Abstract
α-Synuclein is a protein that mainly exists in the presynaptic terminals. Abnormal folding and accumulation of α-synuclein are found in several neurodegenerative diseases, including Parkinson's disease. Aggregated and highly phosphorylated α-synuclein constitutes the main component of Lewy bodies in the brain, the pathological hallmark of Parkinson's disease. For decades, much attention has been focused on the accumulation of α-synuclein in the brain parenchyma rather than considering Parkinson's disease as a systemic disease. Recent evidence demonstrates that, at least in some patients, the initial α-synuclein pathology originates in the peripheral organs and spreads to the brain. Injection of α-synuclein preformed fibrils into the gastrointestinal tract triggers the gut-to-brain propagation of α-synuclein pathology. However, whether α-synuclein pathology can occur spontaneously in peripheral organs independent of exogenous α-synuclein preformed fibrils or pathological α-synuclein leakage from the central nervous system remains under investigation. In this review, we aimed to summarize the role of peripheral α-synuclein pathology in the pathogenesis of Parkinson's disease. We also discuss the pathways by which α-synuclein pathology spreads from the body to the brain.
Key Words: aggregation, autonomic nervous system, barrier receptors, body fluid circulation, in situ generation, Parkinson's disease, phosphorylation, propagation, synucleinopathies, α-synuclein, α-synuclein fibrils
Introduction
α-Synuclein: genes, protein characteristics, and protein behaviors
α-Synuclein (α-Syn) is encoded by the SNCA gene, which is located on the long arm of chromosome 4 (Mizuno et al., 1999). Mutations in the SNCA gene, including A53T, A30P, E46K, G51D, H50Q, duplication, triplication, multiplication, etc., result in their carriers being susceptible to α-Syn pathology, among which A53T carriers exhibit the strongest tendency to form α-Syn pathology in the brain (Ikeuchi et al., 2008; Byers et al., 2011; Porcari et al., 2015; Zhang et al., 2019; Boyer et al., 2020; Joshi et al., 2023; Lau et al., 2023). Compared to healthy controls, A53T carriers had lower levels of serum α-Syn, indicating dysregulated homeostasis of α-Syn caused by SNCA mutation (Emmanouilidou et al., 2020). A53T carriers also show early and persistent accumulation of phosphorylated α-Syn in the enteric nervous system as well as an altered profile of peripheral immune cells, suggesting the potential influence of SNCA mutation on peripheral α-Syn pathology (Bencsik et al., 2014; Idova et al., 2021).
α-Syn has three major domains: N-terminal domain [amino acid (a.a.) 1–60], central domain (a.a. 61–95), and C-terminal domain (a.a. 96–140) (Wang et al., 2019). The N-terminal domain is highly hydrophobic, containing a consensus sequence (a.a. sequence: KTKEGV) consisting of seven imperfect repeats (a.a. 7–87). Deletion of the 13 residues in the N-terminus accelerates the fibrillization of α-Syn (McGlinchey et al., 2021). The central domain is called the non-amyloid-β component, which is indispensable for α-Syn aggregation (Xu et al., 2016). The C-terminus is negatively charged and flexible, which resists aggregation of the protein (Kumari et al., 2021). In vitro and intracellular nuclear magnetic resonance evidence showed that in the normal cellular environment, α-Syn appears as monomeric and disordered (Theillet et al., 2016). Other evidence showed that α-Syn can also form helically folded tetramers that resist aggregation (Selkoe et al., 2014). The aggregation propensity of α-Syn is regulated by the extent of N-terminus exposure (Stephens et al., 2020).
As a synaptic protein, α-Syn regulates synaptic vesicle trafficking and neurotransmitter release. The exact physiological behaviors of α-Syn need to be further investigated (Burré et al., 2010; Butler et al., 2015). The accumulation and hyperphosphorylation of α-Syn play a pivotal role in the pathogenesis of Parkinson's disease (PD) and other synucleinopathies. The already aggregated pathological α-Syn acts as “seeds” to template the aggregation of the remaining soluble counterparts. This abnormal behavior endows it with the characteristics of prion-like proteins (Fink, 2006; Peng et al., 2018; Lau et al., 2020). Aggregated α-Syn is also highly phosphorylated in the brain in PD. The phosphorylation of α-Syn is believed to be mediated by protein kinases including casein kinase 2 and death-associated protein kinase 1. Other kinases may also contribute to the phosphorylation of α-Syn (Fujiwara et al., 2002; Su et al., 2019; Hu et al., 2020; Yu et al., 2022a). The processes of α-Syn aggregation and phosphorylation interact with each other in an ambiguous order of occurrence. Phosphorylation of α-Syn at Ser129, the most commonly observed phosphorylation site, promotes the formation of fibrils, which reversely act on the “soil” of α-Syn monomers and subsequently induce the formation of α-Syn-enriched insoluble inclusions in the cytoplasm of brain cells (Chen and Feany, 2005; Helwig et al., 2016; Froula et al., 2019; Leitão et al., 2021; Yang et al., 2021; Ghanem et al., 2022). Intracerebral injection of α-Syn preformed fibrils (α-Syn PFFs), an artificial analog of α-Syn fibrils, into wild-type mice gave rise to typical α-Syn pathology in the brain, along with loss of dopaminergic neurons, blood-brain barrier (BBB) dysfunction, glial activation, neuroinflammation, and PD-like behavioral deficits (Luk et al., 2012b; Kim et al., 2018; Yun et al., 2018; Bieri et al., 2019; Ding et al., 2021; Butler et al., 2022). Pathological α-Syn can be detected not only in the brain but also in other peripheral organs, body fluids, and autonomic nerves, indicating the flowability and cell-to-cell transmission of pathological α-Syn (Mollenhauer et al., 2011; Wood, 2016; Iranzo et al., 2021; Sharabi et al., 2021; Lobanova et al., 2022; Poggiolini et al., 2022).
Synucleinopathies
PD
Synucleinopathies cover a series of neurodegenerative diseases with α-Syn aggregates, including Lewy body (LB) diseases [including PD, PD with dementia (PDD), and dementia with LBs (DLB)] and multiple system atrophy (MSA) (Koga et al., 2021). PD is the second most common neurodegenerative disease and one of the most studied synucleinopathies, with growing prevalence, disability, and lethality over the years (Pagonabarraga et al., 2015; Bloem et al., 2021). The pathological hallmark of PD is the loss of dopaminergic neurons in the substantia nigra (SN) and the formation of Lewy neurites and LBs consisting mainly of aggregated hyperphosphorylated α-Syn (Spillantini et al., 1997; Yang et al., 2022). Both genetic and environmental factors contribute to the onset of PD (Goldsmith et al., 1997; Polymeropoulos et al., 1997; Menegon et al., 1998; Healy et al., 2008; Tysnes and Storstein, 2017; Blauwendraat et al., 2020). To date, over 8.5 million people have been affected worldwide by PD (Rocca, 2018; GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators, 2019; World Health Organization, 2022).
PD is characterized by both motor and non-motor symptoms (Alarcón et al., 2023). The motor symptoms of PD include static tremor, rigidity, bradykinesia, and changes in posture and gait, resulting from hyperactivity of the acetylcholinergic system relative to deficiency of dopamine (de Bie et al., 1999; Tolosa et al., 2006; Li et al., 2012; Bai and Li, 2021; Nawaz et al., 2022). The non-motor features of PD include sleep disorders, constipation, dysfunction of autonomic nerves, cognitive decline, and depression, which usually occur as atypical symptoms in early stages of PD (Cooper et al., 1992; Wakabayashi and Takahashi, 1997; Williams and Lees, 2005; Camilleri et al., 2022; De Cock et al., 2022; Zhang et al., 2022b).
Other synucleinopathies
In addition to PD, LB diseases also include PDD and DLB. LB diseases share common neuropathological changes: Parkinsonism, cognitive decline, hallucinations, sleep disorders, and fluctuating attention (Donaghy et al., 2018). PD pathology is more confined to the brainstem and limbic regions, while PDD and DLB pathology is more diffuse in the neocortex (Colosimo et al., 2003). Huang et al. (2021) reported that in PDD, α-Syn predominantly causes dementia, while in DLB cases, the cooperation of α-Syn and amyloid β exert the effect. It has been reported that the α-Syn level in the cerebrospinal fluid (CSF) of DLB patients is higher than that of PDD patients (Bougea et al., 2018, 2020). MSA has two major clinical subtypes, MSA-C (MSA with predominant cerebellar ataxia) and MSA-P (MSA with predominant Parkinsonism) (Kalia et al., 2015; Guo et al., 2023). MSA is characterized by the presence of α-Syn-positive glial cytoplasmic inclusions (Papp and Lantos, 1994; Huang et al., 2008; Teil et al., 2022). α-Syn-positive inclusions also invade neurons in MSA, resulting in neuronal cytoplasmic inclusions (Wakabayashi et al., 1998; Hass et al., 2021). The direct correlation between neuronal cytoplasmic inclusions and glial cytoplasmic inclusions remains largely unknown. The density of accumulative glial cytoplasmic inclusions is positively correlated with the clinical manifestations of MSA, typical of which is early and severe autonomic failure, presenting as urinary urge incontinence or retention and orthostatic hypotension (Ozawa et al., 2004; Swaminath et al., 2010; Squair et al., 2022). α-Syn aggregates were also found in the urinary system and correlated with urinary symptoms in MSA (Peelaerts et al., 2023).
‘Brain-first’ and ‘body-first’ transmission mode of α-Syn pathology
The accumulation and propagation patterns of α-Syn have been extensively studied in synucleinopathies. Pathological analysis performed by Braak et al. (2003) showed that α-Syn pathology initially occurs in the dorsal motor nucleus of the glossopharyngeal nerve and the anterior olfactory nucleus, medulla, pontine tegmentum, and midbrain, and finally invades the neocortex, leading to the hypothesis that α-Syn pathology may spread through the nervous system. In some patients with prodromal symptoms of PD, 123I-metaiodobenzylguanidine scintigraphy showed fully developed pathology in the peripheral autonomic nervous system and the locus coeruleus, equal to that in diagnosed PD cases. This peripheral dysfunction of the autonomic nervous system supports that PD pathology initiates from peripheral autonomic nerves and then spreads rostrally to the brainstem in some cases (Knudsen et al., 2018). In addition, using a multimodal imaging method, Jacob Horsager and colleagues further validated the existence of both subtypes of PD pathology: brain-first parkinsonism (pathology found sequentially in the amygdala, SN, locus coeruleus, dorsal motor nucleus, and heart) and body-first parkinsonism (pathology found sequentially in the intestine, heart, dorsal motor nucleus, locus coeruleus, and SN) (Horsager et al., 2020). However, the mechanisms underlying the propagation of α-Syn throughout the body remain under investigation.
In this review, we aimed to summarize the existence and production of α-Syn pathology in the peripheral organs and their possible role as a source of peripheral and central α-Syn pathology. Furthermore, the propagation modes of peripheral α-Syn pathology, including the autonomic nerve pathway and body fluid pathway, and the fibrillization microenvironment in which these two pathways are conducive to the formation of peripheral α-Syn pathology were discussed to provide a peripheral and systemic view of α-Syn pathology as a supplement for recognizing the pathogenesis of synucleinopathies.
Search Strategy
Articles published from the year 1992 to 2023 included in this narrative review were screened and selected from the PubMed database. The search keywords included, but were not limited to, Parkinson's disease (and) α-synuclein (and) synucleinopathy; peripheral α-synuclein; α-synuclein (and) brain; α-synuclein (and) heart; α-synuclein (and) liver; α-synuclein (and) spleen; α-synuclein (and) spleen; α-synuclein (and) intestinal; α-synuclein (and) skin; α-synuclein (and) glands; α-synuclein (and) autonomic nerve; α-synuclein (and) blood. The articles that did not correspond to peripheral α-Syn pathology were excluded.
The Source of Peripheral α-Synuclein Pathology
Solid viscera and glands
α-Syn is abundantly expressed in the central nervous system (Agliardi et al., 2022; Alam et al., 2022a; Pena-DIaz and Ventura, 2022). Thus, most previous studies have focused on α-Syn pathology in the brain (Luk et al., 2012a; Masuda-Suzukake et al., 2013). Pathological α-Syn aggregates can also be detected in peripheral organs, such as the liver and heart, which have a high distribution density of nerves and blood vessels (Navarro-Otano et al., 2013; Javanshiri et al., 2022). Studies have indicated that the liver may help to clear pathological α-Syn, while overexpression of α-Syn in the perivascular nerve fiber lowered norepinephrin-induced contraction of the mouse aorta (Marrachelli et al., 2010; Reyes et al., 2021). In these cases, pathological α-Syn may come from brain-originated α-Syn leakage across the BBB and blood-CSF circulation, which communicates with the autonomic nerve system, or possibly from in situ generation within these solid viscera. The neuroendocrine organs and glands are also affected by α-Syn pathology, which is related to symptoms such as depression in PD. For example, phosphorylated α-Syn can be detected in the posterior pituitary lobe and salivary glands (Homma et al., 2012). Therefore, a biopsy of the salivary glands may facilitate the early diagnosis of PD (Del Tredici et al., 2010; Gelpi et al., 2014; Vilas et al., 2016). According to these findings, the solid viscera and glands can be a potential source of peripheral α-Syn pathology.
Gastrointestinal tract
In the early stage of PD, intestinal inflammation induces dysregulation of the gut microbiota. Gut microbiota dysbiosis is closely related to motor phenotypes observed in PD (Dodiya et al., 2020). The gut microbiota and their secretions may directly promote the aggregation of α-Syn. It is possible that the formation of α-Syn pathology in the gut may alter the gut microbiota (Scheperjans et al., 2015; Sampson et al., 2016, 2020; Wang et al., 2021a). The intestinal bacteria Enterobacteriaceae can secrete the functional amyloid protein major fimbrial subunit of thin curled fimbriae, which is believed to contribute to α-Syn aggregation. Inhibiting the expression of the intestinal major fimbrial subunit of thin curled fimbriae alleviates α-Syn pathology (Sampson et al., 2020; Wang et al., 2021a). Antibiotic-treated mice display less α-Syn pathology; in contrast, recolonization of the microbiota will aggravate α-Syn pathology (Sampson et al., 2016). This is consistent with the observation that the density of Enterobacteriaceae is positively associated with the severity of postural instability and gait difficulty in PD patients (Scheperjans et al., 2015). α-Syn pathology observed in other parts of the digestive tract, such as the esophagus, is also correlated with disease progression (Tanei et al., 2021). Hits from these digestive tract pathological α-Syn and the pathological reactions it causes together contribute to prodromal enteric nervous system dysfunctions, which manifest clinically as hydrostomia, dysphagia, gastroparesis, and constipation (Manfredsson et al., 2018). These observations support the presence of pathological α-Syn in the gastrointestinal tract and its potential in generating in situ α-Syn pathology.
Skin and mucosal tissues
PD is genetically associated with various skin diseases such as melanoma, sweating disorders, dermatophytosis, and seborrheic dermatitis (Dube et al., 2020; Scott et al., 2021). Antemortem skin biopsies conducted by Wang et al. (2020) revealed the existence of pathological α-Syn deposits with seeding activity within the skin among PD and other synucleinopathy cases. Phosphorylated, oligomeric, and aggregated forms of α-Syn are also commonly seen in various skin cells, such as cutaneous nerve cells, indicating communication of PD pathology between the cutaneous nerves and the central nervous system (Spehlmann, 1975; Doppler et al., 2017; Israel and Asch, 2020; Mazzetti et al., 2020; Marano et al., 2022; Nolano et al., 2022; Park et al., 2022). Therefore, detection of pathological α-Syn in the skin and olfactory mucosa is used to diagnose prodromal PD symptoms (Doppler et al., 2022). These results revealed the possibility of pathological α-Syn in the skin and mucosal tissues as a source of peripheral synucleinopathy.
Transmission Pathways of α-Synuclein Pathology from Peripheral Organs to the Brain
The autonomic nerve pathway
Pathological α-Syn accumulates in peripheral tissues many years before the appearance of motor symptoms in synucleinopathies (Palma et al., 2018; Yamada et al., 2020; Camacho et al., 2021; Van Den Berge et al., 2021). Braak et al. (2003) hypothesized that synucleinopathic lesions originate from the peripheral nervous system and spread via the autonomic nerves to the dorsal motor nucleus of the vagus nerve and to the cerebral cortex. Kim et al. (2019) injected α-Syn fibrils into the duodenal and pyloric muscularis layers, which are densely innervated by the vagus nerve, and detected α-Syn lesions in the brain. As expected, pathological changes were first found in the dorsal motor nucleus and then in the caudal portions of the hindbrain. Vagotomy of the autonomic nerve pathway almost completely blocked the propagation from the gastrointestinal tract to the brain (Kim et al., 2019; Chen et al., 2021). Similarly, pathological α-Syn is also enriched in the appendix, and appendectomy may delay PD onset (Killinger et al., 2018). The widespread distribution of α-Syn deposits in autonomic nerves and their upward communication with the central nervous system provide solid evidence supporting the hypothesis that α-Syn may initiate from peripheral tissues. However, considering that α-Syn pathology is predominant in the brain rather than in other tissues, peripheral α-Syn deposits may originate from central nervous system leakage rather than in situ generation. Under in vivo conditions, aggregated α-Syn in the brain may spread to the autonomic nerves, which is then transferred to autonomic nerve-enriched peripheral tissues via mechanisms including macromolecule transport, endocytosis, exocytosis, or neuroendocrine processes (Li et al., 2022). Pathological α-Syn may be further processed in the peripheral organ environment or retained in situ for a long time, thus accounting for nonmotor autonomic symptoms, including hydrostomia, dysphagia, gastroparesis, and constipation (Barboza et al., 2015). Conversely, peripheral pathological α-Syn also crosses the BBB and is transported to the central nervous system, becoming part of the sources leading to central α-Syn pathology. This dual transmission forms a vicious circle between central and peripheral α-Syn pathology (Arotcarena et al., 2020).
The body fluid circulation pathway
The autonomic nerve pathway that pathological α-Syn relies on to propagate between the central nervous system and enteric nervous system could result in PD being considered as a systematic disease. However, the autonomic nervous system is not the only pathway that contributes to the flowability of pathological α-Syn. Early in 2006, El-Agnaf et al. (2006) validated the existence of pathological α-Syn in the CSF. High levels of α-Syn in the CSF are associated with PD symptoms and progression (Mollenhauer et al., 2013; Wurster et al., 2022; Coutinho et al., 2023). In addition to the CSF, pathological α-Syn has also been detected in other bodily fluids, including the saliva, lymph, and blood (Sergeyeva et al., 2011; Kluge et al., 2022; Luan et al., 2022). For instance, plasma α-Syn levels are also reported to be related to some signs of PD (Malec-Litwinowicz et al., 2018). Similar to the autonomic nervous system pathway, the circulation of intracellular bodily fluids, including the plasma, CSF, interstitial fluids, and lymph, also results in the transmission of pathological α-Syn between the brain and the peripheral organs (Kim et al., 2012; Matsui and Matsui, 2017; Bartl et al., 2022).
α-Syn expression in the blood is most abundant in red blood cells (Liu et al., 2021). Erythrocytic α-Syn is expressed at both the mRNA and protein levels throughout the lifetime of red blood cells; therefore, α-Syn possibly influences the hemopoietic system (Nakai et al., 2007). It has been reported that erythrocytic α-Syn levels are linked to the occurrence of constipation, a common autonomic symptom of PD (Martínez-Rodríguez and Rey-Buitrago, 2020). In addition, the level of hemoglobin-binding α-Syn is elevated in patients with α-Syn pathology, which is also reported to be related to some sympathetic symptoms observed in PD (Umehara et al., 2020; Zhang et al., 2022a). In the brain parenchyma, α-Syn is located on the presynaptic membrane, showing high proximity in spatial position with membrane lipid rafts, which may participate in its transmission among brain cells (Perissinotto et al., 2020); in contrast, α-Syn in the blood binds to lipoproteins, thus influencing lipid transport (Emamzadeh and Allsop, 2017; Sinclair et al., 2021).
Phosphorylation is a widely studied post-translational modification of α-Syn that promotes α-Syn aggregation. The levels of phosphorylated α-Syn both inside red blood cells and on the erythrocytic membranes of patients with PD are much higher than those of healthy controls (Tian et al., 2019). Moreover, the level of oligomeric α-Syn in erythrocytes was increased in the early stage of PD (Liu et al., 2022). There is evidence that higher erythrocytic oligomeric α-Syn levels predict accelerated disease progression (Yu et al., 2022b). These blood-oriented phosphorylated α-Syn proteins resist digestion by protein kinase K, similar to α-Syn inclusions extracted from the brain, and are capable of binding phospholipids and plasma proteins (Abd-Elhadi et al., 2015; Iyer et al., 2016). In addition to being transported by plasma proteins, these pathological phosphorylated α-Syn proteins may also be transmitted from the blood to the brain by erythrocytic extracellular vesicles via membrane fusion with the BBB (Matsumoto et al., 2017). Additionally, phosphorylation is not the only post-translational modification found in erythrocytic α-Syn; it has been reported that the lysine residues in erythrocytic α-Syn can be modified by acetylation, glycation, ubiquitination, SUMOylation, and even nitration and acylation, similar to that found in the brain of PD patients. In conclusion, this data supports that erythrocytic α-Syn may play a role in the peripheral formation and propagation of synucleinopathies (Amagai et al., 2023).
On the one hand, the abovementioned erythrocytic normal and pathological α-Syn may originate from the leakage of brain-oriented pathological α-Syn through the BBB or blood-CSF circulation. Many experiments have validated this brain-to-blood propagation. When radio-labeled α-Syn fibrils are injected into certain brain regions or directly into the lateral ventricle, they can be detected in the CSF, peripheral blood, and even in some peripheral tissues (Sui et al., 2014). On the other hand, from the peripheral perspective, other peripheral administration routes of α-Syn PFFs, including oral, intranasal, intraperitoneal, and intramuscular administration, as well as tail vein injection, can also lead to brain α-Syn pathology similar to that induced by intracerebral injection of α-Syn PFFs, proving the existence of α-Syn propagation between the blood and brain through the circulation of bodily fluids (Ayers et al., 2017; Earls et al., 2019; Macdonald et al., 2021; Masuda-Suzukake et al., 2021; Awa et al., 2022).
Multiple Factors Mediate the Propagation of Peripheral α-Synuclein Pathology to the Brain
BBB receptors
The BBB is the main barrier and the most pivotal structural basis blocking the entry of peripheral pathological α-Syn into the brain. The BBB is altered in the brain of PD patients, which results from hits of pathological α-Syn (Dohgu et al., 2019; Tsunemi et al., 2020; Xia et al., 2021; Huang et al., 2022a). Brain microvascular endothelial cells, astrocytes, and pericytes are the main components of the BBB and blood-CSF barrier (Campisi et al., 2018). Peripheral pathological α-Syn is most likely transported across the BBB via interaction with receptors on these cells and extracellular vesicles. These receptors can be divided into three categories according to their affinity to pathological α-Syn: transporters mediating α-Syn transmission by direct binding, facilitators regulating vesicle trafficking of pathological α-Syn, and receptors affecting BBB permeability conducive to α-Syn propagation (Table 1; Calderón-Garcidueñas et al., 2008; Kanekiyo et al., 2011; Jangula and Murphy, 2013; Chen et al., 2015; Mao et al., 2016; Masaracchia et al., 2018; Phillips et al., 2018; Bae and Lee, 2020; Rauch et al., 2020; Emmenegger et al., 2021; Gasca-Salas et al., 2021; Gu et al., 2021; Kim et al., 2021; Pediaditakis et al., 2021; Streubel-Gallasch et al., 2021; Wang et al., 2021b, c; Zhang et al., 2021, 2023a, c; Alam et al., 2022b; Chen et al., 2022; Feng et al., 2022; Huang et al., 2022a; Lan et al., 2022; Prieto Huarcaya et al., 2022; Roshanbin et al., 2022; Ruan et al., 2022; Salman et al., 2022; Shin et al., 2022; Vellingiri et al., 2022). Direct binding was found between α-Syn fibrils and lymphocyte-activation gene 3, as well as amyloid precursor-like protein 1, which are widely expressed in blood, immune, and endothelial cells, thus mediating the transmission of α-Syn fibrils (Mao et al., 2016; Zhang et al., 2021). The second category includes astrocytic vascular endothelial growth factor A (VEGFA), low-density lipoprotein receptor-related protein 1, Ras-related in brain 7 (Rab7), and leucine-rich repeat kinase 2 (LRRK2), which are reported to regulate vesicle trafficking of pathological α-Syn. Blocking astrocyte VEGFA signaling in the in vitro BBB model effectively protects the barrier against the harmful effects of oligomeric α-Syn, while dysregulation of Rab7 signaling and LRRK2 signaling causes abortive clearance of pathological α-Syn, leading to α-Syn accumulation and propagation (Bae and Lee, 2020; Wang et al., 2021b; Alam et al., 2022b; Chen et al., 2022; Lan et al., 2022). When treating in vitro models of the BBB with α-Syn fibrils, a series of targets, including lipoprotein receptor-related protein 1 and LRRK2, have been proven to undergo alterations (Pediaditakis et al., 2021). Physical and environmental hits, such as ultrasound, air pollution, heavy metals, and a ketogenic diet, and chemical factors, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, lipopolysaccharide, cerebrolysin, and tissue plasminogen activator, are also reported to affect other BBB receptors, such as aquaporin 4, thus enhancing BBB permeability and contributing to the entry of pathological α-Syn into the brain (Calderón-Garcidueñas et al., 2008; Jangula and Murphy, 2013; Chen et al., 2015; Phillips et al., 2018; Gasca-Salas et al., 2021; Wang et al., 2021c; Feng et al., 2022; Ruan et al., 2022; Salman et al., 2022; Vellingiri et al., 2022).
Table 1.
BBB receptors interacting with α-Syn and α-Syn fibrils
| Receptor name | Interaction type with α-Syn | Effect on α-Syn transportation |
|---|---|---|
| LAG3 | Direct binding | Transmission |
| APLP1 | ||
| TLR2 | Direct interaction | |
| LRP1 | Indirect interaction | Vesicle trafficking, BBB permeability |
| Changed gene expression by α-Syn monomer | ||
| HSPG | Indirect interaction, synergies with LRP1 | Vesicle trafficking |
| Rab7 | Indirect interaction | |
| VEGFA | ||
| LRRK2 | Indirect interaction | |
| Changed gene expression by α-Syn monomer | ||
| IGF1R | Indirect interaction | Molecular shuttle |
| TfR | ||
| NEK | BBB permeality | |
| AQP4 | ||
| Nurr1 | ||
| M6PR | ||
| LRP2 | Changed gene expression by α-Syn monomer | BBB function |
| ABCB1 | ||
| CLDN4 | Changed gene expression by α-Syn fibril | BBB function |
| CLDN9 | ||
| CLDN1 | ||
| SLC2A6 | ||
| SLC16A6 | ||
| GJA4 |
Data were from studies Calderón-Garcidueñas et al., 2008; Kanekiyo et al., 2011; Jangula and Murphy, 2013; Chen et al., 2015; Mao et al., 2016; Masaracchia et al., 2018; Phillips et al., 2018; Bae and Lee, 2020; Rauch et al., 2020; Emmenegger et al., 2021; Gasca-Salas et al., 2021; Gu et al., 2021; Kim et al., 2021; Pediaditakis et al., 2021; Streubel-Gallasch et al., 2021; Wang et al., 2021b, c; Zhang et al., 2021, 2023a, c; Alam et al., 2022b; Chen et al., 2022; Feng et al., 2022; Huang et al., 2022a; Lan et al., 2022; Prieto Huarcaya et al., 2022; Roshanbin et al., 2022; Ruan et al., 2022; Salman et al., 2022; Shin et al., 2022; Vellingiri et al., 2022. ABCB1: Adenosine triphosphate-binding cassette subfamily B member 1; APLP1: amyloid precursor-like protein 1; AQP4: aquaporin-4; CLDN1: claudin-1; CLDN4: claudin-4; CLDN9: claudin-9; GJA4: gap junction protein alpha 4; SLC2A6: facilitated glucose transporter member 6; HSPG: heparan sulfate proteoglycan; IGF1R: insulin like growth factor 1 receptor; LRRK2: leucine-rich repeat kinase 2; LRP1: low-density lipoprotein receptor-related protein 1; LRP2: low-density lipoprotein receptor-related protein 2; LAG3: lymphocyte activation gene-3; M6PR: mannose-6-phosphate receptor; SLC16A6: monocarboxylate transporter 6; NEK: NimA related kinase; Nurr1: nuclear receptor-related factor 1; Rab7: Ras-related in brain 7; TLR2: Toll-like receptor 2; TfR: transferrin receptor-1; VEGFA: vascular endothelial growth factor A; α-Syn: α-synuclein.
However, it is controversial whether these interactions between pathological α-Syn and the receptors can actually aggravate the α-Syn pathology (Emmenegger et al., 2021; Gu et al., 2021). Often, these receptors have poor selectivity for α-Syn monomers, oligomers, and fibrils, as well as other aggregated proteins (Rauch et al., 2020). Even when confronted with the same protein fibrils, these receptors exhibit distinct binding affinities. α-Syn fibrils with post-translational modifications, such as phosphorylation at Ser129, are believed to have a higher bonding affinity with lymphocyte-activation gene 3 than pure fibrils (Zhang et al., 2023a). Except for poor selectivity, the widespread distribution and functional diversity of these receptors also limits their weight in regulating α-Syn propagation. For instance, lipoprotein receptor-related protein 1 is expressed on various cell types, including neurons, astrocytes, microglia, macrophages, fibroblasts, and smooth muscle cells, and cooperates with other endocytosis-related receptors, such as heparin sulfate proteoglycan, regulating cell-to-cell propagation of not only pathological α-Syn, but also pathological proteins typical of Alzheimer's disease (Kanekiyo et al., 2011). Likewise, in addition to promoting transmission, the LRRK2 and Rab7 pathways are also responsible for normal phagocytosis and clearance of pathological α-Syn (Masaracchia et al., 2018; Streubel-Gallasch et al., 2021). When peripheral pathological α-Syn is attached to a BBB receptor, clearance via lysosomal degradation and autophagy may occur first before it can enter the exocytosis pathway and then be transmitted among brain cells. It can be speculated that there is a receptor-mediated balance among endo- and exocytosis, and the clearance and propagation of pathological proteins, the disturbance of which ultimately leads to successful propagation of α-Syn pathology from the periphery to the central nervous system (Volpicelli-Daley et al., 2011; Rodrigues et al., 2022). Therefore, there is an urgent need to identify the key receptors with specificity that mediate the spreading of pathological α-Syn.
Properties of α-Syn fibrils
The main forms in which pathological α-Syn exists in PD brains are α-Syn oligomers, fibrils, and ribbons (Peelaerts et al., 2015; Rodriguez et al., 2015). The highly aggregated form, α-Syn ribbons, has the strongest seeding activity, while the oligomer and fibril are prone to cause cell toxicity and cell-to-cell transmission, respectively (Mahul-Mellier et al., 2015; Uemura et al., 2023). In different synucleinopathies, aggregated α-Syn possesses different properties. Both insoluble and soluble fractions of α-Syn-enriched brain extracts derived from MSA patients can induce the accumulation of normal α-Syn, while only insoluble fractions derived from patients with PD retain this seeding ability (Yamasaki et al., 2019; Van der Perren et al., 2020). Phosphorylation at Ser129 of α-Syn is another point distinguishing PD from other synucleinopathies (Sonustun et al., 2022). Approximately 90% of α-Syn in LBs in PD is hyperphosphorylated. Ubiquitination, acetylation, nitrification, palmitoylation, etc. also occupy a minority of modifications of α-Syn, influencing the properties of α-Syn fibrils (Sevcsik et al., 2011; Kunadt et al., 2015; Rott et al., 2017; Ho et al., 2023; Zhang et al., 2023b). Among these, phosphorylation of serine, ubiquitination, nitrification, glycation, etc. are believed to promote α-Syn aggregation and propagation (Table 2; Nonaka et al., 2005; Kim et al., 2006; Danielson et al., 2009; Lee et al., 2009; Waxman et al., 2010; Liu et al., 2011; Padmaraju et al., 2011; Izawa et al., 2012; Binolfi et al., 2016; Arawaka et al., 2017; Vicente Miranda et al., 2017; Zhang et al., 2017a, b; Wen et al., 2018; Barinova et al., 2019; Chavarría et al., 2019; Sanyal et al., 2019; Semenyuk et al., 2019; Zhao et al., 2020; Andersen et al., 2021; Dhakal et al., 2021; Hartlage-Rübsamen et al., 2021; Bell et al., 2022; Farzadfard et al., 2022; Jin et al., 2022; Kam et al., 2022; Panigrahi et al., 2023; Zhou et al., 2023). α-Syn can also be modified by other proteins, lipids, and small molecular compounds, which enhance or suppress its propagation or seeding activity (Masaracchia et al., 2018; Kim et al., 2021; Streubel-Gallasch et al., 2021). For example, asparagine endopeptidase cleaves α-Syn at N103, generating the α-Syn (1–103) fragment, which forms aggregates with higher pathogenicity, suggesting that fragmentation of α-Syn influences the properties of fibrils (Zhang et al., 2017b). Furthermore, chemical substances, such as homocysteine derivatives, can also modify α-Syn on the K80 residue, thus forming more toxic fibrils with higher seeding and propagation activity (Zhou et al., 2023). Most of these modified α-Syn proteins are more resistant to digestion by proteinases and are more likely to undergo fibrillation and aggregation; therefore, they are less easily engulfed and degraded by their host cells. In addition, they are more prone to undergo neuron–neuron, glia–neuron, and peripheral cell-brain cell propagation, forming the spreading mechanism of pathological α-Syn among the central nervous system and from the peripheral tissues to the central nervous system (Pluvinage et al., 2019; Yuan et al., 2022).
Table 2.
Posttranslational modifications that affect α-Syn properties
| Modification | Sites | Modification effects |
|---|---|---|
| Phosphorylation | Ser129 | Promoting α-Syn aggregation |
| Ser87 | ||
| Tyr39 | ||
| Tyr136 | ||
| Tyr125 | Suppressing α-Syn aggregation | |
| O-GlcNAcylation | Thr72 | Suppressing α-Syn aggregation |
| Ser87 | ||
| Ubiquitination | Lys6 | Promoting α-Syn aggregation |
| Lys10 | ||
| Lys12 | ||
| Nitrification | Tyr125 | Promoting α-Syn aggregation |
| Tyr133 | ||
| Tyr136 | ||
| Tyr39 | ||
| Glycation | Not applicable | Promoting α-Syn aggregation |
| Arginylation | Glu83 | Suppressing α-Syn aggregation |
| Acetylation | Not applicable | Postponing α-Syn aggregation |
| SUMOylation | Not applicable | Suppressing α-Syn aggregation |
| Nitroalkylation | Not applicable | Suppressing α-Syn aggregation |
| Adenylylation | Not applicable | Suppressing α-Syn aggregation |
| N-homocysteinylation (chemical modification) | Lys80 | Promoting α-Syn aggregation |
| Pyroglutamate (pGlu)79 (chemical modification) | Gln79 | Promoting α-Syn aggregation |
| Glyceraldehyde-3-phosphate (chemical modification) | Not applicable | Preventing α-Syn aggregation |
| 4-Hydroxy-2-nonenal (chemical modification) | His50 | Promoting α-Syn aggregation |
| Dicarbonyl compounds (chemical modification) | Not applicable | Suppressing α-Syn aggregation |
| Tyrosine hydroxylase (chemical modification) | Tyr136 | Promoting α-Syn aggregation |
| Docosahexaenoic acid (chemical modification) | Not applicable | Promoting α-Syn aggregation |
| Asparagine endopeptidase (proteolysis) | Asn103 | Promoting α-Syn aggregation |
Data were from studies Nonaka et al., 2005; Kim et al., 2006; Danielson et al., 2009; Lee et al., 2009; Waxman et al., 2010; Liu et al., 2011; Padmaraju et al., 2011; Izawa et al., 2012; Binolfi et al., 2016; Arawaka et al., 2017; Vicente Miranda et al., 2017; Zhang et al., 2017a, b; Wen et al., 2018; Barinova et al., 2019; Chavarría et al., 2019; Sanyal et al., 2019; Semenyuk et al., 2019; Zhao et al., 2020; Andersen et al., 2021; Dhakal et al., 2021; Hartlage-Rübsamen et al., 2021; Bell et al., 2022; Farzadfard et al., 2022; Jin et al., 2022; Kam et al., 2022; Panigrahi et al., 2023; Zhou et al., 2023. α-Syn: α-Synuclein.
Microenvironment promotes the formation of peripheral α-Syn pathology
The aggregation and propagation of α-Syn requires a specific microenvironment. Thus, we propose that there is a fibrillization microenvironment (FME) composed of pathological α-Syn, the peripheral immune system, the erythrocytic intracellular environment, the autonomic nerve system, soluble and insoluble cytokines, ionic concentration, pH, temperature, hemodynamics, and the BBB (Figure 1). These elements together contribute to the focal enrichment of pathological α-Syn, isolating it from the liquid phase of the intracellular fluid and irreversibly forming agglutinative fibrils and ribbons (Huang et al., 2022b). For instance, dysfunction of ionic homeostasis, disturbance of BBB receptors, acidic pH, and dysregulation of phosphatases promote the formation and cell-to-cell transmission of α-Syn pathology (Bhak et al., 2014; Li et al., 2020, 2023a; Yu et al., 2021). From the peripheral view, the peripheral blood provides an immunity-centered FME that promotes the generation and propagation of peripheral pathological α-Syn. In addition, the adjacency in location between the blood and the autonomic nerves further enhances the effect of FME on the transmissibility of α-Syn pathology throughout the body.
Figure 1.
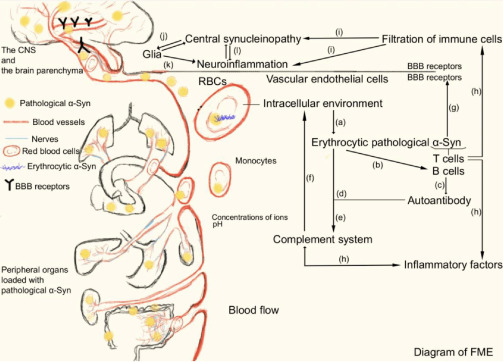
Diagram of FME and the propagation mode of pathological α-Syn from peripheral organs to the brain.
The FME is composed of pathological α-Syn, the peripheral immune system, members of the BBB, the erythrocytic intracellular environment, and soluble and insoluble cytokines. (a) The erythrocytic intracellular environment contributes to the generation of pathological α-Syn. (b) Erythrocytic pathological α-Syn recruits B cells. (c) B cells secrete autoantibodies against α-Syn. (d) Autoantibodies of α-Syn promote clearance of erythrocytic pathological α-Syn. (e) A combination of α-Syn and its antibody leads to activation of the complement system. (f) Members of the complement system reversely aggravate α-Syn pathology. (g) T cells discriminate peptides of α-Syn and perform antigen presentation. (h) Recruiting of B and T cells and vesicle trafficking of pathological α-Syn give rise to infiltration of inflammatory factors and peripheral pathological α-Syn across the BBB. (i–l) Penetration of peripheral immune cells and peripheral pathological α-Syn causes central synucleinopathy and neuroinflammation. Created with Adobe Illustrator. BBB: Blood-brain barrier; CNS: central nervous system; FME: fibrillization microenvironment; RBC: red blood cell; α-Syn: α-synuclein.
Once pathological α-Syn is agglutinated in erythrocytes (possibly taking several decades before the observation of clinical manifestations caused by acute ischemic stroke, thoracic trauma, infection, etc.), it can activate and recruit peripheral immune cells, including B lymphocytes for antibody production and T lymphocytes for antigen presentation prior to neurodegeneration, in line with the clinical detection of α-Syn antibodies in the peripheral blood of PD patients (Xiao et al., 2014; Sulzer et al., 2017; Harms et al., 2018; Tulisiak et al., 2019; Wu et al., 2019; Karikari et al., 2022; Ruf et al., 2022; Li et al., 2023b). Although α-Syn antibodies help to eliminate pathological α-Syn in the peripheral blood and the activated autophagic and lysosomal proteins within these immune cells also promote an active process of proteasomal degradation of pathological α-Syn, the reaction of pathological α-Syn and its antibody still activates the complement system (Papagiannakis et al., 2015; Miki et al., 2018; Gregersen et al., 2021). Thorough activation of the peripheral immune and inflammatory system can directly promote the propagation of α-Syn pathology (Kim et al., 2022). Simultaneously, penetration of active T lymphocytes across the BBB into the brain has also been proven to cause neuroinflammation and aggravation of central α-Syn pathology, again proving the influence of the immune environment on α-Syn transmission (Williams et al., 2021). This in vivo regulation between the peripheral immune system and α-Syn pathology provides an explanation for the failure of antibody therapy in treating PD, owing to the counterforce of the immune system on promoting α-Syn pathology, indicating an immunity-centered FME contributing to the formation and propagation of peripheral α-Syn pathology (Lang et al., 2022; Pagano et al., 2022).
Conclusion
The production of pathological α-Syn in peripheral organs, the crosstalk between the body fluid and autonomic nervous system, the participation of BBB receptors, and the peripheral FME that affects α-Syn properties support peripheral organs as the source of PD pathology and even the initiation of α-Syn pathology. The existence of reverse diffusion from the blood to brain, from peripheral to central tissues, and the circulatory aggravation of α-Syn pathology on either side of the BBB urges us to understand the pathogenesis of PD from a systemic and global perspective. Therapies facilitating the clearance of peripheral α-Syn and inhibiting the forward and reverse transportation of peripheral and central α-Syn, early intervention of peripheral FME, and prevention of the circulatory spread of α-Syn pathology may alleviate the propagation of PD pathology. To date, the autonomic nerve pathway has been the most recognized route that mediates the transmission of pathological α-Syn from the periphery to the brain. Although emerging evidence has proven the existence of the body fluid pathway, there is a lack of feasibility in cutting off the body fluid connection as in the autonomic nerve pathway, bringing difficulties to further studies centering on humoral transmission of α-Syn pathology. In this review, we discussed the autonomic nerve pathway and body fluid circulation pathway as two separate mechanisms; however, we have not extended α-Syn pathology transmission to the crosstalk between the abovementioned two pathways. Whether pathological α-Syn in the bodily fluids can directly reach the brain parenchyma relying on body fluid circulation, or first interact with peripheral autonomic nerves and then indirectly reach the brain parenchyma needs further investigation.
Funding Statement
Funding: This work was supported by the National Natural Science Foundation of China, Nos. 82271447, 81771382; the National Key Research and Development Program of China, No. 2019YFE0115900; and the “New 20 Terms of Universities in Jinan”, No. 202228022 (all to ZZ).
Footnotes
Conflicts of interest: The authors declare no competing interest.
Data availability statement: The data are available from the corresponding author on reasonable request.
C-Editors: Li JY, Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- Abd-Elhadi S, Honig A, Simhi-Haham D, Schechter M, Linetsky E, Ben-Hur T, Sharon R. Total and proteinase K-resistant α-synuclein levels in erythrocytes, determined by their ability to bind phospholipids, associate with Parkinson's disease. Sci Rep. 2015;5:11120. doi: 10.1038/srep11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agliardi C, Guerini FR, Meloni M, Clerici M. Alpha-synuclein as a biomarker in Parkinson's disease: focus on neural derived extracelluar vesicles. Neural Regen Res. 2022;17:1503–1504. doi: 10.4103/1673-5374.330604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MM, Yang D, Li XQ, Liu J, Back TC, Trivett A, Karim B, Barbut D, Zasloff M, Oppenheim JJ. Alpha synuclein, the culprit in Parkinson disease, is required for normal immune function. Cell Rep. 2022a;38:110090. doi: 10.1016/j.celrep.2021.110090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam P, Holst MR, Lauritsen L, Nielsen J, Nielsen SSE, Jensen PH, Brewer JR, Otzen DE, Nielsen MS. Polarized α-synuclein trafficking and transcytosis across brain endothelial cells via Rab7-decorated carriers. Fluids Barriers CNS. 2022b;19:37. doi: 10.1186/s12987-022-00334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón TA, Presti-Silva SM, Simões APT, Ribeiro FM, Pires RGW. Molecular mechanisms underlying the neuroprotection of environmental enrichment in Parkinson's disease. Neural Regen Res. 2023;18:1450–1456. doi: 10.4103/1673-5374.360264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amagai R, Yoshioka S, Otomo R, Nagano H, Hashimoto N, Sakakibara R, Tanaka T, Okado-Matsumoto A. Post-translational modification of lysine residues in erythrocyte α-synuclein. J Biochem. 2023;173:177–184. doi: 10.1093/jb/mvac100. [DOI] [PubMed] [Google Scholar]
- Andersen C, Grønnemose AL, Pedersen JN, Nowak JS, Christiansen G, Nielsen J, Mulder FAA, Otzen DE, Jørgensen TJD. Lipid peroxidation products HNE and ONE promote and stabilize alpha-synuclein oligomers by chemical modifications. Biochemistry. 2021;60:3644–3658. doi: 10.1021/acs.biochem.1c00478. [DOI] [PubMed] [Google Scholar]
- Arawaka S, Sato H, Sasaki A, Koyama S, Kato T. Mechanisms underlying extensive Ser129-phosphorylation in α-synuclein aggregates. Acta Neuropathol Commun. 2017;5:48. doi: 10.1186/s40478-017-0452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arotcarena ML, Dovero S, Prigent A, Bourdenx M, Camus S, Porras G, Thiolat ML, Tasselli M, Aubert P, Kruse N, Mollenhauer B, Trigo Damas I, Estrada C, Garcia-Carrillo N, Vaikath NN, El-Agnaf OMA, Herrero MT, Vila M, Obeso JA, Derkinderen P, et al. Bidirectional gut-to-brain and brain-to-gut propagation of synucleinopathy in non-human primates. Brain. 2020;143:1462–1475. doi: 10.1093/brain/awaa096. [DOI] [PubMed] [Google Scholar]
- Awa S, Suzuki G, Masuda-Suzukake M, Nonaka T, Saito M, Hasegawa M. Phosphorylation of endogenous α-synuclein induced by extracellular seeds initiates at the pre-synaptic region and spreads to the cell body. Sci Rep. 2022;12:1163. doi: 10.1038/s41598-022-04780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers JI, Brooks MM, Rutherford NJ, Howard JK, Sorrentino ZA, Riffe CJ, Giasson BI. Robust central nervous system pathology in transgenic mice following peripheral injection of α-synuclein fibrils. J Virol. 2017;91:e02095–16. doi: 10.1128/JVI.02095-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae EJ, Lee SJ. The LRRK2-RAB axis in regulation of vesicle trafficking and α-synuclein propagation. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165632. doi: 10.1016/j.bbadis.2019.165632. [DOI] [PubMed] [Google Scholar]
- Bai F, Li X. Association of Helicobacter pylori treatment with Parkinsonism and related disorders: a systematic review and meta-analysis. Life Sci. 2021;281:119767. doi: 10.1016/j.lfs.2021.119767. [DOI] [PubMed] [Google Scholar]
- Barboza JL, Okun MS, Moshiree B. The treatment of gastroparesis, constipation and small intestinal bacterial overgrowth syndrome in patients with Parkinson's disease. Expert Opin Pharmacother. 2015;16:2449–2464. doi: 10.1517/14656566.2015.1086747. [DOI] [PubMed] [Google Scholar]
- Barinova K, Serebryakova M, Sheval E, Schmalhausen E, Muronetz V. Modification by glyceraldehyde-3-phosphate prevents amyloid transformation of alpha-synuclein. Biochim Biophys Acta Proteins Proteom. 20191867:396–404. doi: 10.1016/j.bbapap.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Bartl M, Xylaki M, Bähr M, Weber S, Trenkwalder C, Mollenhauer B. Evidence for immune system alterations in peripheral biological fluids in Parkinson's disease. Neurobiol Dis. 2022;170:105744. doi: 10.1016/j.nbd.2022.105744. [DOI] [PubMed] [Google Scholar]
- Bell R, Thrush RJ, Castellana-Cruz M, Oeller M, Staats R, Nene A, Flagmeier P, Xu CK, Satapathy S, Galvagnion C, Wilson MR, Dobson CM, Kumita JR, Vendruscolo M. N-terminal acetylation of α-synuclein slows down its aggregation process and alters the morphology of the resulting aggregates. Biochemistry. 2022;61:1743–1756. doi: 10.1021/acs.biochem.2c00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencsik A, Muselli L, Leboidre M, Lakhdar L, Baron T. Early and persistent expression of phosphorylated α-synuclein in the enteric nervous system of A53T mutant human α-synuclein transgenic mice. J Neuropathol Exp Neurol. 2014;73:1144–1151. doi: 10.1097/NEN.0000000000000137. [DOI] [PubMed] [Google Scholar]
- Bhak G, Lee J, Kim TH, Lee S, Lee D, Paik SR. Molecular inscription of environmental information into protein suprastructures: temperature effects on unit assembly of α-synuclein oligomers into polymorphic amyloid fibrils. Biochem J. 2014;464:259–269. doi: 10.1042/BJ20140723. [DOI] [PubMed] [Google Scholar]
- Bieri G, Brahic M, Bousset L, Couthouis J, Kramer NJ, Ma R, Nakayama L, Monbureau M, Defensor E, Schüle B, Shamloo M, Melki R, Gitler AD. LRRK2 modifies α-syn pathology and spread in mouse models and human neurons. Acta Neuropathol. 2019;137:961–980. doi: 10.1007/s00401-019-01995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binolfi A, Limatola A, Verzini S, Kosten J, Theillet FX, Rose HM, Bekei B, Stuiver M, van Rossum M, Selenko P. Intracellular repair of oxidation-damaged α-synuclein fails to target C-terminal modification sites. Nat Commun. 2016;7:10251. doi: 10.1038/ncomms10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauwendraat C, Nalls MA, Singleton AB. The genetic architecture of Parkinson's disease. Lancet Neurol. 2020;19:170–178. doi: 10.1016/S1474-4422(19)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloem BR, Okun MS, Klein C. Parkinson's disease. Lancet. 2021;397:2284–2303. doi: 10.1016/S0140-6736(21)00218-X. [DOI] [PubMed] [Google Scholar]
- Bougea A, Stefanis L, Emmanouilidou E, Vekrelis K, Kapaki E. High discriminatory ability of peripheral and CFSF biomarkers in Lewy body diseases. J Neural Transm (Vienna) 2020;127:311–322. doi: 10.1007/s00702-019-02137-2. [DOI] [PubMed] [Google Scholar]
- Bougea A, Stefanis L, Paraskevas GP, Emmanouilidou E, Efthymiopoulou E, Vekrelis K, Kapaki E. Neuropsychiatric symptoms and α-Synuclein profile of patients with Parkinson's disease dementia, dementia with Lewy bodies and Alzheimer's disease. J Neurol. 2018;265:2295–2301. doi: 10.1007/s00415-018-8992-7. [DOI] [PubMed] [Google Scholar]
- Boyer DR, Li B, Sun C, Fan W, Zhou K, Hughes MP, Sawaya MR, Jiang L, Eisenberg DS. The α-synuclein hereditary mutation E46K unlocks a more stable, pathogenic fibril structure. Proc Natl Acad Sci U S A. 2020;117:3592–3602. doi: 10.1073/pnas.1917914117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler B, Saha K, Rana T, Becker JP, Sambo D, Davari P, Goodwin JS, Khoshbouei H. Dopamine transporter activity is modulated by α-synuclein. J Biol Chem. 2015;290:29542–29554. doi: 10.1074/jbc.M115.691592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler YR, Liu Y, Kumbhar R, Zhao P, Gadhave K, Wang N, Li Y, Mao X, Wang W. α-Synuclein fibril-specific nanobody reduces prion-like α-synuclein spreading in mice. Nat Commun. 2022;13:4060. doi: 10.1038/s41467-022-31787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B, Cord B, Nguyen HN, Schüle B, Fenno L, Lee PC, Deisseroth K, Langston JW, Pera RR, Palmer TD. SNCA triplication Parkinson's patient's iPSC-derived DA neurons accumulate α-synuclein and are susceptible to oxidative stress. PLoS One. 2011;6:e26159. doi: 10.1371/journal.pone.0026159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, Villarreal-Calderón R, Osnaya N, Stone I, García R, Brooks DM, González-Maciel A, Reynoso-Robles R, Delgado-Chávez R, Reed W. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- Camacho M, Greenland JC, Williams-Gray CH. The gastrointestinal dysfunction scale for Parkinson's disease. Mov Disord. 2021;36:2358–2366. doi: 10.1002/mds.28675. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Subramanian T, Pagan F, Isaacson S, Gil R, Hauser RA, Feldman M, Goldstein M, Kumar R, Truong D, Chhabria N, Walter BL, Eskenazi J, Riesenberg R, Burdick D, Tse W, Molho E, Robottom B, Bhatia P, Kadimi S, et al. Oral ENT-01 targets enteric neurons to treat constipation in Parkinson disease: a randomized controlled trial. Ann Intern Med. 2022;175:1666–1674. doi: 10.7326/M22-1438. [DOI] [PubMed] [Google Scholar]
- Campisi M, Shin Y, Osaki T, Hajal C, Chiono V, Kamm RD. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials. 2018;180:117–129. doi: 10.1016/j.biomaterials.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarría C, Trostchansky A, Durán R, Rubbo H, Souza JM. Nitroalkylation of α-synuclein by nitro-oleic acid: implications for Parkinson's disease. Adv Exp Med Biol. 20191127:169–179. doi: 10.1007/978-3-030-11488-6_11. [DOI] [PubMed] [Google Scholar]
- Chen C, Zhou Y, Wang H, Alam A, Kang SS, Ahn EH, Liu X, Jia J, Ye K. Gut inflammation triggers C/EBPβ/δ-secretase-dependent gut-to-brain propagation of Aβ and Tau fibrils in Alzheimer's disease. EMBO J. 2021;40:e106320. doi: 10.15252/embj.2020106320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Martens YA, Meneses A, Ryu DH, Lu W, Raulin AC, Li F, Zhao J, Chen Y, Jin Y, Linares C, Goodwin M, Li Y, Liu CC, Kanekiyo T, Holtzman DM, Golde TE, Bu G, Zhao N. LRP1 is a neuronal receptor for α-synuclein uptake and spread. Mol Neurodegener. 2022;17:57. doi: 10.1186/s13024-022-00560-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- Chen T, Hou R, Li C, Wu C, Xu S. MPTP/MPP+ suppresses activation of protein C in Parkinson's disease. J Alzheimers Dis. 2015;43:133–142. doi: 10.3233/JAD-140126. [DOI] [PubMed] [Google Scholar]
- Colosimo C, Hughes AJ, Kilford L, Lees AJ. Lewy body cortical involvement may not always predict dementia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2003;74:852–856. doi: 10.1136/jnnp.74.7.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Doherty SM, Jordan N, Tidswell P, Sullivan EV. Different effects of dopaminergic and anticholinergic therapies on cognitive and motor function in Parkinson's disease. A follow-up study of untreated patients. Brain. 1992;115(Pt 6):1701–1725. doi: 10.1093/brain/115.6.1701. [DOI] [PubMed] [Google Scholar]
- Coutinho AM, Ghilardi MG, Campos ACP, Etchebehere E, Fonoff FC, Cury RG, Pagano RL, Martinez RCR, Fonoff ET. Does TRODAT-1 SPECT uptake correlate with cerebrospinal fluid α-synuclein levels in mid-stage Parkinson's disease? Biomedicines. 2023;11:296. doi: 10.3390/biomedicines11020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson SR, Held JM, Schilling B, Oo M, Gibson BW, Andersen JK. Preferentially increased nitration of alpha-synuclein at tyrosine-39 in a cellular oxidative model of Parkinson's disease. Anal Chem. 2009;81:7823–7828. doi: 10.1021/ac901176t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bie RM, de Haan RJ, Nijssen PC, Rutgers AW, Beute GN, Bosch DA, Haaxma R, Schmand B, Schuurman PR, Staal MJ, Speelman JD. Unilateral pallidotomy in Parkinson's disease: a randomised, single-blind, multicentre trial. Lancet. 1999;354:1665–1669. doi: 10.1016/S0140-6736(99)03556-4. [DOI] [PubMed] [Google Scholar]
- De Cock VC, Dodet P, Leu-Semenescu S, Aerts C, Castelnovo G, Abril B, Drapier S, Olivet H, Corbillé AG, Leclair-Visonneau L, Sallansonnet-Froment M, Lebouteux M, Anheim M, Ruppert E, Vitello N, Eusebio A, Lambert I, Marques A, Fantini ML, Devos D, et al. Safety and efficacy of subcutaneous night-time only apomorphine infusion to treat insomnia in patients with Parkinson's disease (APOMORPHEE): a multicentre, randomised, controlled, double-blind crossover study. Lancet Neurol. 2022;21:428–437. doi: 10.1016/S1474-4422(22)00085-0. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson's disease. Acta Neuropathol. 2010;119:703–713. doi: 10.1007/s00401-010-0665-2. [DOI] [PubMed] [Google Scholar]
- Dhakal S, Saha J, Wyant CE, Rangachari V. αS oligomers generated from interactions with a polyunsaturated fatty acid and a dopamine metabolite differentially interact with Aβ to enhance neurotoxicity. ACS Chem Neurosci. 2021;12:4153–4161. doi: 10.1021/acschemneuro.1c00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XB, Wang XX, Xia DH, Liu H, Tian HY, Fu Y, Chen YK, Qin C, Wang JQ, Xiang Z, Zhang ZX, Cao QC, Wang W, Li JY, Wu E, Tang BS, Ma MM, Teng JF, Wang XJ. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson's disease. Nat Med. 2021;27:411–418. doi: 10.1038/s41591-020-01198-1. [DOI] [PubMed] [Google Scholar]
- Dodiya HB, Forsyth CB, Voigt RM, Engen PA, Patel J, Shaikh M, Green SJ, Naqib A, Roy A, Kordower JH, Pahan K, Shannon KM, Keshavarzian A. Chronic stress-induced gut dysfunction exacerbates Parkinson's disease phenotype and pathology in a rotenone-induced mouse model of Parkinson's disease. Neurobiol Dis. 2020;135:104352. doi: 10.1016/j.nbd.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Dohgu S, Takata F, Matsumoto J, Kimura I, Yamauchi A, Kataoka Y. Monomeric α-synuclein induces blood-brain barrier dysfunction through activated brain pericytes releasing inflammatory mediators in vitro. Microvasc Res. 2019;124:61–66. doi: 10.1016/j.mvr.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Donaghy PC, Taylor JP, O'Brien JT, Barnett N, Olsen K, Colloby SJ, Lloyd J, Petrides G, McKeith IG, Thomas AJ. Neuropsychiatric symptoms and cognitive profile in mild cognitive impairment with Lewy bodies. Psychol Med. 2018;48:2384–2390. doi: 10.1017/S0033291717003956. [DOI] [PubMed] [Google Scholar]
- Doppler K, Mammadova S, Kuzkina A, Reetz K, Michels J, Hermann W, Sommerauer M, Volkmann J, Oertel WH, Janzen A, Sommer C. Association between probable REM sleep behavior disorder and increased dermal alpha-synuclein deposition in Parkinson's disease. Parkinsonism Relat Disord. 2022;99:58–61. doi: 10.1016/j.parkreldis.2022.05.010. [DOI] [PubMed] [Google Scholar]
- Doppler K, Jentschke HM, Schulmeyer L, Vadasz D, Janzen A, Luster M, Höffken H, Mayer G, Brumberg J, Booij J, Musacchio T, Klebe S, Sittig-Wiegand E, Volkmann J, Sommer C, Oertel WH. Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson's disease. Acta Neuropathol. 2017;133:535–545. doi: 10.1007/s00401-017-1684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube U, Ibanez L, Budde JP, Benitez BA, Davis AA, Harari O, Iles MM, Law MH, Brown KM, Cruchaga C. Overlapping genetic architecture between Parkinson disease and melanoma. Acta Neuropathol. 2020;139:347–364. doi: 10.1007/s00401-019-02110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls RH, Menees KB, Chung J, Barber J, Gutekunst CA, Hazim MG, Lee JK. Intrastriatal injection of preformed alpha-synuclein fibrils alters central and peripheral immune cell profiles in non-transgenic mice. J Neuroinflammation. 2019;16:250. doi: 10.1186/s12974-019-1636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Schlossmacher MG, Allsop D. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J. 2006;20:419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- Emamzadeh FN, Allsop D. α-Synuclein interacts with lipoproteins in plasma. J Mol Neurosci. 2017;63:165–172. doi: 10.1007/s12031-017-0967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E, Papagiannakis N, Kouloulia S, Galaziou A, Antonellou R, Papadimitriou D, Athanasiadou A, Bozi M, Koros C, Maniati M, Vekrellis K, Ioannou PC, Stefanis L. Peripheral alpha-synuclein levels in patients with genetic and non-genetic forms of Parkinson's disease. Parkinsonism Relat Disord. 2020;73:35–40. doi: 10.1016/j.parkreldis.2020.03.014. [DOI] [PubMed] [Google Scholar]
- Emmenegger M, De Cecco E, Hruska-Plochan M, Eninger T, Schneider MM, Barth M, Tantardini E, de Rossi P, Bacioglu M, Langston RG, Kaganovich A, Bengoa-Vergniory N, Gonzalez-Guerra A, Avar M, Heinzer D, Reimann R, Häsler LM, Herling TW, Matharu NS, Landeck N, et al. LAG3 is not expressed in human and murine neurons and does not modulate α-synucleinopathies. EMBO Mol Med. 2021;13:e14745. doi: 10.15252/emmm.202114745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzadfard A, König A, Petersen SV, Nielsen J, Vasili E, Dominguez-Meijide A, Buell AK, Outeiro TF, Otzen DE. Glycation modulates alpha-synuclein fibrillization kinetics: a sweet spot for inhibition. J Biol Chem. 2022;298:101848. doi: 10.1016/j.jbc.2022.101848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Zhou J, Liu H, Wu X, Li F, Zhao J, Zhang Y, Wang L, Chao M, Wang Q, Qin H, Ge S, Liu Q, Zhang J, Qu Y. Astrocytic NDRG2-PPM1A interaction exacerbates blood-brain barrier disruption after subarachnoid hemorrhage. Sci Adv. 2022;8:eabq2423. doi: 10.1126/sciadv.abq2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink AL. The aggregation and fibrillation of alpha-synuclein. Acc Chem Res. 2006;39:628–634. doi: 10.1021/ar050073t. [DOI] [PubMed] [Google Scholar]
- Froula JM, Castellana-Cruz M, Anabtawi NM, Camino JD, Chen SW, Thrasher DR, Freire J, Yazdi AA, Fleming S, Dobson CM, Kumita JR, Cremades N, Volpicelli-Daley LA. Defining α-synuclein species responsible for Parkinson's disease phenotypes in mice. J Biol Chem. 2019;294:10392–10406. doi: 10.1074/jbc.RA119.007743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Gasca-Salas C, Fernández-Rodríguez B, Pineda-Pardo JA, Rodríguez-Rojas R, Obeso I, Hernández-Fernández F, Del Álamo M, Mata D, Guida P, Ordás-Bandera C, Montero-Roblas JI, Martínez-Fernández R, Foffani G, Rachmilevitch I, Obeso JA. Blood-brain barrier opening with focused ultrasound in Parkinson's disease dementia. Nat Commun. 2021;12:779. doi: 10.1038/s41467-021-21022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelpi E, Navarro-Otano J, Tolosa E, Gaig C, Compta Y, Rey MJ, Martí MJ, Hernández I, Valldeoriola F, Reñé R, Ribalta T. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord. 2014;29:1010–1018. doi: 10.1002/mds.25776. [DOI] [PubMed] [Google Scholar]
- Ghanem SS, Majbour NK, Vaikath NN, Ardah MT, Erskine D, Jensen NM, Fayyad M, Sudhakaran IP, Vasili E, Melachroinou K, Abdi IY, Poggiolini I, Santos P, Dorn A, Carloni P, Vekrellis K, Attems J, McKeith I, Outeiro TF, Jensen PH, et al. α-Synuclein phosphorylation at serine 129 occurs after initial protein deposition and inhibits seeded fibril formation and toxicity. Proc Natl Acad Sci U S A. 2022;119:e2109617119. doi: 10.1073/pnas.2109617119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith JR, Herishanu YO, Podgaietski M, Kordysh E. Dynamics of parkinsonism-Parkinson's disease in residents of adjacent kibbutzim in Israel's Negev. Environ Res. 1997;73:156–161. doi: 10.1006/enrs.1997.3696. [DOI] [PubMed] [Google Scholar]
- Gregersen E, Betzer C, Kim WS, Kovacs G, Reimer L, Halliday GM, Thiel S, Jensen PH. Alpha-synuclein activates the classical complement pathway and mediates complement-dependent cell toxicity. J Neuroinflammation. 2021;18:177. doi: 10.1186/s12974-021-02225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Yang X, Mao X, Xu E, Qi C, Wang H, Brahmachari S, York B, Sriparna M, Li A, Chang M, Patel P, Dawson VL, Dawson TM. Lymphocyte activation gene 3 (Lag3) contributes to α-synucleinopathy in α-synuclein transgenic mice. Front Cell Neurosci. 2021;15:656426. doi: 10.3389/fncel.2021.656426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Shen XN, Huang SY, Chen SF, Wang HF, Zhang W, Zhang YR, Cheng W, Cui M, Dong Q, Yu JT. Head-to-head comparison of 6 plasma biomarkers in early multiple system atrophy. NPJ Parkinsons Dis. 2023;9:40. doi: 10.1038/s41531-023-00481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Thome AD, Yan Z, Schonhoff AM, Williams GP, Li X, Liu Y, Qin H, Benveniste EN, Standaert DG. Peripheral monocyte entry is required for alpha-Synuclein induced inflammation and Neurodegeneration in a model of Parkinson disease. Exp Neurol. 2018;300:179–187. doi: 10.1016/j.expneurol.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartlage-Rübsamen M, Bluhm A, Moceri S, Machner L, Köppen J, Schenk M, Hilbrich I, Holzer M, Weidenfeller M, Richter F, Coras R, Serrano GE, Beach TG, Schilling S, von Hörsten S, Xiang W, Schulze A, Roßner S. A glutaminyl cyclase-catalyzed α-synuclein modification identified in human synucleinopathies. Acta Neuropathol. 2021;142:399–421. doi: 10.1007/s00401-021-02349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass EW, Sorrentino ZA, Lloyd GM, McFarland NR, Prokop S, Giasson BI. Robust α-synuclein pathology in select brainstem neuronal populations is a potential instigator of multiple system atrophy. Acta Neuropathol Commun. 2021;9:80. doi: 10.1186/s40478-021-01173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy DG, Falchi M, O'Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helwig M, Klinkenberg M, Rusconi R, Musgrove RE, Majbour NK, El-Agnaf OM, Ulusoy A, Di Monte DA. Brain propagation of transduced α-synuclein involves non-fibrillar protein species and is enhanced in α-synuclein null mice. Brain. 2016;139:856–870. doi: 10.1093/brain/awv376. [DOI] [PubMed] [Google Scholar]
- Ho GPH, Wilkie EC, White AJ, Selkoe DJ. Palmitoylation of the Parkinson's disease-associated protein synaptotagmin-11 links its turnover to α-synuclein homeostasis. Sci Signal. 2023;16:eadd7220. doi: 10.1126/scisignal.add7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma T, Mochizuki Y, Mizutani T. Phosphorylated α-synuclein immunoreactivity in the posterior pituitary lobe. Neuropathology. 2012;32:385–389. doi: 10.1111/j.1440-1789.2011.01273.x. [DOI] [PubMed] [Google Scholar]
- Horsager J, Andersen KB, Knudsen K, Skjærbæk C, Fedorova TD, Okkels N, Schaeffer E, Bonkat SK, Geday J, Otto M, Sommerauer M, Danielsen EH, Bech E, Kraft J, Munk OL, Hansen SD, Pavese N, Göder R, Brooks DJ, Berg D, et al. Brain-first versus body-first Parkinson's disease: a multimodal imaging case-control study. Brain. 2020;143:3077–3088. doi: 10.1093/brain/awaa238. [DOI] [PubMed] [Google Scholar]
- Hu S, Hu M, Liu J, Zhang B, Zhang Z, Zhou FH, Wang L, Dong J. Phosphorylation of Tau and α-Synuclein Induced Neurodegeneration in MPTP Mouse Model of Parkinson's Disease. Neuropsychiatr Dis Treat. 2020;16:651–663. doi: 10.2147/NDT.S235562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Ding J, Wang X, Gu C, He Y, Li Y, Fan H, Xie Q, Qi X, Wang Z, Qiu P. Transfer of neuron-derived α-synuclein to astrocytes induces neuroinflammation and blood-brain barrier damage after methamphetamine exposure: Involving the regulation of nuclear receptor-associated protein 1. Brain Behav Immun. 2022a;106:247–261. doi: 10.1016/j.bbi.2022.09.002. [DOI] [PubMed] [Google Scholar]
- Huang S, Xu B, Liu Y. Calcium promotes α-synuclein liquid-liquid phase separation to accelerate amyloid aggregation. Biochem Biophys Res Commun. 2022b;603:13–20. doi: 10.1016/j.bbrc.2022.02.097. [DOI] [PubMed] [Google Scholar]
- Huang X, Wang C, Chen L, Zhang T, Leung KL, Wong G. Human amyloid beta and α-synuclein co-expression in neurons impair behavior and recapitulate features for Lewy body dementia in Caenorhabditis elegans. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166203. doi: 10.1016/j.bbadis.2021.166203. [DOI] [PubMed] [Google Scholar]
- Huang Y, Song YJ, Murphy K, Holton JL, Lashley T, Revesz T, Gai WP, Halliday GM. LRRK2 and parkin immunoreactivity in multiple system atrophy inclusions. Acta Neuropathol. 2008;116:639–646. doi: 10.1007/s00401-008-0446-3. [DOI] [PubMed] [Google Scholar]
- Idova GV, Al'perina EL, Gevorgyan MM, Tikhonova MA, Zhanaeva SY. Content of peripheral blood T- and B-cell subpopulations in transgenic A53T mice of different age (a model of Parkinson's disease) Bull Exp Biol Med. 2021;170:401–404. doi: 10.1007/s10517-021-05075-w. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T, Kakita A, Shiga A, Kasuga K, Kaneko H, Tan CF, Idezuka J, Wakabayashi K, Onodera O, Iwatsubo T, Nishizawa M, Takahashi H, Ishikawa A. Patients homozygous and heterozygous for SNCA duplication in a family with parkinsonism and dementia. Arch Neurol. 2008;65:514–519. doi: 10.1001/archneur.65.4.514. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Fairfoul G, Ayudhaya ACN, Serradell M, Gelpi E, Vilaseca I, Sanchez-Valle R, Gaig C, Santamaria J, Tolosa E, Riha RL, Green AJE. Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: a longitudinal observational study. Lancet Neurol. 2021;20:203–212. doi: 10.1016/S1474-4422(20)30449-X. [DOI] [PubMed] [Google Scholar]
- Israel Z, Asch N. Reversing a model of Parkinson's disease with in situ converted nigral neurons. Mov Disord. 2020;35:1955. doi: 10.1002/mds.28306. [DOI] [PubMed] [Google Scholar]
- Iyer A, Roeters SJ, Schilderink N, Hommersom B, Heeren RM, Woutersen S, Claessens MM, Subramaniam V. The impact of N-terminal acetylation of α-synuclein on phospholipid membrane binding and fibril structure. J Biol Chem. 2016;291:21110–21122. doi: 10.1074/jbc.M116.726612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa Y, Tateno H, Kameda H, Hirakawa K, Hato K, Yagi H, Hongo K, Mizobata T, Kawata Y. Role of C-terminal negative charges and tyrosine residues in fibril formation of α-synuclein. Brain Behav. 2012;2:595–605. doi: 10.1002/brb3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangula A, Murphy EJ. Lipopolysaccharide-induced blood brain barrier permeability is enhanced by alpha-synuclein expression. Neurosci Lett. 2013;551:23–27. doi: 10.1016/j.neulet.2013.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanshiri K, Drakenberg T, Haglund M, Englund E. Cardiac alpha-synuclein is present in alpha-synucleinopathies. J Parkinsons Dis. 2022;12:1125–1131. doi: 10.3233/JPD-223161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Matsumoto S, Ayaki T, Yamakado H, Taguchi T, Togawa N, Konno A, Hirai H, Nakajima H, Komai S, Ishida R, Chiba S, Takahashi R, Takao T, Hirotsune S. DOPAnization of tyrosine in α-synuclein by tyrosine hydroxylase leads to the formation of oligomers. Nat Commun. 2022;13:6880. doi: 10.1038/s41467-022-34555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi N, Sarhadi TR, Raveendran A, Nagotu S. Sporadic SNCA mutations A18T and A29S exhibit variable effects on protein aggregation, cell viability and oxidative stress. Mol Biol Rep. 2023;50:5547–5556. doi: 10.1007/s11033-023-08457-7. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Lang AE, Hazrati LN, Fujioka S, Wszolek ZK, Dickson DW, Ross OA, Van Deerlin VM, Trojanowski JQ, Hurtig HI, Alcalay RN, Marder KS, Clark LN, Gaig C, Tolosa E, Ruiz-Martínez J, Marti-Masso JF, Ferrer I, López de Munain A, Goldman SM, et al. Clinical correlations with Lewy body pathology in LRRK2-related Parkinson disease. JAMA Neurol. 2015;72:100–105. doi: 10.1001/jamaneurol.2014.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam TI, Park H, Chou SC, Van Vranken JG, Mittenbühler MJ, Kim H, A M, Choi YR, Biswas D, Wang J, Shin Y, Loder A, Karuppagounder SS, Wrann CD, Dawson VL, Spiegelman BM, Dawson TM. Amelioration of pathologic α-synuclein-induced Parkinson's disease by irisin. Proc Natl Acad Sci U S A. 2022;119:e2204835119. doi: 10.1073/pnas.2204835119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo T, Zhang J, Liu Q, Liu CC, Zhang L, Bu G. Heparan sulphate proteoglycan and the low-density lipoprotein receptor-related protein 1 constitute major pathways for neuronal amyloid-beta uptake. J Neurosci. 2011;31:1644–1651. doi: 10.1523/JNEUROSCI.5491-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikari AA, McFleder RL, Ribechini E, Blum R, Bruttel V, Knorr S, Gehmeyr M, Volkmann J, Brotchie JM, Ahsan F, Haack B, Monoranu CM, Keber U, Yeghiazaryan R, Pagenstecher A, Heckel T, Bischler T, Wischhusen J, Koprich JB, Lutz MB, et al. Neurodegeneration by α-synuclein-specific T cells in AAV-A53T-α-synuclein Parkinson's disease mice. Brain Behav Immun. 2022;101:194–210. doi: 10.1016/j.bbi.2022.01.007. [DOI] [PubMed] [Google Scholar]
- Killinger BA, Madaj Z, Sikora JW, Rey N, Haas AJ, Vepa Y, Lindqvist D, Chen H, Thomas PM, Brundin P, Brundin L, Labrie V. The vermiform appendix impacts the risk of developing Parkinson's disease. Sci Transl Med. 2018;10:eaar5280. doi: 10.1126/scitranslmed.aar5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Kwon S, Iba M, Spencer B, Rockenstein E, Mante M, Adame A, Shin SJ, Fields JA, Rissman RA, Lee SJ, Masliah E. Effects of innate immune receptor stimulation on extracellular α-synuclein uptake and degradation by brain resident cells. Exp Mol Med. 2021;53:281–290. doi: 10.1038/s12276-021-00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Yoo JM, Hwang H, Lee J, Lee SH, Yun SP, Park MJ, Lee M, Choi S, Kwon SH, Lee S, Kwon SH, Kim S, Park YJ, Kinoshita M, Lee YH, Shin S, Paik SR, Lee SJ, Lee S, et al. Graphene quantum dots prevent α-synucleinopathy in Parkinson's disease. Nat Nanotechnol. 2018;13:812–818. doi: 10.1038/s41565-018-0179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Sung JY, Lee HJ, Rhim H, Hasegawa M, Iwatsubo T, Min do S, Kim J, Paik SR, Chung KC. Dyrk1A phosphorylates alpha-synuclein and enhances intracellular inclusion formation. J Biol Chem. 2006;281:33250–33257. doi: 10.1074/jbc.M606147200. [DOI] [PubMed] [Google Scholar]
- Kim KS, Choi YR, Park JY, Lee JH, Kim DK, Lee SJ, Paik SR, Jou I, Park SM. Proteolytic cleavage of extracellular α-synuclein by plasmin: implications for Parkinson disease. J Biol Chem. 2012;287:24862–24872. doi: 10.1074/jbc.M112.348128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, Shen C, Lee H, Kulkarni S, Pasricha PJ, Lee G, Pomper MG, Dawson VL, Dawson TM, Ko HS. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson's disease. Neuron. 2019;103:627–641.e7. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Bae EJ, Jung BC, Choi M, Shin SJ, Park SJ, Kim JT, Jung MK, Ulusoy A, Song MY, Lee JS, Lee HJ, Di Monte DA, Lee SJ. Inflammation promotes synucleinopathy propagation. Exp Mol Med. 2022;54:2148–2161. doi: 10.1038/s12276-022-00895-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge A, Bunk J, Schaeffer E, Drobny A, Xiang W, Knacke H, Bub S, Lückstädt W, Arnold P, Lucius R, Berg D, Zunke F. Detection of neuron-derived pathological α-synuclein in blood. Brain. 2022;145:3058–3071. doi: 10.1093/brain/awac115. [DOI] [PubMed] [Google Scholar]
- Knudsen K, Fedorova TD, Hansen AK, Sommerauer M, Otto M, Svendsen KB, Nahimi A, Stokholm MG, Pavese N, Beier CP, Brooks DJ, Borghammer P. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol. 2018;17:618–628. doi: 10.1016/S1474-4422(18)30162-5. [DOI] [PubMed] [Google Scholar]
- Koga S, Sekiya H, Kondru N, Ross OA, Dickson DW. Neuropathology and molecular diagnosis of Synucleinopathies. Mol Neurodegener. 2021;16:83. doi: 10.1186/s13024-021-00501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari P, Ghosh D, Vanas A, Fleischmann Y, Wiegand T, Jeschke G, Riek R, Eichmann C. Structural insights into α-synuclein monomer-fibril interactions. Proc Natl Acad Sci U S A. 2021;118:e2012171118. doi: 10.1073/pnas.2012171118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunadt M, Eckermann K, Stuendl A, Gong J, Russo B, Strauss K, Rai S, Kügler S, Falomir Lockhart L, Schwalbe M, Krumova P, Oliveira LM, Bähr M, Möbius W, Levin J, Giese A, Kruse N, Mollenhauer B, Geiss-Friedlander R, Ludolph AC, et al. Extracellular vesicle sorting of α-synuclein is regulated by sumoylation. Acta Neuropathol. 2015;129:695–713. doi: 10.1007/s00401-015-1408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan G, Wang P, Chan RB, Liu Z, Yu Z, Liu X, Yang Y, Zhang J. Astrocytic VEGFA: an essential mediator in blood-brain-barrier disruption in Parkinson's disease. Glia. 2022;70:337–353. doi: 10.1002/glia.24109. [DOI] [PubMed] [Google Scholar]
- Lang AE, Siderowf AD, Macklin EA, Poewe W, Brooks DJ, Fernandez HH, Rascol O, Giladi N, Stocchi F, Tanner CM, Postuma RB, Simon DK, Tolosa E, Mollenhauer B, Cedarbaum JM, Fraser K, Xiao J, Evans KC, Graham DL, Sapir I, et al. Trial of cinpanemab in early Parkinson's disease. N Engl J Med. 2022;387:408–420. doi: 10.1056/NEJMoa2203395. [DOI] [PubMed] [Google Scholar]
- Lau A, So RWL, Lau HHC, Sang JC, Ruiz-Riquelme A, Fleck SC, Stuart E, Menon S, Visanji NP, Meisl G, Faidi R, Marano MM, Schmitt-Ulms C, Wang Z, Fraser PE, Tandon A, Hyman BT, Wille H, Ingelsson M, Klenerman D, et al. α-Synuclein strains target distinct brain regions and cell types. Nat Neurosci. 2020;23:21–31. doi: 10.1038/s41593-019-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau HHC, Martinez-Valbuena I, So RWL, Mehra S, Silver NRG, Mao A, Stuart E, Schmitt-Ulms C, Hyman BT, Ingelsson M, Kovacs GG, Watts JC. The G51D SNCA mutation generates a slowly progressive α-synuclein strain in early-onset Parkinson's disease. Acta Neuropathol Commun. 2023;11:72. doi: 10.1186/s40478-023-01570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Park CW, Paik SR, Choi KY. The modification of alpha-synuclein by dicarbonyl compounds inhibits its fibril-forming process. Biochim Biophys Acta. 20091794:421–430. doi: 10.1016/j.bbapap.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Leitão ADG, Rudolffi-Soto P, Chappard A, Bhumkar A, Lau D, Hunter DJB, Gambin Y, Sierecki E. Selectivity of Lewy body protein interactions along the aggregation pathway of α-synuclein. Commun Biol. 2021;4:1124. doi: 10.1038/s42003-021-02624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, Maddalozzo G, Batya SS. Tai chi and postural stability in patients with Parkinson's disease. N Engl J Med. 2012;366:511–519. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhao N, Zhang W, Li P, Yin X, Zhang W, Wang H, Tang B. Assessing the progression of early atherosclerosis mice using a fluorescence nanosensor for the simultaneous detection and imaging of pH and phosphorylation. Angew Chem Int Ed Engl. 2023a;62:e202215178. doi: 10.1002/anie.202215178. [DOI] [PubMed] [Google Scholar]
- Li S, Yue L, Chen S, Wu Z, Zhang J, Hong R, Xie L, Peng K, Wang C, Lin A, Jin L, Guan Q. High clinical diagnostic accuracy of combined salivary gland and myocardial metaiodobenzylguanidine scintigraphy in the diagnosis of Parkinson's disease. Front Aging Neurosci. 2022;14:1066331. doi: 10.3389/fnagi.2022.1066331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yang C, Wang S, Yang D, Zhang Y, Xu L, Ma L, Zheng J, Petersen RB, Zheng L, Chen H, Huang K. Copper and iron ions accelerate the prion-like propagation of α-synuclein: a vicious cycle in Parkinson's disease. Int J Biol Macromol. 2020;163:562–573. doi: 10.1016/j.ijbiomac.2020.06.274. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang T, Meng L, Jin L, Liu C, Liang Y, Ren L, Liu Y, Liu Y, Liu S, Li T, Liang Y, Chen X, Zhang Z. Novel naturally occurring autoantibodies attenuate α-synuclein pathology in a mouse model of Parkinson's disease. Neuropathol Appl Neurobiol. 2023b;49:e12860. doi: 10.1111/nan.12860. [DOI] [PubMed] [Google Scholar]
- Liu G, Yu Z, Gao L, Zheng Y, Feng T. Erythrocytic alpha-synuclein in early Parkinson's disease: A 3-year longitudinal study. Parkinsonism Relat Disord. 2022;104:44–48. doi: 10.1016/j.parkreldis.2022.09.011. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Yang Y, Stewart T, Shi M, Soltys D, Liu G, Thorland E, Cilento EM, Hou Y, Liu Z, Feng T, Zhang J. Reduced erythrocytic CHCHD2 mRNA is associated with brain pathology of Parkinson's disease. Acta Neuropathol Commun. 2021;9:37. doi: 10.1186/s40478-021-01133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Qiang M, Wei Y, He R. A novel molecular mechanism for nitrated {alpha}-synuclein-induced cell death. J Mol Cell Biol. 2011;3:239–249. doi: 10.1093/jmcb/mjr011. [DOI] [PubMed] [Google Scholar]
- Lobanova E, Whiten D, Ruggeri FS, Taylor CG, Kouli A, Xia Z, Emin D, Zhang YP, Lam JYL, Williams-Gray CH, Klenerman D. Imaging protein aggregates in the serum and cerebrospinal fluid in Parkinson's disease. Brain. 2022;145:632–643. doi: 10.1093/brain/awab306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan M, Sun Y, Chen J, Jiang Y, Li F, Wei L, Sun W, Ma J, Song L, Liu J, Liu B, Pei Y, Wang Z, Zhu L, Deng J. Diagnostic value of salivary real-time quaking-induced conversion in Parkinson's disease and multiple system atrophy. Mov Disord. 2022;37:1059–1063. doi: 10.1002/mds.28976. [DOI] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med. 2012a;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012b338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald JA, Chen JL, Masuda-Suzukake M, Schweighauser M, Jaunmuktane Z, Warner T, Holton JL, Grossman A, Berks R, Lavenir I, Goedert M. Assembly of α-synuclein and neurodegeneration in the central nervous system of heterozygous M83 mice following the peripheral administration of α-synuclein seeds. Acta Neuropathol Commun. 2021;9:189. doi: 10.1186/s40478-021-01291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahul-Mellier AL, Vercruysse F, Maco B, Ait-Bouziad N, De Roo M, Muller D, Lashuel HA. Fibril growth and seeding capacity play key roles in α-synuclein-mediated apoptotic cell death. Cell Death Differ. 2015;22:2107–2122. doi: 10.1038/cdd.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malec-Litwinowicz M, Plewka A, Plewka D, Bogunia E, Morek M, Szczudlik A, Szubiga M, Rudzińska-Bar M. The relation between plasma α-synuclein level and clinical symptoms or signs of Parkinson's disease. Neurol Neurochir Pol. 2018;52:243–251. doi: 10.1016/j.pjnns.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Manfredsson FP, Luk KC, Benskey MJ, Gezer A, Garcia J, Kuhn NC, Sandoval IM, Patterson JR, O'Mara A, Yonkers R, Kordower JH. Induction of alpha-synuclein pathology in the enteric nervous system of the rat and non-human primate results in gastrointestinal dysmotility and transient CNS pathology. Neurobiol Dis. 2018;112:106–118. doi: 10.1016/j.nbd.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin JH, Kang HC, Zhang J, Xu J, Chen R, Park H, Andrabi SA, Kang SU, Gonçalves RA, Liang Y, Zhang S, et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 2016;353:aah3374. doi: 10.1126/science.aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marano M, Anzini G, Musumeci G, Magliozzi A, Pozzilli V, Capone F, Di Lazzaro V. Transcutaneous auricular vagus stimulation improves gait and reaction time in Parkinson's disease. Mov Disord. 2022;37:2163–2164. doi: 10.1002/mds.29166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrachelli VG, Miranda FJ, Alabadí JA, Milán M, Cano-Jaimez M, Kirstein M, Alborch E, Fariñas I, Pérez-Sánchez F. Perivascular nerve fiber α-synuclein regulates contractility of mouse aorta: a link to autonomic dysfunction in Parkinson's disease. Neurochem Int. 2010;56:991–998. doi: 10.1016/j.neuint.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Martínez-Rodríguez TY, Rey-Buitrago M. Alpha sinuclein expression in blood and its relationship with chronic constipation in a population from Bogotá, D.C., with problems of alcohol consumption. Biomedica. 2020;40:309–321. doi: 10.7705/biomedica.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaracchia C, Hnida M, Gerhardt E, Lopes da Fonseca T, Villar-Pique A, Branco T, Stahlberg MA, Dean C, Fernández CO, Milosevic I, Outeiro TF. Membrane binding, internalization, and sorting of alpha-synuclein in the cell. Acta Neuropathol Commun. 2018;6:79. doi: 10.1186/s40478-018-0578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Shimozawa A, Hashimoto M, Hasegawa M. Common marmoset model of α-synuclein propagation. Methods Mol Biol. 20212322:131–139. doi: 10.1007/978-1-0716-1495-2_13. [DOI] [PubMed] [Google Scholar]
- Matsui H, Matsui N. Cerebrospinal fluid injection into adult zebrafish for disease research. J Neural Transm (Vienna) 2017;124:1627–1633. doi: 10.1007/s00702-017-1787-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto J, Stewart T, Sheng L, Li N, Bullock K, Song N, Shi M, Banks WA, Zhang J. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson's disease? Acta Neuropathol Commun. 2017;5:71. doi: 10.1186/s40478-017-0470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzetti S, Basellini MJ, Ferri V, Cassani E, Cereda E, Paolini M, Calogero AM, Bolliri C, De Leonardis M, Sacilotto G, Cilia R, Cappelletti G, Pezzoli G. α-Synuclein oligomers in skin biopsy of idiopathic and monozygotic twin patients with Parkinson's disease. Brain. 2020;143:920–931. doi: 10.1093/brain/awaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey RP, Ni X, Shadish JA, Jiang J, Lee JC. The N terminus of α-synuclein dictates fibril formation. Proc Natl Acad Sci U S A. 2021;118:e2023487118. doi: 10.1073/pnas.2023487118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegon A, Board PG, Blackburn AC, Mellick GD, Le Couteur DG. Parkinson's disease, pesticides, and glutathione transferase polymorphisms. Lancet. 1998;352:1344–1346. doi: 10.1016/s0140-6736(98)03453-9. [DOI] [PubMed] [Google Scholar]
- Miki Y, Shimoyama S, Kon T, Ueno T, Hayakari R, Tanji K, Matsumiya T, Tsushima E, Mori F, Wakabayashi K, Tomiyama M. Alteration of autophagy-related proteins in peripheral blood mononuclear cells of patients with Parkinson's disease. Neurobiol Aging. 2018;63:33–43. doi: 10.1016/j.neurobiolaging.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Hattori N, Mori H. Genetics of Parkinson's disease. Biomed Pharmacother. 1999;53:109–116. doi: 10.1016/S0753-3322(99)80075-4. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Döring F, Trenkwalder C, Schlossmacher MG. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol. 2011;10:230–240. doi: 10.1016/S1474-4422(11)70014-X. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Trautmann E, Taylor P, Manninger P, Sixel-Döring F, Ebentheuer J, Trenkwalder C, Schlossmacher MG. Total CSF α-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci Lett. 2013;532:44–48. doi: 10.1016/j.neulet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Nakai M, Fujita M, Waragai M, Sugama S, Wei J, Akatsu H, Ohtaka-Maruyama C, Okado H, Hashimoto M. Expression of alpha-synuclein, a presynaptic protein implicated in Parkinson's disease, in erythropoietic lineage. Biochem Biophys Res Commun. 2007;358:104–110. doi: 10.1016/j.bbrc.2007.04.108. [DOI] [PubMed] [Google Scholar]
- Navarro-Otano J, Gelpi E, Mestres CA, Quintana E, Rauek S, Ribalta T, Santiago V, Tolosa E. Alpha-synuclein aggregates in epicardial fat tissue in living subjects without parkinsonism. Parkinsonism Relat Disord. 2013;19:27–31. doi: 10.1016/j.parkreldis.2012.07.005. discussion 27. [DOI] [PubMed] [Google Scholar]
- Nawaz H, Sargent L, Quilon H, Cloud LJ, Testa CM, Snider JD, Lageman SK, Baron MS, Berman BD, Zimmerman K, Price ET, Mukhopadhyay ND, Barrett MJ. Anticholinergic medication burden in Parkinson's disease outpatients. J Parkinsons Dis. 2022;12:599–606. doi: 10.3233/JPD-212769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolano M, Caporaso G, Manganelli F, Stancanelli A, Borreca I, Mozzillo S, Tozza S, Dubbioso R, Iodice R, Vitale F, Koay S, Vichayanrat E, da Silva FV, Santoro L, Iodice V, Provitera V. Phosphorylated α-synuclein deposits in cutaneous nerves of early Parkinsonism. J Parkinsons Dis. 2022;12:2453–2468. doi: 10.3233/JPD-223421. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Iwatsubo T, Hasegawa M. Ubiquitination of alpha-synuclein. Biochemistry. 2005;44:361–368. doi: 10.1021/bi0485528. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Paviour D, Quinn NP, Josephs KA, Sangha H, Kilford L, Healy DG, Wood NW, Lees AJ, Holton JL, Revesz T. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain. 2004;127:2657–2671. doi: 10.1093/brain/awh303. [DOI] [PubMed] [Google Scholar]
- Padmaraju V, Bhaskar JJ, Prasada Rao UJ, Salimath PV, Rao KS. Role of advanced glycation on aggregation and DNA binding properties of α-synuclein. J Alzheimers Dis 24 Suppl. 2011;2:211–221. doi: 10.3233/JAD-2011-101965. [DOI] [PubMed] [Google Scholar]
- Pagano G, Taylor KI, Anzures-Cabrera J, Marchesi M, Simuni T, Marek K, Postuma RB, Pavese N, Stocchi F, Azulay JP, Mollenhauer B, López-Manzanares L, Russell DS, Boyd JT, Nicholas AP, Luquin MR, Hauser RA, Gasser T, Poewe W, Ricci B, et al. Trial of prasinezumab in early-stage Parkinson's disease. N Engl J Med. 2022;387:421–432. doi: 10.1056/NEJMoa2202867. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga J, Kulisevsky J, Strafella AP, Krack P. Apathy in Parkinson's disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015;14:518–531. doi: 10.1016/S1474-4422(15)00019-8. [DOI] [PubMed] [Google Scholar]
- Palma JA, Norcliffe-Kaufmann L, Kaufmann H. Orthostatic hypotension as a prodromal marker of α-synucleinopathies. JAMA Neurol. 2018;75:1154. doi: 10.1001/jamaneurol.2018.2248. [DOI] [PubMed] [Google Scholar]
- Panigrahi R, Krishnan R, Singh JS, Padinhateeri R, Kumar A. SUMO1 hinders α-Synuclein fibrillation by inducing structural compaction. Protein Sci. 2023;32:e4632. doi: 10.1002/pro.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagiannakis N, Xilouri M, Koros C, Stamelou M, Antonelou R, Maniati M, Papadimitriou D, Moraitou M, Michelakakis H, Stefanis L. Lysosomal alterations in peripheral blood mononuclear cells of Parkinson's disease patients. Mov Disord. 2015;30:1830–1834. doi: 10.1002/mds.26433. [DOI] [PubMed] [Google Scholar]
- Papp MI, Lantos PL. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain. 1994;117(Pt 2):235–243. doi: 10.1093/brain/117.2.235. [DOI] [PubMed] [Google Scholar]
- Park H, Kam TI, Peng H, Chou SC, Mehrabani-Tabari AA, Song JJ, Yin X, Karuppagounder SS, Umanah GK, Rao AVS, Choi Y, Aggarwal A, Chang S, Kim H, Byun J, Liu JO, Dawson TM, Dawson VL. PAAN/MIF nuclease inhibition prevents neurodegeneration in Parkinson's disease. Cell. 2022;185:1943–1959.e21. doi: 10.1016/j.cell.2022.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pediaditakis I, Kodella KR, Manatakis DV, Le CY, Hinojosa CD, Tien-Street W, Manolakos ES, Vekrellis K, Hamilton GA, Ewart L, Rubin LL, Karalis K. Modeling alpha-synuclein pathology in a human brain-chip to assess blood-brain barrier disruption. Nat Commun. 2021;12:5907. doi: 10.1038/s41467-021-26066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- Peelaerts W, Mercado G, George S, Villumsen M, Kasen A, Aguileta M, Linstow C, Sutter AB, Kuhn E, Stetzik L, Sheridan R, Bergkvist L, Meyerdirk L, Lindqvist A, Gavis MLE, Van den Haute C, Hultgren SJ, Baekelandt V, Pospisilik JA, Brudek T, et al. Urinary tract infections trigger synucleinopathy via the innate immune response. Acta Neuropathol. 2023;145:541–559. doi: 10.1007/s00401-023-02562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-DIaz S, Ventura S. One ring is sufficient to inhibit α-synuclein aggregation. Neural Regen Res. 2022;17:508–511. doi: 10.4103/1673-5374.320973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Gathagan RJ, Covell DJ, Medellin C, Stieber A, Robinson JL, Zhang B, Pitkin RM, Olufemi MF, Luk KC, Trojanowski JQ, Lee VM. Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature. 2018;557:558–563. doi: 10.1038/s41586-018-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissinotto F, Stani C, De Cecco E, Vaccari L, Rondelli V, Posocco P, Parisse P, Scaini D, Legname G, Casalis L. Iron-mediated interaction of alpha synuclein with lipid raft model membranes. Nanoscale. 2020;12:7631–7640. doi: 10.1039/d0nr00287a. [DOI] [PubMed] [Google Scholar]
- Phillips MCL, Murtagh DKJ, Gilbertson LJ, Asztely FJS, Lynch CDP. Low-fat versus ketogenic diet in Parkinson's disease: A pilot randomized controlled trial. Mov Disord. 2018;33:1306–1314. doi: 10.1002/mds.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluvinage JV, Haney MS, Smith BAH, Sun J, Iram T, Bonanno L, Li L, Lee DP, Morgens DW, Yang AC, Shuken SR, Gate D, Scott M, Khatri P, Luo J, Bertozzi CR, Bassik MC, Wyss-Coray T. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature. 2019;568:187–192. doi: 10.1038/s41586-019-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggiolini I, Gupta V, Lawton M, Lee S, El-Turabi A, Querejeta-Coma A, Trenkwalder C, Sixel-Döring F, Foubert-Samier A, Pavy-Le Traon A, Plazzi G, Biscarini F, Montplaisir J, Gagnon JF, Postuma RB, Antelmi E, Meissner WG, Mollenhauer B, Ben-Shlomo Y, Hu MT, et al. Diagnostic value of cerebrospinal fluid alpha-synuclein seed quantification in synucleinopathies. Brain. 2022;145:584–595. doi: 10.1093/brain/awab431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Porcari R, Proukakis C, Waudby CA, Bolognesi B, Mangione PP, Paton JF, Mullin S, Cabrita LD, Penco A, Relini A, Verona G, Vendruscolo M, Stoppini M, Tartaglia GG, Camilloni C, Christodoulou J, Schapira AH, Bellotti V. The H50Q mutation induces a 10-fold decrease in the solubility of α-synuclein. J Biol Chem. 2015;290:2395–2404. doi: 10.1074/jbc.M114.610527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto Huarcaya S, Drobny A, Marques ARA, Di Spiezio A, Dobert JP, Balta D, Werner C, Rizo T, Gallwitz L, Bub S, Stojkovska I, Belur NR, Fogh J, Mazzulli JR, Xiang W, Fulzele A, Dejung M, Sauer M, Winner B, Rose-John S, et al. Recombinant pro-CTSD (cathepsin D) enhances SNCA/α-synuclein degradation in α-Synucleinopathy models. Autophagy. 2022;18:1127–1151. doi: 10.1080/15548627.2022.2045534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch JN, Luna G, Guzman E, Audouard M, Challis C, Sibih YE, Leshuk C, Hernandez I, Wegmann S, Hyman BT, Gradinaru V, Kampmann M, Kosik KS. LRP1 is a master regulator of tau uptake and spread. Nature. 2020;580:381–385. doi: 10.1038/s41586-020-2156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JF, Ekmark-Léwen S, Perdiki M, Klingstedt T, Hoffmann A, Wiechec E, Nilsson P, Nilsson KPR, Alafuzoff I, Ingelsson M, Hallbeck M. Accumulation of alpha-synuclein within the liver, potential role in the clearance of brain pathology associated with Parkinson's disease. Acta Neuropathol Commun. 2021;9:46. doi: 10.1186/s40478-021-01136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA. The burden of Parkinson's disease: a worldwide perspective. Lancet Neurol. 2018;17:928–929. doi: 10.1016/S1474-4422(18)30355-7. [DOI] [PubMed] [Google Scholar]
- Rodrigues PV, de Godoy JVP, Bosque BP, Amorim Neto DP, Tostes K, Palameta S, Garcia-Rosa S, Tonoli CCC, de Carvalho HF, de Castro Fonseca M. Transcellular propagation of fibrillar α-synuclein from enteroendocrine to neuronal cells requires cell-to-cell contact and is Rab35-dependent. Sci Rep. 2022;12:4168. doi: 10.1038/s41598-022-08076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JA, Ivanova MI, Sawaya MR, Cascio D, Reyes FE, Shi D, Sangwan S, Guenther EL, Johnson LM, Zhang M, Jiang L, Arbing MA, Nannenga BL, Hattne J, Whitelegge J, Brewster AS, Messerschmidt M, Boutet S, Sauter NK, Gonen T, et al. Structure of the toxic core of α-synuclein from invisible crystals. Nature. 2015;525:486–490. doi: 10.1038/nature15368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshanbin S, Julku U, Xiong M, Eriksson J, Masliah E, Hultqvist G, Bergström J, Ingelsson M, Syvänen S, Sehlin D. Reduction of αSYN pathology in a mouse model of PD using a brain-penetrating bispecific antibody. Pharmaceutics. 2022;14:1412. doi: 10.3390/pharmaceutics14071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott R, Szargel R, Shani V, Hamza H, Savyon M, Abd Elghani F, Bandopadhyay R, Engelender S. SUMOylation and ubiquitination reciprocally regulate α-synuclein degradation and pathological aggregation. Proc Natl Acad Sci U S A. 2017;114:13176–13181. doi: 10.1073/pnas.1704351114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Z, Zhang D, Huang R, Sun W, Hou L, Zhao J, Wang Q. Microglial activation damages dopaminergic neurons through MMP-2/-9-mediated increase of blood-brain barrier permeability in a Parkinson's disease mouse model. Int J Mol Sci. 2022;23:2793. doi: 10.3390/ijms23052793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf WP, Palmer A, Dörfer L, Wiesner D, Buck E, Grozdanov V, Kassubek J, Dimou L, Ludolph AC, Huber-Lang M, Danzer KM. Thoracic trauma promotes alpha-Synuclein oligomerization in murine Parkinson's disease. Neurobiol Dis. 2022;174:105877. doi: 10.1016/j.nbd.2022.105877. [DOI] [PubMed] [Google Scholar]
- Salman MM, Kitchen P, Halsey A, Wang MX, Törnroth-Horsefield S, Conner AC, Badaut J, Iliff JJ, Bill RM. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain. 2022;145:64–75. doi: 10.1093/brain/awab311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Challis C, Jain N, Moiseyenko A, Ladinsky MS, Shastri GG, Thron T, Needham BD, Horvath I, Debelius JW, Janssen S, Knight R, Wittung-Stafshede P, Gradinaru V, Chapman M, Mazmanian SK. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. Elife. 2020;9:e53111. doi: 10.7554/eLife.53111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A, Dutta S, Camara A, Chandran A, Koller A, Watson BG, Sengupta R, Ysselstein D, Montenegro P, Cannon J, Rochet JC, Mattoo S. Alpha-synuclein is a target of Fic-mediated adenylylation/AMPylation: possible implications for Parkinson's disease. J Mol Biol. 2019;431:2266–2282. doi: 10.1016/j.jmb.2019.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- Scott GD, Lim MM, Drake MG, Woltjer R, Quinn JF. Onset of skin, gut, and genitourinary prodromal Parkinson's disease: a study of 1.5 million veterans. Mov Disord. 2021;36:2094–2103. doi: 10.1002/mds.28636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D, Dettmer U, Luth E, Kim N, Newman A, Bartels T. Defining the native state of α-synuclein. Neurodegener Dis. 2014;13:114–117. doi: 10.1159/000355516. [DOI] [PubMed] [Google Scholar]
- Semenyuk P, Barinova K, Muronetz V. Glycation of α-synuclein amplifies the binding with glyceraldehyde-3-phosphate dehydrogenase. Int J Biol Macromol. 2019;127:278–285. doi: 10.1016/j.ijbiomac.2019.01.064. [DOI] [PubMed] [Google Scholar]
- Sergeyeva TN, Sergeyev VG, Tolstolytskaya TO. Effects of bacterial endotoxin on α-synuclein expression in the lymph node leukocytes of rats. Bull Exp Biol Med. 2011;150:348–351. doi: 10.1007/s10517-011-1139-9. [DOI] [PubMed] [Google Scholar]
- Sevcsik E, Trexler AJ, Dunn JM, Rhoades E. Allostery in a disordered protein: oxidative modifications to α-synuclein act distally to regulate membrane binding. J Am Chem Soc. 2011;133:7152–7158. doi: 10.1021/ja2009554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharabi Y, Vatine GD, Ashkenazi A. Parkinson's disease outside the brain: targeting the autonomic nervous system. Lancet Neurol. 2021;20:868–876. doi: 10.1016/S1474-4422(21)00219-2. [DOI] [PubMed] [Google Scholar]
- Shin JW, An S, Kim D, Kim H, Ahn J, Eom J, You WK, Yun H, Lee B, Sung B, Jung J, Kim S, Son Y, Sung E, Lee H, Lee S, Song D, Pak Y, Sandhu JK, Haqqani AS, et al. Grabody B, an IGF1 receptor-based shuttle, mediates efficient delivery of biologics across the blood-brain barrier. Cell Rep Methods. 2022;2:100338. doi: 10.1016/j.crmeth.2022.100338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair E, Trivedi DK, Sarkar D, Walton-Doyle C, Milne J, Kunath T, Rijs AM, de Bie RMA, Goodacre R, Silverdale M, Barran P. Metabolomics of sebum reveals lipid dysregulation in Parkinson's disease. Nat Commun. 2021;12:1592. doi: 10.1038/s41467-021-21669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonustun B, Altay MF, Strand C, Ebanks K, Hondhamuni G, Warner TT, Lashuel HA, Bandopadhyay R. Pathological relevance of post-translationally modified alpha-synuclein (pSer87, pSer129, nTyr39) in idiopathic Parkinson's disease and multiple system atrophy. Cells. 2022;11:906. doi: 10.3390/cells11050906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehlmann R. The effects of acetylcholine and dopamine on the caudate nucleus depleted of biogenic amines. Brain. 1975;98:219–230. doi: 10.1093/brain/98.2.219. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Squair JW, Berney M, Castro Jimenez M, Hankov N, Demesmaeker R, Amir S, Paley A, Hernandez-Charpak S, Dumont G, Asboth L, Allenbach G, Becce F, Schoettker P, Wuerzner G, Bally JF, Courtine G, Bloch J. Implanted system for orthostatic hypotension in multiple-system atrophy. N Engl J Med. 2022;386:1339–1344. doi: 10.1056/NEJMoa2112809. [DOI] [PubMed] [Google Scholar]
- Stephens AD, Zacharopoulou M, Moons R, Fusco G, Seetaloo N, Chiki A, Woodhams PJ, Mela I, Lashuel HA, Phillips JJ, De Simone A, Sobott F, Schierle GSK. Extent of N-terminus exposure of monomeric alpha-synuclein determines its aggregation propensity. Nat Commun. 2020;11:2820. doi: 10.1038/s41467-020-16564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streubel-Gallasch L, Giusti V, Sandre M, Tessari I, Plotegher N, Giusto E, Masato A, Iovino L, Battisti I, Arrigoni G, Shimshek D, Greggio E, Tremblay ME, Bubacco L, Erlandsson A, Civiero L. Parkinson's disease-associated LRRK2 interferes with astrocyte-mediated alpha-synuclein clearance. Mol Neurobiol. 2021;58:3119–3140. doi: 10.1007/s12035-021-02327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Deng MF, Xiong W, Xie AJ, Guo J, Liang ZH, Hu B, Chen JG, Zhu X, Man HY, Lu Y, Liu D, Tang B, Zhu LQ. MicroRNA-26a/death-associated protein kinase 1 signaling induces synucleinopathy and dopaminergic neuron degeneration in Parkinson's disease. Biol Psychiatry. 2019;85:769–781. doi: 10.1016/j.biopsych.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui YT, Bullock KM, Erickson MA, Zhang J, Banks WA. Alpha synuclein is transported into and out of the brain by the blood-brain barrier. Peptides. 2014;62:197–202. doi: 10.1016/j.peptides.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, Dawson VL, Dawson TM, Oseroff C, Pham J, Sidney J, Dillon MB, Carpenter C, Weiskopf D, Phillips E, Mallal S, et al. T cells from patients with Parkinson's disease recognize α-synuclein peptides. Nature. 2017;546:656–661. doi: 10.1038/nature22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminath PV, Ragothaman M, Koshy S, Sarangmath N, Adhyam M, Subbakrishna DK, Mathias CJ, Muthane UB. Urogenital symptoms in Parkinson's disease and multiple system atrophy-Parkinsonism: at onset and later. J Assoc Physicians India. 2010;58:86–90. [PubMed] [Google Scholar]
- Tanei ZI, Saito Y, Ito S, Matsubara T, Motoda A, Yamazaki M, Sakashita Y, Kawakami I, Ikemura M, Tanaka S, Sengoku R, Arai T, Murayama S. Lewy pathology of the esophagus correlates with the progression of Lewy body disease: a Japanese cohort study of autopsy cases. Acta Neuropathol. 2021;141:25–37. doi: 10.1007/s00401-020-02233-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teil M, Dovero S, Bourdenx M, Arotcarena ML, Camus S, Porras G, Thiolat ML, Trigo-Damas I, Perier C, Estrada C, Garcia-Carrillo N, Morari M, Meissner WG, Herrero MT, Vila M, Obeso JA, Bezard E, Dehay B. Brain injections of glial cytoplasmic inclusions induce a multiple system atrophy-like pathology. Brain. 2022;145:1001–1017. doi: 10.1093/brain/awab374. [DOI] [PubMed] [Google Scholar]
- Theillet FX, Binolfi A, Bekei B, Martorana A, Rose HM, Stuiver M, Verzini S, Lorenz D, van Rossum M, Goldfarb D, Selenko P. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature. 2016;530:45–50. doi: 10.1038/nature16531. [DOI] [PubMed] [Google Scholar]
- Tian C, Liu G, Gao L, Soltys D, Pan C, Stewart T, Shi M, Xie Z, Liu N, Feng T, Zhang J. Erythrocytic α-synuclein as a potential biomarker for Parkinson's disease. Transl Neurodegener. 2019;8:15. doi: 10.1186/s40035-019-0155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson's disease. Lancet Neurol. 2006;5:75–86. doi: 10.1016/S1474-4422(05)70285-4. [DOI] [PubMed] [Google Scholar]
- Tsunemi T, Ishiguro Y, Yoroisaka A, Valdez C, Miyamoto K, Ishikawa K, Saiki S, Akamatsu W, Hattori N, Krainc D. Astrocytes protect human dopaminergic neurons from α-synuclein accumulation and propagation. J Neurosci. 2020;40:8618–8628. doi: 10.1523/JNEUROSCI.0954-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulisiak CT, Mercado G, Peelaerts W, Brundin L, Brundin P. Can infections trigger alpha-synucleinopathies? Prog Mol Biol Transl Sci. 2019;168:299–322. doi: 10.1016/bs.pmbts.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysnes OB, Storstein A. Epidemiology of Parkinson's disease. J Neural Transm (Vienna) 2017;124:901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- Uemura N, Marotta N, Ara J, Meymand E, Zhang B, Kameda H, Koike M, Luk K, Trojanowski J, Lee V. Distinct biological activity of Lewy body α-Synuclein strain in mice. Res Sq. 2023 doi: 10.1038/s41467-023-42705-5. doi: 10.21203/rs.3.rs-2579805/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara T, Oka H, Nakahara A, Shiraishi T, Sato T, Matsuno H, Komatsu T, Omoto S, Murakami H, Iguchi Y. Sympathetic nervous activity and hemoglobin levels in de novo Parkinson's disease. Clin Auton Res. 2020;30:273–278. doi: 10.1007/s10286-020-00668-3. [DOI] [PubMed] [Google Scholar]
- Van Den Berge N, Ferreira N, Mikkelsen TW, Alstrup AKO, Tamgüney G, Karlsson P, Terkelsen AJ, Nyengaard JR, Jensen PH, Borghammer P. Ageing promotes pathological alpha-synuclein propagation and autonomic dysfunction in wild-type rats. Brain. 2021;144:1853–1868. doi: 10.1093/brain/awab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Perren A, Gelders G, Fenyi A, Bousset L, Brito F, Peelaerts W, Van den Haute C, Gentleman S, Melki R, Baekelandt V. The structural differences between patient-derived α-synuclein strains dictate characteristics of Parkinson's disease, multiple system atrophy and dementia with Lewy bodies. Acta Neuropathol. 2020;139:977–1000. doi: 10.1007/s00401-020-02157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellingiri B, Suriyanarayanan A, Abraham KS, Venkatesan D, Iyer M, Raj N, Gopalakrishnan AV. Influence of heavy metals in Parkinson's disease: an overview. J Neurol. 2022;269:5798–5811. doi: 10.1007/s00415-022-11282-w. [DOI] [PubMed] [Google Scholar]
- Vicente Miranda H, Szego É M, Oliveira LMA, Breda C, Darendelioglu E, de Oliveira RM, Ferreira DG, Gomes MA, Rott R, Oliveira M, Munari F, Enguita FJ, Simões T, Rodrigues EF, Heinrich M, Martins IC, Zamolo I, Riess O, Cordeiro C, Ponces-Freire A, et al. Glycation potentiates α-synuclein-associated neurodegeneration in synucleinopathies. Brain. 2017;140:1399–1419. doi: 10.1093/brain/awx056. [DOI] [PubMed] [Google Scholar]
- Vilas D, Iranzo A, Tolosa E, Aldecoa I, Berenguer J, Vilaseca I, Martí C, Serradell M, Lomeña F, Alós L, Gaig C, Santamaria J, Gelpi E. Assessment of α-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol. 2016;15:708–718. doi: 10.1016/S1474-4422(16)00080-6. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H. The intermediolateral nucleus and Clarke's column in Parkinson's disease. Acta Neuropathol. 1997;94:287–289. doi: 10.1007/s004010050705. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, Takahashi H. Accumulation of alpha-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol. 1998;96:445–452. doi: 10.1007/s004010050918. [DOI] [PubMed] [Google Scholar]
- Wang C, Sharma SK, Olaluwoye OS, Alrashdi SA, Hasegawa T, Leblanc RM. Conformation change of α-synuclein(61–95) at the air-water interface and quantitative measurement of the tilt angle of the axis of its α-helix by multiple angle incidence resolution spectroscopy. Colloids Surf B Biointerfaces. 2019;183:110401. doi: 10.1016/j.colsurfb.2019.110401. [DOI] [PubMed] [Google Scholar]
- Wang C, Lau CY, Ma F, Zheng C. Genome-wide screen identifies curli amyloid fibril as a bacterial component promoting host neurodegeneration. Proc Natl Acad Sci U S A. 2021a;118:e2106504118. doi: 10.1073/pnas.2106504118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Qi W, Zou C, Xie Z, Zhang M, Naito MG, Mifflin L, Liu Z, Najafov A, Pan H, Shan B, Li Y, Zhu ZJ, Yuan J. NEK1-mediated retromer trafficking promotes blood-brain barrier integrity by regulating glucose metabolism and RIPK1 activation. Nat Commun. 2021b;12:4826. doi: 10.1038/s41467-021-25157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhu Y, Liu Z, Chang L, Bai X, Kang L, Cao Y, Yang X, Yu H, Shi MJ, Hu Y, Fan W, Zhao BQ. Neutrophil extracellular traps promote tPA-induced brain hemorrhage via cGAS in mice with stroke. Blood. 2021c;138:91–103. doi: 10.1182/blood.2020008913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Becker K, Donadio V, Siedlak S, Yuan J, Rezaee M, Incensi A, Kuzkina A, Orrú CD, Tatsuoka C, Liguori R, Gunzler SA, Caughey B, Jimenez-Capdeville ME, Zhu X, Doppler K, Cui L, Chen SG, Ma J, Zou WQ. Skin α-synuclein aggregation seeding activity as a novel biomarker for Parkinson disease. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.3311. doi: 10.1001/jamaneurol.2020.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman EA, Emmer KL, Giasson BI. Residue Glu83 plays a major role in negatively regulating alpha-synuclein amyloid formation. Biochem Biophys Res Commun. 2010;391:1415–1420. doi: 10.1016/j.bbrc.2009.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Zhang QS, Heng Y, Chen Y, Wang S, Yuan YH, Chen NH. NLRP3 inflammasome activation in the thymus of MPTP-induced Parkinsonian mouse model. Toxicol Lett. 2018;288:1–8. doi: 10.1016/j.toxlet.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Williams DR, Lees AJ. Visual hallucinations in the diagnosis of idiopathic Parkinson's disease: a retrospective autopsy study. Lancet Neurol. 2005;4:605–610. doi: 10.1016/S1474-4422(05)70146-0. [DOI] [PubMed] [Google Scholar]
- Williams GP, Schonhoff AM, Jurkuvenaite A, Gallups NJ, Standaert DG, Harms AS. CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson's disease. Brain. 2021;144:2047–2059. doi: 10.1093/brain/awab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. Parkinson disease: Peripheral α-synuclein deposits - prodromal markers for Parkinson disease? Nat Rev Neurol. 2016;12:249. doi: 10.1038/nrneurol.2016.54. [DOI] [PubMed] [Google Scholar]
- World Health Organization Parkinson disease. 2022. https://www.who.int/news-room/fact-sheets/detail/parkinson-disease. https://www.who.int/news-room/fact-sheets/detail/parkinson-disease Accessed June 13, 2022.
- Wu Z, Li X, Zeng M, Qiu H, Feng H, Xu X, Yu S, Wu J. Alpha-synuclein alterations in red blood cells of peripheral blood after acute ischemic stroke. Int J Clin Exp Pathol. 2019;12:1757–1763. [PMC free article] [PubMed] [Google Scholar]
- Wurster I, Quadalti C, Rossi M, Hauser AK, Deuschle C, Schulte C, Waniek K, Lachmann I, la Fougere C, Doppler K, Gasser T, Bender B, Parchi P, Brockmann K. Linking the phenotype of SNCA triplication with PET-MRI imaging pattern and alpha-synuclein CSF seeding. NPJ Parkinsons Dis. 2022;8:117. doi: 10.1038/s41531-022-00379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Zhang G, Kou L, Yin S, Han C, Hu J, Wan F, Sun Y, Wu J, Li Y, Huang J, Xiong N, Zhang Z, Wang T. Reactive microglia enhance the transmission of exosomal α-synuclein via toll-like receptor 2. Brain. 2021;144:2024–2037. doi: 10.1093/brain/awab122. [DOI] [PubMed] [Google Scholar]
- Xiao W, Shameli A, Harding CV, Meyerson HJ, Maitta RW. Late stages of hematopoiesis and B cell lymphopoiesis are regulated by α-synuclein, a key player in Parkinson's disease. Immunobiology. 2014;219:836–844. doi: 10.1016/j.imbio.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Nussinov R, Ma B. Coupling of the non-amyloid-component (NAC) domain and the KTK(E/Q)GV repeats stabilize the α-synuclein fibrils. Eur J Med Chem. 2016;121:841–850. doi: 10.1016/j.ejmech.2016.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Komatsu J, Nakamura K, Sakai K, Samuraki-Yokohama M, Nakajima K, Yoshita M. Diagnostic criteria for dementia with Lewy bodies: updates and future directions. J Mov Disord. 2020;13:1–10. doi: 10.14802/jmd.19052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki TR, Holmes BB, Furman JL, Dhavale DD, Su BW, Song ES, Cairns NJ, Kotzbauer PT, Diamond MI. Parkinson's disease and multiple system atrophy have distinct α-synuclein seed characteristics. J Biol Chem. 2019;294:1045–1058. doi: 10.1074/jbc.RA118.004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wang B, Hoop CL, Williams JK, Baum J. NMR unveils an N-terminal interaction interface on acetylated-α-synuclein monomers for recruitment to fibrils. Proc Natl Acad Sci U S A. 2021;118:e2017452118. doi: 10.1073/pnas.2017452118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shi Y, Schweighauser M, Zhang X, Kotecha A, Murzin AG, Garringer HJ, Cullinane PW, Saito Y, Foroud T, Warner TT, Hasegawa K, Vidal R, Murayama S, Revesz T, Ghetti B, Hasegawa M, Lashley T, Scheres SHW, Goedert M. Structures of α-synuclein filaments from human brains with Lewy pathology. Nature. 2022;610:791–795. doi: 10.1038/s41586-022-05319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Zarate N, White A, Coates D, Tsai W, Nanclares C, Cuccu F, Yue JS, Brown TG, Mansky RH, Jiang K, Kim H, Nichols-Meade T, Larson SN, Gundry K, Zhang Y, Tomas-Zapico C, Lucas JJ, Benneyworth M, Öz G, et al. CK2 alpha prime and alpha-synuclein pathogenic functional interaction mediates synaptic dysregulation in huntington's disease. Acta Neuropathol Commun. 2022a;10:83. doi: 10.1186/s40478-022-01379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Persillet M, Zhang L, Zhang Y, Xiuping S, Li X, Ran G, Breger LS, Dovero S, Porras G, Dehay B, Bezard E, Qin C. Evaluation of blood flow as a route for propagation in experimental synucleinopathy. Neurobiol Dis. 2021;150:105255. doi: 10.1016/j.nbd.2021.105255. [DOI] [PubMed] [Google Scholar]
- Yu Z, Liu G, Li Y, Arkin E, Zheng Y, Feng T. Erythrocytic α-synuclein species for Parkinson's disease diagnosis and the correlations with clinical characteristics. Front Aging Neurosci. 2022b;14:827493. doi: 10.3389/fnagi.2022.827493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Liu H, Zhang H, Wang T, Zheng Q, Li Z. Controlled activation of TRPV1 channels on microglia to boost their autophagy for clearance of alpha-synuclein and enhance therapy of Parkinson's disease. Adv Mater. 2022;34:e2108435. doi: 10.1002/adma.202108435. [DOI] [PubMed] [Google Scholar]
- Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, Kwon SH, Park YJ, Karuppagounder SS, Park H, Kim S, Oh N, Kim NA, Lee S, Brahmachari S, Mao X, Lee JH, Kumar M, An D, Kang SU, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nat Med. 2018;24:931–938. doi: 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lei H, Chen Y, Ma YT, Jiang F, Tan J, Zhang Y, Li JD. Enzymatic O-GlcNAcylation of α-synuclein reduces aggregation and increases SDS-resistant soluble oligomers. Neurosci Lett. 2017a;655:90–94. doi: 10.1016/j.neulet.2017.06.034. [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu YQ, Jia C, Lim YJ, Feng G, Xu E, Long H, Kimura Y, Tao Y, Zhao C, Wang C, Liu Z, Hu JJ, Ma MR, Liu Z, Jiang L, Li D, Wang R, Dawson VL, Dawson TM, et al. Mechanistic basis for receptor-mediated pathological α-synuclein fibril cell-to-cell transmission in Parkinson's disease. Proc Natl Acad Sci U S A. 2021;118:e2011196118. doi: 10.1073/pnas.2011196118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li J, Xu Q, Xia W, Tao Y, Shi C, Li D, Xiang S, Liu C. Conformational dynamics of an α-synuclein fibril upon receptor binding revealed by insensitive nuclei enhanced by polarization transfer-based solid-state nuclear magnetic resonance and cryo-electron microscopy. J Am Chem Soc. 2023a;145:4473–4484. doi: 10.1021/jacs.2c10854. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhu R, Pan B, Xu H, Olufemi MF, Gathagan RJ, Li Y, Zhang L, Zhang J, Xiang W, Kagan EM, Cao X, Yuan C, Kim SJ, Williams CK, Magaki S, Vinters HV, Lashuel HA, Garcia BA, James Petersson E, et al. Post-translational modifications of soluble α-synuclein regulate the amplification of pathological α-synuclein. Nat Neurosci. 2023b;26:213–225. doi: 10.1038/s41593-022-01239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang S, Li X, Li X, Ran W, Liu C, Tian W, Yu X, Wu C, Li P, Li N, Wei Y, Wang Y, Yu S, Chen Z. Hemoglobin-binding α-synuclein levels in erythrocytes are elevated in patients with multiple system atrophy. Neurosci Lett. 2022a;789:136868. doi: 10.1016/j.neulet.2022.136868. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Anwar S, Kim Y, Brown J, Comte I, Cai H, Cai NN, Wade-Martins R, Szele FG. The A30P α-synuclein mutation decreases subventricular zone proliferation. Hum Mol Genet. 2019;28:2283–2294. doi: 10.1093/hmg/ddz057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Roy DS, Zhu Y, Chen Y, Aida T, Hou Y, Shen C, Lea NE, Schroeder ME, Skaggs KM, Sullivan HA, Fischer KB, Callaway EM, Wickersham IR, Dai J, Li XM, Lu Z, Feng G. Targeting thalamic circuits rescues motor and mood deficits in PD mice. Nature. 2022b;607:321–329. doi: 10.1038/s41586-022-04806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang C, He XZ, Li ZH, Meng JC, Mao RT, Li X, Xue R, Gui Q, Zhang GX, Wang LH. Interaction between the glymphatic system and α-synuclein in Parkinson's disease. Mol Neurobiol. 2023c;60:2209–2222. doi: 10.1007/s12035-023-03212-2. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Kang SS, Liu X, Ahn EH, Zhang Z, He L, Iuvone PM, Duong DM, Seyfried NT, Benskey MJ, Manfredsson FP, Jin L, Sun YE, Wang JZ, Ye K. Asparagine endopeptidase cleaves α-synuclein and mediates pathologic activities in Parkinson's disease. Nat Struct Mol Biol. 2017b;24:632–642. doi: 10.1038/nsmb.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Lim YJ, Liu Z, Long H, Sun Y, Hu JJ, Zhao C, Tao Y, Zhang X, Li D, Li YM, Liu C. Parkinson's disease-related phosphorylation at Tyr39 rearranges α-synuclein amyloid fibril structure revealed by cryo-EM. Proc Natl Acad Sci U S A. 2020;117:20305–20315. doi: 10.1073/pnas.1922741117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Guo T, Meng L, Zhang X, Tian Y, Dai L, Niu X, Li Y, Liu C, Chen G, Liu C, Ke W, Zhang Z, Bao A, Zhang Z. N-homocysteinylation of α-synuclein promotes its aggregation and neurotoxicity. Aging Cell. 2023;22:e13745. doi: 10.1111/acel.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]


