Abstract
Idiopathic pulmonary fibrosis (IPF) is an age-related lung interstitial disease that occurs predominantly in people over 65 years of age and for which there is a lack of effective therapeutic agents. It has demonstrated that mesenchymal stem cells (MSCs) including alveolar epithelial cells (AECs) can perform repair functions. However, MSCs lose their repair functions due to their distinctive aging characteristics, eventually leading to the progression of IPF. Recent breakthroughs have revealed that the degree of autophagic activity influences the renewal and aging of MSCs and determines the prognosis of IPF. Autophagy is a lysosome-dependent pathway that mediates the degradation and recycling of intracellular material and is an efficient way to renew the nonnuclear (cytoplasmic) part of eukaryotic cells, which is essential for maintaining cellular homeostasis and is a potential target for regulating MSCs function. Therefore, this review focuses on the changes in autophagic activity of MSCs, clarifies the relationship between autophagy and health status of MSCs and the effect of autophagic activity on MSCs senescence and IPF, providing a theoretical basis for promoting the clinical application of MSCs.
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, irreversible, and fatal lung disease marked by lung scarring, with an average life expectancy of 3–5 years after diagnosis [1–4]. IPF primarily affects middle-aged and older adults; the prevalence of IPF increases with age among the numerous countries studied, with a high rate over 65 years [5]. The pathogenesis of IPF hinges on sustained or repetitive lung epithelial injury, which triggers the activation of fibroblasts and subsequent myofibroblast differentiation [6]. Two new approved therapies by the FDA, namely, pirfenidone and nintedanib, exhibit modest effectiveness in mitigating the decline in lung function over a 1-year follow-up period [7–10]. Nonetheless, these groundbreaking antifibrotic therapies are still in their nascent stages and are not frequently recommended for patients with a milder or stabilized course of the disease, primarily owing to the substantial incidence of side effects [10, 11]. Lung cancer frequently arises as a complication of IPF, with one-fifth experiencing acute exacerbations after treatment [12].
Cellular therapy for pulmonary fibrosis (PF) encompasses the application of mesenchymal stem cells (MSCs) [13]. MSCs are multipotent cells with the ability to differentiate into diverse cell types and bestow immunomodulatory, antiproliferative, and anti-inflammatory effects [14]. However, a multitude of internal and external factors have prompted alterations in the health status of MSCs, thus influencing their capacity to effectively facilitate the repair and regeneration of damaged lung tissue as therapeutic cells [15]. The regulation of autophagy within MSCs stands as a potential mechanism that could influence the properties of MSCs and potentially impact their regenerative and therapeutic potential [16]. Autophagy serves as the principal cellular process for breaking down and recycling intracellular proteins and organelles in various physiological and pathological contexts [17]. Impairment of autophagy fails to efficiently rectify malfunctioning organelles and eliminate detrimental metabolites within MSCs, ultimately resulting in the senescence of MSCs [18]. Excessive autophagy will lead to apoptosis of MSCs, affect the renewal ability of MSCs, and ultimately lead to the inability of MSCs to repair damaged lung tissue, accelerating the occurrence of IPF [19]. Therefore, the change of autophagy activity is closely related to the health status of MSCs.
In recent years, more and more researches have been committed to investigating the regulative network of autophagy in IPF [20, 21]. Autophagy is like a double-edged sword, indicating that autophagy activity may be a significant driving factor for IPF development [22]. Basal autophagy activity maintains pulmonary homeostasis in a cellular protective manner; it can selectively degrade potentially detrimental cytoplasmic substances, uneliminated proteins, and some unfavorable microorganisms, such as damaged organelles, viruses, protists, and bacteria [23]. In this review, this paper provides a focused review of the aging characteristics and functional changes of MSCs in IPF, as well as the mechanisms of autophagic activity affecting the health status of MSCs, to promote a more comprehensive application of MSCs in regenerative medicine.
2. The Emerging Role of Autophagy in IPF
2.1. The Biological Function of Autophagy
Autophagy represents the predominant cellular mechanism not only responsible for a bulk recycling system but also for targeting specific organelles, protein complexes, protein aggregates, and invading pathogens for catabolism [17]. According to the mechanism used to deliver cargo to the lysosome, autophagy can be classified as microautophagy, chaperone-mediated autophagy, and macroautophagy (MA) [24].
The mammalian target of rapamycin (mTOR) kinase is a conserved protein kinase involved in a multitude of cellular processes including nutrient sensing, cell growth, and autophagy, which is a signaling control point downstream of growth factor receptor signaling, hypoxia, ATP levels, and insulin signaling [25, 26]. mTOR kinase is a downstream effector of the PI3K/Akt pathway, signaling in the presence of nutrients and promoting cellular growth by stimulating the expression of ribosomal proteins and enhancing protein translation [27]. Crucially, mTOR also functions to suppress autophagy in these growth-favorable circumstances [28]. The activity of mTOR kinase is inhibited by signals that detect nutrient deficiency, such as hypoxia [29]. Upstream of mTOR, when cellular ATP levels are low, the activation of adenosine 5′-monophosphate (AMP)–activated protein kinase (AMPK) enhances the inhibitory function of the Tsc1/Tsc2 tumor suppressor proteins on Rheb, a small GTPase essential for mTOR function [30]. Consequently, decreased mTOR activity triggers autophagy, thereby ensuring that the cell adapts to its changing environment by slowing down growth and increasing catabolic processes.
Autophagy occurs constitutively in all eukaryotic cells and operates at fundamental levels, assuming a homoeostatic mechanism by regulating the degradation of molecules and the turnover of organelles [16]. In this context, autophagy is directed toward the degradation of misfolded protein cargos, thereby preventing the accumulation of the relevant proteins and consequent toxicity that may ultimately result in cellular damage and mortality [31]. Autophagy is rapidly induced under conditions of glucose or amino acid deprivation, oxidative stress, hypoxia, and exposure to xenobiotics, all of which may initiate or exacerbate cellular injuries [32]. Therefore, autophagy is not only a dynamic adaptation pathway but also safeguarding of proteome integrity and energy metabolism. Paradoxically, excessive autophagy has been observed in association with cell death; controlled autophagy is protective by providing essential substrates [33]. However, to aviod confusion, the term “autophagic cell death” has been restated as “cell death with autophagy” to describe cell death that is suppressed by inhibition of the autophagy pathway and led to a disruption in the autophagic flux [34]. Autophagic flux refers to the whole process of autophagy, and there are various methods to monitor autophagy [35]. An ideal method to assess autophagic activity is measuring the LC3-II levels, but it is crucial to complement this with an examination of substrate degradation (e.g., SQSTM1/p62) [35]. Furthermore, confirming changes in autophagic flux can be achieved through genetic modifications (like using short interfering RNA for ATG genes), using pharmaceutical inhibitors such as 3-methyladenine (3-MA) and chloroquine, or employing inducers like rapamycin [35].
2.2. The Role of Autophagy in IPF
IPF is a fatal chronic interstitial lung disease that impacts both lung mechanical functions and gas exchange. With the emergence of advanced molecular diagnostics, it is increasingly apparent that the pathogenesis of IPF is intricate, involving multiple molecular pathways, and thus is likely to necessitate diverse treatment strategies [6, 36].
Altered autophagy in fibroblasts has also been documented as a crucial factor in the pathogenesis of human IPF [37]. Notably, autophagic activity was abnormally low in IPF fibroblasts, which was attributed to the low expression of FoxO3a leading to a reduced level of LC3B transcription, ultimately causing a decreased autophagic flow in fibroblasts [38, 39]. Defective autophagy is necessary to maintain a cell death-resistant phenotype in fibroblasts within a collagen-rich matrix [20, 38]. The potential profibrotic function of autophagy in IPF fibroblasts necessitates a reevaluation of the utilization of autophagy activators in the treatment of IPF, with a focus on context-specific approaches.
Autophagy is also involved in promoting profibrotic effects in IPF fibroblasts, so the utilization of autophagy activators for the treatment of IPF requires a context-specific approach. Recent evidence highlights the pivotal contributions of disrupted mitochondrial homeostasis in alveolar epithelial type II cells (AECIIs), fibroblasts, and alveolar macrophages (AMs) to the pathogenesis of IPF [40] (Figure 1). For instance, the accumulation of dysmorphic and dysfunctional mitochondria within AECIIs has been reported in the pulmonary of IPF patients [40]. The compromised mitochondria in AECIIs are linked to reduced PINK1 levels and impaired mitophagy. PINK1-deficient mice demonstrate disrupted mitochondrial homeostasis and the onset of PF [40]. The expression of PARK2, another protein associated with mitophagy, is decreased in the lung fibroblasts of IPF patients. PARK2 deficiency exacerbates bleomycin-induced PF in mice by enhancing myofibroblast differentiation and proliferation through the promotion of the PDGFR-PI3K-Akt signaling pathway [41]. Pirfenidone, an FDA-approved therapy and an exciting landmark in the field of IPF treatment, exerts its antifibrotic effects partially through the induction of PARK2-mediated mitophagy and the inhibition of myofibroblast differentiation [42]. Mitophagy, a subtype of macroautophagy, is elevated in profibrotic AMs [43]. During the fibrotic process, Akt1-mediated mitochondrial reactive oxygen species (ROS) induction triggers mitophagy in AMs, thereby influencing macrophage apoptosis resistance and the expression of TGF-β1 [43]. The TGF-β1 derived from AMs is required for PF, which promotes the differentiation of fibroblasts into myofibroblasts and the development of PF [43]. Furthermore, AECIIs treated with TGF-β1 were shown to induce mitophagy but TGF-β1 reduced mitophagy in fibroblasts by activating Akt in IPF lungs [43]. Considering the varying impact of mitophagy on different cell types in the development of IPF targeting cell type-specific mitophagy could lead to more effective therapeutic results in the treatment of IPF.
Figure 1.
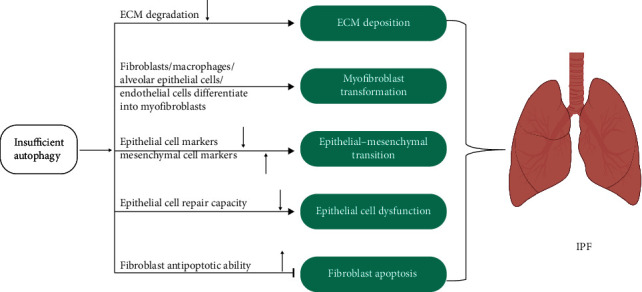
Autophagy process in the IPF. Altered autophagy promotes ECM production, myofibroblast transformation, epithelial–mesenchymal transition, epithelial cell dysfunction, and inhibits fibroblast apoptosis.
3. Role of Autophagy in the Therapeutic Potential of MSCs
Since 1995, first tested MSCs have been gained wide popularity and extensively studied in preclinical model [44]. MSCs afford several advantages, such as easy accessibility, low immunogenicity, and therapeutic potential in regenerative medicine [45]. Due to these properties, MSCs have become very promising tool for therapy in different disease types and ideal cells in the treatment of IPF [46]. Initially, the beneficial effects of MSC-based therapies were attributed to the replacement capacity of MSCs [47]. However, this view has not stood the test of time; studies have revealed that structure and function of injured tissues by direct cell replacement are not the primary property of MSCs [48, 49]. Research to date have demonstrated that MSCs-derived secretome, which comprises a series of bioactive molecules and extracellular vesicles (EVs), plays a key role in immune modulation and promoting tissue repair [50, 51]. The keratinocyte growth factor (KGF), hepatocyte growth factor (HGF), and epidermal growth factor (EGF) derived from MSCs are helpful in tissue repair promoting effects. MSC-derived vascular endothelial growth factor (VEGF) has also been studied extensively for its angiogenic properties, which promote reepithelialization and angiogenesis [52]. MSCs reprogram proinflammatory macrophages (M1) toward an antiinflammatory phenotype (M2) resulting in exerting antifibrotic effects [53]. Furthermore, MSCs exert potent antifibrotic effects via modulating the ratio of metalloproteinases/metalloproteinase tissue inhibitors [54, 55]. Given that IPF is an age-related disease, recent studies have found that MSCs exhibit aging under sustained pathological conditions such as chronic injury and oxidative stress, which affects the therapeutic activity of MSCs and leads to PF [56] (Figure 2).
Figure 2.
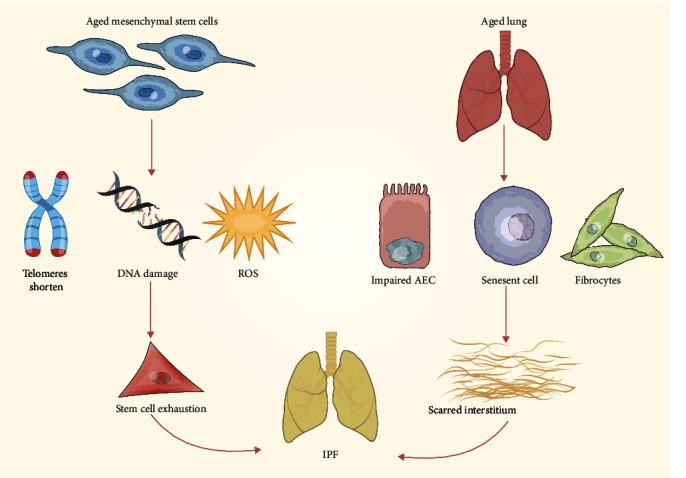
Aging characteristics of MSCs in IPF.
Recently, it has been proposed that autophagy in MSCs is potentially a new approach for improving therapeutic effects of MSCs (Table 1). Autophagy plays a dual role in MSCs: (1) Modulating autophagy in MSCs may control the proliferation, activation, and effector function of MSC; (2) MSCs are able to modulate the autophagy of immune and other cells that play an important role in the pathogenesis of inflammatory lung diseases [67]. Both of these mechanisms eventually affect the efficency of MSC-based therapy. The initial observation indicating the crucial involvement of autophagy in MSC processes was the disparity in autophagosome quantities between undifferentiated MSCs and their differentiated counterparts [68]. Furthermore, the hindrance in the fusion between autophagosomes and lysosomes, resulting in the obstruction of autophagosome degradation, culminates in the accumulation of autophagosomes within undifferentiated MSCs [69].
Table 1.
Role of autophagy in the therapeutic potential of MSCs.
| Disease model | Mechanism | Autophagy effect | Reference |
|---|---|---|---|
| Ischemic stroke | MSCs inhibit autophagy and promote cell survival by transferring miR-25 to support recovery of neurological function after stroke | Negative | [57] |
| Liver fibrosis | Autophagy inhibition via Becn1 downregulation improves the MSCs antifibrotic potential | Negative | [58] |
| Hypoxic-ischemic brain damage | MSCs reduce autophagy in hippocampal neurons partly through the AMPK/mTOR pathway | Negative | [59] |
| Osteoarthritis | MSCs enhance autophagy in chondrocytes via mTOR inhibition and protect articular cartilage from damage | Positive | [60] |
| Acute lung injury | MSCs enhance autophagy and ameliorate acute lung injury partially via delivery of miR-100 | Positive | [61] |
| Idiopathic pulmonary fibrosis | Inhibition of miR-199a-5p enhances autophagy by regulating the Sirt1/AMPK signaling pathway and rejuvenates IPF-MSCs senescence | Positive | [62] |
| Parkinson's disease | MSCs enhance autophagy and exert a neuroprotective effect through the modulation of α-synuclein | Positive | [63] |
| Alzheimer's disease | MSCs enhance autophagy and increase β-amyloid clearance to improve neuronal survival against Aβ toxicity | Positive | [64] |
| Inflammatory bowel disease | Enhancement of autophagy in MSCs improves immunosuppression of MSCs by increasing Pacer levels | Positive | [65] |
| Diabetic kidney disease | MSCs diminish cell death in kidney tissue facing diabetic kidney disease, culminating in podocyte maintenance, and also downregulating the over induction of the autophagy pathway | A double-edged sword | [66] |
A recent study indicates that inhibiting autophagy enhances the immune-suppressing abilities of MSCs [70]. The research reveals that reducing the expression of Becn1 gene in MSCs (short hairpin Becn1-MSCs) strengthens their therapeutic and immune-modulating effects [70]. Notably, when treated with these modified short hairpin Becn1-MSCs, a more pronounced decrease in the populations of CD4+ and CD8+ T cells, as well as a reduced proliferation of MOG (myelin oligodendrocyte glycoprotein)-specific CD4+ T cells, is observed, all without impacting the polarization of T cells [70]. Similar results were achieved when these mice received MSCs that had been pretreated with an autophagy inhibitor [70].
The modulation of MSC autophagy can significantly influence their secretion capacity, thereby impacting their overall functionality [71]. Notably, when MSCs are pretreated with the autophagy-inducer rapamycin and subsequently subcutaneously injected, it results in an augmentation of their wound-healing potential. This enhancement is closely linked to the promotion of angiogenesis, driven by the autophagy-induced secretion of VEGF [71]. Conversely, MSCs in which BECN1 is silenced, causing an early blockade of the autophagic machinery, exhibit a diminished therapeutic effect [71].
Thus, modulation of autophagy in MSCs seems to be a potential target to enhance the therapeutic properties of MSC-based therapy, but great action needs to be taken, and further studies should be conducted.
4. Importance of Autophagy in Maintaining Healthy MSCs
4.1. Excessive Autophagy Promotes Apoptosis of MSCs
MSCs are a heterogeneous population of multipotent stromal stem cells that can be easily isolated from a variety of different sources [72]. MSCs offer diverse benefits that stem from their and the ability to differentiation into osteoblasts, chondrocytes, and adipocytes under appropriate and specific stimuli [73, 74]. Additionlly, MSCs exert an immunomodulatory effect on innate and adaptive immune responses via interaction with the inflammatory microenvironment [75, 76]. Therefore, MSCs have been widely used in clinical trials to treat autoimmune and inflammatory diseases, particularly in the context of lung injuries [77]. However, there is a lack of comprehensive understanding regarding the precise impact of the inflammatory microenvironment on the fate of MSCs. The inflammatory microenvironment plays a key role in mediating immunoregulatory capability of MSCs [76, 78]. MSCs exert enhanced immunosuppressive functions after interaction with inflammatory cytokines, including interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-1α, and IL-β [74, 79] (Figure 3). Related literature has shown that both fetal and adult MSCs are susceptible to lysis by IL2-activated natural killer cells [80]. Furthermore, IFN-γ synergistically amplifies TNFα-induced apoptosis in MSCs, thus impeding their capacity to repair damaged lung tissue, indicating that apoptosis of MSCs could be induced in the inflammatory microenvironment during the development of PF [81].
Figure 3.
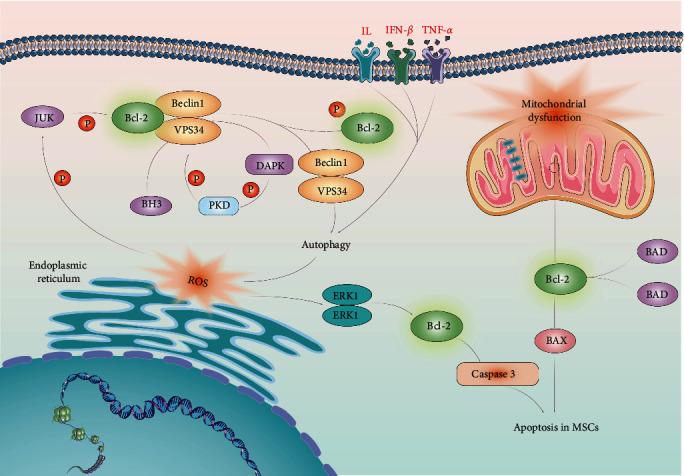
Excessive autophagic activity promotes apoptosis of MSCs under inflammatory microenvironment.
Recent research has demonstrated that TNF-α and IFN-γ inflammatory cytokines such as IFN-γ and TNF-α activate autophagy in MSCs by upregulating Beclin 1 expression, which attenuates the immunosuppressive capacity of MSCs [19]. Although autophagy has been considered a cell survival mechanism, it can also promote cell death depending on the specific physiological and pathological conditions; the dual function of autophagy in prosurvival and prodeath remains incomplete [82, 83]. Autophagy constitutes major adaptive (survival) strategy of cells in response to challenges such as starvation, growth factor withdrawal, and neurodegeneration but is also a critical contributor to the death of certain types of cells [84, 85]. There is evidence to support autophagy promoted TNF-α plus IFN-γ-induced apoptosis of MSCs, highlighting the varied functions of autophagy under conditions of inflammation and nutrient scarcity [19]. Consequently, it is feasible to consider the manipulation of autophagy in MSCs as a means to optimize therapeutic effectiveness.
4.2. Impact of Declined Autophagy on MSCs Aging
As MSC populations with systematic age, they undergo functional deterioration and less effective in vivo or extended culture in vitro, limiting their therapeutic applications [86–88]. The underlying processes that drive MSCs senescence remain unclear, but significant progress has been made in elucidating the aspects of age-related MSCs phenotypic changes as well as possible mechanisms that influence MSCs senescence [89].
Autophagic activity tends to decrease with age across various model organisms, potentially leading to the buildup of autophagic structures and constraining the capacity for maintaining cellular homeostasis in certain contexts [90–92]. Human cell studies have revealed that age-related declines in the breakdown of lysosomal proteins hinder the autophagic flux, worsening cellular damage and playing a role in the onset of age-related diseases [93–96]. Additional evidence has substantiated that aging is linked to a diminished expression of several Atg genes, including Atg2 and Atg8a, which play a crucial role in both the initiation and functionality of autophagy [97]. In normally aged mice, autophagy was significantly reduced, as indicated by decreased levels of Atg7, LC3-II, autophagosome, autophagolysosomal fusion, autophagy substrates, and autophagy receptor [98]. Consistent with this, autophagy was attenuated in both aged rat brain tissue and aged human fibroblasts, as evidenced by significantly decreased levels of autophagy-associated proteins, such as Atg5-Atg12 and Becn1, and significantly increased levels of mTOR and ferritin H [99]. In normal older human brain samples, the expression of key autophagy genes like Atg5 and Atg7 was also reduced [100]. Additionally, several age-related human pathologies are closely linked to deficits in autophagy that develop and progress with age [101–103]. Taken together, compromised autophagy is a characteristic of organismal aging, as autophagy abundance declines with age and cargo is not delivered to the lysosomes as efficiently.
On the contrary, research on long-lived mutant animals has revealed that increased autophagy is linked to delayed aging. Specifically, the prolonged lifespan observed in C. elegans daf-2 loss of function mutants relies on autophagic genes like bec-1, lgg-1, atg-7, and atg-12 [92, 104, 105]. Moreover, the extended longevity in various longevity mutants, including daf-2 mutants with reduced insulin/insulin-like signaling, germline-less glp-1(e2141) mutants, dietary-restricted eat-2 (ad1116) mutants, mitochondrial respiration-defective clk-1(e2519) mutants, and mRNA translation-impaired rsks-1 (sv31) mutants, necessitates the presence of HLH-30 [106]. Activation of autophagy with rapamycin could restore the proliferative function of aged MSCs [107]. These findings align with evidence of reduced induction in autophagosome formation and lysosomal degradation in the absence of HLH-30, suggesting that HLH-30 plays a pivotal role in promoting longevity by regulating the autophagic process downstream of various lifespan-extending mechanisms [106]. Further, the formation of long-lived dauer worms, which correspond to a larval hibernation stage, is correlated with increased autophagy and depends on autophagy genes atg-1, atg-7, lgg-1, and atg-18, demonstrating the importance of autophagy to organismal adaptation in challenging conditions [105]. However, impaired autophagy could increase ROS and lead to MSC aging [108]. Similarly, high glycemic treatment of MSCs increased ROS-mediated autophagy, leading to the formation of Beclin-1, Atg5, Atg7, Atg12, and LC3-II autophagosomes, which induced MSC aging and local inflammation [109].
Together, collective research suggested that (1) autophagy is impaired during as MSCs undergo aging, (2) autophagy dysfunction shortens the lifespan of MSCs, and (3) enhancing or restoring autophagy prolongs the lifespan and extends the healthspan of MSCs (Figure 4). This demonstrates that autophagy regulation is central to the aging of MSCs (Table 2).
Figure 4.
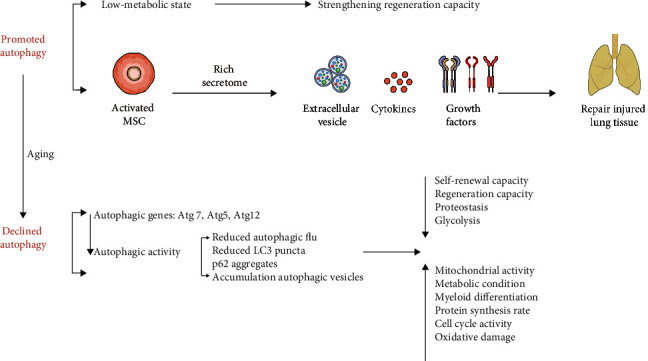
Autophagy influences MSCs activity and aging. In MSCs, promoted autophagy partially reverses the aging of MSCs, while declined autophagy attenuates the biological functions of MSCs.
Table 2.
Autophagy modulation on apoptosis and aging in MSCs.
| Experimental model | Molecular mechanisms | Autophagy effect on MSCs | References |
|---|---|---|---|
| Cecal ligation and puncture mouse model | Inflammatory microenvironment-induced autophagy inhibits the expression of the prosurvival gene Bcl-2 via suppressing reactive oxygen species/mitogen-activated protein kinase 1/3 pathway | Promotes apoptosis | [19] |
| Mice model | Activation of autophagy could reduce the adipogenic differentiation and promote proliferation of aged MSCs | Reverses aging | [107] |
| Mice model | Inhibition of autophagy could turn young MSCs into a relatively aged state by reducing their osteogenic differentiation and proliferation capacity and enhancing their adipogenic differentiation capacity | Promotes aging | [107] |
| Mice model | Impaired autophagy led to increased ROS and further induced the p16INK4a axis | Promotes aging | [108] |
| Cellular experiment | High glycemic treatment of MSCs increased ROS-mediated autophagy, leading to the formation of Beclin-1, Atg5, Atg7, Atg12, and LC3-II autophagosomes, which induced MSCs aging and local inflammation | Promotes aging | [109] |
5. Targeting Autophagy in IPF
Treatment choices for IPF are quite restricted. While recent trials have demonstrated the effectiveness of pirfenidone and nintedanib in slowing the decline of lung function in IPF patients, no medication can reverse or entirely prevent the progression of IPF [110, 111]. IPF has emerged as the most prevalent indication for lung transplantation, with a 5-year survival rate posttransplant just slightly exceeding 50% according to the International Society of Heart and Lung Transplant (ISHLT) registry [112, 113]. However, lung transplants continue to face significant clinical constraints, primarily due to the shortage of available donors [114]. In addition to investigating autophagy mechanisms in IPF, multiple drugs have been introduced to mitigate the progression of the disease [115]. Furthermore, an array of compounds with therapeutic potential in IPF by modulating autophagy are steadily emerging [116, 117].
6. Current Drugs to Treat IPF
In the past 10 years, researchers have spent a lot of effort on IPF drug design, but still only two approved drugs, pirfenidone (Pirfenidone) and nintedanib (NIT), have been used in patients with IPF. Pirfenidone and nintedanib have yielded a discernible elevation in mortality and PF progression among IPF patients under clinical observation [118]. Pirfenidone exerts its antifibrotic effects primarily through inhibition of TGF-β1, a critical mediator involved in IPF development [119, 120]. Pirfenidone, an oral pyridine, reduces extracellular matrix (ECM) deposition via interfering with collagen production and fibrinolytic processes by reducing the production of certain tissue necrosis factors and growth factors [121–123]. Notably, pirfenidone can activate ATG7- and ATG5-dependent canonical autophagy in lung fibroblasts, as a decrease in EGFP-LC3 dot formation as well as LC3 conversion from LC3-I to LC3-II was observed when ATG5 and ATG7 were knocked down [42]. Although pirfenidone induced autophagy has been clearly demonstrated, the precise mechanism of pirfenidone inhibiting lung fibrosis via autophagy during IPF pathogenesis should be futher examined.
Nintedanib is another therapeutic medication possessing antifibrotic attributes, operating as a multityrosine kinase inhibitor (MTKI) [124]. Nintedanib can inhibit the fibrosis process by targeting PDGFRα-β, FGFR1-3, VEGFR1-3, and SFK [125–129]. Nitidanib has shown antifibrotic and anti-inflammatory activity in animal models of lung fibrosis, interfering with fibrotic processes such as fibroblast proliferation, migration, and differentiation and significantly reducing the deposition of lung collagen [130, 131]. In addition, efficacy and safety of nintedanib in patients with IPF have been demonstrated in phase 3 clinical trials, reducing the decline in forced vital capacity (FVC) and slowing the progression of fibrosis [120]. Furthermore, certain studies have substantiated the ability of nintedanib to restrain the growth of specific lung vascular cells, including endothelial cells and pulmonary artery vascular smooth muscle cells [131]. Notably, the research revealed that nintedanib effectively boosted autophagy by assessing the LC3-I/II ratio [132]. Another investigation produced consistent findings, confirming that nintedanib enhanced autophagic flux in fibroblasts confirmed by observing increased LC3-II formation and induced Beclin-1-dependent, ATG7-independent autophagy in fibroblasts [133]. Presently, due to extensive research into autophagy regulation, several autophagy-targeted pulmonary antifibrotic treatments have been identified [134, 135].
7. Potential Compounds to IPF
Amounting research mainly to identify the new molecular targets and therapy choices. Berberine, an important protoberberine alkaloid, shows various pharmacological activities that have been widely used in different therapeutic areas [136]. Berberine is extensively distributed in a variety of herbs and its synthetic derivatives have gained significant interest in clinical applications [136]. Importantly, berberine as an autophagy modulator can be efficient against PF via modulating autophagy [137, 138]. Berberine can remarkably enhance the expression of LC3 and Beclin-1, while significant attenuation of p-mTOR, Akt, and MAPK signaling pathways, thereby stimulating autophagosome formation and initiating autophagy [139, 140].
Spermidine, an autophagy-inducer, enhances Beclin-1-dependent autophagy and autophagy modulators in IPF fibroblasts and bleomycin-induced mouse lungs [141]. Specifically, spermidine upregulated autophagic flux, leading to an increase in the LC3B-I/II ratio and the expression of ATG7 and Beclin-1 in IPF fibroblasts and bleomycin-induced mouse lungs [141]. In addition, spermidine can reverse autophagy impairment by decreasing the expression of p-mTOR in bleomycin-induced lungs [141]. These finding demonstrate that spermidine enhances autophagy and that this effect may hold promise in the treatment of IPF.
Immune checkpoint PD-1 play a critical role in controlling inflammatory response to injury in the normal lung tissues. Programed death ligand-1/programmed cell death 1 (PD-L1/PD-1) axis is one of the most essential immune checkpoints in regulating immunotherapy. In IPF patients, PD-L1 was found to have overexpression on alveolar macrophages (AMs) but was negative on fibroblasts and myofibroblast membranes [142, 143]. Blocking PD-L1 can reverse PF by increasing phagocytosis of profibrotic fibroblasts in vivo mouse model of fibrosis [144]. The anti-PD-L1 monoclonal antibody (anti-PD-L1 mAb) has been discovered to significantly inhibit the proliferation and migration of lung fibroblasts and reduce the deposition of ECM [145]. It can increase the expression of the autophagy-related marker protein SQSTM1 and the accumulation of LC3II, promote the formation of autophagosomes, and ultimately induce autophagy activation in PF [145]. These evidences show that anti-PD-L1 therapy has the potential to alleviate PF, offering a novel approach to treating IPF.
Bergenin, a compound derived from a variety of medicinal plants, is a major component from Bergenia stracheyi (Saxifragaceae) [146]. Bergenin could attenuate bleomycin-induced PF in mice by suppressing the myofibroblast activation and promoting the autophagy and the apoptosis of myofibroblasts [147]. The study revealed that berberine significantly reduced the phosphorylation levels of mTOR, ULK1, and S6 and decreased the expression levels of typical fibroblast activation markers α-SMA and ECM protein collagen I, thus promoting autophagy and alleviating PF [147]. Moreover, bergenin has the potential to maintain normal autophagy and apoptosis balance in IPF fibroblasts by modulating energy metabolism [147]. Overall, there is a pressing requirement for additional investigations and animal model assessments to facilitate the development and validation of novel therapeutic agents for IPF that specifically target autophagy.
8. Conclusion
With the developments in regenerative medicine technology, stem cell therapy has been tested for safety and efficacy in various lung diseases. However, the abnormal health status of MSCs can affect their own therapeutic function, especially in IPF. The new evidence indicates that modulation of autophagy in MSCs plays an important role in the therapeutic action exerted by MSCs. To either induce or inhibit autophagy activity in lung tissue microenvironment can affect the ability of MSCs to repair damaged tissues, specially IPF. Elevating autophagy generally enhances cellular functions and maintains homeostasis, contributing to prolonged lifespan and improved pulmonary health. However, it is crucial to recognize that a substantial increase in autophagy may potentially reduce lifespan and adversely affect lung health. The therapeutic targeting of autophagy in aging and age-related lung diseases is contingent upon the specific autophagic defects present in different cell types. From existing literature, it can be postulated that enhancing autophagic activity to augment MSCs function in IPF represents a promising therapeutic strategy to enhance lung function in the elderly. The sustained health benefits for MSCs are likely to result from achieving an optimal balance of autophagy and are influenced by both lung tissue and organismal age. This review aims to provide more comprehensive insights into how autophagy affects the therapeutic properties of MSCs, thereby broadening the horizon of clinical utilization of MSCs for the treatment of IPF. The development of novel MSC therapies targeting the autophagy signaling pathway may provide an innovative and attractive approach to the field of regenerative medicine.
Acknowledgments
This work was supported by the Natural Science Foundation of Sichuan Province (grant number: 23NSFSC1556), Sichuan Province Youth Innovation Research Project (grant number: Q21003), and Sichuan Province Key Research and Development Project of the Science and Technology Plan (grant number: 2022YFS0023).
Abbreviations
- IPF:
Idiopathic pulmonary fibrosis
- MSCs:
Mesenchymal stem cells
- AECs:
Alveolar epithelial cells
- PF:
Pulmonary fibrosis
- MA:
Macroautophagy
- AMP:
Adenosine 5′-monophosphate
- AMPK:
AMP-activated protein kinase
- 3-MA:
3-methyladenine
- AECIIs:
Alveolar epithelial type II cells
- AMs:
Alveolar macrophages
- ROS:
reactive oxygen species
- EVs:
Extracellular vesicles
- KGF:
Keratinocyte growth factor
- HGF:
Hepatocyte growth factor
- EGF:
Epidermal growth factor
- VEGF:
Vascular endothelial growth factor
- IFN:
Interferon
- IL:
Interleukin
- ISHLT:
International society of heart and lung transplant
- NIT:
Nintedanib
- ECM:
Extracellular matrix
- FVC:
Forced vital capacity
- PD-1:
Programed death-1
- PD-L1:
Programed cell death ligand-1
- AMs:
Alveolar macrophages
- Anti-PD-L1 mAb:
anti-PD-L1 monoclonal antibody.
Contributor Information
Yang Yang, Email: 18981838300@189.cn.
Wei Sun, Email: dlswdoc@163.com.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Wei Sun designed this review. Hongxia Tao wrote the manuscript. Qin Lv and Jing Zhang drew the figures. Lijuan Chen and Yang Yang revised the manuscript. All authors read and approved the final version of the manuscript. All authors approve the submission of the manuscript.
References
- 1.Heukels P., Moor C. C., von der Thüsen J. H., Wijsenbeek M. S., Kool M. Inflammation and immunity in IPF pathogenesis and treatment. Respiratory Medicine . 2019;147:79–91. doi: 10.1016/j.rmed.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Richeldi L., Collard H. R., Jones M. G. Idiopathic pulmonary fibrosis. Lancet . 2017;389(10082):1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 3.Wolters P. J., Blackwell T. S., Eickelberg O., et al. Time for a change: is idiopathic pulmonary fibrosis still idiopathic and only fibrotic? The Lancet. Respiratory Medicine . 2018;6(2):154–160. doi: 10.1016/S2213-2600(18)30007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todd N. W., Atamas S. P., Hines S. E., et al. Demystifying idiopathic interstitial pneumonia: time for more etiology-focused nomenclature in interstitial lung disease. Expert Review of Respiratory Medicine . 2022;16(2):235–245. doi: 10.1080/17476348.2022.2030710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Podolanczuk A. J., Thomson C. C., Remy-Jardin M., et al. Idiopathic pulmonary fibrosis: state of the art for 2023. European Respiratory Journal . 2023;61 doi: 10.1183/13993003.00957-2022.2200957 [DOI] [PubMed] [Google Scholar]
- 6.Sgalla G., Iovene B., Calvello M., Ori M., Varone F., Richeldi L. Idiopathic pulmonary fibrosis: pathogenesis and management. Respiratory Research . 2018;19 doi: 10.1186/s12931-018-0730-2.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalil N., Manganas H., Ryerson C. J., et al. Phase 2 clinical trial of PBI-4050 in patients with idiopathic pulmonary fibrosis. The European Respiratory Journal . 2019;53(3) doi: 10.1183/13993003.00663-2018.1800663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mora A. L., Rojas M., Pardo A., Selman M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nature Reviews Drug Discovery . 2017;16:755–772. doi: 10.1038/nrd.2017.170. [DOI] [PubMed] [Google Scholar]
- 9.Fleetwood K., McCool R., Glanville J., et al. Systematic review and network meta-analysis of idiopathic pulmonary fibrosis treatments. Journal of Managed Care & Specialty Pharmacy . 2017;23(3-b Suppl):S5–S16. doi: 10.18553/jmcp.2017.23.3-b.s5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belhassen M., Dalon F., Nolin M., Van Ganse E. Comparative outcomes in patients receiving pirfenidone or nintedanib for idiopathic pulmonary fibrosis. Respiratory Research . 2021;22 doi: 10.1186/s12931-021-01714-y.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merkt W., Bueno M., Mora A. L., Lagares D. Senotherapeutics: targeting senescence in idiopathic pulmonary fibrosis. Seminars in Cell & Developmental Biology . 2020;101:104–110. doi: 10.1016/j.semcdb.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mei Q., Liu Z., Zuo H., Yang Z., Qu J. Idiopathic pulmonary fibrosis: an update on pathogenesis. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.797292.797292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li D.-Y., Li R.-F., Sun D.-X., Pu D.-D., Zhang Y.-H. Mesenchymal stem cell therapy in pulmonary fibrosis: a meta-analysis of preclinical studies. Stem Cell Research & Therapy . 2021;12 doi: 10.1186/s13287-021-02496-2.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernardo M. E., Locatelli F., Fibbe W. E. Mesenchymal stromal cells: a novel treatment modality for tissue repair. Annals of the New York Academy of Sciences . 2009;1176(1):101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 15.Pereira A. R., Trivanović D., Stahlhut P., Rudert M., Groll J., Herrmann M. Preservation of the naïve features of mesenchymal stromal cells in vitro: comparison of cell- and bone-derived decellularized extracellular matrix. Journal of Tissue Engineering . 2022;13:1–20. doi: 10.1177/20417314221074453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceccariglia S., Cargnoni A., Silini A. R., Parolini O. Autophagy: a potential key contributor to the therapeutic action of mesenchymal stem cells. Autophagy . 2020;16(1):28–37. doi: 10.1080/15548627.2019.1630223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ornatowski W., Lu Q., Yegambaram M., et al. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biology . 2020;36 doi: 10.1016/j.redox.2020.101679.101679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho T. T., Warr M. R., Adelman E. R., et al. Autophagy maintains the metabolism and function of young and old stem cells. Nature . 2017;543(7644):205–210. doi: 10.1038/nature21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang S., Yu Z.-M., Zhang C.-Y., et al. Autophagy promotes apoptosis of mesenchymal stem cells under inflammatory microenvironment. Stem Cell Research & Therapy . 2015;6 doi: 10.1186/s13287-015-0245-4.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero Y., Bueno M., Ramirez R., et al. mTORC1 activation decreases autophagy in aging and idiopathic pulmonary fibrosis and contributes to apoptosis resistance in IPF fibroblasts. Aging Cell . 2016;15(6):1103–1112. doi: 10.1111/acel.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S., Liu G., Gu M., et al. A novel therapeutic approach for IPF: based on the “Autophagy–Apoptosis” balance regulation of Zukamu granules in alveolar macrophages. Journal of Ethnopharmacology . 2022;297 doi: 10.1016/j.jep.2022.115568.115568 [DOI] [PubMed] [Google Scholar]
- 22.Fan G., Liu J., Wu Z., Li C., Zhang Y. Development and validation of the prognostic model based on autophagy-associated genes in idiopathic pulmonary fibrosis. Frontiers in Immunology . 2022;13 doi: 10.3389/fimmu.2022.1049361.1049361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahangari F., Price N. L., Malik S., et al. microRNA-33 deficiency in macrophages enhances autophagy, improves mitochondrial homeostasis, and protects against lung fibrosis. JCI Insight . 2023;8(4) doi: 10.1172/jci.insight.158100.e158100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye W., Xu K., Huang D., et al. Age-related increases of macroautophagy and chaperone-mediated autophagy in rat nucleus pulposus. Connective Tissue Research . 2011;52(6):472–478. doi: 10.3109/03008207.2011.564336. [DOI] [PubMed] [Google Scholar]
- 25.Jewell J. L., Guan K.-L. Nutrient signaling to mTOR and cell growth. Trends in Biochemical Sciences . 2013;38(5):233–242. doi: 10.1016/j.tibs.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zoncu R., Efeyan A., Sabatini D. M. mTOR: from growth signal integration to cancer, diabetes and ageing. Nature Reviews. Molecular Cell Biology . 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pópulo H., Lopes J. M., Soares P. The mTOR signalling pathway in human cancer. International Journal of Molecular Sciences . 2012;13(2):1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabatini D. M. mTOR and cancer: insights into a complex relationship. Nature Reviews Cancer . 2006;6(9):729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 29.Wouters B. G., Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nature Reviews Cancer . 2008;8(11):851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 30.Glick D., Barth S., Macleod K. F. Autophagy: cellular and molecular mechanisms. The Journal of Pathology . 2010;221(1):3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vargas J. N. S., Hamasaki M., Kawabata T., Youle R. J., Yoshimori T. The mechanisms and roles of selective autophagy in mammals. Nature Reviews Molecular Cell Biology . 2023;24:167–185. doi: 10.1038/s41580-022-00542-2. [DOI] [PubMed] [Google Scholar]
- 32.Moore M. N. Autophagy as a second level protective process in conferring resistance to environmentally-induced oxidative stress. Autophagy . 2008;4(2):254–256. doi: 10.4161/auto.5528. [DOI] [PubMed] [Google Scholar]
- 33.Li M., Tan J., Miao Y., Lei P., Zhang Q. The dual role of autophagy under hypoxia-involvement of interaction between autophagy and apoptosis. Apoptosis . 2015;20(6):769–777. doi: 10.1007/s10495-015-1110-8. [DOI] [PubMed] [Google Scholar]
- 34.Nishiyama J., Matsuda K., Kakegawa W., et al. Reevaluation of neurodegeneration in lurcher mice: constitutive ion fluxes cause cell death with, not by, autophagy. Journal of Neuroscience . 2010;30(6):2177–2187. doi: 10.1523/JNEUROSCI.6030-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klionsky D. J., Abeliovich H., Agostinis P., et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy . 2008;4(2):151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hewlett J. C., Kropski J. A., Blackwell T. S. Idiopathic pulmonary fibrosis: epithelial–mesenchymal interactions and emerging therapeutic targets. Matrix Biology . 2018;71-72:112–127. doi: 10.1016/j.matbio.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Dwyer D. N., Ashley S. L., Moore B. B. Influences of innate immunity, autophagy, and fibroblast activation in the pathogenesis of lung fibrosis. American Journal of Physiology-Lung Cellular and Molecular Physiology . 2016;311(3):L590–L601. doi: 10.1152/ajplung.00221.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Im J., Hergert P., Nho R. S. Reduced FoxO3a expression causes low autophagy in idiopathic pulmonary fibrosis fibroblasts on collagen matrices. American Journal of Physiology-Lung Cellular and Molecular Physiology . 2015;309(6):L552–L561. doi: 10.1152/ajplung.00079.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricci A., Cherubini E., Scozzi D., et al. Decreased expression of autophagic beclin 1 protein in idiopathic pulmonary fibrosis fibroblasts. Journal of Cellular Physiology . 2013;228(7):1516–1524. doi: 10.1002/jcp.24307. [DOI] [PubMed] [Google Scholar]
- 40.Bueno M., Lai Y.-C., Romero Y., et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. The Journal of Clinical Investigation . 2015;125(2):521–538. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi K., Araya J., Minagawa S., et al. Involvement of PARK2-mediated mitophagy in idiopathic pulmonary fibrosis pathogenesis. Journal of Immunology . 2016;197(2):504–516. doi: 10.4049/jimmunol.1600265. [DOI] [PubMed] [Google Scholar]
- 42.Kurita Y., Araya J., Minagawa S., et al. Pirfenidone inhibits myofibroblast differentiation and lung fibrosis development during insufficient mitophagy. Respiratory Research . 2017;18(1) doi: 10.1186/s12931-017-0600-3.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larson-Casey J. L., Deshane J. S., Ryan A. J., Thannickal V. J., Carter A. B. Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity . 2016;44(3):582–596. doi: 10.1016/j.immuni.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazarus H. M., Haynesworth S. E., Gerson S. L., Rosenthal N. S., Caplan A. I. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant . 1995;16(4):557–564. [PubMed] [Google Scholar]
- 45.Moghadasi S., Elveny M., Rahman H. S., et al. A paradigm shift in cell-free approach: the emerging role of MSCs-derived exosomes in regenerative medicine. Journal of Translational Medicine . 2021;19(1) doi: 10.1186/s12967-021-02980-6.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu Q., El-Hashash A. H. K. Cell-based therapy for idiopathic pulmonary fibrosis. Stem Cell Investigation . 2019;6 doi: 10.21037/sci.2019.06.09.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kariminekoo S., Movassaghpour A., Rahimzadeh A., Talebi M., Shamsasenjan K., Akbarzadeh A. Implications of mesenchymal stem cells in regenerative medicine. Artificial Cells, Nanomedicine, and Biotechnology . 2016;44(3):749–757. doi: 10.3109/21691401.2015.1129620. [DOI] [PubMed] [Google Scholar]
- 48.Chen J., Li Y., Wang L., et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke . 2001;32(4):1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 49.Chopp M., Li Y. Treatment of neural injury with marrow stromal cells. The Lancet. Neurology . 2002;1(2):92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Y., Yamamoto Y., Xiao Z., Ochiya T. The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. Journal of Clinical Medicine . 2019;8(7) doi: 10.3390/jcm8071025.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rani S., Ryan A. E., Griffin M. D., Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Molecular Therapy . 2015;23(5):812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang S., Liu P., Jiang Y., Wang Z., Dai H., Wang C. Therapeutic applications of mesenchymal stem cells in idiopathic pulmonary fibrosis. Frontiers in Cell and Developmental Biology . 2021;9 doi: 10.3389/fcell.2021.639657.639657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mansouri N., Willis G. R., Fernandez-Gonzalez A., et al. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight . 2019;4(21) doi: 10.1172/jci.insight.128060.e128060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu L., Ding L., Wang L., et al. Umbilical cord-derived mesenchymal stem cells on scaffolds facilitate collagen degradation via upregulation of MMP-9 in rat uterine scars. Stem Cell Research & Therapy . 2017;8(1) doi: 10.1186/s13287-017-0535-0.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu K.-A., Wang S.-Y., Yeh C.-C., et al. Reversal of bleomycin-induced rat pulmonary fibrosis by a xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly. Theranostics . 2019;9(22):6646–6664. doi: 10.7150/thno.33741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cárdenes N., Álvarez D., Sellarés J., et al. Senescence of bone marrow-derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis. Stem Cell Research & Therapy . 2018;9:1–10. doi: 10.1186/s13287-018-0970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuang Y., Zheng X., Zhang L., et al. Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR-25. Journal of Extracellular Vesicles . 2020;10(1) doi: 10.1002/jev2.12024.e12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H. Y., Li C., Liu W. H., et al. Autophagy inhibition via Becn1 downregulation improves the mesenchymal stem cells antifibrotic potential in experimental liver fibrosis. Journal of Cellular Physiology . 2020;235(3):2722–2737. doi: 10.1002/jcp.29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang M., Sun W., Xiao L., et al. Mesenchymal stromal cells suppress hippocampal neuron autophagy stress induced by hypoxic–ischemic brain damage: the possible role of endogenous IL-6 secretion. Neural Plasticity . 2020;2020:12. doi: 10.1155/2020/8822579.8822579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu J., Kuang L., Chen C., et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials . 2019;206:87–100. doi: 10.1016/j.biomaterials.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 61.Chen W. X., Zhou J., Zhou S. S., et al. Microvesicles derived from human Wharton’s jelly mesenchymal stem cells enhance autophagy and ameliorate acute lung injury via delivery of miR-100. Stem Cell Research & Therapy . 2020;11(1) doi: 10.1186/s13287-020-01617-7.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi L., Han Q., Hong Y., et al. Inhibition of miR-199a-5p rejuvenates aged mesenchymal stem cells derived from patients with idiopathic pulmonary fibrosis and improves their therapeutic efficacy in experimental pulmonary fibrosis. Stem Cell Research & Therapy . 2021;12(1) doi: 10.1186/s13287-021-02215-x.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park H. J., Shin J. Y., Kim H. N., Oh S. H., Lee P. H. Neuroprotective effects of mesenchymal stem cells through autophagy modulation in a Parkinsonian model. Neurobiology of Aging . 2014;35(8):1920–1928. doi: 10.1016/j.neurobiolaging.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 64.Shin J. Y., Park H. J., Kim H. N., et al. Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models. Autophagy . 2014;10(1):32–44. doi: 10.4161/auto.26508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bergmann C. A., Beltran S., Vega-Letter A. M., et al. The autophagy protein pacer positively regulates the therapeutic potential of mesenchymal stem cells in a mouse model of DSS-induced colitis. Cells . 2022;11(9) doi: 10.3390/cells11091503.1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sávio-Silva C., Soinski-Sousa P. E., Simplício-Filho A., Bastos R. M. C., Beyerstedt S., Rangel E. B. Therapeutic potential of mesenchymal stem cells in a pre-clinical model of diabetic kidney disease and obesity. International Journal of Molecular Sciences . 2021;22(4) doi: 10.3390/ijms22041546.1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harrell C. R., Sadikot R., Pascual J., et al. Mesenchymal stem cell-based therapy of inflammatory lung diseases: current understanding and future perspectives. Stem Cells International . 2019;2019:14. doi: 10.1155/2019/4236973.4236973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oliver L., Hue E., Priault M., Vallette F. M. Basal autophagy decreased during the differentiation of human adult mesenchymal stem cells. Stem Cells and Development . 2012;21(15):2779–2788. doi: 10.1089/scd.2012.0124. [DOI] [PubMed] [Google Scholar]
- 69.Nuschke A., Rodrigues M., Stolz D. B., Chu C. T., Griffith L., Wells A. Human mesenchymal stem cells/multipotent stromal cells consume accumulated autophagosomes early in differentiation. Stem Cell Research & Therapy . 2014;5(6) doi: 10.1186/scrt530.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dang S., Xu H., Xu C., et al. Autophagy regulates the therapeutic potential of mesenchymal stem cells in experimental autoimmune encephalomyelitis. Autophagy . 2014;10(7):1301–1315. doi: 10.4161/auto.28771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.An Y., Liu W. J., Xue P., et al. Autophagy promotes MSC-mediated vascularization in cutaneous wound healing via regulation of VEGF secretion. Cell Death & Disease . 2018;9(2) doi: 10.1038/s41419-017-0082-8.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao E., Yang H. Q., Gan Y.-H., et al. Brief reports: TRPM7 senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells . 2015;33(2):615–621. doi: 10.1002/stem.1858. [DOI] [PubMed] [Google Scholar]
- 73.Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nature Reviews Immunology . 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 74.Singer N. G., Caplan A. I. Mesenchymal stem cells: mechanisms of inflammation. Annual Review of Pathology . 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 75.Le Blanc K., Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nature Reviews Immunology . 2012;12(5):383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 76.Bernardo M. E., Fibbe W. E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell . 2013;13(4):392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y., Wang L., Kikuiri T., et al. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nature Medicine . 2011;17(12):1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi Y., Su J., Roberts A. I., Shou P., Rabson A. B., Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends in Immunology . 2012;33(3):136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ren G., Zhang L., Zhao X., et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell . 2008;2(2):141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 80.Spaggiari G. M., Capobianco A., Becchetti S., Mingari M. C., Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood . 2006;107(4):1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 81.Németh K., Leelahavanichkul A., Yuen P. S., et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature Medicine . 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fimia G. M., Stoykova A., Romagnoli A., et al. Ambra1 regulates autophagy and development of the nervous system. Nature . 2007;447(7148):1121–1125. doi: 10.1038/nature05925. [DOI] [PubMed] [Google Scholar]
- 83.Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. Autophagy fights disease through cellular self-digestion. Nature . 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Degenhardt K., Mathew R., Beaudoin B., et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell . 2006;10(1):51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu L., Alva A., Su H., et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science . 2004;304(5676):1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 86.Liu J., Ding Y., Liu Z., Liang X. Senescence in mesenchymal stem cells: functional alterations, molecular mechanisms, and rejuvenation strategies. Frontiers in Cell and Developmental Biology . 2020;8 doi: 10.3389/fcell.2020.00258.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y., Ravikumar M., Ling L., Nurcombe V., Cool S. M. Age-related changes in the inflammatory status of human mesenchymal stem cells: implications for cell therapy. Stem Cell Reports . 2021;16(4):694–707. doi: 10.1016/j.stemcr.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Turinetto V., Vitale E., Giachino C. Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. International Journal of Molecular Sciences . 2016;17(7) doi: 10.3390/ijms17071164.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neri S., Borzì R. M. Molecular mechanisms contributing to mesenchymal stromal cell aging. Biomolecules . 2020;10(2) doi: 10.3390/biom10020340.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Del Roso A., Vittorini S., Cavallini G., et al. Ageing-related changes in the in vivo function of rat liver macroautophagy and proteolysis. Experimental Gerontology . 2003;38(5):519–527. doi: 10.1016/s0531-5565(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 91.Terman A. The effect of age on formation and elimination of autophagic vacuoles in mouse hepatocytes. Gerontologia . 1995;41(Suppl. 2):319–326. doi: 10.1159/000213753. [DOI] [PubMed] [Google Scholar]
- 92.Leidal A. M., Levine B., Debnath J. Autophagy and the cell biology of age-related disease. Nature Cell Biology . 2018;20(12):1338–1348. doi: 10.1038/s41556-018-0235-8. [DOI] [PubMed] [Google Scholar]
- 93.Sarkis G. J., Ashcom J. D., Hawdon J. M., Jacobson L. A. Decline in protease activities with age in the nematode caenorhabditis elegans. Mechanisms of Ageing and Development . 1988;45(3):191–201. doi: 10.1016/0047-6374(88)90001-2. [DOI] [PubMed] [Google Scholar]
- 94.Hughes A. L., Gottschling D. E. An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature . 2012;492(7428):261–265. doi: 10.1038/nature11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang J. T., Kumsta C., Hellman A. B., Adams L. M., Hansen M. Spatiotemporal regulation of autophagy during caenorhabditis elegans aging. Elife . 2017;6 doi: 10.7554/eLife.18459.e18459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilhelm T., Byrne J., Medina R., et al. Neuronal inhibition of the autophagy nucleation complex extends life span in post-reproductive C. elegans. Genes & development . 2017;31(15):1561–1572. doi: 10.1101/gad.301648.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simonsen A., Cumming R. C., Brech A., Isakson P., Schubert D. R., Finley K. D. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy . 2008;4(2):176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- 98.Kaushik S., Arias E., Kwon H., et al. Loss of autophagy in hypothalamic POMC neurons impairs lipolysis. EMBO reports . 2012;13(3):258–265. doi: 10.1038/embor.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ott C., König J., Höhn A., Jung T., Grune T. Macroautophagy is impaired in old murine brain tissue as well as in senescent human fibroblasts. Redox Biology . 2016;10:266–273. doi: 10.1016/j.redox.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lipinski M. M., Zheng B., Lu T., et al. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America . 2010;107(32):14164–14169. doi: 10.1073/pnas.1009485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Menzies F. M., Fleming A., Rubinsztein D. C. Compromised autophagy and neurodegenerative diseases. Nature Reviews Neuroscience . 2015;16(6):345–357. doi: 10.1038/nrn3961. [DOI] [PubMed] [Google Scholar]
- 102.Cheon S. Y., Kim H., Rubinsztein D. C., Lee J. E. Autophagy, cellular aging and age-related human diseases. Experimental neurobiology . 2019;28(6):643–657. doi: 10.5607/en.2019.28.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matecic M., Smith D. L., Pan X., et al. A microarray-based genetic screen for yeast chronological aging factors. PLOS Genetics . 2010;6(4) doi: 10.1371/journal.pgen.1000921.e1000921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hansen M., Rubinsztein D. C., Walker D. W. Autophagy as a promoter of longevity: insights from model organisms. Nature Reviews. Molecular Cell Biology . 2018;19(9):579–593. doi: 10.1038/s41580-018-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meléndez A., Tallóczy Z., Seaman M., Eskelinen E.-L., Hall D. H., Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science . 2003;301(5638):1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- 106.Lapierre L. R., De Magalhaes Filho C. D., McQuary P. R., et al. The TFEB orthologue HLH-30 regulates autophagy and modulates longevity in Caenorhabditis elegans. Nature Communications . 2013;4 doi: 10.1038/ncomms3267.2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma Y., Qi M., An Y., et al. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell . 2018;17(1) doi: 10.1111/acel.12709.e12709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.García-Prat L., Martínez-Vicente M., Perdiguero E., et al. Autophagy maintains stemness by preventing senescence. Nature . 2016;529(7584):37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- 109.Zheng Y., Lei Y., Hu C., Hu C. p53 regulates autophagic activity in senescent rat mesenchymal stromal cells. Experimental Gerontology . 2016;75:64–71. doi: 10.1016/j.exger.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 110.Kreuter M., Bonella F., Wijsenbeek M., Maher T. M., Spagnolo P. Pharmacological treatment of idiopathic pulmonary fibrosis: current approaches, unsolved issues, and future perspectives. BioMed Research International . 2015;2015:10. doi: 10.1155/2015/329481.329481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gan Y., Herzog E. L., Gomer R. H. Pirfenidone treatment of idiopathic pulmonary fibrosis. Therapeutics and Clinical Risk Management . 2011;7:39–47. doi: 10.2147/TCRM.S12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Christie J. D., Edwards L. B., Kucheryavaya A. Y., et al. The registry of the international society for heart and lung transplantation: 29th adult lung and heart–lung transplant report-2012. The Journal of Heart and Lung Transplantation . 2012;31(10):1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 113.Hartert M., Senbaklavacin O., Gohrbandt B., Fischer B. M., Buhl R., Vahld C.-F. Lung transplantation: a treatment option in end-stage lung disease. Deutsches Arzteblatt International . 2014;111(7):107–116. doi: 10.3238/arztebl.2014.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leard L. E., Holm A. M., Valapour M., et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. The Journal of Heart and Lung Transplantation . 2021;40(11):1349–1379. doi: 10.1016/j.healun.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chillappagari S., Schwarz J., Kesireddy V., et al. Therapeutic induction of Bcl2-associated athanogene 3-mediated autophagy in idiopathic pulmonary fibrosis. Clinical and Translational Medicine . 2022;12(7) doi: 10.1002/ctm2.935.e935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Romano A., Giallongo C., La Cava P., et al. Proteomic analysis reveals autophagy as pro-survival pathway elicited by long-term exposure with 5-azacitidine in high-risk myelodysplasia. Frontiers in Pharmacology . 2017;8 doi: 10.3389/fphar.2017.00204.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Evans I. C., Barnes J. L., Garner I. M., et al. Epigenetic regulation of cyclooxygenase-2 by methylation of c8orf4 in pulmonary fibrosis. Clinical Science . 2016;130(8):575–586. doi: 10.1042/CS20150697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Raghu G., Anstrom K. J., King T. E., Jr., Lasky J. A., Martinez F. J. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. The New England Journal of Medicine . 2012;366(21):1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.King T. E., Bradford W. Z., Castro-Bernardini S., et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. The New England Journal of Medicine . 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 120.Richeldi L., du Bois R. M., Raghu G., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. The New England Journal of Medicine . 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 121.Ruwanpura S. M., Thomas B. J., Bardin P. G. Pirfenidone: molecular mechanisms and potential clinical applications in lung disease. American Journal of Respiratory Cell and Molecular Biology . 2020;62(4):413–422. doi: 10.1165/rcmb.2019-0328TR. [DOI] [PubMed] [Google Scholar]
- 122.Ma Z., Zhao C., Chen Q., et al. Antifibrotic effects of a novel pirfenidone derivative in vitro and in vivo. Pulmonary Pharmacology & Therapeutics . 2018;53:100–106. doi: 10.1016/j.pupt.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 123.Qin W., Liu B., Yi M., et al. Antifibrotic agent pirfenidone protects against development of radiation-induced pulmonary fibrosis in a murine model. Radiation Research . 2018;190(4):396–403. doi: 10.1667/RR15017.1. [DOI] [PubMed] [Google Scholar]
- 124.Kuwana M., Azuma A. Nintedanib: new indication for systemic sclerosis-associated interstitial lung disease. Modern Rheumatology . 2020;30(2):225–231. doi: 10.1080/14397595.2019.1696505. [DOI] [PubMed] [Google Scholar]
- 125.Chen S., Wei Y., Li S., et al. Zanubrutinib attenuates bleomycin-induced pulmonary fibrosis by inhibiting the TGF-β1 signaling pathway. International Immunopharmacology . 2022;113, Part A doi: 10.1016/j.intimp.2022.109316.109316 [DOI] [PubMed] [Google Scholar]
- 126.Lamb Y. N. Nintedanib: a review in fibrotic interstitial lung diseases. Drugs . 2021;81(5):575–586. doi: 10.1007/s40265-021-01487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roth G. J., Binder R., Colbatzky F., et al. Nintedanib: from discovery to the clinic. Journal of Medicinal Chemistry . 2015;58(3):1053–1063. doi: 10.1021/jm501562a. [DOI] [PubMed] [Google Scholar]
- 128.Macagno F., Varone F., Leone P. M., et al. New treatment directions for IPF: current status of ongoing and upcoming clinical trials. Expert Review of Respiratory Medicine . 2017;11(7):533–548. doi: 10.1080/17476348.2017.1335601. [DOI] [PubMed] [Google Scholar]
- 129.Hughes G., Toellner H., Morris H., Leonard C., Chaudhuri N. Real world experiences: pirfenidone and nintedanib are effective and well tolerated treatments for idiopathic pulmonary fibrosis. Journal of Clinical Medicine . 2016;5(9) doi: 10.3390/jcm5090078.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wollin L., Maillet I., Quesniaux V., Holweg A., Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. The Journal of Pharmacology and Experimental Therapeutics . 2014;349(2):209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- 131.Wollin L., Wex E., Pautsch A., et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. The European Respiratory Journal . 2015;45(5):1434–1445. doi: 10.1183/09031936.00174914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wollin L., Distler J. H. W., Redente E. F., et al. Potential of nintedanib in treatment of progressive fibrosing interstitial lung diseases. The European Respiratory Journal . 2019;54(3) doi: 10.1183/13993003.00161-2019.1900161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rangarajan S., Kurundkar A., Kurundkar D., et al. Novel mechanisms for the antifibrotic action of nintedanib. American Journal of Respiratory Cell and Molecular Biology . 2016;54(1):51–59. doi: 10.1165/rcmb.2014-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kheirollahi V., Wasnick R. M., Biasin V., et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nature Communications . 2019;10(1) doi: 10.1038/s41467-019-10839-0.2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rangarajan S., Bone N. B., Zmijewska A. A., et al. Metformin reverses established lung fibrosis in a bleomycin model. Nature Medicine . 2018;24(8):1121–1127. doi: 10.1038/s41591-018-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jin Y., Khadka D. B., Cho W.-J. Pharmacological effects of berberine and its derivatives: a patent update. Expert Opinion on Therapeutic Patents . 2016;26(2):229–243. doi: 10.1517/13543776.2016.1118060. [DOI] [PubMed] [Google Scholar]
- 137.Deng Y., Xu J., Zhang X., et al. Berberine attenuates autophagy in adipocytes by targeting BECN1. Autophagy . 2014;10(10):1776–1786. doi: 10.4161/auto.29746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chitra P., Saiprasad G., Manikandan R., Sudhandiran G. Berberine inhibits Smad and non-Smad signaling cascades and enhances autophagy against pulmonary fibrosis. Journal of Molecular Medicine . 2015;93(9):1015–1031. doi: 10.1007/s00109-015-1283-1. [DOI] [PubMed] [Google Scholar]
- 139.Mohammadinejad R., Ahmadi Z., Tavakol S., Ashrafizadeh M. Berberine as a potential autophagy modulator. Journal of Cellular Physiology . 2019;234(9):14914–14926. doi: 10.1002/jcp.28325. [DOI] [PubMed] [Google Scholar]
- 140.Zhang Q., Wang X., Cao S., et al. Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomedicine & Pharmacotherapy . 2020;128 doi: 10.1016/j.biopha.2020.110245.110245 [DOI] [PubMed] [Google Scholar]
- 141.Baek A. R., Hong J., Song K. S., et al. Spermidine attenuates bleomycin-induced lung fibrosis by inducing autophagy and inhibiting endoplasmic reticulum stress (ERS)–induced cell death in mice. Experimental & Molecular Medicine . 2020;52(12):2034–2045. doi: 10.1038/s12276-020-00545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ni K., Liu M., Zheng J., et al. PD-1/PD-L1 pathway mediates the alleviation of pulmonary fibrosis by human mesenchymal stem cells in humanized mice. American Journal of Respiratory Cell and Molecular Biology . 2018;58(6):684–695. doi: 10.1165/rcmb.2017-0326OC. [DOI] [PubMed] [Google Scholar]
- 143.Jovanovic D., Milenkovic M. R., Stevuljevic J. K., et al. Membrane PD-L1 expression and soluble PD-L1 plasma levels in idiopathic pulmonary fibrosis-a pilot study. Journal of Thoracic Disease . 2018;10(12):6660–6669. doi: 10.21037/jtd.2018.11.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cui L., Chen S. Y., Lerbs T., et al. Activation of JUN in fibroblasts promotes pro-fibrotic programme and modulates protective immunity. Nature Communications . 2020;11(1) doi: 10.1038/s41467-020-16466-4.2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lu Y., Zhong W., Liu Y., et al. Anti-PD-L1 antibody alleviates pulmonary fibrosis by inducing autophagy via inhibition of the PI3K/Akt/mTOR pathway. International Immunopharmacology . 2022;104 doi: 10.1016/j.intimp.2021.108504.108504 [DOI] [PubMed] [Google Scholar]
- 146.Nazir N., Koul S., Qurishi M. A., et al. Immunomodulatory effect of bergenin and norbergenin against adjuvant-induced arthritis—a flow cytometric study. Journal of Ethnopharmacology . 2007;112(2):401–405. doi: 10.1016/j.jep.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 147.Li X., Wang Y., Liang J., et al. Bergenin attenuates bleomycin-induced pulmonary fibrosis in mice via inhibiting TGF-β1 signaling pathway. Phytotherapy Research: PTR . 2021;35(10):5808–5822. doi: 10.1002/ptr.7239. [DOI] [PubMed] [Google Scholar]


