreased to 45% at the onset of seed filling, the remainder being directed to seeds. Nitrogen remobilization from vegetative parts contributed to 71% of the total N in mature seeds borne on the first two nodes (first stratum). The contribution of remobilized N to total seed N varied, with the highest proportion at the beginning of filling; it was independent of the developmental stage of each stratum of seeds, suggesting that remobilized N forms a unique pool, managed at the whole-plant level and supplied to all filling seeds whatever their position on the plant. Once seed filling starts, N is remobilized from all vegetative organs: 30% of the total N accumulated in seeds was remobilized from leaves, 20% from pod walls, 11% from roots, and 10% from stems. The rate of N remobilization was maximal when seeds of all the different strata were filling, consistent with regulation according to the N demand of seeds. At later stages of seed filling, the rate of remobilization decreases and may become controlled by the amount of residual N in vegetative tissues.
Pea (Pisum sativum) is an important agricultural crop grown primarily for its high seed protein content. However, the protein yield of the pea crop remains too low and variable between cropping area and years (http://apps.fao/faostat/, http://www.prolea.com/unip/) to sustain the needs in plant protein of European countries. To extend the pea crop in Europe and to increase use of pea seed in the feed industry, breeders have to develop varieties with better harvest and nitrogen (N) indices. Toward this aim, a better understanding of the processes involved in the elaboration of seed protein content is needed. The final protein yield of seeds depends both upon the genotype and on environmental factors during seed filling (Lhuillier-Soundélé et al., 1999a). Nitrogen accumulation by seeds during filling depends upon the external N supply: soil mineral N assimilation and/or symbiotic fixation of atmospheric N2. However, exogenous N cannot generally sustain the high N demand of filling seeds, so endogenous N previously accumulated in vegetative parts is largely remobilized to fulfill this demand (Sinclair and de Wit, 1976; Salon et al., 2001). Seed N concentration is correlated to N availability within plants, and the extent of the contribution of N remobilization to seed N yield varies among legumes: 70% in field-grown pea (Atta et al., 2004), 80% to 90% in soybean (Glycine max; Warembourg and Fernandez, 1985; Grandgirard, 2002), 43% to 94% in rain-fed grown lentil (Lens culinaris; Kurdali et al., 1997), 84% in bean plants (Phaseolus vulgaris; Westermann et al., 1985), and 80% in Vicia faba (Dekhuijzen and Verkerke, 1984). Despite this knowledge, N repartition during seed filling within legume plants and in particular within pea plants has not yet been well characterized. All vegetative organs undergo N remobilization, but the efficiency with which it can be transferred to growing seeds and the rate of N remobilization may vary according to the organs (Salon et al., 2001). Leaves and stems are the major contributors to the N supply of seeds (Peoples and Dalling, 1988), while N from pods constitutes a temporary N reserve for seed filling (Pate, 1985) and roots are probably much less involved (Pate, 1985; Peoples and Dalling, 1988). Nevertheless, the order of induction of N remobilization in the different organs and strata during seed filling remains unknown.
The main objective of this study was to understand the process of N remobilization from vegetative organs during seed filling at the level of whole pea plant. The organs and strata involved were identified, and N movement into and between them was quantified. Two N fluxes were distinguished: (1) exogenous N assimilated by the plants during 15N labeling and (2) remobilization of stored endogenous N. These two N fluxes were distinguished by supplying 15N in a nutritive solution. Six successive 3-d 15N-labeling experiments were carried out throughout seed filling, and the harvested plants were divided into several compartments and strata for analysis.
The partitioning of newly assimilated N within plants has been estimated using pulse-chase 15N labeling for pea (Atta et al., 2004), common bean (Westermann et al., 1985), soybean (Warembourg and Fernandez, 1985), and V. faba (Dekhuijzen and Verkerke, 1984). At a particular stage of plant development, such as vegetative growth, flowering, or beginning of seed filling, 15N-labeled N was applied during a given time span from 1 d (Warembourg and Fernandez, 1985) to 1 week (Dekhuijzen and Verkerke, 1984). Nitrogen remobilization was then estimated from 15N redistribution at a given time after labeling. In this article, the fluxes of N remobilized from vegetative tissues were dynamically measured from the exogenous and endogenous N fluxes within plants throughout the seed filling and not from the estimation of the redistribution of exogenous N as it was done previously. By the methodology that we used, the nature of organs and strata where N was remobilized and the order of their solicitation were determined.
First, the characterization of plant growth was described, then the different N pathways (exogenous and endogenous N) were dynamically quantified during seed filling, and finally the contribution of the N remobilization to the total N accumulation by seeds was estimated. Several hypotheses are proposed to account for the regulation of the rate of N remobilization during the seed-filling period.
RESULTS
Plant Growth
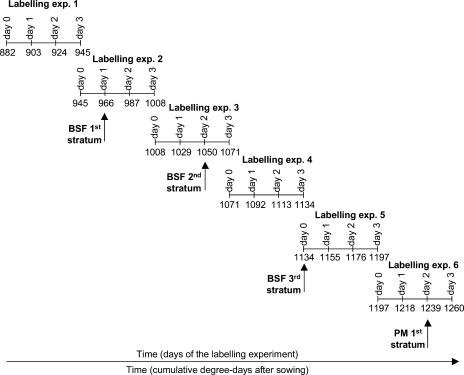
A series of six successive 3-d labeling experiments, starting before the beginning of seed filling, at 882°C days after sowing (DAS), and ending at physiological maturity of seeds from the two lowest reproductive nodes (first stratum), at 1,260°C DAS, was carried out (Fig. 1). The different phases of seed development were defined according to the evolution of seed water concentration. Ney et al. (1993) defined the beginning of seed filling as when seed water concentration decreases under 80% and the end of seed filling as when the water concentration drops below 55%. Physiological maturity then begins and was characterized by a dramatic water loss and by the stop of dry matter accumulation by seeds.
Figure 1.
Experimental setup of the six successive labeling experiments. The correspondence of the labeling experiments with the physiological stages of seeds is indicated. BSF, Beginning of seed filling; PM, physiological maturity. Time is expressed in cumulative degree days after sowing. Each labeling experiment lasted 3 d; except for the first experiment, the beginning of a labeling experiment (i.e. day 0) was concomitant with the end of the preceding labeling experiment (i.e. day 3).
In our experiment, seeds from the first stratum started to fill at around 966°C DAS, i.e. during the second labeling experiment, and ceased filling at around 1,239°C DAS, i.e. during the sixth and last labeling experiment (Fig. 1). Seeds from the second stratum (the third and fourth reproductive nodes) began to fill at around 1,050°C DAS, i.e. during the third labeling experiment (Fig. 1). On average, seeds of the third stratum (all the remaining seeds from higher reproductive nodes than fourth) began to fill at around 1,134°C DAS, i.e. at the end of the fourth labeling experiment (Fig. 1). At the end of the six labeling experiments, only seeds of the first stratum had reached the physiological maturity, the remaining seeds from higher strata (i.e. reproductive nodes higher than two) being still filling.
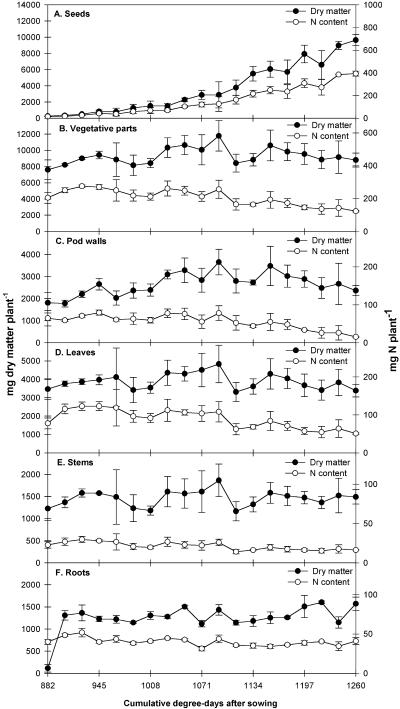
During the developmental period analyzed, about 90% of the dry matter accumulated was allocated to filling seeds (Fig. 2). Seed dry matter increased from 3.3% to 52.3% of total plant dry matter between 882°C and 1,260°C DAS, respectively (Fig. 2A). Vegetative organs, including pod walls, did not significantly accumulate dry matter between 882°C and 1,260°C DAS (Fig. 2B), but a transient dry matter increase was observed for pod walls (Fig. 2C) and leaves (Fig. 2D) up to 1,092°C DAS (i.e. during the fourth labeling experiment). Subsequently, pod walls and leaves from the lower vegetative strata turned yellow and started to lose dry matter. Stem (Fig. 2E) and root (Fig. 2F) dry matter remained constant during this period.
Figure 2.
Changes during the six labeling experiments in dry matter (mg plant−1) represented on the left axis and N content (mg plant−1) represented on the right axis of pea seeds (A) and vegetative plant parts (B) further separated into pods walls (C), leaves (D), stems (E), and roots (F). Time was expressed in cumulative degree days after sowing. Data are means and vertical bars indicate ±se for n = 3 repetitions of each of two plants.
The dynamics of dry matter accumulation differed from those of N incorporation within the various plant compartments during the period covered by the labeling experiments (Fig. 2). Plant N content increased from 220 mg N plant−1 at 882°C DAS to 517 mg N plant−1 at 1,260°C DAS. Nitrogen stored in seeds (Fig. 2A) increased from 14 mg N plant−1 to 393 mg N plant−1 during this period. The amount of N accumulated in vegetative tissues (Fig. 2B) was highest at 945°C DAS (i.e. the beginning of seed filling, second labeling experiment), at around 270 mg N plant−1, and decreased thereafter, indicating that N remobilization was occurring. Pod wall N content (Fig. 2C) decreased throughout the experiment, whereas leaves (Fig. 2D) accumulated N until 945°C DAS and then lost N. Stem (Fig. 2E) and root (Fig. 2F) N content remained constant throughout the period. From the beginning of the second labeling experiment, when seeds from the first stratum began to fill (945°C DAS), up to physiological maturity of seeds from the first stratum (1,260°C DAS), N concentration in vegetative plant tissues decreased from 2.9 to 1.4 mg N g−1 of dry matter, whereas seed N concentration decreased from 5.0 to 4.1 mg N g−1 of dry matter when all seeds had begun to fill (1,134°C DAS) and then remained constant (data not shown).
Measurement of N Fluxes in Pea Plants throughout Seed Filling
The 15N-labeled nutrient solution allowed us to follow the incorporation of exogenous N and to determine the amount of endogenous N remobilized within the plant. The 15N enrichment of the nutrient solution was sufficient to accurately measure exogenous N accumulation and distribution within plants (see “Materials and Methods”), as exemplified by the data of the fourth labeling experiment (Table I). The 15N enrichment of all of the tissues increased continuously throughout the 3-d labeling period.
Table I.
Example of the evolution of the 15N enrichment (expressed as δ15N) of the various plant compartments measured during the duration of the fourth labeling experiment
| Day 1 (1,092°C DAS) | Day 2 (1,113°C DAS) | Day 3 (1,134°C DAS) | |
|---|---|---|---|
| Seeds of the third stratum (S3) | 49 ± 7 | 194 ± 56 | 289 ± 27 |
| Seeds of the second stratum (S2) | 30 ± 6 | 135 ± 38 | 195 ± 21 |
| Seeds of the first stratum (S1) | 29 ± 6 | 105 ± 33 | 129 ± 24 |
| Pod walls of the third stratum (PW3) | 30 ± 11 | 76 ± 29 | 117 ± 25 |
| Pod walls of the second stratum (PW2) | 12 ± 1 | 46 ± 9 | 69 ± 14 |
| Pod walls of the first stratum (PW1) | 15 ± 4 | 50 ± 12 | 71 ± 19 |
| Leaves of the third reproductive stratum (RL3) | 46 ± 11 | 127 ± 29 | 175 ± 5 |
| Leaves of the second reproductive stratum (RL2) | 33 ± 6 | 89 ± 22 | 106 ± 4 |
| Leaves of the first reproductive stratum (RL1) | 30 ± 5 | 99 ± 34 | 109 ± 5 |
| Leaves of the third vegetative stratum (VL3) | 35 ± 5 | 81 ± 14 | 158 ± 40 |
| Leaves of the second vegetative stratum (VL2) | 49 ± 8 | 120 ± 36 | 267 ± 67 |
| Leaves of the first vegetative stratum (VL1) | 70 ± 22 | 276 ± 33 | 324 ± 150 |
| Stem | 134 ± 18 | 375 ± 46 | 554 ± 92 |
| Roots | 131 ± 21 | 432 ± 91 | 523 ± 30 |
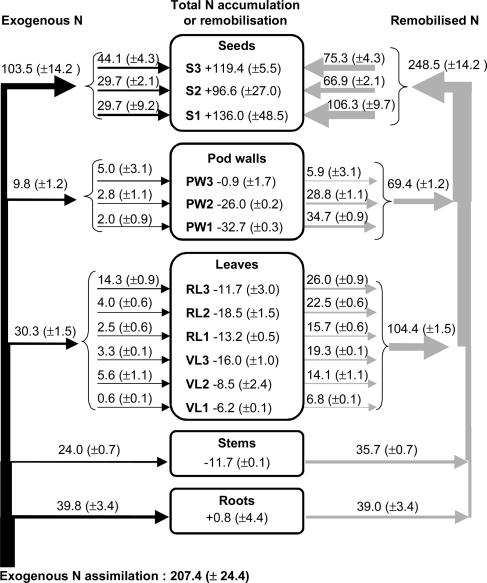
Using 15N enrichment of labeled and unlabeled control plants and plant N content analysis, a flow chart of exogenous N uptake (Fig. 3, left arrows), total N accumulation or remobilization, and endogenous N remobilizations (Fig. 3, right arrows) within the various plant compartments was established for the duration of seed filling of the first stratum of reproductive nodes from 945°C to 1,260°C DAS, i.e. the five last labeling experiments. Exogenous N assimilated by the plants was partitioned into seeds (49.9%), roots (19.2%), leaves (14.6%), stems (11.6%), and pod walls (4.7%).
Figure 3.
Allocation of exogenous N (left arrows) calculated using 15N enrichment (see “Materials and Methods,” Eq. 3) and distribution of endogenous remobilized N (right arrows) from vegetative parts (see “Materials and Methods,” Eq. 4) to seeds during the filling period of the first two reproductive nodes of pea plants (from 945–1,260°C DAS). Values inside the different compartments represent the N accumulation (+) or remobilization (−) in/from a stratum of compartment (see “Materials and Methods,” Eq. 2). Nitrogen fluxes are expressed in mg plant−1; each value is given as the mean ± se for n = 3 repetitions of each of two plants, and the width of arrows is proportional to the N fluxes.
Seeds displayed a net N accumulation of about 352.0 ± 22.1 mg N plant−1 during the period measured, variable between strata: seeds in the first stratum accumulated 136.0 mg N plant−1, whereas those in the second and third strata accumulated 96.6 and 119.4 mg N plant−1, respectively. All vegetative compartments except for the roots underwent a net loss of N during the filling period: 11.7 mg N plant−1 from stems and between 6.2 to 18.5 mg N plant−1 and 0.9 to 32.7 mg N plant−1 for leaves and pod walls, respectively.
Seventy-one percent of the N incorporated in seeds arose from N stored in vegetative tissues prior to 945°C DAS and remobilized during the labeling period (945–1,260°C DAS; Fig. 3); the main sources of remobilized N were leaves, which contributed to 30% of the final N seed yield, followed by pod walls (20%), roots (11%), and stems (10%).
While the rate of N remobilization from leaves increased from the bottom to the top of the canopy, N remobilization from pod walls varied in the opposite direction, the lowest pod walls (PW1) being the main endogenous N supplier to seeds (Fig. 3). The rate of accumulation of remobilized N in a given stratum of seeds was always higher than the rate of N remobilization from vegetative organs of the same stratum (i.e. RLi + PWi); for example, seeds from the first stratum accumulated about 106.3 mg of remobilized N plant−1, whereas only 50.4 mg N plant−1 was remobilized from leaves and pod walls from the first reproductive stratum.
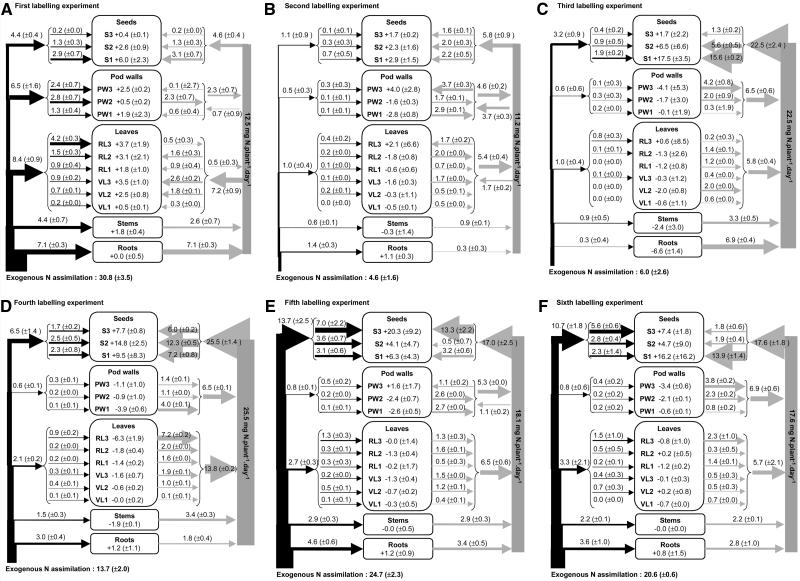
Dynamic Trends of N Fluxes during the Six Labeling Experiments
The flow chart (Fig. 3) represents an integrated view of the various sources of N to filling seeds, which are subjected to change during the labeling period. To obtain a dynamic view of N fluxes during seed filling of the first stratum, the exogenous N supply and the internal cycling of unlabeled N were determined during six successive labeling experiments (Fig. 4).
Figure 4.
Evolution of the allocation of exogenous N (left arrows) and distribution of endogenous remobilized N from vegetative parts (right arrows) to seeds or other vegetative parts during the six successive experiments. Nitrogen fluxes are expressed in mg plant−1 d−1; each value is given as the mean ± se for n = 3 repetitions of each of two plants, and the width of arrows is proportional to the N fluxes.
Exogenous N Supply
The rate of exogenous N assimilation by the plants varied during seed filling: the rate of 30.8 mg N plant−1 d−1 before the beginning of seed filling (i.e. first labeling experiment; Fig. 4A) decreased sharply when seeds started to fill (i.e. second labeling experiment; Fig. 4B), then increased again until all seeds reached the beginning of filling (i.e. third, fourth and fifth labeling experiments; Fig. 4, C–E) and remained constant thereafter (i.e. sixth labeling experiment; Fig. 4F). Although the rates of exogenous N accumulation by seeds and by whole plants followed similar trends throughout seed filling, the proportion of exogenous N acquired by plants that was allocated to seeds varied. The proportion increased from 14% (Fig. 4A) to 24% and 53% when the first (Fig. 4B) and second (Fig. 4C) strata, respectively, of seeds began to fill and remained constant thereafter (Fig. 4, D–F). The proportions of exogenous N received by roots and stems remained at around 20% and 13%, respectively, throughout seed filling (Fig. 4). Allocation of exogenous N to leaves decreased linearly from 27% to 17% between the first (Fig. 4A) and the third (Fig. 4C) labeling experiments and subsequently remained constant at 15% (Fig. 4, D–F). The proportion of exogenous N allocated to pod walls decreased dramatically from 21% to 11% between the first (Fig. 4A) and second labeling experiments (Fig. 4B) and from 10% to 4% between the third (Fig. 4C) and fourth (Fig. 4D) labeling experiments, remaining constant thereafter (Fig. 4, E and F).
Recycling and Remobilization of Endogenous N from Vegetative Organs
Before the beginning of seed filling (Fig. 4A), roots were the main source of remobilized N (57% of the total), while stem, pod wall, and leaf contributions averaged 21%, 18%, and 4%, respectively. During this period of vegetative growth, leaves acquired the bulk of the remobilized N (58%), while seeds and pod walls accumulated the remaining 37% and 5%, respectively.
During the second labeling (Fig. 4B), the recycling of remobilized N between vegetative organs that was observed during the first labeling experiment continued, but leaves became the main source of remobilized N, providing 48% of the remobilized N pool, pod walls and stems providing 41% and 8%, respectively, and roots furnishing only 3% of the remobilized N. The pool of remobilized N was distributed mostly to seeds (52%), pod walls (33%), and the highest reproductive nodes (15%).
During seed filling in the second stratum (i.e. the third labeling experiment; Fig. 4C), the recycling of remobilized N between vegetative tissues ceased, and all remobilized N was allocated to seeds. Stems contributed 15% to the pool of remobilized N, while root contribution declined (Fig. 4, D–F). The contribution of leaves to the pool of remobilized N peaked at around 54% during the fourth labeling experiment (Fig. 4D) and then decreased to 32% (Fig. 4F), while pod walls contributed progressively more and more to the supply of endogenous N remobilized (Fig. 4, C–F). As mentioned above, when the entire period of seed filling was considered (Fig. 3), the rate of remobilized N accumulated in one particular stratum of seeds was always higher than the rate of N remobilized from vegetative organs from the same stratum, whatever the developmental stage of seeds.
The rate of N remobilization from vegetative tissues to filling seeds increased from 11.2 to 25.5 mg N plant−1 d−1 between the second (Fig. 4B) and the fourth (Fig. 4D) labeling experiments and decreased to 17.6 mg N plant−1 d−1 during the last labeling experiment (Fig. 4F).
Contribution of Remobilized N to Total N Accumulation by Seeds
The rate of total N accumulation by all the seeds from the three strata increased from 6.9 mg N plant−1 d−1 at the beginning of filling (Fig. 4B) to 32.0 mg N plant−1 d−1 when all seeds had begun to fill (Fig. 4D) and then remained rather constant.
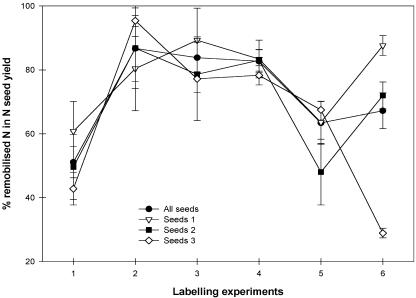
Although the rate of exogenous N accumulation and the rate of remobilized N accumulation by seeds followed similar trends, the differential between their contribution to the total N accumulation by seeds varied according to the developmental stage of the seeds (Fig. 5), increasing from 51% to 84% at the beginning of filling (second labeling experiment), remaining high throughout the period when seeds from the various strata had begun to fill (second, third, and fourth labeling experiments), and finally decreasing to about 60% when all seeds had begun to fill (fifth and sixth labeling experiments). Except during the last labeling experiment when seeds from the first stratum entered physiological maturity, the contribution of remobilized N to total N accumulation by seeds was not significantly different whatever their stratum and, as a consequence, whatever their developmental stage (Fig. 5). Only during the last labeling experiment did the percentage of remobilized N recovered by seeds differ significantly between strata.
Figure 5.
Variation of the contribution of remobilized N to the final N seed yield (see “Materials and Methods,” Eq. 5) during the six labeling experiments indicating the developmental stages of seeds (beginning of seed filling; physiological maturity) and per stratum of seeds. Data are means ± se for n = 3 repetitions of each of two plants. Data were statistically analyzed and classified with a one-way ANOVA (α = 0.05) followed by a Student-Newman-Keuls test (P < 0.05).
DISCUSSION
The aim of this work was to study the processes of N remobilization during pea seed filling by determining and analyzing dynamically N fluxes within the plant. The fluxes of assimilated N (exogenous N) and remobilized N (endogenous N) were measured during a series of successive labeling experiments.
Plants were grown under a high nitrate regime to inhibit symbiotic nitrogen fixation. Therefore, the only exogenous N source was root nitrate assimilation, conditions previously shown not to affect biomass accumulation in the various plant parts or their N concentration (Voisin et al., 2002). Exogenous N flux was measured by 15N-labeled nitrate supply in six successive labeling experiments, starting before seeds had begun to fill and ending when the seeds from the first stratum had reached physiological maturity. Exogenous N uptake was estimated according to the isotopic dilution principle (Deléens et al., 1994; “Materials and Methods”), and Evans (2001) reported that labeled 15N is taken up similarly to unlabeled N. The differential between the amount of N accumulated by a compartment and that arising from exogenous 15N-labeled nitrate allowed us to determine the flux of remobilized N between the various plant compartments.
Rate of Exogenous N Assimilation by Plants
A major decrease in exogenous N assimilation was observed at the transition between vegetative growth and the beginning of the seed-filling period (Fig. 4, A and B). Although plants accumulated N during this period, the overall N accumulation strongly decreased: plant N content increased from 219.2 to 311.6 mg N plant−1 and then from 311.6 to 329.9 mg N plant−1 during the first and the second labeling experiments, respectively. The N content in vegetative tissues increased up to the end of the first labeling experiment and then declined, whereas seed N accumulation remained constant. This dramatic decrease in the rate of N assimilation had already been observed at the onset of pod filling in N2-fixed field-grown pea (Jensen, 1987). In two indeterminate cultivars of cowpea (Vigna unguiculata) and soybean, although the mean net rate of nitrate uptake peaked during early reproductive growth and declined during pod filling, nitrate uptake observed at the transition between the vegetative and early reproductive growth was lower than the mean net rate of nitrate uptake (Imsande and Edwards, 1988). As reported previously, N uptake seems to depend upon plant needs rather than being determined by the N concentration in the rooting medium; indeed, rates of N acquisition seem to be controlled by specific demand-driven regulatory mechanisms (Imsande and Touraine, 1994). The expression and the activity of plant N transport systems may be regulated by soil N availability and by plant demand (Von Wirén et al., 1997), and both NO3− and the products of its assimilation in leaves may serve as long-distance signals indicating the N status of the plant (Muller and Touraine, 1992; King et al., 1993). Therefore, the large decrease in exogenous N assimilation observed at the transition between vegetative growth and the beginning of seed filling may be explained by the decline in N demand by plants at that time. Before seed filling, developing leaves and pod walls were a large N sink and could stimulate exogenous N assimilation by plant. At the onset of seed filling, vegetative tissues become a source for N. So, during a few days there is no major N sink, and N demand is reduced. By contrast, when all the strata of seeds begin to fill, this produces a large N sink that stimulates N assimilation. Nevertheless, the possible regulation of N assimilation by a systemic signal controlled by the source/sink balance may not be applied to all legume species: in lupins, increases of sink strength and N accumulation in the raceme by an exogenous cytokinin application did not increase either seed yield or seed N content (Ma et al., 1998). These authors postulated that N translocation was rate limiting for grain yield rather than sink size or N supply.
Dynamics of Exogenous N Partitioning throughout Seed Filling
To our knowledge, this study is the first report describing the allocation of exogenous 15N-labeled N day by day, from the beginning of seed filling up to their physiological maturity. At the onset of seed filling (second labeling experiment; Fig. 4B), exogenous N was still mainly (76%) accumulated within vegetative organs, and only 24% was accumulated by seeds. For other pea genotypes, although a different method of labeling was used, similar results were obtained (Atta et al., 2004): 20% to 30% of the exogenous N assimilated during 5 d at the beginning of seed filling was recovered by the seeds, the proportion increasing at later stages. In soybean, at an early seed-filling stage, the reproductive parts of the plants attracted 40% of the exogenous N, which increased to 60% during seed filling (Warembourg and Fernandez, 1985). Seeds were also the main sink for exogenous N during filling in Brassica napus (Malagoli et al., 2005), bean (Westermann et al., 1985), and V. faba (Dekhuijzen and Verkerke, 1984).
The proportion of exogenous N allocated to leaves declined from 27% during vegetative growth (Fig. 4A) to 22% and 17% during seed filling (Fig. 4, B and C). The first decrease corresponded to a major decrease in allocation of exogenous N to the upper stratum of leaves upon their growth arrest, the proportion of exogenous N allocated to the other strata of leaves remaining constant. The second decline was observed for all strata of leaves except the upper ones and resulted from a shift of exogenous N allocation from vegetative tissues to seeds. The proportion of exogenous N allocated to pod walls decreased successively throughout seed filling. Despite the sharp decrease at the beginning of seed filling (the three first labeling experiments), the proportion of exogenous N allocated to leaves (15%) remained higher than the allocation to pod walls (4%). The relatively high proportion of exogenous N allocated to leaves during seed filling suggests that leaves may represent a N storage, later remobilized to filling seeds (Peoples and Dalling, 1988), whereas pod walls may be a temporary N storage during early seed filling (Pate, 1985) and thereafter serve principally to transport N to the seeds.
How Can N Remobilization Be Defined?
Nitrogen remobilization could involve different physiological mechanisms according to the organ in which N flux is being considered. In our study, the 15N enrichment of the various plant parts increased throughout the 3-d labeling experiment (Table I), which demonstrates that exogenous N was accumulated in each compartment during this period. However, the nature of the endogenous N flux depends also on the differential between N content before and after the labeling experiment (ΔNtoti,le), the N budget. When the total N content of a compartment is constant (i.e. ΔNtoti,le = 0), the N flux within this compartment (N accumulation and/or N remobilization) can only be determined by isotopic N labeling. When the N budget is nil and the 15N enrichment of a given compartment increases, this demonstrates not only that the compartment accumulates exogenous N but also that the rate of exogenous N accumulation is equal to the efflux rate of N from that compartment. In this case, N remobilization results from the turnover of N compounds within the compartment. When total N content declines in a compartment during a labeling experiment (i.e. ΔNtoti,le < 0) and its 15N enrichment increases, this indicates that the N-efflux rate is higher than that of exogenous N accumulation. In this case, N remobilization from a compartment results from two indistinguishable components: N turnover as quoted above and net N loss. Lastly, when both total N content and 15N enrichment of a compartment increase (ΔNtoti,le > 0), if the rate of total N accumulation within the compartment is higher than the rate of exogenous N accumulation, this means that N is also remobilized from other compartments.
In our study, we considered that N remobilization from a compartment represents only N turnover within that compartment when there is no change in N content. If N content decreases during the time course of a labeling experiment, N remobilization involved both N turnover and net N remobilization.
Remobilized N from Vegetative Organs Constituted a Unique N Pool
At physiological maturity of the first stratum of seeds, 71% of the N in seeds originated from the remobilization of N from vegetative tissues (Fig. 3). This value is similar to those reported using different methods of N labeling: 70% of the total N of seeds in field-grown peas (Atta et al., 2004), 81% in rain-fed chickpea (Kurdali, 1996), 80% to 90% in soybean (Warembourg and Fernandez, 1985; Grandgirard, 2002), and 73% in bean plants (Westermann et al., 1985). The dynamic quantification of endogenous N fluxes showed that the rate of remobilized N accumulated by one particular seed stratum was, whatever the developmental stage, independent of the rate of N remobilization from the same stratum (Fig. 4). This result suggests that remobilized N is a unique N pool controlled at the whole-plant level and redistributed among all filling seeds independent of their position on the plant (Peoples et al., 1983; Lhuillier-Soundélé et al., 1999b) and their developmental stages. This holds true except for the last labeling experiment where the contribution of remobilized N to seed N yield was significantly higher for seeds from the first stratum that had reached the physiological maturity. The reason for this is unclear. As the rate of total N accumulation by the first stratum of seeds was highest between 1,218°C and 1,239°C DAS, just before seeds reached physiological maturity (1,239°C DAS), a specific regulatory mechanism may act during the last part of the seed-filling period that may result from an increase in seed N demand to enhance the final seed N concentration. This hypothesis should be verified for other genotypes.
Rate of N Remobilization from the Vegetative Compartment and Its Regulation
The rate of N remobilization from vegetative tissues to filling seeds first increased (second to fourth labeling experiments) and then decreased (fourth to sixth labeling experiments) consistent with similar observations for soybean (Grandgirard et al., 2001). Grandgirard (2002) suggested that the increase may depend upon the ability of seeds to accumulate N. As the pea cv Caméor is an indeterminate genotype, the number of seeds being filled increases until all nodes have begun to fill. The seed N demand is accompanied by onset of protein degradation in pea leaves, and two chloroplastic protease regulatory subunits increase in relative abundance during seed filling, from 792°C to 1,107°C DAS for the ClpC subunit and from 960°C to 1,107°C DAS for the ClpA subunit (Schiltz et al., 2004). The decline in the rate of N remobilization could result either from a decrease of N demand by seeds, due to the end of seed filling of the lower reproductive nodes, or from a decrease in the amount of N potentially remobilizable, as suggested by Grandgirard (2002). The relation between both the decline of the rate of N remobilization and the amount of N potentially remobilizable could be explained by a concomitant remobilization of both abundant leaf proteins such as Rubisco and photosystems that constitute N potentially remobilizable and the proteolytic enzymes that degrade the abundant proteins. In bean, just after the full leaf expansion, transcripts of the Clp protease subunits C and P, Rubisco, Rubisco activase, and phosphoribulokinase dropped sharply (Crafts-Brandner et al., 1996). In summary, at the beginning of seed filling, the increase of the N remobilization rate appears to be regulated by the seed N demand, whereas the decrease in N remobilization rate when all seeds have started to fill may reflect a limit in the amount of N remobilizable from the vegetative parts.
Contribution of Each Vegetative Organ to the N Seed Yield
Our results showed that all organs undergo N remobilization and contributed to a unique pool of remobilized N. Leaves were the main source of remobilized N as they represent 30% of the total N accumulated by seeds (Fig. 3), similar to the situation for soybean and cowpea where remobilized N from leaves contributed between 28% to 35% and 34% to 38%, respectively, to N seed yield. Less N was remobilized from leaves to seeds in V. faba (between 16%–24%) and Arachis hypogaea (21%; Peoples and Dalling, 1988). While initially behaving as a sink for N, accumulating both exogenous and remobilized N from other vegetative organs, at the onset of seed filling, all leaves except the upper ones still growing shift from being sink to source for remobilized N. In pea, N mobilization from leaves, whatever their age, begins with seed filling. By contrast, in B. napus, the remobilization of leaf N varied according to the developmental stage of plants: from bolting to flowering, N was mobilized from the lower leaf ranks for allocation to new vegetative tissues such as stems and leaf ranks located higher in the canopy; later between flowering and harvest, N remobilized from upper leaves was diverted to flowers and pods (Malagoli et al., 2005). This suggests that growing pea leaves accumulate exogenous and remobilized N from stems and roots but not N remobilized from the lower to the upper stratum of leaves, as observed for B. napus (Malagoli et al., 2005).
Pod walls were the second source of N remobilized to seeds, contributing 20% of the N seed yield. As observed for leaves, before the beginning of seed filling, pod walls were initially a sink for N but switched to being a source during seed development. The uniform allocation of remobilized N to all strata examined implies the existence of a unique N pool.
In our study, N remobilized from pea roots represented about 11% of the total N recovered by seeds, a value at the high end of values reported for legumes: V. faba (3%–5%), cowpea (3%–11%), A. hypogaea (0.4%), and soybean (3%; Peoples and Dalling, 1988). The 15N enrichment of roots increased throughout the 3-d labeling period, but their N content increment was nil, suggesting that N remobilization was only N turnover: newly exogenous N taken up by roots was stored, and N accumulated prior to the labeling experiment was remobilized to shoots. The ability of crude root extracts to degrade endogenous root proteins or hemoglobin has been shown throughout fruit development and root senescence in cowpea (Peoples et al., 1983). Autodigestive activity in root extracts rose to a maximum at flowering and then fell to a low level that was maintained until pod browning and seed desiccation, whereas the rate of hemoglobin degradation also rose prior to flowering but continued to rise slowly until late senescence (Peoples and Dalling, 1988). Increases in the levels of both carboxypeptidase and aminopeptidase and in the activity of enzymes capable of degrading endogenous root proteins (autodigestion) during pea root development are consistent with N remobilization from root proteins during seed filling.
Stems provide remobilized N to the other compartments and contribute 10% of the final N seed yield. This is consistent with results obtained for V. faba (9%–12%), cowpea (15%), A. hypogaea (9%), and soybean (7%–16%; Peoples and Dalling, 1988).
CONCLUSION
This physiological study of the N fluxes in pea plants during seed filling confirms that N remobilization from vegetative parts is the major contributor to seed N. Our results provide a dynamic estimate of the changes of the exogenous and endogenous N fluxes in relation with the nature and the amount of sources and sinks for remobilized N, and the rate of N remobilization was dynamically measured for each organ and strata from the different N fluxes and not deduced from an estimation of the N loss between two dates. This permits a better understanding of the processes of N remobilization at the whole-plant level as well as at the level of either a particular organ or stratum. These experiments show that N supply arising from N remobilized from vegetative parts changes as seed development proceeds.
The fine characterization of the processes of N remobilization provides a physiological basis for further studies that should identify the molecular mechanisms involved in N remobilization and the specific signal(s) activating these processes. These studies could involve comparative proteomics of stems, leaves from vegetative (Schiltz et al., 2004) and reproductive nodes, and pod walls at several stages throughout the seed filling. The genetic variability of N remobilization from vegetative parts should now be studied to identify genotypes in which this mechanism is the most efficient for seed filling.
MATERIAL AND METHODS
A short and broad-leaved genotype (cv Caméor) was cultivated in a growth chamber under controlled conditions: moisture content was about 70%, and using 24°C/16°C as day/night temperatures and a photoperiod of 16 h resulted in a mean value of 21°C day for the thermal time (for review, see Bonhomme, 2000). An amount of 660 μmol m−2 s−1 of photosynthetic active radiation was provided to the plants by sodium lamps (MACS 400 W; Mazda, Dijon, France). Seeds of pea (Pisum sativum) were sown in 5-L pots at a density of 4 seeds per pot. Each pot was filled with a 1:1 (v/v) mixture of sterilized attapulgite and clay balls (diameter 2–6 mm). All plants were regularly watered with a similar volume of nutrient solution to maintain the soil water near pot capacity; the nutrient solution (14 meq NO3−, P, K, and oligoelements) was supplied by regular automatic watering. Time was expressed in cumulative degrees days after sowing.
Labeling Experiments and Sampling
The period of growth cycle that was studied began at 882°C DAS and ended at 1,260°C DAS, i.e. spanning the period from the beginning of seed filling of the first stratum of reproductive node until its physiological maturity. Six successive and overlapping labeling experiments were conducted during this period, each experiment lasting 3 d (Fig. 1). Six homogeneous groups of pots were constituted, each containing 17 pots, and used sequentially for the six labeling experiments.
For each labeling experiment, nine pots out of the 17 were supplied during the 3 d of the experiment with a 14-meq 15NO3− nutritive solution (5% 15N-atom excess enrichment). Three labeled pots were harvested after 24, 48, and 72 h of 15NO3− supply (day 1, 2, and 3, respectively). Concomitantly, to determine the natural 15N enrichment of the plants, the remaining eight pots of the same group were used as unlabeled control: three pots were harvested at the beginning of the labeling experiment (day 0) and at the end of the labeling experiment (day 3), while only one pot was harvested after 24 and 48 h after the start of the labeling. As quoted above, because labeling experiments overlapped except for the first labeling experiment, day 0 of a labeling experiment corresponded to the day 3 of the preceding labeling experiment (Fig. 1). Repeating this protocol six times allowed us to investigate the dynamics of exogenous N allocation to the different plant compartments. The four plants of each control pot and two plants of the labeled pots were harvested. Plants were divided as the whole root system, the stem of the entire plant, leaves from the vegetative and reproductive nodes, pod walls, and seeds. Vegetative nodes were divided into three distinct strata (VL1–3): the first one composed of the first five lowest nodes; the second stratum composed of the four following nodes (sixth, seventh, eighth, and ninth); and the third stratum the three next ones (10th, 11th, and 12th). The first flowering node was most often either the 12th or the 13th node. Organs from the reproductive nodes were also separated into three strata (RL1–3, PW1–3, and S1–3): the first and the second strata included two nodes (13th and 14th and 15th and 16th, respectively), while the third included the remaining upper nodes. Plants held around 18 nodes at the beginning of the experiment and around 22 nodes at the end.
Chemical Analysis and Equations
Control plants and 15N-labeled plants were oven dried at 80°C for 48 h, weighted, and ground for further analysis. For each labeling experiment (le: 1–6) and at each day of sampling (d: 0–3), for each replicate of a compartment i, dry matter (DMi, le, d), N concentration (%Ni, le, d), and 15Ni, le, d enrichment were measured. Total N concentration (14N + 15N) was determined using the Dumas procedure (Allen, 1974), while the 15N enrichment of a compartment was determined using a dual inlet mass spectrometer (Fison Isochrom; Micromass, Lyon, France) operating in line with a CHN analyzer (Carlo Erba, Val de Reuil, France). Seed water concentration was measured to determine the beginning of seed filling and physiological maturity of seeds (Ney et al., 1993): the beginning of seed filling and physiological maturity corresponded to seed water concentrations of 0.80 and 0.55 g g−1, respectively (Le Deunff and Rachidian, 1988; Dumoulin et al., 1994). The water content of a stratum of seeds was a mean value calculated for all seeds from all nodes of this stratum.
Plants always accumulated dry matter and N during seed filling, except between the beginning and the end of the second labeling experiment (i.e. between 945°C and 1,008°C DAS, respectively), where dry matter and N accumulation apparently declined (data not shown). This was in fact an experimental bias due to the lowest size of the plants that were harvested at the end of the second labeling experiment as compared to those harvested at its beginning. To get a continuous growth of plants and N accumulation between two sampling dates and to fit as much as possible to the real growth of plants, we have considered in our calculations that the second labeling experiment spanned 945°C to 1,029°C DAS (4 d) while the third labeling experiment spanned 1,029°C to 1,071°C DAS (2 d).
Using the above plant analysis, total N content of a plant part at a given day “d” of a labeling experiment “le” was calculated as:
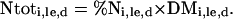 |
(1) |
As such, for any day (different from the day 0) of a labeling experiment, increment in N content of the plant parts was calculated as:
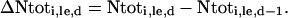 |
(2) |
Supplying plants with 15N-labeled nitrate allowed us to discriminate exogenous N entering the plant during the labeling experiments from the plant unlabeled N (endogenous N) present at the beginning of the labeling experiments. Exogenous N incorporation in the various plant parts during a labeling experiment was calculated using the increment in their N content (ΔNtoti,le), the 15N enrichment of labeled and unlabeled control plant parts (E%i,le,d and E%control i,le,d, respectively), and the 15N enrichment of the labeled nutrient solution (5%):
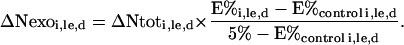 |
(3) |
The amount of endogenous N accumulated in a compartment or remobilized from a compartment to another at a given day (different from day 0) during a labeling experiment was estimated by:
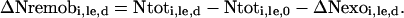 |
(4) |
When Equation 4 yields positive values, this means that the “i” compartment has incorporated endogenous N arising from remobilization of N from other compartments; negative values calculated using Equation 4 indicate in turn that N has been remobilized from the “i” compartment to other compartments.
For each labeling experiment, the contribution of endogenous N remobilized from the various plant parts to the final N seed yield was then estimated by the ratio of the differential in remobilized N to the total N increment between day 0 and 3 of a labeling experiment:
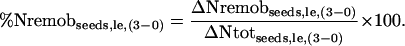 |
(5) |
Mean values resulted from the measurement of three biological replications of two plants. Statistical analyses were performed using the GLM procedure of SAS (SAS Institute, 1987). Means were compared using the Student-Newman-Keuls test at the 0.05 probability level.
Acknowledgments
We thank J. Gonthier for her technical assistance. We also thank O. Delfosse (INRA, Laon, France) for 15N isotopic analysis of the plant material. We are grateful to A.S. Voisin and A. Larmure for helpful discussions and R. Thompson for his critical reading of the manuscript.
This work was partly supported by the Regional Council of Burgundy and the European project Grain Legumes (FOOD–CT–2004–506223).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.056713.
References
- Allen SE (1974) Chemical Analysis of Ecological Materials. John Wiley & Sons, New York
- Atta S, Maltese S, Marget P, Cousin R (2004) 15NO3 assimilation by the field pea Pisum sativum L. Agronomie 4: 85–92 [Google Scholar]
- Bonhomme R (2000) Bases and limits to using “degree.day” units. Eur J Agron 13: 1–10 [Google Scholar]
- Crafts-Brandner SJ, Klein RR, Klein P, Hölzer R, Feller U (1996) Coordination of protein and mRNA abundances of stromal enzymes and mRNA abundances of the Clp protease subunits during senescence of Phaseolus vulgaris (L.) leaves. Planta 200: 312–318 [DOI] [PubMed] [Google Scholar]
- Dekhuijzen HM, Verkerke DR (1984) Uptake, distribution and redistribution of 15nitrogen by Vicia faba under field conditions. Field Crops Res 8: 93–104 [Google Scholar]
- Deléens E, Cliquet J-B, Prioul J-L (1994) Use of 13C and 15N plant label near natural abundance for monitoring carbon and nitrogen partitioning. Aust J Plant Physiol 21: 133–146 [Google Scholar]
- Dumoulin V, Ney B, Etévé G (1994) Variability of seed and plant development in pea. Crop Sci 34: 992–998 [Google Scholar]
- Grandgirard D (2002) Analyse et modélisation du déterminisme de la teneur en azote des graines chez le soja (Glycine max L. Merill): relation entre la remobilisation d'azote vers les graines et l'élaboration du rendement et de sa qualité. PhD thesis. Université de Bourgogne, Dijon, France
- Grandgirard D, Munier-Jolain N, Salon C, Ney B (2001) Nitrogen nutrition level and temperature effects on vegetative remobilisation rate and distribution of canopy N during seed filling period in soybean (Glycine max L. Merr.). In Proceedings of the 4th European Conference on Grain Legumes, July 8–12, 2001, Cracow, Poland. European Association for Grain Legume Research, pp 30–31
- Evans RD (2001) Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci 6: 121–126 [DOI] [PubMed] [Google Scholar]
- Imsande J, Edwards DG (1988) Decreased rates of nitrate uptake during pod filling by cowpea, green gram and soybean. Agron J 80: 789–793 [Google Scholar]
- Imsande J, Touraine B (1994) N demand and the regulation of nitrate uptake. Plant Physiol 105: 3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ES (1987) Seasonal patterns of growth and nitrogen fixation in field-grown pea. Plant Soil 101: 29–37 [Google Scholar]
- King BJ, Siddiqi MY, Ruth TJ, Warner RL, Glass ADM (1993) Feedback regulation of nitrate influx in barley roots by nitrate, nitrite and ammonium. Plant Physiol 102: 1279–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdali F (1996) Nitrogen and phosphorus assimilation, mobilisation and partitioning in rainfed chickpea (Cicer arietinum L.). Field Crops Res 47: 81–92 [Google Scholar]
- Kurdali F, Kalifa K, Al-Shamma M (1997) Cultivar differences in nitrogen assimilation, partitioning and mobilization in rain-fed lentil. Field Crop Res 54: 235–243 [Google Scholar]
- Le Deunff FY, Rachidian Z (1988) Interruption of water delivery at physiological maturity is essential for seed development, germination and seedling growth in pea (Pisum sativum). J Exp Bot 39: 1221–1230 [Google Scholar]
- Lhuillier-Soundélé A, Munier-Jolain NG, Ney B (1999. a) Dependence of seed nitrogen concentration on plant nitrogen availability during the seed filling in pea. Eur J Agron 11: 157–166 [Google Scholar]
- Lhuillier-Soundélé A, Munier-Jolain NG, Ney B (1999. b) Influence of N availability on seed nitrogen accumulation in pea. Crop Sci 39: 1741–1748 [Google Scholar]
- Ma Q, Longnecker N, Atkins C (1998) Exogenous cytokinin and nitrogen do not increase grain yield in narrow-leafed lupins. Crop Sci 38: 717–721 [Google Scholar]
- Malagoli P, Laine P, Rossato L, Ourry A (2005) Dynamics of nitrogen uptake and mobilization in field-grown winter oilseed rape (Brassica napus L.) from stem extension to harvest. I. Global N flows between vegetative and reproductive tissues in relation to leaf fall and their residual N. Ann Bot (Lond) 95: 853–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller B, Touraine B (1992) Inhibition of NO3− uptake by various phloem-translocated amino-acids in soybean seedlings. J Exp Bot 41: 221–241 [Google Scholar]
- Ney B, Duthion C, Fontaine E (1993) Timing of reproductive abortions in relation to cell division, water content, and growth of pea seeds. Crop Sci 33: 267–270 [Google Scholar]
- Pate JS (1985) Physiology of pea—a comparison with other legumes in terms of economy of carbon and nitrogen in whole-plant and organ functioning. In PD Hebblewaite, MC Heath, TCK Dawkins, eds, The Pea Crop. Butterworths, London, pp 279–296
- Peoples MB, Dalling MS (1988) The interplay between proteolysis and amino acid metabolism during senescence and nitrogen reallocation. In LD Nodden, AC Leopold, eds, Senescence and Aging in Plants. Academic Press, New York, pp 182–217
- Peoples MB, Pate JS, Atkins CA (1983) Mobilization of nitrogen in fruiting plants of a cultivar of cowpea. J Exp Bot 34: 563–578 [Google Scholar]
- Salon C, Munier-Jolain NG, Duc G, Voisin AS, Grandgirard D, Larmure D, Emery RJN, Ney B (2001) Grain legume seed filling in relation to nitrogen acquisition: a review and prospects with particular reference to pea. Agronomie 21: 539–552 [Google Scholar]
- SAS Institute (1987) SAS/STAT Guide for Personal Computer, Ed 6. SAS Institute, Cary, NC
- Schiltz S, Gallardo G, Huart M, Negroni L, Sommerer N, Burstin J (2004) Proteome reference maps of vegetative tissues in pea. An investigation of nitrogen mobilization from leaves during seed filling. Plant Physiol 135: 2241–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, de Wit CT (1976) Analysis of the carbon and nitrogen limitations to soybean yield. Agron J 68: 319–324 [Google Scholar]
- Voisin AS, Salon C, Munier-Jolain NG, Ney B (2002) Effect of mineral nitrogen on nitrogen nutrition and biomass partitioning between the shoot and roots of pea (Pisum sativum L.). Plant Soil 242: 251–262 [Google Scholar]
- Von Wirén N, Gazzarrini S, Frommer WB (1997) Regulation of mineral nitrogen uptake in plants. Plant Soil 196: 191–199 [Google Scholar]
- Warembourg FR, Fernandez MP (1985) Distribution and remobilization of symbiotically fixed nitrogen in soybean (Glycine max). Physiol Plant 65: 281–286 [Google Scholar]
- Westermann DT, Porter LK, O'Deen WA (1985) Nitrogen partitioning and mobilization patterns in bean plants. Crop Sci 25: 225–229 [Google Scholar]