Summary
Extrahepatic portal vein obstruction (EHPVO) is a rare disease. Most EHPVO patients are usually referred to a gastroenterologist for intestinal bleeding and hypersplenic thrombocytopenia; however, hypercoagulative diseases may be occult in these patients and require anticoagulation. The purpose of this study was to elucidate the clinical characteristics of EHPVO. We conducted a retrospective analysis of the hospital database, evaluating the medical records of 15 patients (7 males, 8 females, mean age of onset 42.0 years, range 5-74 years). Thirteen of 15 EHPVO patients (86.7%) had intestinal varices. These included 10 esophageal (66.7%), 12 gastric (80.0%), and 6 ectopic varices (40.0%). Nine (60.0%) of 15 had a history of intestinal bleeding. Regarding comorbidities, 5 of 15 (33.3%) suffered from vascular diseases, including acute myocardial infarction, cerebral infarction, pulmonary embolism, Budd-Chiari syndrome, and mesenteric vein thrombosis. The former 3 vascular commodities manifested at less than 32 years of age. Four patients (26.7%) with JAK2V617F mutation were diagnosed as myeloproliferative neoplasm (MPN). 72.3% of EHPVO patients without MPN experienced thrombocytopenic state. No EHPVO patients with MPN experienced thrombo-leukocytopenia. The elevation of white blood cell and platelet counts, and decrease of protein S were seen in EHPVO with MPN, compared with EHPVO without MPN. EHPVO is frequently associated with underlying hypercoagulative factors, causing a dilemma between thrombotic complications and portal hypertensive bleeding. Most EHPVO patients experience an evident thrombocytopenic state due to severe hypersplenism; however, hypersplenic hematologic changes are eliminated in EHPVO with MPN. MPN should be suspected in EHPVO patients negative for thrombo-leukocytopenia.
Keywords: extrahepatic portal vein obstruction, myeloproliferative neoplasm, JAK2V617F mutation
1. Introduction
Extrahepatic portal vein obstruction (EHPVO) is an important cause of non-cirrhotic portal hypertension and a rare disorder with an estimated incidence of 6.1 per 1,000,000 inhabitants in Japan (1). In Europe, the overall gender-specific incidence rates of EHPVO were reportedly 3.78 and 1.73 per 100,000 inhabitants, in males and females, respectively (2). EHPVO is the thrombotic obliteration of the extrahepatic portal vein, with or without involvement of the intrahepatic portal veins or other segments of the splanchnic venous axis. EHPVO is characterized by features of portal hypertension with portal cavernous transformation as a sequel of portal vein obstruction to compensate for the interrupted portal blood flow (2,3). The etiology of EHPVO is diverse: however, an association with underlying risk factors for hypercoagulation has been reported.
The majority of patients with EHPVO are usually referred to a gastroenterologist for intestinal bleeding and hypersplenic thrombocytopenia. Gastroenterologists are often faced with a dilemma between fetal intestinal hemorrhage and thrombotic complications, and forced to simultaneously provide hemostatic control for portal hypertensive bleeding and antithrombotic agent administration. This creates a series of medical care issues for every EVPVO patient, including diagnosis, diverse comorbidities, complications, and treatment. The rarity of EHPVO makes controlled studies impractical, diagnosis difficult, and therapeutic strategies unstandardized.
This retrospective single-center study was conducted to clarify the clinical features of EHPVO and thrombotic comorbidities including hematological and vascular disease to improve diagnostic accuracy and therapeutic efficacy.
2. Patients and Methods
Between January 2000 and July 2023, 15 patients with a diagnosis of EHPVO treated in our hospital were enrolled in this retrospective study. Medical records for all 15 patients were identified and reviewed retrospectively. In all patients, the diagnosis was confirmed by imaging modalities, including ultrasonography, contrast-enhanced computed tomography, angiography, or contrast-enhanced magnetic resonance imaging. Patients with hepatocellular carcinoma, other malignancies, liver cirrhosis or operative history including pancreaticoduodenectomy and choledochotomy were excluded.
The medical records for patient age, gender, body mass index, habit (smoking, and alcohol intake), family history, symptoms at initial onset, comorbidity, laboratory workup, esophagogastroduodenoscopy findings were extracted from patient charts.
Regular blood tests, hepatic and renal function tests were performed in all patients. Thrombotic risk factors of EHPVO, including protein S, protein C, antithrombin III, D-dimer, JAK2V617F mutation, and anti-cardiolipin IgG antibodies, were tested, if needed, by the receiving doctor.
Esophagogastric varices were stratified according to the grading system proposed by the Japan Society for Portal Hypertension (4). Gastric varices in gastric body and antrum were included in ectopic varices. The system did not mention grading for ectopic varices; however, ectopic varices (duodenal varices and gastric body varices) were classified according to the same grading system for gastric varices (4) in this study.
All study participants provided informed consent and the study was carried out in accordance with the ethical standards set by the Declaration of Helsinki. This study was approved by the hospital ethics committee (B-2022- 615).
The data were analyzed using IBM SPSS Statistics® Ver. 28.0.1. Discrete variables were compared using Fisher's exact test. Continuous variables were compared using the Mann-Whitney U-test since the sample sizes were relatively small. A p-value < 0.05 was considered significant.
3. Results and Discussion
Fifteen EHPVO patients were included in the analysis. The demographic data and clinical presentation of the EHPVO patients are presented in Table 1. Seven males (46.6%) and 8 females (53.3%); the age distribution of onset ranged from 5 years to 74 years, with a mean age of onset of 42.0 years. Body mass index (kg/m2) was 23.4 ± 4.2 with a range from 17.6 to 34.2. Of the 15 patients, 6 (40.0%) were smokers, and 7 (46.6%) reported usual alcohol intake.
Table 1. Demographic data and clinical presentation for EHPVO patients.
| Characteristic | All patients (n = 15) | ||
|---|---|---|---|
| Gender | |||
| Male, n (%) | 7 | (46.6%) | |
| Female, n (%) | 8 | (53.3%) | |
| Age of onset | |||
| mean ± SD (range) | 42.0 ± 18.1 | (5–74) | |
| Body mass index (Kg/m2) | |||
| mean ± SD (range) | 23.4 ± 4.2 | (17.6–34.2) | |
| Initial complaint | |||
| hematemesis | 5 | (33.3%) | |
| abdominal pain | 5 | (33.3%) | |
| none | 2 | (13.3%) | |
| bloody stool | 1 | (6.7%) | |
| fullness in the left upper quadrant | 1 | (6.7%) | |
| irregular genital bleeding | 1 | (6.7%) | |
| Disease-associated complications | |||
| upper gastrointestinal varices | 13 | (86.7%) | |
| esophageal varices | 10 | (66.7%) | |
| gastric varices | 12 | (80.0%) | |
| ectopic varices | 6 | (40.0%) | 5 duodenum, 1 gastric body |
| history of intestinal bleeding | 9 | (60.0%) | |
| thrombocytopenia (< 2.0 × 104/uL) | 8 | (53.3%) | |
| portal hypertensive gastropathy | 4 | (26.7%) | |
| ascites | 2 | (13.3%) | |
| portal biliopathy | 2 | (13.3%) | |
| Comorbidities | |||
| vascular disease | 5 | (33.3%) | AMI 1, CI 1, PE 1 , BCS 1, mesenteric vein thrombosis 1 (6.6%) |
| hematological disease | 4 | (26.7%) | MPN 4 (26.7%) |
| diabetes mellitus | 3 | (20.0%) | |
AMI: acute myocardial infarction, BCS: Budd-Chiari Syndrome, CI: cerebral infarction, EHPVO: extrahepatic portal vein obstruction, MPN: myeloproliferative neoplasm, PE: pulmonary embolism.
Initial complaint included 5 hematemesis (33.3%), 5 abdominal pain (33.3%), 2 asymptomatic (13.3%), 1 bloody stool (6.7%), 1 fullness in the left upper quadrant (6.7%), and 1 irregular genital bleeding (6.7%). Regarding disease-associated complications, 13 (86.7%) of 15 EHPVO patients had intestinal varices, including 10 esophageal varices (66.7%), 12 gastric varices in fundus and cardia (80.0%), and 6 ectopic varices (40.0%) with 5 duodenum and 1 gastric body. Nine (60.0%) and 11 (73.3%) of 15 had intestinal bleeding history and variceal red color sign indicating high risk for variceal rupture, respectively. Thrombocytopenia (< 20.0 × 104/ uL), portal hypertensive gastropathy, ascites, and portal biliopathy was noted in 8 (53.3%), 4 (26.7%), 2 (13.3%), and 2 (13.3%) of 15 EHPVO patients, respectively.
Regarding comorbidities, 5 of 15 (33.3%) suffered from vascular diseases, including 1 acute myocardial infarction (6.6%), 1 cerebral infarction (6.6%), 1 pulmonary embolism (6.6%), 1 Budd-Chiari syndrome (6.6%), and 1 mesenteric vein thrombosis (6.6%). The 3 former vascular commodities manifested at less than 32 years of age. None of the 15 EHPVO patients had associated family history of thrombotic diseases. Four (26.7%) and 3 (20.0%) of 15 patients had hematological diseases of myeloproliferative neoplasm (MPN) and diabetes mellitus, respectively.
Laboratory workup for coagulation inhibitor and causative factors with EHPVO patients are shown in Table 2. Antithrombin III and protein S were deficient in 30.8% (4 of 13 tested patients) and 41.7% (5 of 12 tested patients), respectively. However, no deficiency of protein C was noted in any of the tested EHPVO patients (0.0%, 0 of 12 tested patients). Elevation of D-dimer was found in 13 of 14 tested EHPVO patients (92.9%).
Table 2. Coagulation inhibitor and causative factors (APS and MPN) for EHPVO patients.
| Variable | unit (reference range) | Patients tested positive (%) | (range) | |
|---|---|---|---|---|
| Antithrombin III deficiency | % (80-120) | 4/13 (30.8%) | 89.2 ± 12.9 | (65.9-109.0) |
| Protein S deficiency | % (64-149) | 5/12 (41.7%) | 77.5 ± 23.2 | (46.4-117.0) |
| Protein C deficiency | % (64-146) | 0/12 (0.0%) | 90.3 ± 26.2 | (64.0-142.0) |
| D-dimer | ug/mL (<0.5) | 13/14 (92.9%) | 1.0 ± 0.6 | (0.4-2.6) |
| anti-cardiolipin IgG antibody | U/mL (<10) | 0/11 (0.0%) | 4.1 ± 1.5 | (0-6) |
| JAK2V617F mutation | (negative) | 4 /11 (36.3%) |
APS: antiphospholipid syndrome, EHPVO: extrahepatic portal vein obstruction, JAK: Janus kinase, MPN: myeloproliferative neoplasm.
Anti-cardiolipin IgG antibody for screening of antiphospholipid syndrome was negative in all 11 tested patients (0.0%). JAK2V617F mutation, indicating association with MPN, was detected in 36.3% (4 of 11 tested EHPVO patients). All 4 MPN patients diagnosed by a hematologist during EHPVO screening had JAK2V617F mutation. With further hematological scrutiny, 4 MPN patients with EHPVO were diagnosed, respectively, as 2 polycythemia vera (PV), 1 essential thrombocythemia (ET), and 1 myeloproliferative neoplasm, unclassifiable (MPN-U).
Distribution of platelet and white blood cell counts in EHPVO is noted in Figure 1. Eight of 15 EHPVO patients (53.3%) exhibited thrombocytopenia (< 20.0 × 104/uL). Eight of 11 EHPVO patients without MPN (72.3%) exhibited a thrombocytopenic state; however, none of the 4 patients with MPN exhibited thrombo-leukocytopenia. All 4 MPN patients had high counts for both platelets (> 25.5 × 104/uL) and white blood cells (> 8.1 × 103/uL).
Figure 1.
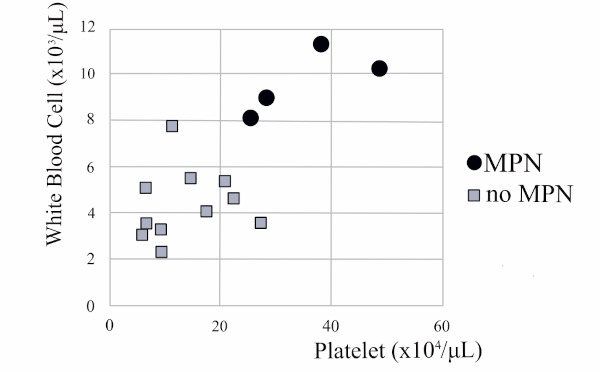
Distribution of platelet and white blood cell counts in EHPVO patients with MPN. Eight of 11 EHPVO patients without MPN (72.3%) exhibited a thrombocytopenic state; however, none of the 4 patients with MPN exhibited thrombo-leukocytopenia. All 4 MPN patients had high counts for both platelets (> 25.5 × 104/uL) and white blood cells (> 8.1 × 103/uL).
As shown in Table 3, white blood cells and platelets were diminished in EHPVO patients without MPN; however, they were significantly elevated in EHPVO patients with MPN. Furthermore, protein S in EHPVO patients with MPN was significantly lower than in patients without MPN. However, Antithrombin III, Protein C, and D-dimer were not statistically significant between the EHPVO patients with or without MPN. The comparison regarding gender, age at initial symptom, red blood cell counts, history of vascular disease and intestinal bleeding revealed no differences between the EHPVO patients with or without MPN.
Table 3. Comparison of clinical character of EHPVO patients with or without MPN.
| Variable | MPN (n = 4) | without MPN (n = 11) | p |
|---|---|---|---|
| Gender (Male/Female) | 1/3 | 6/5 | NS |
| Age at initial symptom | |||
| mean ± SD | 54.3 ± 10.0 | 37.2 ± 18.9 | NS |
| (range) | (42-66) | (5-74) | |
| BMI kg/m2 | |||
| mean ± SD | 20.6 ± 1.7 | 24.6 ± 4.4 | NS |
| WBC (×103/μL) | |||
| mean ± SD | 9.7 ± 1.2 | 4.4 ± 1.6 | < 0.05 |
| RBC (×106/μL) | |||
| mean ± SD | 4.4 ± 1.4 | 4.6 ± 0.4 | NS |
| Platelet (×104/μL) | |||
| mean ± SD | 35.2 ± 9.2 | 13.6 ± 7.0 | < 0.05 |
| Antithrombin III % (80-120) | |||
| mean ± SD | 88.7 ± 10.9 | 89.4 ± 13.8 | NS |
| Protein S % (64-149) | |||
| mean ± SD | 52.9 ± 5.7 | 89.8 ± 18.4 | < 0.05 |
| Protein C % (64-146) | |||
| mean ± SD | 84.5 ± 12.1 | 93.3 ± 30.5 | NS |
| D-dimer ug/mL (< 0.5) | |||
| mean ± SD | 1.20 ± 0.55 | 0.98 ± 0.63 | NS |
| history of vascular disease (yes/no) | 1/3 | 4/7 | NS |
| history of intestinal bleeding (yes/no) | 3/1 | 6/5 | NS |
BMI: body mass index, EHPVO: extrahepatic portal vein obstruction, MPN: myeloproliferative neoplasm, RBC: red blood cell, WBC: white blood cell.
In 2 of 4 EHPVO patients with MPN, Hassab's operation was performed for eradication of esophagogastric varices. Hassab's operation includes splenectomy with devascularization of the upper half of the stomach and distal esophagus. These two patients with MPN caused marked postoperative thrombocytosis of 195 × 104 /uL and 222 × 104 /uL (5), respectively. The increasing platelet counts were thought to be induced by asplenia after splenectomy because hypersplenism preoperatively masked marked thrombocytosis by MPN. The postoperative thrombocytosis due to MPN was treated by administration of cytoreduction therapy by a hematologist.
Various complications due to portal hypertension, including gastropathy, colopathy, biliopathy, intractable ascites, and liver dysfunction, have been reported in adult EHPVO patients (6). The rarity and diverse complications of EHPVO make diagnosis difficult and therapeutic strategies diverse.
Nutritional evaluation in our cases revealed that body mass index was within normal limits at 23.4 kg/ m2 with EHPVO. This result is consistent with other reports indicating favorable prognosis in adult patients with appropriate treatment (7). Conversely, EHPVO in childhood resulted in retardation of growth in around 50% of pediatric patients, possibly due to reduced portal supply to the liver, resistance to growth hormone and reduced insulin-like growth factor (6,8).
EHPVO patients are reported to have a favorable prognosis with a survival rate of 69-86% at 10 years if these portal hypertensive complications are appropriately managed (7,9-11). The fatal presentations in chronic EHPVO patients are mainly variceal bleeding and hypersplenism. Considering the frequency and severity of the various portal hypertensive complications, prophylaxis and treatment for esophagogastric variceal bleeding are of particular importance. EHPVO patients with esophagogastric varices show a favorable prognosis if variceal prophylaxis is performed appropriately (7). In our study, the proportion of subjects with a history of variceal bleeding with EHPVO was 60% and, likewise, the rate of variceal red color sign was 73.3%, indicating the importance of primary or secondary prophylaxis for variceal bleeding. Furthermore, anti-thrombotic therapy for EHPVO should be administered for portal thrombotic prophylaxis. Long-term anticoagulant therapy also should be administered in EHPVO patients with underlying persistent hypercoagulation (3,12). Simultaneous administration for hemorrhagic and thrombotic events complicated treatment for EHPVO by gastroenterologists.
Ectopic varices further complicate the strategy for EHPVO. While ectopic varices account for 1% to 5% of all variceal bleeding in patients with intrahepatic portal hypertension, they account for 20% to 30% of those with EHPVO. In brief, ectopic varices were generally a rare manifestation, but they were a common complication among EHPVO patients (13,14). The most difficult aspect is treatment of the ectopic varices due to the lack of standard guidelines because of their rarity. The treatment strategy for hemorrhagic ectopic varices varies by case based on the portal hemodynamics and the site of ectopic varices.
While it is reported that the major causative disease can be detected in the majority of patients with EHPVO, including MPN, antiphospholipid syndrome, abnormal angiogenesis, and paroxysmal nocturnal hemoglobinuria, none of the risk factors could be identified in a third of patients with EHPVO (15). Among these causative diseases, MPN, including chronic idiopathic myelofibrosis, polycythemia vera, essential thrombocythemia, and unclassifiable type (MPN-U), is the most frequent underlying prothrombotic factor for EHPVO with a reported prevalence of 15-30% (12,16-20). In the West, MPN has been reported in 58% of patients with EHPVO of unknown etiology, and 57% of these go on to develop an overt MPN during follow up (20).
EHPVO in adults is frequently associated with underlying risk factors for hypercoagulation, and the probability of hypercoagulable condition should be considered and screened for (3,21). Protein C, Protein S, and antithrombin III deficiencies are associated with the etiology of EHPVO, and detected in 3.9-20.6%, 2-11.8%, and 0-4.1% of EHPVO cases, respectively (15,22-24). In this study, Protein S was significantly decreased with EHPBO with MPN. The cause of the reduced levels of Protein S in patients with MPN is unknown; however, the mechanism may be related to the ongoing hypercoagulative condition by MPN that causes the consumption of Protein S.
A close relationship between EHPVO and MPN was also confirmed by the high frequency of a clonal mutation of JAK2V617F, which was present in 16-35% of EHPVO patients (16,23,25). Screening for JAK2V617F mutation offers a diagnostic tool for the detection of occult MPN in EHPVO patients (16,23,26). JAK2 tyrosine kinase causes cytokine-independent activation of the JAK- STAT pathway, resulting in proliferation of mature myeloid cells (27). The JAK2V617F gain-of-function mutation increasing the risk of thrombosis is present in up to 95% of patients with polycythemia vera and in about 50% of patients with essential thrombocythemia and myelofibrosis (16,28,29). Furthermore, MPN often sequentially progresses to a fatal condition with an end-stage myelofibrosis or acute myeloid leukemia. These data also suggest the value of screening for JAK2V617F mutation in noninvasive blood sample in EHPVO patients. Patients with splanchnic vein thrombosis, including EHPVO and Budd-Chiari syndrome in adults, show a considerable overlap in etiology; therefore, the JAK2V617F mutation also should be screened for in patients with Budd-Chiari syndrome (16).
The present study demonstrated the significant elevation of white blood cells and platelets, and the decrease of protein S in EHPVO with MPN compared to EHPVO without MPN. This hematological difference is very important in understanding the condition of EHPVO with MPN. In particular, we would like to emphasize the importance of platelet counts in EHPVO patients with MPN. The majority of patients with portal hypertension, including EHPVO patients, are usually in an evident thrombocytopenic state due to severe hypersplenism and are, therefore, referred to gastroenterologists (30); however, EHPVO patients with MPN have a normal or high platelet count caused by stem cell-derived clonal myeloproliferation despite hypersplenism. The actual high platelet count is effectively masked by hypersplenism to show a normal range, making it difficult for general gastroenterologists to properly understand the underlying state of MPN with hypercoagulation.
Although further research on a large series is required to accumulate more detailed data of EHPVO, our results will encourage gastroenterologists to consider MPN in EHPVO patients.
In conclusion, EHPVO is frequently associated with underlying risk factors for hypercoagulation, causing a dilemma between fatal portal hypertensive bleeding and thrombotic complications. EHPVO patients are usually in an evident thrombocytopenic state due to severe hypersplenism; however, hypersplenic hematologic changes are eliminated in EHPVO patients with MPN. MPN should be strongly suspected in EHPVO patients without thrombo-leukocytopenia.
Funding
None.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. Ohfuji S, Furuichi Y, Akahoshi T, Kage M, Obara K, Hashizume M, Matsuura T, Fukushima W, Nakamura Y. Japanese periodical nationwide epidemiologic survey of aberrant portal hemodynamics. Hepatol Res. 2019; 49:890-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ageno W, Dentali F, Pomero F, Fenoglio L, Squizzato A, Pagani G, Re R, Bonzini M. Incidence rates and case fatality rates of portal vein thrombosis and Budd-Chiari Syndrome. Thromb Haemost. 2017; 117:794-800. [DOI] [PubMed] [Google Scholar]
- 3. de Franchis R, Faculty BV. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015; 63:743-52. [DOI] [PubMed] [Google Scholar]
- 4. Tajiri T, Yoshida H, Obara K, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc. 2010; 22:1-9. [DOI] [PubMed] [Google Scholar]
- 5. Shimizu T, Yoshioka M, Matsushita A, Ueda J, Kawashima M, Ono T, Kawano Y, Yoshida H. Esophagogastric varix caused by extrahepatic portal vein obstruction with essential thrombocythemia: A case report. J Nippon Med Sch. 2023.doi: 10.1272/jnms.JNMS.2024_91-601. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6. Sarin SK, Sollano JD, Chawla YK, Amarapurkar D, Hamid S, Hashizume M, Jafri W, Kumar A, Kudo M, Lesmana LA, Sharma BC, Shiha G, Janaka de Silva H; Members of the APASL Working Party on Portal Hypertension. Consensus on extra-hepatic portal vein obstruction. Liver Int. 2006; 26:512-519. [DOI] [PubMed] [Google Scholar]
- 7. Sekimoto T, Maruyama H, Kobayashi K, Kiyono S, Kondo T, Shimada T, Takahashi M, Yokosuka O. Well-tolerated portal hypertension and favorable prognosis in adult patients with extrahepatic portal vein obstruction in Japan. Hepatol Res. 2016; 46:505-513. [DOI] [PubMed] [Google Scholar]
- 8. Sarin SK, Bansal A, Sasan S, Nigam A. Portal-vein obstruction in children leads to growth retardation. Hepatology. 1992; 15:229-233. [DOI] [PubMed] [Google Scholar]
- 9. Bhargava DK, Dasarathy S, Sundaram KR, Ahuja RK. Efficacy of endoscopic sclerotherapy on long-term management of oesophageal varices: A comparative study of results in patients with cirrhosis of the liver, non-cirrhotic portal fibrosis (NCPF) and extrahepatic portal venous obstruction (EHO). J Gastroenterol Hepatol. 1991; 6:471-475. [DOI] [PubMed] [Google Scholar]
- 10. Kitano S, Iso Y, Iwanaga T, Koyanagi N, Sugimachi K. Esophageal transection may well be the approach of choice for patient with portal venous obstruction and esophageal varices. Jpn J Surg. 1989; 19:418-423. [DOI] [PubMed] [Google Scholar]
- 11. Pande GK, Reddy VM, Kar P, Sahni P, Berry M, Tandon BN, Nundy S. Operations for portal hypertension due to extrahepatic obstruction: results and 10 year follow up. Br Med J (Clin Res Ed). 1987; 295:1115-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al. Acute portal vein thrombosis unrelated to cirrhosis: A prospective multicenter follow-up study. Hepatology. 2010; 51:210-218. [DOI] [PubMed] [Google Scholar]
- 13. Sharma B, Raina S, Sharma R. Bleeding ectopic varices as the first manifestation of portal hypertension. Case Reports Hepatol. 2014; 2014:140959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sarin SK, Kumar CKN. Ectopic varices. Clin Liver Dis (Hoboken). 2012; 1:167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amarapurkar P, Bhatt N, Patel N, Amarapurkar D. Primary extrahepatic portal vein obstruction in adults: A single center experience. Indian J Gastroenterol. 2014; 33:19-22. [DOI] [PubMed] [Google Scholar]
- 16. Smalberg JH, Arends LR, Valla DC, Kiladjian JJ, Janssen HL, Leebeek FW. Myeloproliferative neoplasms in Budd- Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood. 2012; 120:4921-4928. [DOI] [PubMed] [Google Scholar]
- 17. Denninger MH, Chaït Y, Casadevall N, Hillaire S, Guillin MC, Bezeaud A, Erlinger S, Briere J, Valla D. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology. 2000; 31:587-591. [DOI] [PubMed] [Google Scholar]
- 18. Janssen HL, Wijnhoud A, Haagsma EB, van Uum SH, van Nieuwkerk CM, Adang RP, Chamuleau RA, van Hattum J, Vleggaar FP, Hansen BE, Rosendaal FR, van Hoek B. Extrahepatic portal vein thrombosis: Aetiology and determinants of survival. Gut. 2001; 49:720-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bayraktar Y, Harmanci O, Büyükasik Y, Shorbagi AI, Sungur AH, Boylu CA, Gürgey A, Balkanci F. JAK2V617F mutation in patients with portal vein thrombosis. Dig Dis Sci. 2008; 53:2778-2783. [DOI] [PubMed] [Google Scholar]
- 20. Valla D, Casadevall N, Huisse MG, Tulliez M, Grange JD, Muller O, Binda T, Varet B, Rueff B, Benhamou JP. Etiology of portal vein thrombosis in adults. A prospective evaluation of primary myeloproliferative disorders. Gastroenterology. 1988; 94:1063-1069. [DOI] [PubMed] [Google Scholar]
- 21. Chait Y, Condat B, Cazals-Hatem D, Rufat P, Atmani S, Chaoui D, Guilmin F, Kiladjian JJ, Plessier A, Denninger MH, Casadevall N, Valla D, Brière JB. Relevance of the criteria commonly used to diagnose myeloproliferative disorder in patients with splanchnic vein thrombosis. Br J Haematol. 2005; 129:553-560. [DOI] [PubMed] [Google Scholar]
- 22. Khanna R, Sarin SK. Idiopathic portal hypertension and extrahepatic portal venous obstruction. Hepatol Int. 2018; 12(Suppl 1):148-167. [DOI] [PubMed] [Google Scholar]
- 23. Primignani M, Barosi G, Bergamaschi G, Gianelli U, Fabris F, Reati R, Dell'Era A, Bucciarelli P, Mannucci PM. Role of the JAK2 mutation in the diagnosis of chronic myeloproliferative disorders in splanchnic vein thrombosis. Hepatology. 2006; 44:1528-1534. [DOI] [PubMed] [Google Scholar]
- 24. Mutreja D, Kotru M, Sazawal S, Ranjan R, Sharma A, Acharya SK, Saxena R. Hereditary and acquired thrombophilia in splanchnic vein thrombosis: A single-center experience. Clin Appl Thromb Hemost. 2015; 21:521-526. [DOI] [PubMed] [Google Scholar]
- 25. Hernández-Gea V, De Gottardi A, Leebeek FWG, Rautou PE, Salem R, Garcia-Pagan JC. Current knowledge in pathophysiology and management of Budd-Chiari syndrome and non-cirrhotic non-tumoral splanchnic vein thrombosis. J Hepatol. 2019; 71:175-199. [DOI] [PubMed] [Google Scholar]
- 26. De Stefano V, Vannucchi AM, Ruggeri M, et al. Splanchnic vein thrombosis in myeloproliferative neoplasms: risk factors for recurrences in a cohort of 181 patients. Blood Cancer J. 2016; 6:e493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R, Bennaceur- Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005; 434:1144-1148. [DOI] [PubMed] [Google Scholar]
- 28. Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR; Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005; 365:1054-1061. [DOI] [PubMed] [Google Scholar]
- 29. Nielsen C, Birgens HS, Nordestgaard BG, Kjaer L, Bojesen SE. The JAK2 V617F somatic mutation, mortality and cancer risk in the general population. Haematologica. 2011; 96:450-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoshida H, Shimizu T, Yoshioka M, Taniai N. Management of portal hypertension based on portal hemodynamics. Hepatol Res. 2021; 51:251-262. [DOI] [PubMed] [Google Scholar]


