Abstract
Sexual selection often favors brighter and exaggerated traits, which also increase the risk of detection by predators. Signals that are preferentially conspicuous to conspecifics would reduce the predation cost of signaling and, therefore, might facilitate the evolution of stronger sexual and social signals. This selective signaling is possible if predators and prey have differently tuned sensory systems. By using a retinal model to compare reflectance from the plumages of Swedish songbirds to the reflectance of their natural backgrounds, we found their color badges to be significantly more conspicuous to other songbirds (which have a UV-tuned visual system) than to raptors and corvids (which have a violet-tuned system) in both coniferous and deciduous forests, consistent with an adaptive private communication system.
Keywords: plumage reflectance, predation, sexual selection, UV vision
In animals subjected to sexual selection, increased exposure to predators will select against conspicuous ornamentation or coloration in both males and females, whereas sexual selection may favor the elaboration of such signals (e.g., ref. 1), with the exception of aposematism (2). To be useful indicators of quality, signals need to be controlled for honesty, through predation, production or social interaction (maintenance) costs (3). However, other members of the species can rely only on their own sensory systems for assessing a signal. In the case of coloration, a high reflectance in some segments of the spectrum may due to differences in sensitivity between the vision systems of the predator and the prey, increase the cost of predation less than it strengthens the signal in the eyes of the intended receiver (4, 5). It has been speculated that UV vision functions as such a “private communication channel” (6) in birds, because mammalian predators are blind to UV (7, 8). However, predation on small birds is at least equally likely to be from raptors (8). UV reflecting plumage ornaments may increase the risk of predation if raptors use these ornaments as hunting cues (9), and at least one bird-feeding raptor (the kestrel Falco tinnunculus) has been shown to use UV cues while foraging (10). However, the risk of avian predation from UV signaling is still unknown because recent work from molecular and retinal studies suggest that avian predators and prey have different spectral sensitivities, especially in the UV range (11).
Retinal microspectrophotometry has revealed two types of color vision in birds. Both types are sensitive to UV light, but there is a pronounced difference in the wavelength of peak absorbance (λ-max) of the opsin in the UV/violet cone [first shortwave-sensitive opsin (SWS1)]. The UV-sensitive (UVS) type (12) has a UV-biased SWS1 with a λ-max between 355 and 380 nm, compared with 402-426 nm (13) in the violet-sensitive (VS) type (12). Furthermore, λ-max of the second shortwave-sensitive opsin (SWS2; 430-463 nm) is significantly shifted toward shorter wavelengths in UVS birds and toward longer wavelengths in VS birds (13). This dramatic difference in SWS1 tuning changes not only the perception of UV or violet colors but also all nonspectral colors with an SWS1 element, and it should have important consequences for foraging, social signaling, and mate choice.
All studies performed to date (12, 14-17) concur that the Passerida (thrush-, sparrow-, and warbler-like songbirds, here-after referred to as songbirds) are of the UVS type, together with the order Psittaciformes and the family Laridae, and that the VS color system is present in most other birds, including raptors and the passeriform group Corvida (ref. 11 and references therein) (18). This distribution shows that two avian groups, which are important predators of songbirds, have a vision system different from that of their prey. The first group includes hawks (in Sweden: sparrowhawk, Accipiter nisus; and goshawk, Accipiter gentilis), which forage primarily in forested environments and are specialized on capturing birds. Prey are often caught while flying, perched on a branch, or in the nest (19). When perching or in the nest, crypsis should be the prey's primary defense. The second group of predators, corvids [in Swedish forests: hooded crow, Corvus corone; common raven, Corvus corax; and European jay, Garrulus glandarius (20)], are important nest predators. There is a positive correlation between parental activity (such as chick provisioning) and nest predation in some birds (21, 22), likely because nest predators can locate nests by detecting parents provisioning their chicks (23). Presumably, increased adult crypsis would also decrease such nest predation.
Given the difference in spectral sensitivities between predator and prey, songbird males theoretically should be able to reduce their signal-color conspicuousness to predators without lowering the attractiveness of the coloration to conspecifics. Therefore, we predict that the color signals of male songbirds will be perceived as more different from the background (more conspicuous) when viewed by a songbird (with a UVS system) than when viewed by a hawk or corvid predator (with a VS system). By using a retinal model, we tested this prediction by measuring plumage reflectance from 18 species of Swedish songbirds, the reflectance of their natural backgrounds (deciduous and/or coniferous forests), and light regimes.
Materials and Methods
The background against which the forest-living birds are to be detected is normally temporally and spatially heterogeneous, consisting of differently colored leaves and branches exposed to ever-changing light conditions. We assumed that the chance of a plumage badge (i.e., a patch that functions as a sexual signal) to be mistaken for a member of the background is proportional to the color distance between them. As a measure of color distance between the plumage signal and the background, we used the discriminability model of Vorobyev et al. (24) in its log form:
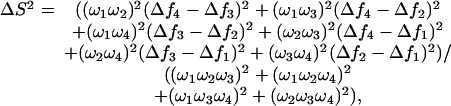 |
[1] |
where
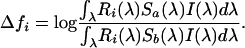 |
[2] |
Here, R is receptor sensitivity, S is the reflectance spectrum, I is ambient light, and i is the cone types [virtually all diurnal birds have four single-cone types (13): SWS1, SWS2, medium-wavelength-sensitive (MWS), and long-wavelength-sensitive (LWS)]. For the noise calculations, we used cone proportions of 1, 1.92, 2.68, and 2.7 for UVS (12) and 1, 1.9, 2.2, and 2.1 for VS (25), and we assumed ω to be independent of light intensity (assuming a Weber fraction of 0.05 for the UVS cone). The spectral calculations were made by using purpose-written software, and the statistics were done by using R (26).
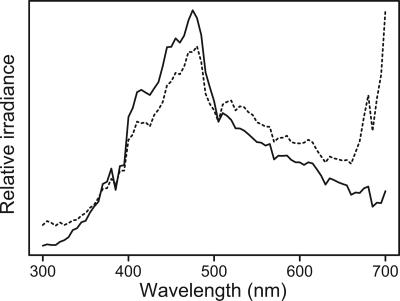
To capture the chromatic variation of the natural background, we took reflectance measurements from breeding habitats. It is reasonable to assume that the plumage of the adults is adapted to provide a maximum signal to crypsis ratio during the breeding season. Mortality of adults due to predation can be expected to be high during this time because the birds have to be active to compete for partners, breeding sites, and subsequently provide for their chicks. For the deciduous habitat, we measured leaves from each of eight species of deciduous trees (wych elm, Ulmus glabra; aspen, Populus tremula; ash, Fraxinus excelsior; silver birch, Betula pendula; pedunculate oak, Quercus robur; hazel, Corylus avellana; Norway maple, Acer platanoides; and rowan, Sorbus aucuparia) and twigs from four species of deciduous trees (elm, ash, birch, and oak). For the coniferous habitat, we measured needles and twigs of Norway spruce Picea abies and scots pine Pinus sylvestris, which is the overwhelmingly dominant coniferous species in Sweden. Also, we collected ambient light measurements in the late morning to noon during one summer day with a clear sky: 12 averaged measurements in a deciduous forest and six averaged measurements in a coniferous forest, both of which were near Uppsala, Sweden. The measurements were taken from elm and maple in deciduous forest and from spruce in coniferous forest, and they were taken on the surface, in the middle of the foliage, and close to the trunk on trees in direct sunlight and in the shade. This light could best be characterized as “forest shade” (27), and it has a peak intensity of ≈400-500 nm because of the sky light and the reflectance of chlorophyll (Fig. 1).
Fig. 1.
Average ambient light in the middle of foliage of sun-exposed trees. The solid line indicates coniferous forest, and the dashed line indicates deciduous forest.
Plumage reflectance was measured on skins from Swedish male birds at the Swedish Museum of Natural History in Stockholm. Three areas of the plumage (forehead, crown, and chest) often function as color signals in songbirds (e.g., refs. 28-32), but we used only the forehead and chest measurements because the crown color in several species correlated with that of the forehead or the back. Humans and birds have very different visual systems (33), so both badges from all species were included (i.e., even badges that appear to the human observer to lack signal colors).
We measured 18 species from nine Passerida families (Table 1), which all breed in Sweden and spend much of their time in the foliage of trees. Information on their habitat was obtained from refs. 19 and 34 and the data of Staffan Ulfstrand (personal communication). The forest type used during the breeding season was classified for each bird species according to the dominating tree species as (i) deciduous, (ii) coniferous, or (iii) mixed deciduous and coniferous (Table 1). Some species may expand their ranges to breed in nonpreferred types of forest during periods of increasing population size. In these cases, the species was classified as belonging to the habitat where it is present under normal conditions. Species were tested in the appropriate light regime mimicking either deciduous or coniferous forest (see below); species belonging to “mixed deciduous and coniferous” were tested in both.
Table 1. Birds measured for the study, with habitat used during breeding season.
| Species | Common name | Habitat |
|---|---|---|
| Aegithalos caudatus | Long-tailed tit | d |
| Bombycilla garrulus | Bohemian waxwing | c |
| Oriolus oriolus | Golden oriole | d |
| Carduelis spinus | Eurasian siskin | c |
| Fringilla coelebs | Chaffinch | c/d |
| Fringilla montifringilla | Brambling | c |
| Pyrrhula pyrrhula | Common bullfinch | c |
| Erithacus rubecula | Robin | c |
| Ficedula hypoleuca | Pied flycatcher | d |
| Luscinia luscinia | Thrush nightingale | d |
| Luscinia svecica | Bluethroat | d |
| Phoenicurus phoenicurus | Common redstart | c |
| Parus caeruleus | Blue tit | d |
| Parus major | Great tit | c/d |
| Parus montanus | Willow tit | c/d |
| Regulus regulus | Goldcrest | c |
| Sitta europaea | Eurasian nuthatch | d |
| Phylloscopus trochilus | Willow warbler | c/d |
c, Coniferous forest; d, deciduous forest; c/d, mixed coniferous and deciduous forest.
Three genera (Fringilla, Luscinia, and Parus) were represented by more than one species but showed high within-genus variability in the color of the examined badges, reducing any risk of taxonomic dependence.
All measurements were taken with an Ocean Optics S2000 diode-array spectrophotometer. The ambient-light measurements were taken vertically with a collection angle of 36°. The surface reflectances were measured at a 60° angle by using a TOP Sensor Systems DH-2000 combined deuterium-halogen light source connected by a fiber optic probe to the spectrophotometer (according to ref. 35).
Because we assumed that the chance of a badge being mistaken for a given patch of the background is proportional to the color distance between them, the crypsis of the badge in its patchy surroundings can be described by the average distance from the badge to all patches of the background in proportion to the average distance between all of the background patches. We calculated the color distance from the badge to the background (relative discriminability, D) as follows:
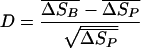 |
[3] |
where ΔSB is the distance from the badge to all background patches and ΔSp is the distance between all background patches. By assuming that the background distances are Poisson distributed (where mean values equal the variance) D will describe the distance from the badge to the average background in standard deviations of the background.
The color of each feather badge was calculated as if the bird were perching in the middle of the foliage of a sun-exposed tree, by using the averaged light from the midfoliage measurements from that habitat. This procedure was repeated by using both the cone sensitivities of a blue tit, P. caeruleus (12), and a peafowl, Pavo cristatus (25), representing birds of the UVS and VS system, respectively. The peafowl, which is an omnivore living in open plains and scrubland, is the best representative for VS birds for which enough data are available. It mainly forages in shaded environments, where there is little need for UV-protective filtration. Therefore, the ocular media of peafowl should transmit UV well compared with raptors, reducing the risk that our calculations exaggerate the differences between the color vision system of raptors and corvids and that of songbirds.
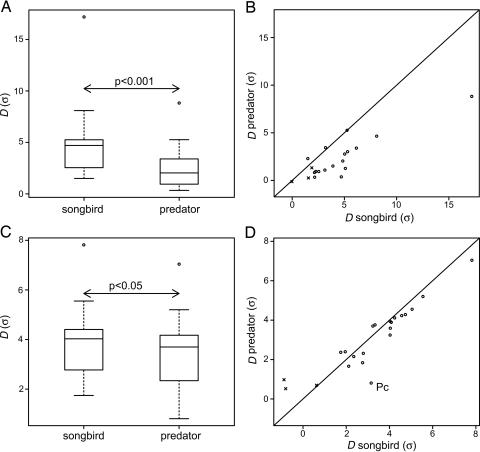
Plumage patches differing from the average background by less than two standard deviations for both color vision systems (Fig. 2 B and D) are not likely to be color signals (badges) and, hence, were omitted from the analysis. Because not all distributions of mean color distances passed the Shapiro-Wilk test for normality, we used Wilcoxon's signed-rank test for all comparisons.
Fig. 2.
Differences in the visibility of songbird plumage colors when viewed by another songbird or an avian predator against coniferous (A and B) or deciduous (C and D) forest backgrounds. Pc, blue tit (P. caeruleus) yellow chest (see Fig. 3); x, colors differing <2 σ from the background (excluded from analysis). Solid line indicates equal distances from the background for both vision systems. Points below the line are more different from the background to songbirds than to their predators. Box plots show medians and quartiles, and the whiskers show the most extreme data points but no more than 1.5 times the interquartile range from the box.
Results and Discussion
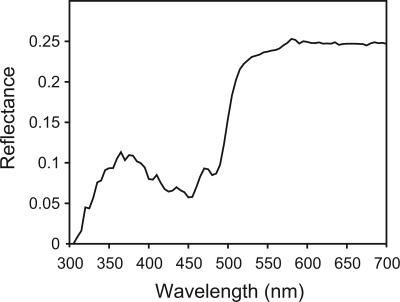
Our results show that male songbirds tune their signals to the vision system of the intended, conspecific receiver while reducing color contrast to the background in spectral parts to which their predators are most sensitive. The colors of the forehead and chest are significantly more conspicuous to other songbirds than to avian predators in both the coniferous and deciduous forests (Fig. 2 A and C). In other words, songbirds use color signals that are more visible to their vision system than to that of their predators, allowing a directed communication channel for displaying male quality. The yellow of the blue tit, P. caeruleus, chest (Figs. 2D, point Pc, and 3) is a good example of a plumage badge that is much less visible to the predators (D = 0.8σ, from the background mean) of the carrier than to the mates of the carrier (D = 3.4σ). In this case, the badge is probably more visible to songbirds because the position of the UV component corresponds to the sensitivity of their vision system to UV, whereas it is positioned too far into UV for the VS system.
Under natural light environments, the strongest stimulus power is in the achromatic dimension (36), i.e., differences in total brightness, which the ΔS model (24) used in this study does not take into account. However, the achromatic signal does not differ significantly between the groups.
Some of the species that we tested also forage extensively on the ground (e.g., great tit, P. major), where the light regime is very different from that in the trees. However, the risk of detection on the ground is probably not primarily influenced by color contrast but by motion against the more homogeneous background, involving signals from the double cones (24, 37). Therefore, if so, the main risk for detection caused by the conspicuous coloration of males during breeding season would be in the variable color environment of the trees.
Using signals that are inconspicuous to predators reduce the predation cost of signaling (cf. ref. 38) and facilitate the evolution of stronger sexual signals by relaxing evolutionary constraints on coloration diversity (1). Structures involved in intraspecific signaling, like song or coloration, are highly developed in specious taxa (39). For example, Barraclough et al. (40) found correlations between species richness and proportion of sexual dichromatism in Passeriformes. This study and other studies (refs. 41 and 42, but see ref. 43) suggest that sexual selection on plumage traits may cause taxa to diversify. A visual communication channel partially concealed from predators may help to explain why more than half of all bird species are passerines.
The realization that birds possess “UV vision” has generated a great deal of behavioral research (e.g., see ref. 8 for review). Unfortunately, much research has ignored the importance of all other differences compared with human color perception throughout the visual spectrum, arising from the differently positioned absorbance maxima of the cone pigments and the absorbance of the ocular media and cone oil droplets. After all, “UV vision” is just an anthropocentric characterization of disparate visual sensitivities of <400 nm, and this distinction makes little sense in biology. Here, we have shown that there is a possibility of a shielded communication channel in songbirds, not simply because of the absence of UV-sensitivity in mammalian predators but also because of the fact that songbirds and their avian predators have differently tuned color receptors. These results emphasize the importance of taking perceptual systems into account when studying animal ecology, behavior, and evolution.
Fig. 3.
Spectrogram of a blue tit's yellow chest (Fig. 2D, point Pc), an example of a color that is cryptic to avian predators but conspicuous to songbirds.
Acknowledgments
We thank Malin Ekstrand for collecting the spectrophotometric data from museum skins; Per Ericson and Göran Frisk at The Swedish Museum of Natural History (NRM) for assisting her in that process; Daniel Osorio for help with the ΔS model; Staffan Ulfstrand, Anna Qvarnström, Göran Arnqvist, Arne Mooers, and anonymous referees for comments on early drafts; and Mats Björklund for various assistance with the project. This work was supported by Zoologiska Stiftelsen; Helge Ax:son-Johnsons Stiftelse, which provided for spectrophotometric equipment (to O.H. and A.Ö); the Swedish Research Council (VR) (A.Ö.); and the Natural Science and Engineering Research Council of Canada (NSERC).
Author contributions: O.H., J.V., and A.Ö. designed research, performed research, contributed new reagents/analytic tools, analyzed data, and wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: VS, violet-sensitive; UVS, UV-sensitive; SWS1/2, first/second shortwave-sensitive opsin.
References
- 1.Endler, J. A. (1980) Evolution (Lawrence, Kans.) 34, 76-91. [DOI] [PubMed] [Google Scholar]
- 2.Siddiqi, A., Cronin, T. W., Loew, E. R., Vorobyev, M. & Summers, K. (2004) J. Exp. Biol. 207, 2471-2485. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, M. (1998) Sexual Selection (Princeton Univ. Press, Princeton).
- 4.Endler, J. A. (1991) Vision Res. 31, 587-608. [DOI] [PubMed] [Google Scholar]
- 5.Cummings, M. E., Rosenthal, G. G. & Ryan, M. J. (2003) Proc. R. Soc. London Ser. B 270, 897-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banks, A. N. (2001) Trends Ecol. Evol. 16, 473-474. [Google Scholar]
- 7.Bowmaker, J. K. (1998) Eye 392, 541-547. [DOI] [PubMed] [Google Scholar]
- 8.Guilford, T. & Harvey, P. H. (1998) Nature 392, 867-869. [Google Scholar]
- 9.Honkavaara, J. M., Koivula, M., Korpimäki, E., Siitari, H. & Viitala, J. (2002) Oikos 98, 505-511. [Google Scholar]
- 10.Viitala, J., Korpimäki, E., Palokangas, P. & Koivula, M. (1995) Nature 373, 425-427. [Google Scholar]
- 11.Ödeen, A. & Håstad, O. (2003) Mol. Biol. Evol. 20, 855-861. [DOI] [PubMed] [Google Scholar]
- 12.Hart, N. S., Partridge, J. C., Cuthill, I. C. & Bennett, A. T. D. (2000) J. Comp. Physiol. 186, 375-387. [DOI] [PubMed] [Google Scholar]
- 13.Hart, N. S. (2001) Prog. Retin. Eye Res. 20, 675-703. [DOI] [PubMed] [Google Scholar]
- 14.Maier, E. J. & Bowmaker, J. K. (1993) J. Comp. Physiol. A 172, 295-301. [Google Scholar]
- 15.Bowmaker, J. K., Heath, L. A., Wilkie, S. E. & Hunt, D. M. (1997) Vision Res. 37, 2183-2194. [DOI] [PubMed] [Google Scholar]
- 16.Das, D., Wilkie, S. E., Hunt, D. M. & Bowmaker, J. K. (1999) Vision Res. 39, 2801-2815. [DOI] [PubMed] [Google Scholar]
- 17.Hart, N. S., Partridge, J. C., Bennett, A. T. D. & Cuthill, I. C. (2000) J. Comp.
- 18.Hart, N. S. (2004) J. Exp. Biol. 207, 1229-1240. [DOI] [PubMed] [Google Scholar]
- 19.Snow, D. W. & Perrins, C. M. (1988) The Birds of the Western Palearctic, Concise Edition (Oxford Univ. Press, Oxford).
- 20.Andrén, H. (1992) Ecology 73, 794-804. [Google Scholar]
- 21.Eggers, S., Griesser, M. & Ekman, J. (2005) Behav. Ecol. 16, 309-315. [Google Scholar]
- 22.Martin, T. E., Scott, J. & Menge C. (2000) Proc. R. Soc. London Ser. B 267, 2287-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alerstam, T. & Högstedt, G. (1981) Ornis. Scand. 12, 188-193. [Google Scholar]
- 24.Vorobyev, M., Osorio, D., Bennett, A. T. D., Marshall, N. J. & Cuthill, I. C. (1998) J. Comp. Physiol. A 183, 621-633. [DOI] [PubMed] [Google Scholar]
- 25.Hart, N. S. (2000) J. Exp. Biol. 205, 3925-3935. [DOI] [PubMed] [Google Scholar]
- 26.Ihaka, R. & Gentleman, R. (1996) J. Comput. Graph. Stat. 5, 299-314. [Google Scholar]
- 27.Endler, J. A. (1993) Ecol. Monogr. 61, 1-27. [Google Scholar]
- 28.Andersson, S., Örnborg J. & Andersson, M. (1998) Proc. R. Soc. London Ser. B 265, 445-450. [Google Scholar]
- 29.Johnsen, A., Lifjeld, J. T., Andersson, S., Örnborg, J. & Amundsen, T. (2001) Behaviour 138, 1371-1390. [Google Scholar]
- 30.Møller, A. P. (1987) Anim. Ecol. 35, 1637-1644. [Google Scholar]
- 31.Norris, K. J. (1990) Behav. Ecol. Sociobiol. 26, 129-138. [Google Scholar]
- 32.Pärt, T. & Qvarnström, A. (1997) Anim. Behav. 54, 839-899. [Google Scholar]
- 33.Goldsmith, T. H. (1990) Q. Rev. Biol. 65, 281-322. [DOI] [PubMed] [Google Scholar]
- 34.Svensson, S., Svensson, M. & Tjernberg, M. (1999) Svensk fågelatlas. Vår Fågelvärld (Sveriges Ornitologiska Förening, Stockholm), Suppl. 31.
- 35.Rintamäki, P. T, Håstad, O., Ödeen, A, Alatalo, R. V., Höglund, J. & Lundberg, A. (2002) Avian Sci. 2, 145-152. [Google Scholar]
- 36.Vorobyev, M. & Osorio, D. (1998) Proc. R. Soc. London Ser. B 265, 351-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campenhausen, M. V. & Kirschfeld, K. (1998) J. Comp. Physiol. A 183, 1-6. [Google Scholar]
- 38.Stuart-Fox, D. M., Moussalli, A., Marshall, N. & Owens, I. P. F. (2003) Anim. Behav. 66, 541-550. [Google Scholar]
- 39.West-Eberhard, M. J. (1983) Q. Rev. Biol. 58, 155-183. [Google Scholar]
- 40.Barraclough, T. G., Harvey, P. H. & Nee, S. (1995) Proc. R. Soc. London Ser. B 259, 211-215. [Google Scholar]
- 41.Møller, A. P. & Cuervo, J. J. (1998) Evolution (Lawrence, Kans.) 52, 859-869. [Google Scholar]
- 42.Owens, I. P. F. Bennett, P. M. & Harvey, P. H. (1999) Proc. R. Soc. London Ser. B 266, 933-939. [Google Scholar]
- 43.Morrow, E. H., Pitcher, T. E. & Arnqvist, G. (2003) Ecol. Lett. 6, 228-234. [Google Scholar]