Abstract
Purpose.
Most patients with intrahepatic cholangiocarcinoma (IHCC) develop recurrence after resection. Adjuvant capecitabine remains the standard of care for resected IHCC. A combination of gemcitabine, cisplatin, and nab-paclitaxel (GAP) was associated with a 45% response rate and 20% conversion rate among patients with unresectable biliary tract cancers. The aim of this study was to evaluate the feasibility of delivering GAP in the neoadjuvant setting for resectable, high-risk IHCC.
Methods.
A multi-institutional, single-arm, phase II trial was conducted for patients with resectable, high-risk IHCC, defined as tumor size > 5 cm, multiple tumors, presence of radiographic major vascular invasion, or lymph node involvement. Patients received preoperative GAP (gemcitabine 800 mg/m2, cisplatin 25 mg/m2, and nab-paclitaxel 100 mg/m2 on days 1 and 8 of a 21-day cycle) for a total of 4 cycles prior to an attempt at curative-intent surgical resection. The primary endpoint was completion of both preoperative chemotherapy and surgical resection. Secondary endpoints were adverse events, radiologic response, recurrence-free survival (RFS), and overall survival (OS).
Results.
Thirty evaluable patients were enrolled. Median age was 60.5 years. Median follow-up for all patients was 17 months. Ten patients (33%) experienced grade ≥ 3 treatment-related adverse events, the most common being neutropenia and diarrhea; 50% required ≥ 1 dose reduction. The disease control rate was 90% (progressive disease: 10%, partial response: 23%, stable disease: 67%). There was zero treatment-related mortality. Twenty-two patients (73%, 90% CI 57–86; p = 0.008) completed all chemotherapy and surgery. Two patients (9%) who successfully underwent resection had minor postoperative complications. Median length of hospital stay was 4 days. Median RFS was 7.1 months. Median OS for the entire cohort was 24 months and was not reached in patients who underwent surgical resection.
Conclusion.
Neoadjuvant treatment with gemcitabine, cisplatin, and nab-paclitaxel is feasible and safe prior to resection of intrahepatic cholangiocarcinoma and does not adversely impact perioperative outcomes.
Intrahepatic cholangiocarcinoma (IHCC) is the second most common primary liver cancer, with a rising annual incidence. Surgical resection is an option in only approximately 40% of patients and is curative in only 30–35% of those who undergo resection due to high disease recurrence rates.1–3 The landmark BILCAP trial established a standard option for adjuvant chemotherapy with monotherapy capecitabine, despite no statistically significant difference in overall survival compared with observation alone in an unadjusted intention-to-treat analysis.4,5 In the metastatic setting, the standard of care regimen remains a combination of gemcitabine and cisplatin, a treatment paradigm unchanged since 2010 following the ABC-02 trial, which demonstrated an improved median progression-free and overall survival of 8.0 months and 11.7 months, respectively, compared with gemcitabine alone.6 Recent data from the TOPAZ-1 trial indicated an overall survival benefit with the addition of durvalumab to gemcitabine and cisplatin.7 Given the overall poor prognosis and limited efficacy of available therapies, the need for improvement in managing resectable and metastatic disease persists.
The addition of nab-paclitaxel to gemcitabine and cisplatin (GAP) has demonstrated a high disease control rate (85%) with improved survival outcomes (median progression-free survival, 11.8 months; median overall survival, 19.2 months) in patients with advanced biliary tract cancers.8 For patients with resectable disease, particularly those with high-risk characteristics, current practice patterns employing a surgery-first approach followed by adjuvant chemotherapy confer limited survival benefits.9 Applying a chemotherapy augmentation approach to the neoadjuvant setting offers a potential opportunity to improve the outcomes of patients with resectable disease. Prior to studying this on a large scale, however, the feasibility and safety of such an approach must first be established. To date, there have been no prospective studies evaluating neoadjuvant chemotherapy in this setting.
Thus, our aim was to assess the feasibility of a neoadjuvant treatment strategy utilizing gemcitabine, cisplatin, and nab-paclitaxel combination chemotherapy prior to curative-intent surgery for resectable, but oncologically high-risk, IHCC.
METHODS
Patient Eligibility
Eligibility criteria included age greater than 18 years; histologically confirmed IHCC; high-quality cross-sectional imaging by computerized tomography (CT) or magnetic resonance imaging (MRI), performed within 6 weeks of trial enrollment, demonstrating high-risk, resectable IHCC confined to the liver, bile duct, and/or regional lymph nodes [high-risk tumors were defined as T-stage Ib or greater; solitary lesion greater than 5 cm; multifocal tumors or satellite lesions confined to the same lobe of the liver as the dominant lesion and technically resectable; presence of major vascular invasion but technically resectable; suspicious or involved regional lymph nodes (N1); and no evidence of extrahepatic disease (M0)]; Eastern Cooperative Oncology Group (ECOG) performance status score less than or equal to 1; and adequate hematologic (defined as absolute neutrophil count ≥ 1500 cells/μl; platelet count ≥ 100,000 cells/μl; and hemoglobin ≥ 9 g/dl), hepatic (defined as serum total bilirubin ≤ 1.5 × ULN; aspartate aminotransferase and alanine aminotransferase ≤ 2.5 × ULN; and albumin ≥ 3 g/dl), and renal (defined as creatinine ≤ 1.5 × ULN) function.
Exclusion criteria included grade 2 or higher peripheral neuropathy defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0;10 any concurrent severe or uncontrolled medical condition that could compromise participation in the study; any known central nervous system disease, except treated brain metastases; and previous (within 5 years) or concurrent diagnosis of other cancer, except non-melanoma skin cancer and in situ carcinomas.
The study protocol was approved by institutional review boards at Winship Cancer Institute, Emory University, Atlanta, Georgia; University of Texas MD Anderson Cancer Center, Houston, Texas; Mayo Clinic, Rochester, Minnesota; and Virginia Mason Medical Center, Seattle, Washington. All patients provided written informed consent prior to study enrollment.
Study Design and Treatments
This was an open label, single-arm, phase II study conducted at Winship Cancer Institute of Emory University, University of Texas MD Anderson Cancer Center, Mayo Clinic Rochester, and Virginia Mason Medical Center. Patients were enrolled between September 2018 and September 2021 and received sequential nab-paclitaxel, cisplatin, and gemcitabine. All three drugs were administered on days 1 and 8 of each 21-day cycle for a total of 4 cycles prior to attempt at curative-intent surgical resection. Patients received pre-cisplatin hydration 30 min prior to receiving the trial drug, which included sodium chloride injection (0.9%, 1000 ml) with mannitol (18.5 g) and magnesium sulfate (2 g, intravenous infusion over 2 h); palonsetron (0.25 mg), fosaprepitant (150 mg), and dexamethasone (12 mg). After treatment, patients received post-cisplatin hydration with sodium chloride injection (0.9%, 1000 ml) over 3 h.
Patients received starting doses of nab-paclitaxel 100 mg/m2, cisplatin 25 mg/m2, and gemcitabine 800 mg/m2, administered over 30, 60, and 30 min, respectively.8 Drug-related toxicity was monitored throughout the course of the study and graded according to the NCI CTCAE, version 4.0. The dose reduction schedule for adverse event management is listed in Supplementary Table 1.
Treatment was discontinued off protocol for any patient with documented disease progression on radiologic scan during the study period. Resection was performed per standard of care and included a portal lymphadenectomy for all cases.
Endpoints and Evaluation
The primary trial endpoint was feasibility of completing all therapy, including neoadjuvant chemotherapy (gemcitabine, cisplatin, and nab-paclitaxel) and surgical resection. Secondary endpoints included chemotherapy tolerability, radiologic response of target lesions based on Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1) [complete response (CR), defined as disappearance of all target lesions for a period of at least 1 month; partial response (PR), defined as at least 30% decrease in the sum of the longest diameter of measured lesion; stable disease (SD), defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD; or progressive disease (PD), defined as a 20% or greater increase in the sum of the longest diameter of measured lesions11], microscopically margin-negative (R0) resection rate, recurrence-free survival (RFS, defined as the time between the date of surgery and the date of disease recurrence, death, or date of last follow-up), and overall survival (OS, defined as the time from date of neoadjuvant treatment initiation to date of death from any cause or date of last-follow-up).
A directed physical exam with neuropathy assessment, vital signs, performance status, and hematologic and serum chemistry assessment was performed on days 1 and 8 of each cycle and within 4 weeks post-resection. Reassessment of tumor extent and response was performed after completion of 4 cycles using the same imaging modality used to establish baseline tumor measurements. Radiologic response was defined according to RECIST.12 Patients were monitored for adverse events from treatment initiation to 4 weeks post-resection. Toxicity was scored using CTCAE, version 4.0, for toxicity and adverse event reporting. Following surgical resection, patients were followed for disease recurrence and survival.
Statistical Analyses
Thirty evaluable patients provided 73% power to reject a null therapy completion rate of 50%, with a target completion rate of 70% using a one-sided exact test with a type I error of 0.05 using an exact binomial test. The power estimation was based on the exact binomial test. Continuous measurements were summarized using median, standard deviation, and interquartile range, and categorical variables were summarized using frequencies and percentages. Exact confidence intervals for proportions were estimated using the Clopper–Pearson method. The observed response proportion was compared with the null value of 50% using a one-sided exact binomial test. The Kaplan-Meier method was used to estimate RFS and OS. Statistical analysis was conducted using SAS 9.4 (SAS Institute Inc., Cary, NC), and statistical significance was assessed at the 0.05 level unless otherwise noted. The method of Thall, Simon, and Estey was used for futility and toxicity monitoring.13 Multc Lean Desktop (version 2.1) design software developed by the Department of Biostatistics at MD Anderson Cancer Center (MDACC) was used to generate stopping boundaries and operating characteristics data for futility and toxicity monitoring. Figures were generated using GraphPad Prism, version 9.4.0.
RESULTS
Patient Population
The trial was sequentially activated at each of the four participating sites from September 2018 to February 2021 and the final patient was enrolled in September 2021. Thirty-seven patients were initially screened, of which 30 were enrolled. Median age was 60.5 years (range 39–78) and 40% (n = 12) of patients were female. Table 1 summarizes demographic characteristics of all patients enrolled in the study. Postoperative adjuvant therapy regimens are listed in Table 3. The trial CONSORT diagram is shown in Fig. 1.
TABLE 1.
Demographic and clinicopathologic characteristics of all patients
| Variable | All patients (n = 30) |
|---|---|
|
| |
| Age (years), median (SD) | 60.5 (9.6) |
| Gender, n (%) | |
| Female | 12 (40) |
| Male | 18 (60) |
| Race, n (%) | |
| White | 20 (67) |
| Black/African American | 3 (10) |
| Asian | 3 (10) |
| Unknown | 4 (13) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 3 (10) |
| Non-Hispanic | 27 (90) |
| Baseline CA19-9 (U/ml), median (IQR) | 14.8 (3.8–258.8) |
| Follow-up (months), median (IQR) | 17 (9–30) |
| NGS completed, n (%) | 19 (63) |
| Positive actionable mutation, n (%)a | 6 (32) |
| Best response to treatment, n (%)b | |
| Partial response | 7 (23) |
| Stable disease | 20 (67) |
| Progression disease | 3 (10) |
NGS next generation sequencing, SD standard deviation, IQR interquartile range, CA19-9 carbohydrate antigen 19–9
Positive mutation for most common actionable targets, FGFR-2 fusion, IDH-1
According to RECIST, version 1.1
TABLE 3.
Perioperative and histopathologic data
| Variable | All patients (n = 22) |
|---|---|
|
| |
| Hospital length of stay (days), median (IQR) | 4 (4–5) |
| Postoperative complication, n (%) | 2 (9) |
| Number of tumors, median (IQR) | 3.5 (2.0–7.0) |
| Size of largest tumor (cm), median (IQR) | 5.5 (3.0–7.4) |
| Number of lymph nodes removed, median (IQR) | 4.0 (3.0–5.8) |
| Lymph node status, n (%) | |
| Negative | 14 (64) |
| Positive | 8 (36) |
| Tumor differentiation, n (%) | |
| Moderately differentiated | 12 (54) |
| Poorly differentiated | 7 (32) |
| Other | 3 (14) |
| Margin status, n (%) | |
| R0 | 16 (73) |
| R1/R2 | 6 (27) |
| Adjuvant chemotherapy, n (%) | 17 (77) |
| Capecitabine | 12 (55) |
| Gemcitabine/cisplatin | 1 (5) |
| Gemcitabine/cisplatin plus nab-paclitaxel | 2 (9) |
| Gemcitabine/capecitabine | 1 (5) |
| FOLFOX | 1 (5) |
| Adjuvant targeted therapy, n (%) | 1 (5) |
| Ivosidenib | 1 (5) |
IQR interquartile range
FIG. 1.
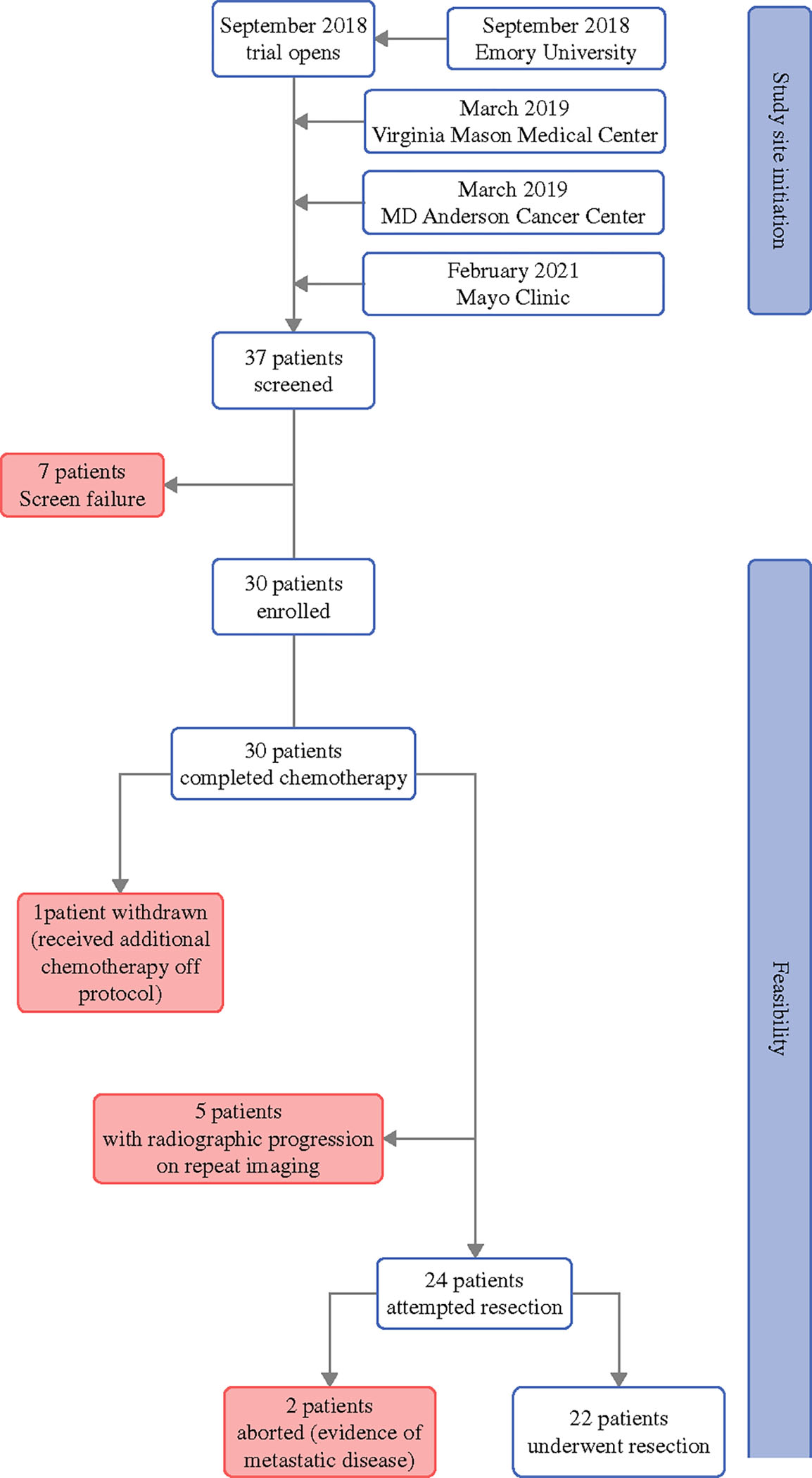
Trial CONSORT diagram
Feasibility
All 30 patients enrolled in the trial completed preoperative chemotherapy (Fig. 1). One patient withdrew from the study in order to receive additional preoperative chemotherapy, but subsequently was able to undergo surgery. Twenty-two patients successfully underwent surgical resection. Thus, of the 30 patients enrolled in the trial, 22 patients (73.3%, 90% CI 57.0–86.0; p = 0.008) completed all preoperative chemotherapy and underwent surgical resection on protocol (Fig. 1).
Tolerability/Toxicity
Ten patients (33%) experienced grade 3 or higher treatment-related adverse events. Neutropenia was the most common, occurring in 17% of patients, followed by diarrhea (7%). All treatment-related adverse events are listed in Table 2.
TABLE 2.
Feasibility and tolerability of study protocol
| Variable | All patients (n = 30) |
|---|---|
|
| |
| Completed all therapy, n (%) | 22 (73) |
| Dose reduction, n (%) | |
| Single dose reduction, any drug | 15 (50) |
| At least 1 gemcitabine dose reduction | 14 (47) |
| At least 1 cisplatin dose reduction | 6 (20) |
| At least 1 nab-paclitaxel dose reduction | 14 (47) |
| 2+ dose reductions, any drug | 5 (17) |
| 2+ gemcitabine dose reductions | 2 (7) |
| 2+ cisplatin dose reductions | 0 (0) |
| 2+ nab-paclitaxel dose reductions | 3 (10) |
| Any grade ≥ 3 AEs, n (%) | 10 (33) |
| Grade ≥ 3 hematologic AEs, n (%) | |
| Anemia | 1 (3) |
| Febrile neutropenia | 1 (3) |
| Leukocytosis | 1 (3) |
| Leukopenia | 1 (3) |
| Neutropenia | 5 (17) |
| Elevated ALT | 1 (3) |
| Grade ≥ 3 gastrointestinal AEs, n (%) | |
| Diarrhea | 2 (7) |
| Treatment-related mortality, n (%) | 0 (0) |
AE adverse event
Fifty percent (n = 15) of patients required a single dose reduction for any drug (Table 2). Seventeen percent (n = 5) of patients required two or greater dose reductions. A greater proportion of patients required single or multiple dose reductions for gemcitabine (47% and 7%, respectively) and nab-paclitaxel (47% and 10%, respectively) compared with cisplatin (20% and 0%, respectively). There was zero treatment-related mortality.
Radiologic Response
Treatment response data were available for all patients. Figure 2 shows percent change in tumor size from baseline to best response for all patients who completed neoadjuvant chemotherapy. According to RECIST 1.1, the partial response rate was 23% and the disease control rate was 90% (PD: 10%, PR: 23%, SD: 67%) (Table 1).
FIG. 2.
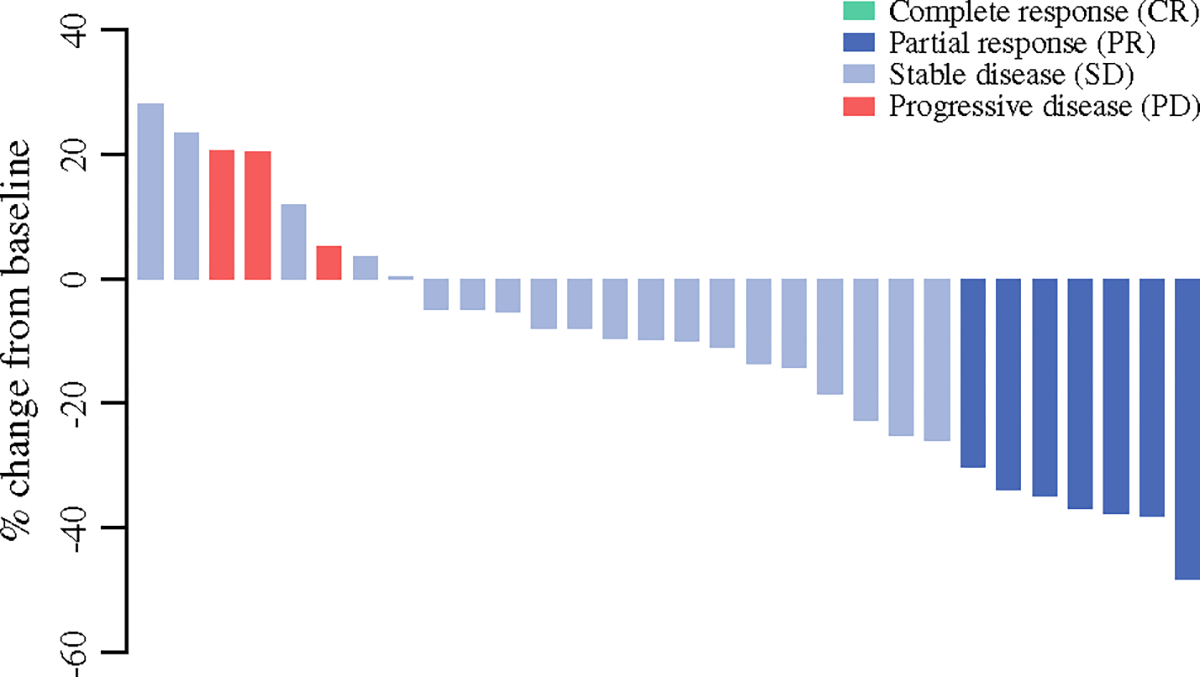
Change in tumor size from baseline to best response among all patients who completed neoadjuvant chemotherapy, according to RECIST criteria (version 1.1) (n = 30)
Operative and Pathologic Data
Of the 22 patients who successfully underwent surgical resection, per Clavien-Dindo classification, there were no major postoperative complications and 2 patients (9%) had minor complications. Median length of hospital stay was 4 days (IQR 4–5) (Table 3). The margin negative (R0) resection rate was 73%.
The median number of tumors resected was 3.5 (IQR 2.0–7.0) and the median size of the largest tumor was 5.5 cm (IQR 3.0–7.4). Thirty-two percent of patients had poorly differentiated and 54% had moderately differentiated tumors. The median number of lymph nodes removed was 4.0 (IQR 3.0–5.8) and 36% (n = 8) of patients had lymph node positive disease (Table 3). There were no patients with a complete pathologic response.
When considering the two most common actionable mutations (FGFR2 fusion and IDH1 mutation), 63% of patients (n = 19) underwent next generation sequencing, of which 6 (32%) were found to have a positive result (Table 1).
Efficacy
The median follow-up for all patients enrolled in the trial (n = 30) was 17 months. Median OS for the entire study population was 24 months (Fig. 3A). Median follow-up for patients who completed all therapy (n = 22) was 16 months. Nine patients (43%) developed disease recurrence after surgery. Median RFS was 7.1 months (Fig. 3B). Median OS was not reached for patients who underwent surgical resection (Fig. 3C).
FIG. 3.
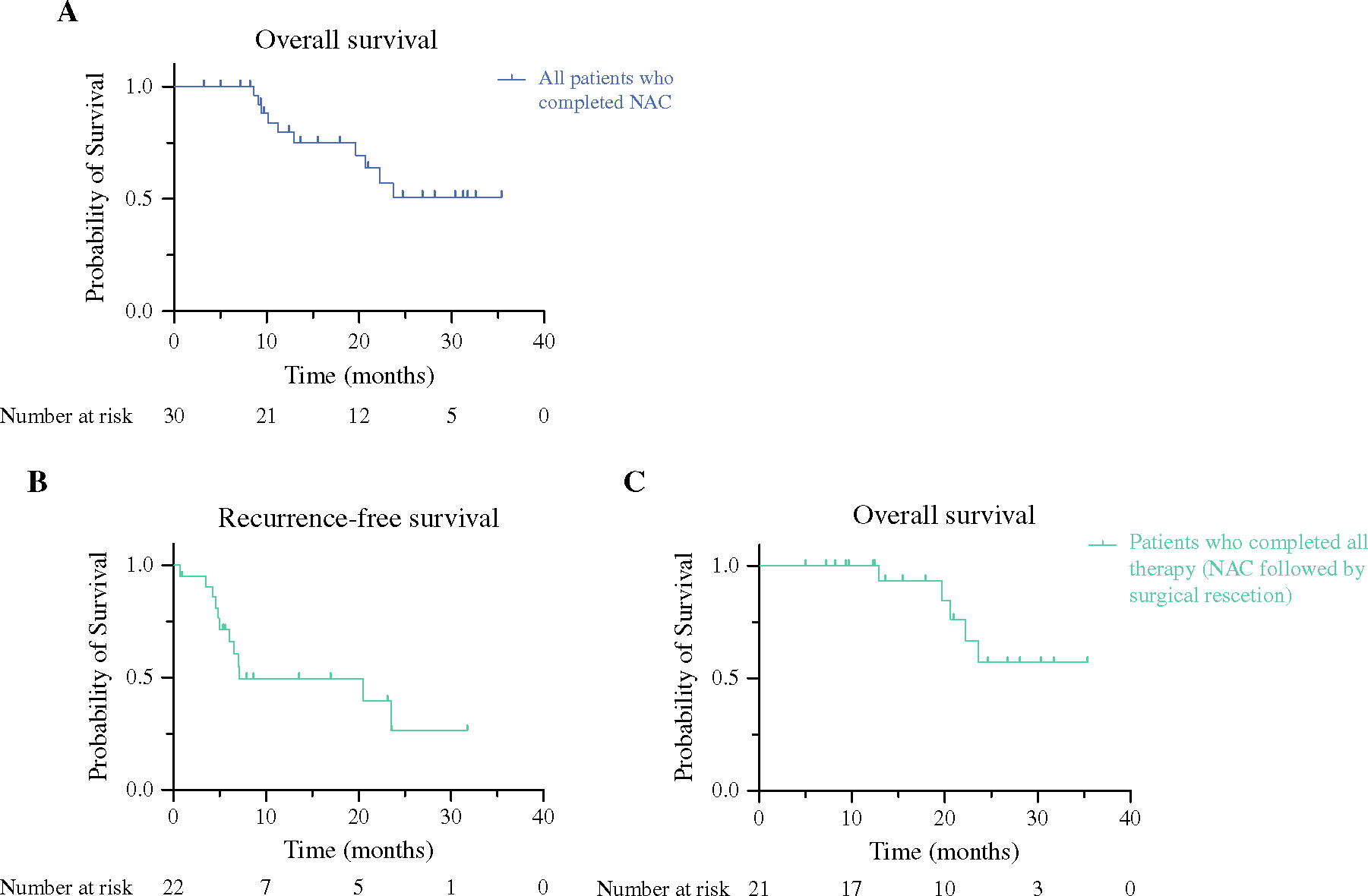
Overall survival among all patients (A n = 30). Recurrence-free and overall survival for all patients who completed all therapy, including neoadjuvant chemotherapy followed by surgical resection (B and C, respectively; n = 22)
DISCUSSION
Our findings suggest that it is feasible to administer neoadjuvant combination chemotherapy with gemcitabine, cisplatin, and nab-paclitaxel prior to curative-intent surgery for patients with resectable, oncologically high-risk IHCC.
As few as one third of patients diagnosed with IHCC are eligible for resection at the time of diagnosis, and as many as two thirds of those who undergo resection experience postoperative disease recurrence. Standard of care adjuvant therapy for resected IHCC remains capecitabine, while other regimens, primarily gemcitabine-based, have shown little to no survival benefit.14,15 Given the natural history of this disease, high recurrence rates, and the dismal outcomes even for patients who present with the best odds of achieving a cure, the management of patients with resectable disease necessitates a change in approach. The transition of chemotherapy to the neoadjuvant setting offers the potential for early treatment of micrometastatic disease, downsizing of tumors to increase margin-negative resection rates, eradication of disease from regional lymph nodes, and improved patient selection for complex resections. Identifying the optimal neoadjuvant chemotherapy backbone remains an unmet need.
Transitioning to a neoadjuvant approach for managing patients with resectable IHCC should extrapolate from and build on the strategies supported in the advanced disease setting. In a phase II trial, chemotherapy augmentation of standard of care gemcitabine and cisplatin with nab-paclitaxel demonstrated an 84% disease control rate, with a median progression-free and overall survival of 11.8 months and 19.2 months, respectively, which compared favorably with historical controls of patients with advanced biliary tract cancers treated with the standard doublet regimen of gemcitabine and cisplatin.8 These data led to the expeditious approval of the ensuing phase III trial, SWOG1815 (NCT03768414), which completed accrual and was recently reported at the 2023 American Society of Clinical Oncology Gastrointestinal Cancers Symposium (ASCO GI). While the trial did not demonstrate a statistically significant improvement in overall survival with gemcitabine, cisplatin, and nab-paclitaxel compared with gemcitabine and cisplatin alone, there was a higher overall response rate and the triplet regimen seemed to be more active in the locally advanced setting compared with the metastatic setting. Although these subset analyses are not adequately powered, it does seem that SWOG1815 suggests that there may be a place for this triplet regimen for localized tumors in the preoperative setting, given the improved response rate. Nab-paclitaxel is an albumin-bound form of paclitaxel currently indicated as first-line treatment of metastatic pancreas adenocarcinoma, in combination with gemcitabine.16 In both preclinical and clinical studies of pancreatic cancer, the addition of nab-paclitaxel to gemcitabine therapy enhanced intratumoral delivery of chemotherapeutic agents by inducing stromal disruption, suggesting a role for this combination in other stromal-rich malignancies, such as cholangiocarcinomas.17,18 Given the strong phase II data with this triplet regimen in the advanced disease state, it was the natural next step at the time of conception of this study to assess its feasibility in the neoadjuvant setting for localized, high-risk tumors.
In the current study, all patients completed preoperative chemotherapy with combination gemcitabine, cisplatin, and nab-paclitaxel. The safety of combining gemcitabine and nab-paclitaxel has been previously described in both biliary tract8 and pancreatic cancers.19 Chemotherapy completion rates for the current study exceeded those of prior studies employing at least one of the study drugs in the adjuvant setting.14,15,20 While 50% of patients required at least one dose reduction, the selected treatment doses were based on the previous phase II study and were well tolerated.8 Only one third of patients experienced grade 3 or higher treatment-related adverse events, the most common being neutropenia, and there was no treatment-related mortality, overall suggesting a favorable safety profile in the neoadjuvant setting. Further, of the 30 patients who completed neoadjuvant chemotherapy, 73% (n = 22) successfully underwent surgical resection (73% R0 resection), of which only 2 patients experienced minor postoperative complications and 36% had lymph node positive disease, suggesting that the postoperative course was not negatively impacted by the administration of preoperative GAP chemotherapy.
It is important to note that the aim of this trial was not to assess the ability of neoadjuvant GAP to ‘convert’ patients from unresectable to resectable disease. Rather, it was to assess the feasibility and safety of delivering this regimen for 3 months prior to resection. Given that this trial only included patients with technically resectable, but oncologically high-risk disease, a surgical completion rate of 73% is above what is expected for a doublet neoadjuvant chemotherapy approach with gemcitabine and cisplatin that is often utilized in clinical practice for patients with disease that is high-risk for early recurrence. In the latter, anecdotal experience estimates a surgical completion rate of only 50% due to disease progression while on a gemcitabine/cisplatin doublet regimen, which is why 50% completion of all therapy was chosen as the null hypothesis for this study. The postoperative outcomes and pathologic data in this current study also compare favorably with the outcomes reported in the BILCAP trial (R0 resection, 62%; lymph node positive rate, 48%).
Although efficacy was not the primary endpoint for the current trial, radiographic disease control rate was 90%, mirroring that which was seen in the advanced disease setting.7 While the partial response rate of 23% is lower than what has previously been reported in the phase II trial of unresectable disease, this may reflect the decreased duration of therapy utilized in the current study.8 With a median follow-up of 16 months, recurrence- free survival was 7.1 months and overall survival was not reached for patients who completed all therapy, including neoadjuvant chemotherapy and surgical resection. Indeed, RFS after surgery was relatively short; however, the trial cohort comprised patients with large or multifocal tumors, major vascular invasion, and preoperative lymph node involvement. These high-risk features likely explain the early disease recurrence after surgical resection.
This treatment paradigm shift that incorporates neoadjuvant therapy needs to also consider the ongoing advances in immunotherapy and molecular profiling and targeted and personalized therapies. The role of immunotherapy in the neoadjuvant and/or adjuvant setting, either alone or in combination with chemotherapy, remains to be defined. Molecular profiling and next-generation sequencing (NGS) have uncovered distinct actionable gene mutations that have been investigated as therapeutic targets, expanding the role of personalized therapy in this disease state. Early phase II studies have led to accelerated FDA approval of three such drugs.21–23 In this study, 32% of the patients who underwent testing had a positive result for the two most common actionable mutations in IHCC. Importantly, NGS was not part of the protocol and most patients were tested after disease progression. Given the efficacy and response rates observed with a targeted approach, the natural next step is to assess the feasibility of employing such an approach in the neoadjuvant setting for patients with resectable disease. A follow-up study is currently underway to assess the feasibility of performing NGS and administering personalized therapy in combination with neoadjuvant chemotherapy in the preoperative setting as we continue to redefine the optimal treatment strategy for patients with resectable IHCC. If this next study proves feasible as well, an umbrella study including multiple arms of personalized therapy strategies based on NGS results in the preoperative setting will be conducted for patients with resectable IHCC.
Limitations
This study was limited by its single arm design, which prevented any intergroup comparisons. The primary endpoint of this trial, however, was feasibility of completing neoadjuvant GAP chemotherapy and surgical resection, and thus the single-arm design mirrored that of other studies attempting to define feasibility and safety. This was also the first prospective study employing neoadjuvant chemotherapy for patients with resectable IHCC. Given that adjuvant chemotherapy was not standardized across patients, differences in adjuvant chemotherapy regimens may affect recurrence rates and survival outcomes; however, the goal of the study was to assess feasibility of administering neoadjuvant GAP chemotherapy. Another limitation is the lack of central review of pathology; thus, details of pathologic response in the surgically resected specimens could not be reported. Finally, while the study included a relatively small number of patients across four participating institutions, it was specifically powered to establish feasibility. Given the feasibility and tolerability of this preoperative regimen in patients with resectable, high-risk IHCC, the current study has set the foundation for future neoadjuvant trials in this disease space.
CONCLUSION
This study met its primary endpoint and demonstrated that neoadjuvant gemcitabine, cisplatin, and nab-paclitaxel is feasible and safe prior to resection for patients with resectable, high-risk intrahepatic cholangiocarcinoma and does not adversely impact perioperative outcomes.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to sincerely thank all the patients and families who participated in this clinical trial. Research reported in this publication was supported in part by the Biostatistics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under Award Number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank Reham Abdel-Wahab for her assistance in protocol development.
FUNDING
Bristol Meyers Squibb (Celgene)
Footnotes
DISCLOSURE Flavio G. Rocha, MD: Consultant for Medtronic; Research funding from Gunze; Advisory board member at Astra Zeneca. Bruce S. Lin, MD: Consultant for Exelixis, QED/Helsinn, Pfizer, Bayer, Daiichi-Sankyo; Clinical research support for Exelixis, Zymeworks, Merck, Incyte, Relay Therapeutics. Sean P. Cleary, MD: Consultant for Ethicon. Mehmet Akce, MD: Research funding (to institution) from Tesaro, RedHill Biopharma Limited, Polaris, Bristol-Myers Squibb-Ono Pharmaceutical, Xencor, Merck Sharp & Dohme, Eisai, GSK. Consulting or advisory role at Eisai, Ipsen, Exelixis, GSK, QED, Isofol, Curio Science, AsrtraZeneca, Genentech. Shishir K. Maithel, MD: Research support for clinical trials for Bristol-Myers Squibb. Milind M. Javle, MD: Research funding (to institution) from Merck, EMD Serono, Novartis, Eli Lilly, Astra Zeneca, Genentech, Transthera, Meclun, BMS, Incyte, QED, Taiho, Servier, Oncosil, Basilea, Nucana and to self or as advisory board/ DSMB member from Incyte, Zymeworks, Mundi Pharma, Nucana, MORE health, Agios, Servier, Helsinnn, Transthera, and Origimed.
Presented: Poster presentation at the American Society of Clinical Oncology 2022 Annual Meeting, Chicago, IL.
SUPPLEMENTARY INFORMATION The online version contains supplementary material available at https://doi.org/10.1245/s10434-023-13809-5.
REFERENCES
- 1.Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17(8):669–80. 10.1111/hpb.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–88. 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma—evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111. 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–73. 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 5.Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol. 2019;37(12):1015–27. 10.1200/JCO.18.02178. [DOI] [PubMed] [Google Scholar]
- 6.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81. 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 7.Oh DY, Lee KH, Lee DW, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol. 2022;7(6):522–32. 10.1016/S2468-1253(22)00043-7. [DOI] [PubMed] [Google Scholar]
- 8.Shroff RT, Javle MM, Xiao L, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol. 2019;5(6):824–30. 10.1001/jamaoncol.2019.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193(4):384–91. 10.1016/s1072-7515(01)01016-x. [DOI] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services NIoH, National Cancer Institute. Common terminology criteria for adverse events (CTCAE), version 4. 2010. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm.
- 11.Schwartz LH, Litiere S, de Vries E, et al. RECIST 1.1—update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7. 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Thall PF, Simon RM, Estey EH. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Stat Med. 1995;14(4):357–79. 10.1002/sim.4780140404. [DOI] [PubMed] [Google Scholar]
- 14.Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12- ACCORD 18-UNICANCER GI): a randomized phase III study. J Clin Oncol. 2019;37(8):658–67. 10.1200/CO.18.00050. [DOI] [PubMed] [Google Scholar]
- 15.Ebata T, Hirano S, Konishi M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg. 2018;105(3):192–202. 10.1002/bjs.10776. [DOI] [PubMed] [Google Scholar]
- 16.Sohal DPS, Kennedy EB, Cinar P, et al. Metastatic pancreatic cancer: ASCO guideline update. J Clin Oncol. 2020. 10.1200/JCO.20.01364. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez R, Musteanu M, Garcia-Garcia E, et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer. 2013;109(4):926–33. 10.1038/bjc.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frese KK, Neesse A, Cook N, et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2(3):260–9. 10.1158/2159-8290.CD-11-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703. 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi S, Nagano H, Tomokuni A, et al. A prospective, randomized phase II study of adjuvant gemcitabine versus S-1 after major hepatectomy for biliary tract cancer (KHBO 1208): Kansai Hepato-Biliary Oncology Group. Ann Surg. 2019;270(2):230–7. 10.1097/SLA.0000000000002865. [DOI] [PubMed] [Google Scholar]
- 21.Javle M, Roychowdhury S, Kelley RK, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol. 2021;6(10):803–15. 10.1016/S2468-1253(21)00196-5. [DOI] [PubMed] [Google Scholar]
- 22.Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–84. 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807. 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


