Abstract
Background
Antimicrobial resistance (AMR) is a global public health problem that is fuelled by the inappropriate prescribing of antibiotics, especially those from the ‘watch’ and ‘reserve’ antibiotic lists. The irrational prescribing of antibiotics is particularly prevalent in developing countries, including Zambia. Consequently, there is a need to better understand prescribing patterns across sectors in Zambia as a basis for future interventions. This study evaluated the prescribing patterns of antibiotics using the WHO prescribing indicators alongside the ‘access, watch and reserve’ (AWaRe) classification system post-COVID pandemic at a faith-based hospital in Zambia.
Methods
A cross-sectional study was conducted from August 2023 to October 2023 involving the review of medical records at St. Francis’ Mission Hospital in Zambia. A WHO-validated tool was used to evaluate antibiotic prescribing patterns alongside the AWaRe classification tool.
Results
Out of 800 medical records reviewed, 2003 medicines were prescribed. Each patient received an average of 2.5 medicines per prescription. Antibiotics were prescribed in 72.3% of encounters, of which 28.4% were injectable. The most frequently prescribed antibiotics were amoxicillin (23.4%—access), metronidazole (17.1%—access), ciprofloxacin (8%—watch) and ceftriaxone (7.4%—watch), with 77.1% overall from the ‘access’ list. Encouragingly, 96.5% of the medicines were prescribed by their generic names and 98% were from the Zambia Essential Medicines List.
Conclusions
There were high rates of antibiotic prescribing, including injectable antibiotics, which needs addressing going forward. It is crucial to implement targeted measures, including antimicrobial stewardship programmes, to improve future antibiotic prescribing in Zambia and reduce the risk of AMR.
Introduction
Antibiotics are a class of medicines used to treat bacterial infections,1,2 and their discovery has revolutionized modern medicine to help save countless lives of patients suffering from infectious diseases.2,3 These medicines represent the most frequently prescribed therapeutic agents in medical facilities, especially in developing countries, where their usage has risen in recent years.4–6 However, their misuse and overuse, especially for self-limiting conditions such as upper respiratory tract infections (URTIs), have contributed to the development of antimicrobial resistance (AMR) worldwide.6–11 AMR arises when pathogenic bacteria undergo genetic changes that lead to a decrease or complete loss of susceptibility to antibiotics.12 AMR is an increasing concern as it is resulting in appreciable morbidity, mortality and costs, with costs rising as a result of extended hospital stays.9,10,13–17 Overall, it was estimated that in 2019, more than 1.27 million deaths globally were directly attributable to bacterial AMR, with potentially up to 4.95 million deaths globally associated with bacterial AMR.10 The morbidity and mortality associated with AMR will grow unless addressed, resulting in AMR increasingly being seen as the next pandemic.18 The greatest burden of infectious diseases and AMR is currently in sub-Saharan Africa.10,19–22
The increasing concerns about the rising rates of AMR, with their impact on mortality and costs, have resulted in a range of global and national initiatives including the development of a Global Action Plan (GAP) on AMR by the WHO to reduce AMR.23–25 The GAP has translated into national action plans (NAPs), with these plans at various stages of development and implementation across Africa.25–31 Alongside the instigation and development of the GAP and NAPs, the WHO also introduced the ‘access, watch and reserve’ (AWaRe) classification of antibiotics and associated guidance, which builds on the Essential Medicines List (EML) concept.32–37 The intention is to reduce the overprescribing of antibiotics, especially in ambulatory care, as well as reduce the prescribing of ‘watch’ and ‘reserve’ antibiotics, with their greater resistance potential.6,32,37–41 The AWaRe classification of antibiotics was developed in 2017 by the WHO to promote the rational prescribing and use of antibiotics, thereby reducing AMR.42,43 The AWaRe classification system has three categories of antibiotics including the ‘access’ (narrow-spectrum antibiotics with fewer side effects and lower risks for selection of AMR, hence recommended for empirical treatment of infections), ‘watch’ (recommended for use in hospital settings for sicker patients due to higher risks for selection of AMR) and ‘reserve’ (last-resort antibiotics reserved to treated infections caused by MDR pathogens).32,33
Before the development of the GAP on AMR, and subsequently NAPs, as well as the WHO AWaRe list and guidance, the WHO developed and implemented a range of prescribing indicators to promote a more rational use of antibiotics and other medicines in ambulatory care.4,44 The WHO prescribing indicators included an average number of medicines per encounter, the percentage of medicines prescribed by generic name, the percentage of encounters with an antibiotic prescribed, the percentage of encounters with an injection prescribed, and the percentage of medicines prescribed from the EML or formulary.4,44,45 The indicators provided a framework for evaluating prescribing practices, measuring antibiotic use, identifying potential areas for improvement, and monitoring changes over time.44,46,47 However, there have been concerns about whether these indicators fully measure the quality of prescribing in ambulatory care.45 This includes concerns with the actual quality of antibiotic prescribing for different infectious disease areas and not just the quantity prescribed, leading to recent initiatives, including the AWaRe classification of antibiotics and the AWaRe guidance by the WHO to reduce inappropriate prescribing of antibiotics and AMR.32,33,37,38 The initial target is that at least 60% of antibiotics prescribed must be from the ‘access’ list.6,33,35,37
There are concerns with the appropriateness of the current prescribing of antibiotics among hospitals across Africa, including hospitals in Zambia and other African countries, with typically high rates of empirical prescribing of antibiotics, especially from the ‘watch’ group.39,40,48,49 This is not helped by concerns of a lack of knowledge regarding antibiotics, AMR and antimicrobial stewardship programmes (ASPs), as well as the implementation of ASPs among healthcare professionals (HCPs) in some hospitals across Africa to improve future use.24,50–53 ASPs have been known to improve antibiotic prescribing in hospitals.54–56 These issues have been exacerbated by the lack of available personnel and resources to implement ASPs in low- and-middle-income countries (LMICs), including among African countries.57 This is now changing, with ASPs being routinely instigated across hospitals in Africa.24,58–61 We are also now seeing improved knowledge regarding antibiotics and AMR among HCPs in Africa, including Zambia, although more needs to be done.62,63
In Zambia, there are also issues and concerns with high rates of antibiotic prescribing in ambulatory care, with repeated high rates of prescribing of ‘watch’ antibiotics.64,65 This situation needs to be monitored and addressed as ambulatory care can account for up to 95% of antibiotic use in humans in LMICs.66 Consequently, this situation concerning antibiotic prescribing in ambulatory care has likely worsened post-COVID-19.67–70 This is because there has been appreciable prescribing of antibiotics for patients with suspected or actual COVID-19 across LMICs, despite limited evidence of secondary bacterial infections or coinfections.67,71–78 This also needs to be addressed going forward as part of the NAPs across Africa, including Zambia, to reduce AMR.
Additionally, studies have reported some deviations from treatment guidelines when antibiotics have been prescribed among healthcare facilities in Zambia.39,48,64,74,79 This is a concern, with adherence to guidelines increasingly seen as providing good-quality care and included in ASPs.24,80–86 Given this, it is important to assess the prescribing patterns of antibiotics in healthcare facilities across Zambia to identify areas for improvement and subsequently develop and implement appropriate strategies to promote their rational use as part of the ongoing NAP.87,88 Consequently, there is a paucity of information in Zambia concerning the prescribing patterns of antibiotics using the WHO prescribing indicators post-COVID-19 pandemic. Therefore, this study aimed to address this information gap by evaluating the antibiotic prescribing patterns in Zambia using the WHO prescribing indicators and the more recent prescribing targets based on the AWaRe classification and guidance post-COVID-19 pandemic.
Materials and methods
Study design, period and site
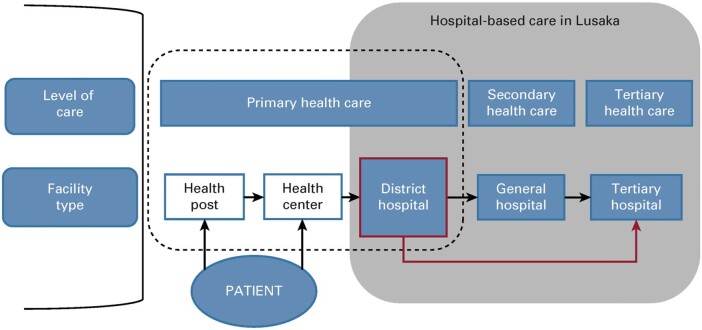
A retrospective cross-sectional study was conducted from August 2023 to October 2023 using patients’ medical records (prescriptions and medical files) at St. Francis’ Mission Hospital located in the Katete district of Eastern Province in Zambia. Zambia is a low-income country located in southern Africa. The country’s economy is highly dependent on the mining and agriculture system, in addition to the construction and trade sectors.89 Zambia’s healthcare system is still developing and is made up of the public and private sectors. The primary healthcare system is composed of rural health posts (usually staffed by community health assistants, pharmacy personnel and nurses), health centres (usually staffed by clinical officers, pharmacy personnel, nurses and midwives) and district hospitals (staffed by specialists including paediatricians, general surgeons, obstetricians and gynaecologists, pharmacists, pharmacy technologists, nurses, physiotherapists, laboratory scientists, dentists, public health specialists, epidemiologists, infectious disease specialists, radiographers and internists).90–92 Regarding the referral system, the district hospitals make patient referrals to general and tertiary hospitals (Figure 1). St. Francis’ Hospital is a 350-bed capacity referral hospital providing medical and surgical services to the population in the Eastern Province of Zambia.93 This rural hospital was chosen for this study since inappropriate prescribing of antibiotics is more likely to occur in such hospitals, with ongoing ASP activities initially being concentrated in urban areas including Lusaka.58 In addition, this hospital accepts referrals from the rest of the Eastern Province (with a population of 1.5 million people) in Zambia, enhancing its importance for assessing current antibiotic prescribing patterns.93,94 Currently, 200 000 outpatients and inpatients access medical services at St. Francis’ Mission Hospital annually, from a previous population of 87 294 patients.94,95
Figure 1.
Structure of the healthcare and referral system in Zambia. Source: Songiso et al.91
Sample size estimation and sampling criteria
In line with the WHO’s recommendations for investigating medicine use in health institutions, a minimum of 600 medical records should be included in a cross-sectional survey.96,97 In our study, 800 medical records were reviewed, thereby enhancing the robustness and representativeness of our findings. Medical records were selected using a systematic random sampling method from a pool of 21 600 prescriptions/medical files dating between 1 January 2023 and 30 June 2023. The 6 month study period was chosen to balance the need for a sufficiently large dataset with the feasibility of timely data collection and analysis post-COVID-19. To determine the sampling interval, we divided the total number of documents by the desired sample size, resulting in a sampling interval of 27. Consequently, every 27th medical record was selected for inclusion in the study. Patients’ medical records from other healthcare facilities were excluded from this study.
Data collection
Data collection was conducted initially using the validated tool developed by the WHO (https://www.who.int/publications/i/item/who-dap-93.1) and used by others,96,98,99 building on earlier studies in Zambia.65,79 The tool collects reliable data on drug use in healthcare facilities and has been used extensively in previous studies.4,47,60,100 Information collected in this part of the study included records of the patient’s age and sex, diagnosis, the name of the antibiotic prescribed, the number of medicines prescribed by generic name, prescriptions with an antibiotic, and prescriptions with injection encounters. Additionally, we also reviewed whether the prescriptions for medicines were contained in the current Zambia Essential Medicines List (ZEML) and Standard Treatment Guideline (STG) and the indications for the prescribed antibiotics.101,102
Some of the indicators that were used in the study to assess the characteristics and the quality of prescribing included: (i) the average number of medicines per encounter; (ii) the percentage of medicines prescribed by their generic name (international non-proprietary name—INN); (iii) the percentage of encounters where an antibiotic was prescribed; (iv) the percentage of encounters where the medicine was given via an injection; and (v) the percentage of medicines prescribed from the EML or formulary.60,96 The rate of prescribing of generic (INN) versus branded medicines is important given anticipated savings.103,104 However, there can be concerns with the quality of generics among African countries, which needs addressing going forward.105–107 There are also concerns about high rates of prescribing of injectable antibiotics in hospitals.108–110 The prescribing of injectable antibiotics also causes possible harm to patients.111 If this prescribing behaviour continues, it will impact the length of hospital stay and associated costs. Therefore, ASPs in hospitals must be strengthened because they are often aimed at de-escalation where this is seen as a problem.112,113
The second part of the study involved a closer evaluation of the different antibiotics prescribed based on the AWaRe classification. The initial target is at least 60% of antibiotics prescribed should be from the ‘access’ list to help limit AMR.6,33 Some of the antibiotics that fall under the ‘access’ group include amoxicillin, amikacin, ampicillin, cloxacillin, clindamycin, metronidazole, tinidazole, benzylpenicillin, doxycycline, gentamicin, chloramphenicol, phenoxymethyl penicillin, cefalexin, sulfamethoxazole/trimethoprim (co-trimoxazole), benzathine penicillin, erythromycin, tetracycline and nitrofurantoin.42,43 Some examples of ‘watch’ group antibiotics include azithromycin, ceftriaxone, cefotaxime, cefepime, cefuroxime, imipenem/cilastatin, meropenem, ertapenem, ciprofloxacin, gatifloxacin, gemifloxacin, levofloxacin, streptomycin, tobramycin, vancomycin, oxytetracycline, kanamycin and neomycin.42,43 Furthermore, some examples of ‘reserve’ group antibiotics include aztreonam, daptomycin, fosfomycin, colistin, linezolid, minocycline and tigecycline.42,43
Data management and analysis
Microsoft Excel (Microsoft, Redmond, WA, USA) was used to enter, sort and clean the data and thereafter analysed using Statistical Package for Social Sciences (SPSS) version 23.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics were performed for demographic characteristics and other prescribing indicators and results were presented in tables and charts as frequencies and percentages.
Ethics
Ethical approval was granted by the University of Zambia School of Health Sciences Research Ethics Committee (UNZASHREC) with a clearance ID of 202301270003. Permission to conduct data collection was obtained from the Senior Medical Superintendent (SMS) at St. Francis’ Mission Hospital. We observed the ethical issues of confidentiality to ensure that all the patient information collected was not accessed by other people. The data collected were stored on a password-protected computer and were only accessible to the research team.
Results
Sociodemographic characteristics of patients and medicine prescriptions
From a total of 800 prescriptions and medical files that were reviewed, the majority were for female patients (57.1%, n = 457), with most prescriptions for patients aged between 16 and 30 years (30.1%, n = 241) (Table 1).
Table 1.
Sociodemographic characteristics of sampled medical records for patients at Saint Francis’ Hospital, Zambia: January 2023—June 2023
| Variable | n | % |
|---|---|---|
| Gender | ||
| Female | 457 | 57.1 |
| Male | 343 | 42.9 |
| Age category (years) | ||
| 0–15 | 210 | 26.2 |
| 16–30 | 241 | 30.1 |
| 31–45 | 167 | 20.9 |
| >45 | 182 | 22.8 |
Number of medicines prescribed per encounter
Of the 800 prescriptions that were included in the study, 33.4% (n = 267) indicated that most patients were prescribed two medicines, followed by 25.4% (n = 203) who were prescribed one medicine per encounter (Figure 2). Only 25.4% of the patients received a single medicine; this shows that most patients (74.6%) received two or more medicines, indicating a high practice of polypharmacy. There was an increased prescribing of medicines by injection at 28.4% (n = 227) of all medicines, which included antibiotics.
Figure 2.
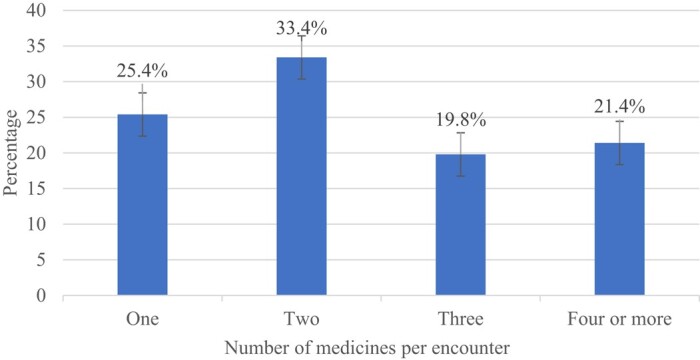
Number of medicines prescribed per encounter at Saint Francis’ Hospital, Zambia: January 2023—June 2023.
Antibiotic prescribing patterns at Saint Francis’ Hospital
Of the 800 medical records reviewed, 51.9% (n = 415) were prescribed a total of 578 antibiotics, of which 34.9% (n = 279) had 1 antibiotic, 13.6% (n = 109) had 2 antibiotics, and 3.4% (n = 27) had 3 antibiotics prescribed (Figure 3). No patient had more than three antibiotics per encounter.
Figure 3.
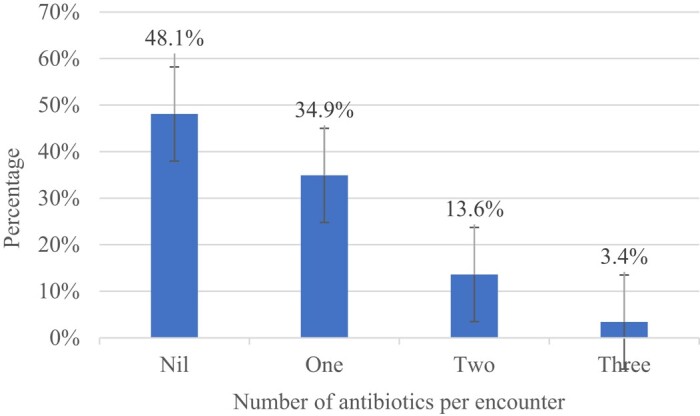
Number of antibiotics prescribed per encounter at Saint Francis’ Hospital, Zambia: January 2023—June 2023.
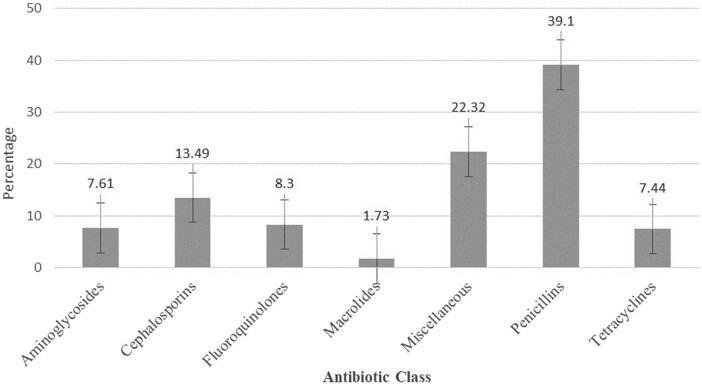
The penicillins accounted for the highest proportion of antibiotics at 39.1% of total encounters, with amoxicillin being the most frequently prescribed antibiotic (23.4%, i.e. 135 encounters) (Table 2, Figure 4). Metronidazole accounted for 17.1% of encounters, followed by ciprofloxacin (8%) with 46 encounters. Overall, among the antibiotics prescribed, the ‘access’ group constituted 77.1% and those from the ‘watch’ group 22.9% (Table 2). The results in Table 2 were presented based on the WHO AWaRe classification of antibiotics.34,37
Table 2.
Distribution of commonly prescribed antibiotics by AWaRe classification at Saint Francis’ Hospital, Zambia: January 2023—June 2023
| Commonly prescribed antibiotics | AWaRe classification | n | % |
|---|---|---|---|
| Amoxicillin | Access | 135 | 23.4 |
| Metronidazole | Access | 99 | 17.1 |
| Ciprofloxacin | Watch | 46 | 8.0 |
| Ceftriaxone | Watch | 43 | 7.4 |
| Benzylpenicillin | Access | 42 | 7.3 |
| Doxycycline | Access | 40 | 6.9 |
| Cefotaxime | Watch | 24 | 4.2 |
| Cloxacillin | Access | 23 | 4.0 |
| Gentamicin | Access | 22 | 3.8 |
| Neomycin | Watch | 19 | 3.3 |
| Chloramphenicol | Access | 16 | 2.8 |
| Phenoxymethyl penicillin | Access | 16 | 2.8 |
| Cefalexin | Access | 14 | 2.4 |
| Co-trimoxazole | Access | 13 | 2.2 |
| Benzathine penicillin | Access | 11 | 1.9 |
| Erythromycin | Access | 10 | 1.7 |
| Tetracycline | Access | 3 | 0.5 |
| Nitrofurantoin | Access | 2 | 0.3 |
Figure 4.
Percentage distribution of commonly prescribed antibiotics by class at Saint Francis’ Hospital, Zambia: January 2023—June 2023.
The most common conditions for which antibiotics were prescribed in this study included RTIs at 40.3% (24.9% for lower RTIs and 15.4% for URTIs) and sexually transmitted infections (STIs) at 9%. Antibiotics were also prescribed for skin and soft-tissue infections (7.6%), conjunctivitis (5.7%), gastrointestinal tract infections (5.2%) and urinary tract infections (UTIs) (5.2%). Antibiotics were also used less frequently for other diseases (Table 3). The high use of penicillin and cephalosporin antibiotics was for RTIs. Nitrofurantoin (0.3%) was rarely prescribed for patients, despite it being highly recommended for the treatment of UTIs in Zambia (Table 3).
Table 3.
Distribution of common conditions treated with antibiotics at Saint Francis’ Hospital, Zambia: January 2023–June 2023
| Diagnosis | n | % |
|---|---|---|
| Lower RTI | 105 | 24.9 |
| URTI | 65 | 15.4 |
| STI | 38 | 9 |
| Skin and soft-tissue infection | 32 | 7.6 |
| Conjunctivitis | 24 | 5.7 |
| Gastrointestinal tract infection | 22 | 5.2 |
| UTI | 22 | 5.2 |
| Obstetric post-Caesarean section prophylaxis | 21 | 5 |
| Sepsis | 14 | 3.3 |
| Prophylaxis in HIV patients | 9 | 2.1 |
| Febrile illness | 7 | 1.7 |
| Incomplete abortion | 7 | 1.7 |
| Pulpitis | 6 | 1.4 |
| Complete abortion | 5 | 1.2 |
| Tooth extraction | 5 | 1.2 |
| Rheumatic heart disease | 4 | 1 |
| Meningitis | 4 | 1 |
| Group 1 diseases | 12 | 3 |
| Group 2 diseases | 19 | 3.8 |
Group 1 infections: septic abortion, post-Caesarean section for cephalopelvic disproportion, peritonitis, corneal abrasion, adult periodontitis and infective endocarditis. Group 2 infections: acute glomerulonephritis, cystic fibrosis, hepatorenal syndrome, malaria, jaundice secondary to cholecystitis, knee arthrotomy, lymphadenitis, lymphogranuloma venereum, pleural effusion, post-dental surgery, post-exploratory laparotomy, post-molar pregnancy extraction, post-operative cataract, proteus syndrome, pseudophakia, severe acute malnutrition with oedema, sickle cell disease, suspected lymphoma and umbilical hernia.
Summary of WHO prescribing indicators of medicines at Saint Francis’ Hospital
The average number of medicines per encounter was 2.5 (2003 medicines prescribed from 800 patient files). Antibiotics were prescribed in 72.3% encounters, of which 28.4% were injectable. Furthermore, 96.5% of medicines were prescribed by generic name and 98% came from the ZEML (Table 4). The findings of the present study indicate that none of the studied indicators met the WHO prescribing indicators of medicines in hospitals (Table 4).
Table 4.
Summary of WHO prescribing indicators of medicines at Saint Francis’ Hospital, Zambia: January 2023–June 2023
| Prescribing indicators | Total number of medicines | Average (n or %) | WHO standard value |
|---|---|---|---|
| Medicines per encounter (n) | 2003 | 2.5 | 1.6–1.8 |
| Encounters with antibiotics (%) | 578 | 72.3% | (20%–26.8%) |
| Encounters with injection (%) | 227 | 28.4% | (13.4%–24.1%) |
| Medicines prescribed by generic name (%) | 1933 | 96.5% | 100% |
| Medicines from the ZEML (%) | 1962 | 98% | 100% |
Discussion
To the best of our knowledge, this is the first study that has evaluated the prescribing of antibiotics using the WHO prescribing indicators, across both ambulatory care and inpatient care in Zambia after the COVID-19 pandemic. The findings reveal a higher-than-average number of medicines being dispensed per patient encounter, with antibiotics constituting a notable 72.3% of the total encounters. This prevalence suggests a potentially alarming reliance on antibiotics, a concern that is amplified by the fact that penicillins, specifically amoxicillin, were the most commonly prescribed. Interestingly, a high proportion of medicines were prescribed by their generic name and sourced from the ZEML, signalling a high adherence to best practices in these specific areas.
The current study found that the average number of medicines prescribed per patient encounter was 2.5, which is higher than the range recommended by the WHO of 1.6–1.8. This finding is consistent with a previous study conducted in Lusaka, Zambia, which also reported an average of 2.5 medicines prescribed per encounter.79 In contrast to our findings, other studies have reported varying average numbers of medicines prescribed for a patient, with an average of 3.4 reported in Nigeria,114 3.2 in Yemen115, 2.91 in India,116 2.8 in Botswana117 and 2.09 in South Sudan.118 On the other hand, a lower average number of medicines prescribed per encounter was reported in other studies, with a 1.12 average reported in India,119 1.14 in Cameroon,120 1.6 in Ethiopia4 and 1.76 in Eritrea.47 The observed discrepancy in the number of prescribed medications per patient encounter may be attributed to differences in the research environments and the specific prescribing practices employed across various medical specialities.121,122 We also assume that the number of medications prescribed for a patient may be informed by patient comorbidities and disease severity, among others.
Furthermore, the present study found that antibiotics were prescribed for 51.9% of the medical records, which translated into 72.3% of total antibiotic encounters. This antibiotic encounter surpassed the WHO-recommended range of 20%–26.8%. Intriguingly, this finding is similar to findings from previous studies, with 53% having antibiotics prescribed in Eritrea,47 52.3% in Ethiopia46 and 52.4% in Pakistan.123 Our findings are higher than the combined findings across the WHO African region of 46.8% of patient encounters in which antibiotics were prescribed44 and 42.7% in Botswana.117 Our findings were lower than the 98.4% to 100% of encounters where antibiotics were prescribed in Niger and Uganda,124 85% in Jordan,125 84.2% in Yemen,115 81.3% in Nigeria114 and 78.8% in Ghana.126 The factors contributing to the excessive use of antibiotics might include health facilities relying on empirical treatment instead of confirmed diagnosis. Furthermore, the lack of essential diagnostic tools to differentiate between bacterial and viral infections, especially in LMICs, is a contributing factor to the inappropriate prescribing of antibiotics in healthcare facilities.127 The present study also found that 13.6% of patients were prescribed two antibiotics, and 3.4% (n = 27) had three antibiotics prescribed. This also needs to be monitored, especially if there was no culture and sensitivity testing undertaken to guide the antibiotic choice. Such prescribing behaviour will increase AMR if not addressed, and impact Zambia achieving the goals within its NAP.87
Our study found that penicillins were the predominant class of antibiotics prescribed, and these are typically from the ‘access’ group, followed by cephalosporins, and fluoroquinolones, similar to an earlier study in Zambia and Jordan, where penicillins were the most widely prescribed antibiotics,65,128 with studies in Botswana, Cameroon, Ghana and Tanzania also showing high rates of prescribing of amoxicillin.86,99,117,120 This was followed by metronidazole and ciprofloxacin, again similar to Botswana, Cameroon, Ghana and Tanzania.86,99,117,120 This may reflect comparatively high rates of STIs as well as UTIs, in addition to RTIs, in these countries, with high rates of RTIs also seen in other countries.99,116,128,129 Respiratory infections are prevalent in Southern Africa, including Zambia, due to the abundance of infectious agents (bacteria, viruses and fungi) and limited healthcare and sanitation access, creating conditions that favour pathogen transmission. Overcrowded living conditions could also worsen the spread of respiratory infections in the region.130,131
The present study findings are different from those in Pakistan, where in one study cephalosporins (81.5%) were the most commonly prescribed antibiotic class, followed by penicillins (6.4%) and fluoroquinolones,123 and in Palestine,132 where ceftriaxone was the most commonly prescribed antibiotic, followed by cefazolin. A similar study in India reported that metronidazole and ceftriaxone were highly prescribed.133 There are also other reports of high rates of prescribing of ceftriaxone in several studies, including those in hospitals in Zambia during the COVID-19 pandemic, enhanced by ceftriaxone being included in treatment guidelines for the management of COVID-19 among African countries.39,64,74,77,134,135 This though was not seen in our study (Table 2), with overall 77.1% of antibiotics encouragingly prescribed from the ‘access’ group and 22.9% from the ‘watch’ group. This finding in our study is higher than an initial target of 60% from the ‘access’ group.6,33 A study conducted in Ghana also found high use of the ‘access’ group antibiotics.126,136,137 Other studies have reported deviations from the WHO AWaRe classification recommendations and reported high use of ‘watch’ antibiotics in hospitals.138–141
The present study found that antibiotics were prescribed mostly for RTIs, STIs and skin and soft-tissue infections. This outcome aligns with findings from other studies, where RTIs were the most common conditions in which antibiotics were often prescribed.99,116,128,129 A similar study conducted in Lusaka, Zambia also reported that most antibiotics were prescribed for RTIs.74
This study found that 28.4% of the prescribed antibiotics were injections, which is significantly higher than the range that the WHO recommends, which is 13.4%–24.1%. This may reflect the fact that our study included both inpatients and ambulatory care patients. This though needs further investigation, especially if there is prolonged use of injections in hospitals without de-escalating to oral antibiotics to hasten discharge. Our figures were higher than the 17.5% of antibiotics given by injection in paediatric outpatient departments in Nigeria,114 6.3% in outpatients in Ethiopia,4 7.8% among community pharmacies in Eritrea47 and 11.8% in a previous study in a University Teaching hospital in Zambia.79 However, they were significantly lower than the 84.8% seen among inpatients in Ethiopia.46 Consequently, the location of care is important when assessing whether there is a high use of injections or not.
Intriguingly, the current study found that 96.5% of antibiotics were prescribed by their generic names, which was slightly lower than the 100% threshold standard recommendation by the WHO. This result was similar to the rate that was reported in Ethiopia (96%).4 However, in our findings the percentage prescribing antibiotics by generic names was higher than that observed in other studies, including 84.4% in Tanzania,99 78.6% in Botswana,117 55% in Palestine,132 51.6% in Nigeria,114 41% in Yemen115 and 10.05% in India.116 In contrast, the results of the current study indicated a slightly lower rate of prescribing antibiotics by their generic names compared to 97.6% and 98.83% reported in two different studies in Ethiopia46,47 and 98.36% in Cameroon.120 Using brand names for medicines can have various consequences, including the potential for medication errors due to the similarity and resemblance of some brand names.
Our study also revealed that 98% of the medicines prescribed came from the current ZEML, which is close to the 100% that is recommended by the WHO. This finding was comparable to those found in earlier research, in which the percentage of medicines given from the national EML was 99.87% in Cameroon,120 98.1% in Zambia,79 98% from the Gaza Strip in Palestine,132 97.6% in Tanzania99 and 97.5% in Ethiopia.100 However, the rate of prescribing antibiotics from the national EML that was found in our study exceeded the results seen in other studies that were similar to it. For example, the prescribing of medicines from the national EML was reported to be 96.1% in Botswana,117 84.8% in India116 and 44% in Yemen.115 Intriguingly, research carried out in Ethiopia and Jordan discovered that all (100%) of the antibiotics prescribed were from the respective national EMLs. This finding is in line with the guidelines made by the WHO and suggests that the EML was followed to its fullest extent.46,129
Overall, the study has highlighted potential areas for improvement that can be part of future ASPs, with ASPs now being routinely instigated across Africa to improve future prescribing. These include the necessity for giving antibiotics by injection, which for inpatients can be extended to assess whether there are delays in de-escalating to oral antibiotics. In addition, the need for routinely documenting the rationale for prescribing antibiotics in the first place across the sectors, especially if more than one antibiotic is being prescribed to patients. Lastly, there is a need to routinely assess the quality of prescribing antibiotics based on the recently available AWaRe book in line with suggestions for other African countries.32,33,142,143 For instance, nitrofurantoin (0.3%) was rarely prescribed for patients with UTIs, despite it being highly recommended for the treatment of UTIs in Zambia and as an alternative antibiotic in the AWaRe book. In the first instance, this can include prescribing targets for percentage adherence to current guidance, with these targets regularly monitored and discussed.142,143
The results of this study highlighted the importance of designing educational interventional activities specifically geared towards the prudent prescription of antibiotics. Educational interventions and positive behavioural change regarding the prescribing of antibiotics are critical in addressing AMR.53,144–150 In addition, our findings point to the necessity of developing and putting into practice measures that encourage the rational prescribing and use of antibiotics, which is consistent with the findings of other research.1,24,25,151–153 In addition, there is a need to promote hospital-based ASPs to assist prescribers in adhering to treatment guidelines and prescribing antibiotics for the appropriate patient, at the appropriate time, in the appropriate dose, for the appropriate duration, via the appropriate route of administration, and for the appropriate indication, as was reported in other studies.60,154–160
We are aware that there are some limitations to our research. Firstly, we only undertook this post-COVID-19 study in one hospital. Secondly, there are always limitations with retrospective studies depending on the details contained within patients’ medical files. Despite these limitations, we believe the findings are noteworthy and provide important insights that can guide potential changes in policy and practice at this facility and others in Zambia to achieve the AMR goals within the Zambian NAP.
Conclusions
This study found a higher than average number of medicines prescribed per patient encounter. Additionally, antibiotics were prescribed in 72.3% of encounters, of which 28.4% were injectable. The most frequently prescribed antibiotics were amoxicillin (23.4%—access), metronidazole (17.1%—access), ciprofloxacin (8%—watch) and ceftriaxone (7.4%—watch), with 77.1% overall from the ‘access’ list. Slight deviations were observed in the prescribing of antibiotics by generic names and from the national EML. Therefore, potential targets for ASPs in this hospital include assessing current prescribing against agreed guidance in the AWaRe book, reducing the need for antibiotics to be given by injection where pertinent, and reducing the extent of multiple antibiotics given to patients without assessing the need through culture and sensitivity testing. There is also a need to follow this up in future studies with the implications for reducing AMR in Zambia in line with the goals of the NAP.
Supplementary Material
Acknowledgements
We are grateful to the leadership and management at Saint Francis’ Mission Central Hospital for allowing us to conduct this study at their institution.
Contributor Information
Steward Mudenda, Department of Pharmacy, School of Health Sciences, University of Zambia, P.O. Box 50110, Lusaka, Zambia.
Robert Chilimboyi, Department of Pharmacy, School of Health Sciences, University of Zambia, P.O. Box 50110, Lusaka, Zambia; Department of Pharmacy, Saint Francis’ Hospital, Private Bag 11, Katete, Zambia.
Scott Kaba Matafwali, Clinical Research Department, Faculty of Infectious and Tropical Diseases, London School of Hygiene & Tropical Medicine, Keppel Street, London WC1E 7HT, UK.
Victor Daka, Department of Public Health, Michael Chilufya Sata School of Medicine, Copperbelt University, P.O. Box 71191, Ndola, Zambia.
Ruth Lindizyani Mfune, Department of Public Health, Michael Chilufya Sata School of Medicine, Copperbelt University, P.O. Box 71191, Ndola, Zambia.
Loriane Arielle Mobou Kemgne, Faculty of Health Sciences of Cotonou, University of Abomey-Calavi, Cotonou, Benin.
Flavien Nsoni Bumbangi, Department of Medicine and Clinical Sciences, School of Medicine, Eden University, P.O. Box 30226, Lusaka, Zambia.
Jimmy Hangoma, Department of Pharmacy, School of Health Sciences, Levy Mwanawasa Medical University, Lusaka, Zambia.
Billy Chabalenge, Department of Medicines Control, Zambia Medicines Regulatory Authority, P.O. Box 31890, Lusaka, Zambia.
Larry Mweetwa, Department of Science and Technology, Ministry of Technology and Science, Maxwell House, Los Angeles Boulevard, P. O. Box 50464, Lusaka, Zambia.
Brian Godman, Department of Public Health Pharmacy and Management, School of Pharmacy, Sefako Makgatho Health Sciences University, Ga-Rankuwa 0208, South Africa; Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow G4 0RE, UK.
Funding
This study was supported by internal funding by the authors and is a part of our routine studies in AMR and antimicrobial stewardship.
Transparency declarations
The authors have no relevant conflicts of interest to declare. All the authors do not have any financial interests or connections that may directly or indirectly raise concerns of bias in the work reported or the conclusions, implications or opinions made in this publication.
Supplementary data
The data collection tool (prescribing indicator form) is available as Supplementary data at JAC-AMR Online.
References
- 1. Sartelli M, Barie PS, Coccolini F et al. Ten golden rules for optimal antibiotic use in hospital settings: the WARNING call to action. World J Emerg Surg 2023; 18: 50. 10.1186/s13017-023-00518-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adedeji WA. The treasure called antibiotics. Ann Ibadan Postgrad Med 2016; 14: 56–7. [PMC free article] [PubMed] [Google Scholar]
- 3. Spellberg B. The future of antibiotics. Crit Care 2014; 18: 228. 10.1186/cc13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yimenu DK, Emam A, Elemineh E et al. Assessment of antibiotic prescribing patterns at outpatient pharmacy using World Health Organization prescribing indicators. J Prim Care Community Health 2019; 10: 2150132719886942. 10.1177/2150132719886942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Browne AJ, Chipeta MG, Haines-Woodhouse G et al. Global antibiotic consumption and usage in humans, 2000–18: a spatial modelling study. Lancet Planet Health 2021; 5: e893–904. 10.1016/S2542-5196(21)00280-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klein EY, Milkowska-Shibata M, Tseng KK et al. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: an analysis of pharmaceutical sales data. Lancet Infect Dis 2021; 21: 107–15. 10.1016/S1473-3099(20)30332-7 [DOI] [PubMed] [Google Scholar]
- 7. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf 2014; 5: 229–41. 10.1177/2042098614554919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Godman B, Haque M, McKimm J et al. Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: findings and implications for the future. Curr Med Res Opin 2020; 36: 301–27. 10.1080/03007995.2019.1700947 [DOI] [PubMed] [Google Scholar]
- 9. Ikuta KS, Swetschinski LR, Robles Aguilar G et al. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the global burden of disease study 2019. Lancet 2022; 400: 2221–48. 10.1016/S0140-6736(22)02185-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murray CJ, Ikuta KS, Sharara F et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–55. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sono TM, Yeika E, Cook A et al. Current rates of purchasing of antibiotics without a prescription across sub-Saharan Africa; rationale and potential programmes to reduce inappropriate dispensing and resistance. Expert Rev Anti Infect Ther 2023; 21: 1025–55. 10.1080/14787210.2023.2259106 [DOI] [PubMed] [Google Scholar]
- 12. Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr 2016; 4: 10.1128/microbiolspec.VMBF-0016–2015. 10.1128/microbiolspec.VMBF-0016-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist 2019; 12: 3903–10. 10.2147/IDR.S234610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. The review on Antimicrobial Resistance. 2016. https://amr-review.org/sites/default/files/160518_Final paper_with cover.pdf.
- 15. Cassini A, Högberg LD, Plachouras D et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: a population-level modelling analysis. Lancet Infect Dis 2019; 19: 56–66. 10.1016/S1473-3099(18)30605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hofer U. The cost of antimicrobial resistance. Nat Rev Microbiol 2019; 17: 3. 10.1038/s41579-018-0125-x [DOI] [PubMed] [Google Scholar]
- 17. Viltsaniuk O, Goliuk A, Sidorova I et al. Antibiotic resistance in medical practice: causes and ways to overcome it in Ukraine. J Periop Med 2023; 6: 4–13. 10.31636/prmd.v6i2.1 [DOI] [Google Scholar]
- 18. Gautam A. Antimicrobial resistance: the next probable pandemic. J Nepal Med Assoc 2022; 60: 225–8. 10.31729/jnma.7174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moyo P, Moyo E, Mangoya D et al. Prevention of antimicrobial resistance in sub-Saharan Africa: what has worked? What still needs to be done? J Infect Public Health 2023; 16: 632–9. 10.1016/j.jiph.2023.02.020 [DOI] [PubMed] [Google Scholar]
- 20. Kariuki S, Kering K, Wairimu C et al. Antimicrobial resistance rates and surveillance in sub-Saharan Africa: where are we now? Infect Drug Resist 2022; 15: 3589–609. 10.2147/IDR.S342753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sartorius B, Gray AP, Davis Weaver N et al. The burden of bacterial antimicrobial resistance in the WHO African region in 2019: a cross-country systematic analysis. Lancet Glob Health 2023; 12: e201–16. 10.1016/S2214-109X(23)00539-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tompkins K, Juliano JJ, van Duin D. Antimicrobial resistance in Enterobacterales and its contribution to sepsis in sub-Saharan Africa. Front Med 2021; 8: 615649. 10.3389/fmed.2021.615649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. WHO . Global action plan on antimicrobial resistance. 2015. https://apps.who.int/iris/handle/10665/193736.
- 24. Saleem Z, Godman B, Cook A et al. Ongoing efforts to improve antimicrobial utilization in hospitals among African countries and implications for the future. Antibiotics 2022; 11: 1824. 10.3390/antibiotics11121824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mudenda S, Chabalenge B, Daka V et al. Global strategies to combat antimicrobial resistance: a one health perspective. Pharmacol Pharm 2023; 14: 271–328. 10.4236/pp.2023.148020 [DOI] [Google Scholar]
- 26. Godman B, Egwuenu A, Wesangula E et al. Tackling antimicrobial resistance across sub-Saharan Africa: current challenges and implications for the future. Expert Opin Drug Saf 2022; 21: 1089–111. 10.1080/14740338.2022.2106368 [DOI] [PubMed] [Google Scholar]
- 27. Iwu CD, Patrick SM. An insight into the implementation of the global action plan on antimicrobial resistance in the WHO African region: a roadmap for action. Int J Antimicrob Agents 2021; 58: 106411. 10.1016/j.ijantimicag.2021.106411 [DOI] [PubMed] [Google Scholar]
- 28. Charani E, Mendelson M, Pallett SJC et al. An analysis of existing national action plans for antimicrobial resistance—gaps and opportunities in strategies optimising antibiotic use in human populations. Lancet Glob Health 2023; 11: e466–74. 10.1016/S2214-109X(23)00019-0 [DOI] [PubMed] [Google Scholar]
- 29. Lota MMM, Chua AQ, Azupardo K et al. A qualitative study on the design and implementation of the national action plan on antimicrobial resistance in the Philippines. Antibiotics 2022; 11: 820. 10.3390/antibiotics11060820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chua AQ, Verma M, Hsu LY et al. An analysis of national action plans on antimicrobial resistance in Southeast Asia using a governance framework approach. Lancet Reg Health West Pac 2021; 7: 100084. 10.1016/j.lanwpc.2020.100084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Munkholm L, Rubin O. The global governance of antimicrobial resistance: a cross-country study of alignment between the global action plan and national action plans. Global Health 2020; 16: 109. 10.1186/s12992-020-00639-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zanichelli V, Sharland M, Cappello B et al. The WHO AWaRe (access, watch, reserve) antibiotic book and prevention of antimicrobial resistance. Bull World Health Organ 2023; 101: 290–6. 10.2471/BLT.22.288614 [DOI] [Google Scholar]
- 33. Sharland M, Zanichelli V, Ombajo LA et al. The WHO essential medicines list AWaRe book: from a list to a quality improvement system. Clin Microbiol Infect 2022; 28: 1533–5. 10.1016/j.cmi.2022.08.009 [DOI] [PubMed] [Google Scholar]
- 34. Sharland M, Pulcini C, Harbarth S et al. Classifying antibiotics in the WHO essential medicines list for optimal use—be AWaRe. Lancet Infect Dis 2018; 18: 18–20. 10.1016/S1473-3099(17)30724-7 [DOI] [PubMed] [Google Scholar]
- 35. Mudenda S, Daka V, Matafwali SK. World Health Organization AWaRe framework for antibiotic stewardship: where are we now and where do we need to go? An expert viewpoint. Antimicrob Steward Healthc Epidemiol 2023; 3: e84. 10.1017/ash.2023.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsia Y, Lee BR, Versporten A et al. Use of the WHO access, watch, and reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. Lancet Glob Health 2019; 7: e861–71. 10.1016/S2214-109X(19)30071-3 [DOI] [PubMed] [Google Scholar]
- 37. Sharland M, Gandra S, Huttner B et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—the new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect Dis 2019; 19: 1278–80. 10.1016/S1473-3099(19)30532-8 [DOI] [PubMed] [Google Scholar]
- 38. Sulis G, Sayood S, Katukoori S et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multi-drug resistant bacteria: a systematic review and meta-analysis. Clin Microbiol Infect 2022; 28: 1193–202. 10.1016/j.cmi.2022.03.014 [DOI] [PubMed] [Google Scholar]
- 39. Kalungia AC, Mukosha M, Mwila C et al. Antibiotic use and stewardship indicators in the first- and second-level hospitals in Zambia: findings and implications for the future. Antibiotics 2022; 11: 1626. 10.3390/antibiotics11111626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. D’Arcy N, Ashiru-Oredope D, Olaoye O et al. Antibiotic prescribing patterns in Ghana, Uganda, Zambia and Tanzania hospitals: results from the global point prevalence survey (G-PPS) on antimicrobial use and stewardship interventions implemented. Antibiotics 2021; 10: 1122. 10.3390/antibiotics10091122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abdelsalam Elshenawy R, Umaru N, Aslanpour Z. WHO AWaRe classification for antibiotic stewardship: tackling antimicrobial resistance—a descriptive study from an English NHS foundation trust prior to and during the COVID-19 pandemic. Front Microbiol 2023; 14: 1298858. 10.3389/fmicb.2023.1298858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. WHO . 2021 AWaRe Classification. 2021. https://www.who.int/publications/i/item/2021-aware-classification.
- 43. WHO . AWaRe classification of antibiotics for evaluation and monitoring of use, 2023. 2023. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04.
- 44. Ofori-Asenso R, Brhlikova P, Pollock AM. Prescribing indicators at primary health care centers within the WHO African region: a systematic analysis (1995–2015). BMC Public Health 2016; 16: 724. 10.1186/s12889-016-3428-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Niaz Q, Godman B, Massele A et al. Validity of World Health Organisation prescribing indicators in Namibia’s primary healthcare: findings and implications. Int J Qual Health Care 2019; 31: 338–45. 10.1093/intqhc/mzy172 [DOI] [PubMed] [Google Scholar]
- 46. Demoz GT, Kasahun GG, Hagazy K et al. Prescribing pattern of antibiotics using who prescribing indicators among inpatients in Ethiopia: a need for antibiotic stewardship program. Infect Drug Resist 2020; 13: 2783–94. 10.2147/IDR.S262104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Amaha ND, Weldemariam DG, Abdu N et al. Prescribing practices using WHO prescribing indicators and factors associated with antibiotic prescribing in six community pharmacies in Asmara, Eritrea: a cross-sectional study. Antimicrob Resist Infect Control 2019; 8: 163. 10.1186/s13756-019-0620-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Masich AM, Vega AD, Callahan P et al. Antimicrobial usage at a large teaching hospital in Lusaka, Zambia. PLoS One 2020; 15: e0228555. 10.1371/journal.pone.0228555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ogunleye OO, Oyawole MR, Odunuga PT et al. A multicentre point prevalence study of antibiotics utilization in hospitalized patients in an urban secondary and a tertiary healthcare facilities in Nigeria: findings and implications. Expert Rev Anti Infect Ther 2022; 20: 297–306. 10.1080/14787210.2021.1941870 [DOI] [PubMed] [Google Scholar]
- 50. Fadare JO, Ogunleye O, Iliyasu G et al. Status of antimicrobial stewardship programmes in Nigerian tertiary healthcare facilities: findings and implications. J Glob Antimicrob Resist 2019; 17: 132–6. 10.1016/j.jgar.2018.11.025 [DOI] [PubMed] [Google Scholar]
- 51. Nabidda S, Ssennyonjo R, Atwaru J et al. Antimicrobial resistance and rational prescription practices: knowledge, perceptions and confidence of health profession interns in Uganda. JAC Antimicrobial Resist 2023; 5: dlad105. 10.1093/jacamr/dlad105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sefah IA, Chetty S, Yamoah P et al. A multicenter cross-sectional survey of knowledge, attitude, and practices of healthcare professionals towards antimicrobial stewardship in Ghana: findings and implications. Antibiotics 2023; 12: 1497. 10.3390/antibiotics12101497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fuller W, Kapona O, Aboderin AO et al. Education and awareness on antimicrobial resistance in the WHO African region: a systematic review. Antibiotics 2023; 12: 1613. 10.3390/antibiotics12111613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ashiru-Oredope D, Garraghan F, Olaoye O et al. Development and implementation of an antimicrobial stewardship checklist in sub-Saharan Africa: a co-creation consensus approach. Healthcare 2022; 10: 1706. 10.3390/healthcare10091706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nathwani D, Varghese D, Stephens J et al. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control 2019; 8: 35. 10.1186/s13756-019-0471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Khadse SN, Ugemuge S, Singh C. Impact of antimicrobial stewardship on reducing antimicrobial resistance. Cureus 2023; 15: e49935. 10.7759/cureus.49935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cox JA, Vlieghe E, Mendelson M et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect 2017; 23: 812–8. 10.1016/j.cmi.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 58. Ashiru-Oredope D, Nabiryo M, Zengeni L et al. Tackling antimicrobial resistance: developing and implementing antimicrobial stewardship interventions in four African commonwealth countries through a health partnership model. J Public Health Africa 2023; 14: 2335. 10.4081/jphia.2023.2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Siachalinga L, Mufwambi W, Lee I-H. Impact of antimicrobial stewardship interventions to improve antibiotic prescribing for hospital inpatients in Africa: a systematic review and meta-analysis. J Hosp Infect 2022; 129: 124–43. 10.1016/j.jhin.2022.07.031 [DOI] [PubMed] [Google Scholar]
- 60. Otieno PA, Campbell S, Maley S et al. A systematic review of pharmacist-led antimicrobial stewardship programs in Sub-Saharan Africa. Int J Clin Pract 2022; 2022: 3639943. 10.1155/2022/3639943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Akpan MR, Isemin NU, Udoh AE et al. Implementation of antimicrobial stewardship programmes in African countries: a systematic literature review. J Glob Antimicrob Resist 2020; 22: 317–24. 10.1016/j.jgar.2020.03.009 [DOI] [PubMed] [Google Scholar]
- 62. Chukwu EE, Oladele DA, Enwuru CA et al. Antimicrobial resistance awareness and antibiotic prescribing behavior among healthcare workers in Nigeria: a national survey. BMC Infect Dis 2021; 21: 22. 10.1186/s12879-020-05689-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tembo N, Mudenda S, Banda M et al. Knowledge, attitudes and practices on antimicrobial resistance among pharmacy personnel and nurses at a tertiary hospital in Ndola, Zambia: implications for antimicrobial stewardship programmes. JAC-Antimicrobial Resist 2022; 4: dlac107. 10.1093/jacamr/dlac107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mudenda S, Chomba M, Chabalenge B et al. Antibiotic prescribing patterns in adult patients according to the WHO AWaRe classification: a multi-facility cross-sectional study in primary healthcare hospitals in Lusaka, Zambia. Pharmacol Pharm 2022; 13: 379–92. 10.4236/pp.2022.1310029 [DOI] [Google Scholar]
- 65. Kalonga J, Hangoma J, Banda M et al. Antibiotic prescribing patterns in paediatric patients at Levy Mwanawasa University Teaching Hospital in Lusaka, Zambia. Int J Pharm Pharmacol 2020; 4: 138. 10.31531/2581-3080.1000138 [DOI] [Google Scholar]
- 66. Duffy E, Ritchie S, Metcalfe S et al. Antibacterials dispensed in the community comprise 85%–95% of total human antibacterial consumption. J Clin Pharm Ther 2018; 43: 59–64. 10.1111/jcpt.12610 [DOI] [PubMed] [Google Scholar]
- 67. Kamara IF, Kumar AMV, Maruta A et al. Antibiotic use in suspected and confirmed COVID-19 patients admitted to health facilities in Sierra Leone in 2020–2021: practice does not follow policy. Int J Environ Res Public Health 2022; 19: 4005. 10.3390/ijerph19074005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yang Z, Bou-Antoun S, Gerver S et al. Sustained increases in antibiotic prescriptions per primary care consultation for upper respiratory tract infections in England during the COVID-19 pandemic. JAC Antimicrobial Resist 2023; 5: dlad012. 10.1093/jacamr/dlad012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fukushige M, Ngo NH, Lukmanto D et al. Effect of the COVID-19 pandemic on antibiotic consumption: a systematic review comparing 2019 and 2020 data. Front Public Health 2022; 10: 946077. 10.3389/fpubh.2022.946077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rabbi F, Banfield L, Munir M et al. Overprescription of antibiotics for treating hospitalized COVID-19 patients: a systematic review & meta-analysis. Heliyon 2023; 9: e20563. 10.1016/j.heliyon.2023.e20563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Alshaikh FS, Godman B, Sindi ON et al. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: a systematic review and meta-analysis. PLoS One 2022; 17: e0272375. 10.1371/journal.pone.0272375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ramzan K, Shafiq S, Raees I et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalized with COVID-19 during the first five waves of the pandemic in Pakistan; findings and implications. Antibiotics 2022; 11: 789. 10.3390/antibiotics11060789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Olamijuwon E, Konje E, Kansiime C et al. Antibiotic dispensing practices during COVID-19 and implications for antimicrobial resistance (AMR): parallel mystery client studies in Uganda and Tanzania. Antimicrob Resist Infect Control 2023; 12: 10. 10.1186/s13756-022-01199-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mudenda S, Nsofu E, Chisha P et al. Prescribing patterns of antibiotics according to the WHO AWaRe classification during the COVID-19 pandemic at a teaching hospital in Lusaka, Zambia: implications for strengthening of antimicrobial stewardship programmes. Pharmacoepidemiology 2023; 2: 42–53. 10.3390/pharma2010005 [DOI] [Google Scholar]
- 75. Bednarčuk N, Golić Jelić A, Stoisavljević Šatara S et al. Antibiotic utilization during COVID-19: are we over-prescribing? Antibiotics 2023; 12: 308. 10.3390/antibiotics12020308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sefah IA, Ogunleye OO, Essah DO et al. Rapid assessment of the potential paucity and price increases for suggested medicines and protection equipment for COVID-19 across developing countries with a particular focus on Africa and the implications. Front Pharmacol 2021; 11: 588106. 10.3389/fphar.2020.588106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Adebisi YA, Jimoh ND, Ogunkola IO et al. The use of antibiotics in COVID-19 management: a rapid review of national treatment guidelines in 10 African countries. Trop Med Health 2021; 49: 51. 10.1186/s41182-021-00344-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dutta S, Kaur R, Bhardwaj P et al. Demand of COVID-19 medicines without prescription among community pharmacies in Jodhpur, India: findings and implications. J Fam Med Prim Care 2022; 11: 503–11. 10.4103/jfmpc.jfmpc_1250_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mudenda W, Chikatula E, Chambula E et al. Prescribing patterns and medicine use at the university teaching hospital, Lusaka, Zambia. Med J Zambia 2016; 43: 94–102. 10.55320/mjz.43.2.344 [DOI] [Google Scholar]
- 80. Versporten A, Zarb P, Caniaux I et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health 2018; 6: e619–29. 10.1016/S2214-109X(18)30186-4 [DOI] [PubMed] [Google Scholar]
- 81. Niaz Q, Godman B, Campbell S et al. Compliance to prescribing guidelines among public health care facilities in Namibia; findings and implications. Int J Clin Pharm 2020; 42: 1227–36. 10.1007/s11096-020-01056-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Owusu H, Thekkur P, Ashubwe-Jalemba J et al. Compliance to guidelines in prescribing empirical antibiotics for individuals with uncomplicated urinary tract infection in a primary health facility of Ghana, 2019–2021. Int J Environ Res Public Health 2022; 19: 12413. 10.3390/ijerph191912413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Govender T, Suleman F, Perumal-Pillay VA. Evaluating the implementation of the standard treatment guidelines (STGs) and essential medicines list (EML) at a public South African tertiary institution and its associated primary health care (PHC) facilities. J Pharm Policy Pract 2021; 14: 105. 10.1186/s40545-021-00390-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Campbell SM, Meyer JC, Godman B. Why compliance to national prescribing guidelines is important especially across sub-Saharan Africa and suggestions for the future. J Biomed Pharm Sci 2021; 4: 316. [Google Scholar]
- 85. Wiedenmayer K, Ombaka E, Kabudi B et al. Adherence to standard treatment guidelines among prescribers in primary healthcare facilities in the Dodoma region of Tanzania. BMC Health Serv Res 2021; 21: 272. 10.1186/s12913-021-06257-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Darkwah TO, Afriyie DK, Sneddon J et al. Assessment of prescribing patterns of antibiotics using national treatment guidelines and World Health Organization prescribing indicators at the Ghana police hospital: a pilot study. Pan Afr Med J 2021; 39: 222. 10.11604/pamj.2021.39.222.29569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Government of the Republic of Zambia . Multi-sectoral National Action Plan on Antimicrobial Resistance, 2017 – 2027. 2017. https://www.afro.who.int/publications/multi-sectoral-national-action-plan-antimicrobial-resistance-2017-2027.
- 88. Kapona O. Zambia successfully launches the first multi-sectoral national action plan on antimicrobial resistance (AMR). Health Press Zambia Bull 2017; 1: 5–7. https://www.flemingfund.org/app/uploads/ec74b8a828168c148bcba3700ace7989.pdf. [Google Scholar]
- 89. International Monetary Fund . Zambia: selected issues. IMF Staff Country Reports 2023; 2023: 1–48. 10.5089/9798400249839.002 [DOI] [Google Scholar]
- 90. Benson AE, Benson MJ, Luke AH. Assessment of maternal referral systems used for a rural Zambian hospital: the development of setting specific protocols for the identification of complications. Afr Health Sci 2019; 19: 1536–43. 10.4314/ahs.v19i1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Songiso M, Pinder LF, Munalula J et al. Minimizing delays in the breast cancer pathway by integrating breast specialty care services at the primary health care level in Zambia. JCO Glob Oncol 2020; 6: 859–65. 10.1200/GO.20.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Atkinson S, Ngwengwe A, Macwan’gi M et al. The referral process and urban health care in sub-Saharan Africa: the case of Lusaka, Zambia. Soc Sci Med 1999; 49: 27–38. 10.1016/S0277-9536(99)00072-6 [DOI] [PubMed] [Google Scholar]
- 93. Saint Francis’ Hospital . Saint Francis’ Hospital, Katete, Zambia—about SFH. 2023. http://www.saintfrancishospital.net/about-1/index.html.
- 94. The Zambia Society Trust . St Francis Hospital, Katete AIDS Orphans Project—Project Update. 2023. https://zambiasocietytrust.org.uk/blog/st-francis-hospital-katete-aids-orphans-project-project-update.
- 95. Kamukwamba AL. St Francis’ Mission Hospital—2015/2016 Annual Report. 2016. https://www.supportstfrancishospital.org/wp-content/uploads/2016/11/2015-2016-Annual-Report.pdf.
- 96. WHO . How to investigate drug use in health facilities: selected drug use indicators. 1993. https://www.who.int/publications/i/item/who-dap-93.1.
- 97. Ghei P. How to investigate drug use in health facilities. Selected drug use indicators. Health Policy 1995; 34: 73–5. 10.1016/0168-8510(95)90068-3 [DOI] [Google Scholar]
- 98. SIAPS . How to Investigate Antimicrobial Use in Hospitals: Selected Indicators. 2012. https://siapsprogram.org/publication/how-to-investigate-antimicrobial-use-in-hospitals-selected-indicators/.
- 99. Kilipamwambu A, Bwire GM, Myemba DT et al. WHO/INRUD core prescribing indicators and antibiotic utilization patterns among primary health care facilities in Ilala district, Tanzania. JAC Antimicrobial Resist 2021; 3: dlab049. 10.1093/jacamr/dlab049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tassew SG, Abraha HN, Gidey K et al. Assessment of drug use pattern using WHO core drug use indicators in selected general hospitals: a cross-sectional study in Tigray region, Ethiopia. BMJ Open 2021; 11: e045805. 10.1136/bmjopen-2020-045805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ministry of Health . Zambia Essential Medicines List (ZEML). 2020. https://www.moh.gov.zm/?wpfb_dl=39.
- 102. Republic of Zambia, Ministry of Health . Zambia Standard Treatment Guidelines 2020. 2020. https://www.moh.gov.zm/? wpfb_dl=32.
- 103. Godman B, Fadare J, Kwon HY et al. Evidence-based public policy making for medicines across countries: findings and implications for the future. J Comp Eff Res 2021; 10: 1019–52. 10.2217/cer-2020-0273 [DOI] [PubMed] [Google Scholar]
- 104. Cameron A, Mantel-Teeuwisse AK, Leufkens HGM et al. Switching from originator brand medicines to generic equivalents in selected developing countries: how much could be saved? Value Health 2012; 15: 664–73. 10.1016/j.jval.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 105. Fadare JO, Adeoti AO, Desalu OO et al. The prescribing of generic medicines in Nigeria: knowledge, perceptions and attitudes of physicians. Expert Rev Pharmacoeconomics Outcomes Res 2016; 16: 639–50. 10.1586/14737167.2016.1120673 [DOI] [PubMed] [Google Scholar]
- 106. Bekoe SO, Ahiabu MA, Orman E et al. Exposure of consumers to substandard antibiotics from selected authorised and unauthorised medicine sales outlets in Ghana. Trop Med Int Health 2020; 25: 962–75. 10.1111/tmi.13442 [DOI] [PubMed] [Google Scholar]
- 107. Osei YA, Oppong Boakye E, Bayor MT et al. Physicochemical equivalence and quality assessment of various brands of gastro-resistant omeprazole capsules in the Kumasi metropolis. Sci World J 2022; 2022: 7924600. 10.1155/2022/7924600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tadesse TY, Molla M, Yimer YS et al. Evaluation of antibiotic prescribing patterns among inpatients using World Health Organization indicators: a cross-sectional study. SAGE Open Med 2022; 10: 205031212210966. 10.1177/20503121221096608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bilal AI, Osman ED, Mulugeta A. Assessment of medicines use pattern using World Health Organization’s prescribing, patient care and health facility indicators in selected health facilities in eastern Ethiopia. BMC Health Serv Res 2016; 16: 144. 10.1186/s12913-016-1414-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kallen MC, Prins JM. A systematic review of quality indicators for appropriate antibiotic use in hospitalized adult patients. Infect Dis Rep 2017; 9: 13–7. 10.4081/idr.2017.6821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Soleymani F, Godman B, Yarimanesh P et al. Prescribing patterns of physicians working in both the direct and indirect treatment sectors in Iran; findings and implications. J Pharm Health Serv Res 2019; 10: 407–13. 10.1111/jphs.12322 [DOI] [Google Scholar]
- 112. GolAli E, Sistanizad M, Salamzadeh J et al. Antibiotic prescribing trends before and after implementation of an audit and feedback program in internal ward of a tertiary hospital in Tehran. Iran J Pharm Res 2019; 18: 2136–43. 10.22037/ijpr.2019.1100833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Khdour MR, Hallak HO, Aldeyab MA et al. Impact of antimicrobial stewardship programme on hospitalized patients at the intensive care unit: a prospective audit and feedback study. Br J Clin Pharmacol 2018; 84: 708–15. 10.1111/bcp.13486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Okoye BI, Udemba JC, Ndugba CA et al. Evaluation of rational prescribing in a hospital paediatric outpatient clinic in Nigeria. BMJ Paediatr Open 2022; 6: e001585. 10.1136/bmjpo-2022-001585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Alshakka M, Said K, Babakri M et al. A study on antibiotics prescribing pattern at outpatient department in four hospitals in Aden-Yemen. J Pharm Pract Community Med 2016; 2: 88–93. 10.5530/jppcm.2016.3.5 [DOI] [Google Scholar]
- 116. Hussain S, Yadav SS, Sawlani KK et al. Assessment of drug prescribing pattern using World Health Organization indicators in a tertiary care teaching hospital. Indian J Public Health 2018; 62: 156–8. 10.4103/ijph.IJPH_429_16 [DOI] [PubMed] [Google Scholar]
- 117. Mashalla Y, Setlhare V, Massele A et al. Assessment of prescribing practices at the primary healthcare facilities in Botswana with an emphasis on antibiotics: findings and implications. Int J Clin Pract 2017; 71: e13042. 10.1111/ijcp.13042 [DOI] [PubMed] [Google Scholar]
- 118. Otim ME, Demaya DK, Al Marzouqi A et al. Are antibiotics prescribed to inpatients according to recommended standard guidelines in South Sudan? A retrospective cross-sectional study in Juba Teaching Hospital. J Multidiscip Healthc 2021; 14: 2871–9. 10.2147/JMDH.S321990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mugada V, Mahato V, Andhavaram D et al. Evaluation of prescribing patterns of antibiotics using selected indicators for antimicrobial use in hospitals and the access, watch, reserve (AWaRe) classification by the World Health Organization. Turkish J Pharm Sci 2021; 18: 282–8. 10.4274/tjps.galenos.2020.11456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chem ED, Anong DN, Akoachere JFKT. Prescribing patterns and associated factors of antibiotic prescription in primary health care facilities of Kumbo East and Kumbo West Health Districts, North West Cameroon. PLoS One 2018; 13: e0193353. 10.1371/journal.pone.0193353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gjelstad S, Straand J, Dalen I et al. Do general practitioners’ consultation rates influence their prescribing patterns of antibiotics for acute respiratory tract infections? J Antimicrob Chemother 2011; 66: 2425–33. 10.1093/jac/dkr295 [DOI] [PubMed] [Google Scholar]
- 122. Bharathiraja R, Sridharan S, Chelliah LR et al. Factors affecting antibiotic prescribing pattern in pediatric practice. Indian J Pediatr 2005; 72: 877–9. 10.1007/BF02731121 [DOI] [PubMed] [Google Scholar]
- 123. Atif M, Azeem M, Sarwar MR et al. WHO/INRUD prescribing indicators and prescribing trends of antibiotics in the accident and emergency department of Bahawal Victoria hospital, Pakistan. Springerplus 2016; 5: 1928. 10.1186/s40064-016-3615-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mambula G, Nanjebe D, Munene A et al. Practices and challenges related to antibiotic use in paediatric treatment in hospitals and health centres in Niger and Uganda: a mixed methods study. Antimicrob Resist Infect Control 2023; 12: 67. 10.1186/s13756-023-01271-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Al-Niemat SI, Aljbouri TM, Goussous LS et al. Antibiotic prescribing patterns in outpatient emergency clinics at Queen Rania Al Abdullah II Children’s Hospital, Jordan, 2013. Oman Med J 2014; 29: 250–4. 10.5001/omj.2014.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Agyare E, Acolatse JEE, Dakorah MP et al. Antimicrobial stewardship capacity and antibiotic utilisation practices in the cape coast teaching hospital, Ghana: a point prevalence survey study. PLoS One 2024; 19: e0297626. 10.1371/journal.pone.0297626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Matee M, Mshana SE, Mtebe M et al. Mapping and gap analysis on antimicrobial resistance surveillance systems in Kenya, Tanzania, Uganda and Zambia. Bull Natl Res Cent 2023; 47: 12. 10.1186/s42269-023-00986-2 [DOI] [Google Scholar]
- 128. Alkhaldi SM, Yaseen NA, Bataineh EA et al. Patterns of antibiotic prescribing and appropriateness for respiratory tract infections in a teaching hospital in Jordan. Int J Clin Pract 2021; 75: e14113. 10.1111/ijcp.14113 [DOI] [PubMed] [Google Scholar]
- 129. Al-Shatnawi SF, AL-Hosban SY, Altawalbeh SM et al. Antibiotic prescribing patterns for childhood infections in ambulatory settings in Jordan. Int J Clin Pract 2021; 75: e14740. 10.1111/ijcp.14740 [DOI] [PubMed] [Google Scholar]
- 130. Boyce MR, Katz R, Standley CJ. Risk factors for infectious diseases in urban environments of sub-Saharan Africa: a systematic review and critical appraisal of evidence. Trop Med Infect Dis 2019; 4: 123. 10.3390/tropicalmed4040123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Loevinsohn G, Hardick J, Sinywimaanzi P et al. Respiratory pathogen diversity and co-infections in rural Zambia. Int J Infect Dis 2021; 102: 291–8. 10.1016/j.ijid.2020.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Alagha HZI, Al Telbani MJ. Investigating antibiotic use in Gaza Strip hospitals: a retrospective cross-sectional analysis. J Infect Dev Ctries 2022; 16: 1739–47. 10.3855/jidc.16985 [DOI] [PubMed] [Google Scholar]
- 133. Negi G, Arjun B, Panda PK. Ground level utility of access, watch, reserve classification: insights from a tertiary care center in north India. World J Exp Med 2023; 13: 123–33. 10.5493/wjem.v13.i5.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ul Mustafa Z, Salman M, Aldeyab M et al. Antimicrobial consumption among hospitalized patients with COVID-19 in Pakistan. SN Compr Clin Med 2021; 3: 1691–5. 10.1007/s42399-021-00966-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Mohamad IN, Wong CKW, Chew CC et al. The landscape of antibiotic usage among COVID-19 patients in the early phase of pandemic: a Malaysian national perspective. J Pharm Policy Pract 2022; 15: 4. 10.1186/s40545-022-00404-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Amponsah OKO, Buabeng KO, Owusu-Ofori A et al. Point prevalence survey of antibiotic consumption across three hospitals in Ghana. JAC Antimicrobial Resist 2021; 3: dlab008. 10.1093/jacamr/dlab008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Labi AK, Obeng-Nkrumah N, Nartey ET et al. Antibiotic use in a tertiary healthcare facility in Ghana: a point prevalence survey. Antimicrob Resist Infect Control 2018; 7: 15. 10.1186/s13756-018-0299-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Peddireddy M, Mahin J, Uppu A et al. Hospital antibiotic prescribing pattern in general surgery specialty: analysis based on the WHO access, watch and reserve (AWaRe) classification. J Pharm Res Int 2021; 33: 7–19. 10.9734/jpri/2021/v33i41B32339 [DOI] [Google Scholar]
- 139. Nunes PHC, Moreira JPL, Thompson AF et al. Antibiotic consumption and deviation of prescribed daily dose from the defined daily dose in critical care patients: a point-prevalence study. Front Pharmacol 2022; 13: 913568. 10.3389/fphar.2022.913568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Thomas AP, Kumar M, Johnson R et al. Evaluation of antibiotic consumption and compliance to hospital antibiotic policy in the surgery, orthopedics and gynecology wards of a tertiary care hospital. Clin Epidemiol Glob Health 2022; 13: 100944. 10.1016/j.cegh.2021.100944 [DOI] [Google Scholar]
- 141. Wieters I, Johnstone S, Makiala-Mandanda S et al. Reported antibiotic use among patients in the multicenter ANDEMIA infectious diseases surveillance study in sub-Saharan Africa. Antimicrob Resist Infect Control 2024; 13: 9. 10.1186/s13756-024-01365-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chigome A, Ramdas N, Skosana P et al. A narrative review of antibiotic prescribing practices in primary care settings in South Africa and potential ways forward to reduce antimicrobial resistance. Antibiotics 2023; 12: 1540. 10.3390/antibiotics12101540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Massele A, Rogers AM, Gabriel D et al. A narrative review of recent antibiotic prescribing practices in ambulatory care in Tanzania: findings and implications. Medicina (B Aires) 2023; 59: 2195. 10.3390/medicina59122195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zheng K, Xie Y, Dan L et al. Effectiveness of educational interventions for health workers on antibiotic prescribing in outpatient settings in China: a systematic review and meta-analysis. Antibiotics 2022; 11: 791. 10.3390/antibiotics11060791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Rocha V, Estrela M, Neto V et al. Educational interventions to reduce prescription and dispensing of antibiotics in primary care: a systematic review of economic impact. Antibiotics 2022; 11: 1186. 10.3390/antibiotics11091186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Shehadeh MB, Suaifan GARY, Hammad EA. Active educational intervention as a tool to improve safe and appropriate use of antibiotics. Saudi Pharm J 2016; 24: 611–5. 10.1016/j.jsps.2015.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kandeel A, Palms DL, Afifi S et al. An educational intervention to promote appropriate antibiotic use for acute respiratory infections in a district in Egypt- pilot study. BMC Public Health 2019; 19: 498. 10.1186/s12889-019-6779-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Nair MM, Mahajan R, Burza S et al. Behavioural interventions to address rational use of antibiotics in outpatient settings of low-income and lower-middle-income countries. Trop Med Int Health 2021; 26: 504–17. 10.1111/tmi.13550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lim JM, Singh SR, Duong MC et al. Impact of national interventions to promote responsible antibiotic use: a systematic review. J Antimicrob Chemother 2020; 75: 14–29. 10.1093/jac/dkz348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kamarudin G, Penm J, Chaar B et al. Educational interventions to improve prescribing competency: a systematic review. BMJ Open 2013; 3: e003291. 10.1136/bmjopen-2013-003291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Godman B, Egwuenu A, Haque M et al. Strategies to improve antimicrobial utilization with a special focus on developing countries. Life 2021; 11: 528. 10.3390/life11060528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Tandan M, Thapa P, Maharjan P et al. Impact of antimicrobial stewardship program on antimicrobial-resistance and prescribing in nursing homes: a systematic review and meta-analysis. J Glob Antimicrob Resist 2022; 29: 74–87. 10.1016/j.jgar.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 153. Sartelli M, Coccolini F, Labricciosa FM et al. Surgical antibiotic prophylaxis: a proposal for a global evidence-based bundle. Antibiotics 2024; 13: 100. 10.3390/antibiotics13010100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Silva JT, Montoro J, Asín MAP-J et al. A joint program of antimicrobial stewardship and hospital-acquired infection control to reduce healthcare-associated infections after kidney transplantation: the Hipomenes study. Am J Transplant 2023; 23: 1949–60. 10.1016/j.ajt.2023.07.009 [DOI] [PubMed] [Google Scholar]
- 155. Sneddon J, Cooper L, Afriyie DK et al. Supporting antimicrobial stewardship in Ghana: evaluation of the impact of training on knowledge and attitudes of healthcare professionals in two hospitals. JAC Antimicrobial Resist 2020; 2: dlaa092. 10.1093/jacamr/dlaa092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev 2005; 18: 638–56. 10.1128/CMR.18.4.638-656.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chetty S, Reddy M, Ramsamy Y et al. Antimicrobial stewardship in public-sector hospitals in KwaZulu-Natal, South Africa. Antibiotics 2022; 11: 881. 10.3390/antibiotics11070881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lakoh S, Bawoh M, Lewis H et al. Establishing an antimicrobial stewardship program in Sierra Leone: a report of the experience of a low-income country in West Africa. Antibiotics 2023; 12: 424. 10.3390/antibiotics12030424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Steinmann KE, Lehnick D, Buettcher M et al. Impact of empowering leadership on antimicrobial stewardship: a single center study in a neonatal and pediatric intensive care unit and a literature review. Front Pediatr 2018; 6: 294. 10.3389/fped.2018.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Campbell A, Borek AJ, McLeod M et al. Impact of the COVID-19 pandemic on antimicrobial stewardship support for general practices in England: a qualitative interview study. BJGP Open 2023; 7: BJGPO.2022.0193. 10.3399/BJGPO.2022.0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.