Abstract
Background.
Recent studies evaluating patients with a positive sentinel lymph node biopsy (SLNB+) show no melanoma-specific survival difference between patients undergoing lymph node basin surveillance and completion lymph node dissection (CLND). This has been broadly applied, despite underrepresentation of head and neck (HN) cutaneous melanoma patients. We evaluated whether this was upheld in the HN melanoma cohort.
Methods.
Patients with HN melanoma with a SLNB+ were selected from the National Cancer Database (NCDB) from 2012 to 2019. Overall survival (OS) of patients who underwent SLNB only versus SLNB + CLND were compared. Subgroup analyses were performed based on pathologic N (pN) and receipt of immunotherapy. Adjusted hazard ratio (aHR) and 95% confidence interval (CI) were calculated.
Results.
Analysis of 634 patients with multivariable Cox regression showed no difference in OS in SLNB only versus SLNB + CLND cohorts (hazard ratio [HR] 1.13; 95% confidence interval [CI] 0.71–1.81; p = 0.610). Charlson–Deyo score (CDS) 1 versus 0 (HR 1.70; 95% CI 1.10–2.63; p = 0.016), pN2+ versus pN1 (HR 1.74; 95% CI 1.23–2.45; p = 0.002), and lymphovascular invasion (LVI) versus no (HR 2.07; 95% CI 1.34–3.19; p = 0.001) were associated with worse prognosis. Subgroup analysis by pN showed no OS benefit for CLND in either pN1 (HR 1.04; 95% CI 0.51–2.10; p = 0.922) or pN2+ (HR 1.31; 95% CI 0.67–2.57; p = 0.427) patients or in patients who received immunotherapy (HR 1.32; 95% CI 0.54–3.22; p = 0.549).
Conclusions.
This study of SLNB + HN melanoma patients showed no OS difference in SLNB only versus SLNB + CLND. Further studies need to be performed to better define the role of CLND.
The majority of melanomas are confined to the skin and have excellent 5-year survival rates of 99%.1 Unfortunately, once melanoma spreads to lymph nodes, survival declines to an average 5-year survival rate of roughly 68%. While there is significant variability within this cohort of patients, ranging from 93% for Stage IIIA patients to 32% for Stage IIID, multiple studies have shown that prognosis is strongly associated with presence of regional nodal disease.1 As such, knowledge of lymph node involvement significantly impacts treatment recommendations and follow-up. Sentinel lymph node biopsy (SLNB) is a staging procedure that allows for identification of lymph node metastasis in patients with clinically node-negative disease.2
For many years, if regional SLNs were involved with metastases, the recommendation was to proceed with completion lymph node dissection (CLND) followed by adjuvant systemic therapy.3,4 Unfortunately, the morbidity of lymph node dissection can be quite high, resulting in lymphedema, neurovascular injury, and neck impairment and the systemic therapies which were available for melanoma only offered limited disease free survival.3–5 In the past decade, the management of SLNB positive patients has dramatically changed. Patients with positive SLNB can either undergo CLND or lymph node basin surveillance with ultrasound. This is based on the results of two large randomized control trials (RCT), the German Dermatologic Cooperative Oncology Group—Selective Lymphadenectomy Trial (DeCOG-SLT) and the Multicenter Selective Lymphadenectomy Trial II (MSLT-II), both showing no survival difference when comparing these surgical approaches in patients with positive SLNs.6,7 Based on these studies, the National Comprehensive Cancer Network (NCCN) guidelines currently include these recommendations for cutaneous melanomas of all primary sites, despite the fact that HN cutaneous melanoma patients were not well represented in these trials.8 In fact, these patients were excluded from DeCOG-SLT and made up only 13.7% of the patients in MSLT-II. Additionally, melanoma of HN origin was the only factor in the multivariable analysis of the MSLT-II trial where there was a trend toward a survival benefit with CLND, although not statistically significant.7 However, given the knowledge that cutaneous melanomas of HN origin are more aggressive and have a worse prognosis compared with those from other primary sites, one questions the utility of CLND in this population.9
From a systemic therapy standpoint, immunotherapy and targeted agents have been adopted as adjuvant treatment, based on several RCTs, which showed recurrence-free survival (RFS) benefit.10–12 However, the majority of these trials were performed before the publication of MSLT-II and DeCOG-SLT and show the effect of treatment on patients who underwent CLND after a positive SLNB but not on patients who underwent nodal basin observation. Conversely, the aforementioned surgical trials were mostly performed before FDA approval of newer immunotherapy treatments. Hence, the impact of new immunotherapy regimens on these patients is unknown. Therefore, we examined a large cohort of HN cutaneous melanoma patients from a national clinical oncology database sourced from hospital registry data to evaluate the role of omission of CLND in patients with positive SLNs, with specific attention to the impact of more advanced nodal disease and immunotherapy on outcomes.
METHODS
Data Source
The National Cancer Database (NCDB) was utilized to perform the analysis. The NCDB is an American College of Surgeons Commission on Cancer and American Cancer Society supported nationwide hospital-based cancer registry, which gathers de-identified data on cancer cases from more than 1500 Commission on Cancer accredited facilities. Institutional Review Board approval was not required for this study, because data in the NCDB are de-identified.
Patient Selection
The study inclusion and exclusion criteria leading to the analytical final sample are outlined in Fig. 1. We included patients from 2012 to 2019 with invasive HN cutaneous melanoma who were aged 18–75 years with clinically negative lymph nodes (N0 disease) who underwent wide local excision (WLE) and SLNB, as was included in both MSLT-II and DeCOG-SLT. We excluded patients with more than one cancer diagnosis, clinically apparent nodal disease, and clinical or pathologic M1 disease. We also excluded patients who did not undergo WLE or SLNB and did not receive treatment at the reporting facility. Of note, even though we included patients through 2019, these patients were not included in our final sample, as the vital status was not reported. We then selected patients who had positive SLNs and stratified patients according to lymph node management, SLNB only, or SLNB + CLND.
FIG. 1.
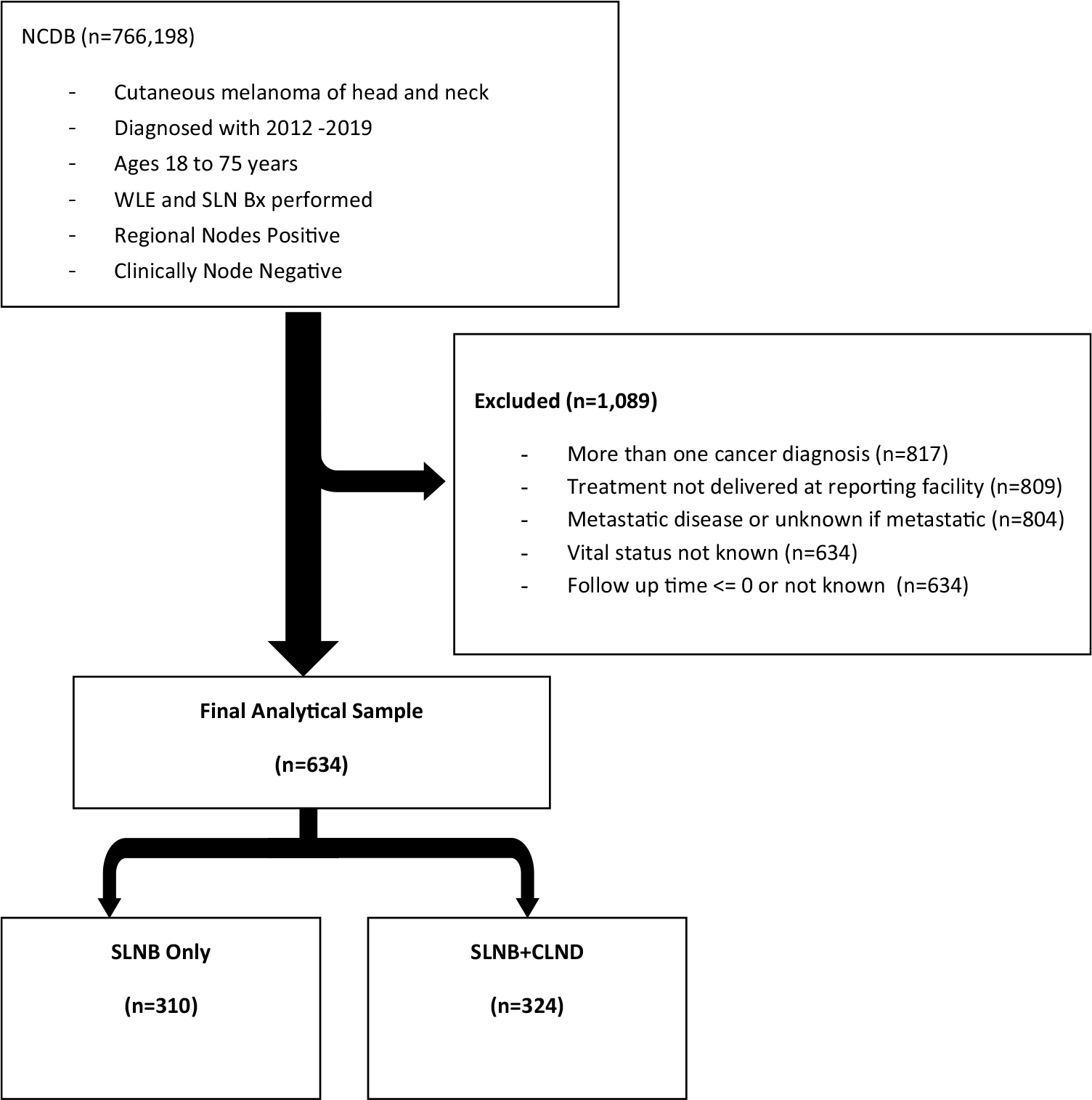
Study flowchart
Demographic and clinicopathologic characteristics were reported for the entire cohort and compared between groups (Table 1). We performed subgroup analysis based on extent of regional lymph node involvement (pN1 vs. pN2+) and receipt of immunotherapy. The NCDB Participant User Files (PUF) 2019 data dictionary describes other variables analyzed.13
TABLE 1.
Patient, tumor, and treatment characteristics
| Variable | All | Cohort | p a | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| SLNB only | SLNB+CLND | ||||||
|
|
|
|
|||||
| N | % | N | % | N | % | ||
|
| |||||||
| All | 634 | 100.0 | 310 | 100.0 | 324 | 100.0 | |
| Age at diagnosis (years) | |||||||
| 18–44 | 144 | 22.7 | 61 | 19.7 | 83 | 25.6 | 0.011 |
| 45–64 | 274 | 43.2 | 126 | 40.6 | 148 | 45.7 | |
| 65+ | 216 | 34.1 | 123 | 39.7 | 93 | 28.7 | |
| Sex | |||||||
| Female | 173 | 27.3 | 88 | 28.4 | 85 | 26.2 | 0.533 |
| Male | 461 | 72.7 | 222 | 71.6 | 239 | 73.8 | |
| Race | |||||||
| White | 622 | 98.1 | 301 | 97.1 | 321 | 99.1 | 0.028 |
| Black/Asian/Other | 11 | 1.7 | 9 | 2.9 | 2 | 0.6 | |
| Unknown | 1 | 0.2 | – | – | 1 | 0.3 | |
| Insurance | |||||||
| Medicaid | 33 | 5.2 | 18 | 5.8 | 15 | 4.6 | 0.008 |
| Medicare | 196 | 30.9 | 111 | 35.8 | 85 | 26.2 | |
| Other government | 19 | 3.0 | 13 | 4.2 | 6 | 1.9 | |
| Not insured | 20 | 3.2 | 10 | 3.2 | 10 | 3.1 | |
| Private | 356 | 56.2 | 151 | 48.7 | 205 | 63.3 | |
| Unknown | 10 | 1.6 | 7 | 2.3 | 3 | 0.9 | |
| Median income | |||||||
| < $40,227 | 70 | 11.0 | 42 | 13.5 | 28 | 8.6 | 0.433 |
| $40,227–$50,353 | 118 | 18.6 | 65 | 21.0 | 53 | 16.4 | |
| $50,354–$63,332 | 110 | 17.4 | 54 | 17.4 | 56 | 17.3 | |
| ≥ $63,333 | 206 | 32.5 | 104 | 33.5 | 102 | 31.5 | |
| Unknown | 130 | 20.5 | 45 | 14.5 | 85 | 26.2 | |
| Year of diagnosis | |||||||
| 2012 | 69 | 10.9 | 22 | 7.1 | 47 | 14.5 | < 0.001 |
| 2013 | 105 | 16.6 | 35 | 11.3 | 70 | 21.6 | |
| 2014 | 81 | 12.8 | 32 | 10.3 | 49 | 15.1 | |
| 2015 | 83 | 13.1 | 37 | 11.9 | 46 | 14.2 | |
| 2016 | 94 | 14.8 | 37 | 11.9 | 57 | 17.6 | |
| 2017 | 79 | 12.5 | 55 | 17.7 | 24 | 7.4 | |
| 2018 | 123 | 19.4 | 92 | 29.7 | 31 | 9.6 | |
| Total Charlson–Deyo score | |||||||
| 0 | 520 | 82.0 | 248 | 80.0 | 272 | 84.0 | 0.098 |
| 1 | 76 | 12.0 | 37 | 11.9 | 39 | 12.0 | |
| ≥ 2 | 38 | 6.0 | 25 | 8.1 | 13 | 4.0 | |
| Pathologic T | |||||||
| pT1 | 5 | 0.8 | 3 | 1.0 | 2 | 0.6 | 0.784 |
| pT2–T4 | 35 | 5.5 | 13 | 4.2 | 22 | 6.8 | |
| Unknown | 128 | 20.2 | 94 | 30.3 | 34 | 10.5 | |
| Pathologic N stage | |||||||
| pN1 | 395 | 62.3 | 231 | 74.5 | 164 | 50.6 | < 0.001 |
| pN2+ | 239 | 37.7 | 79 | 25.5 | 160 | 49.4 | |
| Regional lymph nodes examined | |||||||
| 1–5 | 314 | 49.5 | 269 | 86.8 | 45 | 13.9 | < 0.001 |
| 6–10 | 49 | 7.7 | 22 | 7.1 | 27 | 8.3 | |
| 11–15 | 31 | 4.9 | 7 | 2.3 | 24 | 7.4 | |
| 15+ | 240 | 37.9 | 12 | 3.9 | 228 | 70.4 | |
| Ulceration | |||||||
| No | 68 | 10.7 | 51 | 16.5 | 17 | 5.2 | 0.734 |
| Yes | 43 | 6.8 | 31 | 10.0 | 12 | 3.7 | |
| Unknown | 523 | 82.5 | 228 | 73.5 | 295 | 91.0 | |
| Lymphovascular invasion | |||||||
| No | 444 | 70.0 | 204 | 65.8 | 240 | 74.1 | 0.366 |
| Yes | 94 | 14.8 | 48 | 15.5 | 46 | 14.2 | |
| Unknown | 96 | 15.1 | 58 | 18.7 | 38 | 11.7 | |
| Mitotic rate | |||||||
| 0–1 | 19 | 3.0 | 15 | 4.8 | 4 | 1.2 | 0.797 |
| 2–10 | 74 | 11.7 | 53 | 17.1 | 21 | 6.5 | |
| ≥ 11 | 10 | 1.6 | 7 | 2.3 | 3 | 0.9 | |
| Unknown | 531 | 83.8 | 235 | 75.8 | 296 | 91.4 | |
| Immunotherapy | |||||||
| No | 406 | 64.0 | 190 | 61.3 | 216 | 66.7 | 0.140 |
| Yes | 215 | 33.9 | 114 | 36.8 | 101 | 31.2 | |
| Unknown | 13 | 2.1 | 6 | 1.9 | 7 | 2.2 | |
| Chemotherapy | |||||||
| No | 585 | 92.3 | 283 | 91.3 | 302 | 93.2 | 0.999 |
| Yes | 31 | 4.9 | 15 | 4.8 | 16 | 4.9 | |
| Unknown | 18 | 2.8 | 12 | 3.9 | 6 | 1.9 | |
| Radiation therapy | |||||||
| No | 560 | 88.3 | 280 | 90.3 | 280 | 86.4 | 0.126 |
| Yes | 74 | 11.7 | 30 | 9.7 | 44 | 13.6 | |
| Vital status | |||||||
| Alive | 463 | 73.0 | 232 | 74.8 | 231 | 71.3 | NA |
| Dead | 171 | 27.0 | 78 | 25.2 | 93 | 28.7 | |
Chi–square/Fisher’s exact test excluding unknown
NA not applicable statistical test due to censored observations
Statistical Analysis
For demographic clinical characteristics, descriptive statistics were calculated by using frequencies with percentage for categorical data for overall sample as well as by groups. An analysis of the association of clinicopathologic factors among treatment groups was performed by chi-squared (χ2) or Fisher’s exact tests to show differences in percentages in categorical variables across treatment groups. The variables evaluated included age at diagnosis, sex, race, Hispanic ethnicity, type of insurance, median income, year of diagnosis, Charlson–Deyo Score (CDS), pathologic T (pT) and N (pN) stage, ulceration, lymphovascular invasion (LVI), mitotic rate, receipt of immunotherapy, chemotherapy and/or radiation therapy, and vital status.
The primary clinical endpoint was the overall survival (OS) for patients with SLN positive HN cutaneous melanoma who underwent SLNB only versus SLNB + CLND. Overall survival (OS) was defined as the time in years from cancer diagnosis to death from any cause or last follow-up. Event-free patients were censored at the date of last follow-up. OS were estimated by Kaplan–Meier method and associations with prognostic factors assessed by log-rank test. Univariable and multivariable Cox proportional hazard regression models were used to assess the association between treatment and OS for selected variables. Results were reported as hazard ratios (HR) with 95% confidence intervals (95% CI). Statistical significance was set at a threshold of p < 0.05. A post-hoc sensitivity analysis was performed by excluding the outliers of regional nodes examined. The findings were consistent with those from the primary analysis and lead to similar conclusions about cohort and regional nodes examined effects, meaning that the outliers of regional nodes examined had little or no influence or impact on the primary conclusions.
We found an interaction between treatment and pathologic N (pN) in the whole sample and performed a subgroup analysis on patients within pN1 versus pN2+ disease. Cox proportional hazards regression models were utilized to identify predictors of OS in all and both subsets of patients. All statistical analyses were performed by using SAS version 9.4 (SAS Institute Inc. Cary, NC).
RESULTS
Description of Study Population
A total of 634 patients were identified for analysis (Fig. 1). Table 1 displays the demographics and clinicopathologic features of our patient sample. The majority of patients were > 45 years (77.3%), male (72.8%), white (98.1%), had private insurance (56.4%), CDS of 0 or 1 (94.0%), pT2–4 (73.5%), and pN1 (62.3%). Immunotherapy was utilized in a minority of patients (33.9%), as was chemotherapy (4.9%) and radiation (11.7%). A total of 310 patients (48.9%) underwent SLNB only, and 324 patients (51.1%) underwent SLNB + CLND. In the SLNB only group, a median of 2.0 SLNs were removed, whereas the SLNB + CLND patients had a median of 30.0 lymph nodes removed. Over the study period, there was an increase in omission of CLND (7.1% vs. 29.7% in year 2012 vs. 2018, respectively). The median follow-up time was 3.19 years for the SLNB only group and 4.53 years for the SLNB + CLND patients in alive patients, 2.07 and 2.05 years in deceased patients, respectively. Compared with the SLNB + CLND population, the SLNB only cohort were more likely to be older (> 45 years) (80.3% vs. 74.4%, p = 0.011), Black/Asian/others (2.9% vs. 0.6%, p = 0.028), to have Medicare insurance (35.8 % vs. 26.2%, p = 0.008), to have CDS of ≥ 2 (8.1 vs. 4.0%, p = 0.098), and pN1 disease (74.5% vs. 50.6%, p < 0.001). The SLNB only group had slightly higher utilization of immunotherapy (33.9% vs. 31.2%, p = 0.140), similar rates of chemotherapy (4.8% vs. 4.9%), and slightly less use of radiation (9.7% vs. 13.6%, p = 0.126) (Table 1).
Survival Analysis and Evaluation of Prognostic Factors
On univariable analysis (UVA), age > 65 versus 18–44 (HR 2.45; 95% CI 1.55–3.87; p < 0.001), male sex versus female (HR 1.45; 95% CI 1.01–2.08; p = 0.047), CDS of 1 versus 0 (HR 1.75; 95% CI 1.18–2.59; p = 0005), pT2–T4 versus pT1 (HR 2.96; 95% CI 1.21–7.20; p = 0.017), pN2+ versus pN1 (HR 1.90; 95% CI 1.41–2.57; p < 0.001), LVI (yes vs. no) (HR 2.25; 95% CI 1.56–3.25; p < 0.001), and receipt of radiation therapy (HR 1.62; 95% CI 1.10–2.39; p = 0.015) were associated with worse OS (Table 2; Fig. 2A, B). On multivariable analysis (MVA), we found that total CDS 1 (HR 1.70; 95% CI 1.10–2.63; p = 0.016), pN2+ (HR 1.74; 95% CI 1.23–2.45; p = 0.002), and LVI (HR 2.07; 95% CI 1.32–3.19; p = 0.001) were associated with worse prognosis.
TABLE 2.
Five-year overall survival using Cox proportional hazards regression models
| Variable | Category | Univariable | Multivariable | ||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95% CI) | p | aHR (95% CI) | p | ||
|
| |||||
| Cohort | SLNB only | Ref | Ref | ||
| SLNB+CLND | 0.99 (0.73–1.34) | 0.934 | 1.13 (0.71–1.81) | 0.610 | |
| Age (years) | 18–44 | Ref | Ref | ||
| 45–64 | 1.63 (1.02–2.59) | 0.040 | 1.40 (0.85–2.30) | 0.188 | |
| 65+ | 2.45 (1.55–3.87) | < 0.001 | 1.71 (0.91–3.19) | 0.094 | |
| Sex | Female | Ref | Ref | ||
| Male | 1.45 (1.01–2.08) | 0.047 | 1.32 (0.91–2.01) | 0.132 | |
| Year of diagnosis | 2012 | Ref | Ref | ||
| 2013 | 0.98 (0.62–1.53) | 0.917 | 0.72 (0.44–1.19) | 0.201 | |
| 2014 | 0.83 (0.50–1.37) | 0.466 | 0.72 (0.42–1.23) | 0.235 | |
| 2015 | 0.35 (0.19–0.67) | 0.001 | 0.23 (0.12–0.45) | < 0.001 | |
| 2016 | 0.71 (0.41–1.22) | 0.217 | 0.67 (0.38–1.19) | 0.174 | |
| 2017 | 0.69 (0.37–1.27) | 0.229 | 0.60 (0.31–1.16) | 0.129 | |
| 2018 | 0.38 (0.19–0.79) | 0.010 | 0.16 (0.01–2.09) | 0.163 | |
| Total Charlson–Deyo score | 0 | Ref | Ref | ||
| 1 | 1.75 (1.18– 2.59) | 0.005 | 1.70 (1.10–2.63) | 0.016 | |
| ≥ 2 | 1.79 (0.97–3.32) | 0.065 | 1.57 (0.79–3.10) | 0.196 | |
| Pathologic T | T1 | Ref | Ref | ||
| pT2–pT4 | 2.96 (1.21–7.20) | 0.017 | 1.86 (0.74–4.867) | 0.186 | |
| Unknown | 1.63 (0.57–4.65) | 0.365 | 8.69 (1.58–49.72) | 0.013 | |
| Pathologic N | pN1 | Ref | Ref– | ||
| pN2+ | 1.90 (1.41–2.57) | < 0.001 | 1.74 (1.23–2.45) | 0.002 | |
| Regional nodes examined | 1–5 | Ref | Ref | ||
| 6–10 | 0.93 (0.51–1.70) | 0.806 | 0.87 (0.44–1.72) | 0.695 | |
| 11–15 | 1.60 (0.87–2.93) | 0.130 | 0.90 (0.44–1.84) | 0.774 | |
| 15+ | 0.89 (0.64–1.23) | 0.467 | 0.69 (0.41–1.13) | 0.140 | |
| Lymphovascular invasion | No | Ref | Ref | ||
| Yes | 2.25 (1.56–3.25) | < 0.001 | 2.07 (1.34–3.19) | 0.001 | |
| Unknown | 1.03 (0.65–1.63) | 0.905 | 1.23 (0.76–1.98) | 0.410 | |
| Immunotherapy | No | Ref | Ref | ||
| Yes | 0.79 (0.57–1.12) | 0.183 | 0.81 (0.56–1.18) | 0.267 | |
| Unknown | 0.58 (0.14–2.34) | 0.443 | 0.63 (0.14–2.74) | 0.535 | |
| Radiation therapy | No | Ref | Ref | ||
| Yes | 1.62 (1.10–2.39) | 0.015 | 1.33 (0.86–2.04) | 0.201 | |
MVA without interactions, but when include interaction, we found that there is significant interaction between cohort and pathologic N, and no significant interaction between cohort and immune therapy. Therefore, the effect of cohort should be evaluated from subsample analysis by cohort
Additionally adjusted for race, ethnicity, median income, insurance, ulceration, mitotic rate, chemotherapy
HR Hazard ratio, aHR adjusted hazard ratio, CI confidence interval, Ref reference group
FIG. 2.
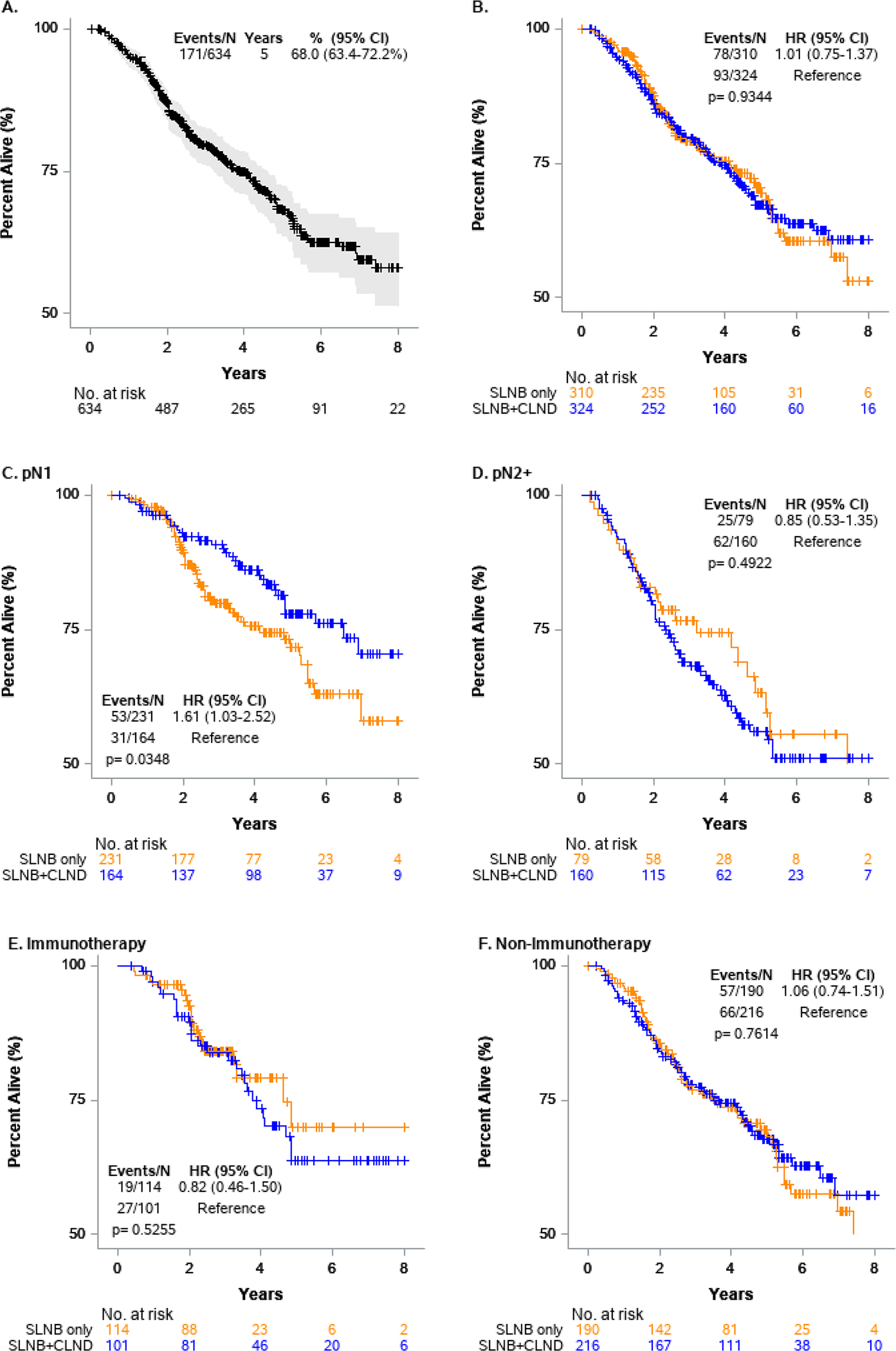
Kaplan–Meier survival analysis of overall cohort and subgroup analyses
Subgroup Analysis: Pathologic Nodal Stage
Because there was a statistically significant interaction between cohort and pathologic N, subgroup analysis by pN was performed to evaluate the cohort effect (Tables 3 and 4). When examining the pN status, 395 (62.3%) patients had pN1 disease and 239 (37.7%) patients had pN2+ disease (Table 3). In both groups, use of CLND decreased over time. Within the pN1 cohort, 164 patients (41.5%) underwent SLNB + CLND. On MVA, patients who underwent SLNB + CLND had similar OS compared with SLNB only patients (Table 4). Factors associated with worse OS in the pN1 cohort were age > 65 years (HR 2.69; 95% CI 1.28–5.64; p = 0.009) and LVI (HR 2.90; 95% CI 1.39–6.04; p = 0.004; Table 4). Within the pN2+ cohort, 160 (66.9%) patients underwent SLNB + CLND. There was no difference in OS between the two treatment arms (p = 0.427; Fig. 2D). Similar prognostic factors were associated with worse OS as in the pN1 group, in addition to median income $40,227–$50,353 (HR 4.16; 95% CI 1.31–13.22; p = 0.016) and CDS 1 (HR 2.16; 95% CI 1.15–4.06, p = 0.017; Table 4).
TABLE 3.
Patient, tumor, and treatment characteristics based upon pathologic N
| Variable | All | Pathologic N | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| pN1 | p a | pN2+ | p a | |||||||||
|
|
|
|||||||||||
| Cohort | Cohort | |||||||||||
|
|
|
|||||||||||
| SLNB only | SLNB+CLND | SLNB only | SLNB+CLND | |||||||||
|
|
|
|
||||||||||
| N | % | N | % | N | % | N | % | N | % | |||
|
| ||||||||||||
| All | 634 | 100.0 | 231 | 100.0 | 164 | 100.0 | 79 | 100.0 | 160 | 100.0 | ||
| Age at diagnosis (years) | ||||||||||||
| 18–44 | 144 | 22.7 | 45 | 19.5 | 42 | 25.6 | 0.319 | 16 | 20.3 | 41 | 25.6 | 0.001 |
| 45–64 | 274 | 43.2 | 102 | 44.2 | 70 | 42.7 | 24 | 30.4 | 78 | 48.8 | ||
| 65+ | 216 | 34.1 | 84 | 36.4 | 52 | 31.7 | 39 | 49.4 | 41 | 25.6 | ||
| Sex | ||||||||||||
| Female | 173 | 27.3 | 72 | 31.2 | 47 | 28.7 | 0.692 | 16 | 20.3 | 38 | 23.8 | 0.543 |
| Male | 461 | 72.7 | 159 | 68.8 | 117 | 71.3 | 63 | 79.7 | 122 | 76.3 | ||
| Insurance | ||||||||||||
| Medicaid | 33 | 5.2 | 14 | 6.1 | 8 | 4.9 | 0.240 | 4 | 5.1 | 7 | 4.4 | 0.002 |
| Medicare | 196 | 30.9 | 73 | 31.6 | 46 | 28.0 | 38 | 48.1 | 39 | 24.4 | ||
| Other government | 19 | 3.0 | 9 | 3.9 | 2 | 1.2 | 4 | 5.1 | 4 | 2.5 | ||
| Not insured | 20 | 3.2 | 7 | 3.0 | 4 | 2.4 | 3 | 3.8 | 6 | 3.8 | ||
| Private | 356 | 56.2 | 122 | 52.8 | 103 | 62.8 | 29 | 36.7 | 102 | 63.8 | ||
| Unknown | 10 | 1.6 | 6 | 2.6 | 1 | 0.6 | 1 | 1.3 | 2 | 1.3 | ||
| Median income | ||||||||||||
| < $40,227 | 70 | 11.0 | 34 | 14.7 | 17 | 10.4 | 0.724 | 8 | 10.1 | 11 | 6.9 | 0.900 |
| $40,227–$50,353 | 118 | 18.6 | 51 | 22.1 | 32 | 19.5 | 14 | 17.7 | 21 | 13.1 | ||
| $50,354–$63,332 | 110 | 17.4 | 39 | 16.9 | 30 | 18.3 | 15 | 19.0 | 26 | 16.3 | ||
| ≥ $63,333 | 206 | 32.5 | 74 | 32.0 | 45 | 27.4 | 30 | 38.0 | 57 | 35.6 | ||
| Unknown | 130 | 20.5 | 33 | 14.3 | 40 | 24.4 | 12 | 15.2 | 45 | 28.1 | ||
| Year of diagnosis | ||||||||||||
| 2012 | 69 | 10.9 | 16 | 6.9 | 30 | 18.3 | < 0.001 | 6 | 7.6 | 17 | 10.6 | 0.009 |
| 2013 | 105 | 16.6 | 24 | 10.4 | 34 | 20.7 | 11 | 13.9 | 36 | 22.5 | ||
| 2014 | 81 | 12.8 | 23 | 10.0 | 23 | 14.0 | 9 | 11.4 | 26 | 16.3 | ||
| 2015 | 83 | 13.1 | 27 | 11.7 | 24 | 14.6 | 10 | 12.7 | 22 | 13.8 | ||
| 2016 | 94 | 14.8 | 27 | 11.7 | 26 | 15.9 | 10 | 12.7 | 31 | 19.4 | ||
| 2017 | 79 | 12.5 | 44 | 19.0 | 14 | 8.5 | 11 | 13.9 | 10 | 6.3 | ||
| 2018 | 123 | 19.4 | 70 | 30.3 | 13 | 7.9 | 22 | 27.8 | 18 | 11.3 | ||
| Total Charlson–Deyo score | ||||||||||||
| 0 | 520 | 82.0 | 187 | 81.0 | 133 | 81.1 | 0.253 | 61 | 77.2 | 139 | 86.9 | 0.127 |
| 1 | 76 | 12.0 | 26 | 11.3 | 24 | 14.6 | 11 | 13.9 | 15 | 9.4 | ||
| ≥ 2 | 38 | 6.0 | 18 | 7.8 | 7 | 4.3 | 7 | 8.9 | 6 | 3.8 | ||
| Pathologic T stage | ||||||||||||
| pT1 | 40 | 6.3 | 15 | 6.5 | 19 | 11.6 | 0.353 | 1 | 1.3 | 5 | 3.1 | 0.675 |
| pT2–pT4 | 466 | 73.5 | 144 | 62.3 | 130 | 79.3 | 56 | 70.9 | 136 | 85.0 | ||
| Unknown | 128 | 20.2 | 72 | 31.2 | 15 | 9.1 | 22 | 27.8 | 19 | 11.9 | ||
| Regional lymph nodes examined | ||||||||||||
| 1–5 | 314 | 49.5 | 210 | 90.9 | 26 | 15.9 | < 0.001 | 59 | 74.7 | 19 | 11.9 | < 0.001 |
| 6–10 | 49 | 7.7 | 13 | 5.6 | 16 | 9.8 | 9 | 11.4 | 11 | 6.9 | ||
| 11–15 | 31 | 4.9 | 5 | 2.2 | 13 | 7.9 | 2 | 2.5 | 11 | 6.9 | ||
| 15+ | 240 | 37.9 | 3 | 1.3 | 109 | 66.5 | 9 | 11.4 | 119 | 74.4 | ||
| Lymphovascular invasion | ||||||||||||
| No | 444 | 70.0 | 157 | 68.0 | 136 | 82.9 | 0.008 | 47 | 59.5 | 104 | 65.0 | 0.701 |
| Yes | 94 | 14.8 | 29 | 12.6 | 9 | 5.5 | 19 | 24.1 | 37 | 23.1 | ||
| Immunotherapy | ||||||||||||
| No | 406 | 64.0 | 145 | 62.8 | 116 | 70.7 | 0.103 | 45 | 57.0 | 100 | 62.5 | 0.341 |
| Yes | 215 | 33.9 | 81 | 35.1 | 45 | 27.4 | 33 | 41.8 | 56 | 35.0 | ||
| Unknown | 13 | 2.1 | 5 | 2.2 | 3 | 1.8 | 1 | 1.3 | 4 | 2.5 | ||
| Chemotherapy | ||||||||||||
| No | 585 | 92.3 | 211 | 91.3 | 154 | 93.9 | 0.645 | 72 | 91.1 | 148 | 92.5 | 0.756 |
| Yes | 31 | 4.9 | 12 | 5.2 | 7 | 4.3 | 3 | 3.8 | 9 | 5.6 | ||
| Unknown | 18 | 2.8 | 8 | 3.5 | 3 | 1.8 | 4 | 5.1 | 3 | 1.9 | ||
| Radiation therapy | ||||||||||||
| No | 560 | 88.3 | 214 | 92.6 | 150 | 91.5 | 0.668 | 66 | 83.5 | 130 | 81.3 | 0.664 |
| Yes | 74 | 11.7 | 17 | 7.4 | 14 | 8.5 | 13 | 16.5 | 30 | 18.8 | ||
| Unknown | 96 | 15.1 | 45 | 19.5 | 19 | 11.6 | 13 | 16.5 | 19 | 11.9 | ||
| Vital status | ||||||||||||
| Alive | 463 | 73.0 | 178 | 77.1 | 133 | 81.1 | NA | 54 | 68.4 | 98 | 61.3 | NA |
| Dead | 171 | 27.0 | 53 | 22.9 | 31 | 18.9 | 25 | 31.6 | 62 | 38.8 | ||
NA not applicable statistical test due to censored observations
Chi-square/Fisher’s exact test excluding unknown
TABLE 4.
Five-year overall survival using Cox proportional hazards analysis of subgroup based upon pathologic N
| Variable | Category | pN1, n = 395 | pN2+, n = 239 | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| UVA | MVA | UVA | MVA | ||||||
|
|
|
|
|
||||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
|
| |||||||||
| Cohort | SLN only | Ref | Ref | Ref | Ref | ||||
| SLNB+CLND | 0.62 (0.40–0.97) | 0.036 | 1.04 (0.51–2.10) | 0.922 | 1.18 (0.74–1.87) | 0.493 | 1.31 (0.67–2.57) | 0.427 | |
| Age (years) | 18–44 | Ref | Ref | Ref | Ref | ||||
| 45–64 | 1.70 (0.83–3.47) | 0.144 | 1.77 (0.85–3.69) | 0.128 | 1.69 (0.91–3.13) | 0.094 | 1.57 (0.82–3.01) | 0.175 | |
| 65+ | 3.01 (1.51–5.99) | 0.002 | 2.69 (1.28–5.64) | 0.009 | 2.19 (1.18–4.06) | 0.013 | 2.06 (1.05–4.01) | 0.035 | |
| Sex | Female | Ref | Ref | Ref | Ref | ||||
| Male | 1.83 (1.06–3.16) | 0.029 | 1.77 (0.98–3.20) | 0.058 | 1.07 (0.66–1.76) | 0.776 | 0.91 (0.52–1.58) | 0.732 | |
| Median income | < $40,227 | Ref | Ref | Ref | Ref | ||||
| $40,227–$50,353 | 0.65 (0.33–1.25) | 0.194 | 0.66 (0.31–1.37) | 0.263 | 2.91 (0.98–8.66) | 0.055 | 4.16 (1.31–13.22) | 0.016 | |
| $50,354–$63,332 | 0.53 (0.25–1.13) | 0.099 | 0.53 (0.23–1.18) | 0.120 | 2.52 (0.85–7.50) | 0.096 | 2.86 (0.91–8.99) | 0.071 | |
| ≥ $63,333 | 0.40 (0.21–0.79) | 0.008 | 0.43 (0.21–0.88) | 0.021 | 1.55 (0.54–4.45) | 0.413 | 1.74 (0.58–5.27) | 0.324 | |
| Unknown | 0.62 (0.31–1.24) | 0.175 | 0.77 (0.37–1.64) | 0.333 | 2.08 (0.72–6.03) | 0.176 | 2.98 (0.97–9.17) | 0.057 | |
| Year of diagnosis | 2012 | Ref | Ref | Ref | Ref | ||||
| 2013 | 0.79 (0.43–1.45) | 0.443 | 0.82 (0.43–1.58) | 0.560 | 1.10 (0.55–2.18) | 0.785 | 0.59 (0.26–1.32) | 0.200 | |
| 2014 | 0.73 (0.37–1.44) | 0.361 | 0.65 (0.32–1.32) | 0.234 | 0.88 (0.41–1.89) | 0.744 | 0.56 (0.25–1.27) | 0.163 | |
| 2015 | 0.13 (0.04–0.45) | 0.001 | 0.08 (0.02–0.29) | < .001 | 0.61 (0.27–1.39) | 0.242 | 0.36 (0.14–0.89) | 0.027 | |
| 2016 | 0.44 (0.19–1.01) | 0.052 | 0.35 (0.14–0.83) | 0.018 | 1.01 (0.47–2.18) | 0.973 | 0.78 (0.35–1.77) | 0.559 | |
| 2017 | 0.63 (0.28–1.41) | 0.260 | 0.51 (0.22–1.20) | 0.122 | 0.93 (0.36–2.38) | 0.875 | 0.72 (0.27–1.90) | 0.505 | |
| 2018 | 0.70 (0.30–0.59) | 0.388 | 0.41 (0.16–1.02) | 0.056 | 0.08 (0.01–0.64) | 0.017 | 0.06 (0.01–0.52) | 0.010 | |
| Total Charlson–Deyo score | 0 | Ref | Ref | Ref | Ref | ||||
| 1 | 1.82 (1.05–3.15) | 0.034 | 1.69 (0.90–3.18) | 0.102 | 1.80 (1.03–3.14) | 0.040 | 2.16 (1.15–4.06) | 0.017 | |
| ≥ 2 | 2.80 (1.27–6.16) | 0.011 | 2.09 (0.86–5.08) | 0.105 | 1.04 (0.38–2.85) | 0.941 | 1.64 (0.54–4.98) | 0.382 | |
| Regional nodes examined | 1–5 | Ref | Ref | Ref | Ref | ||||
| 6–10 | 0.76 (0.33–1.78) | 0.533 | 0.63 (0.24–1.67) | 0.348 | 0.97 (0.40–2.36) | 0.952 | 0.93 (0.33–2.64) | 0.896 | |
| 11–15 | 1.90 (0.86–4.17) | 0.111 | 1.34 (0.51–3.53) | 0.557 | 1.14 (0.44–2.96) | 0.789 | 0.74 (0.23–2.39) | 0.612 | |
| 15+ | 0.44 (0.25–0.77) | 0.004 | 0.34 (0.14–0.79) | 0.012 | 1.08 (0.68–1.73) | 0.744 | 1.06 (0.53–2.12) | 0.877 | |
| Lymphovascular invasion | No | Ref | Ref | Ref | Ref | ||||
| Yes | 2.14 (1.12–4.09) | 0.021 | 2.90 (1.39–6.04) | 0.004 | 1.81 (1.14–2.88) | 0.012 | 1.84 (1.09–3.11) | 0.022 | |
| Unknown | 1.09 (0.60–1.98) | 0.784 | 1.18 (0.62–2.25) | 0.610 | 0.96 (0.47–1.96) | 0.918 | 1.36 (0.65–2.84) | 0.420 | |
Additionally adjusted for age, sex, chemotherapy, radiation
NE not estimable
Subgroup Analysis: Immunotherapy
We also performed a subgroup analysis by receipt of immunotherapy (Table 5). A total of 215 patients received immunotherapy. Compared with the entire cohort, patients who received immunotherapy were younger (< 45 years 29.8% vs. 22.7%), had higher median income (≥ $63,333 34.9% vs. 32.5%), had private insurance (62.8% vs. 56.2%), more frequently had pN2+ disease (41.4% vs. 37.7%), had more tumors with LVI (19.5% vs. 14.8%), and were treated in later years (2017–2018, 50.7% vs. 2012–2016, 27.9%). Within the immunotherapy cohort, 114 (53.0%) underwent SLNB only and 101 (47.0%) underwent SLNB + CLND (Table 5). Patients receiving immunotherapy had comparable survival to the total cohort (Fig. 2E). Subgroup analysis of patients not receiving immunotherapy also was performed (Table 5). There was no significant difference in OS between SLNB alone and SLNB + CLND for both subgroups (Table 6). No prognostic factors were identified on multivariable models to be associated with worse survival in patients receiving immunotherapy.
TABLE 5.
Patient and disease characteristics based upon receipt of immunotherapy
| Variable | All | Immunotherapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Yes | p a | No | p a | |||||||||
|
|
|
|||||||||||
| Cohort | Cohort | |||||||||||
|
|
|
|||||||||||
| SLNB only | SLNB+CLND | SLNB only | SLNB+CLND | |||||||||
|
|
|
|
|
|
||||||||
| N | % | N | % | N | % | N | % | N | % | |||
|
| ||||||||||||
| All | 621 | 100.0 | 114 | 100.0 | 101 | 100.0 | 190 | 100.0 | 216 | 100.0 | ||
| Age at diagnosis (years) | ||||||||||||
| 18–44 | 141 | 22.7 | 27 | 23.7 | 37 | 36.6 | 0.044 | 33 | 17.4 | 44 | 20.4 | 0.063 |
| 45–64 | 268 | 43.2 | 50 | 43.9 | 44 | 43.6 | 73 | 38.4 | 101 | 46.8 | ||
| 65+ | 212 | 34.1 | 37 | 32.5 | 20 | 19.8 | 84 | 44.2 | 71 | 32.9 | ||
| Sex | ||||||||||||
| Female | 169 | 27.2 | 31 | 27.2 | 21 | 20.8 | 0.274 | 54 | 28.4 | 63 | 29.2 | 0.869 |
| Male | 452 | 72.8 | 83 | 72.8 | 80 | 79.2 | 136 | 71.6 | 153 | 70.8 | ||
| Insurance | ||||||||||||
| Medicaid | 31 | 5.0 | 10 | 8.8 | 5 | 5.0 | 0.066 | 8 | 4.2 | 8 | 3.7 | 0.020 |
| Medicare | 192 | 30.9 | 34 | 29.8 | 19 | 18.8 | 75 | 39.5 | 64 | 29.6 | ||
| Other government | 19 | 3.1 | 2 | 1.8 | 1 | 1.0 | 11 | 5.8 | 5 | 2.3 | ||
| Not insured | 20 | 3.2 | 6 | 5.3 | 2 | 2.0 | 4 | 2.1 | 8 | 3.7 | ||
| Private | 350 | 56.4 | 62 | 54.4 | 73 | 72.3 | 86 | 45.3 | 129 | 59.7 | ||
| Unknown | 9 | 1.4 | – | – | 1 | 1.0 | 6 | 3.2 | 2 | 0.9 | ||
| Median income | ||||||||||||
| < $40,227 | 66 | 10.6 | 11 | 9.6 | 10 | 9.9 | 0.301 | 30 | 15.8 | 15 | 6.9 | 0.169 |
| $40,227–$50,353 | 114 | 18.4 | 26 | 22.8 | 13 | 12.9 | 36 | 18.9 | 39 | 18.1 | ||
| $50,354–$63,332 | 110 | 17.7 | 22 | 19.3 | 20 | 19.8 | 32 | 16.8 | 36 | 16.7 | ||
| ≥ $63,333 | 202 | 32.5 | 36 | 31.6 | 39 | 38.6 | 67 | 35.3 | 60 | 27.8 | ||
| Unknown | 129 | 20.8 | 19 | 16.7 | 19 | 18.8 | 25 | 13.2 | 66 | 30.6 | ||
| Year of diagnosis | ||||||||||||
| 2012 | 68 | 11.0 | 4 | 3.5 | 16 | 15.8 | < 0.001 | 18 | 9.5 | 30 | 13.9 | < 0.001 |
| 2013 | 103 | 16.6 | 4 | 3.5 | 20 | 19.8 | 31 | 16.3 | 48 | 22.2 | ||
| 2014 | 78 | 12.6 | 5 | 4.4 | 8 | 7.9 | 27 | 14.2 | 38 | 17.6 | ||
| 2015 | 82 | 13.2 | 8 | 7.0 | 14 | 13.9 | 28 | 14.7 | 32 | 14.8 | ||
| 2016 | 91 | 14.7 | 11 | 9.6 | 16 | 15.8 | 23 | 12.1 | 41 | 19.0 | ||
| 2017 | 77 | 12.4 | 19 | 16.7 | 11 | 10.9 | 35 | 18.4 | 12 | 5.6 | ||
| 2018 | 122 | 19.6 | 63 | 55.3 | 16 | 15.8 | 28 | 14.7 | 15 | 6.9 | ||
| Total Charlson–Deyo score | ||||||||||||
| 0 | 507 | 81.6 | 91 | 79.8 | 86 | 85.1 | 0.139 | 151 | 79.5 | 179 | 82.9 | 0.482 |
| 1 | 76 | 12.2 | 12 | 10.5 | 12 | 11.9 | 25 | 13.2 | 27 | 12.5 | ||
| 2+ | 38 | 6.1 | 11 | 9.6 | 3 | 3.0 | 14 | 7.4 | 10 | 4.6 | ||
| Pathologic T stage | ||||||||||||
| pT1 | 38 | 6.1 | 2 | 1.8 | 10 | 9.9 | 0.131 | 13 | 6.8 | 13 | 6.0 | 0.565 |
| pT2–pT4 | 457 | 73.6 | 49 | 43.0 | 73 | 72.3 | 148 | 77.9 | 187 | 86.6 | ||
| Unknown | 126 | 20.3 | 63 | 55.3 | 18 | 17.8 | 29 | 15.3 | 16 | 7.4 | ||
| Pathologic N stage | ||||||||||||
| pN1 | 387 | 62.3 | 81 | 71.1 | 45 | 44.6 | < 0.001 | 145 | 76.3 | 116 | 53.7 | < 0.001 |
| pN2+ | 234 | 37.7 | 33 | 28.9 | 56 | 55.4 | 45 | 23.7 | 100 | 46.3 | ||
| Ulceration | ||||||||||||
| No | 67 | 10.8 | 34 | 29.8 | 8 | 7.9 | 0.659 | 16 | 8.4 | 9 | 4.2 | 1.000 |
| Yes | 43 | 6.9 | 23 | 20.2 | 7 | 6.9 | 8 | 4.2 | 5 | 2.3 | ||
| Unknown | 511 | 82.3 | 57 | 50.0 | 86 | 85.1 | 166 | 87.4 | 202 | 93.5 | ||
| Lymphovascular invasion | ||||||||||||
| No | 434 | 69.9 | 70 | 61.4 | 68 | 67.3 | 0.466 | 130 | 68.4 | 166 | 76.9 | 0.765 |
| Yes | 94 | 15.1 | 24 | 21.1 | 18 | 17.8 | 24 | 12.6 | 28 | 13.0 | ||
| Unknown | 93 | 15.0 | 20 | 17.5 | 15 | 14.9 | 36 | 18.9 | 22 | 10.2 | ||
| Mitotic rate | ||||||||||||
| 0–1 | 19 | 3.1 | 11 | 9.6 | 2 | 2.0 | 0.780 | 4 | 2.1 | 2 | 0.9 | 1.000 |
| 2–10 | 74 | 11.9 | 37 | 32.5 | 10 | 9.9 | 16 | 8.4 | 11 | 5.1 | ||
| 11+ | 10 | 1.6 | 4 | 3.5 | 2 | 2.0 | 3 | 1.6 | 1 | 0.5 | ||
| Unknown | 518 | 83.4 | 62 | 54.4 | 87 | 86.1 | 167 | 87.9 | 202 | 93.5 | ||
| Regional lymph nodes examined | ||||||||||||
| 1–5 | 308 | 49.6 | 99 | 86.8 | 17 | 16.8 | < 0.001 | 192 | 47.3 | 165 | 86.8 | < 0.001 |
| 6–10 | 48 | 7.7 | 12 | 10.5 | 9 | 8.9 | 27 | 6.7 | 9 | 4.7 | ||
| 11–15 | 30 | 4.8 | 1 | 0.9 | 4 | 4.0 | 25 | 6.2 | 6 | 3.2 | ||
| 15+ | 235 | 37.8 | 2 | 1.8 | 71 | 70.3 | 162 | 39.9 | 10 | 5.3 | ||
| Chemotherapy | ||||||||||||
| No | 575 | 92.6 | 109 | 95.6 | 95 | 94.1 | 0.737 | 170 | 89.5 | 201 | 93.1 | 0.703 |
| Yes | 31 | 5.0 | 4 | 3.5 | 5 | 5.0 | 11 | 5.8 | 11 | 5.1 | ||
| Unknown | 15 | 2.4 | 1 | 0.9 | 1 | 1.0 | 9 | 4.7 | 4 | 1.9 | ||
| Radiation therapy | ||||||||||||
| No | 548 | 88.2 | 101 | 88.6 | 86 | 85.1 | 0.453 | 174 | 91.6 | 187 | 86.6 | 0.109 |
| Yes | 73 | 11.8 | 13 | 11.4 | 15 | 14.9 | 16 | 8.4 | 29 | 13.4 | ||
| Vital status | 121 | 29.8 | 99 | 86.8 | 17 | 16.8 | 111 | 58.4 | 10 | 4.6 | ||
| Alive | 452 | 72.8 | 95 | 83.3 | 74 | 73.3 | NA | 133 | 70.0 | 150 | 69.4 | NA |
| Dead | 169 | 27.2 | 19 | 16.7 | 27 | 26.7 | 57 | 30.0 | 66 | 30.6 | ||
Chi–square/Fisher’s exact test excluding unknown
TABLE 6.
Five-year overall survival using Cox proportional hazards analysis of subgroup based upon receipt of immunotherapy
| Variable | Category | Immunotherapy, n = 215 | No immunotherapy n = 406 | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| UVA | MVA | UVA | MVA | ||||||
|
|
|
|
|
||||||
| HR (95%CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
|
| |||||||||
| Cohort | SLN only | Ref | Ref | Ref | Ref | ||||
| SLNB+CLND | 1.21 (0.67–2.20) | 0.526 | 1.32 (0.54–3.22) | 0.549 | 0.95 (0.66–1.35) | 0.760 | 1.16 (0.65–2.07) | 0.608 | |
| Age (years) | 18–44 | Ref | Ref | Ref | Ref | ||||
| 45–64 | 1.49 (0.71–3.11) | 0.291 | 2.05 (0.92–4.60) | 0.08 | 1.93 (1.02–3.63) | 0.042 | 1.58 (0.81–3.06) | 0.178 | |
| 65+ | 1.91 (0.87–4.16) | 0.104 | 1.59 (0.59–4.25) | 0.359 | 2.89 (1.56–5.36) | < 0.001 | 2.46 (1.27–4.77) | 0.008 | |
| Sex | Female | Ref | Ref | Ref | Ref | ||||
| Male | 1.18 (0.59–2.39) | 0.636 | 1.19 (0.50–2.83) | 0.686 | 1.60 (1.04–2.47) | 0.032 | 1.39 (0.87–2.22) | 0.167 | |
| Median income | < $40,227 | Ref | Ref | Ref | Ref | ||||
| $40,227–$50,353 | 0.91 (0.33–2.51) | 0.856 | 1.84 (0.57–5.88) | 0.307 | 1.09 (0.57–2.09) | 0.800 | 1.22 (0.60–2.46) | 0.585 | |
| $50,354–$63,332 | 0.58 (0.20–1.68) | 0.319 | 0.73 (0.22–2.42) | 0.610 | 1.24 (0.63–2.43) | 0.541 | 1.14 (0.53–2.44) | 0.737 | |
| ≥ $63,333 | 0.60 (0.23–1.56) | 0.296 | 0.70 (0.24–2.01) | 0.507 | 0.71 (0.37–1.33) | 0.284 | 0.65 (0.32–1.32) | 0.233 | |
| Unknown | 0.53 (0.18–1.57) | 0.252 | 0.67 (0.20–2.20) | 0.509 | 1.15 (0.61–2.15) | 0.668 | 1.40 (0.70–2.80) | 0.348 | |
| Year of diagnosis | 2012 | Ref | Ref | Ref | Ref | ||||
| 2013 | 0.48 (0.19–1.19) | 0.114 | 0.35 (0.10, 1.20) | 0.094 | 1.26 (0.74–2.15) | 0.388 | 0.89 (0.50–1.60) | 0.693 | |
| 2014 | 0.27 (0.07–0.97) | 0.045 | 0.35 (0.08, 1.49) | 0.156 | 1.10 (0.62–1.97) | 0.747 | 0.95 (0.52, 1.75) | 0.867 | |
| 2015 | 0.18 (0.05–0.64) | 0.008 | 0.11 (0.02, 0.52) | 0.005 | 0.46 (0.22–0.95) | 0.036 | 0.29 (0.13, 0.63) | 0.002 | |
| 2016 | 0.50 (0.19–1.29) | 0.152 | 0.46 (0.14, 1.51) | 0.200 | 0.73 (0.37–1.46) | 0.374 | 0.78 (0.38, 1.61) | 0.503 | |
| 2017 | 0.89 (0.36–2.21) | 0.802 | 0.97 (0.30, 3.12) | 0.959 | 0.54 (0.23–1.29) | 0.164 | 0.46 (0.18, 1.16) | 0.100 | |
| 2018 | 0.23 (0.08–0.70) | 0.009 | NE | 0.71 (0.26–1.91) | 0.496 | 0.44 (0.02, 8.73) | 0.590 | ||
| Total Charlson–Deyo score | 0 | Ref | Ref | Ref | Ref | ||||
| 1 | 1.46 (0.65–3.28) | 0.362 | 1.11 (0.43, 2.89) | 0.827 | 1.84 (1.17–2.88) | 0.008 | 1.82 (1.11, 2.98) | 0.017 | |
| 2+ | 0.95 (0.23–3.96) | 0.944 | 0.49 (0.08, 2.86) | 0.425 | 2.20 (1.10–4.37) | 0.025 | 1.81 (0.83, 3.92) | 0.133 | |
| Pathologic T | T1 | Ref | Ref | Ref | Ref | ||||
| pT2–pT4 | 4.55 (0.62–33.19) | 0.135 | 3.74 (0.46–30.37) | 0.216 | 2.41 (0.89–6.55) | 0.083 | 1.38 (0.49–3.91) | 0.541 | |
| 1 | Unknown | 2.08 (0.24–17.61) | 0.503 | NE | 2.12 (0.59–7.59) | 0.248 | NE | ||
| Pathologic N | pN1 | Ref | Ref | Ref | Ref | ||||
| pN2+ | 1.57 (0.88–2.80) | 0.127 | 1.91 (0.86, 4.22) | 0.111 | 2.08 (1.46–2.96) | < 0.001 | 1.82 (1.20, 2.76) | 0.005 | |
| Lymphovascular invasion | No | Ref | Ref | Ref | Ref | ||||
| Yes | 1.65 (0.84–3.24) | 0.148 | 1.49 (0.67, 3.32) | 0.332 | 2.83 (1.83–4.40) | < 0.001 | 2.49 (1.48, 4.19) | < 0.001 | |
| Unknown | 0.92 (0.38–2.22) | 0.847 | 1.10 (0.41, 2.98) | 0.851 | 1.16 (0.68–1.98) | 0.590 | 1.21 (0.69, 2.14) | 0.500 | |
| Mitotic rate | 0–1 | Ref | Ref | NA | NA | ||||
| 2–10 | 0.91 (0.09–8.79) | 0.938 | 0.36 (0.03, 5.07) | 0.453 | |||||
| 11+ | 3.25 (0.20–52.08) | 0.404 | 0.38 (0.01, 16.07) | 0.611 | |||||
| Unknown | 2.12 (0.29–15.63) | 0.460 | NE | ||||||
| Regional nodes examined | 1–5 | Ref | Ref | Ref | Ref | ||||
| 6–10 | 1.34 (0.50, 3.59) | 0.565 | 0.81 (0.24, 2.77) | 0.741 | 0.83 (0.38, 1.82) | 0.650 | 0.71 (0.28, 1.78) | 0.461 | |
| 11–15 | 4.43 (1.30, 15.08) | 0.017 | 4.91 (0.89, 27.02) | 0.068 | 1.25 (0.62, 2.52) | 0.530 | 0.70 (0.29, 1.66) | 0.418 | |
| 15+ | 1.18 (0.62, 2.25) | 0.608 | 0.59 (0.21, 1.68) | 0.325 | 0.81 (0.55, 1.18) | 0.273 | 0.58 (0.31, 1.07) | 0.083 | |
| Chemotherapy | No | Ref | Ref | Ref | Ref | ||||
| Yes | 1.60 (0.49–5.17) | 0.433 | 1.69 (0.40, 7.22) | 0.476 | 0.65 (0.24–1.77) | 0.403 | 0.77 (0.27, 2.22) | 0.625 | |
| Unknown | 5.36 (0.73–39.43) | 0.099 | 58.53 (3.15, 1088.88) | 0.006 | 1.10 (0.41–2.99) | 0.849 | 1.55 (0.54, 4.44) | 0.413 | |
| Radiation therapy | No | Ref | Ref | Ref | Ref | ||||
| Yes | 0.95 (0.42–2.14) | 0.904 | 0.53 (0.19, 1.48) | 0.224 | 1.92 (1.22–3.02) | 0.005 | 1.73 (1.04, 2.88) | 0.034 | |
| Unknown | 3.42 (0.81–14.44) | 0.094 | NE | 2.02 (0.49–8.22) | 0.329 | NE | |||
DISCUSSION
In this study, we analyzed the impact of the omission of CLND to SLNB on OS of patients with HN cutaneous melanoma with positive SLNs. When examining the entire cohort of patients, there was no significant difference in OS between those who underwent SLNB only compared with SLNB + CLND. This finding is consistent with the results of MSLT-II and DeCOG-SLT, which showed no statistically significant difference in melanoma-specific survival in patients with cutaneous melanoma of all sites when comparing SLNB only to SLNB + CLND.6,7 These results also are consistent with a previous analysis of HN cutaneous melanoma patients from the NCDB 2012–2014 and a retrospective review of patients from the SEER database who were diagnosed with HN cutaneous melanoma from 1998 through 2007.14,15 Both of these studies showed no OS benefit to the addition of CLND.14,15 Our study is unique as it included a larger population, and it evaluated patients who were managed both before and after the publication of DeCOG-SLT in 2016 and MSLT-II in 2017.7,16 This is reflected by the trend toward more frequent omission of CLND in the more recent years. The reports of these major studies impacted practice patterns as shown in our study with an increasing proportion of patients undergoing SLNB only from 2017 and beyond. Our study also highlights a longer follow-up of Huang et al.’s population and shows that the addition of CLND in the overall study group did not add an additional long-term survival benefit.14 As previous studies have shown, roughly 75% of the time there are no non-SLNs with metastatic disease, and SLNB alone is providing durable regional control.17
In our analysis, CDS of 1, pN2+, and LVI were factors associated with worse OS. Given that the majority of patients had CDS of 0 (82.0%), the worse survival seen in patients with CDS of 1 may reflect additional comorbid conditions. The association of advanced nodal disease with worse OS is consistent with previously reported studies.18 The association of LVI with OS is less consistent in the literature, although multiple studies have shown that LVI is associated with higher risk features and increased likelihood of identifying positive lymph nodes.19
Given the association of survival with nodal burden, we performed a subgroup analysis to further evaluate the role of CLND in patients depending on extent of nodal disease found on pathology. An OS benefit was not observed in those patients with either pN1 or pN2+ disease who underwent SLNB + CLND compared with those who had SLNB alone. This is consistent with the findings of both the MSLT-II and DeCOG-SLT trials.6,7 A majority of patients in these two trials had low-volume nodal disease, with 71% of patients in MSLT-II and 92% of patients in DeCOG-SLT having only one positive node.6,7 Therefore, the pN1 patients in our analysis are similar in terms of nodal burden to those included in MSLT-II and DeCOG-SLT, and as suggested by the recent follow-up study of MSLT-II, SLNB alone is playing a therapeutic role in controlling the regional nodal basin without the need for CLND.17 For the patients with an even higher nodal burden (pN2), there is an increased risk of distant metastasis and poor overall prognosis. This likely explains the absence of a survival benefit with the addition of CLND in patients with pN2+ disease in our analysis. Furthermore, follow-up studies of the MSLT-II trial show that even when patients have regional recurrence, they are being effectively managed with CLND. This effect is likely further enhanced with the addition of systemic therapy, such as immunotherapy or targeted agents.17 Additional institutional studies that contain granular data on burden of lymph node disease are required to better characterize which patients may benefit from upfront CLND.
In our study, a total of 215 patients (33.9%) received immunotherapy and use of immunotherapy increased over time, particularly after 2017. This is an increase from the study by Huang et al. where only 22.3% of patients received immunotherapy.14 This likely reflects that fact that current immunotherapeutic agents, which are utilized for treatment of advanced melanoma were approved by the FDA for use in the adjuvant setting during our study period (2012–2019), including the approval of ipilimumab in 2015 based on a large RCT showing a RFS benefit and the approval of Nivolumab in 2017 and of pembrolizumab in 2019 based on findings from CHECKMATE-238 (NCT02388906) and EORTC1325/KEYNOTE-054 (NCT02362594) respectively.20–22 We showed that in patients who received immunotherapy, there was no difference in OS in patients who underwent SLNB alone compared with SLNB + CLND. In addition, unlike the entire cohort, there were no prognostic factors on MVA that were associated with worse OS, including pathologic nodal burden and LVI. This suggests that immunotherapy may help to overcome some of these poor prognostic factors.
There are several limitations to our study. Most significantly is the retrospective nature and the associated selection bias that comes from individual physician choices regarding extent of surgery and utilization of immunotherapy. It is notable that in our study the patients in the SLNB + CLND group had a longer median, follow-up time than the SLNB only group. While this may not have captured some of the patient deaths, recent studies have shown that the majority of melanoma recurrences occurred within the first 3 years of diagnosis.17 Considering that most of the melanoma-specific deaths occur in patients with recurrences, one could extrapolate that this differential would not contribute substantially to an overall survival difference. While the NCDB is a great resource to provide a large population of patients with cancer, its shortcoming is the lack of specific features regarding characteristics of node positivity (including more specific data on size of nodal metastases, extranodal extension, number of sentinel vs. nonsentinel lymph nodes in the CLND, etc.), location or timing of recurrence, or cause of death, and in turn limits the ability to determine melanoma-specific survival. The NCDB also does not provide treatment data, such as the specifics of single-agent immunotherapy and how many cycles were delivered, both of which have been found to have an impact on survival. This population of patients also does not reflect patients who had neoadjuvant immunotherapy. Recent studies have shown this approach leads to an improvement in event-free survival.23 Furthermore, the NCDB only includes patients from Commission on Cancer (CoC) accredited hospitals, which may not accurately reflect all stage III patients treated throughout the United States.
CONCLUSIONS
Our study shows that in a large population from the NCDB, there is no OS benefit for patients with HN cutaneous melanoma who undergo SLNB + CLND compared with SLNB only. This is consistent with the large, published studies of cutaneous melanoma of other primary sites. Larger studies that include more specific data on lymph node burden and utilization of systemic therapy are needed to evaluate which patients may benefit from CLND.
Footnotes
DISCLOSURE The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC’s NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
REFERENCES
- 1.Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krag DN, Meijer SJ, Weaver DL, et al. Minimal-access surgery for staging of malignant melanoma. Arch Surg. 1995;130(6):654. [DOI] [PubMed] [Google Scholar]
- 3.Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16(1):5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farlow JL, McLean SA, Peddireddy N, et al. Impact of completion lymphadenectomy on quality of life for head and neck cutaneous melanoma. Otolaryngol Head Neck Surg. 2022;166(2):313–20. [DOI] [PubMed] [Google Scholar]
- 6.Leiter U, Stadler R, Mauch C, German Dermatologic Cooperative Oncology Group, et al. Final analysis of DeCOG-SLT trial: no survival benefit for complete lymph node dissection in patients with melanoma with positive sentinel node. J Clin Oncol. 2019;37(32):3000–8. [DOI] [PubMed] [Google Scholar]
- 7.Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. Melanoma: Cutaneous (Version 3.2022). https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf. Accessed 4 June 2022. [Google Scholar]
- 9.Shashanka R, Smitha BR. Head and neck melanoma. ISRN Surg. 2012;2012:948302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ascierto PA, Del Vecchio M, Mandalá M, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(11):1465. [DOI] [PubMed] [Google Scholar]
- 11.Eggermont AMM, Blank CU, Mandala M, et al. Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage III melanoma: Updated results from the EORTC 1325-MG/KEYNOTE-054 trial. J Clin Oncol. 2020;38(33):3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauschild A, Dummer R, Schadendorf D, et al. Longer follow-up confirms relapse-free survival benefit with adjuvant dabrafenib plus trametinib in patients with resected BRAF V600-mutant stage III melanoma. J Clin Oncol. 2018;36(35):3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. https://www.facs.org/media/aq3aummh/puf_data_dictionary_2019.pdf. [Google Scholar]
- 14.Huang K, Misra S, Lemini R, et al. Completion lymph node dissection in patients with sentinel lymph node positive cutaneous head and neck melanoma. J Surg Oncol. 2020;122(6):1057–65. [DOI] [PubMed] [Google Scholar]
- 15.Smith VA, Cunningham JE, Lentsch EJ. Completion node dissection in patients with sentinel node-positive melanoma of the head and neck. Otolaryngol Head Neck Surg. 2011;146:591–9. [DOI] [PubMed] [Google Scholar]
- 16.Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–67. [DOI] [PubMed] [Google Scholar]
- 17.Anu A, Crystal JS, Thompson JF, et al. Therapeutic value of sentinel lymph node biopsy in patients with melanoma: a randomized clinical trial. JAMA Surg. 2022;157:835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woeste MR, McMasters KM, Egger ME. Stage IIIa melanoma and impact of multiple positive lymph nodes on survival. J Am Coll Surg. 2021;232(4):517–24.e1. [DOI] [PubMed] [Google Scholar]
- 19.Namikawa K, Aung PP, Gershenwald JE, Milton DR, Prieto VG. Clinical impact of ulceration width, lymphovascular invasion, microscopic satellitosis, perineural invasion, and mitotic rate in patients undergoing sentinel lymph node biopsy for cutaneous melanoma: a retrospective observational study at a comprehensive cancer center. Cancer Med. 2018;7(3):583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–30. [DOI] [PubMed] [Google Scholar]
- 21.Webber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–35. [DOI] [PubMed] [Google Scholar]
- 22.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–801. 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 23.Patel S, Othus M, Prieto V, Lowe M, Buchbinder E. LBA6—neoadjvuant versus adjuvant pembrolizumab for resected stage III–IV melanoma (SWOG S1801). Ann Oncol. 2022;33:7S. [Google Scholar]


