Abstract
A role for endocytosis and exocytosis in cell migration has been proposed but not yet demonstrated. Here, we show that cellubrevin (Cb), an early endosomal v-SNARE, mediates trafficking in the lamellipod of migrating epithelial cells and partially colocalizes with markers of focal contacts. Expression of tetanus neurotoxin, which selectively cleaves Cb, significantly reduced the speed of migrating epithelial cells. Furthermore, expression of tetanus neurotoxin enhanced the adhesion of epithelial cells to collagen, laminin, fibronectin, and E-cadherin; altered spreading on collagen; and impaired the recycling of β1 integrins. These results suggest that Cb-dependent membrane trafficking participates in cell motility through the regulation of cell adhesion.
Keywords: membrane traffic, exocytosis, endocytosis, clostridial neurotoxin
Cell migration and cell adhesion are two interdependent, well conserved mechanisms of foremost importance during embryonic development, and both are pathologically altered in metastatic cancer cells (1, 2). In order for a cell to migrate, it first develops a polarized phenotype with a lamellipod at the front of the cell, whereby the migration front leads the movement, attaching to the substrate and propelling the rest of the cell forward. Cell migration can be reconstituted in vitro by treating cells with motogenic stimuli, such as hepatocyte growth factor (HGF, also called scatter factor) (3), or by mechanically wounding a monolayer of epithelial cells, such as Madin-Darby canine kidney (MDCK) cells, stimulating cell migration on each side of the wound toward the opposite side, thereby closing the gap (4).
Two nonexclusive models have been proposed to explain the progression of the migration front of motile cells. The first model is based on a large body of evidence from studies in monocellular and multicellular eukaryotes that demonstrates a fundamental role for the cytoskeleton in the migration front. Dynamic reorganization of the actin cytoskeleton is controlled by the small GTPases of the Rho subfamily (5, 6), and these GTPases, as well as actin and tubulin, play a major role in cell motility (7). A second model has emerged that proposes a role for exocytosis and endocytosis in cell motility. Endocytosis and exocytosis occur in the lamellipods of migrating cells (8-10) and could contribute to the extension of the cell border (11) and/or could bring receptors necessary for the binding of chemotactic ligands and for cell attachment to substrates (12, 13).
Cellubrevin (Cb) is a vesicular soluble N-ethylmaleimide sensitive factor attachment protein receptor (v-SNARE) homologous to the neuronal synaptobrevins (Sybs) 1/2 and is also a substrate of tetanus neurotoxin (TeNT) (14). Syb and Cb are the only known substrates of TeNT, and TeNT is considered to be among the most selective toxins selected by pathological prokaryotes during evolution (15, 16). The function of Cb has been explored by treating cells with TeNT and by homologous recombination. The first approach demonstrated an important function of Cb in the recycling of plasma membrane receptors, including transferrin receptors (17) and T cell receptors, to the immunological synapse (18). In addition, Cb is involved in pathways implicating early endosomes, such as apical transport of H+-ATPase (19), focal exocytosis at sites of phagocytosis (20, 21), release of retroviruses that assemble in endosomes (22), and the endosome to trans-Golgi network retrograde transport of Shiga toxin (23). In contrast, Cb knockout mice showed a lack of phenotype (24-26) that could result from functional redundancy with Sybs 1/2, endobrevin/VAMP8, TI-VAMP/VAMP7, or yet another v-SNARE that acute TeNT treatment may circumvent. In this study, we set out to understand the role of Cb in cell migration and adhesion by testing the effect of TeNT in the epithelial MDCK cell line.
Materials and Methods
Antibodies and DNA Constructs. Mouse monoclonal antibody anti-GFP (clone 7.1 and 13.1) was obtained from Roche Diagnostics, anti-Talin (clone 8d4) was from Sigma, anti-focal adhesion kinase (FAK) (clone 4.47) was from Upstate Biotechnology (Lake Placid, NY), and anti-phosphotyrosine (P-Tyr-100) was from Cell Signaling Technology (Beverly, MA). Monoclonal antibodies against TeNT and brevins (Cl10.1) were generous gifts from H. Niemann (Hannover Medical School, Hannover, Germany) and R. Jahn (Max-Planck-Institut, Gottingen, Germany), respectively. The mouse monoclonal antibody anti-β1 integrin (Cl18) from Transduction Laboratories (Lexington, KY) was used for Western blotting, and the purified hamster monoclonal antibody (clone Ha2/5) was used for anti-β1 integrin antibody (BD Biosciences, Franklin Lakes, NJ) uptake experiments. Alexa Fluor 488- and Alexa Fluor 568-coupled phalloidin was from Molecular Probes. GFP-Cb stably expressing MDCK cells are described in ref. 27. WT and E234Q inactive TeNT in pCMV are described in ref. 14. The GFP-tagged VW Cb mutant, resistant to the proteolytic action of TeNT (GFP-Cb VW) was kindly provided by R. Regazzi (University of Lausanne, Lausanne, Switzerland) (28).
Cell Culture and Transfection. MDCK cells were cultured in DMEM with 7% FCS and transfected by electroporation. MDCK cells expressing GFP-Cb were cotransfected with a pCMV vector expressing either WT or E234Q inactive light chains of TeNT and a pPur vector conferring puromycin resistance (Clontech). Cells were selected in medium containing 200 μg/ml G418 and 4 μg/ml puromycin.
For HGF treatment, cells were cultured for 16-24 h in DMEM with 1% FCS supplemented by 20 ng/ml human HGF recombinant (Calbiochem).
Treatment of Cell Extracts with Neurotoxins. CaCo-2, PC12, and MDCK cells were resuspended in 0.32 M sucrose/10 mM Hepes/1 mM MgCl2 plus protease inhibitors and passed through a ball-bearing cell cracker. Postnuclear supernatants (PNSs) were obtained by recovering the supernatant after centrifugation (5 min at 1,000 × g). The recombinant light chains of TeNT and botulinum neurotoxin were produced and purified as by Galli et al. (17). The PNSs were treated with the indicated toxin (200 nM) for 30 min at 37°C in the resuspension buffer. The brevins were revealed by Western blotting of the treated and untreated extracts with the anti-brevin antibody Cl10.1.
Immunocytochemistry. Cells were fixed with 3% paraformaldehyde and processed for immunofluorescence as described in ref. 29. Confocal laser scanning microscopy was performed by using an SP2 confocal microscope (Leica, Vienna). Images were assembled without modification by using photoshop (Adobe Systems, San Jose, CA).
Wound Healing Experiments. Cells were plated and cultured for 3 days to allow for the formation of monolayers. Cells were wounded by scratching with a bevel-edged 0.6 × 25-mm needle (Terumo, Leuven, Belgium).
Time-Lapse Video-Microscopy and Velocity Measurement. All studies were performed with an inverted microscope (Leica) placed in a temperature-controlled enclosure set at 37°C either with a 20× objective, 50-ms exposure, and a rate of 20 exposures per hour or with a 63× oil objective for stream aquisition of vesicle movement. In this case, DMEM without phenol red and without riboflavin (Invitrogen) was used. We used a Cascade amplified camera (Roper Scientific, Trenton, NJ), which allows an amplification of the transmitted signal up to 3,000×. For vesicle observations, the amplification used was 2,500×. The digital images were recorded and viewed by using metamorph software (Universal Imaging, Downingtown, PA). Analysis of video sequences was done with metamorph and excel (Microsoft).
After cell monolayer injuries, velocities were measured by the displacement of individual cells over time. A minimum of 60 cells was analyzed per condition (20 cells per injury; 10 at each border of the wound).
Statistical analyses were performed by using the Kruskal-Wallis nonparametric test with statview software (SAS Institute, Cary, NC).
Cell Adhesion Assays. All substrates were prepared by incubating overnight at room temperature, washing in PBS, and saturating with PBS-1% BSA (ultrapure BSA, Sigma), 2 μg of collagen (rat tail collagen in 30% ethanol, Roche Diagnostics), 2 μg of fibronectin [human fibronectin in 0.1 M borate buffer (pH 8.1), PAA, Linz, Austria), 2 μg of mouse laminin [prepared from Engelbreth-Holm-Swarm mouse tumors, kindly provided by M. Vigny (Institut du Fer-à-Moulin, Paris), in 0.1 M borate buffer (pH 8.1)], and 0.3 μg of polyornithine (Sigma) in PBS as a control. Alternatively, 1 μg of anti-human Fcγ fragment antibody (Jackson ImmunoResearch) was incubated overnight before coating with 0.5 μg of E-cadherin-Fc chimera (human E-cadherin extracellular domain fused to human Fc fragment, Ecad-Fc, R & D Systems).
MDCK cells expressing the transgenes indicated were mechanically dissociated in PBS/1% BSA/0.5 mM EDTA, and 5 × 105 cells were plated in 200 μl of DMEM in 48-well tissue culture plates in the presence or absence of 10% FCS (PAA). Cells were allowed to adhere to the plates at 37°C for the times indicated, and then the medium was entirely removed, and the cells were washed three times with 1 ml of PBS and dissociated with 100 μl of trypsin for direct cell counting. Three independent experiments were carried out; in each case, two different cell clones were used in duplicate for each transgene. For immunofluorescence experiments, the cells were plated on collagen-coated glass coverslips and cultured for the indicated times at 37°C, washed with PBS, fixed, and processed for immunofluorescence as above.
Antibody Uptake Experiments. Monolayers of MDCK cells were wounded by scratching. Two hours later, cells were incubated for 1.5 h in DMEM/10% FCS containing 5 μg/ml anti-β1 integrin antibody. Cells were then fixed and processed for immunofluorescence as previously described. In three independent experiments, cells from both sides of wounds were scored for intracellular labeling (cells comparable to the cells marked with an asterisk in Fig. 5C were considered positive for intracellular labeling). A minimum of 450 cells per wound were counted (at least 1,750 cells per condition).
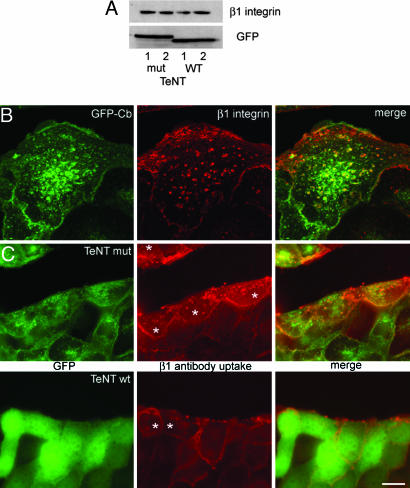
Fig. 5.
Cb regulates β1 integrin internalization. (A) (Upper) There is no significant difference in β1 integrin expression in cells expressing WT or mut-TeNT. (Lower) Western blot showing the cleavage of GFP-Cb in cells expressing WT TeNT. (B) β1 integrin and GFP-Cb colocalize in cells coexpressing GFP-Cb (green) and mut-TeNT at the border of a wound after anti-β1 integrin antibody uptake experiments (β1 integrin labeling in red). (C) Cells expressing WT TeNT at the border of a wound (Lower) show a less intense intracellular labeling after anti-β1 integrin antibody uptake (β1 integrin labeling in red) than cells expressing mut-TeNT. Cells with intracellular labeling as scored in our quantification are marked with an asterisk. (Scale bar: 39 μm for B and 20 μm for C.)
Results
TeNT Impairs Cell Migration. We first investigated whether the v-SNARE Cb was expressed in MDCK cells, which, indeed, is the case; a 12-kDa band was recognized by the monoclonal antibody Cl10.1, previously shown to specifically detect Cb and the neuronal synaptic v-SNAREs Sybs 1/2 (14) (Fig. 1A). Furthermore, canine Cb (cCb), as well as human Cb (hCb) and rat Syb 2, is sensitive to TeNT, because treatment of MDCK cell extract with TeNT cleaved the12-kDa band recognized by Cl10.1.
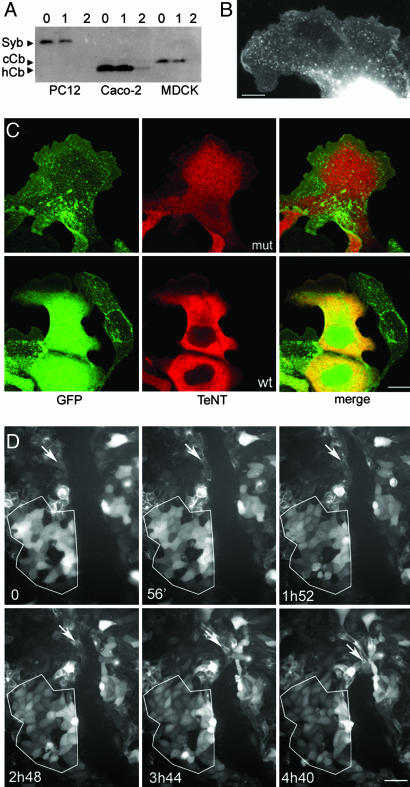
Fig. 1.
TeNT cleaves cCb and inhibits cell migration. (A) Western blot showing Syb, human Cb (hCb), and cCb in extracts from PC12 (rat cells), Caco-2 (human cells), and MDCK cells (canine cells) treated with botulinum C1 (lanes marked “1”) or tetanus (lanes marked “2”) neurotoxin in comparison with untreated cell extracts (lanes marked “0”). hCb, cCb, and Syb are TeNT-sensitive. Only one band (corresponding to cCb) is detected by Cl10.1, a pan-brevin monoclonal antibody, in MDCK cells. None of the brevins are sensitive to botulinum neurotoxin C1, as expected. (B) MDCK cells expressing GFP-Cb. GFP-Cb is localized in vesicles dispersed throughout the lamellipod. (Scale bar: 5 μm.) (C) MDCK cells expressing GFP-Cb and either mutant (mut) or WT TeNT (red) are able to form lamellipods. Cells expressing mutant TeNT (Upper) contain vesicular GFP-Cb (green), whereas cells expressing WT TeNT (Lower) show a diffuse labeling due to the cleavage of Cb and the liberation of soluble GFP. (Scale bar: 13 μm.) (D) Cell border migration of MDCK cells expressing GFP-Cb, with or without coexpression of WT TeNT, after monolayer injuries. Cells expressing WT TeNT show a diffuse labeling of GFP due to the release of GFP by toxin cleavage. One area particularly rich in cells expressing WT TeNT is outlined (lower left). One cell not expressing WT TeNT is marked by an arrow. Note that this cell is moving faster than WT TeNT-expressing cells. These images were extracted from Movie 1. Times indicated correspond to the time elapsed from the beginning of the film. (Scale bar: 31 μm.)
To study the dynamic localization and function of Cb in MDCK cells, we used a stable cell line expressing GFP-Cb (27). We found that GFP-Cb-containing vesicles were largely present throughout the lamellipod of MDCK cells treated with HGF (Fig. 1B), suggesting that Cb may play a role in cell migration or at least in some function of the lamellipods of migrating cells. To study the function of Cb-mediated trafficking in cell migration, we expressed the light chain of WT or inactive (E234Q, mut) TeNT (14) in our stable GFP-Cb-expressing MDCK cell line (27). Twenty-four-hour treatment of the transfected cells with HGF revealed no obvious change in phenotype; both the cells expressing WT TeNT and mut-TeNT showed large lamellipods (Fig. 1C). Cells expressing the mutated form maintain their punctate labeling, whereas cells expressing WT TeNT have a diffuse GFP staining, owing to the cleavage of Cb and the resulting release of the GFP into the cytoplasm. This difference enabled us to directly detect the cells expressing WT TeNT in videomicroscopy experiments (Fig. 1D; see also Movie 1, which is published as supporting information on the PNAS web site). We then tested the ability of TeNT-transfected epithelial cells to heal a mechanical wound. As a first approach, we studied the migration of GFP-Cb-expressing cells after transient transfection with WT TeNT. We found a striking difference between cells showing a punctate pattern for GFP-Cb, thus being TeNT negative, and those showing a soluble pattern, thus being TeNT positive, because the latter did not seem to participate in the closing of the wound (Fig. 1D and Movie 1). Because we were concerned that this difference could be the result of overexpression, we generated double stable cell lines coexpressing GFP-Cb and either WT TeNT or mut-TeNT. Stable cell lines were selected on the basis of the GFP pattern (punctate when GFP-Cb was uncleaved and soluble when it was cleaved) and staining for TeNT by using a monoclonal antibody raised against the light chain of TeNT (Fig. 1C). We repeated the wound healing experiments in the resulting double stable cell lines by using two independent clones expressing WT TeNT and two independent clones expressing mut-TeNT (Fig. 2 A and B; see also Movie 2, which is published as supporting information on the PNAS web site). In cell tracking experiments, we were able to show that the expression of WT TeNT resulted in a decrease in the migration speed of ≈50% (P < 0.0001 for each pair of clones expressing WT versus inactive TeNT; the same P value is obtained when the two clones of each category are pooled). Indeed, GFP-Cb/WT TeNT-expressing cell lines migrated at a speed of ≈8 μm/h, whereas GFP-Cb/mut-TeNT cell lines migrated at a speed of ≈17 μm/h (Fig. 2C). These results demonstrate that Cb plays an essential role in MDCK cell migration.
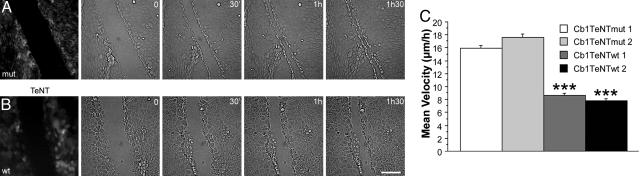
Fig. 2.
Stable expression of TeNT impairs cell migration. (A) Cell border migration of MDCK cells coexpressing GFP-Cb and mut-TeNT after monolayer injuries. (B) Cell border migration of MDCK cells coexpressing GFP-Cb and WT TeNT after monolayer injuries. The images in A and B were extracted from Movie 2; times indicated correspond to the time elapsed from the beginning of the film. The first image corresponds to the direct fluorescence pattern of the cells. (Scale bar: 40 μm.) (C) The migration of MDCK cells expressing WT TeNT is severely impaired. Quantification of the migration of MDCK cells expressing GFP-Cb and either WT or mut-TeNT after monolayer injuries. (Cells counted: 60-80 per clone.) Bars indicate SD (*, P < 0.0001).
GFP-Cb Dynamics in Migrating Cells. The study of GFP-Cb dynamics in migrating cells showed several types of behavior: (i) immobile vesicular structures, (ii) highly mobile vesicles, and (iii) transient accumulations at the leading edge. As an example of case ii, two mobile vesicles that are reaching the plasma membrane are indicated by an arrow or circled in Fig. 6 and Movie 3, which are published as supporting information on the PNAS web site. Interestingly, we found that some of these transient accumulations of GFP-Cb at the leading edge did not move with the leading edge but became immobile while the lamellipod moved forward (Fig. 3A; see also Movie 4, which is published as supporting information on the PNAS web site). Quantification of the fluorescence intensity showed that there was a moderate increase of fluorescence in these GFP-Cb domains, followed by an abrupt decline in fluorescence as the leading edge advanced (unpublished data). Such transient accumulations were not seen in GFP-expressing cells and did not have the same dynamics as 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI), a lipidic marker used to follow the dynamics of the entire plasma membrane (unpublished data); thus, they are not likely to result from the dynamics of membrane ruffles. Instead, these spots were reminiscent of focal contacts, and, in fact, we found colocalization of some accumulations of GFP-Cb with talin and FAK by confocal microscopy of immunostained, fixed MDCK cells. However, in these domains, GFP-Cb seems to concentrate at the distal extremity of the focal contact (Fig. 3B). These results indicated that Cb may participate in cell migration by regulating the trafficking at focal contacts.
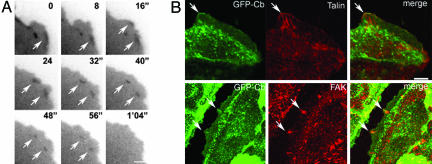
Fig. 3.
GFP-Cb-labeled subdomains of lamellipod in migrating cells. (A) A lamellipod of a migrating cell expressing GFP-Cb after a monolayer injury. Two transient accumulations of GFP-Cb are marked by arrows (time 0 and 16″). These accumulations remain immobile while the leading edge moves forward (from 16″ to 56″) and then finally disappear (1′04″). These images were extracted from Movie 4; times indicated correspond to the time elapsed from the beginning of the film ′, min; ″, s. (Scale bar: 2.7 μm.) (B) Colocalization of GFP-Cb subdomains with the focal adhesion markers talin (Upper) and FAK (Lower). A concentration of GFP-Cb (green) colocalizing with talin (red) is indicated by an arrow (Upper). Two domains of colocalization of GFP-Cb (green) with FAK (red) are also indicated by arrows (Lower). Note that in these domains, the GFP-Cb seems to be more distal than FAK. (Scale bar: Upper,7 μm; Lower, 8.2 μm.)
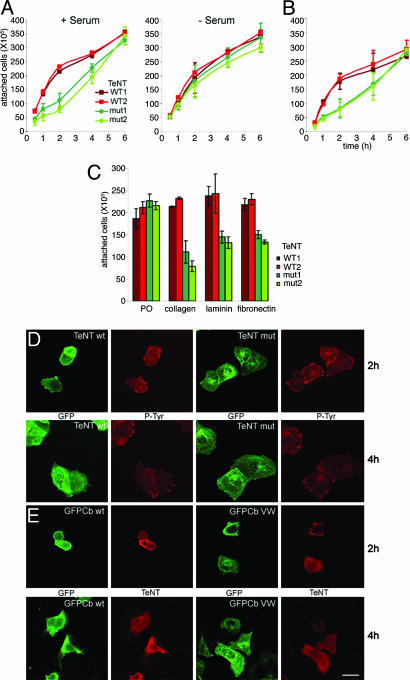
TeNT Impairs Cell-Substrate Adhesion. The enrichment of GFP-Cb close to focal contacts prompted us to test the hypothesis that Cb may be implicated in cell adhesion by assaying the ability of the different clones to adhere to different substrates. Cells interact with the extracellular matrix by means of integrins, a family of cell surface heterodimeric receptors composed of various α- and β-subunits (30). GFP-Cb/WT TeNT-expressing cell lines adhered faster to collagen-coated plates than GFP-Cb/mut-TeNT cell lines (Fig. 4A Left) with a maximum difference after 2 h in the presence of serum, suggesting that the effect seen on migration may be related to modification in integrin expression or recycling. Interestingly, in the absence of serum, a known activator of integrin recycling (31), we found no more difference between cells expressing WT TeNT and mut-TeNT, suggesting that the effect seen in the adhesion to collagen may be linked to integrin recycling (Fig. 4A Right). The adhesion to collagen is mediated by a variety of integrin dimers containing the β1-subunits (32, 33). It has been reported that receptors implicated in adhesion to collagen also promote adhesion to laminin, whereas cells adhere to fibronectin with αvβ3 and α5β1 integrin heterodimers (34, 35). Accordingly, we found a similar difference of adhesion on laminin- and fibronectin-coated plates at 2 h after plating but no difference on polyornithine, to which adhesion is considered to be mediated by electrostatic interaction of membrane lipid (Fig. 4C). Interestingly, adhesion to an E-cadherin-Fc susbtrate was also accelerated in GFP-Cb/WT TeNT-expressing cell lines (Fig. 4B). This acceleration could be attributed to homophilic E-cadherin-Fc/MDCK endogenous E-cadherin interaction or to E-cadherin-Fc/MDCK α2β1 heterophilic interaction. Indeed, the collagen ligand α2β1 has also been shown to interact with the cell-cell adhesion molecule E-cadherin (36).
Fig. 4.
Cb regulates β1 integrin-dependent cell-substrate adhesion. (A) Cells expressing WT TeNT (red) adhere faster to collagen-coated plates than cells expressing mut-TeNT (green) in the presence of 10% serum (Left) but not in the absence of serum (Right). (B) Cells expressing WT TeNT (red) adhere faster to human E-cadherin-Fc-coated plates than cells expressing mut-TeNT (green). Graphs in A and B represent numbers of cells adhered after different elapsed times. Values shown are mean ± SD calculated from three independent experiments using the same cell clones; each experiment was performed in duplicate. (C) Cells expressing WT TeNT (red) adhere faster to collagen-, laminin-, and fibronectin-coated plates than cells expressing mut-TeNT (green). No difference was observed with polyornithine-coated plates (PO). Graph bars show mean values ± SD calculated from three independent experiments at time 2 h, the point with the highest difference between clones according to A and B; each experiment was performed in duplicate. (D) Cells expressing WT TeNT spread slower on collagen than cells expressing mut-TeNT. Cells expressing WT TeNT (Upper Left, red) are more rounded and less spread and show a more diffuse phosphotyrosine labeling (Right) than cells expressing mut-TeNT (Upper, red) 2 h after plating on collagen. No significant difference is seen 4 h after plating (Lower). In this experiment, anti-GFP labeling is detected in green and anti-phosphotyrosine in red. (E) Expression of a TeNT-resistant mutant of Cb (GFPCb VW) rescues the phenotype induced by TeNT (red). Cells expressing WT TeNT and GFP-Cb VW (Right) are more spread than cells coexpressing WT TeNT and GFP-Cb (GFPCb wt, Left) 2 h after plating on collagen. (Scale bar: 24 μm.)
We then asked whether the effects observed on cell adhesion would affect the morphology of cells 2 h after plating on collagen, the time point for which the difference was maximal in the adhesion assay (Fig. 4A). We found that cells expressing WT TeNT are still round, whereas cells expressing mut-TeNT show a normal spreading in these conditions. Such a strong difference was not seen 4 h after plating (Fig. 4D). To demonstrate that the effect of TeNT was specifically due to the cleavage of Cb, we studied the spreading on collagen of WT MDCK cells cotransfected by WT TeNT plus either GFP-Cb WT or GFP-Cb VW, a TeNT-resistant mutant of Cb (28). Cells coexpressing TeNT and GFP-Cb VW spread more on collagen than those coexpressing TeNT and GFP-Cb WT (Fig. 4E).
Taken together, these results suggest that Cb may regulate cell migration by mediating the fast recycling of integrins, particularly β1 integrin, with an additional possible direct or indirect implication in E-cadherin-dependent adhesion. We tested this hypothesis by comparing the endocytosis of an antibody directed against β1 integrin in our cell lines. We first checked that there is no significant difference in the expression of β1 integrin between our cell lines (Fig. 5A). We found that GFP-Cb and endocytosed β1 integrin (detected by antibody uptake) partially colocalized in cells located at the border of wounds (Fig. 5B). In similar antibody uptake experiments, the labeling at the plasma membrane was similar when we compared the different clones (Fig. 5C), but cells expressing WT TeNT showed a much weaker intracellular signal than mut-TeNT-expressing cells located at the border of a wound. The ratio of cells with punctuate intracellular staining of anti-β1 integrin internalization (asterisk in Fig. 5C) was strongly reduced in cells expressing WT TeNT (48.2 ± 5%) compared with cells expressing mut-TeNT (72.4 ± 7.64%; three independent experiments, χ2 P value < 0.0001). Together, these results suggest that TeNT inhibits the recycling of β1 integrins.
Discussion
In this study, we were able to demonstrate the involvement of the endosomal vesicular SNARE Cb in cell migration and integrin-dependent adhesion. This conclusion is supported by the following evidence: (i) Cb traffics in lamellipods of migrating cells, (ii) impairment of Cb function by TeNT leads to a 2-fold reduction in the migration speed, and (iii) TeNT treatment alters cell-matrix adhesion and spreading and β1 integrin recycling. Therefore, our findings strongly suggest that exo-endocytosis is a key process in cell migration and adhesion and that maximal migration speed requires a fine regulation of cell adhesion.
As highlighted in the introduction, several important functions have been attributed to Cb, particularly in the recycling of plasma membrane receptors (17) and in phagocytosis in macrophages (20, 21), a process in which endosomal membranes are targeted in a polarized fashion to the site of particle binding and engulfment. Here, we identify another function of Cb and early endosomes in cell migration and adhesion. We show that Cb-dependent trafficking regulates cell-substrate adhesion to a specific set of substrates including collagen, laminin, and fibronectin, all known to involve β1 integrins (37). The expression of TeNT resulted in a decrease in the migration speed, a faster adhesion to collagen-, laminin-, and fibronectin-coated plates, and a decrease in the recycling of β1 integrin. Thus, Cb may regulate cell migration by promoting the fast recycling of proteins implicated in cell adhesion, particularly β1 integrins, thereby decreasing cell-substrate adhesion and enabling the lamellipod to move forward. Our data are reminiscent of findings on the cell adhesion molecule L1. Indeed, the neuronal form of L1 has an additional exon that encodes an AP-2-binding site, thereby promoting fast recycling of the protein (38) and weaker adhesion (39). Thus, our data are compatible with the concept that optimal migration is obtained with submaximal cell-matrix adhesion. The involvement of Cb in cell migration is in agreement with the need for the recycling of membrane proteins and receptors, as previously suggested (11, 13).
Previous work has shown that αvβ3 integrin recycles by means of a rab4-dependent mechanism and that expression of dominant-negative rab4 compromises αvβ3-dependent cell adhesion (35). Furthermore, rab11 stimulates the recycling of β1 integrins in HeLa cells (31). In agreement with these studies, we demonstrate here a role for the early endosomal v-SNARE Cb that partially colocalizes with rab4 (40) and rab11 (41) in the recycling of β1 integrins and in cell adhesion and migration. Similar to the effect of the Syb 2 knockout, which impairs both exocytosis and endocytosis of synaptic vesicles (42), the elimination of Cb by TeNT may affect exocytosis or endocytosis of β1 integrins or both. The present study suggests that Cb is required for the efficient, fast recycling of plasma membrane proteins such as β1 integrin in migrating cells. The fact that β1 integrins are still expressed at the plasma membrane of TeNT-expressing MDCK cells indicates that β1 integrins are also able to reach the plasma membrane in a TeNT-insensitive manner. Further work will be required to elucidate the coordination and regulation of these different pathways of β1 integrin trafficking. Our results are reminiscent of previous work that shows that FAK-/- fibroblast-like cells cultured from E8 embryos have a reduced motility, a rounder morphology, and a poorer spreading than FAK+/+ cells but nevertheless have an enhanced attachment to collagen and laminin (43). Thus, our data suggest that Cb-dependent recycling of β1 integrins may regulate the dynamics of focal adhesion contacts.
Our data also suggest that TeNT may have an effect on neuronal cell adhesion in addition to the well known block of neurotransmitter release. Several botulinum neurotoxins were shown to stimulate axonal sprouting (16). This phenomenon may result from the incapacity of the affected neurites to properly regulate their substrate adhesion. Furthermore, the recycling of synaptic vesicles was recently shown to involve talin (44), a protein known to be involved in the regulation of integrins (45), thus suggesting that our observations in MDCK cells may also have implications in neuronal cells.
Supplementary Material
Acknowledgments
We are grateful to Romano Regazzi for the VW mutant of Cb; Mark Bretscher, Daniel Louvard, Bernard Hofflack, and Rachel Rudge for critical reading of the manuscript; André Sobel (Institut du Fer-à-Moulin, Paris), who generously provided lab space and reagents for initial experiments; Jean-Antoine Girault for constant support; and all members of the Institut National de la Santé et de la Recherche Médicale (INSERM) Units 706 and 536, especially Elodie Charbaut, Isabelle Jourdain, Sonia Martinez-Arca, and Hervé Enslen, for advice and encouragement. V.P.-G. was supported by the Association pour la Recherche sur le Cancer (ARC). This work was supported in part by grants from the INSERM Avenir Program, the European Community (“Signaling and Traffic” STREP 503229), the Human Frontier Science Program (RGY0027/2001-B101), ARC (nos. 5873 and 4762), the Association Française Contre les Myopathies, and the Ministère de la Recherche (Action Concertée Incitative-Biologie du développement et Physiologie Integrative) (to T.G.).
Author contributions: T.G. designed research; V.P.-G. and J.G. performed research; J.G. contributed new reagents/analytic tools; V.P.-G., T.I., R.-M.M., and T.G. analyzed data; and V.P.-G. and T.G. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Cb, cellubrevin; Syb, synaptobrevin; TeNT, tetanus neurotoxin; HGF, hepatocyte growth factor; MDCK, Madin-Darby canine kidney; FAK, focal adhesion kinase; cCb, canine Cb.
References
- 1.Ridley, A. J., Schwartz, M. A., Burridge, K., Firtel, R. A., Ginsberg, M. H., Borisy, G., Parsons, J. T. & Horwitz, A. R. (2003) Science 302, 1704-1709. [DOI] [PubMed] [Google Scholar]
- 2.Montell, D. J. (2003) Nat. Rev. Mol. Cell Biol. 4, 13-24. [DOI] [PubMed] [Google Scholar]
- 3.Stoker, M. (1989) J. Cell. Physiol. 139, 565-569. [DOI] [PubMed] [Google Scholar]
- 4.Rosen, P. & Misfeldt, D. S. (1980) Proc. Natl. Acad. Sci. USA 77, 4760-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall, A. (1998) Science 279, 509-514. [DOI] [PubMed] [Google Scholar]
- 6.Kjoller, L. & Hall, A. (1999) Exp. Cell. Res. 253, 166-179. [DOI] [PubMed] [Google Scholar]
- 7.Waterman-Storer, C. M. & Salmon, E. (1999) Curr. Opin. Cell Biol. 11, 61-67. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins, C. R., Gibson, A., Shipman, M., Strickland, D. K. & Trowbridge, I. S. (1994) J. Cell Biol. 125, 1265-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rappoport, J. Z. & Simon, S. M. (2003) J. Cell Sci. 116, 847-855. [DOI] [PubMed] [Google Scholar]
- 10.Schmoranzer, J., Kreitzer, G. & Simon, S. M. (2003) J. Cell Sci. 116, 4513-4519. [DOI] [PubMed] [Google Scholar]
- 11.Bretscher, M. S. & Aguado-Velasco, C. (1998) Curr. Opin. Cell Biol. 10, 537-541. [DOI] [PubMed] [Google Scholar]
- 12.Bretscher, M. S. (1992) EMBO J. 11, 383-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawson, M. A. & Maxfield, F. R. (1995) Nature 377, 75-79. [DOI] [PubMed] [Google Scholar]
- 14.McMahon, H. T., Ushkaryov, Y. A., Edelmann, L., Link, E., Binz, T., Niemann, H., Jahn, R. & Sudhof, T. C. (1993) Nature 364, 346-349. [DOI] [PubMed] [Google Scholar]
- 15.Niemann, H., Blasi, J. & Jahn, R. (1994) Trends Cell Biol. 4, 179-185. [DOI] [PubMed] [Google Scholar]
- 16.Humeau, Y., Doussau, F., Grant, N. J. & Poulain, B. (2000) Biochimie 82, 427-446. [DOI] [PubMed] [Google Scholar]
- 17.Galli, T., Chilcote, T., Mundigl, O., Binz, T., Niemann, H. & De Camilli, P. (1994) J. Cell Biol. 125, 1015-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das, V., Nal, B., Dujeancourt, A., Thoulouze, M. I., Galli, T., Roux, P., Dautry-Varsat, A. & Alcover, A. (2004) Immunity 20, 577-588. [DOI] [PubMed] [Google Scholar]
- 19.Breton, S., Nsumu, N. N., Galli, T., Sabolic, I., Smith, P. J. S. & Brown, D. (2000) Am. J. Physiol. 278, F717-F725. [DOI] [PubMed] [Google Scholar]
- 20.Bajno, L., Peng, X. R., Schreiber, A. D., Moore, H. P., Trimble, W. S. & Grinstein, S. (2000) J. Cell Biol. 149, 697-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun, V., Fraisier, V., Raposo, G., Hurbain, I., Sibarita, J. B., Chavrier, P., Galli, T. & Niedergang, F. (2004) EMBO J. 23, 4166-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basyuk, E., Galli, T., Mougel, M., Blanchard, J. M., Sitbon, M. & Bertrand, E. (2003) Dev. Cell 5, 161-174. [DOI] [PubMed] [Google Scholar]
- 23.Mallard, F., Tang, B. L., Galli, T., Tenza, D., Saint-Pol, A., Yue, X., Antony, C., Hong, W., Goud, B. & Johannes, L. (2002) J. Cell Biol. 156, 653-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schraw, T. D., Rutledge, T. W., Crawford, G. L., Bernstein, A. M., Kalen, A. L., Pessin, J. E. & Whiteheart, S. W. (2003) Blood 102, 1716-1722. [DOI] [PubMed] [Google Scholar]
- 25.Allen, L. A. H., Yang, C. M. & Pessin, J. E. (2002) J. Leukocyte Biol. 72, 217-221. [PMC free article] [PubMed] [Google Scholar]
- 26.Yang, C. M., Mora, S., Ryder, J. W., Coker, K. J., Hansen, P., Allen, L. A. & Pessin, J. E. (2001) Mol. Cell. Biol. 21, 1573-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Arca, S., Proux-Gillardeaux, V., Alberts, P., Louvard, D. & Galli, T. (2003) J. Cell Sci. 116, 2805-2816. [DOI] [PubMed] [Google Scholar]
- 28.Regazzi, R., Sadoul, K., Meda, P., Kelly, R. B., Halban, P. A. & Wollheim, C. B. (1996) EMBO J. 15, 6951-6959. [PMC free article] [PubMed] [Google Scholar]
- 29.Coco, S., Raposo, G., Martinez, S., Fontaine, J. J., Takamori, S., Zahraoui, A., Jahn, R., Matteoli, M., Louvard, D. & Galli, T. (1999) J. Neurosci. 19, 9803-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hynes, R. O. (1992) Cell 69, 11-25. [DOI] [PubMed] [Google Scholar]
- 31.Powelka, A. M., Sun, J., Li, J., Gao, M., Shaw, L. M., Sonnenberg, A. & Hsu, V. W. (2004) Traffic 5, 20-36. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto, K. & Yamamoto, M. (1994) Exp. Cell Res. 214, 258-263. [DOI] [PubMed] [Google Scholar]
- 33.Velling, T., Risteli, J., Wennerberg, K., Mosher, D. F. & Johansson, S. (2002) J. Biol. Chem. 277, 37377-37381. [DOI] [PubMed] [Google Scholar]
- 34.Belkin, A. M. & Stepp, M. A. (2000) Microsc. Res. Tech. 51, 280-301. [DOI] [PubMed] [Google Scholar]
- 35.Roberts, M., Barry, S., Woods, A., van der Sluijs, P. & Norman, J. (2001) Curr. Biol. 11, 1392-1402. [DOI] [PubMed] [Google Scholar]
- 36.Whittard, J. D., Craig, S. E., Mould, A. P., Koch, A., Pertz, O., Engel, J. & Humphries, M. J. (2002) Matrix Biol. 21, 525-532. [DOI] [PubMed] [Google Scholar]
- 37.Lafrenie, R. M. & Yamada, K. M. (1996) J. Cell Biochem. 61, 543-553. [DOI] [PubMed] [Google Scholar]
- 38.Kamiguchi, H., Long, K. E., Pendergast, M., Schaefer, A. W., Rapoport, I., Kirchhausen, T. & Lemmon, V. (1998) J. Neurosci. 18, 5311-5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long, K. E., Asou, H., Snider, M. D. & Lemmon, V. (2001) J. Biol. Chem. 276, 1285-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daro, E., van der Sluijs, P., Galli, T. & Mellman, I. (1996) Proc. Natl. Acad. Sci. USA 93, 9559-9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilcke, M., Johannes, L., Galli, T., Mayau, V., Goud, B. & Salamero, J. (2000) J. Cell Biol. 151, 1207-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deak, F., Schoch, S., Liu, X., Sudhof, T. C. & Kavalali, E. T. (2004) Nat. Cell Biol. 6, 1102-1108. [DOI] [PubMed] [Google Scholar]
- 43.Ilic, D., Furuta, Y., Kanazawa, S., Takeda, N., Sobue, K., Nakatsuji, N., Nomura, S., Fujimoto, J., Okada, M. & Yamamoto, T. (1995) Nature 377, 539-544. [DOI] [PubMed] [Google Scholar]
- 44.Di Paolo, G., Pellegrini, L., Letinic, K., Cestra, G., Zoncu, R., Voronov, S., Chang, S., Guo, J., Wenk, M. R. & De Camilli, P. (2002) Nature 420, 85-89. [DOI] [PubMed] [Google Scholar]
- 45.Tadokoro, S., Shattil, S. J., Eto, K., Tai, V., Liddington, R. C., de Pereda, J. M., Ginsberg, M. H. & Calderwood, D. A. (2003) Science 302, 103-106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.