Abstract
In support of the Integrated Risk Information System (IRIS), the U.S. Environmental Protection Agency (EPA) completed an evaluation of the inhalation carcinogenicity of ethylene oxide (EtO) in December 2016. This article reviews key findings and scientific issues regarding the carcinogenicity of EtO in EPA’s Carcinogenicity Assessment. EPA’s assessment critically reviewed and characterized epidemiologic, laboratory animal, and mechanistic studies pertaining to the human carcinogenicity of EtO, and addressed some key scientific issues such as the analysis of mechanistic data as part of the cancer hazard evaluation and to inform the quantitative risk assessment. The weight of evidence from the epidemiologic, laboratory animal, and mechanistic studies supports a conclusion that EtO is carcinogenic in humans, with the strongest human evidence linking EtO exposure to lymphoid and breast cancers. Analyses of the mechanistic data establish a key role for genotoxicity and mutagenicity in EtO-induced carcinogenicity and reveal little evidence supporting other mode-of-action hypotheses. In conclusion, EtO was found to be carcinogenic to humans by inhalation, posing a potential human health hazard for lymphoid and breast cancers.
Keywords: Assessment, Cancer/tumors, Breast Cancer, Epidemiology, Review, Integrated Risk Information System, Mode of Action, Ethylene Oxide, Mutagenesis, Genotoxicity
Introduction
Ethylene oxide (C2H4O; EtO) is a gas at room temperature. It is derived from ethylene and used primarily as a chemical intermediate in the production of ethylene glycol (for the manufacture of antifreeze and polyester fibers). EtO is also used in the derivation of numerous other chemicals (e.g., various ethoxylation products, polyethylene glycols, glycol ethers, and ethanolamines), which are employed in the production of a variety of industrial and consumer products (e.g., surfactants, detergents, solvents, plasticizers, lubricants, personal care products, and pharmaceuticals) (IARC 2008). A small proportion (<1%) of EtO is used for the sterilization of medical equipment, spices, and other items.
Occupational exposures can occur in worksites that produce or use EtO, including sterilization facilities and hospitals. Similarly, the main environmental sources of EtO are chemical plants, commercial sterilization operations, and medical facilities. Total releases to the environment reported to the U.S. Environmental Protection Agency’s (EPA’s) Toxics Release Inventory have declined from over 4 million pounds in 1988 to about 0.3 million pounds in 2015 (U.S. EPA 2017). EtO in the atmosphere degrades primarily by reaction with hydroxyl radicals, with a half-life on the order of months. Based on EPA’s 2005 National-scale Air Toxics Assessment data, the average environmental exposure concentration of EtO in the United States from all sources (including concentrations near known sources) is 0.0062 μg/m3; the average background concentration excluding concentrations near known sources of EtO is 0.0044 μg/m3 (http://www.epa.gov/ttn/atw/nata2005/tables.html).
From a regulatory perspective, EPA has an interest in air concentrations of EtO because EtO is one of the 188 hazardous air pollutants listed in the 1990 Clean Air Act Amendments. In addition, EPA’s Office of Pesticide Programs has an interest in both environmental and occupational exposures resulting from the sterilization and fumigation uses of EtO because the EPA is responsible for pesticide labeling and registration decisions under the Federal Insecticide, Fungicide, and Rodenticide Act.
EPA’s Integrated Risk Information System (IRIS) Program released a Carcinogenicity Assessment of EtO in December 2016 (U.S. EPA 2016a, b). Because EtO is a gas at room temperature and inhalation is the primary route of human exposure, the assessment focused only on inhalation exposure. The Carcinogenicity Assessment presents conclusions on the carcinogenic hazard and mode of action (MOA) of EtO, as well as a unit risk estimate (i.e., a plausible upper bound on the estimate of extra cancer risk per μg EtO/m3 air breathed) for lifetime environmental exposures, and estimates of extra cancer risk for a range of occupational exposure scenarios. The assessment was developed over the span of more than 10 years and underwent many stages of both internal and external peer review as well as public comment. EPA’s final EtO assessment incorporated input from two independent peer reviews by EPA’s Science Advisory Board (SAB) (SAB 2007, 2015), other federal agencies, and the public.
This article describes key findings and scientific issues addressed in EPA’s 2016 Carcinogenicity Assessment of EtO, covering the following topics: (1) characterization of EtO carcinogenicity, based on the weight of the evidence from epidemiological, laboratory animal, and mechanistic studies; (2) MOA analysis, including the support for a mutagenic MOA and the lack of support for other proposed MOAs; and (3) consideration of mechanistic information in view of deriving quantitative risk estimates for low exposures. For more details on the topics discussed in this article and for findings and issues related to other topics (e.g., the exposure-response modeling of the lymphoid cancer and the breast cancer data), readers are referred to the relevant chapters and appendices of EPA’s Carcinogenicity Assessment of EtO (U.S. EPA 2016a, b).
Discussion
Absorption, Distribution, Metabolism, and Excretion
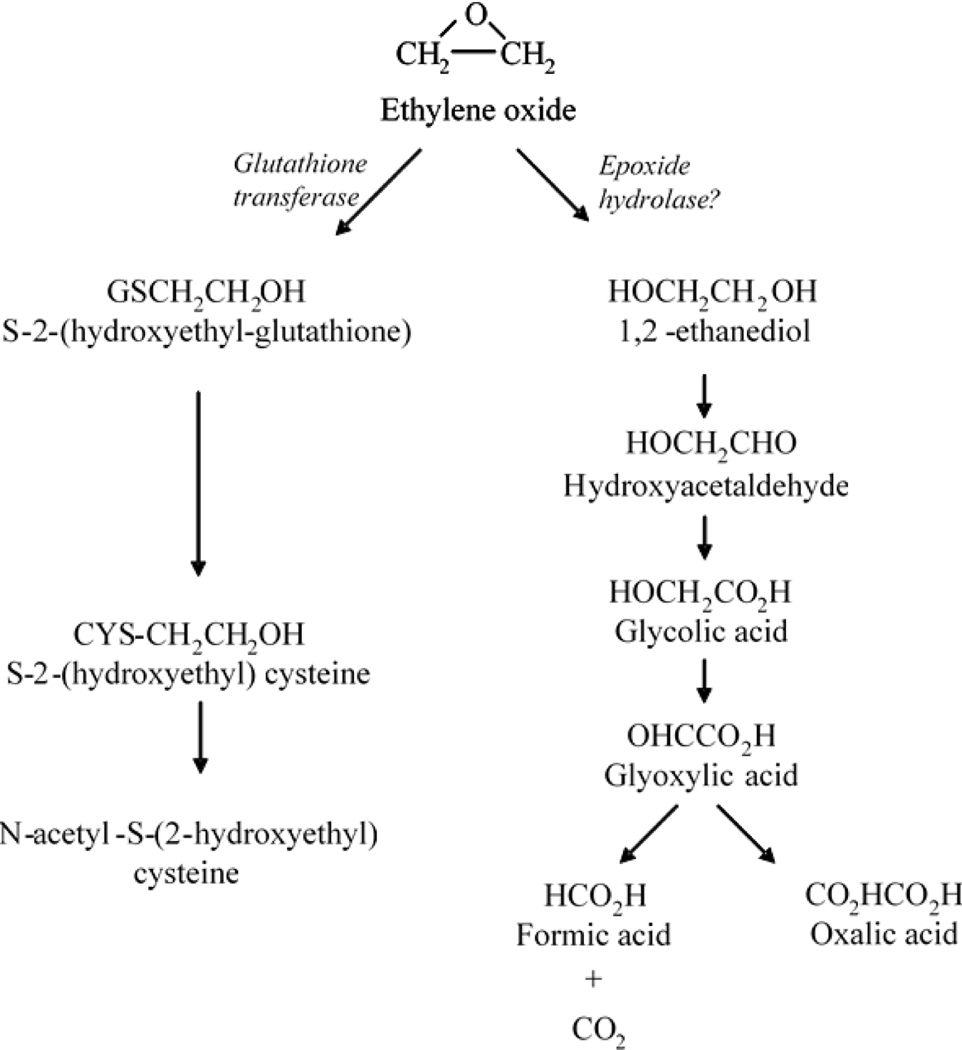
Following inhalation, EtO is readily absorbed into the blood and rapidly distributed throughout the body. As illustrated in Figure 1, EtO is metabolized primarily by two pathways: glutathione conjugation, which predominates in mice and, to a lesser extent, in rats; and hydrolysis, which is the predominant pathway in humans (Fennell and Brown 2001). Because EtO is an epoxide capable of reacting directly with cellular macromolecules, both pathways are considered to be detoxifying. Available studies suggest that tissue concentrations in mice, rats, and humans exposed to a particular air concentration of EtO are approximately equal and that they are linearly related to inhalation concentration, at least in the range of exposures used in the rodent cancer bioassays (i.e., 100 ppm and below) (Brown et al. 1996; Fennell and Brown 2001).
Figure 1.
Metabolism of ethylene oxide. Major routes of mammalian metabolism involve either glutathione conjugation facilitated by glutathione-S-transferase, or oxidation, potentially via epoxide hydrolase (see U.S. EPA, 2016a; U.S. EPA, 2016b).
Carcinogenicity in Rodent Bioassays
Three chronic inhalation cancer bioassays have been conducted – one in B6C3F1 mice (NTP 1987), one in male F344 rats (Lynch et al. 1984), and one in male and female F344 rats (Garman et al. 1985, 1986; Snellings et al. 1984); their results are summarized in Table 1. In mice, findings included concentration-dependent increases in incidence of malignant lymphomas and adenocarcinomas in the mammary glands and the uterus in females and of lung neoplasms in both sexes. In both rat studies, concentration-dependent increases in the incidence of splenic mononuclear cell leukemia (a type of lymphoid cancer), brain tumors, and peritoneal mesothelioma in the testes were observed in male rats, and in the study that included female rats, increases in the former two cancer types were also observed in females.
Table 1.
Summary of tumor incidence findings in rodent bioassays
| Gender/tumor type | EtO concentration (time-weighted average)a | |||
|---|---|---|---|---|
| NTP (1987) 2-year bioassay of B6C3F1 miceb,c | ||||
| 0 ppm | 50 ppm (16.3 mg/m3) | 100 ppm (32.7 mg/m3) | ||
| Males | ||||
| Lung adenomas plus carcinomas | 11/49 | 19/49 | 26/49* | |
| Females | ||||
| Lung adenomas plus carcinomas | 2/44 | 5/44 | 22/49* | |
| Malignant lymphoma | 9/44 | 6/44 | 22/49* | |
| Uterine carcinoma | 0/44 | 1/44 | 5/49j | |
| Mammary carcinoma | 1/44 | 8/44* | 6/49 | |
| Lynch et al. (1984) 2-year bioassay of male F344 ratsd,e | ||||
| 0 ppm | 50 ppm (19.1 mg/m3) | 100 ppm (38.1 mg/m3) | ||
| Splenic mononuclear cell leukemia | 24/77 | 38/79* | 30/76 | |
| Testicular peritoneal mesothelioma | 3/78 | 9/79 | 21/79* | |
| Brain mixed-cell glioma | 0/76 | 2/77 | 5/79* | |
| (Garman et al. (1985); Snellings et al. (1984)) 2-year bioassay of F344 ratsa,f,g | ||||
| 0 ppmh | 10 ppm (3.27 mg/m3) | 33 ppm (10.8 mg/m3) | 100 ppm (32.7 mg/m3) | |
| Males | ||||
| Splenic mononuclear cell leukemia | 13/97 (13%)i | 9/51 (18%) | 12/39* (32%) | 9/30* (30%) |
| Testicular peritoneal mesothelioma | 2/97 (2.1%) | 2/51 (3.9%) | 4/39 (10%) | 4/30* (13%) |
| Primary brain tumors | 1/181 (0.55%) | 1/92 (1.1%) | 5/85* (5.9%) | 7/87* (8.1%) |
| Females | ||||
| Splenic mononuclear cell leukemia | 11/116 (9.5%) | 11/54* (21%) | 14/48* (30%) | 15/26* (58%) |
| Primary brain tumors | 1/188 (0.53%) | 1/94 (1.1%) | 3/92 (3.3%) | 4/80* (5%) |
Adjusted to continuous exposure; 1 ppm = 1.83 mg/m3.
Exposed 6 hr/d, 5 d/wk.
Incidence data were adjusted by the EPA by eliminating the animals that died prior to the occurrence of the first tumor or prior to 52 wk, whichever was earlier. No treatment-related effects on survival or body weight were observed.
Exposed 7 hr/d, 5 d/wk.
Mean body weights were statistically significantly decreased in both treated groups compared with controls, and increased mortality was observed in the treated groups, with the increase statistically significant in the 100-ppm exposure group (p < 0.01). The individual animal data for this study were not available.
Significant decreases in mean body weight were observed in the 100-ppm exposure group in males and in the 100-ppm and 33-ppm exposure groups in females. Mortality was statistically significantly increased in the 100-ppm exposure groups of both sexes.
Denominators refer to the number of animals for which histopathological diagnosis was performed. For brain tumors, Garman et al. (1985) included animals in the 18-month and the 24-month kills and found dead or euthanized moribund of those alive at the time of the first brain tumor, whereas for the other sites, Snellings et al. (1984) included animals only at the 24-month kill.
Results for two control groups combined.
Numbers in parentheses indicate percentage incidence values.
p = 0.058 by pairwise Fisher’s exact test compared to concurrent controls; however, uterine carcinomas are rare tumors in female B6C3F1 mice, and p < 0.0001 by pairwise Fisher’s exact test compared to the NTP historical control incidence of 1/1,077 for inhalation (air) for female B6C3F1 mice fed with the NIH-07 diet.
p < 0.05 by pairwise Fisher’s exact test.
Carcinogenicity in Human Studies
The strongest evidence for associations between EtO exposure and cancer in humans is for lymphohematopoietic cancers in both sexes and for breast cancer in females, similar to some of the findings in rodents.
Numerous epidemiological studies of the carcinogenic effects of EtO in occupational cohorts have been conducted, and these studies were critically reviewed by the EPA in its Carcinogenicity Assessment of EtO (Section 3.1 and Appendix A of U.S. EPA 2016a, b). The studies evaluate 11 independent study populations, some with multiple follow-ups or analyses. These study populations spanned 7 different countries and several types of operations involving EtO – either work in chemical-manufacturing facilities that produced or used EtO, which may have also involved exposure to other chemicals, and/or work in sterilization operations, either commercial or in hospitals, which were generally free of exposure to other chemicals. Five of the study populations were men only, 5 were a mix of men and women, and 1 small study was of women only. The largest study population by far was a National Institute for Occupational Safety and Health (NIOSH) cohort of over 18,000 workers. The 10 remaining study populations ranged in size from 299 to 2,876 workers.
The NIOSH studies (latest mortality follow-up by Steenland et al. 2004; breast cancer incidence study by Steenland et al. 2003) are of note because of their large cohort, with individual worker exposure estimates from a high-quality exposure assessment, allowing for exposure-response modeling, and other attributes. The NIOSH cohort includes 18,235 workers (45% male and 55% female) in 14 commercial sterilization plants. Individual exposure estimates were derived for the 17,530 workers from 13 of the plants using a regression model developed by NIOSH from available data on EtO measurements in commercial sterilization facilities (Greife et al. 1988; Hornung et al. 1994). The model incorporates a variety of plant and production variables to allow estimation of exposure levels for time periods, facilities, and operations for which industrial hygiene data were unavailable. Other strengths of the NIOSH studies include the cohort study design, sufficient follow-up time, inclusion of males and females in the cohort, absence of any known confounding exposures, and use of internal comparisons for the estimation of relative risks. The breast cancer incidence study, involving a subcohort of female workers with interviews, had the additional attribute of having investigated and controlled for several breast cancer risk factors, such as parity (Steenland et al. 2003).
The largest epidemiologic database is for cancers of the lymphohematopoietic system. Increases in the risk of lymphohematopoietic cancer, sometimes reported as increases in leukemia or lymphoid cancer subtypes, were present in most (9 of 11) of the study populations. The few studies that failed to observe any increased risks of lymphohematopoietic cancer had study limitations, such as small numbers of cases or inadequate exposure information, that undermined confidence in the apparent lack of an effect. The evidence of lymphohematopoietic cancer was strongest in the large, high-quality NIOSH study (Steenland et al. 2004). In this study, statistically significant exposure-response relationships with cumulative exposure to EtO were seen, particularly for lymphoid cancers (n = 53 deaths). However, the magnitude of the effect was not large (relative risk [RR] estimates of about 3). In addition, Steenland et al. (2004) found stronger exposure-response trends in males than in females, although the sex difference was not statistically significant (Appendix D of U.S. EPA 2016b). In most of the other studies, the increased risks were similarly modest in magnitude, and in some of the workplaces, other chemicals cannot be ruled out as possible confounders. Thus, although strong and consistent overall, the epidemiological evidence alone was not considered sufficient to conclusively establish causality for the association between EtO exposure and lymphohematopoietic cancer in humans.
Fewer data sets are available on EtO exposure and breast cancer in females because only six of the study populations included females. All of the women were sterilizer workers. The largest study of women, the NIOSH study, reported statistically significant exposure-response relationships for breast cancer incidence (Steenland et al. 2003; n = 319 cases, 233 in the subcohort with interviews) and mortality (Steenland et al. 2004; n = 103 deaths). A more recent follow-up of the next largest study with women (Mikoczy et al. 2011; n = 41 breast cancer cases) reported statistically significant increases in the incidence rate ratio in the two highest cumulative exposure quartiles relative to the lowest 50% of exposures, adding important corroborating support to the findings of the NIOSH study. The four remaining studies of women had small numbers of subjects and/or short follow-up times, yielding few breast cancer events, the next highest number being 12 deaths. Nonetheless, two of these underpowered studies were also supportive of an increased risk of breast cancer in the sterilizer workers (Kardos et al. 2003; Norman et al. 1995). Overall, although the database is limited, the available human evidence for a causal association between EtO exposure and breast cancer is strong and consistent.
Some relevant study features and results from the epidemiological studies of EtO exposures are summarized in Supplemental Material Tables S1 (lymphohematopoietic cancers) and S2 (breast cancer in women).
Genotoxicity
EtO is a direct-acting alkylating agent that forms adducts with cellular macromolecules such as proteins (e.g., hemoglobin) and DNA (e.g., Walker et al. 1992a; 1992b; see Table 2). However, endogenous EtO, formed by the cytochrome P450-mediated conversion of ethylene, which itself is produced during a variety of normal physiological processes, also contributes significantly to hemoglobin and DNA adduct levels, making it difficult to detect the impacts of low levels of exogenous EtO exposure using those markers.
Table 2.
Weight of evidence for relevant key characteristics of carcinogens in proposed mode-of-action hypotheses for ethylene oxide carcinogenicity
| Relevant Key Characteristic of Carcinogensa | Category of Effect | Weight-of-evidence Summary: Laboratory Animals | Weight-of-evidence Summary: Humans | Summary, Evidence Integration |
|---|---|---|---|---|
| Mutagenic MOA – Sufficient Evidence | ||||
| Is Electrophilic or Can Be Metabolically Activated | Protein adductsb | In rats and mice, Hb adducts are linearly correlated with exposures up to at least 33 ppm, with increased Hb adduction at exposures ≥ 100 ppm, consistent with decreased GSH metabolism. | In humans, Hb adducts can be used as biomarkers of recent exposure to EtO, and exposure-response relationships have been reported in several studies. | EtO is a direct-acting alkylating agent. Strong and consistent evidence demonstrates protein adduction in humans and rodents. Strong and consistent evidence supports DNA adduction in rodents, along with weak but not inconsistent evidence from the small number of human DNA adduct studies. |
| DNA adductsc | In rats and mice, N7-HEG adducts increased in multiple tissues following repeated exposures to ≥ 3 ppm. In rats exposed to 300 ppm, O6-HEG and N3-HEA adducts were also detected; in mice exposed to 100 or 200 ppm, O6-HEG and N1- and N6-HEA adducts were observed and increased in an exposure-related manner. | In two human studies, N7-HEG adducts were non-significantly elevated in white blood cells. | ||
| Is Genotoxic | Point mutations in reporter genes or surrogate markersc | In rats and mice, Hprt or LacI mutation incidences were increased in several tissues and dominant lethal effects were observed in germ cells. | In one study with some of the higher and longer exposures, HPRT mutant frequency was significantly increased in human PBLs. | Strong and consistent evidence supports the induction of point mutations and chromosomal effects in rodent tissues, consistent with strong and consistent evidence of chromosomal effects in humans. Most mutations in proto-oncogenes or tumor suppressor genes in tumors of EtO-exposed mice occurred at purine nucleotides, which is consistent with the DNA adduct pattern noted in the text. |
| Point mutations in proto-oncogenes or tumor suppressor genesc | In lung and Harderian gland tumors of EtO-exposed mice, Kras mutation incidence was higher and the mutational spectra differed, compared to those tumors from control mice. In mammary gland carcinomas, the Trp53 and Hras mutational spectra were likewise different, mutations of the two genes were frequently concurrent, and Trp53 mutations and protein expression were induced in an exposure-dependent manner. | No evidence available | ||
| Chromosomal effectsd | In monkeys, rabbits, and rats, SCEs were induced in lymphohematopoietic tissues; in mice, CA incidence increased in similar tissues, as well as in germ cells. | CAs and SCEs were elevated in human PBLs, particularly in populations with the highest and/or longest exposures. | ||
| Oxidative Stress MOA – Insufficient Evidence | ||||
| Induces Oxidative Stress | ROS or lipid peroxidation products | No evidence available | No evidence available | There is no evidence of DNA or GSH oxidation following subchronic EtO exposure. No direct measures of ROS were reported, and only limited evidence supports increased levels of an oxidized lipid-DNA adduct (CrotondG). |
| DNA oxidatione | In mice, lung levels of CrotondG increased in an exposure-related manner with subchronic exposure ≤ 200 ppm, while 8-OHdG levels were unaffected. | In human lung epithelial cells, keratinocytes and PBLs exposed in vitro, oxidative DNA damage (Fpg-dependent comet assay) was not increased. | ||
| Glutathione species levelsf | In mice, lung levels of both GSH and GSSG decreased in an exposure-related manner with subchronic exposures ≤ 200 ppm, while levels of EtO-conjugated glutathione (HESG) increased. The GSH:GSSG ratio and total glutathione content were not affected. | No evidence available | ||
Hb = hemoglobin; PBL = peripheral blood lymphocyte; CA = chromosomal aberration; Fpg = formamidopyrimidine DNA-glycosylase.
Evidence for key characteristics of carcinogens was evaluated, as described by Smith et al. (2016), in the context of proposed MOA hypotheses. Beyond the three characteristics summarized herein, there was no evidence from the identified human or laboratory animal data to support EtO altering cell proliferation, cell death or nutrient supply, and there was insufficient evidence available to evaluate support for the remaining six key characteristics.
Protein adducts summarized from discussion in Section 3.3.2 of U.S. EPA (2016a).
DNA adducts and point mutations adapted from Table 3–6 in Section 3.3.3 of U.S. EPA (2016a).
Chromosomal effects adapted from Tables 3–7 and 3–8 in Section 3.3.3.3 of U.S. EPA (2016a).
DNA oxidation in human cells was reported by Nagy et al. (2013) and in laboratory animals by Zhang et al. (2015b), adapted from Sections J.3.2 and J.4.1 and Table J-7 of Appendix J in U.S. EPA (2016b).
Glutathione species levels in laboratory animals were reported by Zhang et al. (2015a), adapted from Section J.4.1 and Table J-6 in U.S. EPA (2016b).
The predominant DNA adduct formed by EtO is N7-(2-hydroxyethyl)guanine (N7-HEG). O6-hydroxyethylguanine (O6-HEG) and N1-, N3- and N6-(2-hydroxyethyl)adenine (N1, N3, or N6-HEA, respectively) have also been identified, but in much lower amounts. Recently, using sensitive LC/pESI/MS-MS techniques, Zhang et al. (2015b) observed quantifiable levels of O6-HEG in all 5 samples from the lungs of male mice following 12 weeks of exposure to 100 ppm EtO, a concentration used in the cancer bioassays, as well as in 3 of 5 samples from control mice, while N1- and N6-HEA were only detectable in EtO-exposed groups. Although the N7-HEG adducts are abundant, their mutagenic potential may be minimal; conversely, O6-HEG and the adenine adducts, though rare, likely have a greater mutagenic potential (Tompkins et al. 2008). At present, both the identity of the responsible adduct(s) and the mechanism(s) by which such DNA adducts may be responsible for EtO-induced mutations are unknown (some possibilities are discussed in Section 3.4.1.1 of U.S. EPA 2016a).
Of note is an EtO-DNA adduct rat study by Marsden et al. (2009) that used sensitive detection techniques and an approach designed to separately quantify both endogenous N7-HEG adducts and exogenous N7-HEG adducts induced by EtO treatment (LC-MS/MS combined with [HPLC-]AMS analysis), for a range of intraperitoneal doses all below the lowest-observed-adverse-effect levels (LOAELs) from the EtO cancer bioassays. These investigators reported increases in exogenous adducts in DNA of spleen and liver tissue at the lowest dose administered and statistically significant linear dose-response relationships for exogenous adducts in all three tissues examined (spleen, liver, and stomach), although some of the adduct levels induced at low EtO concentrations were below the limit of accurate quantitation (Marsden et al. 2009).
Numerous studies have reported positive genotoxic activity by EtO in a wide range of biological systems (e.g., see summaries in IARC 2008, U.S. EPA 2016a, b). In mammalian (including human) cells, genotoxic effects induced by in vitro and/or in vivo exposures include unscheduled DNA synthesis, gene mutations, sister chromatid exchanges (SCEs), and chromosomal aberrations. Increases in frequencies of gene mutations have been reported in the lung, T-lymphocytes, bone marrow, and testes of EtO-exposed rats and/or mice. In particular, two studies (Hong et al. 2007; Houle et al. 2006) investigated the mutations in specific genes (the proto-oncogenes Kras and Hras and the tumor-suppressor gene Trp53) from several tumor types in EtO-exposed mice from the National Toxicology Program (NTP) cancer bioassay (NTP 1987) and from spontaneous tumors of the same types in control mice from various NTP bioassays from the same time period, and their results suggest that EtO-induced mutations in these cancer-related genes play a role in EtO-induced carcinogenesis (see Table 2). Subchronic inhalation studies in laboratory animals have demonstrated that EtO exposure levels in the range of those used in the rodent bioassays induce SCEs in rats and chromosomal aberrations in mice (these different endpoints have been examined to different extents in the two rodent species; see Section 3.3.3.3 of U.S. EPA 2016a for more discussion).
There are few studies of point mutations in EtO-exposed humans and the available studies are insensitive; thus, the evidence for this endpoint is limited. However, there is clear evidence from multiple studies of EtO-exposed workers that EtO causes chromosomal aberrations, SCEs, and micronuclei in peripheral blood lymphocytes (e.g., Tates et al. 1991), and one study has reported increased levels of micronuclei in bone marrow cells (Högstedt et al. 1983). Chromosomal aberrations (e.g., Boffetta et al. 2007; Hagmar et al. 2004) and, to a lesser extent, micronucleus frequency (Bonassi et al. 2007) have been linked to an increased risk of cancer in several large prospective general population studies.
Mode-of-Action Considerations
After a comprehensive analysis of the mechanistic data and consideration of MOA hypotheses, the EPA concluded that EtO-induced carcinogenicity has a mutagenic MOA and that there is little evidence for other potential MOAs (Section 3.4 of U.S. EPA 2016a). The analysis reviewed possible mechanisms by which EtO might be inducing carcinogenesis (both in general and specifically for lymphohematopoietic and breast cancers), and evaluated the evidence regarding a mutagenic MOA under the MOA framework in the EPA’s Guidelines for Carcinogen Risk Assessment (U.S. EPA 2005a). Mechanistic information regarding hypothesized MOAs is summarized in Table 2, organized around the key characteristics of carcinogens proposed by Smith et al. (2016).
Although the precise mechanisms by which EtO exposure induces multisite carcinogenicity in mice, rats, and humans are unknown, EtO clearly causes genotoxicity and mutagenicity, and these are well-established factors in carcinogenesis. As described above, EtO directly interacts with DNA, causing concentration- and duration-dependent increases in DNA adducts, genetic mutations, and chromosome damage in various rodent tissues and human peripheral blood cells. Moreover, EtO-induced genotoxicity is observed after shorter exposure durations and at lower exposure concentrations than those associated with tumor induction in both rodents and occupationally exposed humans. The weight of evidence is strongly supportive of a mutagenic MOA involving gene mutations and/or chromosomal aberrations (translocations, deletions, or inversions) that critically alter the function of oncogenes or tumor suppressor genes. This mutagenic MOA is presumed to apply to all tumor types associated with EtO exposure.
Furthermore, there are no other compelling MOAs proposed for EtO carcinogenicity. For example, there is no evidence of cytotoxicity or other cellular dysfunction indicative of regenerative proliferation or some other toxicity-related MOA. Oxidative stress has been hypothesized as a MOA (Parsons et al. 2013), but EPA found insufficient evidence supporting this hypothesis and concluded that the role of oxidative stress in EtO-induced carcinogenicity is speculative at this time (Table 2 and U.S. EPA 2016a, b).
Parsons et al. (2013) investigated oxidative stress as a MOA for EtO carcinogenicity by exposing Big Blue B6C3F1 mice to various concentrations (up to 200 ppm) of EtO by inhalation for various durations (up to 12 weeks) and analyzing the levels of three specific Kras codon-12 mutations in lung DNA samples. Parsons et al. (2013) posited that because the majority of the codon-12 mutations in the lung cancers from EtO-exposed mice evaluated by Hong et al. (2007) were GGT→GTT mutations and because 8-hydroxy-2′-deoxyguanosine (8-OHdG) adducts may preferentially cause G:C→T:A mutations, an early increase in GGT→GTT (and/or GGT→TGT) mutations relative to GGT→GAT mutations would support the hypothesis that EtO causes oxidative stress in the mouse lung, resulting in the formation of 8-OHdG adducts. Their findings, however, did not conform with this hypothesis, and so they revised their hypothesis, speculating that EtO induces reactive oxygen species (ROS) that modulate the clonal growth of pre-existing Kras mutations, although ROS levels were not measured. (No studies directly evaluating ROS levels following EtO exposure were identified.)
The EPA found the data and hypotheses of Parsons et al. (2013) to be unconvincing for a number of reasons (Section J.3.2 of Appendix J of U.S. EPA, 2016b). For example, the high degree of variability in most of the exposure group mutation frequencies and the instability of the control results across different exposure durations suggest that the assay results might be unreliable. The EPA also notes that G:C→T:A mutations are not just markers of oxidative stress; a variety of mutagens are known to cause these mutations (DeMarini 2000). Moreover, other recent studies report no increases in more direct markers of oxidative stress (Nagy et al. 2013; Zhang et al. 2015a; 2015b; see Table 2). Nagy et al. (2013) exposed different human cell types to EtO in vitro and investigated their relative susceptibility to different types of DNA damage. These investigators reported that lung epithelial cells were relatively sensitive to the DNA alkylating effects of EtO yet relatively resistant to oxidative DNA damage. In fact, EtO did not induce oxidative DNA damage in any cell type.
Zhang et al. (2015a) exposed male B6C3F1 mice to various concentrations (up to 200 ppm) of EtO for 4 weeks and then measured the lung levels of glutathione species (reduced glutathione [GSH], oxidized glutathione [GSSG], and 2-hydroxyethylated glutathione [HESG], resulting from EtO alkylation of GSH). Lung GSH and GSSG levels decreased in an exposure-related manner, while HESG levels, unquantifiable in control lungs, increased. Overall, levels of non-oxidized glutathione (GSH + HESG) and of total glutathione (GSH + GSSG + HESG), as well as the ratio of GSH:GSSG, remained largely unchanged. These findings suggest that the lung GSH depletion resulted from direct EtO alkylation to HESG, and not oxidation to GSSG. In male mice similarly exposed for 12 weeks, Zhang et al. (2015b) used sensitive measurement techniques to evaluate lung levels of the direct purine alkylation adducts O6-HEG, N1-HEA, and N6-HEA, as well as levels of guanine adducts likely to result from ROS activity either directly (8-OHdG) or indirectly following lipid peroxidation (N2-propano-2′-deoxyguanosine [CrotondG]). As discussed above, EtO exposure increased levels of O6-HEG, N1-HEA, and N6-HEA in mouse lung. In contrast, 8-OHdG levels were not increased by EtO exposure, and CrotondG levels were statistically significantly increased at 200 ppm exposure by about 60%, which is much lower than the threefold or greater increases observed for O6-HEG, N1-HEA, and N6-HEA.
Although neither ROS nor oxidized lipids were measured directly, the lack of decrease in the ratio of GSH:GSSG (Zhang et al. 2015a) or increase in 8-OHdG levels (Zhang et al. 2015b), both of which are routinely evaluated as markers of cellular oxidative stress, coupled with the limited increase in CrotondG adducts possibly formed following lipid peroxidation, indicated to the EPA that oxidative stress is not appreciably induced in the lungs of mice following 4–12 weeks of exposure up to 200 ppm EtO. Furthermore, the significant exposure-related increases in O6-HEG and other potentially mutagenic purine adducts (e.g., N1-HEA and N6-HEA) observed by (Zhang et al. 2015b) is consistent with the general preference for mutations involving purine nucleotides in proto-oncogenes in lung and other tumors from EtO-exposed mice (Hong et al. 2007; Houle et al. 2006). This supports the conclusion that EtO-induced tumors arise via a mutagenic MOA following the direct formation of mutagenic EtO-DNA adducts.
As the U.S. EPA (2016a) concluded, although oxidative stress or other processes might contribute to the development of EtO-induced cancers, the available evidence provides sufficient support for a primarily mutagenic MOA in EtO carcinogenicity. The conclusion that a mutagenic MOA is instrumental in EtO carcinogenesis has multiple implications in the EPA’s Carcinogenicity Assessment. First, it bolsters the cancer hazard conclusion (see below). Second, it supports the use of linear low-exposure extrapolation in the quantitative cancer risk assessment (U.S. EPA 2005a, 2016a). Finally, it provides the basis for an assumption of increased early-life susceptibility to EtO exposure and the recommendation to apply age-dependent adjustment factors to the unit risk estimate to account for this increased susceptibility (U.S. EPA 2005b, 2016a).
The EPA also evaluated some mechanism-related proposals that claimed that a linear low-exposure extrapolation should not be used, mutagenic MOA notwithstanding. For example, a mechanistically motivated exposure-response modeling approach for leukemia based solely upon chromosomal aberrations, as the presumed initiating events in acute leukemia, has been proposed whereby EtO must induce two nearly simultaneous DNA adducts, resulting in a dose-squared (quadratic) relationship between EtO exposure and leukemia risk (Kirman et al. 2004). However, the EPA (U.S. EPA 2016a) concluded that chromosomal aberrations need not represent the sole initiating event and noted that there is evidence that genetic mutations are involved in certain types of leukemia (U.S. EPA 1997, 2016a). In addition, in the large NIOSH study, lymphoid cancer, not acute leukemia, was the lymphohematopoietic cancer subtype most strongly associated with EtO exposure (Steenland et al. 2004). Furthermore, even if two reactions with DNA resulting in chromosomal aberrations are early-occurring events in some EtO-induced lymphohematopoietic cancers, it is not necessary that both events be associated with EtO exposure (e.g., background errors in repair or exposure to other alkylating agents may contribute). Moreover, EtO could also produce translocations indirectly by forming DNA or protein adducts that affect the normally occurring recombination activities of lymphocytes or the repair of spontaneous double-strand breaks (DSBs). Lymphocytes may be more sensitive to EtO-induced DNA fragmentation than other cell types (Adám et al. 2005), especially in populations with polymorphisms in DSB-repair components (Godderis et al. 2006). In light of these broader MOA considerations, the EPA did not find sufficient support for the hypothesis that the exposure-response relationship is quadratic.
A minority opinion in the 2007 SAB review (SAB 2007) expressed another hypothesis arguing against linear low-exposure extrapolation. This opinion maintained that linear extrapolation is a conservative assumption, given EtO’s reactivity (which would diminish the amount reaching the nucleus) coupled with the fact that it is generated endogenously and the expectation that some repair occurs (SAB 2007). As discussed above, however, there is substantial evidence that EtO from both endogenous and exogenous sources reaches the nucleus and forms adducts, and more recent data from Marsden et al. (2009) specifically demonstrate (nonsignificant) increases of DNA adducts for very low doses of exogenous EtO. Any diminution of the amount of EtO reaching the nucleus is expected to affect the slope of the low-dose linear relationship but not linearity per se. In addition, the facts that endogenous EtO is present and that some repair takes place are not considered evidence against low-dose linearity because small amounts of exogenous EtO are expected to contribute to background carcinogenic processes for the common cancers, lymphoid cancer and breast cancer, associated with EtO exposure. Moreover, the data from Marsden et al. (2009), with low doses of EtO, are consistent with a linear exposure-response relationship for EtO exposure and DNA adducts. Similarly, the EPA’s analysis of the two EtO-specific mutation data sets presented in the 2007 SAB report in support of nonlinearity showed that those data are also consistent with low-dose linearity (Appendix H of U.S. EPA 2016b).
Overall, the EPA’s analysis of studies of dose-response patterns for adduct formation and mutagenesis by EtO found the data to be supportive of the inferences made in the EtO Carcinogenicity Assessment (and more broadly in the EPA’s Guidelines for Carcinogen Risk Assessment [U.S. EPA 2005a]) regarding the plausibility of linear, nonthreshold, low-dose dose-response relationships for the biological effects of EtO, which is mutagenic and directly damages DNA. The EPA further concluded that there was insufficient support to warrant also including a non-linear quantitative approach. In the second external review of the EPA’s Carcinogenicity Assessment, the SAB (2015) agreed with the EPA’s conclusion.
Synthesis of the Evidence and Cancer Hazard Characterization
The synthesis of the epidemiologic evidence for lymphohematopoietic cancer and for breast cancer in females was organized according to the key considerations proposed by Hill (1965). Temporality, coherence, biological plausibility, and analogy were readily satisfied, and consistency, biological gradient, and strength of association were satisfied to varying degrees, as summarized in Table 3. Chance, bias, and confounding were also considered in evaluating the weight of the epidemiological evidence. Given the consistency of the findings across studies, the exposure-response relationships observed in the large NIOSH study, the absence of coexposures in the sterilization workers (Steenland et al. 1991), and the inclusion of significant breast cancer risk factors in the RR models for the subcohort with interviews in the NIOSH breast cancer incidence study (Steenland et al. 2003), the EPA determined that chance, bias, or confounding were unlikely to explain the observed associations between EtO exposure and lymphohematopoietic cancer or breast cancer in females. Overall, the EPA judged the epidemiological evidence for causal associations between EtO exposure and lymphohematopoietic cancer as well as female breast cancer to be strong but less than conclusive.
Table 3.
Evaluation of the epidemiological database for EtO using the key considerations proposed by Hill (1965) for causality determination
| Consideration | Weight-of-Evidence Summary |
|---|---|
|
| |
| Temporality | • Strong evidence – the subjects of all the epidemiology studies of EtO were workers who were exposed to EtO before the cancers of interest were observed (i.e., exposure preceded the development of the disease). |
|
| |
| Consistency of observed association | • Moderate evidence of consistency for lymphohematopoietic (LHP) cancers – ○ About 9 of 11 studies reported an increased risk of LHP cancers or a subgroup thereof, although not all were statistically significant (In the large NIOSH study, the strongest evidence was for lymphoid cancers, including lymphocytic leukemia; none of the other studies considered a lymphoid category or subcategorized leukemia into its distinct myeloid and lymphocytic subtypes). ○ The studies that did not report a significant LHP cancer effect generally had major limitations, such as small numbers of cases, inadequate exposure information, and/or reliance on external analyses. • Moderate evidence of consistency for female breast cancer – ○ Statistically significant increased risks of breast cancer mortality and/or incidence in the 2 studies with the largest number of breast cancer cases. ○ Two other studies suggest an increased risk of breast cancer despite their small size. ○ No elevated risks were seen in the only other study reporting breast cancer results; however, that study had few cases (n = 11). |
|
| |
| Strength of observed association | • Strength of association is limited. For example, in the large NIOSH study, the RR estimate for lymphoid cancer mortality in the highest exposure quartile is about 3.0, and the RR estimate for breast cancer incidence in the highest exposure quintile in the subcohort with interviews is on the order of 1.9. The modest RR estimates may, in part, reflect the relatively high background rates of these cancers, particularly of breast cancer incidence. |
|
| |
| Biological gradient (exposure-response relationship) | • Limited evidence - only a few epidemiologic studies examined exposure-response relationships. ○ In the large, high-quality NIOSH study, statistically significant exposure-response relationships were observed for the risk of all LHP and for lymphoid cancers (Steenland et al. 2004). In the Swaen et al. (2009) study, no statistically significant exposure-response relationships were observed for leukemia or lymphoid cancer using a model which notably did not yield statistically significant trends in the NIOSH study either. Similarly, no exposure-response relationship was observed for LHP cancers in internal analyses in the Mikoczy et al. (2011) study, but this study was limited by a small number of cases (10 exposed cases of all LHP cancers) and the lack of a nonexposed referent group. ○ For breast cancer, statistically significant trends for both mortality and incidence were observed in the NIOSH study (Steenland et al. 2003; Steenland et al. 2004). The Mikoczy et al. (2011) study reported significant increases in the incidence rate ratios in the highest two cumulative exposure quartiles compared to the workers with cumulative exposures below the median, with the highest RR estimate for the highest exposure quartile. |
|
| |
| Biological plausibility, coherence, and analogy | • Strong evidence – ○ EtO is genotoxic and mutagenic, which are common mechanistic features of many carcinogens. ○ EtO is carcinogenic in rodents, with LHP cancers being observed in both rats and mice and mammary carcinomas being observed in female mice. ○ EtO is an epoxide, and epoxides are capable of directly interacting with DNA and are the active metabolites of many carcinogens. |
| Specificity | • Specificity is not expected for an agent like EtO, which is widely distributed across tissues and is a direct-acting, multisite mutagen. |
| Experimental evidence | • Experimental evidence is seldom available for observational studies of human populations and is not available in the case of human exposures to EtO. |
LHP = lymphohematopoietic
Regarding the laboratory animal data on EtO carcinogenicity, the EPA concluded that there was sufficient evidence that EtO causes cancer in laboratory animals based on clear findings of tumors at multiple sites and in both sexes of both rats and mice. Tumor types included mononuclear cell leukemia in male and female rats and malignant lymphoma and mammary carcinoma in female mice, suggesting some site concordance with the lymphohematopoietic and breast cancers associated with EtO exposure in humans.
Similarly, EPA found the evidence of EtO genotoxicity and mutagenicity to be unequivocal, with EtO inducing mutations or other genotoxic effects in a wide variety of in vitro and in vivo test systems and in EtO-exposed workers.
Conclusion
Integrating the different types of evidence, the EPA concluded that EtO is “carcinogenic to humans” (U.S. EPA 2016a), in accordance with the EPA’s 2005 Guidelines for Carcinogen Risk Assessment (U.S. EPA 2005a,). The cancer hazard descriptor “carcinogenic to humans” is generally used when there is convincing epidemiologic evidence of a causal association between human exposure and cancer. This descriptor is also appropriate with a lesser weight of epidemiologic evidence that is strengthened by other specific lines of evidence set forth in the Guidelines (U.S. EPA 2005a), conditions that are satisfied for EtO. The lines of evidence supporting the characterization of “carcinogenic to humans” include the following: (1) there is strong, although less than conclusive independently, evidence of cancer in humans associated with EtO exposure via inhalation, specifically, evidence of lymphohematopoietic cancers and of female breast cancer in EtO-exposed workers; (2) there is extensive evidence of EtO-induced carcinogenicity in laboratory animals, including lymphohematopoietic cancers in rats and mice and mammary carcinomas in mice following inhalation exposure; (3) EtO is a direct-acting alkylating agent whose genotoxic and mutagenic capabilities have been well established in a variety of experimental systems, and a mutagenic MOA has been identified in laboratory animals involving the key precursor events of DNA adduct formation and subsequent DNA damage, including point mutations and chromosomal effects; and (4) there is strong evidence that the key precursor events are anticipated to occur in humans and progress to tumors, including evidence of chromosome damage, such as chromosomal aberrations, SCEs, and micronuclei in EtO-exposed workers.
This hazard characterization conclusion of “carcinogenic to humans” is similar to the EtO listing of “known to be a human carcinogen” made by the NTP in its 9th Report on Carcinogens in 1999 (NTP 1999) and the evaluation of “carcinogenic to humans” reaffirmed by the International Agency for Research on Cancer (IARC) in its Monograph 100 in 2012, also relying on the genotoxicity data (IARC 2012). Characterizing and quantifying potential human health hazards following EtO exposure, such as reaching a conclusion of “carcinogenic to humans” and developing cancer risk estimates (U.S. EPA, 2016a), are early steps in the regulation of EtO exposures by EPA. EtO is pending Special Review for pesticide uses (https://www.epa.gov/pesticide-reevaluation/reregistration-and-other-review-programs-predating-pesticide-registration), and because EtO is listed as a hazardous air pollutant, facilities producing it or using it may be subject to regulation under the National Emission Standards for Hazardous Air Pollutants (NESHAP), including hospitals (https://www.epa.gov/stationary-sources-air-pollution/hospital-ethylene-oxide-sterilizers-national-emission-standards) and as well as other sterilization or fumigation facilities (https://www.epa.gov/stationary-sources-air-pollution/ethylene-oxide-emissions-standards-sterilization-facilities), and such rules are reviewed and updated on a periodic basis.
Supplementary Material
Acknowledgements:
This work has benefitted from comments from a number of scientific reviewers, including members of two EPA Science Advisory Board review panels, scientists at various federal agencies (including EPA), and scientists who prepared public comments. We also thank D. Bussard, P. White, and V. Morozov for providing EPA management support for the assessment and this manuscript and David DeMarini and Anu Mudipalli for their technical review of a draft manuscript.
List of abbreviations and acronyms:
- AMS
accelerator mass spectrometry
- CrotondG
N2-propano-2′-deoxyguanosine
- DSB
double-strand break
- EPA
Environmental Protection Agency
- EtO
ethylene oxide
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HESG
2-hydroxyethylated glutathione
- IRIS
Integrated Risk Information System
- LC/pESI/MS-MS
liquid chromatography-positive electrospray ionization tandem mass spectrometry
- N7-HEG
N7-(2-hydroxyethyl)guanine
- N1-HEA
N1-(2-hydroxyethyl)adenine
- N3-HEA
N3-(2-hydroxyethyl)adenine
- N6-HEA
N6-(2-hydroxyethyl)adenine
- MOA
mode of action
- NIOSH
National Institute for Occupational Safety and Health
- NTP
National Toxicology Program
- 8-OHdG
8-hydroxy-2´-deoxyguanosine
- O6-HEG
O6-hydroxyethylguanine
- ROS
reactive oxygen species
- RR
relative risk
- SAB
Science Advisory Board
- SCE
sister chromatid exchange
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
Disclosure of interest: The authors report no conflicts of interest.
References
- Adám B, Bárdos H, Adány R. 2005. Increased genotoxic susceptibility of breast epithelial cells to ethylene oxide. Mutat Res, 585, 120–126. [DOI] [PubMed] [Google Scholar]
- Boffetta P, van der Hel O, Norppa H, Fabianova E, Fucic A, Gundy S, et al. 2007. Chromosomal aberrations and cancer risk: Results of a cohort study from Central Europe. Am J Epidemiol, 165, 36–43. [DOI] [PubMed] [Google Scholar]
- Bonassi S, Znaor A, Ceppi M, Lando C, Chang WP, Holland N, et al. 2007. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis, 28, 625–631. [DOI] [PubMed] [Google Scholar]
- Brown CD, Wong BA, Fennell TR. 1996. In vivo and in vitro kinetics of ethylene oxide metabolism in rats and mice. Toxicol Appl Pharmacol, 136, 8–19. [DOI] [PubMed] [Google Scholar]
- DeMarini DM. 2000. Influence of DNA repair on mutation spectra in Salmonella. Mutat Res, 450, 5–17. [DOI] [PubMed] [Google Scholar]
- Fennell TR, Brown CD. 2001. A physiologically based pharmacokinetic model for ethylene oxide in mouse, rat, and human. Toxicol Appl Pharmacol, 173, 161–175. [DOI] [PubMed] [Google Scholar]
- Garman RH, Snellings WM, Maronpot RR. 1985. Brain tumors in F344 rats associated with chronic inhalation exposure to ethylene oxide. Neurotoxicology, 6, 117–137. [PubMed] [Google Scholar]
- Garman RH, Snellings WM, Maronpot RR. 1986. Frequency, size and location of brain tumours in F-344 rats chronically exposed to ethylene oxide. Food Chem Toxicol, 24, 145–153. [DOI] [PubMed] [Google Scholar]
- Godderis L, Aka P, Matecuca R, Kirsch-Volders M, Lison D, Veulemans H. 2006. Dose-dependent influence of genetic polymorphisms on DNA damage induced by styrene oxide, ethylene oxide and gamma-radiation. Toxicology, 219, 220–229. [DOI] [PubMed] [Google Scholar]
- Greife AL, Hornung RW, Stayner LG, Steenland KN. 1988. Development of a model for use in estimating exposure to ethylene oxide in a retrospective cohort mortality study. Scand J Work Environ Health, 1, 29–30. [PubMed] [Google Scholar]
- Hagmar L, Strömberg U, Bonassi S, Hansteen IL, Knudsen LE, Lindholm C, et al. 2004. Impact of types of lymphocyte chromosomal aberrations on human cancer risk: Results from Nordic and Italian cohorts. Cancer Res, 64, 2258–2263. [DOI] [PubMed] [Google Scholar]
- Hill AB. 1965. The environment and disease: Association or causation? Proc R Soc Med, 58, 295–300. [PMC free article] [PubMed] [Google Scholar]
- Högstedt B, Gullberg B, Hedner K, Kolnig A,-M, Mitelman F, Skerfving S, et al. 1983. Chromosome aberrations and micronuclei in bone marrow cells and peripheral blood lymphocytes in humans exposed to ethylene oxide. Hereditas, 98, 105–113. [DOI] [PubMed] [Google Scholar]
- Hong H,-HL, Houle CD, Ton T,-VT, Sills RC. 2007. K-ras mutations in lung tumors and tumors from other organs are consistent with a common mechanism of ethylene oxide tumorigenesis in the B6C3F1 mouse. Toxicol Pathol, 35, 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Greife AL, Stayner LT, Steenland NK, Herrick RF, Elliott LJ, et al. 1994. Statistical model for prediction of retrospective exposure to ethylene oxide in an occupational mortality study. Am J Ind Med, 25, 825–836. [DOI] [PubMed] [Google Scholar]
- Houle CD, Ton T,-VT, Clayton N, Huff J, Hong H,-HL, Sills RC. 2006. Frequent p53 and H-ras mutations in benzene- and ethylene oxide-induced mammary gland carcinomas from B6C3F1 mice. Toxicol Pathol, 34, 752–762. [DOI] [PubMed] [Google Scholar]
- IARC. 2008. Ethylene oxide. (IARC monographs on the evaluation of carcinogenic risks to humans: Volume 97, 1,3-butadiene, ethylene oxide and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide)). Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- IARC. 2012. Ethylene oxide. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 100F: Chemical agents and related occupations. Lyon, France, World Health Organization. [PMC free article] [PubMed] [Google Scholar]
- Kardos L, Széles G, Gombkötö G, Szeremi M, Tompa A, Ádány R. 2003. Cancer deaths among hospital staff potentially exposed to ethylene oxide: An epidemiological analysis. Environ Mol Mutagen, 42, 59–60. [DOI] [PubMed] [Google Scholar]
- Kirman CR, Sweeney LM, Teta MJ, Sielken RL, Valdez-Flores C, Albertini RJ, et al. 2004. Addressing nonlinearity in the exposure-response relationship for a genotoxic carcinogen: Cancer potency estimates for ethylene oxide. Risk Anal, 24, 1165–1183. [DOI] [PubMed] [Google Scholar]
- Lynch DW, Lewis TR, Moorman WJ, Burg JR, Groth DH, Khan A, et al. 1984. Carcinogenic and toxicologic effects of inhaled ethylene oxide and propylene oxide in F344 rats. Toxicol Appl Pharmacol, 76, 69–84. [DOI] [PubMed] [Google Scholar]
- Marsden DA, Jones DJ, Britton RG, Ognibene T, Ubick E, Johnson GE, et al. 2009. Dose-response relationships for N7-(2-hydroxyethyl)guanine induced by low-dose [14C]ethylene oxide: Evidence for a novel mechanism of endogenous adduct formation. Cancer Res, 69, 3052-3059. [DOI] [PubMed] [Google Scholar]
- Mikoczy Z, Tinnerberg H, Björk J, Albin M. 2011. Cancer incidence and mortality in Swedish sterilant workers exposed to ethylene oxide: Updated cohort study findings 1972–2006. Int J Environ Res Public Health, 8, 2009–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy K, Ádány R, Szűcs S, Ádám B. 2013. Susceptibility of lung epithelial cells to alkylating genotoxic insult. Environ Mol Mutagen, 54, 682–689. [DOI] [PubMed] [Google Scholar]
- Norman SA, Berlin JA, Soper KA, Middendorf BF, Stolley PD. 1995. Cancer incidence in a group of workers potentially exposed to ethylene oxide. Int J Epidemiol, 24, 276–284. [DOI] [PubMed] [Google Scholar]
- NTP. 1987. Toxicology and carcinogenesis studies of ethylene oxide (CAS no 75–21-8) in B6C3F1 mice (inhalation studies). Natl Toxicol Program Tech Rep Ser, 326, 1–114. [PubMed] [Google Scholar]
- NTP. 1999. Report on carcinogens (9th): 1999, Full report. Research Triangle Park, NC. [Google Scholar]
- Parsons BL, Manjanatha MG, Myers MB, McKim KL, Shelton SD, Wang Y, et al. 2013. Temporal changes in K-ras mutant fraction in lung tissue of big blue B6C3F mice exposed to ethylene oxide. Toxicol Sci, 136, 26–38. [DOI] [PubMed] [Google Scholar]
- SAB. 2007. Review of Office of Research and Development (ORD) draft assessment entitled “Evaluation of the carcinogenicity of ethylene oxide”. Washington, DC, U.S. Environmental Protection Agency, Science Advisory Board. [Google Scholar]
- SAB. 2015. Science Advisory Board Review of the EPAs evaluation of the inhalation carcinogenicity of ethylene oxide: Revised external review draft - August 2014. EPA-SAB-15-012. Washington, DC, U.S. Environmental Protection Agency, Science Advisory Board. [Google Scholar]
- Smith MT, Guyton KZ, Gibbons CF, Fritz JM, Portier CJ, Rusyn I, et al. 2016. Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ Health Perspect, 124, 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellings WM, Weil CS, Maronpot RR. 1984. A two-year inhalation study of the carcinogenic potential of ethylene oxide in Fischer 344 rats. Toxicol Appl Pharmacol, 75, 105–117. [DOI] [PubMed] [Google Scholar]
- Steenland K, Stayner L, Greife A, Halperin W, Hayes R, Hornung R, et al. 1991. Mortality among workers exposed to ethylene oxide. N Engl J Med, 324, 1402–1407. [DOI] [PubMed] [Google Scholar]
- Steenland K, Whelan E, Deddens J, Stayner L, Ward E. 2003. Ethylene oxide and breast cancer incidence in a cohort study of 7576 women (United States). Cancer Causes Control, 14, 531-539. [DOI] [PubMed] [Google Scholar]
- Steenland K, Stayner L, Deddens J. 2004. Mortality analyses in a cohort of 18 235 ethylene oxide exposed workers: Follow up extended from 1987 to 1998. Occup Environ Med, 61, 2–7. [PMC free article] [PubMed] [Google Scholar]
- Swaen GMH, Burns C, Teta JM, Bodner K, Keenan D, Bodnar CM. 2009. Mortality study update of ethylene oxide workers in chemical manufacturing: A 15 year update. J Occup Environ Med, 51, 714–723. [DOI] [PubMed] [Google Scholar]
- Tates AD, Grummt T, Törnqvist M, Farmer PB, van Dam FJ, van Mossel H, et al. 1991. Biological and chemical monitoring of occupational exposure to ethylene oxide. Mutat Res, 250, 483–497. [DOI] [PubMed] [Google Scholar]
- Tompkins EM, Jones DJL, Lamb JH, Marsden DA, Farmer PB, Brown K. 2008. Simultaneous detection of five different 2-hydroxyethyl-DNA adducts formed by ethylene oxide exposure, using a high-performance liquid chromatography/electrospray ionisation tandem mass spectrometry assay. Rapid Commun Mass Spectrom, 22, 19–28. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. 1997. Chemical and radiation leukemogenesis in humans and rodents and the value of rodent models for assessing risks of lymphohematopoietic cancers. EPA/600/R-97/090. Washington, DC, Office of Research and Development, National Center for Environmental Assessment. [Google Scholar]
- U.S. EPA. 2005a. Guidelines for carcinogen risk assessment. EPA/630/P-03/001F. Washington, DC, U.S. Environmental Protection Agency, Risk Assessment Forum. [Google Scholar]
- U.S. EPA. 2005b. Supplemental guidance for assessing susceptibility from early-life exposure to carcinogens. EPA/630/R-03/003F. Washington, DC, U.S. Environmental Protection Agency, Risk Assessment Forum. [Google Scholar]
- U.S. EPA. 2016a. Evaluation of the inhalation carcinogenicity of ethylene oxide (CASRN 75–218): In support of summary information on the Integrated Risk Information System (IRIS). EPA/635/R-16/350Fa. Washington, DC, U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment. [Google Scholar]
- U.S. EPA. 2016b. Evaluation of the inhalation carcinogenicity of ethylene oxide - appendices (CASRN 75–21-8): In support of summary information on the Integrated Risk Information System (IRIS). EPA/635/R-16/350Fb. Washington, DC, U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment. [Google Scholar]
- U.S. EPA. 2017. TRI Explorer (2015 dataset released October 2016). Washington, DC. [Google Scholar]
- Walker VE, Fennell TR, Upton PB, Skopek TR, Prevost V, Shuker DEG, et al. 1992a. Molecular dosimetry of ethylene oxide: Formation and persistence of 7-(2-hydroxyethyl)guanine in DNA following repeated exposures of rats and mice. Cancer Res, 52, 4328–4334. [PubMed] [Google Scholar]
- Walker VE, MacNeela JP, Swenberg JA, Turner MJ Jr, Fennell TR. 1992b. Molecular dosimetry of ethylene oxide: Formation and persistence of N-(2-hydroxyethyl)valine in hemoglobin following repeated exposures of rats and mice. Cancer Res, 52, 4320–4327. [PubMed] [Google Scholar]
- Zhang F, Bartels MJ, LeBaron MJ, Schisler MR, Gollapudi BB, Moore NP. 2015a. A novel approach for concurrent quantitation of glutathione, glutathione disulfide, and 2-hydroxyethylated glutathione in lungs of mice exposed to ethylene oxide, using liquid chromatography-positive electrospray tandem mass spectrometry. Biomed Chromatogr, 29, 1364–1374. [DOI] [PubMed] [Google Scholar]
- Zhang F, Bartels MJ, LeBaron MJ, Schisler MR, Jeong Y,-C, Gollapudi BB, et al. 2015b. LC-MS/MS simultaneous quantitation of 2-hydroxyethylated, oxidative, and unmodified DNA nucleosides in DNA isolated from tissues of mice after exposure to ethylene oxide. J Chromatogr B Analyt Technol Biomed Life Sci, 976–977, 33–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.