Abstract
Objective:
To understand the impact of interictal spikes on brain connectivity in patients with Self-Limited Epilepsy with Centrotemporal Spikes (SeLECTS).
Methods:
Electroencephalograms from 56 consecutive SeLECTS patients were segmented into periods with and without spikes. Connectivity between electrodes was calculated using the weighted phase lag index. To determine if there are chronic alterations in connectivity, we compared spike-free connectivity to connectivity in 65 matched controls. To understand the acute impact of spikes, we compared connectivity immediately before, during, and after spikes versus baseline, spike-free connectivity. We explored whether behavioral state, spike laterality, or antiseizure medications affected connectivity.
Results:
Children with SeLECTS had markedly higher connectivity than controls during sleep but not wakefulness, with greatest difference in the right hemisphere. During spikes, connectivity increased globally; before and after spikes, left frontal and bicentral connectivity increased. Right hemisphere connectivity increased more during right-sided spikes than left; left hemisphere connectivity was equally affected by right and left spikes.
Conclusions:
SeLECTS patient have persistent increased connectivity during sleep; connectivity is further elevated during the spike and perispike periods.
Significance:
Testing whether increased connectivity impacts cognition or seizure susceptibility in SeLECTS and more severe epilepsies could help determine if spikes should be treated.
Keywords: Rolandic Epilepsy, Benign Epilepsy with Centrotemporal Spikes (BECTS), Childhood Epilepsy with Centrotemporal Spikes (CECTS), Connectivity, Sleep, Interictal epileptiform discharges (IEDs)
1. Introduction1
Self-limited epilepsy with centrotemporal spikes (SeLECTS), also known as Benign Rolandic Epilepsy, is the most common focal childhood epilepsy syndrome (Specchio et al., 2022; Stephen et al., 2020; Wirrell, 1998). SeLECTS is characterized by self-limited nocturnal focal sensorimotor seizures and sleep-activated centrotemporal spikes. Not every child with SeLECTS takes anti-seizure medications, and spikes are not a treatment target (Panayiotopoulos et al., 2008; Wirrell, 1998). Most children outgrow SeLECTS but they often have cognitive and behavioral comorbidities, especially affecting language and attention (Kavros et al., 2008; Smith et al., 2015, 2012; Teixeira and Santos, 2018; Vannest et al., 2015; Wickens et al., 2017). Some posit that SeLECTS is an epileptic encephalopathy that disrupts brain function during childhood (Berg et al., 2010; Kramer et al., 2021; Spencer et al., 2022; Vannest et al., 2015) with potentially lasting cognitive consequences (Monjauze et al., 2011). Understanding the impact of SeLECTS on brain function, and specifically the role that spikes play, has important clinical implications.
Evidence suggests that SeLECTS subtly changes structural and functional brain connectivity. Imaging demonstrates reduced structural connectivity between the Rolandic regions and the frontal and temporal language regions (Besseling et al., 2014, 2013a) as well as the thalami (Thorn et al., 2020). Longitudinal studies suggest that divergence in structural connectivity from the healthy population increases over the course of the epilepsy and that this correlates with neurocognitive difficulties (Garcia-Ramos et al., 2019, 2015). Functional magnetic resonance imaging (fMRI) identifies altered connectivity between Rolandic and language regions (Besseling et al., 2013b), as well as default mode network differences (Oser et al., 2014) that also evolves over the course of the disease (Zeng et al., 2015). Whether spikes drive connectivity abnormalities remains an open question. Several fMRI studies found that spike-related connectivity alterations (Li et al., 2017; Ofer et al., 2018; Xiao et al., 2016) correlate with cognitive performance. Few studies have looked at connectivity in SeLECTS with electroencephalogram (EEG). EEG directly measures brain activity with high temporal sensitivity, and thus may be well-suited to isolate the impact of spikes. Two EEG studies (Adebimpe et al., 2015; Ghantasala and Holmes, 2019) found widespread increases in connectivity during spikes, but one (Ghantasala and Holmes, 2019) did not control for volume conduction, which can inflate connectivity estimations. EEG studies comparing spike-free periods in children with SeLECTS vs. children without epilepsy (Adebimpe et al., 2016, 2015; Clemens et al., 2016; Varotto et al., 2018) have focused on wakefulness, even though the majority of seizures and spikes occur in sleep. A single EEG study (Varotto et al., 2018) found more prominent connectivity differences in sleep than wakefulness.
Here we aimed to: 1) compare connectivity between children with and without SeLECTS during both sleep and wakefulness; and 2) assess the topology and duration of connectivity changes associated with spikes. We analyzed clinical EEGs obtained from children with SeLECTS and compared connectivity during spike-free periods both awake and asleep with that of age- and sex-matched children without epilepsy. To understand the duration of spike-related effects, we compared connectivity in the 500ms preceding, during, and following spikes to spike-free periods. We used weighted Phase Lag Index (wPLI), a phase-based connectivity measurement that minimizes volume conduction (Vinck et al., 2011). We focused on beta-frequency band connectivity which can be estimated in short epochs (Cohen, 2014) and has been reported abnormal in SeLECTS (Adebimpe et al., 2016, 2015; Clemens et al., 2016; Song et al., 2019). We hypothesized that children with SeLECTS would have connectivity differences compared to children without epilepsy in both the awake and sleep state and that centrotemporal spikes would increase connectivity.
2. Methods
2.1. Inclusion Criteria:
The Stanford University Institutional Review Board approved this study. Using Stanford Research Repository Tools, we searched all clinical records using a combination of International Classification of Disease (ICD)-9 and ICD-10 codes, procedural codes, and keywords to identify children born between January 2005 – December 2015 who underwent a routine EEG before October 2019 at Lucile Packard Children’s Hospital with a possible diagnosis of SeLECTS. We reviewed resulting medical records to confirm age of onset between 3–15 years old, appropriate seizure semiology (hypersalivation, facial/hemibody twitching, or nocturnal tonic-clonic), and centrotemporal spikes on EEG (Fisher et al., 2017). We excluded children with a history of: prematurity (<35 weeks); abnormal brain MRI; other epilepsy syndromes; neurosurgery; severe brain injury; diagnosed genetic disorder; profound intellectual disability; or severe medical conditions (i.e. congenital heart disease, cancer, chronic immunomodulation). Sex and age (within 1.25 years) matched controls were identified by searching for children born between July 2001-December 2017 evaluated for syncope, headache, or altered consciousness and for whom, after evaluation, there was no concern for epilepsy or serious neurologic problem. We applied the same exclusion criteria and additionally excluded children on antiseizure or other psychoactive medications. Since not every child slept, we included additional controls to obtain matched sleep data.
2.2. EEG Acquisition:
EEGs were recorded using the Nihon-Kohden acquisition system with a standard 10–20 montage with 19 scalp electrodes at 200 or 500Hz.
2.3. EEG Annotation (Figure 1):
Figure 1: Creating Electroencephalogram Epochs for Analysis.
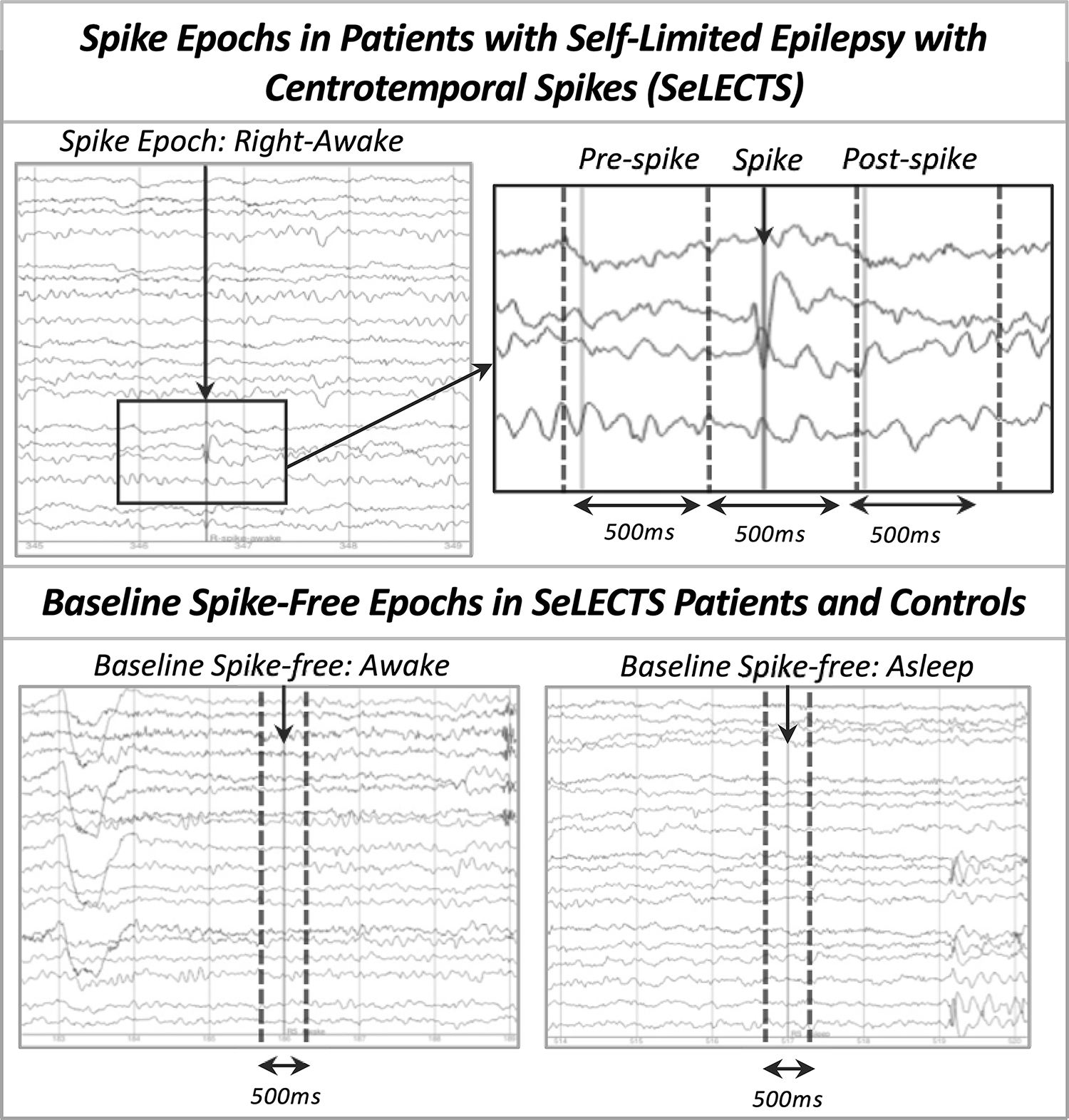
The top panel shows annotation of the spike and definition of the three spike-related (pre-spike, during spike, post-spike) epochs based on this annotation. The lower panel shows identification of awake (left) and asleep (right) baseline, spike-free epochs separated by a minimum of 2 seconds from artifacts and from sleep architecture.
EEGs were manually annotated by a research assistant (BG) and reviewed by a board-certified child neurologist/epileptologist (FMB).
2.3.1. Spike-Related Epochs:
Spikes were identified only in the SeLECTS group. We annotated the point of maximal negativity and classified each spike as falling into one of four categories (left-awake; left-asleep; right-awake; right-asleep) based on hemisphere of maximum amplitude and behavioral state. We defined sleep by loss of the posterior dominant rhythm plus presence of sleep architecture; sleep was limited to Stage II in these 30–60 minute studies. A spike category was included only if the patient had at least 18 such examples of that spike type; this number was chosen to maximize data included while ensuring stable connectivity estimates, which can be sensitive to low trial numbers (Cohen, 2014). Each annotation was used to generate 3 distinct spike-related epochs: pre-spike (−700 to −200ms), spike (−200 to 300ms), and post-spike (300 to 800ms). Spikes and any after going slow-wave fell within the spike epoch. We only annotated spikes separated by at least 1.5 seconds to prevent overlapping epochs.
2.3.2. Baseline/Spike-Free Epochs:
For the SeLECTS and control groups, we identified 500ms epochs at least 2 seconds away from any artifact and, for the SeLECTS group, at least 2 seconds away from spikes (preferentially >5 seconds away). We classified baseline epochs as awake or asleep as above and excluded spindles, vertex waves, or K-complexes in sleep epochs. For SeLECTS patients, we matched the number of spike-free to spike-related epochs (i.e. 23 right-awake spikes to 23 awake spike-free epochs). We also matched the number of spike-free epochs contributed by the SeLECTS and control subjects at the group level.
2.4. Calculation of Connectivity Measures:
The EEG was band pass filtered between 12–30Hz using a zero-phase, acausal filter (firwin design, Hamming window from the mne-python library) to extract the beta band, down-sampled to 200 Hz, and then the Hilbert transform was applied (Cohen, 2014). The analytic signal was segmented into 500ms epochs described above. Using mne-python (version 0.21) package (Gramfort et al., 2013), the wPLI over time was calculated between each of the 171 electrode pairs for every epoch using custom python code (Cohen, 2014; Lee-Messer, 2021; Vinck et al., 2011). Data was prepared for statistical analysis using the Pandas (Mckinney, 2018; Reback et al., 2022) python software package facilitated by the PyArrow (Apache Arrow Team, 2022), fastparquet (Durant, 2022) and RAPIDS libraries (RAPIDS Development Team, 2018; for overview see Raschka et al., 2020) in order to process the data more quickly and store it more efficiently. We quantified connectivity in 2 ways. First, we calculated the “pairwise connectivity” for each of the 171 unique electrode pairs by averaging the wPLI values for all epochs of a given type (i.e. all right-awake pre-spike epochs). We then calculated an “average connectivity” for each of the 19 electrodes by averaging the pairwise connectivity values between that electrode and the other 18 electrodes. We included the average measurement as it improves stability of connectivity estimates in pediatric data (Haartsen et al., 2021).
2.5. Statistical Analysis
Statistical analyses were performed using Statistical Analysis System (SAS) OnDemand for Academics (Cary, NC). We tested for significant differences in age and sex between the SeLECTS and control groups using the student t-test and chi-square test respectively. We used a 1-way ANOVA to assess for significant differences in epoch numbers for any of the groups/conditions being compared, specifically: (1) between groups (SeLECTS and controls), in the number of spike-free awake and asleep epochs; and (2) within the SeLECTS group, in the number of epochs for each of the four spike categories (left-awake; left-asleep; right-awake; right-asleep).
All further analyses focused on connectivity. Since subjects contributed multiple connectivity outcome measurements (i.e. awake and asleep spike-free epochs) to each model, we used a generalized estimating equations (GEE) with an independent correlation matrix (Zeger and Liang, 1986; Zeger et al., 1988) to account for repeated-measures and correlation within individuals.
To test if baseline, spike-free connectivity in children with and without SeLECTS differed in sleep or wakefulness, we fit a GEE model with connectivity as the dependent variable and group (SeLECTS/controls), behavioral state (awake/asleep), and the interaction between the two as the independent variables and performed analyses stratified by behavioral state. For average connectivity, we ran separate models for each of the 19 electrodes and after Bonferroni correction, considered p-values <0.0026 significant. For pairwise connectivity, we ran separate models for each of the 171 unique electrode pairs and, after Bonferroni correction, considered p-values <0.0003 significant. We adjusted models for age and sex as we were comparing two independent groups. We also adjusted for antiseizure medication use as not all SeLECTS children were medicated and furthermore conducted a sensitivity analysis comparing controls to unmedicated patients.
To assess the impact of spikes on connectivity, we calculated the change in connectivity from the spike-free baseline (Connectivity in Spike Epoch – Connectivity in Spike-Free Epoch) as the dependent variable matching for behavioral state. We modeled each of the three spike-related epochs (pre, during, post) separately. We fit a GEE model with only an intercept where the intercept quantified change in either: 1) the average connectivity of each electrode; or 2) the pairwise connectivity between all electrodes, with Bonferroni correction as above. We used a GEE model to account for repeated measures as each subject could contribute up to four potential categories of spikes (left-awake; left-asleep; right-awake; right-asleep). Given that we investigated the ability of a spike to perturb connectivity from baseline, and hence individuals were compared to themselves, we did not adjust for age, sex or medication use. In exploratory analyses, we tested if behavioral state, spike laterality, and antiseizure medication use altered spike-related connectivity changes.
3. Results
3.1. Subjects (Figure 2):
Figure 2: Selection Criteria for Self-Limited Epilepsy with Centrotemporal Spikes (SeLECTS) Patients.
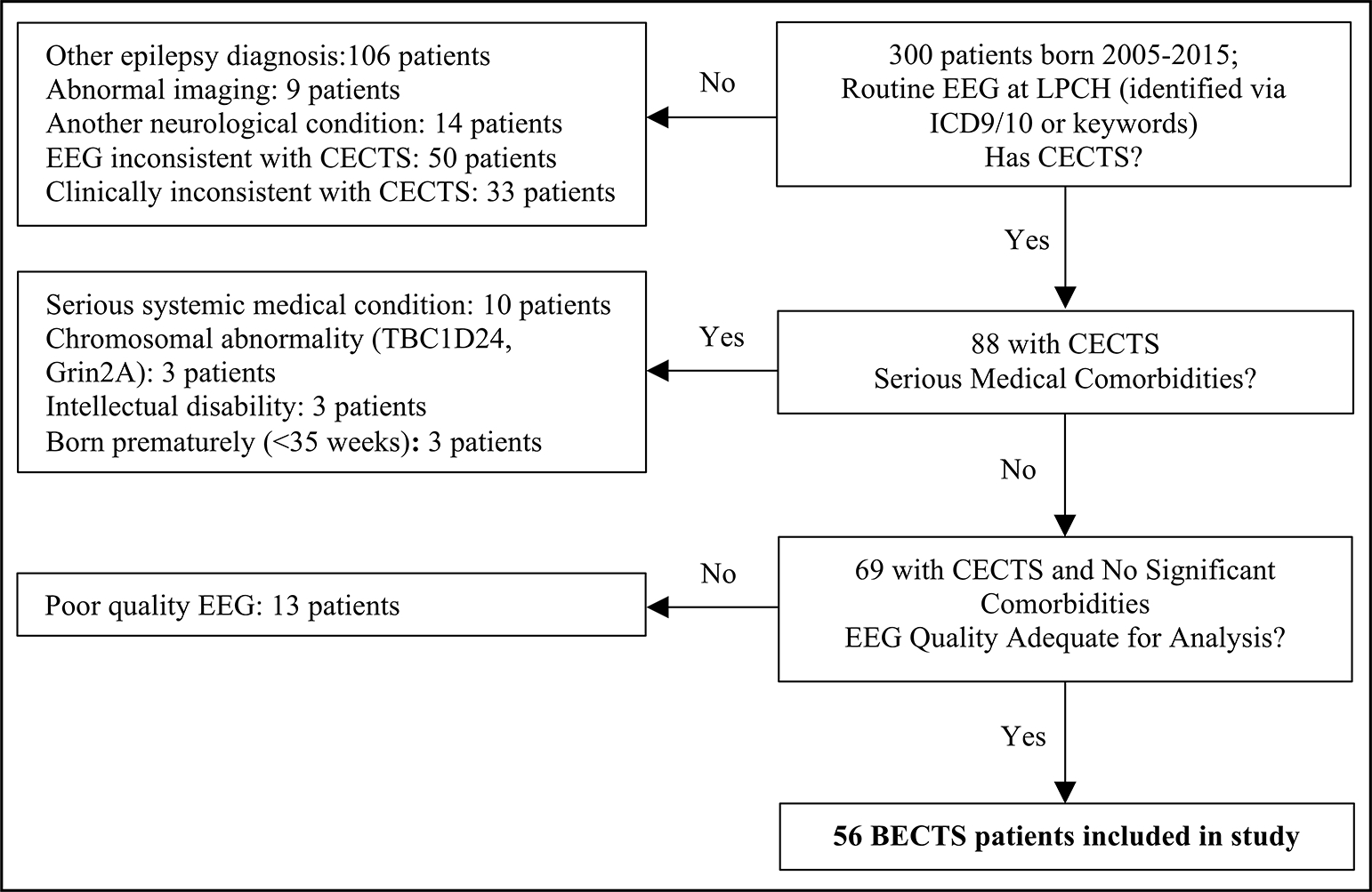
Children with SeLECTS were identified as above. Charts from 1019 patients evaluated for syncope, headache, or altered consciousness were reviewed to identify age- and sex-matched controls with electroencephalograms but without a diagnosis of epilepsy or significant neurological disorders.
Of the initial 300 patients reviewed, 56 met inclusion and exclusion criteria. We reviewed 1019 charts to identify 56 controls who were 1:1 age and sex matched with children in the SeLECTS group. As only 32 controls slept during their EEG as compared to 41 SeLECTS patients, we included an additional 9 controls with sleep EEGs who otherwise met inclusion criteria, including 65 controls in total.
3.2. Clinical Information:
There were no group differences in age at EEG (SeLECTS 8.69+/− 2.03yrs; controls 8.80+/−2.40yrs; t(120)=0.44, p =0.80) or sex distribution (SeLECTS 40/56, 71% male; controls 43/65, 66% male, X2(1, n=121)=0.39, p=0.53). The average age of SeLECTS onset was 8.69 +/− 2.04 years and the median time between first seizure and EEG was 0.08 years (IQR 0.77 years). Fifteen (27%) SeLECTS patients were taking antiseizure medication: 6 oxcarbazepine, 7 levetiracetam, 1 sulthiame, and 1 valproate. Indications for control EEGs included syncopal spells (46/65, 71%), staring spells (9/65, 14%), nocturnal waking with vomiting (1/65, 1.5%), episodes of confusion (3/65, 4.5%), inattention (4/65, 6%), tremors (1/65, 1.5%), and nausea (1/65, 1.5%).
3.3. Epoch Counts:
Fifty-three SeLECTS and 65 controls contributed awake spike-free epochs; 41 SeLECTS and 41 controls contributed asleep epochs. Number of spike-free awake (SeLECTS = 26 +/−8; controls=26 +/− 5; t(116)=1.73, p=0.09) or asleep epochs (SeLECTS=24+/−3; controls=24+/−4; t(80)=0.17, p=0.86) did not differ between groups. Of the 56 SeLECTS patients, 19 had only left hemispheric spikes on the EEG, 21 only had only right hemispheric spikes, and 16 had bilateral independent spikes. We were able to extract both left and right-sided spike epochs from 11 of the 16 patients with bilateral independent spikes; for the remaining 5 patients, we were only able to extract right spike epochs from 4 and left spike epochs from 1. We excluded bilaterally synchronous spikes (spikes of equal amplitude in both hemispheres) from the analysis as there were not enough epochs nor enough patients with synchronous spikes to generate meaningful connectivity estimates. In total, 20 SeLECTS patients contributed left-awake spikes (mean 26+/−8 epochs), 18 left-asleep spikes (23+/−3 epochs), 20 right-awake spikes (24+/−6 epochs), and 29 right-asleep spikes (24+/−4 epochs). There was no significant difference in the number of epochs for each spike category (F(3,83)=1.03, p=0.38).
3.4. Group Differences in Baseline (Spike-Free) Connectivity (Figure 3; Table 1:
Figure 3: Spike-Free Baseline Connectivity in Children with Self-Limited Epilepsy with Centrotemporal Spikes (SeLECTS) vs. Children without Epilepsy.
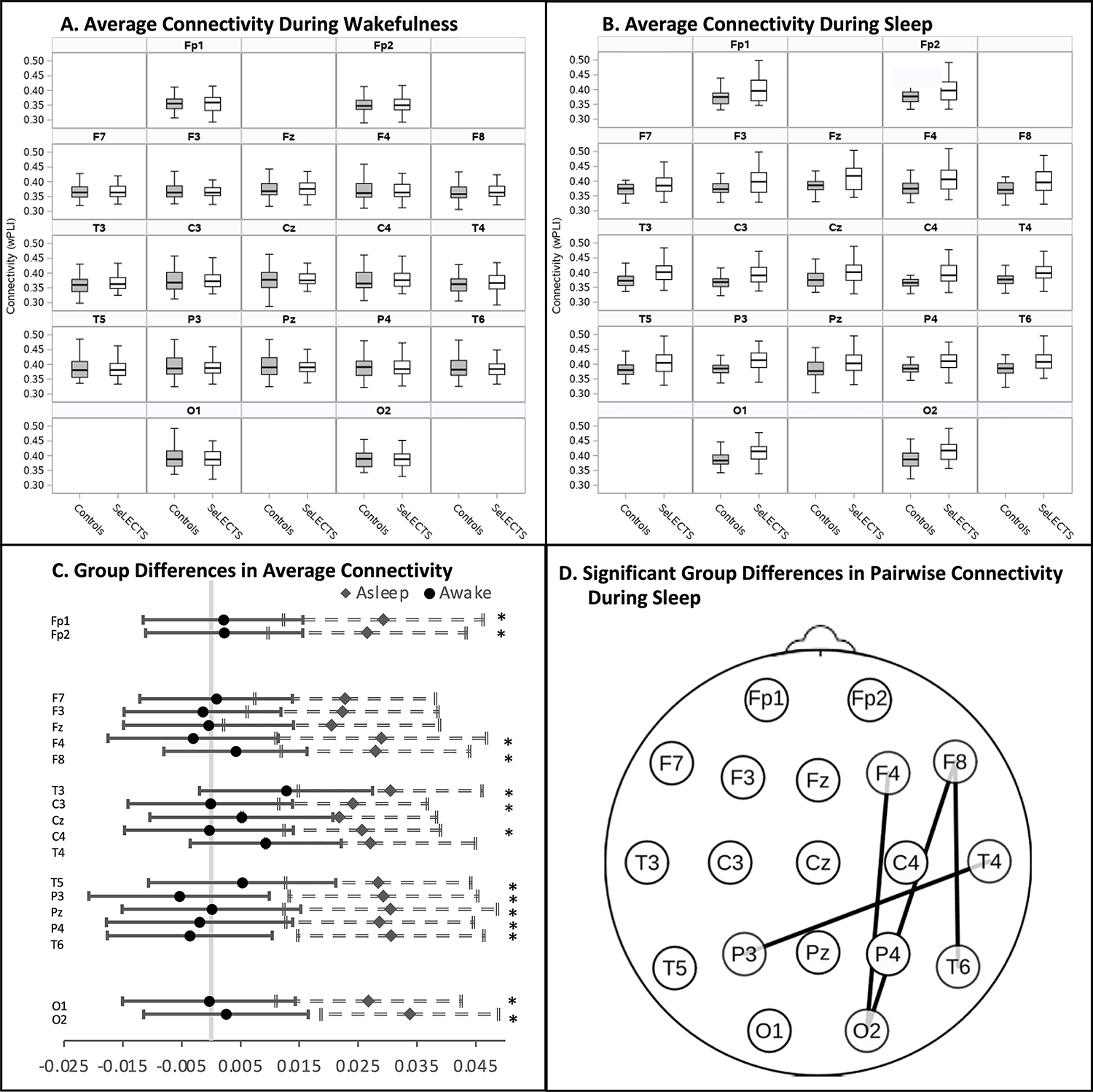
Average baseline connectivity in spike free epochs of children with and without SeLECTS in the (A) waking and (B) sleeping state, averaged by electrode. Box plots represent median, interquartile range and 95% distribution of data. C. Forest plot show group differences in average connectivity in the waking (circle) and sleep (diamond) states by electrode. Significant differences in the sleep state indicated by * (p<0.0026); no differences were noted in the awake state. D. Topographic plot showing electrode pairs with significantly increased pairwise connectivity in SeLECTS patients vs. controls (p<0.0003) in the sleeping state; there were no significant group differences in pairwise connectivity in wakefulness. Connectivity was quantified using the weighted Phase Lag Index (wPLI).
Table 1:
Difference in Average Connectivity Between Children with Self-Limited Epilepsy with Centrotemporal Spikes (SeLECTS) and Controls During Spike-Free Baseline Epochs
| AWAKE | ASLEEP | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean Difference (SE) | Z | p | * | Mean Difference (SE) | Z | p | * | |
|
| ||||||||
| Fp1 | 0.002 (0.007) | 0.29 | 0.77 | 0.03 (0.009) | 3.38 | 0.001 | * | |
| Fp2 | 0.002 (0.007) | 0.33 | 0.74 | 0.03 (0.009) | 3.08 | 0.002 | * | |
| F3 | −0.001 (0.007) | −0.22 | 0.83 | 0.02 (0.008) | 2.70 | 0.007 | ||
| F4 | −0.003 (0.007) | −0.41 | 0.68 | 0.03 (0.009) | 3.16 | 0.002 | * | |
| F7 | 0.0008 (0.007) | 0.13 | 0.90 | 0.02 (0.008) | 2.90 | 0.004 | ||
| F8 | 0.004 (0.006) | 0.67 | 0.51 | 0.03 (0.008) | 3.41 | 0.001 | * | |
| Fz | −0.0004 (0.007) | −0.06 | 0.95 | 0.02 (0.009) | 2.19 | 0.029 | ||
| C3 | −0.0002 (0.007) | −0.02 | 0.98 | 0.02 (0.006) | 3.74 | 0.0002 | * | |
| C4 | −0.0004 (0.007) | −0.05 | 0.96 | 0.03 (0.007) | 3.78 | 0.0002 | * | |
| Cz | 0.005 (0.008) | 0.65 | 0.52 | 0.02 (0.008) | 2.58 | 0.010 | ||
| P3 | −0.005 (0.008) | −0.70 | 0.49 | 0.03 (0.008) | 3.58 | 0.0003 | * | |
| P4 | −0.002 (0.008) | −0.24 | 0.81 | 0.03 (0.008) | 3.52 | 0.0004 | * | |
| Pz | 0.00005 (0.008) | 0.01 | 0.99 | 0.03 (0.009) | 3.29 | 0.001 | * | |
| T3 | 0.01 (0.008) | 1.70 | 0.09 | 0.03 (0.008) | 3.82 | 0.0001 | * | |
| T4 | 0.009 (0.007) | 1.42 | 0.16 | 0.03 (0.009) | 2.98 | 0.003 | * | |
| T5 | 0.005 (0.008) | 0.65 | 0.52 | 0.03 (0.008) | 3.54 | 0.0004 | * | |
| T6 | −0.004 (0.007) | −0.51 | 0.61 | 0.03 (0.008) | 3.78 | 0.0002 | * | |
| O1 | −0.0004 (0.007) | −0.05 | 0.96 | 0.03 (0.008) | 3.33 | 0.001 | * | |
| O2 | 0.003 (0.007) | 0.35 | 0.73 | 0.03 (0.008) | 4.38 | <.0001 | * | |
Standard Error (SE). Positive numbers indicate that SeLECTS patients have higher connectivity than controls.
Indicates that the difference in connectivity between controls and SeLECTS patients is significant after Bonferroni correction for multiple comparisons (p < 0.0026).
We compared connectivity between SeLECTS and controls during baseline spike-free epochs, adjusting for age, sex, and medication use.
3.4.1. Average Connectivity of Each Electrode:
There were no group differences in the average connectivity of each electrode during wakefulness. In contrast, there were widespread group differences during sleep, with the SeLECTS group showing significantly higher connectivity in all but five electrodes (F3, F7, Fz, Cz, T4).
Pairwise Connectivity between All Electrodes:
SeLECTS patients showed higher connectivity than controls during sleep between 4 electrode pairs: F8 to T6 (0.04, CI 0.02 to 0.07, p<0.0001); F8 to O2 (0.05, CI 0.02 to 0.07, p<0.0001); F4 to O2 (0.06; CI 0.03 to 0.09, p<0.0001); and T4 to P3 (0.05, CI 0.02 to 0.08, p=0.0002). Connectivity did not differ between groups during wakefulness.
3.4.2. Sensitivity Analysis of Unmedicated Patients (Supplemental Table 1):
Unmedicated SeLECTS patients (41/56, 73%) had elevated average connectivity compared to controls at 6 electrodes (Fp1, P4, T3, T5, T6, and O2). Pairwise connectivity was elevated in SeLECTS vs. controls in sleep between F8 to T6 (0.05, CI 0.02 to 0.07, p=0.0001); F8 to O2 (0.05, CI 0.03 to 0.08, p <0.0001); F4 to O2 (0.06, CI 0.02 to 0.08, p=0.0003).
3.5. Duration and Topography of Spike-Related Connectivity Changes (Figure 4, Table 2):
Figure 4: Change in Connectivity from the Spike-Free Baseline during the Pre-Spike, Spike, and Post- Spike Epochs in Children with Self-Limited Epilepsy with Centrotemporal Spikes (SeLECTS).
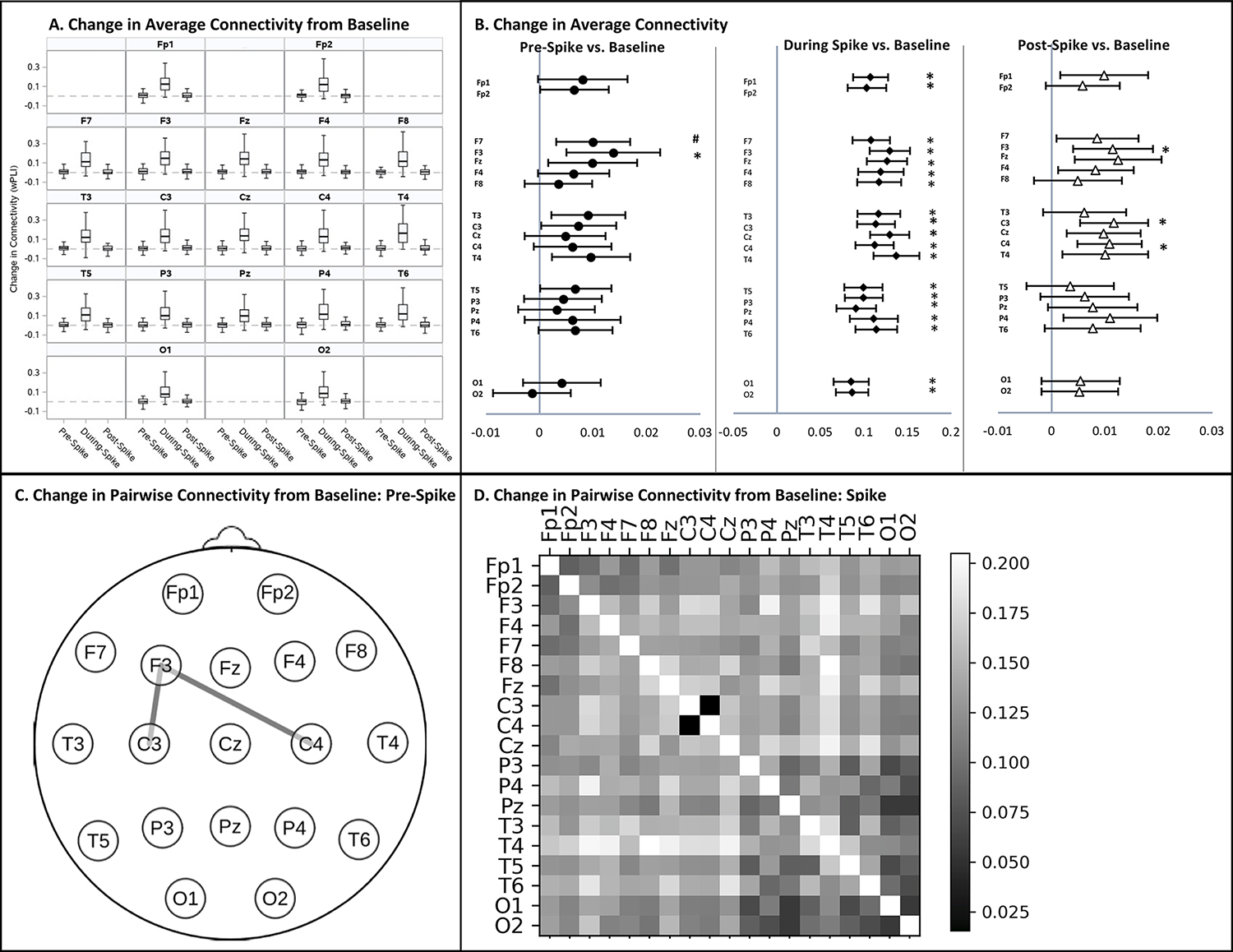
A. Median change in average connectivity between the pre-spike, spike, and post-spike epochs from the remote, spike-free baseline by electrode. Dotted line indicates no change from baseline. Box plots illustrate the median, interquartile range and 95% distribution of data. B. Forest plots show electrodes with significant increases in average connectivity from baseline during the pre- (circles), spike- (diamonds), and post-spike (triangles) epochs. The vertical gray line indicates no change from baseline. Significant increases in connectivity indicated by * (p<0.0026); connectivity increases trending toward significance indicated by # (p<0.005). No electrodes showed a significant decrease in connectivity from baseline. C. Topographic plot showing electrode pairs with a significant increase in pairwise connectivity from baseline (p<0.0003) in the pre-spike epoch. D. Heat map illustrating increases in connectivity from baseline between all electrode pairs, with brighter boxes indicating greater connectivity increases. The diagonal has been left white. No electrode pairs experienced a decrease in connectivity compared to baseline. The increase in connectivity was significant during the spike epoch for all pairs (p<0.0003) except C3-C4. Changes in pairwise connectivity in the post-spike epoch are not depicted as they were not significant. Connectivity was quantified using the weighted Phase Lag Index (wPLI).
Table 2:
Difference in Average Connectivity in the Time Periods Around Spikes compared to the Spike-Free Baseline
| Pre Spike (vs. Baseline) | During Spike (vs. Baseline) | Post Spike (vs. Baseline) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Difference (SE) | Z | p | * | Mean Difference (SE) | Z | p | * | Mean Difference (SE) | Z | p | * | |
|
| ||||||||||||
| Fp1 | 0.008 (0.004) | 1.89 | 0.06 | 0.13 (0.01) | 12.1 | <.0001 | * | 0.009 (0.004) | 2.06 | 0.04 | ||
| Fp2 | 0.007 (0.003) | 1.99 | 0.05 | 0.13 (0.01) | 11.0 | <.0001 | * | 0.006 (0.004) | 1.78 | 0.08 | ||
| F3 | 0.014 (0.005) | 3.08 | 0.002 | * | 0.15 (0.01) | 12.8 | <.0001 | * | 0.01 (0.004) | 3.31 | 0.001 | * |
| F4 | 0.006 (0.003) | 1.86 | 0.06 | 0.15 (0.01) | 10.7 | <.0001 | * | 0.007 (0.004) | 1.80 | 0.07 | ||
| F7 | 0.01 (0.004) | 2.85 | 0.004 | 0.13 (0.01) | 11.3 | <.0001 | * | 0.007 (0.004) | 1.82 | 0.07 | ||
| F8 | 0.004 (0.003) | 1.09 | 0.27 | 0.15 (0.01) | 10.7 | <.0001 | * | 0.006 (0.004) | 1.37 | 0.17 | ||
| Fz | 0.01 (0.004) | 2.34 | 0.02 | 0.15 (0.01) | 12.4 | <.0001 | * | 0.01 (0.004) | 2.92 | 0.004 | * | |
| C3 | 0.007 (0.004) | 2.06 | 0.04 | 0.14 (0.01) | 11.9 | <.0001 | * | 0.01 (0.003) | 3.35 | 0.0008 | * | |
| C4 | 0.006 (0.004) | 1.67 | 0.10 | 0.14 (0.01) | 11.7 | <.0001 | * | 0.01 (0.003) | 3.31 | 0.00 | ||
| Cz | 0.005 (0.004) | 1.24 | 0.22 | 0.15 (0.01) | 12.8 | <.0001 | * | 0.008 (0.004) | 2.27 | 0.02 | ||
| P3 | 0.004 (0.004) | 1.18 | 0.24 | 0.12 (0.01) | 11.1 | <.0001 | * | 0.007 (0.004) | 1.68 | 0.09 | ||
| P4 | 0.006 (0.005) | 1.35 | 0.18 | 0.14 (0.01) | 9.5 | <.0001 | * | 0.01 (0.004) | 2.48 | 0.01 | ||
| Pz | 0.003 (0.004) | 0.87 | 0.38 | 0.11 (0.01) | 9.4 | <.0001 | * | 0.008 (0.004) | 1.88 | 0.06 | ||
| T3 | 0.009 (0.004) | 2.58 | 0.01 | 0.14 (0.01) | 10.9 | <.0001 | * | 0.005 (0.004) | 1.22 | 0.22 | ||
| T4 | 0.01 (0.004) | 2.57 | 0.01 | 0.17 (0.01) | 11.9 | <.0001 | * | 0.01 (0.004) | 2.24 | 0.03 | ||
| T5 | 0.007 (0.003) | 1.98 | 0.05 | 0.12 (0.01) | 10.7 | <.0001 | * | 0.003 (0.004) | 0.77 | 0.44 | ||
| T6 | 0.007 (0.004) | 1.91 | 0.06 | 0.14 (0.01) | 11.0 | <.0001 | * | 0.007 (0.004) | 1.66 | 0.10 | ||
| O1 | 0.004 (0.004) | 1.12 | 0.26 | 0.10 (0.01) | 10.8 | <.0001 | * | 0.005 (0.003) | 1.57 | 0.12 | ||
| O2 | −0.001 (0.004) | −0.39 | 0.70 | 0.11 (0.01) | 9.8 | <.0001 | * | 0.006 (0.004) | 1.49 | 0.14 | ||
Standard Error (SE). Positive numbers indicate that connectivity is increased compared to baseline.
Indicates a significant difference in connectivity after Bonferroni correction for multiple comparisons (p < 0.0026).
We next assessed if connectivity in the pre-, during-, or post-spike epochs differed from connectivity in the spike-free epochs.
3.5.1. Change in Average Connectivity of Each Electrode:
Connectivity during the spike epoch was significantly elevated compared to the spike free baseline at all electrodes. In the pre-spike epoch, connectivity was elevated from baseline significantly at F3 (0.01, CI 0.005 to 0.02, p=0.002) and at a trend level at F7 (0.01, CI 0.003 to 0.02, p=0.004). In the post-spike epoch, connectivity was elevated at C3 (0.01, CI 0.005 to 0.02, p=0.0008), C4 (0.01, CI 0.004 to 0.02, p=0.0004), and F3 (0.01, CI 0.005 to 0.02, p=0.0009). As we were surprised that only the left frontal and not the bifrontal regions showed increased pre-spike connectivity, we tested if baseline connectivity differences between the hemispheres specific to SeLECTS could explain the left frontal finding using a mixed-factor ANOVA. Connectivity was higher at F8 than F7 (F(1,80)=4.59, p=0.04) and trended higher at F4 vs. F3 (F(1,80)=3.02, p=0.09), but this asymmetry did not differ between SeLECTS vs. controls (Group*Hemisphere interaction for F7/F8, F(1,80)=0.56, p=0.458; for F3/F4, F(1,80)=1.41, p=0.24). There were no other baseline connectivity differences between the hemispheres (Supplemental Table 2).
3.5.2. Change in Pairwise Connectivity between All Electrodes:
Connectivity was significantly elevated during the spike compared to spike-free baseline between all electrode pairs except C3 to C4. In the pre-spike epoch, connectivity was elevated between the left frontal (F3) and bicentral regions (F3 to C3 increased by 0.03, CI 0.02 to 0.05, p<0.0001; F3 to C4 by 0.03, CI 0.02 to 0.05, p<0.0001). In the post-spike epoch, there was no significant change in connectivity between any electrode pairs.
3.6. Factors Modifying Spike Connectivity:
We assessed if behavioral state (sleep/wake), medication use, or spike lateralization (left/right) influenced the degree that connectivity changed in the pre-spike, spike, or post-spike epochs.
3.6.1. Behavioral state & Antiseizure Medications (Supplemental Tables 3–4):
Behavioral state did not influence the increases in average connectivity or pairwise connectivity from baseline in pre-spike, spike, or post-spike epochs. Antiseizure medications did not subdue the increases in average connectivity in the pre-spike, spike or post-spike epochs. In the pairwise analysis, Fz to Cz connectivity was greater in medicated than unmedicated children (0.05, CI 0.02–0.08, Z=3.68, p=0.0002).
3.6.2. Spike Laterality (Figure 5, Table 3):
Figure 5: Impact of Spike Laterality on Connectivity During the Spike Epoch.
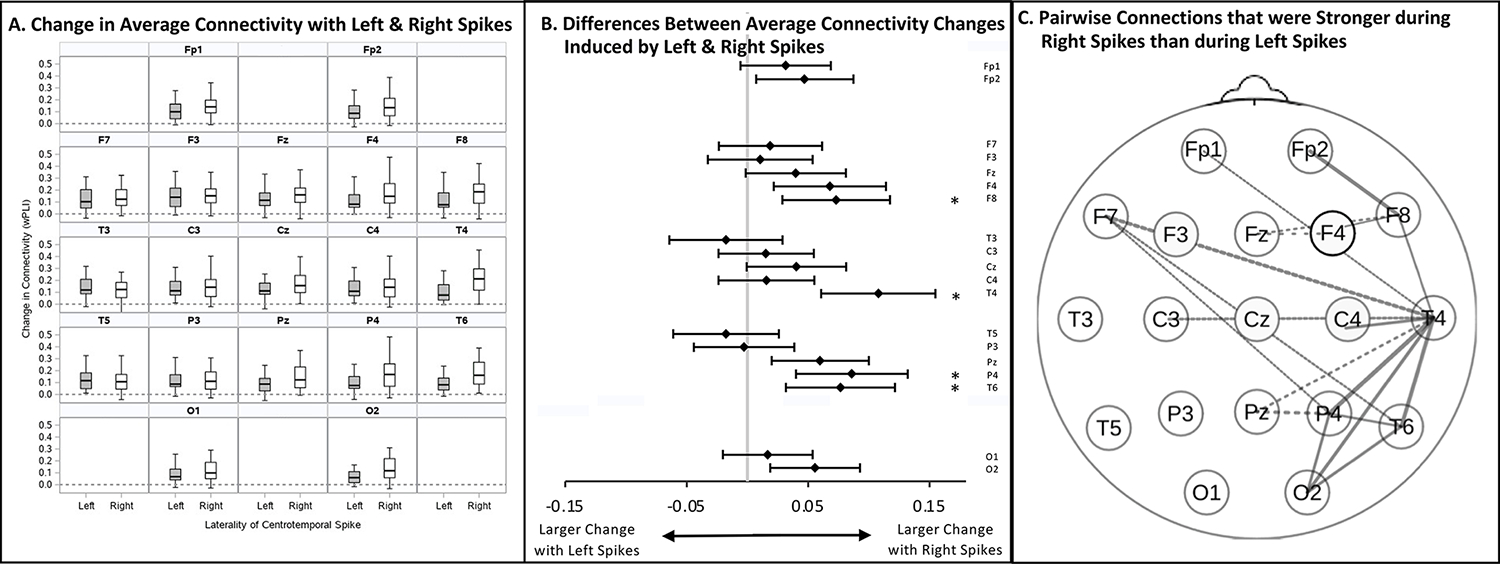
A. Median change in average connectivity from baseline of each electrode during left vs. right centrotemporal spikes. The dotted line represents no change from baseline. Box plots illustrate the median, interquartile range and 95% distribution of data. B. Forest plot show whether left or right centrotemporal spike have a greater impact on connectivity. The gray vertical line indicates that connectivity is equally affected by left and right spikes. Electrodes showing a significantly greater increase in average connectivity from baseline with right rather than left spikes are indicated by * (p<0.0026). No electrodes showed a significantly greater increase from baseline with left-sided spikes. C. Topographic plot showing electrode pairs with a significantly greater increase in pairwise connectivity (p<0.0003) after right than left centrotemporal spikes. Solid lines represent connections within the right hemisphere; dotted lines represent connections between the right hemisphere and vertex; beveled lines represent connections between the right and left hemispheres. No electrode or electrode pairs showed a significantly greater increase in connectivity during left than right centrotemporal spikes. Connectivity was quantified using the weighted Phase Lag Index (wPLI).
Table 3:
Influence of Spike Laterality on Change in Average Connectivity from Baseline during the Peri-Spike Epochs
| SPIKE LATERALITY (Right vs. Left) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Spike | During-Spike | Post-Spike | ||||||||||
| Mean Difference (SE) | Z | p | * | Mean Difference (SE) | Z | p | * | Mean Difference (SE) | Z | p | * | |
|
| ||||||||||||
| Fp1 | −0.001 (0.007) | −0.13 | 0.89 | 0.03 (0.02) | 1.66 | 0.10 | 0.007 (0.008) | 0.99 | 0.32 | |||
| Fp2 | −0.006 (0.006) | −0.96 | 0.34 | 0.05 (0.02) | 2.31 | 0.02 | 0.003 (0.007) | 0.47 | 0.64 | |||
| F3 | −0.01 (0.009) | −1.21 | 0.23 | 0.01 (0.02) | 0.48 | 0.63 | −0.004 (0.007) | −0.66 | 0.51 | |||
| F4 | −0.005 (0.007) | −0.75 | 0.46 | 0.07 (0.02) | 2.88 | 0.004 | # | −0.004 (0.006) | −0.67 | 0.50 | ||
| F7 | −0.009 (0.006) | −1.47 | 0.14 | 0.02 (0.02) | 0.88 | 0.38 | 0.006 (0.008) | 0.82 | 0.41 | |||
| F8 | −0.004 (0.006) | −0.71 | 0.48 | 0.07 (0.02) | 3.24 | 0.001 | * | 0.0001 (0.007) | 0.01 | 0.99 | ||
| Fz | −0.009 (0.008) | −1.12 | 0.26 | 0.04 (0.02) | 1.89 | 0.06 | −0.007 (0.008) | −0.83 | 0.41 | |||
| C3 | −0.006 (0.007) | −0.83 | 0.41 | 0.02 (0.02) | 0.77 | 0.44 | 0.003 (0.006) | 0.50 | 0.62 | |||
| C4 | −0.005 (0.007) | −0.72 | 0.47 | 0.02 (0.02) | 0.78 | 0.44 | 0.002 (0.006) | 0.42 | 0.68 | |||
| Cz | −0.009 (0.007) | −1.15 | 0.25 | 0.04 (0.02) | 1.93 | 0.05 | −0.003 (0.008) | −0.33 | 0.74 | |||
| P3 | −0.005 (0.007) | −0.67 | 0.51 | −0.003 (0.02) | −0.13 | 0.90 | −0.004 (0.007) | −0.62 | 0.53 | |||
| P4 | −0.01 (0.009) | −1.70 | 0.09 | 0.09 (0.02) | 3.67 | 0.0002 | * | −0.006 (0.008) | −0.75 | 0.45 | ||
| Pz | −0.02 (0.007) | −2.20 | 0.03 | 0.06 (0.02) | 2.94 | 0.003 | # | −0.008 (0.008) | −1.02 | 0.31 | ||
| T3 | −0.005 (0.007) | −0.74 | 0.46 | −0.02 (0.02) | −0.75 | 0.46 | −0.0004 (0.008) | −0.05 | 0.96 | |||
| T4 | −0.003 (0.007) | −0.49 | 0.62 | 0.11 (0.02) | 4.49 | <.0001 | * | −0.005 (0.007) | −0.76 | 0.44 | ||
| T5 | −0.01 (0.007) | −1.39 | 0.16 | −0.02 (0.02) | −0.79 | 0.43 | −0.01 (0.008) | −1.55 | 0.12 | |||
| T6 | −0.003 (0.006) | −0.44 | 0.66 | 0.08 (0.02) | 3.35 | 0.001 | * | 0.002 (0.008) | 0.26 | 0.80 | ||
| O1 | −0.008 (0.007) | −1.10 | 0.27 | 0.02 (0.02) | 0.89 | 0.37 | −0.004 (0.007) | −0.57 | 0.57 | |||
| O2 | −0.01 (0.007) | −1.82 | 0.07 | 0.06 (0.02) | 2.96 | 0.003 | # | −0.007 (0.007) | −0.95 | 0.34 | ||
Standard Error (SE). Positive numbers indicate that right centrotemporal spikes induce a greater increase in connectivity from baseline than left centrotemporal spikes; negative numbers indicate that left centrotemporal spikes induce a greater increase in connectivity from baseline than right spikes.
Indicates that right and left spikes have significantly different effects on connectivity after Bonferroni correction for multiple comparisons (p < 0.0026).
indicates that the differential impact of right vs. left spikes on connectivity trends toward significance after Bonferroni correction (p <0.005).
Spike laterality modified the topographical distribution of connectivity changes. As might be expected, connectivity in the right hemisphere increased more with right than left spikes. Interestingly, left and right spikes increased left hemisphere connectivity to a similar extent.
3.6.2.1. Average Connectivity of Each Electrode:
During the spike epoch, connectivity in the right hemisphere increased more with right than left centrotemporal spikes, significantly so at F8, P4, T4 and T6 and trending at F4, O2, and Pz. In contrast, no region showed larger increases with left spikes than right ones. Connectivity in the pre- and post-spike epochs was not modified by spike laterality.
3.6.2.2. Pairwise Connectivity between All Electrodes:
Connectivity between 19 electrode pairs increased more with right than left centrotemporal spikes. All 19 pairs involved at least one right hemispheric electrode: 10 pairs were fully within the right hemisphere (Fp2-F8, F4-F8, F8-T4, C4-T4, T4-O2, T4-P4, T4-T6, T6-P4, T6-O2, P4-O2); 4 pairs were between the right hemispheric and vertex (F4-Fz, F8-Fz, T4-Pz, P4-Pz), and 5 pairs were between right and left hemispheres (Fp1-T4, F7-T4, F7-T6, F7-P4, C3-T4). No pairs showed a greater increase in connectivity with left vs. right spikes. Spike laterality did not affect the increase in pre- or post-spike epoch connectivity.
4. Discussion
Prior studies of children with SeLECTS (Adebimpe et al., 2015; Ghantasala and Holmes, 2019; Li et al., 2017; Ofer et al., 2018; Xiao et al., 2016) found that spikes induce an immediate and diffuse increase in brain connectivity, but whether spikes lead to chronic changes is not understood. This large retrospective study addressed this important question in two ways. First, we compared brain connectivity in children with SeLECTS during spike-free epochs to that of age and sex-matched children without epilepsy and found increased connectivity during sleep but not wakefulness. Second, we measured the topographical distribution and duration of connectivity changes associated with individual spikes. We confirmed that spikes are associated with large-scale increase in brain connectivity, similar to (Ghantasala and Holmes, 2019) and furthermore identified frontal and central connectivity changes that precede visible spikes and persist after the spike. Finally, we found differences between the right and left hemisphere connectivity during both spike- and spike-free epochs. We measured connectivity with wPLI, because it can estimate connectivity using short epochs proximate to spikes and is robust against volume conduction (Vinck et al., 2011).
4.1. Baseline Spike-Free Connectivity
Prior studies in SeLECTS have found functional connectivity changes, but location and direction of these changes has not been consistent across studies. Inconsistencies can be attributed to imaging modality (EEG, magnetoencephalogram, fMRI), analytic methods (i.e. connectivity measures) used, or patients’ behavioral state. While most studies examine only wakefulness, our study also examines sleep, when EEG abnormalities and seizures are most prominent. We find robust and diffuse increases in connectivity in SeLECTS compared to controls limited to sleep. Given that we analyzed epochs remote from spikes, we think this represents a chronic state change rather than an acute effect of spikes. A potential explanation is that hypersynchronization during sleep is fundamental to the disorder, with a pathologic sleep state permitting increased spikes and seizures in SeLECTS. An alternative hypothesis is that frequent spiking drives increased connectivity. Several existing lines of evidence suggest that pathologic sleep is a feature of SeLECTS. First, a prior EEG study showed more profound connectivity differences in sleep than wakefulness (Varotto et al., 2018). This study found reduced connectivity during sleep as measured by partial directed coherence, a directed connectivity measure that assesses if prediction of future signal in one region is enhanced by information about past signal in a different region. In contrast, wPLI captures only phase synchronization between regions. If brain regions oscillate in a highly synchronous way, leading to an elevated wPLI, each region may contribute less “unique” information to a prediction model and decrease directed coherence. Taken together, these studies suggest that children with SeLECTS have diminished variability in brain oscillations during sleep. Kramer et al. (2021) also concluded that SeLECTS may be a disorder of abnormal sleep after finding that spindle deficits correlate more strongly with cognitive outcomes than spikes and improve as seizures resolve. Electrical status epilepticus of sleep, a related but more severe condition, is also associated with pathologically elevated synaptic strength in sleep (Bölsterli Heinzle et al., 2014).
Pairwise analysis found that connectivity was significantly elevated compared to controls only between four electrode pairs. The remaining electrode pairs also showed higher connectivity than controls, but did not meet the stringent criteria for significance. Notably, three significant pairs included a right frontal electrode, suggesting that SeLECTS may have a particularly large impact on the right frontal region even in the absence of spikes. Consistent with this, fMRI identified altered right inferior frontal gyrus connectivity in SeLECTS (Besseling et al., 2013c). The elevated connectivity may relate to previously described structural changes. A longitudinal imaging study (Garcia-Ramos et al., 2019) found more synchronized changes in cortical volumes in SeLECTS than controls throughout the brain. The increased functional connectivity we see even at time of diagnosis could drive these synchronous structural changes or alternatively be due to them, a hypothesis that could be tested with paired longitudinal EEG and MRI measurements.
Children with SeLECTS had similar connectivity to controls during wakefulness, suggesting that excessive synchrony is a state-specific rather than constant feature of SeLECTS. A single EEG study (Varotto et al., 2018) also found minimal connectivity differences during wakefulness compared to sleep. Two other studies identified altered connectivity during wakefulness. One found increased beta-band connectivity between the bifrontal and fronto-temporal regions (Clemens et al., 2013) whereas the second, performed on a small homogenous group of children with right centrotemporal spikes (Adebimpe et al., 2016), noted globally increased alpha and decreased beta phase synchronization. These studies may be more sensitive to connectivity changes during wakefulness as they analyzed connectivity in source space, whereas we were limited to less sensitive channel space analyses (Song et al., 2019) by the low-density nature of clinical recordings. Furthermore, because our connectivity measure, wPLI, is stringent against volume conduction, it can be less sensitive to true differences. The single prior study using wPLI in SeLECTS (Choi et al., 2019) is not directly comparable as some EEGs were recorded sedated. While our methodology may miss true differences between children with and without SeLECTS in wakefulness, it signifies that the increased connectivity seen in sleep is profound.
4.2. Duration and Topography of Spike-Related Connectivity Changes
Our findings support prior studies showing global increases in connectivity during centrotemporal spikes (Adebimpe et al., 2015; Ghantasala and Holmes, 2019) and demonstrate that this is unlikely to be an artifact of volume conduction (Vinck et al., 2011). The widespread impact of spikes is limited to the 500ms spike epoch, but more focal changes are evident before and after spikes. Preceding the spike, left frontal (F3) connectivity increases, most notably between F3 and the bicentral (C3 and C4) electrodes. Average connectivity of F3, C3, and C4 also remain elevated after the spike. This is interesting as SeLECTS patients have language dysfunction. Though spatially-imprecise, F3 roughly overlays Broca’s expressive language area (left inferior frontal gyrus) whereas the central electrodes (C3, C4) overlay motor cortex. The fMRI literature has identified differences in connectivity between the sensorimotor and left frontal regions. One study identified reduced connectivity between the left inferior frontal gyrus and motor regions in children with SeLECTS (Besseling et al., 2013b). A second study found increased regional homogeneity (a measure of blood oxygen level-dependent [BOLD] signal synchronization, potentially representing local neuronal activity) in the sensorimotor and left frontal language regions in new-onset SeLECTS, which remain elevated only in the left frontal regions in long-standing epilepsy (Zeng et al., 2015). Our findings generally align with previously reported differences between the left frontal and sensorimotor regions, even though scalp EEG lacks the spatial specificity of fMRI.
4.3. Laterality
Ghantasala and Holmes (2019) argued that SeLECTS behaves like a generalized epilepsy because they observed that widespread increase in connectivity were not modified by spike laterality. In contrast, we found several lateralized effects. First, connectivity in the right hemisphere increases more with right than left spikes; however, the converse is not true in the left hemisphere, where right and left spikes increase connectivity to a similar degree. Second, as previously described, connectivity increases in the left frontal region preceding spikes and remains elevated after spike resolution. Given the frontal trajectory of centrotemporal spikes’ tangential dipole, alterations in frontal connectivity are expected, but it is surprising that significant differences are only seen in the left frontal region. One explanation for this constellation of findings, supported by our exploratory findings, is that the right (typically non-dominant) hemisphere is more highly connected than the left at baseline, such that it experiences a smaller increase in connectivity with each spike but may be more prone to generating spikes. In support of this, some authors (Vannest et al., 2016) have noted a higher preponderance of children with right-hemispheric spikes, which we also find in this clinical population, raising the question of whether baseline connectivity contributes to spike lateralization.
4.4. Limitations
Several limitations warrant discussion. First, as we used clinical EEGs, we did not have control over the child’s activity during the recording and thus lack a controlled “resting state” EEG as obtained during research recordings. We chose quiet, artifact-free periods, but variability in patients’ mental states could limit our ability to detect group differences in wakefulness. Second, we limited analysis to the beta frequency band, and thus may be missing connectivity changes in other bands. We made this decision a priori, because the beta connectivity can be estimated using shorter epochs which is important for investigating temporally clustered spikes seen in SeLECTS. A third consideration is that our epoch numbers were low, limited by the number of available spikes, which can inflate connectivity estimates. To limit the impact of small trial numbers on our inferences, we matched the number of spike-free epochs between groups and the number of spike- and spike-free epochs within the SeLECTS group. Fourth, several of our analytic choices – including the use of stringent but undirected wPLI, the channel space analysis (Song et al., 2019), and the focus on bivariate relationships – may be less sensitive or may overlook non-linear connectivity patterns. However, even with our constrained methods, we still found robust differences in connectivity between groups and with spikes and peri-spike periods that shed light on this common epilepsy syndrome. Finally, it is possible that future studies will demonstrate that the connectivity differences we describe as well as spikes do not contribute to cognitive differences or seizure susceptibility in SeLECTS, but rather that all three are secondary to a common etiology. Notably, even in epilepsies with a well-described etiology (like specific genetic changes in tuberous sclerosis), clinical outcomes can be variable and other biomarkers including EEG-connectivity can be helpful for prognostication of cognitive (Dickinson et al., 2019) or seizure (Davis et al., 2019) outcomes.
4.5. Conclusion:
Whether and how interictal spikes alter neurocognition, behavior and mood is poorly understood and often not addressed by clinicians. Treatments specifically targeting spikes are lacking. We find that SeLECTS patients experience large increases in connectivity not only during spikes, but also during peri-spike periods as well as during spike-free segments of Stage II sleep. Longitudinal studies could help determine whether spike-related increases in connectivity cause the connectivity changes seen during spike-free sleep. Further studies are also needed to understand the mechanism and impact of spikes on epilepsy patients’ quality of life. Here we demonstrate that clinical EEG identifies meaningful connectivity differences in SeLECTS and could thus be an important tool for such longitudinal studies.
Supplementary Material
Acknowledgements:
FMB receives funding for her research efforts from the NINDS K23NS116110. BEP has received support from TESS Research Foundation, Eisai, NIH and FDA. CLM has received support from the Wu Tsai Neuroscience Institute and LVIS Inc. These funding sources had no involvement in study design; collection, analysis and interpretation of data; in writing of the report; or in the decision to submit the article for publication.
Footnotes
Declarations of Interest: None.
Ethics in Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The study was approved by the Stanford University IRB with waiver of patient consent given the retrospective nature of the research; there is no clinical trial registration. There are no reproduced materials.
Functional Magnetic Resonance Imaging (fMRI), Generalized estimating equations (GEE); International Classification of Disease (ICD), Self-limited Epilepsy with Centrotemporal Spikes (SeLECTS), weighted Phase Lag Index (wPLI)
Data Availability Statement:
The data is clinical data that has not been deidentified and hence is not publicly available, but the code used for the analysis is publicly available and cited in the manuscript.
References
- Adebimpe A, Aarabi A, Bourel-Ponchel E, Mahmoudzadeh M, Wallois F. EEG Resting State Functional Connectivity Analysis in Children with Benign Epilepsy with Centrotemporal Spikes. Front Neurosci 2016;10:143. 10.3389/fnins.2016.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebimpe A, Aarabi A, Bourel-Ponchel E, Mahmoudzadeh M, Wallois F. Functional Brain Dysfunction in Patients with Benign Childhood Epilepsy as Revealed by Graph Theory. PLoS One 2015;10:e0139228. 10.1371/journal.pone.0139228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apache Arrow Team, Apache Arrow. PyArrow python bindings to the Apache Arrow project, a development platform for in-memory analytics. 2022. (PyArrow version 5.0.0) [Online accessed Oct 2020, updated Dec 2021, Aug 2022] Apache Software Foundation. https://arrow.apache.org/docs/python/index.html [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010;51:676–85. 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Besseling RMH, Jansen JFA, Overvliet GM, Van Der Kruijs SJM, Ebus SCM, De Louw A, et al. Reduced structural connectivity between sensorimotor and language areas in rolandic epilepsy. PLoS One 2013a;8:1–7. 10.1371/journal.pone.0083568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besseling RMH, Jansen JFA, Overvliet GM, van der Kruijs SJM, Ebus SCM, de Louw AJA, et al. Delayed convergence between brain network structure and function in rolandic epilepsy. Front Hum Neurosci 2014;8:704. 10.3389/fnhum.2014.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besseling RMH, Jansen JFA, Overvliet GM, Van Der Kruijs SJM, Vles JSH, Ebus SCM, et al. Reduced functional integration of the sensorimotor and language network in rolandic epilepsy. NeuroImage Clin 2013b;2:239–46. 10.1016/j.nicl.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besseling RMH, Overvliet GM, Jansen JFA, van der Kruijs SJM, Vles JSH, Ebus SCM, et al. Aberrant functional connectivity between motor and language networks in rolandic epilepsy. Epilepsy Res 2013c;107:253–62. 10.1016/j.eplepsyres.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Bölsterli Heinzle BK, Fattinger S, Kurth S, Lebourgeois MK, Ringli M, Bast T, et al. Spike wave location and density disturb sleep slow waves in patients with CSWS (continuous spike waves during sleep). Epilepsia 2014;55:584–91. 10.1111/EPI.12576. [DOI] [PubMed] [Google Scholar]
- Choi HS, Chung YG, Choi SA, Ahn S, Kim H, Yoon S, et al. Electroencephalographic Resting-State Functional Connectivity of Benign Epilepsy with Centrotemporal Spikes. J Clin Neurol 2019;15:211–20. 10.3988/JCN.2019.15.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens B, Puskás S, Besenyei M, Spisák T, Emri M, Fekete I. Remission of benign epilepsy with rolandic spikes: an EEG-based connectivity study at the onset of the disease and at remission. Epilepsy Res 2013;106:128–35. 10.1016/j.eplepsyres.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Clemens B, Puskás S, Spisák T, Lajtos I, Opposits G, Besenyei M, et al. Increased resting-state EEG functional connectivity in benign childhood epilepsy with centro-temporal spikes. Seizure 2016;35:50–5. 10.1016/j.seizure.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Cohen MX. Analyzing neural time series data: Theory and practice. Cambridge, MA: MIT Press; 2014. [Google Scholar]
- Davis PE, Kapur K, Filip-Dhima R, Trowbridge SK, Little E, Wilson A, et al. Increased electroencephalography connectivity precedes epileptic spasm onset in infants with tuberous sclerosis complex. Epilepsia 2019;60:1721–32. 10.1111/EPI.16284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Varcin KJ, Sahin M, Nelson CA, Jeste SS. Early patterns of functional brain development associated with autism spectrum disorder in tuberous sclerosis complex. Autism Res 2019;12:1758–73. 10.1002/AUR.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant M. Fastparquet. A python implementation of the parquet format. 2022. https://github.com/dask/fastparquet/. [Google Scholar]
- Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:522–30. 10.1111/EPI.13670. [DOI] [PubMed] [Google Scholar]
- Garcia-Ramos C, Dabbs K, Lin JJ, Jones JE, Stafstrom CE, Hsu DA, et al. Network analysis of prospective brain development in youth with benign epilepsy with centrotemporal spikes and its relationship to cognition. Epilepsia 2019;60:1838–48. 10.1111/epi.16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ramos C, Jackson DC, Lin JJ, Dabbs K, Jones JE, Hsu DA, et al. Cognition and brain development in children with benign epilepsy with centrotemporal spikes. Epilepsia 2015;56:1615–22. 10.1111/EPI.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghantasala R, Holmes GL. Benign Rolandic epilepsy: widespread increases in connectivity in a focal epilepsy syndrome. Epileptic Disord 2019;21:567–78. 10.1684/epd.2019.1111. [DOI] [PubMed] [Google Scholar]
- Gramfort A, Luessi M, Larson E, Engemann DA, Strohmeier D, Brodbeck C, et al. MEG and EEG data analysis with MNE-Python. Front Neurosci 2013;7:267. 10.3389/fnins.2013.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haartsen R, Mason L, Braithwaite EK, Del Bianco T, Johnson MH, Jones EJH. Reliability of an automated gaze-controlled paradigm for capturing neural responses during visual and face processing in toddlerhood. Dev Psychobiol 2021;63:e22157. 10.1002/DEV.22157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavros PM, Clarke T, Strug LJ, Halperin JM, Dorta NJ, Pal DK. Attention impairment in rolandic epilepsy: Systematic review. Epilepsia 2008;49:1570–80. 10.1111/j.1528-1167.2008.01610.x. [DOI] [PubMed] [Google Scholar]
- Kramer MA, Stoyell SM, Chinappen D, Ostrowski LM, Spencer ER, Morgan AK, et al. Focal Sleep Spindle Deficits Reveal Focal Thalamocortical Dysfunction and Predict Cognitive Deficits in Sleep Activated Developmental Epilepsy. J Neurosci 2021;41:1816–29. 10.1523/JNEUROSCI.2009-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Messer C. EEG Connectivity Implementation 2021. https://github.com/cleemesser/eeg-connectivity-2022.
- Li R, Ji G-J, Yu Y, Yu Y, Ding M-P, Tang Y-L, et al. Epileptic Discharge Related Functional Connectivity Within and Between Networks in Benign Epilepsy with Centrotemporal Spikes. Int J Neural Syst 2017;27:1750018. 10.1142/S0129065717500186. [DOI] [PubMed] [Google Scholar]
- Mckinney W. Python for Data Analysis, Data Wrangling with Pandas, NumPy, and IPython. Second. Sebastopol, CA: O’Reilly Media, Inc; 2018. [Google Scholar]
- Monjauze CC, Broadbent H, Boyd SG, Neville BGR, Baldeweg T. Language deficits and altered hemispheric lateralization in young people in remission from BECTS. Epilepsia 2011;52:79–84. 10.1111/j.1528-1167.2011.03105.x. [DOI] [PubMed] [Google Scholar]
- Ofer I, Jacobs J, Jaiser N, Akin B, Hennig J, Schulze-Bonhage A, et al. Cognitive and behavioral comorbidities in Rolandic epilepsy and their relation with default mode network’s functional connectivity and organization. Epilepsy Behav 2018;78:179–86. 10.1016/j.yebeh.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Oser N, Hubacher M, Specht K, Datta AN, Weber P, Penner IK. Default mode network alterations during language task performance in children with benign epilepsy with centrotemporal spikes (BECTS). Epilepsy Behav 2014;33:12–7. 10.1016/j.yebeh.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Panayiotopoulos CP, Michael M, Sanders S, Valeta T, Koutroumanidis M. Benign childhood focal epilepsies: assessment of established and newly recognized syndromes. Brain 2008;131:2264–86. 10.1093/BRAIN/AWN162. [DOI] [PubMed] [Google Scholar]
- RAPIDS Development Team. RAPIDS: a Collection of Libaries for End-To-End GPU Data 2018. (version 0.16 Oct 2020 updated to 21.12 Dec. 2021) [Online docmentation accessed Aug 2022] https://rapids.ai
- Raschka S, Patterson J, Nolet C. Machine Learning in Python: Main Developments and Technology Trends in Data Science, Machine Learning, and Artificial Intelligence. Inf 2020; 11:193. 10.3390/INFO11040193. [DOI] [Google Scholar]
- Reback J, jbrockmendel, McKinney W, Bossche J Van den, Augspurger T, Roeschke M, et al. pandas-dev/pandas: Pandas 1.4.2 2022. 10.5281/ZENODO.6408044. [DOI] [Google Scholar]
- Smith AB, Bajomo O, Pal DK. A meta-analysis of literacy and language in children with rolandic epilepsy. Dev Med Child Neurol 2015;57:1019–26. 10.1111/dmcn.12856. [DOI] [PubMed] [Google Scholar]
- Smith AB, Kavros PM, Clarke T, Dorta NJ, Tremont G, Pal DK. A neurocognitive endophenotype associated with rolandic epilepsy. Epilepsia 2012;53:705–11. 10.1111/j.1528-1167.2011.03371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DY, Stoyell SM, Ross EE, Ostrowski LM, Thorn EL, Stufflebeam SM, et al. Beta oscillations in the sensorimotor cortex correlate with disease and remission in benign epilepsy with centrotemporal spikes. Brain Behav 2019;9:e01237. 10.1002/brb3.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specchio N, Wirrell EC, Scheffer IE, Nabbout R, Riney K, Samia P, et al. International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022;63:1398–442. 10.1111/EPI.17241. [DOI] [PubMed] [Google Scholar]
- Spencer ER, Chinappen D, Emerton BC, Morgan AK, Hämäläinen MS, Manoach DS, et al. Source EEG reveals that Rolandic epilepsy is a regional epileptic encephalopathy. NeuroImage Clin 2022;33. 10.1016/J.NICL.2022.102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen J, Weir CJ, Chin RFM. Temporal trends in incidence of Rolandic epilepsy, prevalence of comorbidities and prescribing trends: birth cohort study. Arch Dis Child 2020;105:569–74. 10.1136/ARCHDISCHILD-2019-318212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira J, Santos ME. Language skills in children with benign childhood epilepsy with centrotemporal spikes: A systematic review. Epilepsy Behav 2018;84:15–21. 10.1016/j.yebeh.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Thorn EL, Ostrowski LM, Chinappen DM, Jing J, Westover MB, Stufflebeam SM, et al. Persistent abnormalities in Rolandic thalamocortical white matter circuits in childhood epilepsy with centrotemporal spikes. Epilepsia 2020. 10.1111/epi.16681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannest J, Tenney JR, Altaye M, Byars AW, Spencer C, Maloney TC, et al. Impact of frequency and lateralization of interictal discharges on neuropsychological and fine motor status in children with benign epilepsy with centrotemporal spikes. Epilepsia 2016;57:e161–7. 10.1111/epi.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannest J, Tenney JR, Gelineau-Morel R, Maloney T, Glauser TA. Cognitive and behavioral outcomes in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav 2015;45:85–91. 10.1016/j.yebeh.2015.01.041. [DOI] [PubMed] [Google Scholar]
- Varotto G, Franceschetti S, Caputo D, Visani E, Canafoglia L, Freri E, et al. Network characteristics in benign epilepsy with centro-temporal spikes patients indicating defective connectivity during spindle sleep: A partial directed coherence study of EEG signals. Clin Neurophysiol 2018;129:2372–9. 10.1016/j.clinph.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Vinck M, Oostenveld R, van Wingerden M, Battaglia F, Pennartz CMA. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 2011;55:1548–65. 10.1016/j.neuroimage.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Wickens S, Bowden SC, D’Souza W. Cognitive functioning in children with self-limited epilepsy with centrotemporal spikes: A systematic review and meta-analysis. Epilepsia 2017;58:1673–85. 10.1111/EPI.13865. [DOI] [PubMed] [Google Scholar]
- Wirrell EC. Benign epilepsy of childhood with centrotemporal spikes. Epilepsia 1998;39 Suppl 4:S32–41. 10.1111/j.1528-1157.1998.tb05123.x. [DOI] [PubMed] [Google Scholar]
- Xiao F, An D, Lei D, Li L, Chen S, Wu X, et al. Real-time effects of centrotemporal spikes on cognition in rolandic epilepsy. Neurology 2016;86:544–51. 10.1212/WNL.0000000000002358. [DOI] [PubMed] [Google Scholar]
- Zeger S, Liang K. Longitudinal Data Analysis for Discrete and Continuous Outcomes. Biometrics 1986;42:121–30. [PubMed] [Google Scholar]
- Zeger SL, Liang K-Y, Albert PS. Models for Longitudinal Data: A Generalized Estimating Equation Approach. Biometrics 1988;44:1049. 10.2307/2531734. [DOI] [PubMed] [Google Scholar]
- Zeng H, Ramos CG, Nair VA, Hu Y, Liao J, La C, et al. Regional homogeneity (ReHo) changes in new onset versus chronic benign epilepsy of childhood with centrotemporal spikes (BECTS): A resting state fMRI study. Epilepsy Res 2015;116:79–85. 10.1016/J.EPLEPSYRES.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is clinical data that has not been deidentified and hence is not publicly available, but the code used for the analysis is publicly available and cited in the manuscript.


