Abstract
Objective
To compare the laboratory tests conducted in real-life settings for patients with anemia with the expected prescriptions derived from an optimal checkup.
Methods
A panel of experts formulated an “optimal laboratory test assessment" specific to each anemia profile. A retrospective analysis was done of the laboratory tests conducted according to the type of anemia (microcytic, normocytic or macrocytic). Using an algorithmic system, the laboratory tests performed in real-life practice were compared with the recommendations suggested in the “optimal laboratory test assessment” and with seemingly “unnecessary” laboratory tests.
Results
In the analysis of the “optimal laboratory test assessment”, of the 1179 patients with microcytic anemia, 269 (22.8%) had had one of the three tests recommended by the expert system, and only 33 (2.8%) had all three tests. For normocytic anemia, 1054 of 2313 patients (45.6%) had one of the eleven recommended tests, and none had all eleven. Of the 384 patients with macrocytic anemia, 196 (51%) had one of the four recommended tests, and none had all four. In the analysis of “unnecessary laboratory tests", one lab test was unnecessarily done in 727/3876 patients (18.8%), i.e. 339 of 1179 (28.8%) microcytic, 171 of 2313 (7.4%) normocytic, and 217 of 384 (56.5 %) macrocytic anemias.
Conclusion
Laboratory investigations of anemia remain imperfect as more than half of the cases did not receive the expected tests. Analyzing other diagnostic domains, the authors are currently developing an artificial intelligence system to assist physicians in enhancing the efficiency of their laboratory test prescriptions.
Keywords: Anemia, Laboratory tests, Expert system, Artificial intelligence
1. Introduction
Anemia is a widespread condition seen in daily medical practice and it is recognized as a risk factor for several adverse outcomes, including hospitalization, morbidity and mortality. In 2010, the global prevalence of anemia was 32.9%, affecting over 2.2 billion people, with iron deficiency the most common cause [1]. When diagnosing anemia, numerous laboratory tests may be conducted, following various classification systems [2,3]. One of the classification systems of anemia is based on observations that red blood cell size can help to differentiate the potential etiology, and this resulted in the concept of “microcytic,” “normocytic,” and “macrocytic” anemia. Another classification system for anemia focuses on the underlying mechanism, distinguishing between an increase in red blood cell loss or a decrease in red blood cell production. If the reticulocyte count increases, then hemolysis and blood loss are primary considerations. Conversely, when the reticulocyte count is low, potential causes of impaired marrow production should be considered; such causes include nutritional deficiencies (iron, vitamin B12, folate, copper), marrow failure (aplastic anemia, pure red cell aplasia, myelodysplasia, leukemia), lack of growth factors (lack of erythropoietin owing to chronic renal disease), and myelopathic processes (cancer, infection). A blood smear can also offer valuable diagnostic clues as to the etiology of anemia.
Based on these classifications, after anemia is diagnosed, testing for specific causes might include a comprehensive range of laboratory tests. These tests can include evaluations of kidney function, inflammation, nutritional deficiencies, thalassemia, sickle cell disease, hemolysis and myeloma [[2], [3], [4], [5], [6]]. In daily practice, we often encounter challenges when faced with both the extensive range of underlying diseases that can potentially cause anemia and the need for comprehensive laboratory tests to identify the etiological factors. Due to the lack of studies outlining the ideal prescription for anemia detection, we have developed an expert system to help physicians prescribe appropriate tests to investigate the different types of anemia. This study aimed to compare, in the context of anemia, the effectiveness of laboratory tests conducted in a real-life setting with the recommended prescriptions for an optimal checkup as defined by an expert system.
2. Methods
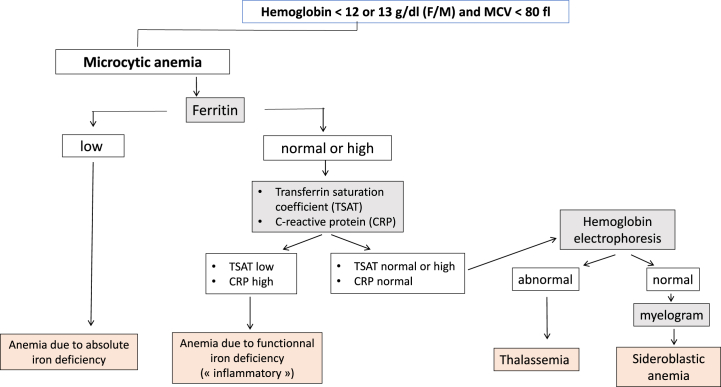
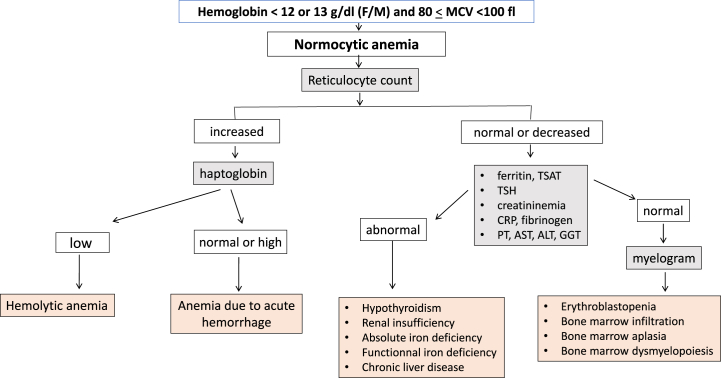
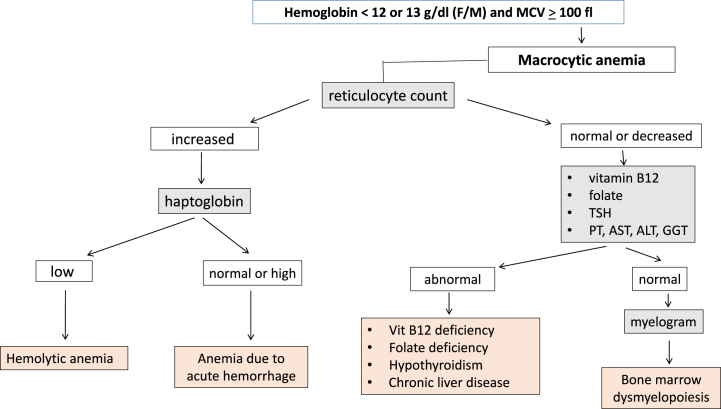
Based on a review of the literature and the established guidelines from medical societies [[6], [7], [8], [9], [10], [11], [12], [13],[14], [15], [16], [17], [18]], a group of experts including both biologists and clinicians [PH, NB, SB, PC] defined an “optimal laboratory test assessment" for each of the following anemia profiles. For microcytic anemia, tests included serum ferritin, transferrin saturation coefficient (TSAT) and C-reactive protein (CRP). Tests for normocytic anemia included serum ferritin, TSAT, CRP, reticulocyte count, haptoglobin, serum creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), bilirubin, prothrombin time (PT) and thyreostimulin hormone (TSH). And for macrocytic anemia, the tests were reticulocyte count, haptoglobin, serum creatinine, ALT, AST, GGT, bilirubin, PT, TSH, folate and vitamin B12 (Fig. 1, Fig. 2, Fig. 3). A list of laboratory tests that appeared to be “unnecessary” for each type of anemia was also defined. For microcytic anemia these laboratory tests were reticulocyte count, haptoglobin, TSH, serum creatinine, PT, AST, ALT, GGT, folate and vitamin B12; for normocytic anemia, they were folate and vitamin B12; and for macrocytic anemia, serum ferritin, TSAT, serum creatinine and CRP.
Fig. 1.
Algorithm describing the step-by-step laboratory tests in microcytic anemia.
For each tree branch, gray boxes indicate the lab tests to be done, and orange boxes indicate the main diagnoses.
(For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Algorithm describing the step-by-step laboratory tests in normocytic anemia.
Fig. 3.
Algorithm describing the step-by-step laboratory tests in macrocytic anemia. Laboratory checkup recommended by ChatGPT4 for microcytic, normocytic or macrocytic anemia. At the bottom, the blue boxes indicate laboratory tests suggested by the expert system but not by ChatGPT. The orange boxes indicate laboratory tests suggested by ChatGPT, which appeared to be unnecessary according to the expert system. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
A retrospective analysis was done of the medical records of patients who came to the Alphabio Laboratory (European Hospital site, Marseille, France) between January 1st, 2021 and March 1st, 2021 and who presented with anemia (hemoglobin <12 g/dL in women and <13 g/dL in men). The measurement of mean corpuscular volume (MCV) was used to determine the specific type of anemia, i.e. microcytic (MCV <80 fl), normocytic (80≤MCV<100 fl) or macrocytic (MCV ≥100 fl). Using the laboratory database, we obtained the list of all additional laboratory tests conducted in the same laboratory for each anemic patient over a period ranging from seven days to two months after the initial assessment (i.e. January 8th, 2021–May 1st, 2021).
Utilizing our expert algorithmic system, we compared the laboratory tests performed for each anemic patient within the study period with those recommended by the expert system (known as “optimal laboratory test assessment”), based on the specific anemia profile. In addition, we assessed the concordance between the “unnecessary” laboratory tests done for each anemic patient and the “optimal laboratory test assessment”.
Data were extracted from the Odancio Version 2017.R2.2 laboratory computer system (Dedalus, Le Plessis Robinson, France) and processed using SAS Version 9.4 statistical analysis software (SAS Institute Inc., Cary, NC, USA). The study was reported to the Health Data Hub (MR-004 studies) under the reference F20220128113241.
3. Results
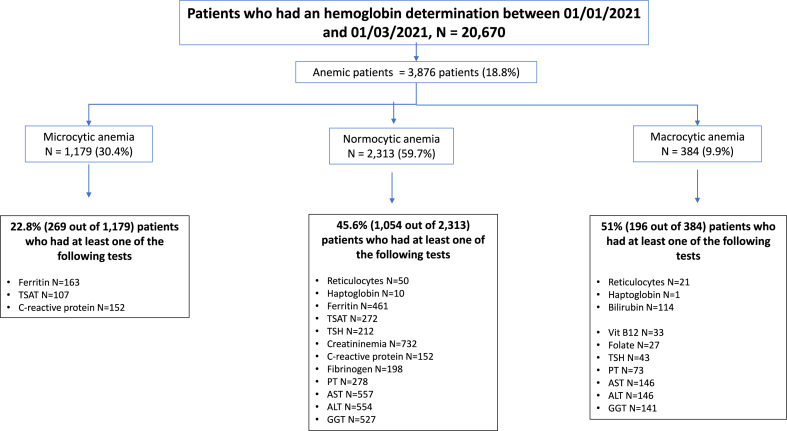
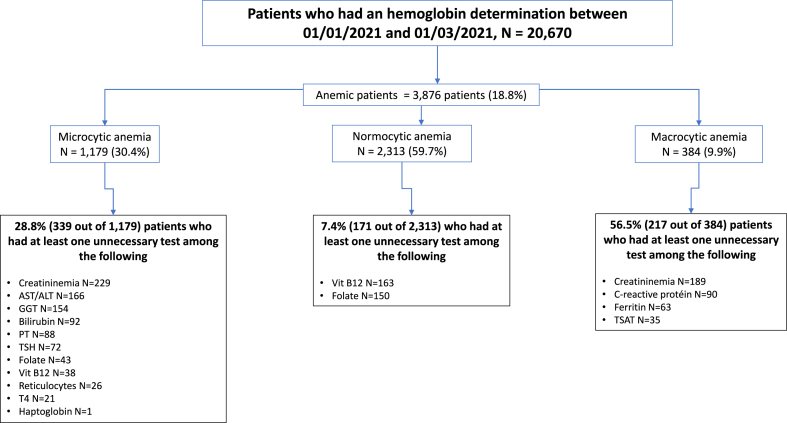
Out of the 20,670 patients who underwent hemoglobin determination within the specified period, 3876 (18.8%) were diagnosed with anemia (mean age 56 years (standard deviation [Sd] [24], 62% male). Among these anemic patients, 1179 (30.4%) exhibited microcytic anemia, 2313 (59.7%) normocytic anemia and 384 (9.9%) macrocytic anemia (Fig. 4). The mean age was 44 [24] years for the microcytic patients, 60 [19] years for the normocytic patients, and 69 [18] years for those with macrocytic anemia (p<0.0001). The male to female ratio was 27% in microcytic, 39% in normocytic and 59% in macrocytic patients (p<0.0001).
Fig. 4.
Analyses of laboratory tests done in patients with anemia, from a real-life database, categorized by the type of anemia (microcytic, normocytic or macrocytic) and considering the optimal laboratory test checkup (recommended by the algorithms, see Fig. 1, Fig. 2, Fig. 3).
3.1. Analysis of “optimal laboratory test assessment”
Out of the 1179 patients with microcytic anemia, 269 (22.8%) patients had had at least one of the recommended tests as suggested by the expert system, whereas only 33 (2.8%) patients had completed all three recommended tests (Fig. 4). For patients with normocytic anemia, 1054 of the 2313 (45.6%) patients had had at least one of the eleven recommended tests, and none had had all eleven tests. Of the 384 patients with macrocytic anemia, 196 (51%) had had one of the recommended tests, and none had completed all four recommended tests. Overall, a large proportion of anemic patients (ranging from 49% to 74.4%) did not undergo any portion of the “optimal laboratory test assessment” as recommended by the expert system. Conversely, only zero to 2.8% of patients received the complete “optimal laboratory test assessment” as prescribed.
3.2. Analysis of “unnecessary” laboratory tests
At least one “unnecessary” laboratory analysis was conducted in 727 of the 3876 (18.8%) patients diagnosed with anemia; these included 339 of 1179 (28.8%) patients with microcytic anemia, 171 of 2313 (7.4%) patients with normocytic anemia and 217 of 384 (56.5 %) patients with macrocytic anemia (Fig. 5).
Fig. 5.
Analyses of laboratory tests done in patients with anemia, from a real-life database, categorized by the type of anemia (microcytic, normocytic or macrocytic) and which appeared to be unnecessary (i.e. not recommended by the algorithms, see Fig. 1, Fig. 2, Fig. 3).
4. Discussion
In this large retrospective study, we found that laboratory tests performed in a real-life setting for the investigation of anemia often deviated from the recommendations provided by our expert system. On one hand, over half of the patients did not receive any portion of the expected laboratory tests, which may imply a potential “lost chance” for accurate diagnosis and treatment. On the other hand, we also found that a fifth of patients had laboratory tests that appeared to be unnecessary, suggesting possible “unjustified expenses”.
Remarkably, in the context of a common clinical condition like anemia, a very high percentage of patients did not undergo the recommended optimal laboratory tests. Once anemia is diagnosed, testing for specific causes may include an extensive list of laboratory tests, including those for kidney function (serum creatinine, BUN, erythropoietin level), anemia of chronic disease (erythropoietin, ferritin, CRP), nutritional deficiencies (iron, ferritin, TSAT, vitamin B12, methylmalonic acid, folate, homocysteine, serum copper, ceruloplasmin), thalassemia (hemoglobin electrophoresis, DNA sequencing), sickle cell disease (sickle solubility test, hemoglobin electrophoresis), hemolysis (haptoglobin, lactate dehydrogenase, indirect bilirubin, reticulocyte count) and myeloma (serum protein electrophoresis and immunofixation, serum free light chain analysis) [[2], [3], [4], [5], [6], [7], [8], [9], [10],[14], [15], [16], [17], [18]]. Using this approach, we designed an automatic algorithmic system to assist in guiding the selection of appropriate laboratory tests based on the specific anemia profile (Fig. 1, Fig. 2, Fig. 3). Implementation of this system should potentially reduce the overall number of required laboratory tests. For instance, in the case of microcytic anemia, the algorithm suggests measuring ferritin, TSAT and CRP. Although iron deficiency is the leading cause of microcytic anemia, our study showed a surprisingly low proportion of patients with this anemia profile who had all three recommended measurements. This situation is concerning as it deprives these patients of the opportunity for early diagnosis and treatment of their iron deficiency, consequently increasing the risks of morbidity and mortality.
In this study, up to a fifth of cases had lab tests that appeared to not be indicated based on the specific type of anemia. Some laboratory tests might be useful for the exploration of various anemia profiles. For example, laboratory tests helpful for the diagnosis of both normocytic and macrocytic anemia include initially a reticulocyte count, followed subsequently by liver functions tests and TSH level. However, in this study, the high number of prescriptions found for vitamin B12 and folate testing in the investigation of normocytic anemia, as well as ferritin and transferrin saturation for macrocytic anemia, did not appear reasonable.
The rapid advancement of artificial intelligence systems designed to assist physicians in patient care should be considered within the scope of our chosen model, namely the optimization of laboratory tests for investigating anemia. As an example, we posed the following question to ChatGPT: “What kind of laboratory testing do you suggest in the presence of an anemia?” We obtained of generic response that merely described anemia and its primary underlying causes, providing an extensive list of laboratory tests to be done. However, ChatGPT exhibited numerous flaws, such as failing to stratify the response based on the anemia profile (micro-, normo- or macrocytic). It also omitted several important tests, and suggested some unnecessary tests given the specific anemia profile (see Supplementary Figure 1). As an example, ChatGPT failed to suggest helpful tests such as TSAT, CRP and hemoglobin electrophoresis for microcytic anemia, whereas it suggested tests such as vitamin B12, folate and genetic analyses, which are not recommended for this condition. Based on the findings of this study, we highlight the limitations of chatbots like GPT4 in providing accurate and relevant recommendations for diagnostic work-ups.
Other expert systems and artificial intelligence on investigating anemia have been proposed. An app has been developed to estimate hemoglobin levels by analyzing color and metadata of fingernail bed smartphone photos. It showed an ability to detect anemia with an accuracy of ±2.4 g/dL and a sensitivity of 97% when compared with complete blood count hemoglobin levels [20]. An intelligent system, based on machine learning, that supports the automated diagnosis of anemia was trained on a dataset that contains two different areas of the mucous membrane of the eye conjunctiva photos of patients. When appropriately trained on palpebral conjunctiva images, this system showed good performance in classifying anemic and non-anemic patients [21]. A decision support system using artificial neural network has been proposed for the diagnosis of anemia including 26 blood values for the proposed structure accepted as system input [22]. Although interesting by their different approaches, researchers and clinicians should be aware that reliance on uncontrolled artificial intelligence-based decision support systems without proper oversight may result in misdiagnosis [23,19].
This study has several noteworthy strengths including its originality (no similar study exists in the literature), the large study sample size, the wide variety of anemia types and finally the construction of an expert system that defines the “optimal laboratory tests” specific to each type of anemia. We acknowledge certain limitations associated with this work. Firstly, although the sample size is large, the data analyzed were derived from a single laboratory database over a limited time period. Additionally, in the analysis of the “optimal laboratory tests”, we assumed that all patients had undergone follow-up laboratory examinations in the same laboratory. It is possible that some patients may have had these tests done at a different laboratory. In such cases, we may have underestimated the quantity and type of laboratory analyses conducted in real-life compared to those suggested by the expert system. As a result, this limitation may have also led to an underestimation of the frequency of “unnecessary” laboratory tests. Caution must be used in the analysis of seemingly “unnecessary tests", as without comprehensive clinical data, these laboratory tests may have been prescribed for reasons unrelated to the specific investigation of anemia.
5. Conclusion
In real-life settings, laboratory investigations of anemia remain imperfect, with over half of the cases lacking at least one of the expected tests. Conversely, in a fifth of cases the laboratory investigations included seemingly unnecessary tests. To gain a better understanding of our preliminary results, additional prospective multicenter studies conducted on a larger scale and incorporating clinical data would be useful. Such studies should also include a medico-economic analysis in order to improve the patient care pathway. Based on these data and from insight gained from various algorithmic systems in other diagnostic domains, the authors are currently developing an artificial intelligence system to assist physicians in enhancing the efficiency of their laboratory test prescriptions.
Ethics approval and consent to participate
The study was reported to the Health Data Hub (MR-004 studies) under the reference F20220128113241.
Consent for publication
All authors read and approved the final paper.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
No funding for this study.
CRediT authorship contribution statement
Philippe Halfon: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. Guillaume Penaranda: Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. Dan Ringwald: Formal analysis. Frederique Retornaz: Methodology, Writing – review & editing. Nicolas Boissel: Methodology, Validation, Writing – review & editing. Sylvain Bodard: Methodology, Validation, Writing – review & editing. Jean Marc Feryn: Validation, Writing – review & editing. David Bensoussan: Conceptualization, Methodology, Validation, Writing – review & editing. Patrice Cacoub: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.plabm.2024.e00357. the comment of the supplementary figure: Laboratory checkup recommended by ChatGPT4 for microcytic, normocytic or macrocytic anemia. At the bottom, the blue boxes indicate laboratory tests suggested by the expert system but not by ChatGPT. The orange boxes indicate laboratory tests suggested by ChatGPT, which appeared to be unnecessary according to the expert system.
Abbreviations
- CRP
C-reactive protein
- TSAT
transferrin saturation coefficient
- TSH
thyreostimulin hormone
- PT
prothrombin time
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- GGT
gamma glutamyl transpeptidase
Appendix A. Supplementary data
The following is the supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Kassebaum N.J. GBD 2013 anemia collaborators. The global burden of anemia. Hematol. Oncol. Clin. N. Am. 2016 Apr;30(2):247–308. doi: 10.1016/j.hoc.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Milovanovic T., Dragasevic S., Nikolic A.N., Markovic A.P., Lalosevic M.S., Popovic D.D., et al. Anemia as a problem: GP approach. Dig. Dis. 2022;40(3):370–375. doi: 10.1159/000517579. [DOI] [PubMed] [Google Scholar]
- 3.Vieth J.T., Lane D.R. Anemia. Hematol. Oncol. Clin. N. Am. 2017 Dec;31(6):1045–1060. doi: 10.1016/j.hoc.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Lopez A., Cacoub P., Macdougall I.C., Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016 Feb 27;387(10021):907–916. doi: 10.1016/S0140-6736(15)60865-0. [DOI] [PubMed] [Google Scholar]
- 5.Cacoub P., Vandewalle C., Peoc'h K. Using transferrin saturation as a diagnostic criterion for iron deficiency: a systematic review. Crit. Rev. Clin. Lab Sci. 2019 Dec;56(8):526–532. doi: 10.1080/10408363.2019.1653820. [DOI] [PubMed] [Google Scholar]
- 6.Cacoub P., Choukroun G., Cohen-Solal A., Luporsi E., Peyrin-Biroulet L., Peoc'h K., et al. Towards a common definition for the diagnosis of iron deficiency in chronic inflammatory diseases. Nutrients. 2022 Feb 28;14(5):1039. doi: 10.3390/nu14051039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLoughery T.G. Microcytic anemia. N. Engl. J. Med. 2014;371(14):1324–1331. doi: 10.1056/NEJMra1215361. [DOI] [PubMed] [Google Scholar]
- 8.Goddard A.F., James M.W., McIntyre A.S., Scott B.B. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309–1316. doi: 10.1136/gut.2010.228874. [DOI] [PubMed] [Google Scholar]
- 9.Camaschella C. Iron-deficiency anemia. N. Engl. J. Med. 2015;372(19):1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 10.Cacoub P., Nicolas G., Peoc'h K. Iron deficiency markers in patients undergoing iron replacement therapy: a 9-year retrospective real-world evidence study using healthcare databases. Sci. Rep. 2020 Sep 11;10(1):14983. doi: 10.1038/s41598-020-72057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cacoub P., Choukroun G., Cohen-Solal A., Luporsi E., Peyrin-Biroulet L., Peoc'h K., et al. Iron deficiency screening is a key issue in chronic inflammatory diseases: a call to action. J. Intern. Med. 2022 Oct;292(4):542–556. doi: 10.1111/joim.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peyrin-Biroulet L., Bouguen G., Laharie D., Pellet G., Savoye G., Gilletta C., et al. Iron deficiency in patients with inflammatory bowel diseases: a prospective multicenter cross-sectional study. Dig. Dis. Sci. 2022 Dec;67(12):5637–5646. doi: 10.1007/s10620-022-07474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen-Solal A., Philip J.L., Picard F., Delarche N., Taldir G., Gzara H., et al. Iron deficiency in heart failure patients: the French CARENFER prospective study. ESC Heart Fail. 2022 Apr;9(2):874–884. doi: 10.1002/ehf2.13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss G., Ganz T., Goodnough L.T. Anemia of inflammation. Blood. 2019 Jan 3;133(1):40–50. doi: 10.1182/blood-2018-06-856500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donker A.E., Raymakers R.A.P., Vlasveld T., van Barneveld T., Terink R., Dors N., et al. Practice guidelines for the diagnosis and management of microcytic anemias due to genetic disorders of iron metabolism or heme synthesis. Blood. 2014;123(25):3873–3886. doi: 10.1182/blood-2014-01-548776. [DOI] [PubMed] [Google Scholar]
- 16.Green R., Dwyre D.M. Evaluation of macrocytic anemias. Semin. Hematol. 2015;52(4):279–286. doi: 10.1053/j.seminhematol.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Nagao T., Hirokawa M. Diagnosis and treatment of macrocytic anemias in adults. J Gen Fam Med. 2017;18(5):200–204. doi: 10.1002/jgf2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore C.A., Adil A. StatPearls [Internet]. Treasure Island (FL) StatPearls Publishing; 2022 Jan. Macrocytic anemia.https://www.ncbi.nlm.nih.gov/books/NBK459295/ [Updated 2021 Jul 15] [Google Scholar]
- 19.Beam A.L., Drazen J.M., Kohane I.S., et al. Artificial intelligence in medicine. N. Engl. J. Med. 2023;388:1220–1221. doi: 10.1056/NEJMe2206291. [DOI] [PubMed] [Google Scholar]
- 20.Mannino R.G., Myers D.R., Tyburski E.A., Caruso C., Boudreaux J., Leong T., et al. Smartphone app for non-invasive detection of anemia using only patient-sourced photos. Nat. Commun. 2018 Dec 4;9(1):4924. doi: 10.1038/s41467-018-07262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimauro G., Griseta M.E., Camporeale M.G., Clemente F., Guarini A., Maglietta R., et al. An intelligent non-invasive system for automated diagnosis of anemia exploiting a novel dataset. Artif. Intell. Med. 2023;136 doi: 10.1016/j.artmed.2022.102477. [DOI] [PubMed] [Google Scholar]
- 22.Saputra D.C.E., Sunat K., Ratnaningsih T. A new artificial intelligence approach using extreme learning machine as the potentially effective model to predict and analyze the diagnosis of anemia. Healthcare. 2023;11:697. doi: 10.3390/healthcare11050697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee P., Bubeck S., Petro J. Benefits, limits, and risks of GPT-4 as an AI chatbot for medicine. N. Engl. J. Med. 2023 Mar 30;388(13):1233–1239. doi: 10.1056/NEJMsr2214184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data will be made available on request.