Abstract
Background:
Infliximab (IFX) use is limited by loss of response often due to the development of anti-infliximab antibodies and low drug levels.
Methods:
We performed a single center prospective observational cohort study of pediatric and young adult subjects with IBD on infliximab with over 3 years of follow-up. Infliximab levels (IFXL) and antibodies to infliximab (ATI) were measured throughout the study. Subjects were followed until infliximab was discontinued.
Results:
We enrolled 219 subjects with IBD (184: Crohn Disease; 33: Ulcerative Colitis and 2 Indeterminant Colitis; 84 female, median age 14.4 years, 37 % on concomitant immunomodulator). 919 serum samples [mean 4.2 ± 2.1 per patient] were tested for IFXL and ATI. During the study, 31 (14%) subjects discontinued infliximab. 60 patients had ATI. 22 of those 60 patients with ATI discontinued infliximab; 14 of 31 patients who discontinued infliximab had detectable ATI at study onset. The combination of ATI and IFXL <5μg/mL at study entry was associated with the highest risk of drug discontinuation [(hazard ratios (HR) ATI 4.27 (p<0.001) and IFXL <5μg/mL [(HR 3.2) p=0.001]. Patients with IFXL 5-10 μg/mL had the lowest rate of discontinuation (6%). Infliximab dose escalation eliminated ATI in 21 of 60 subjects.
Conclusions:
ATI is a strong predictor of needing to stop infliximab use and inversely correlates with IFXL. Detection of ATI during therapeutic drug monitoring post induction but also periodically during maintenance therapy identifies individuals who may benefit from infliximab dose escalation and/or the addition of an immunomodulator, as these interventions may reduce or eliminate ATI.
Keywords: infliximab level, antibodies to infliximab, pediatric inflammatory bowel disease, Crohn disease, ulcerative colitis
Introduction:
Infliximab, a chimeric IgG1 monoclonal antibody against human tumor necrosis factor alpha (TNFα) is a highly effective therapy for both Crohn disease (CD) and ulcerative colitis (UC) [1] [2, 3]. Unfortunately, infliximab use is limited by loss of response (LOR) over time with rates of 13-46% annually [4, 5]. The precise mechanisms of LOR are unknown. Possibilities include formation of antibodies to infliximab (ATI), leading to more rapid drug clearance, mechanistic failure of blocking TNFα and low infliximab drug levels (IFXL) [6]. We and others have shown ATI development in IBD patients leads to lower IFXL, increased risk of surgery, decreased drug efficacy and loss of response [7, 8]. Combination therapy of infliximab with an immunomodulator (6-mercaptopurine or methotrexate) reduces ATI formation and increase IFXL [7, 9]. In adults with inflammatory bowel disease (IBD), IFXL correlate with clinical disease activity scores, serum acute phase reactants, and mucosal healing[8, 10] [11, 12]. Less is known about the correlation between IFXL and clinical outcomes in children [13-15]. Therefore, we studied a large prospective cohort of children and adolescents with IBD receiving infliximab to: 1) evaluate the relationship among IFXL, ATI to infliximab discontinuation (ID); and 2) identify clinical risk factors associated with ID. Our initial cross sectional study including some members of this cohort described the prevalence of ATI in children receiving infliximab, and the impact of combination therapy on IFXL [16] [7]. This paper highlights the impact of ATI and IFXL on infliximab discontinuation, in a larger prospective cohort.
Materials and Methods:
This was a prospective observational study of children and adolescents with IBD who received infliximab at Boston Children’s Hospital between June 2012 and October 2015. Inclusion criteria included any patient followed in our practice with IBD who was receiving infliximab and was willing to provide informed consent for additional serum to be obtained for research drug monitoring. Some subjects from our prior cross-sectional study were followed longitudinally for this study [16]. All participants in the study had research IFXL and ATI drawn by the study team, at the time of enrollment. A subset of patients had additional levels at subsequent infusions. Treating clinicians did not receive the results of the assays conducted by the research team (they were blinded as to the results of the research assays). Therefore, IFXL and ATI were obtained irrespective of disease activity. Treating clinicians ordered clinical IFXL and ATI based on the preference of the individual clinicians. IFXL and ATI ordered by treating clinicians were not included in the study, because assays were conducted at multiple different laboratories other than Prometheus which would have made it challenging to compare results. Combination therapy with immunomodulators was also at the discretion of the managing clinician.
Participants were followed until infliximab was discontinued, or until January 2017. Charts of those who discontinued infliximab were reviewed to determine the reason for infliximab discontinuation (ID). Serial serum IFXL and ATI were drawn immediately prior to scheduled infusions. Our study design was modeled after a prior adult study [5]. Clinical data abstracted from the medical record included: demographics, IBD type (UC, CD, or indeterminate colitis (IC)) and phenotype, age at diagnosis, use of immunomodulators, ESR, CRP, albumin, and duration and dosing of infliximab therapy. Disease activity was characterized using the Pediatric Crohn’s Disease Activity Index (PCDAI) or Pediatric Ulcerative Colitis Activity Index (PUCAI).
Serum IFXL and ATI levels were quantified using a homogenous mobility shift assay (HMSA) at Prometheus Laboratories Inc. (San Diego, CA). The limit of detection for ATI was 3.1 U/mL and the dynamic range for IFXL was 1.0-34.0 μg/mL. Lack of efficacy was determined by the primary treating physician necessitating a change in therapy. Combination therapy was defined as any use of thiopurines or methotrexate and categorized as prior (as combination therapy prior to study enrollment); current (combination therapy); or never use of an immunomodulator at study enrollment.
The incidence of infliximab discontinuation based upon disease type, IFXL and ATI was compared using survival analysis. Cox proportional Hazard models were used to examine the association between ID and the following risk factors (age, sex, BMI, ESR >20 mm/h, CRP >0.5 mg/dL, immunomodulator use, detectable ATI and IFXL <5μg/mL). An IFXL <5μg/mL was chosen because level was associated with clinical and biological response in both adult[12, 17] and pediatric studies[18] [19].
Frequency and percentages with t-tests for comparisons are reported for categorical variables, and median and IQR for continuous variables which were not normally distributed. Chi-squared tests were used for categorical variables. Cox proportional hazard models were used to examine the associations between combination therapy and IFXL with the risk of development of detectable ATI; combination therapy, IFXL, and detectable ATI with risk of ID. Any additional risk factors cited above, and albumin levels (continuous) and IBD type were included as covariates in the regression models. Statistical analyses were performed with SAS (release 9.4) and significance tests were 2-sided with P < 0.05 considered significant.
Ethical Considerations:
The study was approved by the Boston Children’s Hospital Institutional Review Board.
Results:
Patient Population
The 219 participants (38% female) had CD (n=184, 84%), UC (33, 15%) and IC (2, 1%). The mean age was 14.4 ± 3.8 years, and the mean follow-up duration was 38.8 months. The duration of treatment with infliximab at the time of enrollment for the entire cohort was (median 32.6 months, IQR 10.5-45.6): CD (median 34.9, IQR11.8-47.8), IC (median 11.7, IQR 1.3-22.0) and UC (median 20.9, IQR 6.7-31.3). For subsequent analysis the UC and IC groups were pooled. Most patients were primary responders to IFX on maintenance therapy. Specifically, 34 (15%) patients were enrolled within 6 months of starting infliximab and 17 were within 2 months of starting IFX so 202 of 219 patients (93%) were on maintenance therapy. A total of 919 serum samples [mean 4.2 ± 2.1 per patient] were assayed for ATI and IFXL. The median interval between samples was 7 months. Two patients had 10 serial samples (0.9% of our study population); 2 had 9 samples (0.9%); 10 had 8 samples (4.6%), 24 had 7 samples (11%), 24 had 6 samples (11%), 24 had 5 samples (11%), 39 had 4 samples (17.8%), 43 had 3 samples (15%), 33 had 2 samples (4.6%), and 18 had 1 sample (8%).
Thirty-one participants (14%) discontinued infliximab (20 with CD, 11 with UC/IC) during the follow up period. Seventeen had lack of efficacy determined by their treating clinician, 11 had infusion reactions (9 acute, 2 serum sickness), and one each developed infliximab induced lupus, psoriasis, and elevated transaminases.
Among the entire cohort, duration of infliximab therapy prior to enrollment was associated with staying on infliximab (p<0.01, Table 1). Given the lower-than-expected infliximab discontinuation rates, we examined dose escalation (increased mg/kg dose or more frequent infusions) prior to study entry. The rate of infliximab discontinuation among those who had their dose escalated prior to study entry was similar to those without dose adjustment (14/104, 13% vs. 17/115, 15%; p=0.78). Serum albumin concentration did not differ between groups.
Table 1.
Study population according to infliximab discontinuation (n=219)
| IFX discontinuation during the follow-up | |||
|---|---|---|---|
| No (n=188) | Yes (n=31) | P value | |
| Age at diagnosis, yrs, median (IQR) | 12.1(9.7,14.4) | 11.2(9.8,15.4) | 0.83 |
| Age at enrollment, yrs, median (IQR) | 14.3(12.0,19.0) | 14.9(11.3,17.8) | 0.80 |
| Female, n (%) | 69(36.7%) | 15(48.3%) | 0.21 |
| Body mass index, kg/m2, median (IQR) | 21.5(19.324.2) | 21.5(20.1,23.9) | 0.80 |
| Antibodies to infliximab >3.1 U/mL, n (%) | 22(11.7%) | 14(45.2%) | <0.001 |
| Antibodies to infliximab level categories, n (%) | <0.001 * | ||
| Undetectable | 165(88.2%) | 17(54.8%) | |
| >3.1-<7 U/mL | 3(1.6%) | 3(9.7%) | |
| ≥7 U/mL | 19(10.2%) | 11(35.5%) | |
| Months on infliximab at enrollment, median (IQR) | 24.5(12.3,46.8) | 14.3(3.7,27.0) | <0.01 |
| Duration of follow-up, months, median (IQR) | 48.2(32.9,50.2) | 13.6(4.4,18.5) | <0.001 |
| Infliximab level, μg/mL, median (IQR) | 10.0(5.1,23.5) | 4.4(1.0,17.7) | 0.01 |
| Infliximab<3.5 μg/mL, n (%) | 34(18.1%) | 14(45.2%) | <0.001 |
| Infliximab<5.0 μg/mL, n (%) | 46(24.5%) | 16(51.6%) | 0.002 |
| Albumin, g/dL, median (IQR) | 4.40(4.20,4.50) | 4.20(4.00,4.50) | 0.23 |
| Albumin<3 g/dL, n (%) | 0 | 0 | |
| CRP, mg/dL | 0.10(0.10,0.24) | 0.19(0.10,0.58) | 0.02 |
| CRP>0.5mg/dL, n (%) | 20(10.9%) | 11(29.7%) | 0.004 * |
| Type of IBD, n (%) | 0.003 * | ||
| Crohn's Disease | 164(87.2%) | 20(64.5%) | |
| Ulcerative Colitis | 24(12.8%) | 11(35.5%) | |
| Combination therapy, n (%) | <0.05 | ||
| Current | 71(37.8%) | 10(32.3%) | |
| Prior | 94(50.0%) | 12(38.7%) | |
| Never | 23(12.2%) | 9(29.0%) | |
| Crohn's Disease Location, Paris classification, n (%) | |||
| L1: distal 1/3 ileum +/− limited cecal disease | 17(10.4%) | 3(15.0%) | 0.46* |
| L2: Colonic | 8(4.9%) | 3(15.0%) | 0.10* |
| L3: Ileocolonic | 135(82.3%) | 13(65.0%) | 0.08* |
| L4: Isolated upper disease | 0 | 0 | |
| L4a: upper disease proximal to Ligament of Treitz | 110(67.1%) | 11(55.0%) | 0.28 |
| L4b: upper disease distal to Ligament of Treitz and proximal to distal 1/3 ileum | 20(12.2) | 1(5.0%) | 0.48* |
| Crohn's Disease Behavior, Paris classification, n (%) | 0.09* | ||
| Non-stricturing, non-penetrating | 122(74.4%) | 13(68.4%) | |
| Stricturing only | 28(17.1%) | 2(10.5%) | |
| Penetrating only | 6(3.7%) | 0 | |
| Sticturing and Penetrating | 8(4.9%) | 4(21.1%) | |
| Perianal, n (%) | 57(34.8%) | 8(40.0%) | 0.64 |
| Pediatric Crohn’s Disease Activity Index score, median (IQR) | 5.0(0,10.0) | 5.0(2.5,11.3) | 0.11 |
| Ulcerative Colitis Disease Location, Paris classification, n (%) | 0.24* | ||
| E1: Ulcerative proctitis | 0 | 0 | |
| E2: Left-sided UC (distal to splenic flexure) | 4(17.4%) | 0 | |
| E3: Extensive (hepatic flexure distally) | 2(8.7%) | 0 | |
| E4: Pancolitis (proximal to hepatic flexure) | 17(73.9%) | 10(100.0%) | |
| Ulcerative Colitis Disease Severity, Paris classification, n (%) | 0.72* | ||
| never severe | 12(52.2%) | 6(60.0%) | |
| ever severe | 11(47.8%) | 4(40.0%) | |
| PUCAI score, median (IQR) | 7.5(0,10.0) | 20.0(5.0,25.0) | 0.08 |
Fisher's exact test was used to get P value due to more than 25% cells with expected count less than 5.
IQR – Interquartile range
The study cohort included 3 patients who had previously been on adalimumab, and 1 had been on certolizumab. None of these patients had ATI at enrollment nor did they develop ATI during the study.
Risk factors for infliximab discontinuation
In univariate analysis, enrollment characteristics associated with infliximab discontinuation were a diagnosis of UC [hazard ratio (HR) 2.98, confidence interval (CI): 1.36-6.55, p = 0.007], CRP >0.5mg/dL (HR 1.31, 95% CI: 1.05-1.63, p = 0.02), IFXL<5.0 μg/mL (HR 3.18, CI 1.56-6.49, p = 0.001 and detectable ATI (HR 4.27, CI: 2.09-8.73, p = < 0.001) (Table 2). All those who discontinued infliximab in the UC/IC group had pancolitis. Age, gender, BMI and combination therapy were not associated with drug discontinuation. Detectable ATI at enrollment correlated with discontinuation even after adjusting for confounders including age, sex, BMI, CRP>0.5mg/dL, albumin level and disease type.
Table 2.
Risk factors at enrollment for IFX discontinuation (n=219)
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR(95%CI) | P value |
HR(95%CI) | P value | |
| Age | 1.02(0.93-1.12) | 0.63 | 1.00(0.89-1.11) | 0.92 |
| Female vs male | 1.62(0.80-3.28) | 0.18 | 1.71(0.78-3.76) | 0.18 |
| Body Mass Index | 1.03(0.95-1.11) | 0.49 | 1.04(0.95-1.14) | 0.39 |
| Combined immunotherapy | ||||
| Never | 1.00 | 1.00 | ||
| Prior | 0.64(0.25-1.62) | 0.34 | 1.29(0.45-3.65) | 0.63 |
| Current | 0.45(0.18-1.13) | 0.09 | 1.25(0.41-3.78) | 0.69 |
| Detectable antibodies to Infliximab (ATI)a | 4.27(2.09-8.73) | <0.001 | 3.22(1.35-7.65) | 0.008 |
| Infliximab<5.0 μg/mL | 3.18(1.56-6.49) | 0.001 | 2.10(0.85-5.19) | 0.11 |
| CRP | 1.31(1.05-1.63) | 0.02 | 1.30(0.92-1.85) | 0.14 |
| Albumin | 0.44(0.15-1.27) | 0.13 | 1.29(034-4.99) | 0.71 |
| Type of IBD | ||||
| Crohn's Disease | 1.00 | 1.00 | ||
| Ulcerative Colitis | 2.96(1.41-6.20) | 0.004 | 2.98(1.36-6.55) | 0.007 |
Detectable Antibodies To Infliximab >3.1 U/mL
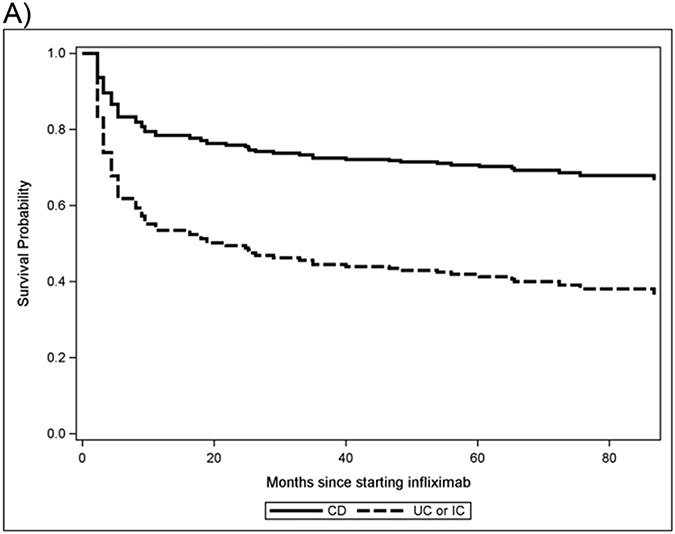
In multivariate analysis, the HR for detectable ATI at enrollment was HR 3.22 (CI: 1.35-7.65) and HR for UC/IC was similar at 2.98 (CI: 1.36-6.55). Neither IFXL <5 μg/mL nor CRP >0.5 mg/dL were significant in the multivariate model. UC/IC patients were more likely to discontinue infliximab in survival analysis compared to CD (p=0.007) Figure 1).
Figure 1a. Survival curve of IFXL discontinuation by IBD disease type.
Survival probability is defined as the likelihood that a subject will remain on the current treatment (infliximab) for the duration of the study, resulting in a higher or lower rates of drug discontinuation. Subjects with ulcerative colitis (UC) and indeterminate colitis (IC) have a lower survival probability than patients with Crohn’s disease (CD), indicating a higher rate of drug discontinuation (P=0.006).
Dynamics of ATI, IFXL and infliximab discontinuation
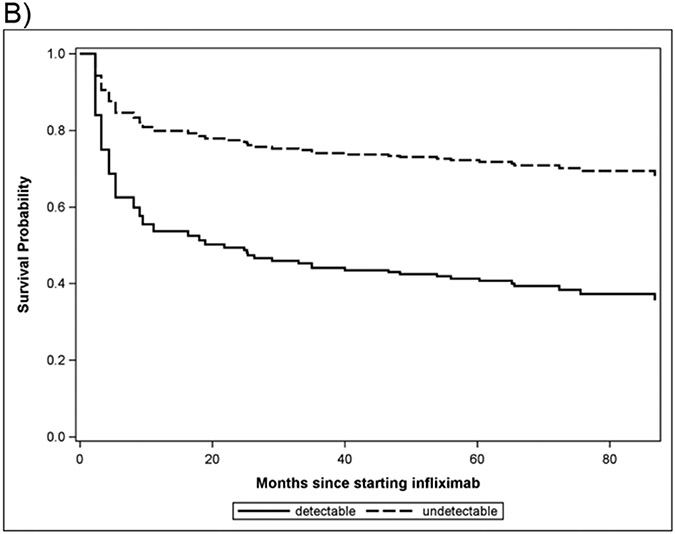
This was a blinded study, where treating clinicians could manage patients according to their best practices (including ordering drug levels clinically) but were unaware of the results of the research levels. Therefore, the 36 participants with detectable ATI at enrollment continued to receive infliximab as prescribed by their treating clinicians who were blinded to the study drug levels but were able to order levels based on their clinical judgement. This group with positive ATI had a higher rate of drug discontinuation (45%, 14/31) than those who did not have detectable ATI at enrollment (11% 22/188) (Table 1 p = <0.001 and Survival Figure 1b; p = 0.02). Since some investigators have suggested that low level ATI may be transient [20], and have less of an effect on loss of response, we subdivided ATI into categories by level [undetectable level(≤3.1 U/mL), low level ATIs (>3.1-<7U/mL) and high level (>7 U/mL)]. 31 participants with ATI at enrollment continued to receive infliximab but only 14 (45%) discontinued infliximab.
Figure 1b. Survival curve of IFXL discontinuation by presence of ATI at enrollment.
Survival probability is defined as the likelihood that a subject will remain on infliximab for the duration of the study. Detectable antibodies to infliximab (ATI) are associated with lower survival probability and thus higher rates of drug discontinuation (P=0.02).
Sixty patients had ATI at enrollment or developed ATI during the study. Thirty participants had ATI at only one time point, and the remaining 30 had ATI at two or more timepoints. Twenty-two of 60 discontinued infliximab use. Eight of 30 patients identified once with detectable ATI stopped infliximab during the study. Fourteen of 30 patients identified two or more times with detectable ATI ultimately discontinued infliximab. The time from detection of ATI to discontinuing infliximab was 0-48 months (median:10, IQR: 4.4-16 months). Half of those 60 with ATI continued IFX this suggests that loss of response even with antibodies may not be immediate.
We recorded interventions in the 60 patients that had ATI at enrollment or developed ATI during the study (Supplemental Fig 1). Twenty-nine underwent dose escalation 21 of those (75%) which resulted in successful elimination of ATI (p=0.01 when compared to no intervention). Two had dose escalation and 6MP added and one of them no longer had ATI (50%, p = 1.0). Four had 6MP added alone and none had elimination of ATI (p = 0.29). Twenty-one had no intervention and of those 7 had spontaneous resolution of ATI (33%, reference group) (Supplemental Figure 1). Four had detectable ATI but only one ATI measurement so we cannot speak to the result of dose adjustments or the addition of immunomodulator.
Twenty-four of the 188 participants who did not have detectable ATI at enrollment developed ATI during the study. In multivariable analysis, no association was found between low levels at study entry (IFXL<5.0μg/mL) and risk of developing ATI during the study (HR 0.30, p=0.16). The statistical model adjusted for age, sex, disease type (CD or UC/IC), BMI, CRP, albumin which were not significant. Prior or current combination therapy was protective (HR 0.11, p=0.004; HR 0.22, p=0.03). Eight (33.3%) of 24 patients (who developed ATI) stopped IFX.
The median IFXL at enrollment for the entire cohort was 13.3μg/mL (IQR 4.3-22.2). Lower IFXL was noted in those that discontinued drug compared to those that continued IFX (4.4 vs 10 μg/mL, p= 0.01, Table 1). Levels of 5-10 μg/mL had the lowest rates of drug discontinuation (6%). Nearly half (45%) of those with a level of <5 μg/mL discontinued drug during study. At higher levels of 10-15 μg/mL 10% discontinued infliximab and at >15 μg/mL 12% stopped infliximab; potentially because clinicians were dose escalating in the setting of mechanistic failure (meaning that blocking TNFα was no longer effective at treating inflammation regardless of IFXL or ATI). Overall, 52% of patients who discontinued infliximab had an enrollment IFXL level <5 μg/mL, compared to 25% of patients who continued to receive infliximab (p=0.002). In survival analysis, discontinuing infliximab with IFXL <5 μg/mL compared to those with ≥5 μg/mL was not statistically significant (p=0.14, Figure 1c)
Figure 1c. Survival curve of IFXL discontinuation by concentration<5μg/mL.
Survival probability is defined as the likelihood that a subject will remain on infliximab for the duration of the study. Subjects with IFXL levels <5.0μg/mL or IFXL ≥5.0μg/mL did not differ with respect to remaining on infliximab (P=0.14).
Immunomodulators, development of ATI and drug discontinuation
In our cohort, 10/81 (12%) participants on current combination therapy at enrollment (17 thiopurines, 64 methotrexate) discontinued infliximab during the study. There were only 32 patients who were never on an immunomodulator, 9 (28%) of whom discontinued infliximab. The remaining 106 patients had been exposed to at least one immunomodulator prior to study enrollment (91 thiopurine, 24 methotrexate), 12 (11%) of whom discontinued infliximab. Immunomodulator-naïve subjects had the highest prevalence of ATI. Specifically, 22/81 current (26%; 9 at enrollment, 12 subsequently), 23/106 prior (21%, 16 at enrollment, 12 subsequently) and 16/32 never (48%, 11 at enrollment, 5 subsequently) combination therapy users developed ATI. For the 183 with undetectable ATI at study enrollment, prior and current use of immunomodulator therapy was associated with decreased risk of developing ATI (prior HR 0.10, p=0.002, current HR 0.21, p=0.02, Table 3).
Table 3.
The association of combination therapy and plasma IFX concentrations with the risk of development of detectable Antibodies To Infliximab (n=183) a
| Univariate | Multivariate b | ||||
|---|---|---|---|---|---|
| event/N | HR(95%CI) | P value | HR(95%CI) | P value | |
| Age | 1.10(0.98,1.23) | 0.11 | 1.17(1.01,1.37) | 0.04 | |
| Female vs male | 1.13(0.49,2.60) | 0.77 | 0.62(0.24,1.61) | 0.33 | |
| Body Mass Index | 1.09(1.01,1.18) | 0.03 | 1.08(0.99,1.18) | 0.08 | |
| CRP | 0.19(0.01,2.88) | 0.23 | 0.05(0.002,1.47) | 0.08 | |
| Albumin | 0.51(0.13,1.94) | 0.32 | 0.20(0.04, 1.11) | 0.07 | |
| Type of IBD | |||||
| Crohn's Disease | 19/155 | 1.00 | 1.00 | ||
| Ulcerative Colitis or Indeterminate Colitis | 5/28 | 2.13(0.78,5.80) | 0.14 | 1.28(0.43,3.88) | 0.66 |
| Combination therapy | |||||
| Never | 5/21 | 1.00 | 1.00 | ||
| Prior | 7/90 | 0.27(0.08,0.88) | 0.03 | 0.10(0.02,0.44) | 0.002 |
| Current | 12/72 | 0.49(0.17,1.43) | 0.19 | 0.21(0.05,0.78) | 0.02 |
| Infliximab<5.0 μg/mL | |||||
| No | 21/145 | 1.00 | 1.00 | ||
| Yes | 3/38 | 0.63(0.19, 2.11) | 0.45 | 0.46(0.11,1.90) | 0.28 |
The analysis was conducted using Cox proportional hazard model, those (n=36) with detectable Antibodies To Infliximab at enrollment were excluded from the analysis
Adjusted for Age (continuous), sex (male vs. female), Body Mass Index (continuous), CRP, albumin levels, and IBD disease types
Combined effect of IFXL and ATI on infliximab discontinuation
To further explore the relationship between IFXL and ATI and stopping infliximab, the study population was categorized based upon enrollment IFXL level and whether ATI were detected at enrollment (Table 4). Compared to those with IFXL level ≥5 μg/mL and no detectable ATI as the index group, individuals with an undetectable ATI with IFXL level <5 μg/mL did not have a statistically significant risk of discontinuing infliximab [HR 2.30(0.71-7.51 CI, p=0.17)]. The risk of discontinuing infliximab with detectable ATI and IFXL level ≥5 μg/mL also did not reach statistical significance [HR (3.65(0.95,13.96 CI, p=0.06)]. Those with both low IFXL <5 μg/mL and detectable ATI had the highest risk of infliximab discontinuation in both univariate analysis and multivariate analysis adjusted for age, gender, BMI, combination therapy, CRP, albumin, ESR and IBD type (HR 6.07(2.26-16.32 CI, p<0.001).
Table 4.
The combined effect of lower infliximab level and detectable Antibodies to Infliximab (ATI) on the risk of infliximab discontinuation.
| Univariate | Multivariatea | ||||
|---|---|---|---|---|---|
| HR(95%CI) | P value | HR(95%CI) | P value | ||
| Undetectable ATI b | |||||
| Infliximab≥5.0 μg/mL | 12/145 | 1.00 | 1.00 | ||
| Infliximab<5.0 μg/mL | 5/38 | 1.84(0.65-5.26) | 0.25 | 2.30(0.71-7.51) | 0.17 |
| Detectable ATI b | |||||
| Infliximab≥5.0 μg/mL | 3/12 | 2.62(0.74-9.30) | 0.14 | 3.65(0.95,13.96) | 0.06 |
| Infliximab<5.0 μg/mL | 11/24 | 6.68(2.88-15.46) | <0.001 | 6.07(2.26-16.32) | <0.001 |
Adjusted for Age (continuous), sex, Body Mass Index (continuous), combination therapy (never, prior, current), CRP>0.5 mg/dL (yes/no), albumin (continuous) and IBD type (CD or UC)
Detectable Antibodies To Infliximab >3.1 U/mL undetectable <3.1U/mL
Discussion:
In summary, in this prospective patient and prescriber blinded observational study, we found that the presence of ATI strongly correlates with future discontinuation of infliximab, but drug discontinuation was not immediate. While low infliximab levels, particularly at enrollment, also correlated with drug discontinuation, the presence of ATI was a stronger predictor of stopping treatment. Unexpectedly, 36 participants with ATI at study entry continued to receive infliximab but only 14 (39%) discontinued infliximab. Additionally, 60 patients had ATI at one or more time points during the study but only 22 discontinued infliximab use during the study follow up period averaging just over 3 years. This suggests that in a subset of patients, the presence of ATI either has no clinical significance, or there is a longer period between the development of ATI and loss of response. The specific binding site of the antibodies that develop to infliximab may lead to more clearance or less drug efficacy. Additional studies are needed to define ATI epitopes and their clinical significance. Our study expands on the current knowledge of therapeutic drug monitoring (TDM) in pediatric IBD, by supporting the temporal association of development of ATI and decreased IFXL with discontinuation of infliximab. In our study infliximab dose escalation eliminated ATI in 38% of subjects which has been demonstrated in a few other studies in low numbers of subjects [21] [22]. Our study also suggests early combination therapy may improve infliximab durability and a trend towards concomitant combination therapy also reducing antibody formation and, in some patients, eliminating antibodies which is shown by some studies but not by others [23, 24].
Our study design in which participants and their prescribing providers were blinded to prospectively obtained IFXL with years of follow-up provides a unique opportunity to assess the dynamics of ATI and treatment discontinuation. This is not possible in unblinded studies where treating providers reflexively adjust dosing or concomitant immunomodulators based on real-time ATI detection. Additionally, providers were able to obtain levels when they thought it was clinically indicated. Although serum samples were obtained prospectively, this is not a study of proactive drug monitoring because clinicians were blinded to study results. While prior studies of IFXL and ATI in pediatric IBD exist, our study represents a large prospective cohort rather than a retrospective analysis. The majority of our patients were primary responders on maintenance therapy for years with years of follow-up, whereas many other studies examine post induction levels and have much shorter follow-up time[13] [25]. Physicians in our study were blinded to the study results and managed patients without knowledge of the research levels, and outside of the setting of a clinical trial, reflecting “real world” practice prior to the era of prospective TDM. Our study builds upon our prior work, by demonstrating that ATI development in pediatric and young adult patients with IBD strongly correlates with infliximab discontinuation [7]. Furthermore, early combination therapy with 6-mercaptopurine, or methotrexate was associated with a lower risk of ATI development and monotherapy was associated with the highest rates of ATI development, which was also seen in our cross-sectional study [26]. After ATI was detected dose escalation (as well as 6MP in one subject) eliminated antibodies in some of our population which others have also reported ([27] [28]). Since elimination of ATI improves drug durability our study supports periodic measurement of ATI and IFXL during maintenance therapy.
We were also able to demonstrate low IFXL correlated with infliximab discontinuation, which has been reported by other investigators [29] [30]. Our prospective blinded pediatric study is also important because it demonstrates that IFXL < 5 μg/mL is associated with drug discontinuation in children and young adults during long-term maintenance therapy. Most prior adult and pediatric studies have focused on IFXL post induction or only in the first year [13] [14] [25]. In contrast, most of the patients enrolled in our prospective study had been on infliximab for at least 2 years. Thus, data from our cohort supports maintaining infliximab levels above 5 μg/mL during maintenance therapy to reduce the likelihood of treatment discontinuation.
Some investigators have hypothesized that low IFXL may be immunogenic and promote ATI development [25, 31]. Our prospective study design enabled us to examine this temporal relationship. We could not demonstrate that low IFXL led to subsequent development of ATI. Although low IFXL at study enrollment was not independently associated with the development of ATI, this may simply reflect insufficient follow-up duration to detect the influence of low IFXL on ATI development, and/or lack of power to detect such a difference. Proactive TDM with dose adjustment to maintain a post induction and at least one maintenance level of 5ug/mL was shown by others to decrease the development of high titer ATI in children. [19, 25].
Our prospective observational cohort with blinded proactive measurement of IFXL and ATI had some limitations. Firstly, the median duration of infliximab therapy at enrollment was 2 years, thus the rate of drug discontinuation was low as many who had early mechanistic failure (blocking TNF α is not effective at controlling their disease), inadequate initial dosing, or early loss of response were not enrolled. Notably, rates of infliximab discontinuation did not differ between those who did and did not have dose adjustments prior to study enrollment. Also, this was an observational study so compliance with treatments other than infliximab was not monitored. Therefore, non-compliance may have attenuated the effect of combination treatment with immunomodulators. Additionally, only 14% were on infliximab monotherapy group because many physicians in our center utilize combination therapy based on the results of the SONIC and COMMIT trials [1, 32]. The time between ATI detection and IFX discontinuation was prolonged and highly variable. Although our follow-up period of years is longer than in many prior studies, had we followed those with ATI that continued IFX for even longer they eventually may have lost response. While serial IFXL and ATI were obtained, the number of samples per patient and the intervals between measurements varied which limited conclusions on when levels should be drawn. Finally, despite the large sample size of this cohort, certain groups (such as those patients with low levels of antibodies to infliximab) and those who discontinued due to lack of drug efficacy had small sample sizes, making it difficult to definitively ascertain patient outcomes in these groups.
Although providers in our study did not use proactive TDM as it was not yet standard of care, our results provide support for this practice. Patients with ATI are at significantly increased risk of drug discontinuation, yet many patients with ATI have no clinical symptoms, possibly because there is a time delay between the development of ATI, reduction of IFXL levels, and subsequent intestinal inflammation. Our study suggests that periodic monitoring of IFXL and ATI during maintenance therapy can benefit patients by detecting ATI formation before patients lose response to infliximab. The detection of ATI may in turn allow for dose escalation or the addition of an immunomodulator, which may in turn reduce ATI and prolong response to infliximab. The data in this study support performing periodic measurement of IFXL and ATI in IBD patients receiving maintenance infliximab. [33]. There is no evidence or studies determining how often TDM should be performed in children in remission receiving maintenance therapy, and the assay is costly. However, the authors have adapted a practice of measuring of IFXL and ATI approximately once per year.
Supplementary Material
Supplemental Figure 1. Subjects with ATI at enrolment and those that developed ATI during the study. Interventions were recorded and whether ATI resolved.
What is known:
Infliximab use is limited by loss of response, which can be associated with antibodies to infliximab (ATI) and/or low infliximab drug levels (IFXL).
Current target IFXL (5-12 μg/mL) are mostly based on retrospective studies and post induction levels.
What is new:
This prospective longitudinal study followed ATI and IFXL mainly in primary responders on long term maintenance therapy.
IFXL 5-10 μg/mL and the absence of ATI were associated with continued infliximab use.
ATI had a larger impact on drug discontinuation compared to low IFXL alone.
Dose escalation after ATI detection can eliminate ATI.
This study supports the need for periodic post induction testing of IFXL and ATI to improve drug durability.
Acknowledgements:
We would like to thank the MacInnes, Rasmussen, Wolfman, Balise, Wolpow, Ward, Clark, Rubin and Pasculano families for their support in our Inflammatory Bowel Disease (IBD) Center at Boston Children's Hospital.
Funding:
Infliximab levels and antibody to infliximab assays were performed through a grant provided by Prometheus laboratories to Athos Bousvaros and Naamah Zitomersky. Research coordinator support and data analysis was provided by the Pediatric IBD Center, Division of Gastroenterology, Hepatology and Nutrition, Boston Children’s Hospital. This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Numbers P30DK034854, NIH K23 DK 119584 and T32DK07760. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References:
- 1.Colombel JF, et al. , Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med, 2010. 362(15): p. 1383–95. [DOI] [PubMed] [Google Scholar]
- 2.Targan SR, et al. , A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med, 1997. 337(15): p. 1029–35. [DOI] [PubMed] [Google Scholar]
- 3.Hanauer SB, et al. , Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet, 2002. 359(9317): p. 1541–9. [DOI] [PubMed] [Google Scholar]
- 4.Gisbert JP and Panes J, Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. Am J Gastroenterol, 2009. 104(3): p. 760–7. [DOI] [PubMed] [Google Scholar]
- 5.Afif W, et al. , Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol, 2010. 105(5): p. 1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baert F, et al. , Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med, 2003. 348(7): p. 601–8. [DOI] [PubMed] [Google Scholar]
- 7.Zitomersky NL, et al. , Antibodies to infliximab are associated with lower infliximab levels and increased likelihood of surgery in pediatric IBD. Inflamm Bowel Dis, 2015. 21(2): p. 307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levesque BG, et al. , A prospective cohort study to determine the relationship between serum infliximab concentration and efficacy in patients with luminal Crohn's disease. Aliment Pharmacol Ther, 2014. 39(10): p. 1126–35. [DOI] [PubMed] [Google Scholar]
- 9.Vermeire S, et al. , Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut, 2007. 56(9): p. 1226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maser EA, et al. , Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol, 2006. 4(10): p. 1248–54. [DOI] [PubMed] [Google Scholar]
- 11.Seow CH, et al. , Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut, 2010. 59(1): p. 49–54. [DOI] [PubMed] [Google Scholar]
- 12.Papamichael K, et al. , Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh N, et al. , Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis, 2014. 20(10): p. 1708–13. [DOI] [PubMed] [Google Scholar]
- 14.Stein R, et al. , Serum Infliximab, Antidrug Antibodies, and Tumor Necrosis Factor Predict Sustained Response in Pediatric Crohn's Disease. Inflamm Bowel Dis, 2016. 22(6): p. 1370–7. [DOI] [PubMed] [Google Scholar]
- 15.Lega S, et al. , Proactively Optimized Infliximab Monotherapy Is as Effective as Combination Therapy in IBD. Inflamm Bowel Dis, 2019. 25(1): p. 134–141. [DOI] [PubMed] [Google Scholar]
- 16.Chi LY, et al. , The Impact of Combination Therapy on Infliximab Levels and Antibodies in Children and Young Adults With Inflammatory Bowel Disease. Inflamm Bowel Dis, 2018. 24(6): p. 1344–1351. [DOI] [PubMed] [Google Scholar]
- 17.Ungar B, et al. , Optimizing Anti-TNF-alpha Therapy: Serum Levels of Infliximab and Adalimumab Are Associated With Mucosal Healing in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol, 2016. 14(4): p. 550–557 e2. [DOI] [PubMed] [Google Scholar]
- 18.Clarkston K, et al. , Development of Infliximab Target Concentrations During Induction in Pediatric Crohn Disease Patients. J Pediatr Gastroenterol Nutr, 2019. 69(1): p. 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyles JL, et al. , Effect of a Practice-wide Anti-TNF Proactive Therapeutic Drug Monitoring Program on Outcomes in Pediatric Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis, 2021. 27(4): p. 482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ungar B, et al. , The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut, 2014. 63(8): p. 1258–64. [DOI] [PubMed] [Google Scholar]
- 21.Kothari MM, Nguyen DL, and Parekh NK, Strategies for overcoming anti-tumor necrosis factor drug antibodies in inflammatory bowel disease: Case series and review of literature. World J Gastrointest Pharmacol Ther, 2017. 8(3): p. 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pariente B, et al. , Trough levels and antibodies to infliximab may not predict response to intensification of infliximab therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis, 2012. 18(7): p. 1199–206. [DOI] [PubMed] [Google Scholar]
- 23.Grossi V, et al. , Concomitant Use of Immunomodulators Affects the Durability of Infliximab Therapy in Children With Crohn's Disease. Clin Gastroenterol Hepatol, 2015. 13(10): p. 1748–56. [DOI] [PubMed] [Google Scholar]
- 24.Vermeire S, et al. , Immunogenicity of biologics in inflammatory bowel disease. Therap Adv Gastroenterol, 2018. 11: p. 1756283X17750355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy NA, et al. , Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol, 2019. 4(5): p. 341–353. [DOI] [PubMed] [Google Scholar]
- 26.Barnich N, et al. , CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest, 2007. 117(6): p. 1566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben-Horin S, et al. , Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol, 2013. 11(4): p. 444–7. [DOI] [PubMed] [Google Scholar]
- 28.Battat R, et al. , Immunogenicity of Tumor Necrosis Factor Antagonists and Effect of Dose Escalation on Anti-Drug Antibodies and Serum Drug Concentrations in Inflammatory Bowel Disease. Inflamm Bowel Dis, 2021. 27(9): p. 1443–1451. [DOI] [PubMed] [Google Scholar]
- 29.Liefferinckx C, et al. , Infliximab Trough Levels at Induction to Predict Treatment Failure During Maintenance. Inflamm Bowel Dis, 2017. 23(8): p. 1371–1381. [DOI] [PubMed] [Google Scholar]
- 30.Hofmekler T, et al. , Infliximab Optimization Based on Therapeutic Drug Monitoring in Pediatric Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr, 2017. 64(4): p. 580–585. [DOI] [PubMed] [Google Scholar]
- 31.Vande Casteele N, et al. , The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn's disease. Gut, 2015. 64(10): p. 1539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feagan BG, et al. , Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn's disease. Gastroenterology, 2014. 146(3): p. 681–688 e1. [DOI] [PubMed] [Google Scholar]
- 33.Vande Casteele N, et al. , Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology, 2015. 148(7): p. 1320–9 e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Subjects with ATI at enrolment and those that developed ATI during the study. Interventions were recorded and whether ATI resolved.