Abstract
Background and study aims Gastroesophageal reflux disease (GERD) is a widespread chronic gastrointestinal condition with an increasing worldwide prevalence. This research was a systematic review and meta-analysis evaluating the efficacy, safety, and long-term outcomes of endoscopic full-thickness plication (EFTP) for the treatment of GERD.
Methods A comprehensive search of databases was conducted for studies published up to April 2023. We included randomized controlled trials (RCTs) and prospective observational studies that examined the use of EFTP in treating GERD among adult patients. We calculated pooled effect estimates using a random-effects model.
Results EFTP significantly improved GERD Health-Related Quality of Life (GERD-HRQL) scores at 3-, 6-, and 12-month follow-up intervals. A considerable proportion of patients discontinued proton pump inhibitors, with cessation rates of 59% (95% confidence interval [CI]: 0.47–0.71), 68% (95% CI: 0.58–0.78), and 67% (95% CI: 0.46–0.89,) at 3, 6, and 12 months, respectively. At 3 and 6 months, 61% (95% CI: 0.54–0.68) and 66% (95% CI: 0.56–0.76) of patients experienced ≥50% improvement in GERD-HRQL scores. EFTP demonstrated a favorable safety profile, with a low rate of severe adverse events. We observed a 6.76% reduction (95% CI: –14.53–1.02) in the percentage of time with esophageal pH <4, a decrease in DeMeester scores, and fewer total reflux episodes. The average procedure time was 22.75 minutes (95% CI: 22.03–23.48). Subgroup analyses suggest that both the GERDx system and the NDO Plicator are effective and safe in treating GERD.
Conclusions The findings from our study reveal that EFTP is a safe and effective treatment for GERD patients who have not responded adequately to conventional therapies. Given its minimally invasive nature, effectiveness, and limited adverse effects, EFTP emerges as a compelling alternative to conventional surgical procedures.
Keywords: Reflux disease, GI surgery, Endoscopy Upper GI Tract, Motility / achalasia
Introduction
Gastroesophageal reflux disease (GERD) is a prevalent chronic gastrointestinal disorder that has experienced a considerable increase globally, with a 77.53% growth from 441.57 million cases in 1990 to 783.95 million in 2019 1 2 . GERDs symptoms such as heartburn and regurgitation impact life and work, potentially leading to Barrett's esophagus and esophageal cancer if untreated 3 4 . Conventional therapies involve lifestyle changes and medications, primarily proton pump inhibitors (PPIs), but limitations exist including non-responsiveness and side effects, prompting the need for surgical or endoscopic alternatives 5 6 7 8 9 10 11 .
Surgical interventions can effectively alleviate GERD symptoms but may cause complications such as structural laxity of fundoplication, stenosis, swallowing difficulties, and gas bloat syndrome 12 13 . Endoscopic approaches, such as radiofrequency ablation, endoscopic fundoplication, and endoscopic mucosal resection, have been developed for GERD management. These methods, notably endoscopic full-thickness plication (EFTP), are recognized for their effectiveness in symptom reduction and safety, often presenting fewer complications compared to surgical options 14 15 . Short-term outcomes post-procedure, such as immediate symptom relief and reduced medication dependency, are generally positive 15 .
Nevertheless, certain aspects of endoscopic treatment for GERD, including EFTP, are yet to be fully understood. There is a notable lack of long-term efficacy data, particularly concerning sustained symptom relief and ongoing quality of life improvements. The comparative effectiveness of different endoscopic techniques and their performance relative to surgical treatments remain underexplored, creating gaps in treatment selection guidelines. The effectiveness of these endoscopic methods in diverse patient groups, especially those with severe or complicated GERD, also requires further study 16 . Moreover, the cost-effectiveness of endoscopic treatments in comparison to conventional therapies is an area in need of more comprehensive research.
EFTP represents a significant advance in minimally invasive GERD management. The technique involves creating a functional anti-reflux valve by applying transmural sutures at the gastroesophageal junction, folding and connecting gastric fundus segments to the esophagus, ultimately enhancing the valvular mechanism and reducing gastroesophageal reflux 14 15 16 . The long-term effectiveness and safety of EFTP have been supported by several randomized controlled trials (RCTs) and prospective studies 17 18 19 20 . With EFTP increasingly being used in GERD management, it is imperative to critically evaluate the existing literature through a systematic review and meta-analysis. Our objective is to thoroughly examine the effectiveness, safety, and long-term results of EFTP for GERD treatment, ultimately offering valuable guidance for clinical decision-making.
Methods
Search strategy and data sources
A comprehensive literature search adhering to the Meta-analysis of Observational Studies in Epidemiology guidelines was conducted in MEDLINE, Embase, Scopus, Web of Science, and the Cochrane Library databases from their inception to April 2023. We employed a combination of relevant words and medical subject headings (MeSH) terms. In addition, the search was conducted using a combination of these keywords: „endoscopic full-thickness plication“, „EFTP“, „gastroesophageal reflux disease“, and „GERD“. The search was limited to articles published in English. Furthermore, we screened the reference lists of included articles and relevant reviews to identify any additional eligible studies.
Eligibility criteria
We included RCTs and prospective observational studies investigating EFTP application in treating GERD among adult patients. Eligible studies were required to report outcomes such as symptom relief, medication use, esophageal pH, and/or adverse events (AEs). Conference abstracts, reviews, case reports, retrospective studies, and studies with insufficient data were excluded.
Inclusion criteria encompassed patients aged 18 years or older, with a history of at least one typical reflux symptom (e.g., heartburn, regurgitation, epigastric pain), treated with EFTP procedure.
Exclusion criteria were as follows: American Society of Anesthesiologist physical status >II; pregnancy; severe esophagitis (Los Angeles grade D); esophageal stricture; Barrett's esophagus; hiatal hernia greater than 3 cm; and Hill’s II classification.
Data extraction
Two independent reviewers (JZ, CN) screened the titles and abstracts of the identified articles. Full-text articles were assessed for eligibility based on the predefined inclusion and exclusion criteria. Any disagreements were resolved by consensus or by consulting a third reviewer. Data extraction was performed independently by two reviewers (JZ, CN) using a standardized data extraction form. Extracted data included study characteristics (e.g., authors, year of publication, study design), patient demographics, EFTP procedure details, and outcome measures. Any discrepancies in data extraction were resolved through discussion or by involving a third reviewer (CI).
Quality assessment
Two independent reviewers (JZ, CN) assessed the risk of bias in the included RCTs using the Cochrane Risk of Bias tool and in observational studies using the Newcastle-Ottawa Scale 21 22 . Any disagreements in the quality assessment were resolved by consensus or by consulting a third reviewer.
Outcomes assessment
The primary outcomes focused on ≥50% improvement in GERD health-related quality of life (GERD-HRQL) score, PPI cessation rate, and the requirement for laparoscopic fundoplication post-EFTP. Secondary outcomes involved changes in GERD-HRQL score, DeMeester score, esophageal acid exposure time (average percentage of time with pH <4 over 24 hours), total reflux events, Gastrointestinal Quality of Life Index (GIQLI), overall procedure duration, and severe procedure-associated AEs.
Definitions
The GERD-HRQL score is derived from patient responses to a 16-item Likert-type GERD-HRQL questionnaire 23 . Individual responses range from 0 (absence of symptoms) to 5 (severe symptoms) for each question. The total score ranges from 0 to 75 points, with higher scores signifying a worse GERD symptom. The GIQLI is a survey divided into five categories and 36 items: gastrointestinal symptoms (0–76 points), physical functionality (0–28 points), emotional well-being (0–20 points), social engagement (0–16 points), and a single item assessing the stress of medical treatment (0–4 points) 24 . Total reflux episodes identified by impedance were categorized as acidic (pH <4) based on pH monitoring 25 . Severe AEs are defined as incidents necessitating hospitalization, an emergency procedure, early termination of the procedure, bleeding that requires blood transfusion, perforation, or death.
Statistical analysis
We conducted a meta-analysis using a random-effects model to pool the data from the included studies, as this model accounts for both within- and between-study variability. For dichotomous outcomes, we calculated pooled odds ratios (ORs) and their corresponding 95% confidence intervals (Cis), while for continuous outcomes, we calculated weighted mean differences (WMDs) with 95% CIs. We assessed heterogeneity between studies using the I² statistic, with I² ≥ 50% indicating substantial heterogeneity. In cases of substantial heterogeneity, we explored potential sources by conducting subgroup and sensitivity analyses based on factors such as sample size and procedure device type. All statistical analyses were performed using Stata software, version 17.0 (StataCorp LLC). We considered P <0.05 to indicate statistical significance for all tests, except for Egger's test, where P <0.10 was used.
Publication bias
Due to the small sample size of the outcomes, which did not include 10 or more studies, publication bias was assessed using funnel plots and Egger's test. If the P value for the Egger’s test had been <0.10, it would have indicated the possibility of bias.
Sensitivity analysis
To assess the robustness of the results, a “leave-one-out” sensitivity analysis was performed. Each study was individually excluded and the meta-analysis was recalculated to observe its influence on the overall results. This approach helped identify any single study's disproportionate impact and confirmed the stability and consistency of our conclusions.
Results
Search results and characteristics of included studies
The initial literature search identified 1,847 articles across various databases. After thoroughly screening titles and abstracts, 324 articles were considered potentially eligible. A subsequent full-text review led to the inclusion of 13 studies in our systematic review and meta-analysis 17 18 19 20 26 27 28 29 30 31 32 33 34 . These studies consisted of four RCTs and nine prospective cohort studies, totaling 429 patients who underwent EFTP and 146 patients who underwent sham surgery for GERD treatment. We would like to underscore those studies, specifically those conducted by Renteln D (2008 and 2009), and Pleskow D (2004 and 2005), employed the same patient population 28 29 . Despite this shared population, these studies presented different outcomes that were consistent with our inclusion criteria. Consequently, we elected to include both studies in our analysis. For sections that reported overlapping outcomes, we chose to incorporate the 6-month follow-up data from the Renteln 2008 study, as well as the Pleskow 6-month follow-up data, into our overall analysis 28 30 . In addition, we applied different follow-up periods for primary outcomes when applicable, which included 3 months, 6 months, and 12 months. The flowchart and characteristics of the included studies can be found in Supplementary Fig. 1 and Table 1 , respectively. The quality assessment for the involved articles is demonstrated in Table 2 and Table 3 .
Table 1 Baseline characteristics.
| Author | Publication year | Country | Design | Follow-up months | Device | Refractory GERD on PPI | No. patients | Female (N/%) | Age | ||||||
| EFTP | Sham | EFTP | Sham | EFTP | Sham | ||||||||||
| EFTP, endoscopic full-thickness plication; PPI, proton pump inhibitor; N/A, not applicable; RCT, randomized controlled trial. | |||||||||||||||
| Kalapala R | 2022 | India | RCT | 12 | GERDx | Yes | 35 | 35 | 10/28.6 | 10/28.6 | 35 (29–41) | 37 (29–45) | |||
| Weitzendorfer M | 2018 | Germany | Prospective | 3 | GERDx | Yes | 40 | N/A | 22/55 | N/A | 49.75 (13.8) | N/A | |||
| Maydeo A | 2023 | India | RCT | 6 | GERDx | Yes | 30 | 30 | 13/43.3 | 14/46.7 | 40.5 (33.7–45) | 40 (35.7–45.2) | |||
| Antoniou S | 2011 | Australia | RCT | 12 | NDO | 28/29on PPI | 29 | N/A | N/A | N/A | 46.5 | N/A | |||
| Jeansonne L | 2009 | US | Prospective | 6 | NDO | 55/58 on PPI | 58 | 68 | 48/82.8 | N/A | 41.75 (15.2) | N/A | |||
| Rothstein R | 2006 | US | RCT | 3 | NDO | Yes | 78 | 81 | 46/59.0 | 38/46.9 | 48.1 (13.1) | 46.3 (13.8) | |||
| Pleskow D | 2004 | US | prospective | 6 | NDO single plication | Yes | 64 | 3F | N/A | N/A | 46.28 (13.27) | N/A | |||
| Pleskow D | 2005 | US | Prospective | 12 | NDO | Yes | 64 | N/A | 33/51.6 | N/A | 46.28 (13.27) | N/A | |||
| Renteln D | 2008 | Germany | Prospective | 6 | NDO | Yes | 41 | N/A | 17/41.5 | N/A | 49.8 (10.23) | N/A | |||
| Renteln D | 2009 | Germany | Prospective | 12 | NDO | Yes | 41 | N/A | 17/41.5 | N/A | 49.8 (10.23) | N/A | |||
| Koch O | 2013 | Australia | Prospective | 12 | NDO | Yes | 36 | N/A | 9/25 | N/A | 46.5 (12.8) | N/A | |||
| Chuttani R | 2003 | USA | Prospective | 6 | NDO | Yes | 6 | N/A | N/A | N/A | 31 (8) | N/A | |||
| Renteln D | 2009 | Germany | Prospective | 6 | NDO | Yes | 12 | N/A | 6/50 | N/A | 47 (9.02) | N/A | |||
| Author | Publication year | ≥50% improvement in GERD-HRQL scores | GERD-HRQL scores | PPI cessation after EFTP | Necessity for laparoscopic fundoplication | Severe adverse effect | Total reflux episodes | Percentage of time the pH <4 (%) | |||||||
| Pre-EFTP | Post-EFTP | Pre-EFTP | Post-EFTP | Pre-EFTP | Post-EFTP | ||||||||||
| Kalapala R | 2022 | 23/35 | 40 (23–51) | 92.3% reduction | 22/35 | 0 | 0 | 90(65–115) | 54.5(33–100) | 4.4(2–8.7) | 3.4 (0.6–5.5) | ||||
| Weitzendorfer M | 2018 | 30/40 | 49.84 (24.83) | 23.93 (15.63) | 19/30 | 7/40 | 4 | 148.42(108.91) | 69.59(63.87) | N/A | N/A | ||||
| Maydeo A | 2023 | 16/29 | 11 (9–12) | N/A | 20/29 | 0 | 0 | 148(120.2–178.2) | 45(24–80) | 28.6(24.6–35.6) | 4.7 (3.4–6.5) | ||||
| Antoniou S | 2011 | N/A | N/A | N/A | 25/29 | N/A | N/A | N/A | N/A | ||||||
| Jeansonne L | 2009 | N/A | N/A | N/A | 12/23 | 3/58 | N/A | N/A | N/A | 10 | 6.1 | ||||
| Rothstein R | 2006 | 44/78 | 25.7 (7.1) | 12.5 (11.1) | 39/78 | 1/78 | 4 | 168(108–236) | 124(89–193) | 10 | 7 | ||||
| Pleskow D | 2004 | 41/64 | 20.2 (6.91) | 8.2 (7.67) | 40/53 | N/A | 4 | 159.0 (87.71) | 122.9(78.79) | 12.4(7.81) | 9.3 (6.82) | ||||
| Pleskow D | 2005 | N/A | 20.1 (6.81) | 9.8 (9.21) | 36/53 | N/A | 0 | 128(47–462) | 100 (18–402) | 10 | 8 | ||||
| Renteln D | 2008 | 30/40 | 26.0 (7.1) | 8 (7.84) | 28/40 | N/A | N/A | N/A | N/A | 11 | 9 | ||||
| Renteln D | 2009 | N/A | 25.4 (6.9) | 7.8 (7.5) | 24/35 | 2/41 | 2 | 202.2(109.39) | 172.4(144.20) | N/A | N/A | ||||
| Koch O | 2013 | N/A | N/A | N/A | 13/26 | 3/36 | 1 | 78.1(42.71) | 41.29(23.01) | N/A | N/A | ||||
| Chuttani R | 2003 | N/A | 18.8 (5.9) | 4.6 (5.6) | 3/5 | 1/6 | 0 | N/A | N/A | N/A | N/A | ||||
| Renteln D | 2009 | N/A | N/A | N/A | N/A | N/A | 1 | 118(76) | 63(22) | 6.8 | 3.4 | ||||
Table 2 Quality assessment.
| Newcastle-Ottawa Quality Assessment scale for Cohort Studies | |||||||||
| Author | Selection | Comparability | Outcome | Total | |||||
| Representativeness of exposed cohort | Selection of non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohort on the basis of the design or analysis | Ascertainment of Outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow-up cohorts | ||
| Weitzendorfer M 2018 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Jeansonne L 2009 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Koch O 2013 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Renteln D 2008 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Renteln D 2009 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Renteln D2009 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Pleskow D 2004 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Pleskow D 2005 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Chuttani R 2003 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
Table 3 The Cochrane Risk of Bias Tool for RCTs.
| Author | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias | |
| Random sequence | Allocation concealment | Blinding of participants and personnel | Blinging of outcome assessment | Incomplete outcome data | Selective reporting | ||
| Kalapala R 2022 | Low risk | Low risk | High risk (Investigator who performed procedure was not blinded) | Low risk (investigator who followed patients and patients were blinded) | Low risk | Low risk | Low risk |
| Maydeo A 2023 | Low risk | Low risk | High risk | Low risk (assessor blinded) | Low risk | Low risk | Low risk |
| Antoniou S 2011 | Low risk | Low risk | High risk | High risk | Low risk | Low risk | Low risk |
| Rothstein R 2006 | Low risk | Low risk | High risk | High risk | Low risk | Low risk | Low risk |
Meta-analysis outcomes
Primary outcomes
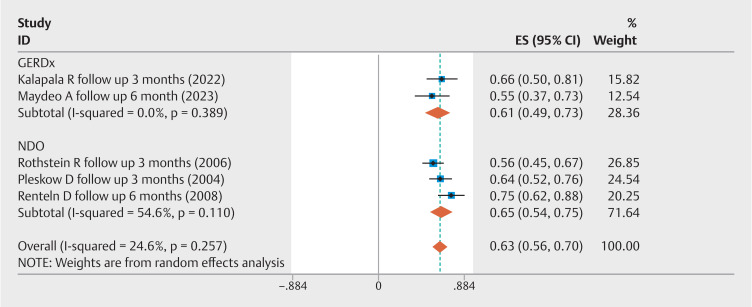
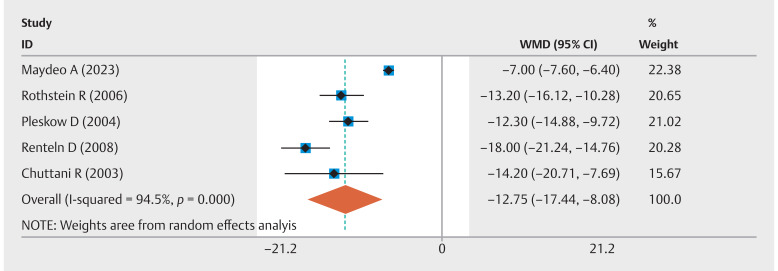
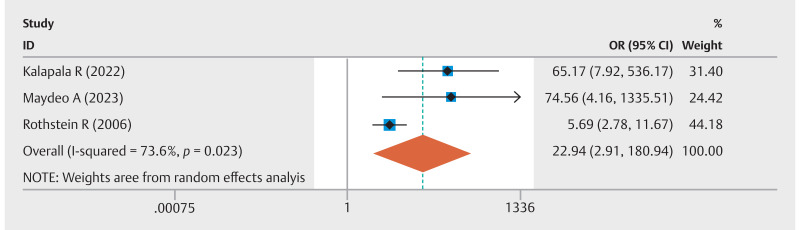
Five studies analyzed the rate of ≥ 50% improvement in GERD-HRQL scores. The combined event rate was 63% (95% CI 0.56–0.70; I 2 =24.6%) ( Fig. 1 ). In addition, five studies compared pre-EFTP and post-EFTP GERD-HRQL scores, revealing a significant improvement with an overall pooled WMD of –12.75 (95% CI –17.44 to –8.07; I 2 =94.5%) ( Fig. 2 ). Three studies compared EFTP with sham procedures, with a pooled OR of 22.94 (95% CI 2.91–180.94; I 2 =73.6%) for achieving at least a 50% improvement in GERD-HRQL scores ( Fig. 3 ). Subgroup analyses were performed by device type and study methodology. In the device-based cohorts, GERDx (n=2 studies) exhibited an event rate of 61% (95% CI 0.49–0.73; I 2 =0%) for achieving at least a 50% enhancement in GERD-HRQL scores, whereas the NDO cohort (n=3 studies) demonstrated a comparable rate of 65% (95% CI 0.54–0.75; I 2 =54.6%). Methodologically, prospective observational studies (n=2) yielded an event rate of 69% (95% CI 0.58–0.80; I 2 =30.7%), in contrast to RCTs (n=3), which reported a rate of 59% (95% CI 0.51–0.67; I 2 =0%) ( Supplementary Fig. 2 ).
Fig. 1.
Forest plot of the proportion of patients with a ≥ 50% improvement in GERD-HRQL scores following EFTP.
Fig. 2.
Forest plot of the change in GERD-HRQL scores following EFTP.
Fig. 3.
Forest plot of pooled odds ratio of the rate of GERD-HRQL scores in EFTP group compared to sham procedure group.
Two studies had a 3-month follow-up, four had a 6-month follow-up, and two had a 12-month follow-up. The results are as follows.
In the studies with 3-month follow-up, tThe pooled event rate for at least a 50% improvement in GERD-HRQL scores was 61% (95% CI 0.54–0.68; I 2 =0%) ( Supplementary Fig. 3 ). The pooled WMD in GERD-HRQL scores after EFTP showed a significant improvement of –12.69 (95% CI –14.63 to –10.76; I 2 =0%) ( Supplementary Fig. 4 ).
In the studies with 6-month follow-up, the pooled event rate for at least a 50% improvement in GERD-HRQL scores was 66% (95% CI 0.56–0.76; I 2 =37.6%) ( Supplementary Fig. 5 ). The pooled WMD in GERD-HRQL scores after EFTP showed a significant improvement of –12.58 (95% CI –18.10 to –7.06; I 2 =94.9%) ( Supplementary Fig. 6 ).
Only Rentelen D et al. (2009) and Pleskow D (2005) reported a 12-month follow-up for at least a 50% improvement in GERD-HRQL scores. The pooled WMD in GERD-HRQL scores after EFTP showed a significant improvement of –13.93 (95% CI –21.09 to –6.78; I 2 =90.9%) ( Supplementary Fig. 7 ).
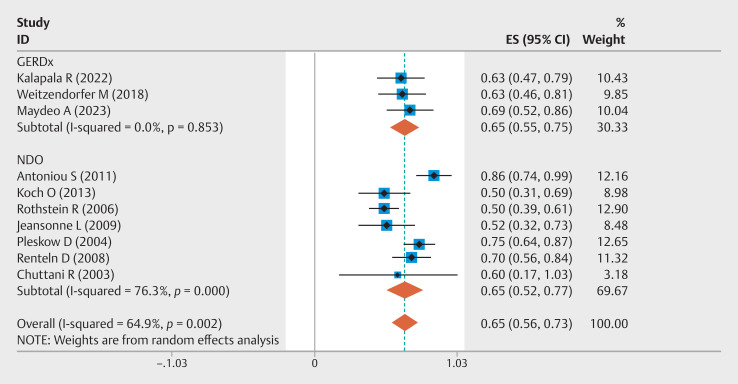
Ten studies reported the PPI cessation rate after EFTP. The overall event rate was 65% (95% CI 0.56–0.73, I²=64.9%) ( Fig. 4 ). Three studies compared EFTP with sham procedures, resulting in a pooled OR of 5.83 (95% CI 2.54–13.38; I 2 =51.4%) for PPI cessation rate ( Supplementary Fig. 8 ). Subgroup analyses delineated by device type and study design revealed distinct patterns. In the device-based classification, the GERDx group (n=3 studies) and the NDO group (n=7 studies) both demonstrated a PPI cessation rate of 65%, with CIs of 0.55–0.75 (I²=0%) and 0.52–0.77 (I²=76.3%), respectively. Methodological stratification showed that RCTs (RCTs, n=4) had a PPI cessation rate of 67% (95% CI 0.50–0.84, I²=83.5%), whereas prospective observational studies (n=6) reported a rate of 64% (95% CI 0.56–0.73, I²=33.0%) ( Supplementary Fig. 9 ). Three studies had a 3-month follow-up, four had a 6-month follow-up, and three had a 12-month follow-up, with overall PPI cessation event rates of 59% (95% CI 0.47–0.71, I²=49.5%), 68% (95% CI 0.58–0.78, I²=24.6%), and 67% (95% CI 0.46–0.89, I²=82.1%), respectively ( Supplementary Fig. 10 , Supplementary Fig. 11 , Supplementary Fig. 12 ). Eight studies reported the necessity for laparoscopic fundoplication, with an overall event rate of 7% (95% CI 0.02–0.13, I²=58.8%) ( Supplementary Fig. 13 ).
Fig. 4.
Forest plot of PPI cessation rates following EFTP.
Secondary outcomes
Six studies reported the procedure time. The average procedure time was 22.75 minutes (95% CI 22.03–23.48), with significant heterogeneity among the studies (97.1%, P < 0.0001) ( Supplementary Fig. 14 ). The overall WMD of the percentage of time with pH < 4 was –6.76% (95% CI –14.53 to 1.02) ( Supplementary Fig. 15 ). Seven studies reported a decrease in the DeMeester score following EFTP, with a WMD of –16.44 (95% CI –29.73 to –3.15, I 2 =98.8%). ( Supplementary Fig. 16 ) Subgroup analysis was performed based on device type. Two studies utilized the GERDx and three studies utilized the NDO. The overall event rate for DeMeester score in GERDx was –22.32 (95% CI –52.58 to 7.94, I 2= 99.5%), while the NDO group had a rate of –9.36 (95% CI –11.07 to 7.65, I 2 =0%). Two studies had a 3-month follow-up, four had a 6-month follow-up, and three had a 12-month follow-up. At 3, 6, and 12-month follow-up, the DeMeester score decreased following EFTP, with WMDs of –12.98 (95% CI –37.98 to 12.02, I 2 =93.9%), –19.67 (95% CI –40.25 to 0.92, I 2 =99.2%), and –5.72 (95% CI –19.43 to 7.99, I 2 =94.4%), respectively ( Supplementary Fig. 17 , Supplementary Fig. 18 , Supplementary Fig. 19 ).
Two studies reported improvements in the GIQLI following EFTP, with an WMD of 20.55 (95% CI 14.72–26.39, I 2 =0%) ( Supplementary Fig. 20 ). A reduction in total reflux episodes was reported in eight studies after EFTP, with a WMD of –51.36 (95% CI, –78.12 to –24.60, I²=98.8%) ( Supplementary Fig. 21 ). We performed a subgroup analysis based on the device type, with two studies utilizing GERDx and three studies using the NDO. The overall event rate for total reflux episodes in the GERDx group was –68.18 (95% CI, –131.41 to –4.96, I²=99.3%), while the NDO group had a rate of –42.87 (95% CI, –48.38 to –37.36, I²=0%). Four studies had a 3-month follow-up, two had a 6-month follow-up, and four had a 12-month follow-up. At 3-, 6-, and 12-month follow-ups, the total reflux rate decreased following EFTP with WMDs of –61.52 (95% CI –100.25 to –22.79, I²=99.0%), –41.66 (95% CI –65.93 to –17.38, I²=0%), and –35.39 (95% CI –41.60 to –29.18, I²=0%), respectively ( Supplementary Fig. 22 , Supplementary Fig. 23 , Supplementary Fig. 24 ). The overall procedure-related severe adverse effects rate was 5% (95% CI, 0.03- 0.08, I 2 =0%) ( Supplementary Fig. 25 ).
Validation of meta-analysis results
Sensitivity analysis
We conducted sensitivity analyses to assess the robustness of our findings. These analyses involved removing individual studies one by one and reanalyzing the data, and the results remained consistent regardless of which studies were included or excluded. The sensitivity analysis for the primary outcome of ≥ 50% improvement in GERD-HRQL scores and PPI cessation rate after EFTP was shown in Supplementary Fig. 26 and Supplementary Fig. 27 .
Heterogeneity
All outcomes exhibiting substantial heterogeneity were further examined. We discovered that for the results concerning the percentage of time with pH <4, eliminating the study by Maydeo A et al. reduced heterogeneity from 99.7% to 62.1%, and the result became significant WMD –1.97 (95%CI –2.94 to –1.01). The result of leave-one-out sensitivity analysis was shown in Supplementary Fig. 29 . Because Maydeo's study was the only one that performed EFTP after the failure of peroral endoscopic myotomy procedure, it is reasonable that its removal would lead to a decrease in heterogeneity and a significant result 18 . In addition, we conducted subgroup analyses for outcomes with higher heterogeneity, and found that heterogeneity within the same subgroups dramatically decreased.
The heterogeneity observed in the DeMeester score for GERDx devices is primarily attributed to the variations in the baseline scores and outcomes reported across the different studies included in our analysis. Notably, the study by Kalapala R et al. on PPI-dependent GERD patients using GERDx demonstrated significant variability in baseline DeMeester scores 17 . This suggests a diverse range of GERD severity within the study population, contributing to the heterogeneity observed in our analysis. In contrast, studies using the NDO system showed more homogeneity, likely due to more consistent patient profiles and study methodologies, leading to less variability in outcomes. Similarly, the high heterogeneity in GIQLI scores for GERDx could be attributed to the same factors affecting the DeMeester score.
Publication bias
In assessing publication bias, we analyzed the funnel plots for our primary outcomes: the ≥ 50% improvement in GERD-HRQL scores and PPI cessation rate post-EFTP, as illustrated in Supplementary Fig. 29 and Supplementary Fig. 30 . These funnel plots demonstrated symmetry. In addition, Egger's test results for these outcomes were 0.865 and 0.528, respectively. Together, the symmetry of the funnel plots and the Egger's test P values clearly indicate an absence of evidence for publication bias in our study.
Discussion
Our systematic review and meta-analysis of 13 prospective studies reveal substantial symptom amelioration post-EFTP treatment, with over half the patients (63%) demonstrating a ≥ 50% improvement in their GERD-HRQL scores. A significant reduction in PPI dependency was noted, with an overall cessation event rate of 65%. Furthermore, EFTP was associated with a reduction in the percentage of time with esophageal pH below 4, alongside a substantial decrease in DeMeester scores, showcasing an average WMD of –16.44. The procedure also led to fewer total reflux episodes, with a notable reduction in reflux rates across different follow-up intervals.
The average procedure time for EFTP was found to be 22.75 minutes, suggesting a relatively brief duration of the procedure. The safety profile of EFTP was favorable, with a low rate of severe AEs reported. Although previous studies have reported positive outcomes, our analysis is the first to systematically assess the available data on EFTP for GERD. Our results contribute to the existing literature and suggest that EFTP could be a viable alternative for patients who have not had adequate symptom relief with conventional therapies.
Our study highlights that EFTP considerably reduces PPI dependence in GERD patients, with 59%, 68%, and 67% of patients ceasing PPI usage at 3, 6, and 12-month follow-up intervals, respectively. This clinical relevance is important, because many patients face insufficient symptom control or complications from long-term PPI use, such as increased infection susceptibility, secondary hypergastrinemia, impaired micronutrient absorption, and osteoporosis 35 . In addition, 61% of patients at 3 months and 66% at 6 months experienced a minimum of 50% improvement in GERD-HRQL scores, indicating that EFTP effectively alleviates common symptoms like heartburn, regurgitation, and dysphagia. We also observed a significant decrease in GERD-HRQL scores following EFTP. Moreover, there was a substantial enhancement in the rate of at least 50% improvement in GERD-HRQL scores and PPI cessation rate after EFTP treatment when compared to the sham procedure group.
Following EFTP, we observed a 6.76% reduction in the percentage of time with esophageal pH < 4, although this difference was not statistically significant. However, after conducting a sensitivity analysis and removing the study by Maydeo A et al., the result became statistically significant 18 . Maydeo A et al. was the only study that applied EFTP in treating GERD patients after the peroral endoscopic myotomy procedure. Therefore, it can be concluded that EFTP effectively reduces the percentage of time with esophageal pH <4 in patients without a history of esophageal procedures. Furthermore, we noted a reduction in both the DeMeester score and the total number of reflux episodes after EFTP. This could be due to EFTP capacity to create a mechanical barrier at the gastroesophageal junction, enhance lower esophageal sphincter pressure, and potentially decrease transient LES relaxations, ultimately reducing gastric reflux into the esophagus 18 19 20 26 .
Our analysis demonstrated that EFTP has a favorable safety profile with a low rate of severe AEs. Although we observed a high rate of minor AEs, such as hoarseness, chest pain, dysphagia, and abdominal pain, these were predominantly mild and self-limiting 17 18 19 20 26 27 28 29 30 31 32 33 34 . In our assessment, we identified two cases of perforation, one Mallory-Weiss lesion, two significant bleeding events, one pneumomediastinum, three pneumoperitoneum, and one loosened needle bracket during the procedure, but no deaths related to these incidents. Weitzendorfer M et al., the first study to use the GERDx device, reported a high rate of severe AEs, with four patients suffering severe AEs 19 . In the same study, seven of 40 patients needed follow-up laparoscopic fundoplication within a 3-month period. However, later studies by Kalapala R et al. and Maydeo A et al. did not report the need for subsequent surgery or severe AEs 17 18 . This could be due to the initial development stage of the GERDx device and the improvement of surgical skills over time, resulting in a reduced need for additional surgery and a decrease in severe procedure-related AEs.
The EFTP procedures utilizing the GERDx system and the NDO Plicator share similarities, as both are performed under general anesthesia and involve a standard upper endoscopy, Savary-guidewire placement, and the introduction of the EFTP device into the stomach 17 18 19 20 26 27 28 29 30 31 32 33 34 . Both devices deploy pre-tied transmural pledgeted sutures to create a tissue valve at the lower esophageal sphincter around the endoscope. Our study's subgroup analyses suggest that both the GERDx system and the NDO Plicator are effective and safe in the treatment of GERD. Although the NDO Plicator is not commercially available, conducting a meta-analysis combining the NDO Plicator and GERDx is still valuable, given their shared mechanism and similar procedural processes.
Furthermore, the average procedure time for EFTP was 22.75 minutes. A meta-analysis revealed that the procedure time for transoral incisionless fundoplication (TIF) ranges from 33.4 to 100 minutes 36 . A shorter operation time could be a valuable feature of this innovative EFTP device, as it reflects the technical simplicity of the procedure. Future research should also compare EFTP with other minimally invasive GERD treatment options like TIF 37 . Studies directly comparing the efficacy, safety, and cost-effectiveness of these interventions will be essential for guiding clinical decision-making and optimizing GERD management.
In addition to our current findings, it is important to address the inherent challenges in long-term monitoring of EFTP effectiveness for GERD treatment. Long-term follow-up is essential to fully understand the durability of therapeutic effects and to identify potential late-onset complications or symptom recurrence 28 29 . However, acquiring such data is often complicated by issues like patient attrition, inconsistent follow-up protocols, and the continuous evolution of endoscopic techniques. To enhance the quality and reliability of long-term data, we suggest adopting standardized follow-up protocols across different studies, which would aid in comparison and analysis. Furthermore, establishing a comprehensive patient registry and conducting post-market surveillance could offer valuable insights into the real-world, long-term outcomes of EFTP. Encouraging longitudinal studies with extended monitoring periods can also provide crucial information about the long-term impact of this treatment, thereby guiding clinical decisions and improving patient care.
A potential limitation of this meta-analysis is the heterogeneity among included studies; despite no significant differences in primary outcomes from subgroup and sensitivity analyses, it may affect applicability across diverse patient populations and clinical settings. Moreover, our study encountered limitations in obtaining long-term data concerning EFTP durability as a GERD treatment. Previous long-term follow-up studies on surgical treatments for GERD have indicated that a significant percentage of patients experienced relapse after 5 years 38 . Concurrently, research has shown that the average GERD-HRQL score decreased substantially after 1 year following the TIF procedure for GERD treatment 39 40 . Pleskow D et al. (2008), however, revealed that EFTP can reduce GERD symptoms and medication use for at least 5 years post-procedure with no long-term procedure-related AEs 41 . It is still essential to examine long-term outcomes after performing EFTP with GERDx (more than 1 year). An additional limitation is that not all participating patients were undergoing long-term PPI therapy and unresponsive to this treatment before the procedure. However, the majority of patients were indeed resistant to long-term PPI treatment, with only a few patients in the Antonious et al., Jeansonne et al., and Pleskow D et al. studies not taking PPIs daily 20 26 27 28 .
Conclusions
In conclusion, our systematic review and meta-analysis demonstrate that EFTP techniques are safe and effective in the treatment of GERD. EFTP techniques are safe and effective in the treatment of GERD, in a 12-month follow-up. However, further research is essential to compare EFTP with other minimally invasive GERD treatments like TIF. Such comparative studies should evaluate not only efficacy in symptom relief but also differences in procedure techniques, recovery times, and safety profiles. This research will help determine the most suitable treatment options for individual patient profiles and inform clinical decisions. In addition, assessing the cost-effectiveness of these procedures is crucial, particularly in optimizing patient care and resource allocation in healthcare settings.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Supplementary Material
References
- 1.Zhang D, Liu S, Li Z et al. Global, regional and national burden of gastroesophageal reflux disease, 1990–2019: update from the GBD 2019 study. Ann Med. 2022;54:1372–1384. doi: 10.1080/07853890.2022.2074535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richter JE, Rubenstein JH. Presentation and epidemiology of gastroesophageal reflux disease. Gastroenterology. 2018;154:267–276. doi: 10.1053/j.gastro.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P, Shaheen NJ, Katzka D et al. AGA clinical practice update on endoscopic treatment of Barrett’s esophagus with dysplasia and/or early cancer: expert review. Gastroenterology. 2020;158:760–769. doi: 10.1053/j.gastro.2021.06.078. [DOI] [PubMed] [Google Scholar]
- 4.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308–328. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 5.Mehta RS, Staller K, Chan AT. Review of gastroesophageal reflux disease. JAMA. 2021;325:1472. doi: 10.1001/jama.2021.1438. [DOI] [PubMed] [Google Scholar]
- 6.Ness-Jensen E, Hveem K, El-Serag H et al. Lifestyle intervention in gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2016;14:175–182. doi: 10.1016/j.cgh.2015.04.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan BA, Sodhi JS, Zargar SA et al. Effect of bed head elevation during sleep in symptomatic patients of nocturnal gastroesophageal reflux. J Gastroenterol Hepatol. 2012;27:1078–1082. doi: 10.1111/j.1440-1746.2011.06968.x. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita Y, Ishimura N, Ishihara S. Advantages and disadvantages of long-term proton pump inhibitor use. J Neurogastroenterol Motil. 2018;24:182–196. doi: 10.5056/jnm18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma P, Shaheen NJ, Perez MC et al. Clinical trials: healing of erosive oesophagitis with dexlansoprazole MR, a proton pump inhibitor with a novel dual delayed-release formulation-results from two randomized controlled studies. Aliment Pharmacol Ther. 2009;29:731–741. doi: 10.1111/j.1365-2036.2009.03933.x. [DOI] [PubMed] [Google Scholar]
- 10.Sigterman KE, van Pinxteren B, Bonis PA et al. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2013;2013(05):CD002095. doi: 10.1002/14651858.CD002095.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKinley SK, Dirks RC, Walsh D et al. Surgical treatment of GERD: systematic review and meta-analysis. Surg Endosc. 2021;35:4095–4123. doi: 10.1007/s00464-021-08358-5. [DOI] [PubMed] [Google Scholar]
- 12.Lundell L, Bell M, Ruth M. Systematic review: laparoscopic fundoplication for gastroesophageal reflux disease in partial responders to proton pump inhibitors. World J Gastroenterol. 2014;20:804–813. doi: 10.3748/wjg.v20.i3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadlapati R, Hungness ES, Pandolfino JE. Complications of antireflux surgery. Am J Gastroenterol. 2018;113:1137–1147. doi: 10.1038/s41395-018-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuribayashi S, Hosaka H, Nakamura F et al. The role of endoscopy in the management of gastroesophageal reflux disease. DEN Open. 2021;2:e86. doi: 10.1002/deo2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DP, Chang KJ. EndoscopicmManagement of GERD. Dig Dis Sci. 2022;67:1455–1468. doi: 10.1007/s10620-022-07390-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslam N, Telese A, Sehgal V et al. Minimally invasive endoscopic therapies for gastro-oesophageal reflux disease. Frontline Gastroenterol. 2023;14:249–257. doi: 10.1136/flgastro-2022-102343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalapala R, Karyampudi A, Nabi Z et al. Endoscopic full-thickness plication for the treatment of PPI-dependent GERD: Results from a randomised, sham controlled trial. Gut. 2022;71:686–694. doi: 10.1136/gutjnl-2020-321811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maydeo A, Patil G, Kamat N et al. Endoscopic full-thickness plication for the treatment of gastroesophageal reflux after peroral endoscopic myotomy: a randomized sham-controlled study. Endoscopy. 2023 doi: 10.1055/a-2040-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weitzendorfer M, Spaun GO, Antoniou SA et al. Clinical feasibility of a new full-thickness endoscopic plication device (GERDx) for patients with GERD: results of a prospective trial. Surg Endosc. 2018;32:2541–2549. doi: 10.1007/s00464-018-6153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antoniou SA, Koch OO, Kaindlstorfer A et al. Endoscopic full-thickness plication versus laparoscopic fundoplication: a prospective study on quality of life and symptom control. Surg Endosc. 2012;26:1063–1068. doi: 10.1007/s00464-011-1999-0. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Altman DG, Gøtzsche PC et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 23.Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus. 2007;20:130–134. doi: 10.1111/j.1442-2050.2007.00658.x. [DOI] [PubMed] [Google Scholar]
- 24.Sailer M, Bussen D, Debus ES et al. Quality of life in patients with benign anorectal disorders. Br J Surg. 1998;85:1716–1719. doi: 10.1046/j.1365-2168.1998.00958.x. [DOI] [PubMed] [Google Scholar]
- 25.Uno K, Iijima K, Hatta W et al. Direct measurement of gastroesophageal reflux episodes in patients with squamous cell carcinoma by 24-h pH-impedance monitoring. Am J Gastroenterol. 2011;106:1923–1929. doi: 10.1038/ajg.2011.282. [DOI] [PubMed] [Google Scholar]
- 26.Jeansonne LO, White BC, Nguyen V et al. Endoluminal full-thickness plication and radiofrequency treatments for GERD: An outcomes comparison. Arch Surg. 2009;144:19–24. doi: 10.1001/archsurg.144.1.19. [DOI] [PubMed] [Google Scholar]
- 27.Rothstein R, Filipi C, Caca K et al. Endoscopic full-thickness plication for the treatment of gastroesophageal reflux disease: a randomized, sham-controlled trial. Gastroenterology. 2006;131:704–712. doi: 10.1053/j.gastro.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Pleskow D, Rothstein R, Lo S et al. Endoscopic full-thickness plication for the treatment of GERD: a multicenter trial. Gastrointest Endosc. 2004;59:163–171. doi: 10.1016/s0016-5107(03)02542-2. [DOI] [PubMed] [Google Scholar]
- 29.Pleskow D, Rothstein R, Lo S et al. Endoscopic full-thickness plication for the treatment of GERD: 12-month follow-up for the North American open-label trial. Gastrointest Endosc. 2005;61:643–649. doi: 10.1016/s0016-5107(04)02648-3. [DOI] [PubMed] [Google Scholar]
- 30.von Renteln D, Schiefke I, Fuchs KH et al. Endoscopic full-thickness plication for the treatment of GERD by application of multiple Plicator implants: a multicenter study (with video) Gastrointest Endosc. 2008;68:833–844. doi: 10.1016/j.gie.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Von Renteln D, Schiefke I, Fuchs KH et al. Endoscopic full-thickness plication for the treatment of gastroesophageal reflux disease using multiple Plicator implants: 12-month multicenter study results. Surg Endosc. 2009;23:1866–1875. doi: 10.1007/s00464-009-0490-7. [DOI] [PubMed] [Google Scholar]
- 32.Koch OO, Kaindlstorfer A, Antoniou SA et al. Subjective and objective data on esophageal manometry and impedance pH monitoring 1 year after endoscopic full-thickness plication for the treatment of GERD by using multiple plication implants. Gastrointest Endosc. 2013;77:7–14. doi: 10.1016/j.gie.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 33.von Renteln D, Schmidt A, Riecken B et al. Evaluating outcomes of endoscopic full-thickness plication for gastroesophageal reflux disease (GERD) with impedance monitoring. Surg Endosc. 2010;24:1040–1048. doi: 10.1007/s00464-009-0723-9. [DOI] [PubMed] [Google Scholar]
- 34.Chuttani R. Endoscopic full-thickness plication: the device, technique, pre-clinical and early clinical experience. Gastrointest Endosc Clin N Am. 2003;13:109–116. doi: 10.1016/s1052-5157(02)00109-5. [DOI] [PubMed] [Google Scholar]
- 35.Haastrup PF, Thompson W, Søndergaard J et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123:114–121. doi: 10.1111/bcpt.13023. [DOI] [PubMed] [Google Scholar]
- 36.McCarty TR, Itidiare M, Njei B et al. Efficacy of transoral incisionless fundoplication for refractory gastroesophageal reflux disease: a systematic review and meta-analysis. Endoscopy. 2018;50:708–725. doi: 10.1055/a-0576-6589. [DOI] [PubMed] [Google Scholar]
- 37.Haseeb M, Brown JRG, Hayat U et al. Impact of second-generation transoral incisionless fundoplication on atypical GERD symptoms: A systematic review and meta-analysis. Gastrointest Endosc. 2023;97:394–406. doi: 10.1016/j.gie.2022.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundell L, Bell M, Ruth M. Systematic review: laparoscopic fundoplication for gastroesophageal reflux disease in partial responders to proton pump inhibitors. World J Gastroenterol. 2014;20:804–813. doi: 10.3748/wjg.v20.i3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witteman BPL, Conchillo JM, Rinsma NF et al. Randomized controlled trial of transoral incisionless fundoplication vs. proton pump inhibitors for treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2015;110:531–542. doi: 10.1038/ajg.2015.28. [DOI] [PubMed] [Google Scholar]
- 40.Testoni PA, Testoni S, Mazzoleni G et al. Long-term efficacy of transoral incisionless fundoplication with Esophyx (Tif 2.0) and factors affecting outcomes in GERD patients followed for up to 6 years: a prospective single-center study. Surg Endosc. 2015;29:2770–2780. doi: 10.1007/s00464-014-4008-6. [DOI] [PubMed] [Google Scholar]
- 41.Pleskow D, Rothstein R, Kozarek R et al. Endoscopic full-thickness plication for the treatment of GERD: five-year long-term multicenter results. Surg Endosc. 2008;22:326–332. doi: 10.1007/s00464-007-9667-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.