Abstract
Objective:
We aim to compare the psychiatric antecedents of schizophrenia (SZ) and bipolar disorder (BD).
Methods:
Using the Rochester Epidemiology Project, we searched for residents of Olmsted County that had a diagnosis of SZ or BD. We confirmed each case using DSM-5 criteria and obtained the psychiatric antecedents.
Results:
We identified 205 cases with first episode psychosis or mania (SZ = 131; BD = 74). The mean age at first visit for mental health reasons was 12.3 ± 6.3 years for SZ and 13.9 ± 5.6 years for BD. The duration of the initial prodrome (time from first mental health visit to first episode) was similar for both groups (SZ 8.3 ± 6.2 years vs BD 7.3 ± 5.9 years). We found that SZ and BD have overlapping antecedents, but SZ was more common in males and in foreign born and had more learning deficits before the first episode. BD was more common in white population and had higher rates of depressive and adjustment disorders prior to first episode. BD also had more affective symptoms, nightmares, and panic attacks before the first episode. Both groups had similarly high rates of substance use (SZ 74 % vs BD 74.3 %), prescription of antidepressants (SZ 46.6 % vs BD 55.4 %) and stimulants (SZ 30.5 % vs BD 22.9 %).
Conclusions:
The psychiatric antecedents of SZ and BD usually start during adolescence, overlap, and present in unspecific ways. The initial prodromes are more alike than distinct. Further studies are encouraged to continue looking for specific factors that distinguish the antecedents of these two disorders.
Keywords: Bipolar disorder, Schizophrenia, Prodrome, Cohort studies, First episode
1. Introduction
More than a century ago, German psychiatrist Emil Kraepelin suggested a categorical distinction between the two major psychiatric illnesses now known as schizophrenia (SZ) and bipolar disorder (BD). Known as the Kraepelinian dichotomy (Craddock and Owen, 2010) he operationalized and developed the concept of dementia praecox, now SZ, where a premature mental impairment was detectable in the early stage of the syndrome. The course of SZ as he described was characterized by significant deterioration of the mental state resulting in “unmistakable symptoms of weak-mindedness” after a few years of the illness (Kendler, 1986; Kraepelin, 1919). This entity was clinically distinct from manic-depressive illness, now BD, in which a “happy, sunny disposition” was noted prior to the illness and episodes of periodic and circular changes of emotion, energy, volition, and cognition characterized the acute episodes but without “any considerable injury” to the mental state of the patients during the intervals between the episodes (Kendler, 1986; Kraepelin, 1923). These two descriptions by Kraepelin of the antecedents of SZ and BD suggest that he considered that prior to the onset of the illness SZ and BD had distinct features.
To current day, the tradition of studying these two dimensions, the affective and the psychotic, as having separate boundaries remains (Kotov et al., 2013; Rybakowski, 2019). However, the validity of Kraepelinian dichotomy has been criticized as subsequent research has shown that SZ and BD overlap in multiple domains including genetic risk, clinical symptoms, neuroimaging correlates, and cognitive profiles (Birur et al., 2017; Craddock and Owen, 2010; Hill et al., 2013; Pradhan et al., 2008; Rybakowski, 2019; Zivanovic and Nedic, 2012). While evidence accumulates demonstrating similarities between SZ and BD in multiple domains, less attention has been paid to how the clinical antecedents or prodromes of these two disorders compare (Hartmann et al., 2019).
In psychiatry, the concept of prodrome is used in retrospective research to understand the antecedents of the people that have developed a specific illness or episode. A prodrome has been defined as the “period of disturbance which represents a deviation from a person’s previous experience and behavior prior to the development of the florid features of a disorder” (Conus et al., 2008). Therefore, in the study of SZ and BD, the initial prodrome entails the clinical antecedents that occur prior to the first episode of psychosis or mania, alternatively, the relapse prodrome are the clinical antecedents that precede the recurrence of a psychotic or manic episode (Andrade-González et al., 2020).
Most of the studies regarding prodromes, have studied the initial prodromes of SZ and BD independently (Correll et al., 2010; Gourzis et al., 2002; Skjelstad et al., 2010; Van Meter et al., 2016); therefore, our understanding of how the initial prodromes of these two disorders compare is limited. The few studies comparing the initial prodromes of SZ and BD have found resemblances between them with similarities in number of symptoms, cognitive measures, premorbid adjustment, and historical diagnoses (Chan et al., 2019; Olvet et al., 2010; Rietdijk et al., 2011; Verdolini et al., 2022). Some authors have considered that the initial prodromes between these two disorders are “indistinguishable” (Olvet et al., 2010).
Therefore, to further extend our understanding of the prodromes of SZ and BD and how they relate, we aim to describe and compare the psychiatric diagnoses, symptoms, substance use and exposure to psychiatric medications in people with SZ and BD prior to the first episode using a unique American population-based cohort in Olmsted County, Minnesota. Studying clinical antecedents is essential as it can improve early detection, therefore increasing the possibility of early intervention, reducing the duration of untreated illness, and improving outcomes (Bechdolf et al., 2012; Norman et al., 2005).
2. Methods
This study was approved by the institutional review boards (IRBs) of the Mayo Clinic (IRB # 20–006720 and 20–008773) and Olmsted Medical Center (Approval dates: 11/20/2020 and 04/22/2021) who provided a Health Insurance Portability and Accountability Act (HIPAA) waiver, in line with state, federal, and international recommendations. Hence, written informed consent was not required for passive medical record review in the Rochester Epidemiology Project (Rocca et al., 2018).
2.1. The Rochester Epidemiology Project (REP)
The Rochester Epidemiology Project (REP), a population-based registry study provides an opportunity to retrospectively analyse the history of individuals who developed an illness while mitigating risks of sampling, recall, referral, and response biases (Allebeck, 2009; Maret-Ouda et al., 2017). The REP is a population registry that links records from multiple health care facilities in Olmsted County, Minnesota, USA. It contains nearly all records for residents of Olmsted County who have received medical care in the region since 1966.
The REP population shares similar demographic characteristics with the State of Minnesota, the Upper Midwest, and much of the U.S. population (Rocca et al., 2018). The potential of using the Rochester Epidemiology Project to understand diseases and improve community health has been described elsewhere (Rocca et al., 2012).
Different studies have shown replicability of the findings of research conducted in Olmsted County in other populations in the USA and the world (Rocca et al., 2018). The REP has been used for previous studies on SZ (Capasso et al., 2008) and BD (Foroughi et al., 2022; Prieto et al., 2016).
2.2. Search strategy
Using the REP, a specialist searched for all residents of Olmsted County between the ages of 13 to 29 years of age at the time of initial diagnosis with SZ or BD (See Table S1 and S2 for diagnostic codes used for SZ and BD). This search was chosen to provide high sensitivity while sacrificing specificity as the ascertainment of the case did not burden the study subjects.
We limited the search to the period between 1985 and 2019 given practical limitations in conducting a more comprehensive search a with longer time-period. Finally, after reviewing multiple charts, we decided to exclude individuals who were solely identified based on the ICD code 296.90 (unspecified episodic mood disorder), due to the lack of specificity of the code.
2.3. Case ascertainment, variables obtained and definitions
The process of identifying cases was split into two phases. During the first phase, the potential cases identified by diagnostic codes (N = 1335), were manually reviewed by one of two psychiatrists (JOO or MGR). The review was independent of any previous diagnoses in the subject’s medical history and the case was reclassified using the available data in the records and DSM-5 criteria (American Psychiatric Association, 2013). After the review, the case was classified as SZ, BD, or neither.
During phase 2, we searched for the first episode of psychosis (FEP) or first episode of mania (FEM) in the cases that had been confirmed in phase one as meeting criteria of SZ or BD. The first episode was defined as the clinical encounter in which a person was first observed to have symptoms of psychosis or mania. This definition is simple, reliable, and intuitive (Breitborde et al., 2009).
Once we identified the SZ and BD cases with a first episode, we collected data prior to the first episode using a standardized version we created with RedCap (Harris et al., 2019). We collected demographic data, psychiatric history (diagnoses, medications, and substance use), symptoms and data from the first episode. Codes for mental health problems were classified according to the spectrums of DSM-5 (American Psychiatric Association, 2013). Specific neurodevelopmental spectrum diagnoses included autism spectrum disorder, attention deficit hyperactivity disorder, intellectual disability, and other neurodevelopmental disorders were extracted separately from the spectrum. Conduct disorder and oppositional defiant disorder were also extracted separately from their respective spectrums. Substance use was noted as positive if there was any exposure noted in the record not necessarily a substance use disorder.
We defined the onset of the initial prodrome as the date in which the first psychiatric problem, including neurodevelopmental problems, were first coded in the patients record or the first time a mental health problem was written in the problem list of a clinical note. Our definition of prodrome onset did not require that psychiatric symptoms be contiguous with the first episode (Geoffroy and Scott, 2017). This approach has been used by other authors (Powers et al., 2020). The end of the initial prodrome was at the time of the first episode.
2.4. Statistical analysis
We first tested for differences in demographics, diagnoses, symptoms, and substance use between BD and SZ using two-sample t-tests for continuous variable and either chi-square or Fisher’s exact tests for categorical variables.
We then used Cox proportional hazards models to test for associations with age of onset and duration of illness between first mental health contact and FEP/FEM and adjusting for age at first contact, sex, race (white vs. non-white), and birthplace (US-born vs. outside of US). For this analysis, we combined the SZ and BD groups due to overlap of the initial prodromes and examined the correlation between demographic and antecedent diagnosis, substance use and exposure to medications with age of onset and duration of the initial prodrome. We created the survival curve with R Studio using ggplot2 (Team, 2013; Wickham, 2016).
3. Results
3.1. Search
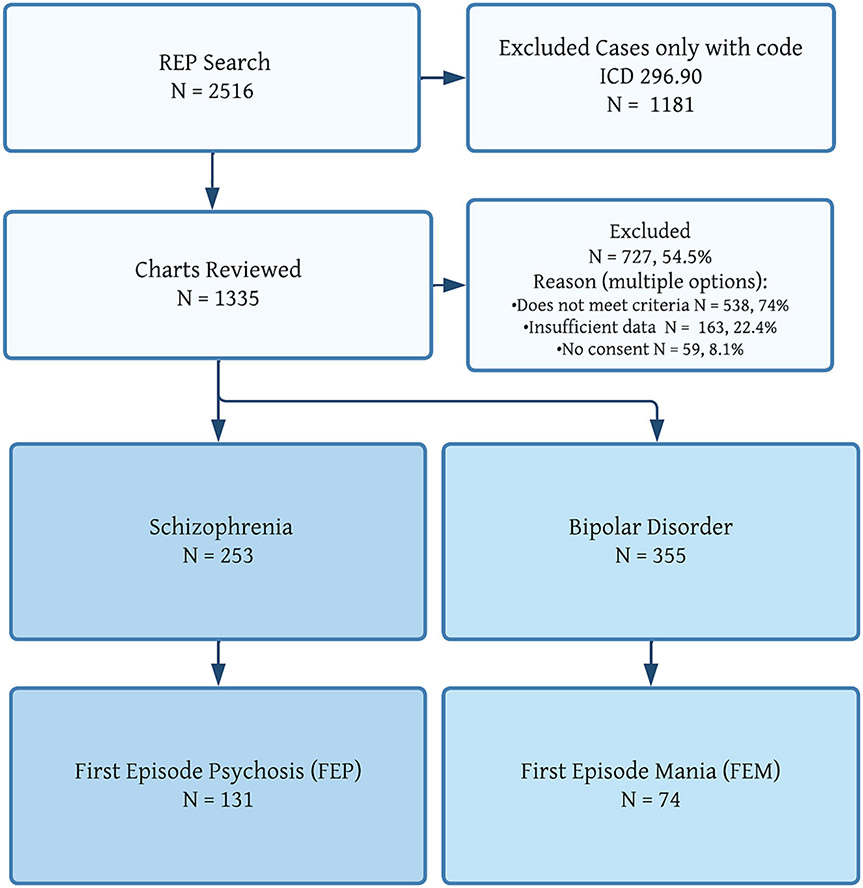
As presented in Fig. 1, the initial search in the REP system yielded 2516 patients. After removal of the patients identified with only an ICD code 296.90 (unspecified episodic mood disorder), we manually inspected 1335 individual patient charts. In the context of the sensitive search, we excluded most cases (727 individuals, 54.5 % of the sample). The reasons for exclusion are shown in Fig. 1.
Fig. 1.
Patient flow.
We confirmed 253 patients to meet criteria for SZ and 355 patients to meet criteria for BD. Of the SZ cases, 131 subjects (51 % of those with confirmed SZ) had an identifiable FEP. Of the BD cases, 74 subjects (20 % of those with confirmed BD) had an identifiable FEM.
3.2. Characteristics of the population
Table 1 shows the characteristics of the population identified with FEP or FEM. Most of both groups were male but there was a higher rate of males in the SZ groups versus males in the BD group (80.15 % vs 60.8 %, respectively; p = 0.003). There were no statistically significant differences between the races of the two groups; however, the proportion of White patients was higher in the BD group compared to the SZ group (SZ 61.1 % vs BD 75.7 %).
Table 1.
Comparison of demographics and psychiatric antecedents.
| Schizophrenia | Bipolar disorder |
p- value |
|||
|---|---|---|---|---|---|
|
|
|
||||
| |
(N = 131) |
(N = 74) |
|
||
| Characteristics of the population | N | % | N | % | |
| Male | 105 | 80.2 | 45 | 60.8 | 0.003 |
| Race | 0.48 | ||||
| White | 80 | 61.1 | 56 | 75.7 | |
| Black/African/African American | 31 | 23.7 | 13 | 17.6 | |
| Indian (American) | 1 | 0.8 | 0 | 0 | |
| Asian/Asian American | 3 | 2.3 | 1 | 1.4 | |
| Other | 11 | 8.4 | 3 | 4.1 | |
| Not specified | 5 | 3.8 | 1 | 1.4 | |
| Hispanic ethnicity | 10 | 7.6 | 3 | 4.1 | 0.38 |
| Foreign born | 28 | 22.1 | 4 | 5.1 | 0.005 |
| Initial presentation for a neurodevelopmental problem | 41 | 41.8 | 16 | 27.1 | 0.09 |
| Patients with an initial prodrome | 99 | 75.6 | 59 | 79.7 | 0.61 |
| Psychiatric diagnoses before first episode | |||||
| ADHD | 50 | 38.2 | 24 | 31.1 | 0.5 |
| Intellectual disability | 9 | 6.9 | 2 | 2.7 | 0.33 |
| Autism spectrum disorder | 7 | 5.3 | 0 | 0 | 0.05 |
| Other neurodevelopmental disorder † | 41 | 31.3 | 15 | 20.3 | 0.12 |
| Conduct disorder | 15 | 11.5 | 7 | 9.5 | 0.81 |
| Oppositional-defiant disorder | 13 | 9.9 | 6 | 8.1 | 0.80 |
| Depressive disorders | 62 | 47.3 | 47 | 63.5 | 0.04 |
| Anxiety disorder | 42 | 32.1 | 31 | 41.9 | 0.21 |
| Adjustment disorders | 27 | 20.6 | 27 | 36.5 | 0.02 |
| Medications before first episode | |||||
| Any psychotropic before first episode | 78 | 59.5 | 48 | 64.9 | 0.55 |
| Antidepressant | 61 | 46.6 | 41 | 55.4 | 0.28 |
| Mood stabilizer | 14 | 10.7 | 4 | 5.4 | 0.3 |
| Antipsychotic | 27 | 20.6 | 7 | 9.5 | 0.06 |
| ADHD Medication (stimulant) | 40 | 30.5 | 17 | 22.9 | 0.24 |
| Substance use before first episode | |||||
| Any substance use | 97 | 74 | 55 | 74.3 | 0.97 |
| Alcohol | 55 | 42 | 39 | 52.7 | 0.18 |
| Stimulants | 30 | 22.9 | 13 | 18.9 | 0.36 |
| Cannabis | 90 | 68.7 | 48 | 64.9 | 0.68 |
ADHD = attention deficit hyperactivity disorder, USA = United States of America.
Other neurodevelopmental disorder include tics, learning, reading, speech, and other DSM-5 neurodevelopmental spectrum disorder.
The SZ group had a significantly higher rate of birth outside of the United States (SZ 22.1 % vs BD 5.1 %; p = 0.005). Of the 32 patients born outside of the United States, sixteen were from Somalia, seven were from Mexico, four were from Sudan, two were from Ethiopia, two from Kenya and one case was from Saudi Arabia.
3.3. First episode characteristics
The age of onset of the FEP for SZ was 20.4 years (SD 3.8) and the FEM for BD was 21.3 (SD 3.6). Most of the first episodes for both groups were identified in the emergency department (SZ 79 % vs BD 82 %), which resulted in hospitalization (SZ 87.7 % vs BD 79.7 %), and treatment with antipsychotics (SZ 84 % vs BD 77 %). There were no significant differences between BD and SZ for these events.
3.4. Mental health antecedents prior to the first episode
Most individuals (SZ 75.6 % vs BD 79.7 %) in our sample had an initial prodrome. Of those that had a psychiatric visit prior to the first episode, most started seeking help before the age of eighteen (SZ 80.8 % vs BD 79.6 %). The mean age at first visit for mental health reasons 12.3 years (SD 6.3) years for SZ and 13.9 year (SD 5.6) for BD. The duration of the prodrome was similar for both groups (SZ 8.3 ± 6.2 years vs BD 7.3 ± 5.9 years; p = 0.33).
As shown in Table 1, we found significant overlap between the antecedent diagnoses of SZ and BD with only some differences in their characteristics. Before their first episode, patients with BD had a greater rate of diagnostic antecedents of depressive disorders and adjustment disorders (63.5 % and 36.5 % respectively) compared to patients with SZ (47.3 % and 20.6 % respectively). While not statistically significant, patients with SZ were more likely to have antecedent diagnoses of neurodevelopmental problems, such as ADHD, intellectual disability, and autism spectrum disorders.
We did not find a large difference between groups in the rate of prescription of any psychotropic medication (SZ 59.5 % vs BD 64.9 %; p = 0.55), prescription of antidepressants (SZ 46.6 % vs BD 55.4 %; p = 0.28), mood stabilizers (SZ 10.7 % vs BD 5.4 %; p = 0.3), and stimulants (SZ 30.5 % vs BD 22.9 %; p = 0.24) during the prodrome. There was a higher rate of antipsychotic use in the (SZ 20.6 % vs BD 9.5 %) group. Common reasons given for antipsychotic prescription in both groups included behavioral control or management of aggression (38 %), sleep (23 %), anxiety (14 %), diagnosis of autism (14 %), irritability (14 %), and mood augmentation (14 %).
Before the first episode, substance use during the lifetime was common among both samples (SZ 74 % vs BD 74.3 %). The most frequently used substances were cannabis followed by alcohol and nicotine. We did not find differences in the frequency of substance use between the two groups.
3.5. Symptoms before the first episode
Prior to the first episode, we found that SZ and BD have a wide range of symptoms and that the symptoms they exhibit overlap (Table 2). However, while the symptoms overlap, the differences in symptoms include SZ having higher rates of cases with learning deficits compared to BD. Contrarily, BD is characterized by a greater prevalence of any affective symptoms and has higher rates of low self-esteem, irritability, hyperthymia, nightmares, and panic attacks. Also, during the initial prodrome BD has higher rate of subsyndromal manic symptoms such as, euphoria, increased goal-directed behaviors, and episodes of sleep-deprived energy enhancement. Both disorders had similar rates of suicidal spectrum symptoms and behavioral symptoms.
Table 2.
Symptoms prior to the first episode.
| Schizophrenia |
Bipolar Disorder |
p-value | Schizophrenia |
Bipolar Disorder |
p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(N = 131) |
(N = 74) |
|
|
(N = 131) |
(N = 74) |
|
||||
| Symptoms | N | % | N | % | Symptoms | N | % | N | % | ||
| Any affective symptom | 73 | 55.73 | 61 | 82.43 | <0.001 | Any developmental symptom | 63 | 48.09 | 28 | 37.84 | 0.20 |
| Sadness | 53 | 40.46 | 38 | 51.35 | 0.17 | Intelligence deficits | 13 | 9.92 | 6 | 8.11 | 0.80 |
| Anhedonia | 25 | 19.08 | 20 | 27.03 | 0.25 | Attention deficits | 46 | 35.11 | 22 | 29.73 | 0.52 |
| Low self esteem | 27 | 20.61 | 26 | 35.14 | 0.03 | Learning deficits | 34 | 25.95 | 8 | 10.81 | 0.01 |
| Decreased energy | 27 | 20.61 | 23 | 31.08 | 0.13 | Language deficits | 21 | 16.03 | 7 | 9.46 | 0.27 |
| Mood swings | 20 | 15.27 | 20 | 27.03 | 0.06 | Motor deficits | 9 | 6.87 | 2 | 2.7 | 0.33 |
| Euphoria | 0 | 0.0 | 8 | 10.81 | <0.001 | Individualized Education Plan | 28 | 21.37 | 10 | 13.51 | 0.22 |
| Irritability | 53 | 40.46 | 47 | 63.51 | 0.002 | Completed IQ testing | 31 | 23.66 | 13 | 17.57 | 0.39 |
| Hyperthymia | 1 | 0.76 | 6 | 8.11 | 0.01 | ||||||
| Increased goal directed behaviors | 2 | 1.53 | 10 | 13.51 | <0.001 | Any behavioral symptom | 63 | 48.09 | 28 | 37.84 | 0.20 |
| Hypersexuality | 1 | 0.76 | 3 | 4.05 | 0.13 | Classroom disruptiveness | 19 | 14.5 | 12 | 16.22 | 0.90 |
| Cognitive problems | 7 | 5.34 | 4 | 5.41 | >0.99 | Runaway | 4 | 3.05 | 1 | 1.35 | 0.65 |
| Changes in appetite | 10 | 7.63 | 12 | 16.22 | 0.06 | Truancy | 11 | 8.4 | 6 | 8.11 | >0.99 |
| Hypersomnia | 6 | 4.58 | 2 | 2.7 | 0.71 | Fighting | 16 | 12.21 | 15 | 20.27 | 0.17 |
| Insomnia | 48 | 36.64 | 36 | 48.65 | 0.12 | Bullying | 6 | 4.58 | 2 | 2.7 | 0.71 |
| Parasomnia | 0 | 0.0 | 2 | 2.7 | 0.12 | Aggression | 29 | 22.14 | 25 | 33.78 | 0.09 |
| Sleep deprived energy-enhancement | 3 | 2.29 | 11 | 14.86 | 0.001 | Inappropriate sexual behaviors | 5 | 3.82 | 4 | 5.41 | 0.72 |
| Any anxious-trauma-obsessive symptom | 60 | 45.8 | 36 | 48.65 | 0.80 | Destructiveness | 9 | 6.87 | 7 | 9.46 | 0.59 |
| Excessive worries | 41 | 31.3 | 26 | 35.14 | 0.68 | Criminal behavior | 19 | 14.5 | 14 | 18.92 | 0.53 |
| Hypervigilance | 5 | 3.82 | 3 | 4.05 | >0.99 | Stealing | 16 | 12.21 | 9 | 12.16 | >0.99 |
| Restlessness | 23 | 17.56 | 14 | 18.92 | 0.95 | Any Suicidality | 57 | 43.51 | 35 | 47.3 | 0.70 |
| Social anxiety | 21 | 16.03 | 11 | 14.86 | 0.98 | Suicide ideation | 50 | 38.17 | 32 | 43.24 | 0.57 |
| Excessive fear or phobias | 13 | 9.92 | 11 | 14.86 | 0.36 | Suicidal ideation with plan | 29 | 22.14 | 18 | 24.32 | 0.85 |
| Nightmares | 4 | 3.05 | 9 | 12.16 | 0.015 | Suicide attempt | 23 | 17.56 | 17 | 22.97 | 0.44 |
| Flashbacks | 3 | 2.29 | 2 | 2.7 | >0.99 | ||||||
| Obsessions | 4 | 3.05 | 4 | 5.41 | 0.46 | Self-Injurious Behaviors | 29 | 22.14 | 22 | 29.73 | 0.29 |
| Panic attacks | 10 | 7.63 | 13 | 17.57 | 0.03 | ||||||
BD = bipolar disorder, SZ = Schizophrenia, IQ = intelligence quotient.
3.6. Predictors of age of onset and duration of initial prodrome
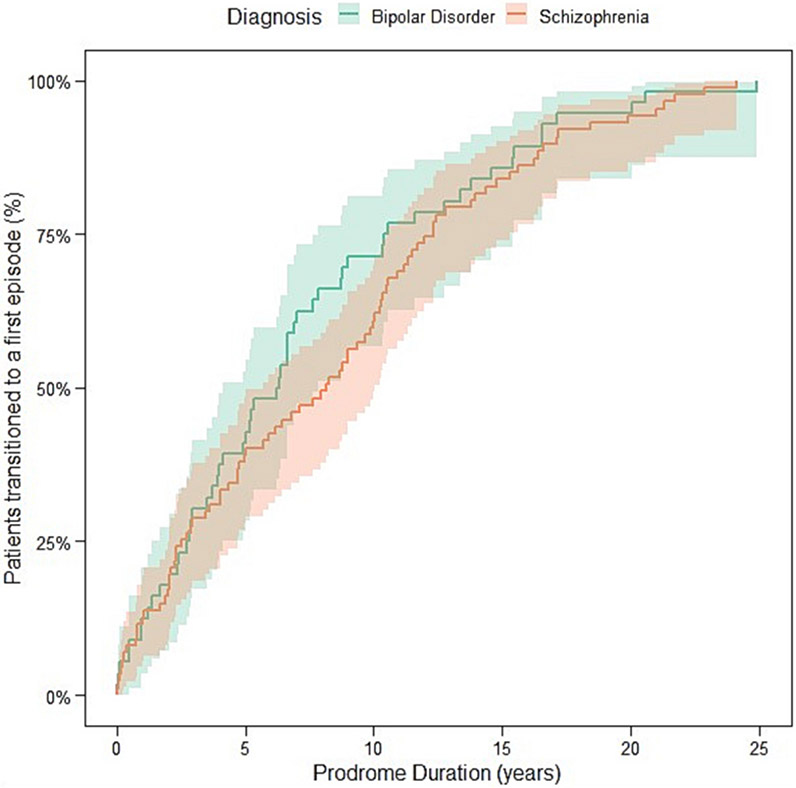
After adjusting for sex, race, and place of birth, we did not find evidence that age of onset (HR = 0.89; 95 CI 0.66 to 1.19; p = 0.44) or the duration of the initial prodromic phase (HR = 1.08; 95 CI 0.76 to 1.54; p = 0.65) was different between BD versus SZ individuals. These results remain essentially the same in the unadjusted mode (Fig. 2).
Fig. 2.
Unadjusted survival curve comparing the initial duration of prodrome of SZ and BD. Prodrome duration was defined as the time from first mental health visit to the first episode of psychosis or mania.
As shown in Table 3, The seven patients with a diagnosis of autism prior to the first episode of psychosis had an earlier onset of the illness (HR = 3.80; 95 CI from 1.75 to 8.24; p < 0.001). Otherwise, white race, a history of depression and anxiety, and use of antidepressants were related to a later age of onset of the illness after adjusting for confounders.
Table 3.
Factors associated with age of onset and duration of prodrome.
| |
Age of onset |
Duration of initial prodrome |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics of the population | N | HR | LCI | UCI | p-value | N | HR | LCI | UCI | p-value |
| Diagnosis of BD vs SZ | 205 | 0.89 | 0.66 | 1.19 | 0.44 | 143 | 1.08 | 0.76 | 1.54 | 0.65 |
| Male | 205 | 0.98 | 0.71 | 1.34 | 0.90 | 143 | 0.90 | 0.62 | 1.29 | 0.56 |
| White race vs non-white | 205 | 0.61 | 0.44 | 0.86 | < 0.005 | 143 | 1.03 | 1.01 | 1.06 | 0.01 |
| Hispanic ethnicity | 205 | 1.60 | 0.89 | 2.87 | 0.11 | 143 | 0.64 | 0.42 | 0.95 | 0.03 |
| Foreign born | 205 | 1.29 | 0.83 | 2.01 | 0.25 | 143 | 0.88 | 0.41 | 1.91 | 0.75 |
| Initial presentation for a neurodevelopmental problem | 158 | 1.06 | 0.75 | 1.50 | 0.73 | 143 | 0.38 | 0.26 | 0.57 | < 0.001 |
| Diagnoses before first episode | ||||||||||
| ADHD | 205 | 1.04 | 0.75 | 1.45 | 0.80 | 143 | 0.43 | 0.29 | 0.63 | < 0.001 |
| Intellectual disability | 205 | 1.11 | 0.60 | 2.08 | 0.74 | 143 | 0.52 | 0.27 | 0.98 | 0.04 |
| Autism spectrum disorder | 205 | 3.80 | 1.75 | 8.24 | < 0.001 | 143 | 1.04 | 0.45 | 2.40 | 0.92 |
| Other neurodevelopmental disorder † | 205 | 1.05 | 0.75 | 1.48 | 0.76 | 143 | 0.40 | 0.27 | 0.59 | < 0.001 |
| Conduct disorder | 205 | 1.48 | 0.94 | 2.34 | 0.090 | 143 | 0.66 | 0.41 | 1.07 | 0.09 |
| Oppositional-defiant disorder | 205 | 1.48 | 0.90 | 2.41 | 0.12 | 143 | 0.61 | 0.36 | 1.03 | 0.07 |
| Depressive disorders | 205 | 0.65 | 0.48 | 0.88 | < 0.005 | 143 | 0.98 | 0.67 | 1.46 | 0.94 |
| Anxiety disorder | 205 | 0.73 | 0.54 | 1.00 | 0.05 | 143 | 0.98 | 0.68 | 1.41 | 0.91 |
| Adjustment disorders | 205 | 1.10 | 0.79 | 1.54 | 0.57 | 143 | 1.08 | 0.76 | 1.54 | 0.66 |
| Medications | ||||||||||
| Any psychotropic before first episode | 205 | 0.82 | 0.60 | 1.12 | 0.21 | 143 | 0.77 | 0.50 | 1.20 | 0.25 |
| Antidepressant | 205 | 0.74 | 0.54 | 1.00 | 0.05 | 143 | 0.96 | 0.67 | 1.39 | 0.84 |
| Mood Stabilizer | 205 | 0.69 | 0.41 | 1.17 | 0.17 | 143 | 0.66 | 0.40 | 1.09 | 0.10 |
| Antipsychotic | 205 | 0.81 | 0.54 | 1.21 | 0.30 | 143 | 0.94 | 0.62 | 1.42 | 0.77 |
| ADHD medication (stimulant) | 205 | 1.00 | 0.71 | 1.40 | 0.99 | 143 | 0.40 | 0.27 | 0.60 | < 0.001 |
| Substance use | ||||||||||
| Any substance use | 205 | 0.69 | 0.50 | 0.95 | 0.02 | 143 | 1.23 | 0.83 | 1.81 | 0.30 |
| Alcohol | 205 | 0.76 | 0.57 | 1.01 | 0.06 | 143 | 1.01 | 0.72 | 1.43 | 0.93 |
| Stimulants | 205 | 0.71 | 0.49 | 1.04 | 0.08 | 143 | 0.83 | 0.55 | 1.26 | 0.39 |
| Cannabis | 205 | 0.78 | 0.58 | 1.05 | 0.10 | 143 | 1.07 | 0.75 | 1.53 | 0.70 |
BD = bipolar disorder, SZ = Schizophrenia, ADHD = attention deficit hyperactivity disorder, LCI = lower confidence interval, UCI = upper confidence interval, HR = Hazard Ratio, USA = United States of America.
Other neurodevelopmental disorder include tics, learning, reading, speech, and other DSM-5 neurodevelopmental spectrum disorder.
The significant factors associated with longer initial prodromes were clinical problems that we expect to start in early life (i.e., neurodevelopmental disorders) and White race having a longer duration of prodrome. We also found that initially seeking help for neurodevelopmental disorders (e.g., speech disorder, unspecified neurodevelopmental problems or learning disorders), a history of ADHD, and prescription of stimulants were associated with longer initial prodrome (see Table 3). Of note, we did not find that antipsychotics, mood stabilizers, cannabis use, or non-prescription stimulant use were associated with the duration of the prodrome or the age of onset.
4. Discussion
Our main findings show that most individuals with SZ and BD have psychiatric antecedents before the first episode. The antecedents of these two disorders usually starts during adolescence, overlap, and present in unspecific ways. We found that the data gathered in a regular clinical practice is insufficient to easily differentiate between the antecedents of SZ and BD given the overlap. However, some differences between the two initial prodromes do appear when looking at larger samples.
Our results add to the accumulating evidence suggesting that the prodromes of SZ and BD are similar but not identical as they have overlap in diagnoses received, symptoms, medications prescribed, and substances used before the first episode of SZ and BD (Correll et al., 2007; Kafali et al., 2019; Olvet et al., 2010; Salvatore et al., 2014; Skjelstad et al., 2010; Verdolini et al., 2022).
While in most measures the psychiatric antecedents were similar, they were not identical. Patients with BD were diagnosed more frequently with depressive and adjustment disorders prior to their first episode. These results align with previous reports that have identified that over 50 % of patients who develop BD experienced mood symptoms or major depression in youth, versus 23 % in SZ (Addington et al., 2002; Faedda et al., 2019; Kalman et al., 2021). We also found that prior to the first episode, patients with BD have more subsyndromal symptoms resembling mania including euphoria, hyperthymia, and sleep deprived energy enhancement. Similar results were found in other studies in which this subsyndromal manic symptoms accumulate more in the prodromes of BD (Van Meter et al., 2016; Verdolini et al., 2022) suggesting specificity of these symptoms for BD during the prodromal phase.
We found that SZ was diagnosed more frequently in immigrant and in non-white populations, adding to the accumulating evidence linking immigration and race as more commonly associated with SZ than with BD (Hollander et al., 2016; Schwartz and Blankenship, 2014). It is worth noting that in our sample, 16 (50 %) of the foreign born population come from Somalia, a reflection of Minnesota being the state with the largest population of Somalis in the US (Gambino et al., 2012) The Somali population might introduce a confounder as this report does not account for other factors previously reported in Somali population that also increase the risk for SZ or BD such as a history of trauma, malnutrition, and/or head trauma (Kroll et al., 2011). Finally, while there was also a higher prevalence of all neurodevelopmental diagnoses in SZ; we did not find a statistically significant difference between the two groups, likely due to insufficient power to detect differences. However, we did find that people with SZ had more learning deficits during the initial prodrome. The predominance of a neurodevelopmental component in SZ adds to the accumulating literature supporting the notion that SZ has a higher prevalence of cognitive problems and neuroanatomical changes compared to BD (Demjaha et al., 2012; Verdolini et al., 2022; Zanelli et al., 2010).
We also found that regardless of the diagnosis, prior to the FEP/FEM there is a long period of health care utilization for mental health reasons. This period starts at around 13-years of age and is characterized by psychiatric morbidity, a wide range of symptoms, prescription of psychotropics medications, and high rates of substance use. This period should continue to be used for early detections endeavours and early interventions given the importance of early treatment in improving outcomes (Correll et al., 2018).
Our results replicate previous finding that autism has been previously associated with earlier onset of schizophrenia (Rapoport et al., 2009). As expected, longer prodromes are associated with disorders characterized by seeking help during childhood such as neurodevelopmental disorders, ADHD, and stimulant prescription for ADHD. Notably, we did not find that cannabis was associated with longer prodromes or an earlier age of onset. This contrast with the findings of a meta-analysis that showed that most substances of abuse, especially cannabis, causes an earlier onset of psychosis in people with SZ (Large et al., 2011), but replicates the findings of the Finnish Cohort in BD (Denissoff et al., 2022).
Lastly, diagnoses of depression, anxiety, and use of antidepressants were related to a later of onset of the first episode. Given the significant correlation of these three factors, it is hard to distinguish if there is a unique factor driving the later age of onset or if they independently affect age of onset. Furthermore, in the context of the combination of the SZ and BD for this analysis, we are also we are unable to distinguish if the effect affects a particular group or both. The current evidence indicates that antidepressant use in the schizophrenia prodrome might have some protective effects (Cornblatt et al., 2007; Fusar-Poli et al., 2015) and in contrast the exposure to antidepressant may precipitate mania and accelerate the age of onset (Chengappa et al., 2003; Perlis et al., 2004).
The strength of this study lies in the REP being a unique database in the United States that permits population-based research with medical, and continuity of care data collected by health care professionals (St. Sauver et al., 2012). Similar databases in the United States, that have been used to describe antecedents of people with psychosis include the Kaiser Permanente database; however, this database is limited as it does not provide complete coverage to the population that they serve and more importantly it excludes the uninsured population, a crucial limiting factor when studying people with SZ or BD (Kaiser Permanente Research Bank, n.d.; St. Sauver et al., 2012). Furthermore, each case was ascertained manually by a trained psychiatrist using DSM 5 criteria, this method of ascertainment offers a precision that studies using algorithms with imperfect specificity to detect psychosis from claims data do not (Simon et al., 2017). Also, the sample avoids the referral bias of clinical series or specialized programs (Powers et al., 2020). Finally, this dataset contained adequate data in clinical notes from healthcare encounters to allow assessment of illness trajectories, thus minimizing the risk of recall bias compared to studies with similar aims that depend on retrospective assessments (Kafali et al., 2019; Verdolini et al., 2022).
This study has several limitations. First, it is a retrospective study, which means that the data was collected from medical records. This type of study is prone to information bias, as the data in the medical records may not be accurate or complete. We suspect that this bias affects more prominently the symptom data (Table 2) as this data tends to not be systematically documented when compared to diagnostic codes, substance use, and medications Also, while the REP has shown to be generalizable for most diseases, the generalizability for BD and SZ might be limited as the REP population is less diverse (mostly white) and has a higher socioeconomical status compared to the rest of the United States (St. Sauver et al., 2012). Another factor is the sample size deficits resulting in some of the analyses being underpowered and the differences in sample size between SZ and BD. Finally, the REP is missing data from a few small private clinics that provide mental health services as they are not included REP; therefore, we expect some missing data from patients using these services.
5. Conclusion
Starting at around adolescence, people with SZ and BD present to clinical settings with unspecific and heterogeneous psychiatric problems and symptoms which result in a wide range of diagnoses and prescriptions of psychiatric medications. Despite the tradition of splitting SZ and BD, our data shows that the antecedents or prodromes of SZ and BD are more alike than different; however, they are not “indistinguishable” (Olvet et al., 2010) at the population level. Further studies are encouraged to continue looking for specific factors that distinguish the antecedents of these two disorders.
Supplementary Material
Acknowledgments/Funding source
This study was supported, in part, by the Luther Automotive Foundation and Mayo Foundation; neither had a role in the design, conduct, analysis, or submission of the study. Manuel Gardea-Resendez received salary support from Mayo Foundation. Santiago Castiello-de Obeso received salary support from the University of Guadalajara, Mexico.
This study was also made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This study was presented, in part, at the 2021 American Psychiatric Association Annual Meeting, the Minnesota Psychiatric Society 2022 Spring Meeting, the 2022 Mental Health Services Conference and the 2022 International Society for Bipolar Disorders Annual Meeting.
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Mark A. Frye received grant support from Assurex Health and Mayo Foundation, received CME travel and honoraria from Carnot Laboratories, and has Financial Interest/Stock ownership/Royalties from Chymia LLC.
No other declaration of interests from other authors.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jad.2023.07.106.
References
- Addington J, van Mastrigt S, Hutchinson J, Addington D, 2002. Pathways to care: help seeking behaviour in first episode psychosis. Acta Psychiatr. Scand 106 (5), 358–364. 10.1034/j.1600-0447.2002.02004.x. [DOI] [PubMed] [Google Scholar]
- Allebeck P. (2009). The use of population based registers in psychiatric research. In Acta Psychiatrica Scandinavica (Vol. 120, issue 5, pp. 386–391). doi: 10.1111/j.1600-0447.2009.01474.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders. American Psychiatric Association, 10.1176/appi.books.9780890425596. [DOI] [Google Scholar]
- Andrade-González N, Álvarez-Cadenas L, Saiz-Ruiz J, Lahera G, 2020. Initial and relapse prodromes in adult patients with episodes of bipolar disorder: A systematic review. European Psychiatry 63 (1), e12. 10.1192/J.EURPSY.2019.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechdolf A, Ratheesh A, Wood SJ, Tecic T, Conus P, Nelson B, Cotton SM, Chanen AM, Amminger GP, Ruhrmann S, Schultze-Lutter F, Klosterkotter J, Fusar Poli P, Yung AR, Berk M, McGorry PD, 2012. Rationale and first results of developing at-risk (prodromal) criteria for bipolar disorder. Curr. Pharm. Des 18 (4), 358–375. 10.2174/138161212799316226. [DOI] [PubMed] [Google Scholar]
- Birur B, Kraguljac NV, Shelton RC, Lahti AC, 2017. Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder—a systematic review of the magnetic resonance neuroimaging literature. NPJ Schizophr. 3 (1), 15. 10.1038/s41537-017-0013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitborde NJ, Srihari VH, Woods SW, 2009. Review of the operational definition for first-episode psychosis. Early Interv Psychiatry 3 (4), 259–265. 10.1111/j.1751-7893.2009.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso RM, Lineberry TW, Bostwick JM, Decker PA, St Sauver J, 2008. Mortality in schizophrenia and schizoaffective disorder: an Olmsted County, Minnesota cohort: 1950-2005. Schizophr. Res 98 (1–3), 287–294. 10.1016/j.schres.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Chan CC, Shanahan M, Ospina LH, Larsen EM, Burdick KE, 2019. Premorbid adjustment trajectories in schizophrenia and bipolar disorder: A transdiagnostic cluster analysis. Psychiatry Res. 272, 655–662. 10.1016/j.psychres.2018.12.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chengappa KNR, Kupfer DJ, Frank E, Houck PR, Grochocinski VJ, Cluss PA, Stapf DA, 2003. Relationship of birth cohort and early age at onset of illness in a bipolar disorder case registry. Am. J. Psychiatr 160 (9), 1636–1642. 10.1176/APPI.AJP.160.9.1636/ASSET/IMAGES/LARGE/L014F6.JPEG. [DOI] [PubMed] [Google Scholar]
- Conus P, Ward J, Hallam KT, Lucas N, Macneil C, McGorry PD, Berk M, 2008. The proximal prodrome to first episode mania a new target for early intervention. Bipolar Disord. 10 (5), 555–565. 10.1111/j.1399-5618.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Lencz T, Smith CW, Olsen R, Auther AM, Nakayama E, Lesser ML, Tai JY, Shah MR, Foley CA, Kane JM, Correll CU, 2007. Can antidepressants be used to treat the schizophrenia prodrome? Results of a prospective, naturalistic treatment study of adolescents. The Journal of Clinical Psychiatry 68 (4), 546–557. 10.4088/JCP.V68N0410. [DOI] [PubMed] [Google Scholar]
- Correll CU, Penzner JB, Frederickson AM, Richter JJ, Auther AM, Smith CW, Kane JM, Cornblatt BA, 2007. Differentiation in the preonset phases of schizophrenia and mood disorders: evidence in support of a bipolar mania prodrome. Schizophr. Bull 33 (3), 703–714. 10.1093/schbul/sbm028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Hauser M, Auther AM, Cornblatt BA, 2010. Research in people with psychosis risk syndrome: a review of the current evidence and future directions. J. Child Psychol. Psychiatry 51 (4), 390–431. 10.1111/J.1469-7610.2010.02235.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Galling B, Pawar A, Krivko A, Bonetto C, Ruggeri M, Craig TJ, Nordentoft M, Srihari VH, Guloksuz S, Hui CLM, Chen EYH, Valencia M, Juarez F, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, Kane JM, 2018. Comparison of early intervention services vs treatment as usual for early-phase psychosis. JAMA Psychiatry 75 (6), 555. 10.1001/jamapsychiatry.2018.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, Owen MJ, 2010. The Kraepelinian dichotomy – going, going but still not gone. Br. J. Psychiatry 196 (2), 92–95. 10.1192/bjp.bp.109.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demjaha A, MacCabe JH, Murray RM, 2012. How genes and environmental factors determine the different neurodevelopmental trajectories of schizophrenia and bipolar disorder. Schizophr. Bull 38 (2), 209–214. 10.1093/schbul/sbr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denissoff A, Mustonen A, Alakokkare AE, Scott JG, Sami MB, Miettunen J, Niemelä S, 2022. Is early exposure to cannabis associated with bipolar disorder? Results from a Finnish birth cohort study. Addiction 117 (8), 2264–2272. 10.1111/ADD.15881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faedda GL, Baldessarini RJ, Marangoni C, Bechdolf A, Berk M, Birmaher B, Conus P, DelBello MP, Duffy AC, Hillegers MHJ, Pfennig A, Post RM, Preisig M, Ratheesh A, Salvatore P, Tohen M, Vázquez GH, Vieta E, Yatham LN, Correll CU, 2019. An International Society of Bipolar Disorders task force report: precursors and prodromes of bipolar disorder. Bipolar Disord. 21 (8), 720–740. 10.1111/bdi.12831. [DOI] [PubMed] [Google Scholar]
- Foroughi M, Medina Inojosa JR, Lopez-Jimenez F, Saeidifard F, Suarez L, Stokin GB, Prieto ML, Rocca WA, Frye MA, Morgan RJ, 2022. Association of Bipolar Disorder with Major Adverse Cardiovascular Events: A population-based historical cohort study. Psychosom. Med 84 (1), 97–103. 10.1097/PSY.0000000000001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Frascarelli M, Valmaggia L, Byrne M, Stahl D, Rocchetti M, Codjoe L, Weinberg L, Tognin S, Xenaki L, McGuire P, 2015. Antidepressant, antipsychotic and psychological interventions in subjects at high clinical risk for psychosis: OASIS 6-year naturalistic study. Psychol. Med 45 (6), 1327–1339. 10.1017/S003329171400244X. [DOI] [PubMed] [Google Scholar]
- Gambino C, Trevelyan E, Fitzwater JT, 2012. The Foreign-Born Population from Africa: 2008–2012. [Google Scholar]
- Geoffroy PA, Scott J, 2017. Prodrome or risk syndrome: what’s in a name? Int J Bipolar Disord 5 (1), 7. 10.1186/s40345-017-0077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourzis P, Katrivanou A, Beratis S, 2002. Symptomatology of the initial prodromal phase in schizophrenia. Schizophr. Bull 28 (3), 415–429. 10.1093/OXFORDJOURNALS.SCHBUL.A006950. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, Consortium, R. Edc, 2019. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inform 95, 103208 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann JA, Nelson B, Ratheesh A, Treen D, & McGorry PD (2019). At-risk studies and clinical antecedents of psychosis, bipolar disorder and depression: a scoping review in the context of clinical staging. In Psychological Medicine (Vol. 49, Issue 2, pp. 177–189). Cambridge University Press. doi: 10.1017/S0033291718001435. [DOI] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RSE, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, 2013. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the bipolar-schizophrenia network on intermediate phenotypes (B-SNIP) study. Am. J. Psychiatr 170 (11), 1275–1284. 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander AC, Dal H, Lewis G, Magnusson C, Kirkbride JB, Dalman C, 2016. Refugee migration and risk of schizophrenia and other non-affective psychoses: cohort study of 1.3 million people in Sweden. BMJ 352, i1030. 10.1136/bmj.i1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafali HY, Bildik T, Bora E, Yuncu Z, Erermis HS, 2019. Distinguishing prodromal stage of bipolar disorder and early onset schizophrenia spectrum disorders during adolescence. Psychiatry Res. 275, 315–325. 10.1016/j.psychres.2019.03.051. [DOI] [PubMed] [Google Scholar]
- Kaiser Permanente Research Bank. (n.d.). Retrieved 20 September 2022, from https://researchbank.kaiserpermanente.org/our-research/for-researchers/.
- Kalman JL, Olde Loohuis LM, Vreeker A, McQuillin A, Stahl EA, Ruderfer D, Grigoroiu-Serbanescu M, Panagiotaropoulou G, Ripke S, Bigdeli TB, Stein F, Meller T, Meinert S, Pelin H, Streit F, Papiol S, Adams MJ, Adolfsson R, Adorjan K, Ophoff RA, 2021. Characterisation of age and polarity at onset in bipolar disorder. Br. J. Psychiatry 219 (6), 659–669. 10.1192/bjp.2021.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, 1986. Kraepelin and the differential diagnosis of dementia praecox and manic-depressive insanity. Compr. Psychiatry 27 (6), 549–558. 10.1016/0010-440X(86)90059-3. [DOI] [PubMed] [Google Scholar]
- Kotov R, Leong SH, Mojtabai R, Erlanger ACE, Fochtmann LJ, Constantino E, Carlson GA, Bromet EJ, 2013. Boundaries of schizoaffective disorder. JAMA. Psychiatry 70 (12), 1276. 10.1001/jamapsychiatry.2013.2350. [DOI] [PubMed] [Google Scholar]
- Kraepelin E., 1919. Dementia Praecox and Paraphrenia. Livingstone. [Google Scholar]
- Kraepelin E., 1923. Clinical Psychiatry: A Textbook for Students and Physicians. MacMillan. [Google Scholar]
- Kroll J, Yusuf AI, Fujiwara K, 2011. Psychoses, PTSD, and depression in Somali refugees in Minnesota. Soc. Psychiatry Psychiatr. Epidemiol 46 (6), 481–493. 10.1007/s00127-010-0216-0. [DOI] [PubMed] [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O, 2011. Cannabis use and earlier onset of psychosis. Arch. Gen. Psychiatry 68 (6), 555. 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- Maret-Ouda J, Tao W, Wahlin K, Lagergren J, 2017. Nordic registry-based cohort studies: possibilities and pitfalls when combining Nordic registry data. Scand J Public Health 45 (17_suppl), 14–19. 10.1177/1403494817702336. [DOI] [PubMed] [Google Scholar]
- Norman RMG, Lewis SW, Marshall M, 2005. Duration of untreated psychosis and its relationship to clinical outcome. Br. J. Psychiatry 187 (S48), s19–s23. 10.1192/BJP.187.48.S19. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Stearns WH, McLaughlin D, Auther AM, Correll CU, Cornblatt BA, 2010. Comparing clinical and neurocognitive features of the schizophrenia prodrome to the bipolar prodrome. Schizophr. Res 123 (1), 59–63. 10.1016/j.schres.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, Bowden CL, Sachs GS, Nierenberg AA, 2004. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol. Psychiatry 55 (9), 875–881. 10.1016/J.BIOPSYCH.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Powers AR, Addington J, Perkins DO, Bearden CE, Cadenhead KS, Cannon TD, Cornblatt BA, Mathalon DH, Seidman LJ, Tsuang MT, Walker EF, McGlashan TH, Woods SW, 2020. Duration of the psychosis prodrome. Schizophr. Res 216, 443–449. 10.1016/j.schres.2019.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan BK, Chakrabarti S, Nehra R, Mankotia A, 2008. Cognitive functions in bipolar affective disorder and schizophrenia: comparison. Psychiatry Clin. Neurosci 62 (5), 515–525. 10.1111/j.1440-1819.2008.01844.x. [DOI] [PubMed] [Google Scholar]
- Prieto ML, Schenck LA, Kruse JL, Klaas JP, Chamberlain AM, Bobo W, v, Bellivier F, Leboyer M, Roger VL, Brown RD Jr., Rocca WA, & Frye MA, 2016. Long-term risk of myocardial infarction and stroke in bipolar I disorder: A population-based cohort study. J. Affect. Disord 194, 120–127. 10.1016/j.jad.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport J, Chavez A, Greenstein D, Addington A, Gogtay N, 2009. Autism Spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J. Am. Acad. Child Adolesc. Psychiatry 48 (1), 10–18. 10.1097/CHI.0b013e31818b1c63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietdijk J, Hogerzeil SJ, van Hemert AM, Cuijpers P, Linszen DH, van der Gaag M, 2011. Pathways to psychosis: help-seeking behavior in the prodromal phase. Schizophr. Res 132 (2–3), 213–219. 10.1016/j.schres.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Yawn BP, St. Sauver JL, Grossardt BR, & Melton LJ, 2012. History of the Rochester epidemiology project: half a century of medical records linkage in a US population. Mayo Clin. Proc 87 (12), 1202–1213. 10.1016/J.MAYOCP.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Brue SM, Bock-Goodner CM, Chamberlain AM, Wilson PM, Finney Rutten LJ, St Sauver JL, 2018. Data resource profile: expansion of the Rochester epidemiology project medical records-linkage system (E-REP). Int. J. Epidemiol 47 (2) 10.1093/ije/dyx268, 368–368j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakowski JK, 2019. 120th anniversary of the Kraepelinian dichotomy of psychiatric disorders. Current Psychiatry Reports 21 (8), 65. 10.1007/s11920-019-1048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore P, Baldessarini RJ, Khalsa HM, Vazquez G, Perez J, Faedda GL, Amore M, Maggini C, Tohen M, 2014. Antecedents of manic versus other first psychotic episodes in 263 bipolar I disorder patients. Acta Psychiatr. Scand 129 (4), 275–285. 10.1111/acps.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RC, Blankenship DM, 2014. Racial disparities in psychotic disorder diagnosis: A review of empirical literature. World J Psychiatry 4 (4), 133–140. 10.5498/wjp.v4.i4.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE, Coleman KJ, Yarborough BJH, Operskalski B, Stewart C, Hunkeler EM, Lynch F, Carrell D, & Beck A (2017). First presentation with psychotic symptoms in a population-based sample. Psychiatr Serv. Doi: 10.1176/APPI.PS.201600257, 68(5), 456–461. doi: 10.1176/APPI.PS.201600257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjelstad DV, Malt UF, Holte A, 2010. Symptoms and signs of the initial prodrome of bipolar disorder: A systematic review. J. Affect. Disord 126 (1–2), 1–13. 10.1016/J.JAD.2009.10.003. [DOI] [PubMed] [Google Scholar]
- St. Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, Rocca WA, 2012. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester epidemiology project. Mayo Clin. Proc 87 (2), 151–160. 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, R.C., 2013. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://Www.R-Project.Org/. [Google Scholar]
- Van Meter AR, Burke C, Youngstrom EA, Faedda GL, Correll CU, 2016. The bipolar Prodrome: Meta-analysis of symptom prevalence prior to initial or recurrent mood episodes. J. Am. Acad. Child Adolesc. Psychiatry 55 (7), 543–555. 10.1016/J.JAAC.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Verdolini N, Borras R, Sparacino G, Garriga M, Sague-Vilavella M, Madero S, Palacios-Garran R, Serra M, Forte MF, Salagre E, Aedo A, Salgado-Pineda P, Salvatierra IM, Sanchez Gistau V, Pomarol-Clotet E, Ramos-Quiroga JA, Carvalho AF, Garcia-Rizo C, Undurraga J, Amoretti S, 2022. Prodromal phase: differences in prodromal symptoms, risk factors and markers of vulnerability in first episode mania versus first episode psychosis with onset in late adolescence or adulthood. Acta Psychiatr. Scand 146 (1), 36–50. 10.1111/acps.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. (2016). Programming with ggplot2 (pp. 241–253). doi: 10.1007/978-3-319-24277-4_12. [DOI] [Google Scholar]
- Zanelli J, Reichenberg A, Morgan K, Fearon P, Kravariti E, Dazzan P, Morgan C, Zanelli C, Demjaha A, Jones PB, Doody GA, Kapur S, Murray RM, 2010. Specific and generalized neuropsychological deficits: a comparison of patients with various first-episode psychosis presentations. Am. J. Psychiatry 167 (1), 78–85. 10.1176/appi.ajp.2009.09010118. [DOI] [PubMed] [Google Scholar]
- Zivanovic O, Nedic A, 2012. Kraepelin’s concept of manic-depressive insanity: one hundred years later. J. Affect. Disord 137 (1–3), 15–24. 10.1016/j.jad.2011.03.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.