Abstract
The technology is now available for commercial cloning of farm animals for food production, but is the food safe for consumers? Here, we provide data on >100 parameters that compare the composition of meat and milk from beef and dairy cattle derived from cloning to those of genetic- and breed-matched control animals from conventional reproduction. The cloned animals and the comparators were managed under the same conditions and received the same diet. The composition of the meat and milk from the clones were largely not statistically different from those of matched comparators, and all parameters examined were within the normal industry standards or previously reported values. The data generated from our match-controlled experiments provide science-based information desired by regulatory agencies to address public concerns about the safety of meat and milk from somatic animal clones.
Keywords: cloned cattle, food safety, clone health
Somatic cell cloning by nuclear transfer has potential agricultural applications for duplicating food animals with desired genetic merit. However, somatic cloned animals have been associated with aberrant gene expression (1-3), as well as developmental abnormalities and high neonatal death rates. These findings suggest the incomplete reactivation of some inactivated genes from the differentiated somatic donor cells. Because of limited knowledge of the nature of gene dysregulation in clones, public debate has arisen as to whether food products from animal clones are safe for human consumption. In the United States, the Food and Drug Administration's Center for Veterinary Medicine has asked companies not to introduce animal clones, their progeny, or their food products, such as milk or meat, into the human or animal food supply (www.fda.gov/cvm/index/updates/clones.htm). The U.S. Food and Drug Administration requested that producers abstain from placing edible products from clones into the food supply until the agency considers the safety of their products based on scientific information gained from the direct evaluation of safety. To date, no animals cloned from somatic nuclei, or their products, have been permitted to enter the food chain in any country (4). Information on the composition of meat and milk from somatic clones of food animals is extremely limited and highly desired by federal regulatory agencies concerned with food safety. Commissioned by the U.S. Food and Drug Administration, the National Academy of Sciences was charged to identify any safety concerns that animal clones might present to humans, animals, and the environment. The National Academy of Sciences report concludes that clones are not likely to pose a food consumption risk, but the National Academy of Sciences states that information on compositions of the products of animal clones is needed to decrease food safety uncertainties (www.nap.edu/catalog/10418.html). Thus, we have conducted extensive comparisons of the composition of milk and meat from somatic cloned animals to those from naturally reproduced comparator animals. Here, we provide data on >100 parameters that compare the composition of meat and milk from beef and dairy cattle derived from somatic cloning to those of genetic- and breed-matched comparator animals from conventional reproduction. All of the experimental animals used for the comparisons were managed under the same conditions and received the same diet. This report addresses the scientific and public concerns of the physiology and safety of the meat and milk products from beef and dairy animal clones.
Methods
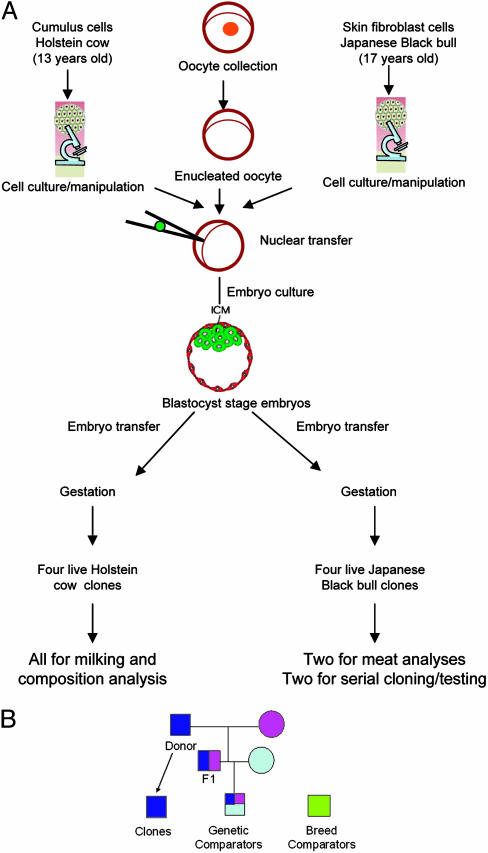
Cloned Beef and Dairy Cattle. Our beef and dairy animal clones were produced by somatic cell nuclear transfer by using cultured skin fibroblasts or cumulus cells from an adult Japanese Black beef bull or a Holstein dairy cow (Fig. 1). Our beef clones were produced in 1998; male clones of a farm animal species had not been produced previously. The donor bull (Kamitakafuku), a superior breeding stud bull with superior marbling traits, was 17 years old when we produced six bull clones of him (5). Four clones survived normally, and two of these clones were selected randomly for serial cloning, semen quality, and breeding performance analyses (6). The other two beef clones were slaughtered and subjected to standard meat analyses in this study. We produced 10 diary clones from skin fibroblast (n = 4) and cumulus cells (n = 6) of a Holstein cow at 13 years of age, between June and August 1999 (7). Four of these clones, all derived from cumulus cells, survived and are healthy. We have studied their telomere lengths (7), expression of X-linked genes (1), onset of puberty (8), growth endocrinology (9), and behavior (10). All animal use was approved by the institutional animal care and use committees at the University of Connecticut (dairy) or the Kagoshima Prefectural Institute of Cattle Breeding and Development (beef).
Fig. 1.
Somatic cloning and clones. (A) Source and production procedure of the cloned dairy and beef cattle used for this study. (B) The genetic relationship among the beef clones and their comparators. Squares indicate males, and circles indicate females. The same colors indicate identical genetic makeup. The genetic comparators share 25% of genetic identity with the clones, whereas the breed comparators are not related genetically to the clones or the donor animal.
Comparison of Milk Production. In the present study, the four live dairy clones and four age and parity-matched comparator heifers from natural reproduction were raised in the same facility from 2 months of age. All animals were subjected to the same diet and management protocols and were bred by artificial insemination or natural breeding starting at 14-15 months of age. Immediately after calving, we monitored milk production by collecting samples three times daily during the entire first lactation. The total amount of milk produced in the first 305 days of lactation, the standard lactation period in dairy cattle, was compared among the clones, to their matched comparators and to the production records of the clones' genetic donor cow.
Comparison of Milk Composition. To compare the milk compositions, two milk samples were collected from each of the three milkings on a given day of each week, throughout the entire first lactation. One of these milk samples was delivered to Dairy One Cooperative (Ithaca, NY), a Dairy Herd Improvement Association (DHIA)-designated laboratory, for analyses of total protein (percentage), total fat (percentage), lactose (percentage), total solids (percentage), milk urea nitrogen (mg/dl), and somatic cell counts (× 103 per ml). All these parameters are routinely monitored for the dairy industry by the DHIA (www.dhia.org). We used the second set of the milk samples (frozen at -20°C) for monthly analyses of protein profiles by denaturing SDS/PAGE stained with Coomassie blue. The gel images were scanned, and the relative quantities of each band were determined by using the quantity one software program (Bio-Rad). Additionally, we also measured antobody concentrations (IgM, IgA, and IgG) in the colostrum from the first milking by using the Single Radial ImmunoDiffusion kit (VMRD, Pullman, WA).
Comparison of Meat Composition. We compared our two beef clones with eight genetically matched comparators by using the same analyses protocols (Fig. 1B). All animals were raised in the same facility and subjected to the same diet and management. The genetic matched comparator bulls were produced by artificial insemination using semen from the son of the original donor bull (Kamitakafuku), and thus, they are genetic “nephews” and share 25% of their genetic makeup with the clones (Fig. 1B). Additionally, 20 mature Japanese Black beef cattle (referred to as breed comparators), at the Kagoshima Prefectural Livestock Station or Japan Meat Grading Association, were also used to establish the normal range for each parameter analyzed. All bulls in this study were castrated at 3 months of age and were then given a standard growing ration from 8 to 26 months of age, according to the normal practice for the beef breed in Japan. The animals were then slaughtered and examined to determine meat quality and carcass composition by using beef industry standard protocols (www.acess.gpo.gov/nara/cfr/waisidx_99/9cfrv2_99.html). The following parameters were analyzed and compared between the beef clones and their genetic and breed comparators: (i) organ or body part weights; (ii) total proportion of meat and fat in the dressed carcass; (iii) cross section of the left dressed carcass between the sixth and seventh rib, following the standard methods of the Japan Meat Grading Association (11); (iv) the moisture, crude protein, and crude fat contents of six muscles (infraspinatus, longissimus thoracis, latissimus dorsi, adductor, biceps femoris, and semitendinosus); measured by the Kjeldahl analysis method from the Official Methods of Analysis of AOAC International by the Soxtec method (12); (v) fatty acid composition (lauric acid, myristic acid, palmitic acid, palmitoleic acid, stearic acid, oleic acid, linoleic acid, and linolenic acid) of five major fat tissues (s.c. fat, intra- and inter-muscular fats, celom fat, and kidney leaf fat); analyzed by gas chromatography after lipid extraction at the Japan Food Research Laboratories; (vi) amino acid composition of the longissimus thoracis muscle; determined by an amino acid analysis system (Shimadzu) at Kagoshima Prefectural Livestock Station (three controls were used for this analysis); and (vii) histopathology of all organs, examined at the National Institute of Animal Health, Kyusyu, Japan.
Data Analyses. Milk production and composition data were subjected to a mixed model analysis by using the General Linear Model (sas 9.0, SAS Institute, Cary, NC) with week as a repeated measure. The somatic cell count data were analyzed after a log transformation. Data presented in figures are least-square means. We compared 90% confidence intervals of each parameter of meat composition in a pairwise manner to determine any significant difference of biological relevance.
Results
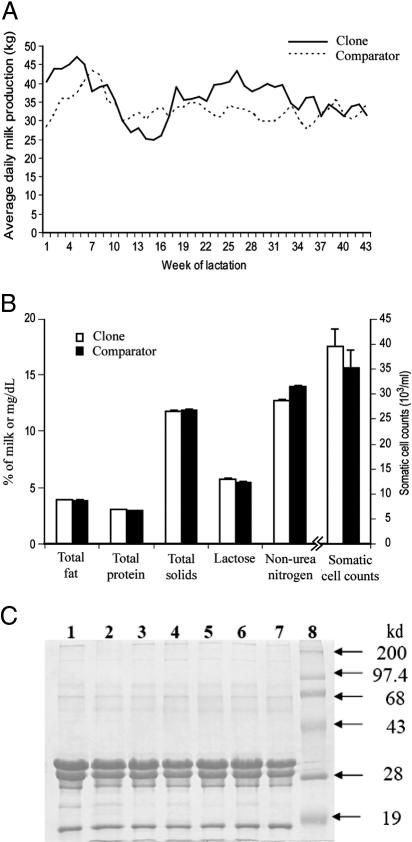
Milk Production. All cloned and comparator animals were bred with semen from different bulls and delivered normal calves during three consecutive parturitions at the expected due dates, with the exception of one parturition. The exception was that one of the clones (clone B) gave birth to a stillborn calf, 2 weeks prematurely at her first parturition, and did not have full udder development at the commencement of lactation. All of the other pregnancies produced normal calves in terms of their gestation lengths, ease of calving, and birth weights. Together, we collected >1,000 milk samples, and the representative production curves of a clone and a comparator animal are shown in Fig. 2A. All of the clones and their matched comparators showed similar, normal lactation curves (13); milk production increased during the first month of lactation and then declined progressively for the remainder of the lactation period. The amount of milk produced by the four clones (8,646.1 ± 743.8 kg) in the first lactation was not significantly different compared with that of matched comparator cows (9,507.8 ± 743.8 kg). Clone B, who gave birth prematurely, produced 30% less milk (6,339.3 kg) than the average of the other three clones (9,378.4 kg). The reason that the donor cow was cloned was that she held one of the highest production records in the herd in her best lactation period (15,875.9 kg). As expected, the production of the three clones in their first lactation (9,378.4 kg) was similar to that of the donor animal in her first lactation (8,990.7 kg; P > 0.05).
Fig. 2.
Analyses of milk production and compositions of somatic clones and matched comparator cows. (A) Representative first lactation curves of a clone and a comparator cow. (B) Milk total protein (percentage), total fat (percentage), lactose (percentage), total solids (percentage), milk urea nitrogen (mg/dl) and somatic cell count (× 103/ml). (C) A representative image of protein profile analysis of milk samples from clones (lanes 1-3) and comparator cows (lanes 4-7) by SDS/PAGE. Lane 8, molecular mass markers. The four major bands are (from top to bottom identified on the basis on their molecular mass): α-caseins, β-caseins, κ-caseins, and β-lactoglobulins.
Milk Composition. No significant difference was detected between the composition of milk from the clones and the matched comparator cows (Fig. 2B). A representative image of the protein profile analysis is shown in Fig. 2C. Four major bands, ranging from ≈17 to 35 kDa and representing α-caseins, β-caseins, κ-caseins, and β-lactoglobulins, were observed to be consistent in all milk samples from either the clones or their comparators. Minor bands were also present at high and low molecular masses in all samples, indistinguishable among clones and comparators. There was no significant difference in the percentages of each major constituent protein between milk samples from the clones and their comparators. Antibodies in the colostrum from the clones ranged from 2,000 to 15,000 mg/dl, 70 to 360 mg/dl, and 125 to 500 mg/dl for IgG, IgA, and IgM, respectively. These values for antibody concentrations were in the typical range for antibody composition of colostrums (14), and the concentrations of antibodies in colostrum from the clones and their comparators appeared similar. These results indicate that the quality of colostrum from clones is sufficient for the nutritional and health requirements of their calves.
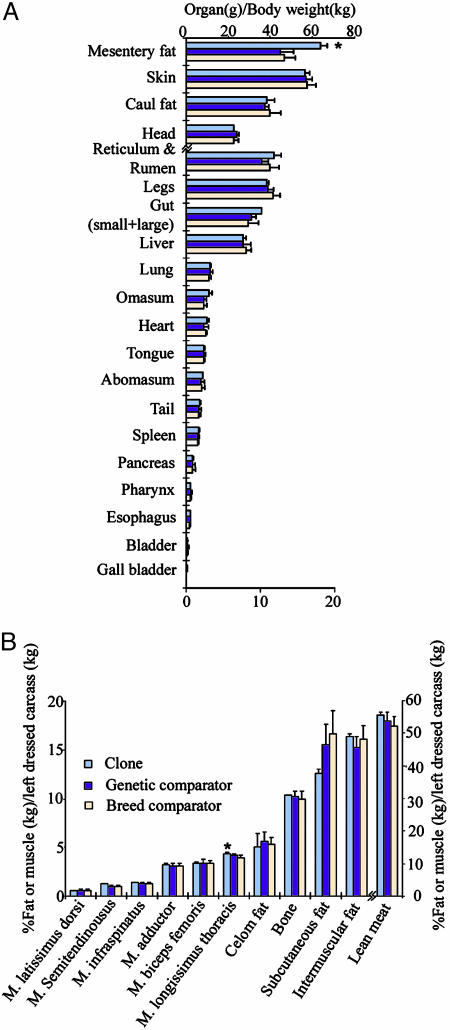
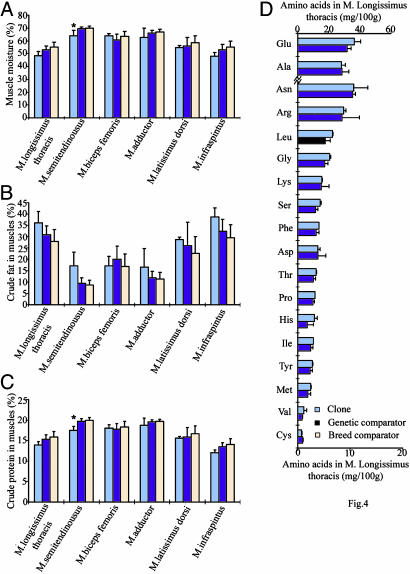
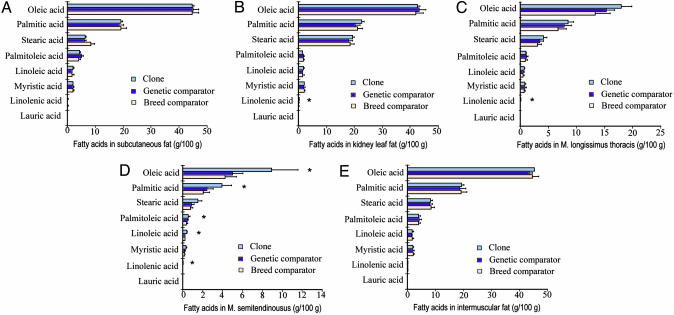
Meat Composition. We analyzed >100 parameters concerning the quality of meat from our beef clones and matched comparator animals, using the standard analysis methods of the industry. The 90% confidence intervals (C.I.) of each parameter of meat composition in a pairwise manner were used to determine any significant difference of biological relevance. Overlap of C.I.s for each paired comparison (clone vs. genetic comparator and clone vs. breed comparator) were not significantly different. Our results indicated that no significant difference was detected in >90% of all parameters examined (Figs. 3, 4, 5 and Table 1). There were, however, 12 instances in which the clones and genetic comparators showed differences, and these were as follows: the amount of mesentery fat (Fig. 3A); yield score (Table 1); the proportion of longissimus thoracis muscle over body weight (Fig. 3B); the muscle moisture (Fig. 4A) and the amount of crude protein in the semitendinosus muscle (Fig. 4C); the amount of linolenic acid in kidney leaf fat (Fig. 5B) in the longissimus thoracis (Fig. 5C) and semitendinosus (Fig. 5D) muscles; and the amount of oleic acid, palmitic acid, palmitoleic acid, and linoleic acid in the semitendinosus muscle (Fig. 5D). All these parameters concerning the amount of mesentery fat and fatty acids in the meat/fat were significantly higher in the clones than in their genetic or breed comparators, except for crude protein or muscle moisture in semitendinosus muscle.
Fig. 3.
Body organ parameters for clones (n = 2), genetic comparators (n = 8, except in A where n = 3), and breed comparators (n = 20). (A) Proportions (percentage; means ± SD) of organ or body part (g) over body weight (kg). (B) The proportions over body weight of various muscles or fat tissues (percentage; means ± SD). *, Significant difference was detected between clones and comparators.
Fig. 4.
Parameters (percentage; means ± SD) for clones (n = 2), genetic comparators (n = 8), and breed comparators (n = 20). (A) Muscle moisture. (B) Muscle crude fat. (C) Muscle crude protein. (D) Amino acid composition of longissimus thoracis muscle (mg/100 g of muscle. Results are means ± SD; *, significant difference was detected between clones and comparators.
Fig. 5.
Comparisons of fatty acid composition (g/100 g) among clones (n = 2), genetic comparators (n = 8), and breed comparators (n = 20) in various tissues. (A) Subcutaneous fat. (B) Kidney leaf fat. (C) Intramuscular fat in longissimus thoracis muscle. (D) Intramuscular fat in semitendinosus muscle. (E) Intermuscular fat. Results are means ± SD; *, significant difference was detected between clones and comparators.
Table 1. Parameters of the left dressed carcass.
| Parameter | Clone (n = 2) | Genetic comparator (n = 8) | Breed comparator (n = 20) |
|---|---|---|---|
| Carcass weight, kg | 425.5 ± 16.8 | 457.4 ± 30.4 | 457.0 ± 43.7 |
| Rib eye area, cm2 | 57.0 ± 1.41 | 58.9 ± 5.1 | 55.4 ± 6.4 |
| Rib thickness, cm | 8.7 ± 0.00 | 7.8 ± 0.6 | 7.6 ± 0.6 |
| s.c. fat thickness, cm | 3.3 ± 0.00 | 3.1 ± 0.4 | 3.3 ± 0.5 |
| Yield score, % | 74.4 ± 0.00* | 73.8 ± 0.3 | 73.0 ± 0.8 |
| Beef marbling standard | 8.0 ± 1.41 | 6.5 ± 0.9 | 5.2 ± 1.5 |
| Beef color standard | 3.0 ± 0.00 | 2.9 ± 0.4 | 3.3 ± 0.7 |
| Beef fat standard | 3.0 ± 0.00 | 2.9 ± 0.4 | 3.1 ± 0.5 |
Results are presented as mean ± SD. *, Significant difference was detected between clones and comparators.
To determine the comparative health and pathology of all organs from the two clones used for meat analyses, the organs were subjected to histological analyses after slaughter. Both clones were normal in all their organs, including liver, kidney, lung, heart, spleen, and the adrenal and thyroid glands (Table 2). No macroscopical or microscopical abnormalities were observed in the clones, except for the kidney urinary calculi (collecting ducts). However, these calculi are often detected in the usual beef cattle because of a feeding peculiarity (15).
Table 2. Pathological observations on the clones.
| Clone
|
||||
|---|---|---|---|---|
| Organ | Examination level | Observation | 1 | 2 |
| Liver | Gross appearance | Normal | Normal | |
| Histological findings | Irregular arrangement of hepatic cords | — | — | |
| Focal necrosis | — | — | ||
| Inflammation | — | — | ||
| Fibrosis | — | — | ||
| Kidney | Gross appearance | Structure | Normal | Normal |
| Urinary calculus (collecting duct) | +* | +* | ||
| Histological findings | ||||
| Cortex | ||||
| Glomerulus | Varying size | — | — | |
| Immature form | — | — | ||
| Proliferation of the mesangial cells | — | — | ||
| Thickened basement membrane | — | — | ||
| Renal tubules | Cystic tubules | — | — | |
| Urinary casts | — | — | ||
| Atrophied or immature tubules | — | — | ||
| Thickened basement membrane | — | — | ||
| Abnormal cell infiltration | — | — | ||
| Medulla | Urinary cast | — | — | |
| Cystic tubule | — | — | ||
| Inflammation | — | — | ||
| Lung | Gross appearance | Normal | Normal | |
| Histological findings | Atelectasis | — | — | |
| Inflammation | — | — | ||
| Heart | Gross appearance | Normal | Normal | |
| Histological findings | ||||
| Cardiac muscle cells | Irregular arrangement | — | — | |
| Necrosis | — | — | ||
| Inflammation | — | — | ||
| Spleen | Gross appearance | Normal | Normal | |
| Histological findings | ||||
| Inflammation | — | — | ||
| Hyperplasia | — | — | ||
| Adrenal gland | Gross appearance | Normal | Normal | |
| Histological findings | Irregular arrangement of cortex cells | — | — | |
| Hyperplasia | — | — | ||
| Thyroid | Gross appearance | Normal | Normal | |
| Histological findings | Abnormal follicles | — | — | |
| Abnormal follicular epithelium | — | — | ||
Significant difference was detected between clones and comparators.
Discussion
In a recent report, the composition of milk from somatic cow clones was analyzed, but the findings were confounded with different diets and management (16), which are known to affect milk production and composition. In the present study, we compared the composition of meat and milk from our somatic beef (5) and dairy (7) cattle clones to those of age-, genetic-, and breed-matched naturally reproduced comparator animals, using standard protocols well established in the beef and dairy industries. We found no significant differences in the composition of milk from cloned animals compared with the comparator animals managed under the same conditions. Our results of the milk analyses using the DHIA standards suggest that healthy clones not only are normal themselves based on previously examined parameters, such as telomere lengths (7), onset of puberty (8), reproduction and lactation (17, 18), growth endocrinology (9), expression of X-linked genes (1), and behavior (10) but also appear to have normal gene expression in their mammary tissues. This normality is because the production of each milk protein constituent involves the elaborate regulatory function of many proteins and enzymes, and any abnormal gene expression would likely be reflected by imbalances in the constituents of the milk. Furthermore, our finding that there were no differences in somatic cell counts, which is a parameter used to detect subclinical mastitis, demonstrates that these clones were not more susceptible than the comparator animals to this mammary gland disease that is associated with lactation.
For the milk production comparison, we found that all clones and their matched comparators showed similar and normal lactation curves (13). The slight improvement in the milk production of the clones over the donor cow at their first lactation was likely due to improved nutrition and management practices that have evolved in the last 13 years in the dairy industry (www.usda.gov/nass/pubs/histdata.htm). Overall, our data on comparing various aspects of milk, including protein profiles, antibody levels, composition, and production from somatic cloned animals with naturally reproduced comparator animals are very comprehensive and convincing.
One of our reasons for cloning the Japanese Black beef breeding bull was his top-ranking breeding value due to an excellent meat marbling score. The popularity of this donor bull was shown by the fact that >350,000 cows were inseminated with the bull's semen and that he was the sire of >165,000 offspring at the time we cloned him in 1998. The two clones of this bull were found to have an average marbling score of 8 of 12, whereas the average score for the breed is only 5.2. The genetic comparators in this study had a marbling score of 6.5, which is between the clones and the breed average (Table 1). This score is what might be expected, considering the fact that the genetic comparators were also descendents of and had some genetic influence (25%) from the donor bull. Furthermore, the two clones had nearly identical marbling patterns of the muscles imaged at the sixth and seventh rib (photos not shown), using the standard comparison for this breed, suggesting a strong genetic influence on this production trait in the Japanese Black Beef cattle.
In the present study, we analyzed >100 parameters concerning the quality of meat from our beef clones, and the prevailing majority of these parameters did not differ from those of the matched comparator animals. Among the 12 parameters differentially detected between the clones and comparator animals, 8 were related to the amount of fat or fatty acids in the meat/fat (high levels in clones). Animals with more fat or fatty acids in meat/fat are more valuable in Japanese Black beef and have been selected for. The fact that both clones had consistently higher amounts of mesentery fat and fatty acids compared with the comparators is hardly surprising because these two clones are genetic copies of a top breeding bull and they both exhibited the most preferable values as expected (19, 20). The other four parameters found different between clones and comparators: yield score, the proportion of longissimus thoracis muscle to body weight, the muscle moisture, and the amount of crude protein in the semitendinosus muscle, all fall within the normal range of the previously recorded industry standards (19, 21, 22). Therefore, none of these parameters are of public concern.
To our knowledge, there have been no previous reports on the organ histology, composition, and quality of meat from somatic cloned animals for potential human consumption. Previously, there has been one study evaluating the meat of animals cloned from embryonic cells (23). Those results, however, do not provide insight into the products from animals cloned from adult somatic cells and could not be fully justified to serve as the scientific basis to address public concerns on the food safety of somatic animal clones. This is because embryonic animal clones are produced from blastomeres of fertilized embryos at a very early stage of development, and thus, embryonic clones may undergo little gene reprogramming during their development. This is likely why food products from embryonic animal clones have been used for human consumption and their safety has not been a public concern.
In summary, we conclude that most parameters of the composition of the meat and milk from somatic animal clones were not significantly different from those of their genetically matched comparators or industry breed comparators, and that all parameters examined in this study were within the normal range of beef and dairy products approved for human consumption. It is important to note that this study was conducted with a relatively small number of diary and beef clones, and the clones of each breed were derived from a single genetic source. The experiments presented here, however, are a pilot study to provide guidelines for more conclusive studies with larger numbers of clones from different genetic backgrounds, to further increase the consumers' confidence concerning product safety of somatic cloned food animals.
Acknowledgments
We thank K. Kouri, C. Moyes, D. Yang, P. Amond, K. Kawabata, M. Ozono, M. Yonemaru, and the farm crew at the Kellogg Dairy Center at the University of Connecticut for help with milk sample collection and/or shipping; the farm crew at the Kagoshima Prefectural Livestock Experiment Station for taking care of cloned bulls; T. Onitsuka for histopathological analyses; T. Koga, Y. Da, L. Kuo, J. Riesen, and T. Hoagland for help with statistical analyses; and C. Faustman, R. Foote, and M. Julian for critical reading of the manuscript. This work was supported by grants from the U.S. Department of Agriculture and Connecticut Innovations, Inc. (to X.Y. and X.C.T.); and from the Kagoshima Prefecture and the National Institute of Agrobiological Science (to C.K.).
Author contributions: X.C.T., C.K., and X.Y. designed research; X.C.T., C.K., K.S., Y.I., R.O., N.T., C.C., L.J., Y.Z., S.S., C.B., M.S., S.A., and X.Y. performed research; C.K. contributed new reagents/analytic tools; X.C.T., C.K., J.X., S.A., and X.Y. analyzed data; and X.C.T. and X.Y. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: DHIA, Dairy Herd Improvement Association.
References
- 1.Xue, F., Tian, X. C., Du, F., Kubota, C., Taneja, M., Dinnyes, A., Dai, Y., Levine, H., Pereira, L. V. & Yang, X. (2002) Nat. Genet. 31, 216-220. [DOI] [PubMed] [Google Scholar]
- 2.Humpherys, D., Eggan, K., Akutsu, H., Hochedlinger, K., Rideout, W. M., III, Biniszkiewicz, D., Yanagimachi, R. & Jaenisch, R. (2001) Science 293, 95-97. [DOI] [PubMed] [Google Scholar]
- 3.Humpherys, D., Eggan, K., Akutsu, H., Friedman, A., Hochedlinger, K., Yanagimachi, R., Lander, E. S., Golub, T. R. & Jaenisch, R. (2002) Proc. Natl. Acad. Sci. USA 99, 12889-12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galli, C., Duchi, R., Lagutina, I. & Lazzari, G. (2004) IEEE Eng. Med. Biol. Mag. 23, 52-54. [DOI] [PubMed] [Google Scholar]
- 5.Kubota, C., Yamakuchi, H., Todoroki, J., Mizoshita, K., Tabara, N., Barber, M. & Yang, X. (2000) Proc. Natl. Acad. Sci. USA 97, 990-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubota, C., Tian, X. C. & Yang, X. (2004) Nat. Biotechnol. 22, 693-694. [DOI] [PubMed] [Google Scholar]
- 7.Tian, X. C., Xu, J. & Yang, X. (2000) Nat Genet. 26, 272-273. [DOI] [PubMed] [Google Scholar]
- 8.Enright, B. P., Taneja, M., Schreiber, D., Riesen, J., Tian, X. C., Fortune, J. E. & Yang, X. (2002) Biol. Reprod. 66, 291-296. [DOI] [PubMed] [Google Scholar]
- 9.Govoni, K. E., Tian, X. C., Kazmer, G. W., Taneja, M., Enright, B. P., Rivard, A. L., Yang, X. & Zinn, S. A. (2002) Biol. Reprod. 66, 1293-1298. [DOI] [PubMed] [Google Scholar]
- 10.Savage, A. F., Maull, J., Tian, X., Taneja, M., Katz, L., Darre, M. & Yang, X. (2003) Theriogenology 60, 1097-1110. [DOI] [PubMed] [Google Scholar]
- 11.Japan Meat Grading Association (1988) New Beef Carcass Grading Standards (Japan Meat Grading Assoc., Tokyo).
- 12.Horwitz, W. (2000) Official Methods of Analysis of AOAC International (AOAC International, Gaithersburg, MD), 17th Ed.
- 13.Touchberry, R. W. (1974) in Nutrition and Biochemistry of Milk/Maintenance, Lactation, a Comprehensive Treatise, eds. Larson, B. L. & Smith, V. R. (Academic, New York), Vol. 3, pp. 349-381. [Google Scholar]
- 14.Devery-Pocius, J. E. & Larson, B. L. (1983) J. Dairy Sci. 66, 221-226. [DOI] [PubMed] [Google Scholar]
- 15.Huntington, G. B. & Emerick, R. J. (1984) Am. J. Vet. Res. 45, 180-182. [PubMed] [Google Scholar]
- 16.Walsh, M. K., Lucey, J. A., Govindasamy-Lucey, S., Pace, M. M. & Bishop, M. D. (2003) Cloning Stem Cells 5, 213-219. [DOI] [PubMed] [Google Scholar]
- 17.Lanza, R. P., Cibelli, J. B., Faber, D., Sweeney, R. W., Henderson, B., Nevala, W., West, M. D. & Wettstein, P. J. (2001) Science 294, 1893-1894. [DOI] [PubMed] [Google Scholar]
- 18.Pace, M. M., Augenstein, M. L., Betthauser, J. M., Childs, L. A., Eilertsen, K. J., Enos, J. M., Forsberg, E. J., Golueke, P. J., Graber, D. F., Kemper, J. C., et al. (2002) Biol. Reprod. 67, 334-339. [DOI] [PubMed] [Google Scholar]
- 19.Ozutsumi, K. (1994) Farming Jpn. 28, 10-30. [Google Scholar]
- 20.Oka, A., Iwaki, F., Dohgo, T., Ohtagaki, S., Noda, M., Shiozaki, T., Endoh, O. & Ozaki, M. (2002) J. Anim. Sci. 80, 1005-1011. [DOI] [PubMed] [Google Scholar]
- 21.Yamada, T., Kawakami, S. & Nakanishi, N. (2003) Anim. Sci. J. 74, 95-100. [Google Scholar]
- 22.Tsuchiya, H. (1962) Bull. Chugoku Natl. Agric. Exp. Station Ser. B 19, 15-39. [Google Scholar]
- 23.Diles, J. J. B., Green, R. D., Hughes, L. J., Mathiews, G. L. & Miller, M. F. (1996) Prof. Anim. Sci. 12, 244-249. [Google Scholar]