Abstract
Mast cells are not only important effector cells in immediate hypersensitivity reactions and immune responses to pathogens but also can contribute to T cell-mediated disorders. However, the mechanisms by which mast cells might influence T cells in such settings are not fully understood. We find that mast cells can enhance proliferation and cytokine production in multiple T cell subsets. Mast cell-dependent enhancement of T cell activation can be promoted by FcεRI-dependent mast cell activation, TNF production by both mast cells and T cells, and mast cell-T cell contact. However, at high concentrations of cells, mast cells can promote T cell activation independent of IgE or TNF. Finally, mast cells also can promote T cell activation by means of soluble factors. These findings identify multiple mechanisms by which mast cells can influence T cell proliferation and cytokine production.
Keywords: allergy, asthma, autoimmunity, cytokines, immune response
Gell and Coombs (1) placed “immediate” and “delayed” hypersensitivity reactions at opposite ends of their new classification scheme, as “type I” and “type IV” reactions. Subsequently, it became clear that IgE-dependent mast cell activation represents a major effector mechanism in many immediate hypersensitivity reactions, whereas various T cell subsets are the major effector cells in delayed hypersensitivity responses (2).
Although the Gell and Coombs classification retains heuristic value, it is now apparent that both mast cell- and effector T cell-dependent mechanisms contribute to the expression of some examples of host defense or immunological disorders. For example, studies in genetically mast cell-deficient mice have shown that mast cells can enhance the development and/or magnitude of certain T cell-associated responses. These responses include those elicited by exogenous antigens (Ags), such as in models of contact hypersensitivity (CHS) (3-5), delayed-type hypersensitivity (6), and asthma (7, 8), and in models of autoimmune diseases, such as experimental autoimmune encephalomyelitis (9), Ab-induced arthritis (10), and inflammatory bowel disease (11). Moreover, in some of these settings, including models of asthma or CHS, mast cell activation may reflect, at least in part, the recognition of specific Ags by IgE (7, 8, 12) or IgG1 (7, 8, 10, 13) Abs bound to FcεRI or FcγRIII receptors on the mast cell surface.
During certain immunological responses, mast cells and T cells may be activated in parallel by independent mechanisms. In addition, mast cells and T cells can influence each other's function. Thus, contact with activated T cells can induce certain mast cells to secrete histamine, TNF, and metalloproteinase 9, and to exhibit enhanced IL-4 mRNA transcription (14-17). Also, purified populations of mast cells can present Ags to T cells by either MHC class I- or class II-restricted mechanisms in vitro (18-21).
Moreover, it is known that certain mast cell products can influence T cell function. Mast cells represent a major potential source of TNF (22, 23), which can have a number of effects on T cell recruitment, activation, and function (24-27), and juxtacrine effects of TNF can contribute to the mechanism by which T cell contact induces mast cells to secrete metalloproteinase 9 (17). Histamine, a major product of mast cells, can promote Th1 and Th2 cell activation (28). Activated mast cells also can secrete many chemokines, including CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), and CCL-5 (RANTES) (29, 30), which can enhance T cell recruitment to sites of inflammation; and recent evidence indicates that leukotriene B4 produced by mast cells also may have a role in regulating T cell migration (31). By using cDNA microarray analysis, we showed that human umbilical cord blood-derived mast cells can express certain costimulatory molecules, including OX40 ligand and 4-1BB ligand, which can promote T cell activation through cell-cell contact, and that stimulation of such mast cells through FcεRI aggregation enhances the expression of mRNA for these molecules (30).
In the present study, we investigated the ability of mast cells to enhance T cell activation and identified multiple mechanisms by which mast cells can stimulate T cell proliferation.
Materials and Methods
Mice. TNF-/- mice were generated from C57BL/6 ES cells (32). FcRγ-/-, C57BL/6 and BALB/c mice were purchased from Taconic Farms (FcRγ-/-) and The Jackson Laboratory (the others). KitW-sh/KitW-sh mice on the C57BL/6 background (33) were kindly provided by P. Besmer (Memorial Sloan-Kettering Cancer Center and Cornell University Graduate School of Medical Sciences, New York). All mice were housed at the animal care facilities at Stanford University Medical Center (Stanford, CA) and were kept under standard temperature, humidity, and timed lighting conditions, provided mouse chow and water ad libitum, and were treated in a humane manner in compliance with National Research Council and Stanford Institutional Animal Care and Use Committee guidelines.
Preparation of Bone Marrow-Derived Cultured Mast Cells (BMCMCs). BMCMCs were obtained by culturing mouse femoral BM cells in 20% WEHI-3 conditioned medium (containing IL-3) for 6-12 weeks, at which time the cells were >98% c-kithighFcεRIαhigh by flow cytometry analysis (data not shown).
Preparation of T Cells. A single-cell suspension of spleen cells was prepared, and red blood cells were lysed in RBC lysing buffer (Sigma). For CD3+ T cell purification, spleen cells were incubated with biotinylated anti-mouse B220 (RA3-6B2), Gr-1 (RB6-8C5), CD11b (M1/70), CD11c (N418), CD49b (DX5), Ter119 (Ter119), and c-kit/CD117 (2B8) for 20 min at 4°C. All Abs were from eBioscience (San Diego). The cells were then washed and incubated with streptavidin beads (Miltenyi Biotec, Auburn, CA) for 20 min at 4°C, and washed again and passed through a magnetic cell-sorting column (Miltenyi Biotec) (>95% CD3+ T cells).
T Cell Mast Cell Coculture. BMCMCs were sensitized with 1 μg/ml H1-ε-26 monoclonal IgE anti-DNP Ab (34) at 37°C overnight. After IgE sensitization, BMCMCs were treated with mitomycin C (50 μg per 107 cells) for 30 min at 37°C. Preliminary experiments showed that mitomycin C treatment did not reduce mast cell survival; also, such mitomycin C-treated mast cells did not proliferate in response to IgE and Ag challenge (e.g., see Figs. 1 and 2 A and C).
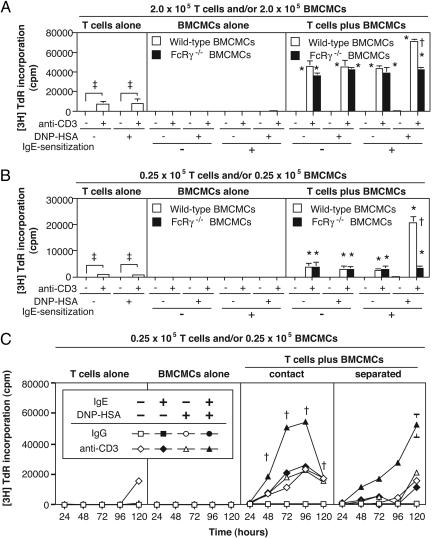
Fig. 1.
Enhancement of T cell proliferation by mast cells. (A) Proliferation of T cells (2.0 × 105 cells) cocultured with WT or FcRγ-/- BMCMCs (2.0 × 105 cells) for 48 h. (B) Proliferation of T cells (0.25 × 105 cells) cocultured with WT or FcRγ-/- BMCMCs (0.25 × 105 cells) at 72 h. Here and in all subsequent figures, “anti-CD3-” indicates treatment with a control hamster IgG mAb. (A and B)†, P < 0.05 vs. corresponding values for FcRγ-/- BMCMCs; *, P < 0.05 vs. corresponding values for BMCMCs alone or T cells alone; ‡, P < 0.05 for comparisons indicated by brackets. Data are mean ± SD (triplicate wells). (C) Proliferation of T cells cocultured with mitomycin C-treated BMCMCs or separated from them by a transwell membrane. †, P < 0.05 vs. corresponding values for cultures of separated T cells and BMCMCs. All values after 24 h in T cells plus BMCMCs (either in contact or separated) are P < 0.05 vs. corresponding values for BMCMCs alone. Data in C are mean ± SD (triplicate wells). All results show representative results from at least three experiments using three different batches of BMCMCs.
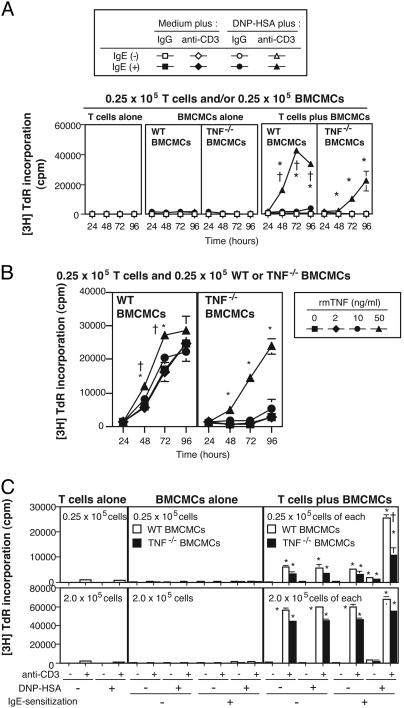
Fig. 2.
Mast cell-derived TNF is required for optimal T cell activation mediated by IgE/Ag-stimulated mast cells. (A) Proliferation of CD3+ T cells cocultured with WT or TNF-/- BMCMCs. *, P < 0.05 vs. corresponding values for any other group in that box; †, P < 0.05 vs. corresponding values for T cells plus TNF-/- BMCMCs. (B) Proliferation of CD3+ T cells cocultured with IgE-sensitized WT or TNF-/- BMCMCs in the presence of DNP-HSA and various concentrations of rmTNF. rmTNF restored the reduced T cell proliferation observed in experiments by using TNF-/- instead of WT mast cells. *, P < 0.05 vs. corresponding values for any other group in that box; †, P < 0.05 vs. corresponding values for T cells plus TNF-/- BMCMCs. (C) Proliferation of CD3+ T cells cocultured with WT or TNF-/- BMCMCs at low (Upper) or high (Lower) cell concentrations. [3H]thymidine incorporation was measured at 72 h at low, and at 48 h at high, cell concentrations. †, P < 0.05 vs. corresponding values for T cells plus TNF-/- BMCMCs; *, P < 0.05 vs. corresponding values for BMCMCs alone. Data in A-C are mean ± SD (A and B) or + SD (C) (triplicate wells) and show representative results from at least three experiments using at least two different batches of BMCMCs.
BMCMCs and T cells were suspended in RPMI medium 1640 (Cellgro), including 50 μM 2-mercaptoethanol (Sigma), 50 μg/ml streptomycin (Invitrogen), 50 units/ml penicillin (Invitrogen), and 10% heat-inactivated FCS (Sigma). T cells were plated on a 96-well flat-bottom plates (BD Falcon) coated with 1 μg/ml anti-mouse CD3 (145-2C11) or hamster IgG (eBioscience) (in some experiments, “anti-CD3 (-)” means the substitution of control IgG for anti-CD3), with mitomycin C (Sigma)-treated, IgE-sensitized, or nonsensitized BMCMCs in the presence or absence of 5 ng/ml 2,4-dinitrophenol-conjugated human serum albumin (DNP-HSA) (Sigma) at 37°C for 24-120 h. In some experiments, recombinant mouse TNF (rmTNF) (PeproTech, Rocky Hill, NJ), anti-CD120a/TNFRI (55R-170, BD Pharmingen), anti-mouse CD120b/TNFRII (TR75-32, BD Pharmingen), and hamster IgG (eBioscience) were added. For coculture of cells in the absence of T cell-mast cell contact, T cells were plated in the lower section of the system, by placing them in Ab-coated MultiScreen 96-well cell culture trays (Millipore). A transwell membrane containing a MultiScreen-HV 96-well filtration plate (MAHVS4510, Millipore) was then placed over the lower plate. BMCMCs were plated in the upper well with or without DNP-HSA. Proliferation was assessed by pulsing with 0.25 μCi of [3H]thymidine (Amersham Biosciences, Piscataway, NJ) for 6 h, harvesting the cells by using Harvester 96 Mach IIIM (Tomtec, Hamden, CT) and measuring incorporated [3H]thymidine by using the Micro Beta system (Amersham Biosciences).
Induction of Mast Cell- and T Cell-Dependent Cutaneous Inflammation in Vivo. CD3+ T cells from C57BL/6 mice were stimulated with 1 μg/ml plate-coated anti-CD3 mAb (145-2C11) at 37°C for 72 h. C57BL/6 BMCMCs were sensitized with 1 μg/ml H1-ε-26 monoclonal IgE anti-DNP Ab (34) at 37°C overnight. Activated T cells (1 × 106 cells), IgE-sensitized BMCMCs (1 × 106 cells), or both (1 × 106 activated T cells and 1 × 106 IgE-sensitized BMCMCs) were injected intradermally into both ear pinnae of naïve C57BL/6 mice. Approximately 30 min after injection of cells, we treated the dorsal surface of the skin of one ear with 20 μl of 0.2% 2,4-dinitrofluorobenzene (DNFB) in acetone:olive oil (4:1 ratio), and the other ear with 20 μl of vehicle alone. After challenge with DNFB or vehicle, ear thickness was measured at multiple intervals by using a dial caliper (G-1A, Ozaki, Tokyo). Data are expressed as: Δ ear thickness (μm) = (thickness of ear treated with DNFB) - (thickness of ear treated with vehicle).
Statistics. A two-tailed Student t test was used for statistical evaluation of the results. Unless otherwise specified, all results are shown as mean ± SD.
Results
T Cells Exhibit Close Associations with Mast Cells at Sites of CHS. CHS is an allergic inflammatory response mediated by hapten-specific T cells. As noted in the Introduction, mast cells can contribute to the expression of CHS under certain circumstances but not in others. In mice sensitized to express CHS responses to 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (oxazolone), by using a protocol in which mast cells contribute to both the sensitization and effector phases of the response (5), many inflammatory cells, including CD3+ T cells, were observed in oxazolone-treated, but not in vehicle-treated, ear skin (Fig. 4, which is published as supporting information on the PNAS web site). Moreover, many CD3+ T cells were colocalized with mast cells in oxazolone-treated ear skin, but not in vehicle-treated sites (Fig. 4C). At 24 and 48 h after oxazolone challenge, 12.4 ± 3.4% (n = 4) and 5.7 ± 1.7% (n = 4) of CD3+ T cells in the dermis appeared to be in contact with resident mast cells. The numbers of cells per mm2 of dermis at these times were 479 ± 204 and 676 ± 240 CD3+ T cells and 135 ± 31 and 163 ± 24 mast cells.
We are not aware of prior reports that quantified mast cell-T cell associations in CHS reactions. However, other investigators have reported that mast cells and T cells can occur in close proximity in CHS and other T cell-associated immune responses (35, 36). These findings support the hypothesis that mast cells and T cells can functionally interact at sites of such T cell-associated immune responses.
Mast Cells Can Enhance T Cell Proliferation. To search for specific effects of mast cells on T cells, we turned to in vitro coculture systems. T cell proliferation was induced when relatively high concentrations of purified splenic T cells (2.0 × 105 cells per well) were stimulated with plate-bound anti-CD3 mAb (Fig. 5A, which is published as supporting information on the PNAS web site); the responses were not affected by DNP-HSA (Fig. 5A). BMCMC proliferation also was most strongly induced at relatively high cell concentrations (1.0 or 2.0 × 105 cells per well), but this response required both IgE and specific Ag, DNP-HSA; the presence or absence of anti-CD3 mAb had no effect on BMCMC proliferation (Fig. 5B).
We then cocultured a small number of T cells with a small number of mitomycin C-treated BMCMCs (0.25 × 105 of each cell type per well). IgE-sensitized BMCMCs markedly enhanced T cell proliferation, but only in the presence of both DNP-HSA and anti-CD3 mAb (Fig. 5C). These results indicate that mast cells activated by means of IgE and specific Ag can significantly enhance T cell proliferation when the T cells are also stimulated suboptimally by means of the T cell receptor (TCR)/CD3 complex.
We next cocultured different numbers of T cells and mitomycin-C treated BMCMCs with or without anti-CD3 mAb, IgE, and specific Ag (Fig. 6, which is published as supporting information on the PNAS web site). At low cell concentrations, T cell proliferation in the presence of anti-CD3 mAb was only potentiated by IgE/Ag-stimulated BMCMCs, whereas, at high cell concentrations, the response was also enhanced by nonsensitized BMCMCs, either in the presence or absence of specific Ag (Fig. 6). However, even at high cell concentrations, the responses to IgE/Ag-stimulated BMCMCs were still greater than those observed in the absence of IgE or DNP-HSA (Fig. 6).
We observed a similar phenomenon by using FcRγ-/- BMCMCs, which cannot signal through the FcεRI in response to IgE and Ag. At high cell concentrations, FcRγ-/- BMCMCs, like WT BMCMCs, strongly enhanced T cell proliferation in the presence of anti-CD3, an effect that was enhanced by IgE and DNP-HSA only in WT BMCMCs (Fig. 1 A). By contrast, at low cell concentrations, only IgE/Ag-stimulated WT BMCMCs strongly enhanced T cell proliferation, whereas either FcRγ-/- or WT BMCMCs could weakly enhance T cell proliferation independent of IgE and/or DNP-HSA (Fig. 1B).
Taken together, these results indicate that there are at least two distinct pathways by which mast cells can enhance T cell proliferation; an FcεRI-dependent pathway, and a mechanism that is independent of signaling through FcεRI.
It has been reported that mast cells can present Ags by MHC class I- or II-dependent mechanisms (18-21). However, we detected no I-Ab expression on unchallenged or IgE- and/or Ag-incubated C57BL/6 BMCMCs (Fig. 7A, which is published as supporting information on the PNAS web site), for 24, 48 or 72 h (data not shown). IgE-sensitized BMCMCs that were cocultured with T cells for 72 h, under conditions the same as those for the experiments shown in Fig. 1 A, expressed low levels of I-Ab; however, such mast cell I-Ab expression was much less than that on freshly isolated splenic CD11c+ DCs (Fig. 7B).
We then tested the ability of mitomycin C-treated BMCMCs to mediate Ag presentation, assessed as their ability to enhance T cell proliferation, in cocultures with CD4+ T cells from the spleens of C57BL/6 OTII transgenic mice, which express a TCR that recognizes ovalbumin (OVA) (37). These mast cell populations weakly enhanced the proliferation of OTII T cells at the highest concentration of OVA tested (5 μM), but these responses were markedly lower (<1%) than those induced in OTII spleen cells by a 10-fold lower concentration of OVA (Fig. 8 A and B, which is published as supporting information on the PNAS web site).
Although our results indicate that these BMCMCs preparations can weakly express Ag presentation function, the conditions examined favor the detection of such an effect, given the very high concentration of Ag-specific T cells in these cultures. It is even possible that some or all of the Ag presentation function in these BMCMC populations might reside in the <2% of the cells in these preparations which are not identifiable as mast cells. These experiments thus argue against the possibility that mast cell Ag presentation function significantly contributes to the high levels of T cell proliferation induced by BMCMCs under our usual conditions of coculture (Fig. 1 A and B).
Role of Cell-Cell Proximity. To investigate the importance of cell-cell proximity for mast cell-dependent enhancement of T cell proliferation, we cocultured T cells with mitomycin C-treated BMCMCs with or without a transmembrane filter to separate the two cell populations. When T cell-mast cell proximity was permitted, peak levels of T cell proliferation were reached at 72 and 96 h, whereas lower responses were observed at these intervals in cocultures in which T cells and mast cells were separated; however, T cell proliferation in the latter setting was substantially increased at 120 h (Fig. 1C).
Thus, both cell-cell proximity and soluble factors can contribute to enhancement of T cell proliferation by IgE/Ag-stimulated mast cells. Moreover, mast cell-dependent enhancement of T cell proliferation, unlike mast cell-dependent Ag presentation, can occur in the absence of mast cell-T cell proximity.
Importance of TNF. We next used WT vs. TNF-/- BMCMCs to assess whether mast cell-derived TNF is required for mast cell-derived T cell proliferation. At low cell concentrations (0.25 × 105 cells per well), IgE/Ag-stimulated TNF-/- BMCMCs did enhance T cell proliferation, but the response was significantly decreased compared with that observed with WT BMCMCs (Fig. 2 A). Moreover, this diminished response was largely reconstituted by the addition of rmTNF (50 ng/ml) (Fig. 2B). By contrast, at high cell concentrations (2.0 × 105 cells per well), TNF-/- BMCMCs were almost as efficient as WT BMCMCs in enhancing T cell proliferation, and, in this setting, the effect was independent of IgE and specific Ag (Fig. 2C).
Therefore, at low concentrations of cells, optimal enhancement of T cell proliferation by mast cells requires IgE/Ag-FcεRI-mediated production of TNF by mast cells and cell-cell contact, whereas, at high cell concentrations, substantial mast cell-dependent T cell proliferation can occur independent of IgE/Ag and mast cell TNF production.
Either CD4+ or CD8+ T cells also can produce TNF after anti-CD3 stimulation (data not shown). Although we used WT T cells in Fig. 2, any T cell-derived TNF in the cultures did not rescue the reduced responses observed by using TNF-deficient mast cells. To assess further the potential contribution of T cell-derived TNF in our coculture systems, we cocultured WT or TNF-/- T cells with WT or TNF-/- BMCMCs in the presence or absence of stimuli.
In accord with our other observations (Fig. 2), at low cell concentrations, IgE/Ag-stimulated TNF-/- BMCMCs did not efficiently enhance WT T cell proliferation in the presence of anti-CD3 Ab (Fig. 9A, which is published as supporting information on the PNAS web site). Interestingly, the proliferation of TNF-/- T cells cocultured with IgE/Ag-stimulated WT BMCMCs was reduced compared with that of WT T cells cocultured with IgE/Ag-stimulated WT BMCMCs in the presence of anti-CD3 mAb (Fig. 9A). Moreover, the response of TNF-/- T cells cocultured with IgE/Ag-stimulated TNF-/- BMCMCs was markedly impaired compared with the other T cell-mast cell coculture combinations (Fig. 9A). These results indicate that both mast cell- and T cell-derived TNF are required for optimal enhancement of T cell proliferation mediated by IgE/Ag-activated mast cells.
TNF binds to two receptors, TNFRI and TNFRII. To assess which receptors might contribute to the enhancement of T cell proliferation by IgE/Ag-activated mast cells, we added a neutralizing Ab to either TNFRI or TNFRII during T cell-mast cell cocultures at low cell concentrations. Either anti-TNFRI or -TNFRII mAbs inhibited the response, reducing it to the levels observed when T cells were cocultured with IgE/Ag-stimulated TNF-/- BMCMCs (Fig. 9B). These results indicate that signaling from both TNF-TNFRI and -TNFRII can contribute to optimal enhancement of T cell proliferation by IgE/Ag-stimulated mast cells.
Mast Cells Can Activate Multiple T Cell Subsets. We next examined the effect of TNF produced by mast cells on CD4+ and CD8+ T cells in T cell-mast cell cocultures. IgE/Ag-stimulated WT BMCMCs enhanced the proliferation of both CD4+ and CD8+ T cells in the presence of anti-CD3 mAb at low cell concentrations, but the responses induced by IgE/Ag-stimulated TNF-/- BMCMCs were markedly reduced (Fig. 10 A and B, which is published as supporting information on the PNAS web site). The levels of the Th1-type cytokine, IFN-γ, in culture supernatants from CD4+ or CD8+ T cells cocultured with IgE/Ag-stimulated TNF-/- BMCMCs were also significantly reduced compared with those in cocultures with WT BMCMCs. The Th2-type cytokine, IL-4, was below the limit of detection in all culture conditions (Fig. 10 A and B). However, the non-Th1/Th2-type cytokine IL-17 (38), produced by activated CD4+ T cells, was also decreased in cocultures of CD4+ T cells plus IgE/Ag-stimulated TNF-/- BMCMCs (Fig. 10A). These results indicate that mast cell-derived TNF is required for optimal expression of the IgE/Ag-FcεRI-dependent mechanism of mast cell enhancement of activation of both CD4+ and CD8+ T cells.
CD4+ T cells differentiate into Th1 cells in the presence of rIL-12 or to Th2 cells in the presence of rIL-4 in vitro; under the same conditions, CD8+ T cells can differentiate into Tc1 or Tc2 cells. To generate Th and Tc cells from naïve T cells, naïve T cells are stimulated through the TCR in the presence of cytokines. Once the T cells are so stimulated, T cell proliferation can be easily promoted by a very small amount of anti-CD3 mAb during secondary stimulation. Therefore, to observe optimal T cell activation by IgE/Ag-activated mast cells, we changed the concentration of anti-CD3 mAb from 1 to 0.03 μg/ml, and the concentration of Th/Tc cells from 0.25 × 105 to 0.05 × 105 cells.
In cocultures with Th1 or Th2 cells in the presence of anti-CD3 mAb, IgE/Ag-stimulated WT BMCMCs were significantly more effective than IgE/Ag-stimulated TNF-/- BMCMCs in enhancing both Th1 and Th2 cell proliferation (Fig. 11 A, which is published as supporting information on the PNAS web site). IFN-γ levels in supernatants from Th1 cells and IL-4 levels in supernatants from Th2 cells cocultured with IgE/Ag-stimulated TNF-/- BMCMCs were also significantly reduced compared with those in cocultures containing IgE/Ag-stimulated WT BMCMCs (data not shown). Proliferation of Tc1 and/or Tc2 cells (Fig. 11B) and IFN-γ levels in supernatants from Tc1 cells and IL-4 levels in supernatants from Tc2 cells (data not shown) cocultured with IgE/Ag-stimulated TNF-/- BMCMCs were also significantly reduced compared with those in cocultures containing IgE/Ag-stimulated BMCMCs (Fig. 11B).
These results indicate that mast cell-derived TNF contributes to the optimal expression of the IgE/Ag-FcεRI-dependent mechanism of mast cell enhancement of activation of both Th1/Th2 cells and Tc1/Tc2 cells. Very similar results were obtained when we assessed the ability of IgE/Ag-stimulated BMCMCs to enhance the proliferation of CD4+CD62L- memory type T cells or γδTCR+ T cells (Fig. 11 C and D). The purity of the T cell subsets analyzed is shown in Fig. 12 and Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Mast Cells Can Act Synergistically with T Cells in Vivo. It has been reported that TNF-/- mice exhibit reduced CHS responses (39), and that mast cell-derived TNF can contribute to the development of the effector phase of CHS responses, apparently by enhancing the recruitment of neutrophils to reactions elicited in sensitized mice (4).
We were interested in investigating whether mast cell-derived TNF might also contribute to mast cell-dependent T cell activation, specifically, in settings in which the mast cells are activated by FcεRI-dependent mechanisms, i.e., by IgE and specific Ag. We therefore assessed whether mast cells activated via IgE- and Ag-dependent mechanisms and T cells suboptimally stimulated through the TCR/CD3 complex can interact in enhancing expression of inflammatory responses in vivo.
To control for possible effects of mast cells on T cell recruitment to sites of cutaneous inflammation, we injected anti-CD3-treated T cells and anti-DNP IgE-sensitized BMCMCs intradermally into both the right and left ear pinnae of C57BL/6 mice, and then the ear pinnae were treated epicutaneously with DNFB or vehicle. Ear thickness was not substantially increased after DNFB treatment in mice that had been injected with PBS alone, activated T cells alone, or IgE-sensitized BMCMCs alone (Fig. 3A). By contrast, ear thickness was markedly increased after DNFB treatment in mice that had been injected with both activated T cells and WT BMCMCs (Fig. 3A).
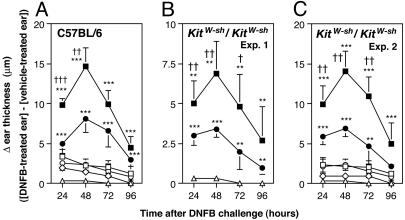
Fig. 3.
IgE/Ag-stimulated mast cells can act synergistically with anti-CD3-stimulated T cells to promote cutaneous inflammation. (A-C) Vehicle (PBS) alone [▵: A, n = 6; B, n = 4; C, n = 5], anti-CD3-stimulated CD3+ T cells alone [⋄: A, n = 6; C, n = 5], anti-DNP IgE-sensitized WT BMCMCs alone [□: A, n = 8; C, n = 6], anti-DNP IgE-sensitized TNF-/- BMCMCs alone [○: A, n = 8; C, n = 6], both anti-CD3-stimulated CD3+ T cells and anti-DNP IgE-sensitized WT BMCMCs [▪: A, n = 10; B, n = 7; C, n = 10] or both anti-CD3-stimulated CD3+ T cells and anti-DNP IgE-sensitized TNF-/- BMCMCs [•: A, n = 10; B, n = 7; C, n = 10] were injected intradermally in the ear skin of C57BL/6WT (A) or mast cell-deficient KitW-shKit/W-sh (B and C) mice. Mice were then challenged by treatment with 0.2% DNFB in acetone:olive oil (4:1 ratio), or, as a control, vehicle alone. DNFB-induced changes in ear thickness (thickness in DNFB-treated ear minus thickness in contralateral vehicle-treated ear) were measured at multiple intervals after challenge. Data in A-C are mean + SD. †, P < 0.05; ††, P < 0.01; †††, P < 0.005 for mice injected with both anti-CD3-stimulated CD3+ T cells and anti-DNP IgE-sensitized WT BMCMCs vs. mice injected with both anti-CD3-stimulated CD3+ T cells and anti-DNP IgE-sensitized TNF-/- BMCMCs; *, P < 0.05, **, P < 0.01; ***, P < 0.005 vs. corresponding values for mice injected with either T cells alone or mast cells alone.
Notably, the corresponding responses in mice injected with both activated T cells and IgE-sensitized TNF-/- BMCMCs were significantly (P < 0.05) reduced compared with those in mice that had been injected with both activated T cells and IgE-sensitized WT BMCMCs (Fig. 3A). Similar observations were obtained when we tested either KitW-sh/KitW-sh mast cell-deficient mice (Fig. 3 B and C) or KitW/KitW-v mice (data not shown), in which all mast cell function in the skin could reflect only the contribution of the adoptively transferred mast cells. Moreover, by using immunohistochemistry, we observed that many T cells were colocalized with mast cells in DNFB-treated ear skin of the KitW-sh/KitW-sh mice that had been injected with these cells (data not shown).
Taken together, these results show that, in an adoptive transfer model, mast cells activated through IgE and Ag can act synergistically with activated T cells to orchestrate the local development of cutaneous inflammation, and that mast cell-derived TNF contributes significantly to this response.
Discussion
We found that mast cells markedly enhanced proliferation and cytokine production in T cells that had been suboptimally stimulated through the CD3/TCR complex in vitro. Moreover, mast cells stimulated via the FcεRI enhanced the activation of all of the T cell subsets tested, including CD4+, CD8+, Th1, Th2, Tc1, Tc2, γδTCR+, and CD4+CD62L- T cells. By contrast, under the conditions tested, even IgE/Ag-stimulated mast cells had little or no effect on resting splenic T cells. Taken together, these observations suggest that mast cell-dependent enhancement of T cell activation may occur most readily in vivo in those contexts in which T cells already are undergoing some level of activation, such as at sites of Ag exposure in sensitized individuals.
Mast cell-dependent enhancement of T cell activation was promoted by IgE- and FcεRI-dependent mast cell activation, TNF production by both mast cells and T cells, and mast cell-T cell proximity. However, it appears that mast cells can enhance T cell activation in the mouse by additional mechanisms as well. For example, at high cell concentrations, mast cells can enhance the T cell response in the absence of signaling through the FcεRIγ chain (Fig. 1B). Also, when mast cells are stimulated with IgE and Ag, substantial enhancement of T cell proliferation can occur in the absence of mast cell-T cell proximity (Fig. 1C).
In the skin in vivo, our adoptive transfer studies showed that IgE/Ag-activated mast cells and T cells stimulated suboptimally through anti-CD3 Ab treatment can act synergistically to enhance cutaneous inflammation (Fig. 3A). Moreover, analyses in both WT (Fig. 3A) and genetically mast cell-deficient mice (Fig. 3 B and C) indicated that optimal expression of this effect, like many of those observed in vitro, required mast cell-derived TNF.
In summary, our findings have revealed both cell-proximity-dependent and -independent mechanisms through which mast cells can enhance the proliferation and/or cytokine production of T cells that have also been stimulated through the CD3/TCR complex. Our findings also have shown the importance of TNF as a mediator of mast cell-dependent enhancement of T cell activation. These observations clearly demonstrate that mast cells, and mast cell-derived TNF, can enhance T cell proliferation and function, and suggest that such mechanisms may contribute to the expression of immune responses in host defense and allergic or autoimmune disorders.
Supplementary Material
Acknowledgments
We thank Fu-Tong Liu (University of California at Davis, Sacramento) and David H. Katz (Medical Biology Institute, La Jolla, CA) for generously providing hybridoma cells that produce the H1-ε-26 anti-DNP monoclonal IgE Ab and all of the members of the laboratory for helpful discussions. This work was supported by National Institutes of Health Grants AI23990, CA72074, and HL67674, Project 1 (to S.J.G.).
Author contributions: S.N., J.D.S., M.T., and S.J.G. designed research; S.N., H.S., and M.K. performed research; J.D.S. contributed new reagents/analytic tools; S.N., H.S., M.K., J.D.S., M.T., and S.J.G. analyzed data; and S.N., H.S., M.K., J.D.S., M.T., and S.J.G. wrote the paper.
Abbreviations: BMCMC, bone marrow-derived cultured mast cell; CHS, contact hypersensitivity; Ag, antigen; DNP-HSA, 2,4-dinitrophenol-conjugated human serum albumin; rmTNF, recombinant mouse TNF; DNFB, 2,4-dinitrofluorobenzene; oxazolone; 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one; TCR, T cell receptor.
References
- 1.Coombs, R. P. A. & Gell, P. G. H. (1963) in Clinical Aspects of Immunology, ed. Gell, P. G. H. (Blackwell Scientific, Oxford), pp. 317-337.
- 2.Janeway, C. A., Travers, P., Walport, M. & Capera, J. D. (1999) in Immunobiology: The Immune System in Health and Disease (Current Biology Publications, London), 4th Ed., pp. 461-488.
- 3.Askenase, P. W., Van Loveren, H., Kraeuter-Kops, S., Ron, Y., Meade, R., Theoharides, T. C., Nordlund, J. J., Scovern, H., Gerhson, M. D. & Ptak, W. (1983) J. Immunol. 131, 2687-2694. [PubMed] [Google Scholar]
- 4.Biedermann, T., Kneilling, M., Mailhammer, R., Maier, K., Sander, C. A., Kollias, G., Kunkel, S. L., Hultner, L. & Rocken, M. (2000) J. Exp. Med. 192, 1441-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryce, P. J., Miller, M. L., Miyajima, I., Tsai, M., Galli, S. J. & Oettgen, H. C. (2004) Immunity 20, 381-392. [DOI] [PubMed] [Google Scholar]
- 6.von Stebut, E., Metz, M., Milon, G., Knop, J. & Maurer, M. (2003) Blood 101, 210-215. [DOI] [PubMed] [Google Scholar]
- 7.Williams, C. M. & Galli, S. J. (2000) J. Exp. Med. 192, 455-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi, T., Miura, T., Haba, T., Sato, M., Serizawa, I., Nagai, H. & Ishizaka, K. (2000) J. Immunol. 164, 3855-3861. [DOI] [PubMed] [Google Scholar]
- 9.Secor, V. H., Secor, W. E., Gutekunst, C. A. & Brown, M. A. (2000) J. Exp. Med. 191, 813-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee, D. M., Friend, D. S., Gurish, M. F., Benoist, C., Mathis, D. & Brenner, M. B. (2002) Science 297, 1689-1692. [DOI] [PubMed] [Google Scholar]
- 11.Araki, Y., Andoh, A., Fujiyama, Y. & Bamba, T. (2000) Clin. Exp. Immunol. 119, 264-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ptak, W., Geba, G. P. & Askenase, P. W. (1991) J. Immunol. 146, 3929-3936. [PubMed] [Google Scholar]
- 13.Robbie-Ryan, M., Tanzola, M. B., Secor, V. H. & Brown, M. A. (2003) J. Immunol. 170, 1630-1634. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharyya, S. P., Drucker, I., Reshef, T., Kirshenbaum, A. S., Metcalfe, D. D. & Mekori, Y. A. (1998) J. Leukocyte Biol. 63, 337-341. [DOI] [PubMed] [Google Scholar]
- 15.Frandji, P., Mourad, W., Tkaczyk, C., Singer, M., David, B., Colle, J. H. & Mecheri, S. (1998) Eur. J. Immunol. 28, 844-854. [DOI] [PubMed] [Google Scholar]
- 16.Inamura, N., Mekori, Y. A., Bhattacharyya, S. P., Bianchine, P. J. & Metcalfe, D. D. (1998) J. Immunol. 160, 4026-4033. [PubMed] [Google Scholar]
- 17.Baram, D., Vaday, G. G., Salamon, P., Drucker, I., Hershkoviz, R. & Mekori, Y. A. (2001) J. Immunol. 167, 4008-4016. [DOI] [PubMed] [Google Scholar]
- 18.Eager, K. B., Hackett, C. J., Gerhard, W. U., Bennink, J., Eisenlohr, L. C., Yewdell, J. & Ricciardi, R. P. (1989) J. Immunol. 143, 2328-2335. [PubMed] [Google Scholar]
- 19.Frandji, P., Oskeritzian, C., Cacaraci, F., Lapeyre, J., Peronet, R., David, B., Guillet, J. G. & Mecheri, S. (1993) J. Immunol. 151, 6318-6328. [PubMed] [Google Scholar]
- 20.Fox, C. C., Jewell, S. D. & Whitacre, C. C. (1994) Cell Immunol. 158, 253-264. [DOI] [PubMed] [Google Scholar]
- 21.Frandji, P., Tkaczyk, C., Oskeritzian, C., David, B., Desaymard, C. & Mecheri, S. (1996) Eur. J. Immunol. 26, 2517-2528. [DOI] [PubMed] [Google Scholar]
- 22.Gordon, J. R. & Galli, S. J. (1990) Nature 346, 274-276. [DOI] [PubMed] [Google Scholar]
- 23.Gordon, J. R. & Galli, S. J. (1991) J. Exp. Med. 174, 103-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tartaglia, L. A., Goeddel, D. V., Reynolds, C., Figari, I. S., Weber, R. F., Fendly, B. M. & Palladino, M. A., Jr. (1993) J. Immunol. 151, 4637-4641. [PubMed] [Google Scholar]
- 25.Bazzoni, F. & Beutler, B. (1996) N. Engl. J. Med. 334, 1717-1725. [DOI] [PubMed] [Google Scholar]
- 26.Wajant, H., Pfizenmaier, K. & Scheurich, P. (2003) Cell Death Differ. 10, 45-65. [DOI] [PubMed] [Google Scholar]
- 27.McLachlan, J. B., Hart, J. P., Pizzo, S. V., Shelburne, C. P., Staats, H. F., Gunn, M. D. & Abraham, S. N. (2003) Nat. Immunol. 4, 1199-1205. [DOI] [PubMed] [Google Scholar]
- 28.Jutel, M., Watanabe, T., Klunker, S., Akdis, M., Thomet, O. A., Malolepszy, J., Zak-Nejmark, T., Koga, R., Kobayashi, T., Blaser, K., et al. (2001) Nature 413, 420-425. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima, T., Inagaki, N., Tanaka, H., Tanaka, A., Yoshikawa, M., Tamari, M., Hasegawa, K., Matsumoto, K., Tachimoto, H., Ebisawa, M., et al. (2002) Blood 100, 3861-3868. [DOI] [PubMed] [Google Scholar]
- 30.Sayama, K., Diehn, M., Matsuda, K., Lunderius, C., Tsai, M., Tam, S. Y., Botstein, D., Brown, P. O. & Galli, S. J. (2002) BMC Immunol. 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ott, V. L., Cambier, J. C., Kappler, J., Marrack, P. & Swanson, B. J. (2003) Nat. Immunol. 4, 974-981. [DOI] [PubMed] [Google Scholar]
- 32.Korner, H., Cook, M., Riminton, D. S., Lemckert, F. A., Hoek, R. M., Ledermann, B., Kontgen, F., Fazekas de St. Groth, B. & Sedgwick, J. D. (1997) Eur. J. Immunol. 27, 2600-2609. [DOI] [PubMed] [Google Scholar]
- 33.Duttlinger, R., Manova, K., Chu, T. Y., Gyssler, C., Zelenetz, A. D., Bachvarova, R. F. & Besmer, P. (1993) Development (Cambridge, U.K.) 118, 705-717. [DOI] [PubMed] [Google Scholar]
- 34.Liu, F. T., Bohn, J. W., Ferry, E. L., Yamamoto, H., Molinaro, C. A., Sherman, L. A., Klinman, N. R. & Katz, D. H. (1980) J. Immunol. 124, 2728-2737. [PubMed] [Google Scholar]
- 35.Dvorak, A. M., Mihm, M. C., Jr., & Dvorak, H. F. (1976) Lab. Invest. 34, 179-191. [PubMed] [Google Scholar]
- 36.Mekori, Y. A. & Metcalfe, D. D. (1999) J. Allergy Clin. Immunol. 104, 517-523. [DOI] [PubMed] [Google Scholar]
- 37.Barnden, M. J., Allison, J., Heath, W. R. & Carbone, F. R. (1998) Immunol. Cell Biol. 76, 34-40. [DOI] [PubMed] [Google Scholar]
- 38.Infante-Duarte, C., Horton, H. F., Byrne, M. C. & Kamradt, T. (2000) J. Immunol. 165, 6107-6115. [DOI] [PubMed] [Google Scholar]
- 39.Pasparakis, M., Alexopoulou, L., Episkopou, V. & Kollias, G. (1996) J. Exp. Med. 184, 1397-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.