Microglial cells are the only resident immune cells in the central nervous system and constitute its frontline guardian. They are extremely reactive against infections, trauma, or toxins, but are also responsible for mediating inflammation, taking part in the pathogenic course of many neuropathologies (Sierra et al., 2019). Cell-specific staining, ultrastructural analysis by transmission electron microscopy (TEM), or two-photon-microscopy imaging have been relevant for the characterization of microglia as well as their cell-cell interactions, which have led to a better understanding of microglial roles in health and disease.
Nowadays, we know that microglia are very dynamic cells that require a well-developed endomembrane system to respond to a plethora of stimuli and perform their major biological functions, e.g., self-renewal, migration to the damaged area, phagocytic activity, production, and release of anti- or pro-inflammatory cytokines, etc. According to the type and severity of the stimulation trigger, microglia can drastically change in morphology in the adult brain: They transform from branched tiny cells with homeostatic functions to cells with an amoeboid appearance due to retraction of their processes and enlargement of the cell body, which are more correlated with a pro-inflammatory phenotype. Among all the endomembranous compartments in microglia, the endoplasmic reticulum (ER) can be considered as a key organelle governing cellular metabolism, but recent evidence also points to a crucial role in modulating specific microglial functions.
Ultrastructural features of the ER in microglia: The ER constitutes a large and dynamic compartment that forms a continuous network of sheet-like cisternae with ribosomes on the cytosolic face of the membrane, the rough ER, and tubules lacking ribosomes, the smooth ER. Both differ in physical and functional characteristics. In microglial cells, the content of the rough ER is very abundant, and its ultrastructure can be easily visualized by TEM in ultrathin sections of brain tissue (Savage et al., 2018) or of primary microglial cell cultures (Figure 1). Cisternae may appear as long stretches arranged in parallel stacks or form extensive concentric systems resembling fingerprints. The lumen of the cisternae is quite narrow, but it might appear dilated by elevated production and secretion of cytokines and other factors, especially in the inflammatory microglial phenotype (Figure 1C). We can also find dilated ER under stress or pathological conditions (Bisht et al., 2016). Functionally, it is well known that the ER is involved in the synthesis, folding, modification, and transport of proteins and lipid metabolism, but the ER also represents the largest intracellular compartment for Ca2+ storage in all cells, including microglia. However, many questions remain unanswered about how this reservoir can participate in modulating Ca2+ signals in response to extra- or intracellular cues, microglial activation states, lesions, or pathologies.
Figure 1.
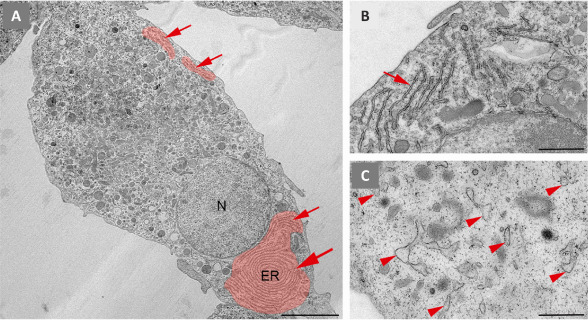
Ultrastructural features of the endoplasmic reticulum in primary microglia in culture.
We isolated microglia from cerebral cortex of neonatal mice. Subsequently, they were cultured in vitro and processed for transmission electron microscopy (TEM) visualization. (A) Ultrathin section showing a euchromatic nucleus (N) and a cytoplasm heavily filled with components of the endomembrane system, including dispersed long stretches (arrows) of rough endoplasmic reticulum (ER), that can adopt a characteristic fingerprint shape (big arrowhead). The ER was pseudocoloured in red. (B) High magnification of unstimulated microglia showing narrow long ER stretches (arrow). (C) High magnification of microglia stimulated with 100 ng/mL lipopolysaccharide for 24 hours to induce an inflammatory phenotype before TEM processing. The cell contains dilated and dispersed ER (arrowheads). Sourced from the authors' laboratory (unpublished data). Scale bars: 5 µm in A; 1 µm in B and C.
ER as Ca2+ store in microglia: Ca2+ as a secondary messenger is involved in many signaling networks. While in resting microglia the variations in the cytosolic Ca2+ concentration are minimal (nM range), high oscillations happen during microglial stimulation (Olmedillas Del Moral et al., 2019). As mentioned before, the ER is the main Ca2+ store in microglia, reaching intraluminal ER Ca2+ concentrations in the mM range. Thus, the ER plays a key role in regulating cytosolic Ca2+ levels, but it also contains intraluminal functions that are Ca2+-dependent. This high Ca2+ concentration in the ER compared to the cytosol is due to the active Ca2+ transport of the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) and the presence of luminal Ca2+-binding chaperones for sequestering free Ca2+. The flux of Ca2+ from the ER to the cytosol is mainly mediated by inositol 1,4,5-trisphosphate receptors and ryanodine receptors, and rapid transient depletion of Ca2+ from the ER can also be accompanied by store-operated Ca2+ entry at the plasma membrane. These events result in complex spatial-temporal Ca2+ signals that may control microglial functions in a stimulus-depending manner. Ca2+ dysregulation, in the cytosol as well as in Ca2+ stores, can drastically affect microglial physiology. Therefore, selective ligands and blockers of proteins involved in Ca2+ homeostasis are currently considered as novel compounds that might reduce the Ca2+-mediated chronic microglial activation that contributes to neuroinflammation in many diseases.
We recently reported the relevance of the SERCA Ca2+ transporter as a target to modulate intracellular Ca2+ dynamics in microglia (Morales-Ropero et al., 2021). We found an upregulated expression of SERCA2b, the main isoform in the brain, in lipopolysaccharide-stimulated microglia in culture, and, interestingly, in amoeboid microglia of post mortem human brains with Alzheimer's disease. This upregulation might be associated with a cellular aim to restore a maintained high cytosolic Ca2+ concentration caused by cell responses to strong inflammatory overstimulation. The role of SERCA as a major player regulating microglial functions was supported by our data demonstrating that its inhibition affects differentially specific functions, i.e. it stimulated microglial migration but inhibited phagocytosis (Morales-Ropero et al., 2021). Likewise, studies with the well-known microglial marker Ionized Ca2+-binding adaptor molecule 1 (Iba1), an actin-crosslinking protein, showed that its silencing inhibited actin dynamics involved in migration but stimulated phagocytic functions (Gheorghe et al., 2020). Altogether, these data clearly support that many cellular processes in microglia can be specifically, and differentially, switched on or off depending on cytosolic Ca2+ concentrations. Thus, the activity of Ca2+ transporters in the ER can be determinant to modulate Ca2+ signals in the cytosol. Nevertheless, the ER, although the largest one, is not the only Ca2+ store in microglia, and the role of other reservoirs in modulating cytosolic Ca2+ signaling remains to be addressed.
ER stress: The role of the ER in cellular protein metabolism is so important that proteins that fail to fold correctly within the ER are eliminated by several quality control mechanisms, e.g., chaperone-mediated folding or ER-associated degradation. However, disturbed Ca2+ homeostasis in the ER and/or overwhelming accumulation of misfolded proteins can induce a robust activation of unfolded protein response (UPR) genes. Although mild UPR is beneficial as it helps to restore cellular homeostasis, robust UPR can even drive to ER stress-associated cell death when prolonged over time (Almanza et al., 2019). Aberrant Ca2+ depletion in the ER can also result in the mass departure of ER-resident proteins in a process recently termed exodosis. Alterations in the ER proteome, chronic ER stress, and UPR can impact cellular functions and viability, often contributing to inflammatory response and disease pathogenesis. This is critical in many neurodegenerative diseases that include aberrant protein aggregation, but it is also a trigger for microglial cell death in spinal cord injury, hyperglycemia, sepsis, and other neuropathologies (Yi et al., 2023).
ER crosstalk with other organelles: The view of organelles as independent units in the cell has been left behind. New techniques show that the ER establishes functional contact sites with nearly all other membranous organelles (Wu et al., 2018). However, most of the attention has been focused on mitochondria-associated ER membranes. This inter-organellar communication is particularly interesting by its participation in lipid transfer, autophagy, ROS generation, or inflammation and because defective communication between ER and mitochondria is critical in cell survival, especially in neurodegenerative diseases. In addition, ER-mitochondria contact sites seem to play a crucial role in Ca2+ homeostasis, allowing Ca2+ flux between both of them to modulate their subcellular functions (de Ridder et al., 2023). However, pathological Ca2+ overload in mitochondria can cause cell death. This ER-mitochondria communication has recently been reported in microglia, where it appears to be involved in inflammasome activation (Pereira et al., 2022). Microglial ER stress and mitochondrial damage are present in several pathological conditions; thus, further research will support and clarify the functional importance of these contacts in microglia physiology.
ER in microglia-associated pathologies: Despite the main neuroprotective role of microglia as immune cells in the brain, it is also well reported that persistent microglial overstimulation can contribute to the inflammation present in several neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, or amyotrophic lateral sclerosis. This dual role seems to be disease phase-dependent, as early stages of the disease show a higher content of neuroprotective microglia, while pro-inflammatory microglia predominate at later stages. Interlinking pathways between ER stress, inflammation, and oxidative stress seem to be an important part of shifting the microglia population between different phenotypes (Asveda et al., 2023).
Searching for evidence to distinguish different microglial activation states, TEM studies uncovered a special microglial phenotype named dark microglia (Bisht et al., 2016). These cells are found in areas with neural dystrophy in different models of neurodegenerative diseases, in particular, related to Alzheimer's disease, and are characterized by chromatin condensation and an electro-dense cytoplasm that appears dark under TEM. Interestingly, another ultrastructural feature is the dilation of the ER that could be due to ER stress, including Ca2+ overload in the lumen. As a consequence, disturbances in the ER might affect Ca2+-dependent microglial functions that could be correlated with the hyperactive dark microglia, which exhibit high phagocytic activity at the synapse. These cells can be immunostained for the triggering receptor expression on myeloid cells-2 (Bisht et al., 2016), which is required to switch on the apolipoprotein E pathway to convert homeostatic microglia into dysfunctional ones in neurodegenerative diseases, characterized by a specific transcriptional signature (Krasemann et al., 2017). In addition, dark microglia are immunonegative for the homeostatic microglial marker P2ry12 and, thus, are likely to resemble these neurodegenerative P2ry12-negative and Clec7a-positive microglia found close to amyloid-β plaques (Krasemann et al., 2017). Although the transcriptomic data shed new light on this phenotype, we are still far from fully understanding its role in pathology.
In summary, these new insights about the ER attribute novel functions to this compartment in microglia, in addition to its canonical ones. A better knowledge of the subcellular biology of this glial-cell type may contribute in the future to disclosing potential therapeutic targets for neurodegenerative diseases associated with dysfunctional microglia.
We are grateful to Professor Miguel Ángel Cuadros from the Department of Cell Biology, University of Granada (UGR), Spain for critical reading and suggestions.
This work was funded by grants PPJIA2022.29 (to VEN) and PP2022.PP.29 (to MRS) from University of Granada Research Program, Spain.
Footnotes
Open peer reviewer: Elliot James Glotfelty, NIH, USA.
P-Reviewer: Glotfelty EJ; C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- Almanza A, Carlesso A, Chintha C, Creedican S, Doultsinos D, Leuzzi B, Luis A, McCarthy N, Montibeller L, More S, Papaioannou A, Puschel F, Sassano ML, Skoko J, Agostinis P, de Belleroche J, Eriksson LA, Fulda S, Gorman AM, Healy S, et al. Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J. 2019;286:241–278. doi: 10.1111/febs.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asveda T, Talwar P, Ravanan P. Exploring microglia and their phenomenal concatenation of stress responses in neurodegenerative disorders. Life Sci. 2023;328:121920. doi: 10.1016/j.lfs.2023.121920. [DOI] [PubMed] [Google Scholar]
- Bisht K, Sharma KP, Lecours C, Sanchez MG, El Hajj H, Milior G, Olmos-Alonso A, Gomez-Nicola D, Luheshi G, Vallieres L, Branchi I, Maggi L, Limatola C, Butovsky O, Tremblay ME. Dark microglia: a new phenotype predominantly associated with pathological states. Glia. 2016;64:826–839. doi: 10.1002/glia.22966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ridder I, Kerkhofs M, Lemos FO, Loncke J, Bultynck G, Parys JB. The ER-mitochondria interface, where Ca(2+) and cell death meet. Cell Calcium. 2023;112:102743. doi: 10.1016/j.ceca.2023.102743. [DOI] [PubMed] [Google Scholar]
- Gheorghe RO, Deftu A, Filippi A, Grosu A, Bica-Popi M, Chiritoiu M, Chiritoiu G, Munteanu C, Silvestro L, Ristoiu V. Silencing the cytoskeleton protein Iba1 (ionized calcium binding adapter protein 1) interferes with BV2 microglia functioning. Cell Mol Neurobiol. 2020;40:1011–1027. doi: 10.1007/s10571-020-00790-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O'Loughlin E, Xu Y, Fanek Z, Greco DJ, Smith ST, Tweet G, Humulock Z, Zrzavy T, Conde-Sanroman P, Gacias M, Weng Z, Chen H, Tjon E, et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47:566–581. doi: 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Ropero JM, Arroyo-Urea S, Neubrand VE, Martin-Oliva D, Marin-Teva JL, Cuadros MA, Vangheluwe P, Navascues J, Mata AM, Sepulveda MR. The endoplasmic reticulum Ca(2+) -ATPase SERCA2b is upregulated in activated microglia and its inhibition causes opposite effects on migration and phagocytosis. Glia. 2021;69:842–857. doi: 10.1002/glia.23931. [DOI] [PubMed] [Google Scholar]
- Olmedillas Del Moral M, Asavapanumas N, Uzcategui NL, Garaschuk O. Healthy brain aging modifies microglial calcium signaling in vivo. Int J Mol Sci. 2019;20:589. doi: 10.3390/ijms20030589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, De Pascale J, Resende R, Cardoso S, Ferreira I, Neves BM, Carrascal MA, Zuzarte M, Madeira N, Morais S, Macedo A, do Carmo A, Moreira PI, Cruz MT, Pereira CF. ER-mitochondria communication is involved in NLRP3 inflammasome activation under stress conditions in the innate immune system. Cell Mol Life Sci. 2022;79:213. doi: 10.1007/s00018-022-04211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JC, Picard K, Gonzalez-Ibanez F, Tremblay ME. A brief history of microglial ultrastructure: distinctive features, phenotypes, and functions discovered over the past 60 years by electron microscopy. Front Immunol. 2018;9:803. doi: 10.3389/fimmu.2018.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Paolicelli RC, Kettenmann H. Cien Anos de microglia: milestones in a century of microglial research. Trends Neurosci. 2019;42:778–792. doi: 10.1016/j.tins.2019.09.004. [DOI] [PubMed] [Google Scholar]
- Wu H, Carvalho P, Voeltz GK. Here, there, and everywhere: the importance of ER membrane contact sites. Science. 2018;361:eaan5835. doi: 10.1126/science.aan5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Duan Y, Song R, Zhou Y, Cui Y, Liu C, Mao Z, Hu J, Zhou F. Activation of glucagon-like peptide-1 receptor in microglia exerts protective effects against sepsis-induced encephalopathy via attenuating endoplasmic reticulum stress-associated inflammation and apoptosis in a mouse model of sepsis. Exp Neurol. 2023;363:114348. doi: 10.1016/j.expneurol.2023.114348. [DOI] [PubMed] [Google Scholar]


