Abstract
Background
Which patients benefit from the addition of immune checkpoint inhibitors (ICIs) to chemotherapy for small cell lung cancer (SCLC) remains unclear. There have been few reports on the efficacy of ICIs based on conventional immunohistochemical neuroendocrine (NE) markers (synaptophysin, chromogranin A, and neural cell adhesion molecule [NCAM]). In the present study, we aimed to analyze the relationship between the expression of immunohistochemical NE markers and the efficacy of ICIs in patients with extensive disease (ED)‐SCLC, to assess whether conventional NE markers are predictive of ICIs.
Methods
Patients with untreated ED‐SCLC who received first‐line therapy at the Shizuoka Cancer Center between November 2002 and July 2021 were retrospectively reviewed. We evaluated the efficacy of first‐line chemotherapy according to the expression status of each immunohistochemical NE marker in patients treated with ICI plus chemotherapy (ICI‐chemo group) and with chemotherapy alone (chemo group).
Results
A total of 227 patients were included in the ICI‐chemo and chemo groups, respectively. The progression‐free survival (PFS) tended to be better in patients in the ICI‐chemo group than those treated with chemotherapy alone in patients with NE marker‐positive SCLC. In particular, it was statistically significant in patients with chromogranin A‐positive SCLC (p = 0.036). In patients with NE marker‐negative SCLC, no significant differences were observed in PFS between the two groups. There were no significant differences in overall survival (OS), regardless of the expression of any conventional NE marker.
Conclusion
Our study suggests that the efficacy of ICIs in addition to chemotherapy may be poor in patients with NE marker‐negative SCLC.
Keywords: chemotherapy, immune checkpoint inhibitor, immunohistochemical, neuroendocrine, small cell lung cancer
We retrospectively analyzed the relationship between the efficacy of ICIs and the expression patterns of conventional NE markers in ED‐SCLC. PFS tended to be better in the ICI‐chemo group than in the chemo‐group in any NE marker‐positive SCLC. However, no significant differences were observed in any of the NE marker‐negative SCLC patients between the groups. Our study suggests that the efficacy of ICIs in addition to chemotherapy may not be demonstrated in conventional NE marker‐negative SCLC.
INTRODUCTION
Lung cancer is the most common cause of cancer‐related deaths worldwide. 1 Small cell lung cancer (SCLC) accounts for 13% of all lung cancers and has a poor prognosis. 1 Approximately 60%–70% of patients have extensive disease (ED)‐SCLC, and the median survival time (MST) for ED‐SCLC is 8–13 months. 2 Although no drugs have shown promising results in improving the prognosis of patients with ED‐SCLC in recent decades, the results of the IMPower 133 study, which compared carboplatin, etoposide, and atezolizumab with carboplatin, etoposide, and placebo in patients with previously untreated ED‐SCLC, were reported in 2018. 3 The primary endpoints of the trial were investigator‐assessed progression‐free survival (PFS) and overall survival (OS). The MST was 12.3 months in the atezolizumab group and 10.3 months in the placebo group (hazard ratio [HR], 0.70; 95% confidence interval [CI]: 0.54–0.91, p = 0.007). The median PFS was 5.2 months and 4.3 months, respectively (HR: 0.77, 95% CI: 0.62–0.96, p = 0.02). Additionally, the results of the CASPIAN study, which has a similar design, were reported in 2019. 4 The results of these studies have shown that the addition of immune checkpoint inhibitors (ICI) to chemotherapy is effective in patients with ED‐SCLC and non‐small cell lung cancer (NSCLC). Based on these trials, platinum agents plus etoposide combined with atezolizumab or durvalumab are recommended as the first‐line treatment for patients with ED‐SCLC.
Many guidelines recommend that patients with NSCLC should be treated based on the subtype, according to the expression level of programmed death ligand 1 (PD‐L1) and driver gene mutations. 5 , 6 , 7 , 8 The expression level of PD‐L1 is a predictive marker for the efficacy of ICI in patients with NSCLC, although the expression is incomplete. In SCLC, there are no subgroups related to treatment efficacy. Moreover, PD‐L1 expression was not a predictive marker of ICI efficacy. Most SCLCs express conventional neuroendocrine (NE) markers (synaptophysin, chromogranin A, and neural cell adhesion molecule [NCAM]). Furthermore, there are few reports on the efficacy of ICIs based on conventional immunohistochemical NE markers. Thus, in this study, we aimed to analyze the relationship between the expression of immunohistochemical NE markers and the efficacy of ICIs in patients with ED‐SCLC, to assess whether conventional NE markers are predictive of ICIs.
METHODS
Patients
We retrospectively analyzed the clinical data of patients with ED‐SCLC treated with first‐line therapy between November 2002 and July 2021 at the Shizuoka Cancer Center. The inclusion criteria were as follows: (1) histological confirmation of SCLC; (2) diagnosis of ED‐SCLC; (3) received carboplatin plus etoposide plus atezolizumab or cisplatin/carboplatin plus etoposide plus durvalumab (ICI‐chemo group) or cisplatin plus irinotecan, cisplatin plus etoposide, or carboplatin plus etoposide (chemo group) as first‐line chemotherapy; (4) patient tumor samples were assessed using at least one of synaptophysin, chromogranin A, and NCAM; and (5) no history of interstitial lung disease. To assess the relationship between the immunostaining status of conventional NE markers and the efficacy of ICIs in first‐line chemotherapy, we investigated the differences in the efficacy of first‐line chemotherapy between the ICI‐chemo and chemo groups according to the expression of immunostaining conventional NE markers.
Immunohistochemical analysis
Formalin‐fixed paraffin‐embedded samples were sectioned at a thickness of 3 μm, mounted onto glass slides, and incubated with an antimouse monoclonal antibody against synaptophysin (clone 27G12) (Leica Biosystems) at a dilution of 1:100, NCAM (clone CD564, Leica Biosystems) at a dilution of 1:100, and anti‐rabbit polyclonal antibody chromogranin A (Rabbit Polyclonal, Nichirei Bioscience), ready to use, for immunohistochemical analysis. All slides were processed on the Autostainer Bond‐III platform (Leica Biosystems Inc.) and visualized using a Leica Bond Polymer Refine Detection kit (DS9800, Bond Polymer Refine Detection, Leica) according to protocol F. Deparaffinization, rehydration, and antigen retrieval were performed by Bond Epitope Retrieval Solution 1 (ER1) (prediluted; pH 6.0) for synaptophysin and chromogranin A in 20 min and Bond Epitope Retrieval Solution 2 (ER2) (prediluted; pH 9.0) for NCAM in 10 min. Nuclei were lightly counterstained with Mayer's hematoxylin.
Immunohistochemical staining was evaluated independently by a pathologist and a physician (TK and YI, respectively). The expression of each marker Ab (synaptophysin, NCAM, and chromogranin A) in a tumor was defined as positive when at least 1% of the tumor cells were stained and negative when less than 1% were stained.
Evaluation and statistical analysis
We evaluated tumor response to chemotherapy in accordance with the Response Evaluation Criteria in Solid Tumors by performing chest and abdominal computed tomography, head magnetic resonance imaging, bone scintigraphy, or positron emission tomography–computed tomography. 9 All categorical variables were analyzed using the chi‐squared test or Fisher's exact test, as appropriate. Clinical evaluation of PFS and OS after the initiation of first‐line chemotherapy was conducted using the Kaplan–Meier method to assess the time of recurrence and death, respectively. Survival analyses were performed using the log‐rank test. The follow‐up period was estimated using the Kaplan–Meier method. All tests were two‐sided, and p‐values <0.05 were considered statistically significant. All the statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical interface for R (The R Foundation for Statistical Computing, Vienna, Austria). 10 Our study was approved by the Institutional Review Board of the Shizuoka Cancer Center.
RESULTS
Patient characteristics
A total of 227 patients diagnosed with ED‐SCLC between November 2002 and July 2021 were included. A total of 30 patients received ICI plus chemotherapy (ICI‐chemotherapy group), and 197 patients received chemotherapy alone (chemotherapy group). Patient characteristics in the ICI‐chemo group were as follows: median age, 68 years (range, 50–84 years); 86.7% males; 93.3% had a performance status (PS) of 0 or 1; and all patients were past or current smokers. Carboplatin, etoposide, and atezolizumab were the most used drugs and were administered to 25 patients (83.3%). All SCLC tumors in the ICI‐chemo group were stained with synaptophysin, chromogranin A, and NCAM.
Patient characteristics in the chemotherapy group were as follows: median age, 70 years (range, 48–89 years); 80.7% males; 70.3% had a PS of 0 or 1; and 98.0% were past or current smokers. Carboplatin plus etoposide was the most used drug, administered to 114 patients (57.9%). There were no significant differences in age, sex, PS, smoking status, expression of conventional NE markers, or subsequent chemotherapy between the ICI‐chemotherapy and chemotherapy groups (Table 1).
TABLE 1.
Characteristics of patients.
| ICI‐chemo group (n = 30) | Chemotherapy alone group (n = 197) | p‐value | |
|---|---|---|---|
| Age (years) | |||
| Median/range | 68/50–84 | 70/48–86 | 0.945 |
| Gender, n (%) | |||
| Male | 26 (86.7%) | 159 (80.7%) | 0.614 |
| Female | 4 (13.3%) | 38 (19.3%) | |
| PS at first‐line treatment, n (%) | |||
| 0–1 | 28 (93.3%) | 139 (70.5%) | 0.0788 |
| 2–4 | 2 (6.7%) | 58 (29.5%) | |
| Smoking status, n (%) | |||
| Current or former | 30 (100%) | 193 (98.0%) | 1.000 |
| Never | 0 (0%) | 2 (1.0%) | |
| Unknown | 0 (0%) | 2 (1.0%) | |
| Histological classification | |||
| Small cell carcinoma | 28 (93.3%) | 193 (98.0%) | 0.181 |
| Combined small cell carcinoma | 2 (6.7%) | 4 (2.0%) | |
| Regimen of first‐line treatment, n (%) | |||
| Carboplatin plus etoposide plus atezolizumab | 25 (83.3%) | ‐ | |
| Cisplatin plus etoposide plus durvalumab | 4 (13.3%) | ‐ | |
| Carboplatin plus etoposide plus durvalumab | 1 (3.3%) | ‐ | |
| Carboplatin plus etoposide | ‐ | 114 (57.9%) | |
| Cisplatin plus irinotecan | ‐ | 60 (30.5%) | |
| Cisplatin plus etoposide | ‐ | 23 (11.7%) | |
| Expression of synaptophysin | |||
| Positive | 26 (86.7%) | 153 (77.7%) | 0.465 |
| Negative | 4 (13.3%) | 38 (19.3%) | |
| Expression of chromogranin A | |||
| Positive | 22 (73.3%) | 100 (50.8%) | 0.072 |
| Negative | 8 (26.7%) | 84 (42.6%) | |
| Expression of NCAM | |||
| Positive | 28 (93.3%) | 182 (92.4%) | 0.668 |
| Negative | 2 (6.7%) | 10 (5.1%) | |
| Subsequent chemotherapy, n (%) | |||
| Yes | 19 (63.3%) | 139 (70.6%) | 0.400 |
| No | 11 (36.7%) | 57 (28.9%) |
Abbreviations: ICI, immune checkpoint inhibitor; NCAM, neural cell adhesion molecule; PS, performance status.
Efficacy of first‐line therapy between groups according to the expression of NE marker status
No significant differences in patient characteristics such as age, sex, PS, smoking status, and subsequent chemotherapy were observed between the ICI‐chemo and chemo groups, regardless of the expression of each conventional NE marker. The best overall response rate (ORR) for the first‐line treatment is shown in Table 2. There were no significant differences in the ORR between the ICI‐chemo and ICI‐chemo groups, regardless of synaptophysin expression (84.6% and 83.1%, respectively, in the synaptophysin‐positive group, p = 0.166; and 75.0% and 76.3%, respectively, in the synaptophysin‐negative group, p = 0.457). Regardless of chromogranin A expression, there were no significant differences in ORR between the ICI‐chemo and chemo groups (81.8% and 83.1%, respectively, in the chromogranin A‐positive group, p = 0.313; and 87.5% and 81.0%, respectively, in the chromogranin A‐negative group, p = 0.669). Regardless of the expression of NCAM, there were no significant differences in the ORR between the ICI‐chemotherapy and chemotherapy groups (85.7% and 81.9%, respectively, in the NCAM‐positive group, p = 0.149; and 50.0% and 80.0%, respectively, in the NCAM‐negative group, p = 0.182).
TABLE 2.
Best overall response to first‐line treatment.
| All | Synaptophysin | Chromogranin A | NCAM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |||||||||
| ICI‐c | c | ICI‐c | c | ICI‐c | c | ICI‐c | c | ICI‐c | c | ICI‐c | c | |||
| N | 30 | 197 | 26 | 153 | 4 | 38 | 22 | 100 | 8 | 84 | 28 | 182 | 2 | 10 |
| Response, % | ||||||||||||||
| CR | 0 (0%) | 1 (0.5%) | 0 (0%) | 1 (0.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.6%) | 0 (0%) | 0 (0%) |
| PR | 25 (83.3%) | 160 (81.2%) | 22 (84.6%) | 126 (82.4%) | 3 (75.0%) | 29 (76.3%) | 18 (81.8%) | 82 (82.0%) | 7 (87.5%) | 68 (81.0%) | 24 (85.7%) | 148 (81.3%) | 1 (50.0%) | 8 (80.0%) |
| SD | 0 (0%) | 16 (8.1%) | 0 (0%) | 13 (8.5%) | 0 (0%) | 2 (5.3%) | 0 (0%) | 7 (7.0%) | 0 (0%) | 7 (8.3%) | 0 (0%) | 16 (8.8%) | 0 (0%) | 0 (0%) |
| PD | 3 (10.0%) | 10 (5.1%) | 1 (3.8%) | 8 (5.2%) | 1 (25.0%) | 2 (5.3%) | 2 (9.1%) | 6 (6.0%) | 1 (12.5%) | 4 (4.8%) | 2 (7.1%) | 10 (5.5%) | 1 (50.0%) | 0 (0%) |
| NE | 1 (3.3%) | 10 (5.1%) | 2 (7.7%) | 5 (3.3%) | 0 (0%) | 5 (13.1%) | 1 (4.5%) | 4 (4.0%) | 0 (0%) | 5 (6.0%) | 1 (3.6%) | 7 (3.8%) | 0 (0%) | 2 (20.0%) |
| N‐CR/N‐PD | 1 (3.3%) | 0 (0%) | 1 (3.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3.6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| ORR, % | 83.3% | 81.7% | 84.6% | 83.1% | 75.0% | 76.3% | 81.8% | 83.0% | 87.5% | 81.0% | 85.7% | 81.9% | 50.0% | 80.0% |
| p‐value | 0.106 | 0.166 | 0.457 | 0.313 | 0.669 | 0.149 | 0.182 | |||||||
Abbreviations: c, chemotherapy; CR, complete response; ICI‐c, immune checkpoint inhibitor plus chemotherapy; NCAM, neural cell adhesion molecule; N‐CR/N‐PD, not complete response/not progressive disease; NE, neuroendocrine; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
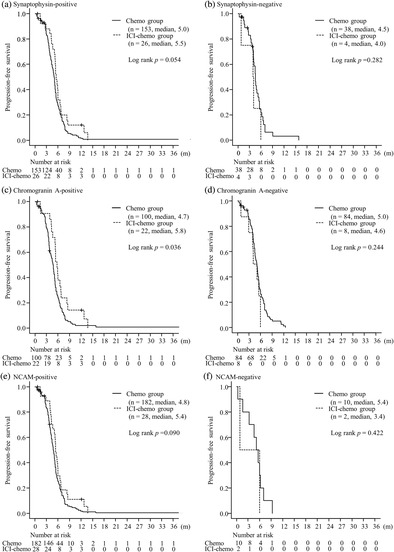
The median PFS was significantly longer in the ICI‐chemo group than in the chemo group in chromogranin A‐positive group (5.8 months in the ICI‐chemo group and 4.7 months in the chemo group; p = 0.036). The median PFS was 5.5 months in the ICI‐chemo group and 5.0 months in the chemo group; in Synaptophysin‐positive group (p = 0.054) and was 5.4 months in ICI‐chemo group and 4.8 months in the chemo group; NCAM‐positive groups, respectively (p = 0.090). There were no significant differences in PFS between the two groups in any NE marker‐negative SCLC (p = 0.282 in the synaptophysin‐negative group, p = 0.244 in the chromogranin A‐negative group, p = 0.422 in the NCAM‐negative group). No significant differences were observed in OS between the two groups regardless of the expression of any NE markers (p = 0.213 in the synaptophysin‐positive group, p = 0.317 in the synaptophysin‐negative group, p = 0.116 in the chromogranin A‐positive group, p = 0.688 in the chromogranin A‐negative group, p = 0.449 in the NCAM‐positive group, p = 0.472 in NCAM‐negative group, respectively) (Figures 1 and 2). In the population with PS 0–1, the median PFS was significantly longer in the ICI‐chemo group than in the chemo group in the synaptophysin‐ and chromogranin A‐positive groups (p = 0.043 and p = 0.013, respectively). The median PFS in the NCAM‐positive group tended to be longer in the ICI‐chemo group than in the chemo group (p = 0.077). Further, in the population with PS 0–1, the median OS for chromogranin A‐positive group was significantly longer in the ICI‐chemo group than in the chemo group (p = 0.016). There were no significant differences in the synaptophysin‐ and NCAM‐positive groups (p = 0.154 and p = 0.212, respectively). Moreover, no significant differences were found between the two groups in any NE marker‐negative SCLC in the population with PS 0–1 for both PFS (p = 0.546 in the synaptophysin‐negative group, p = 0.158 in the chromogranin A‐negative group, p = 0.624 in the NCAM‐negative group) and OS (p = 0.752 in the synaptophysin‐negative group, p = 0.563 in the chromogranin A‐negative group, p = 0.643 in the NCAM‐negative group).
FIGURE 1.
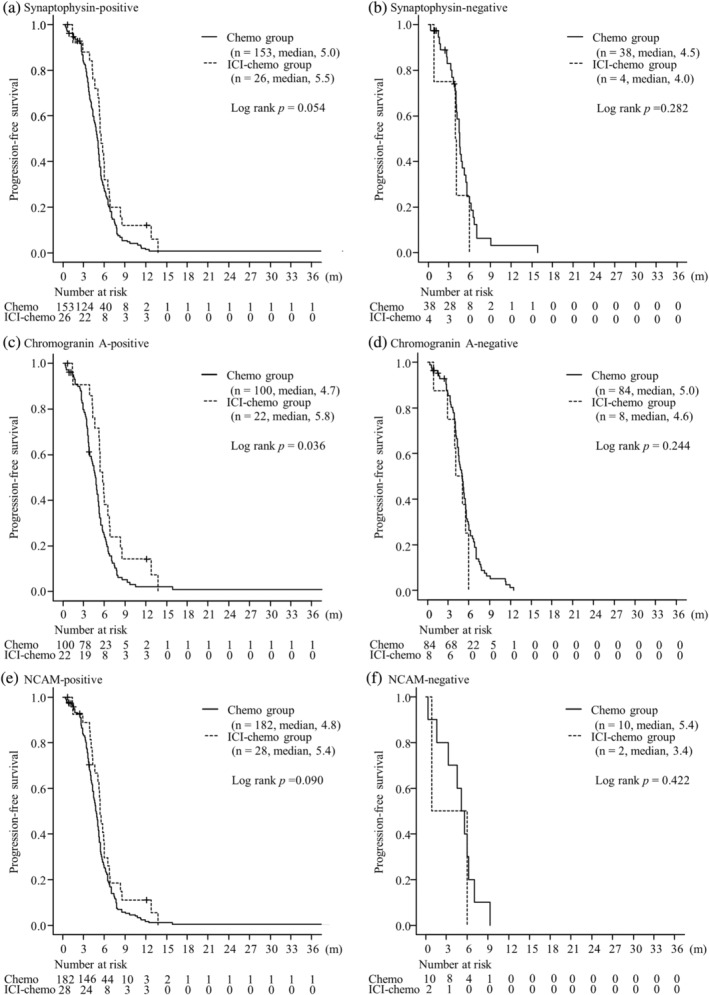
Progression‐free survival of patients with tumors stained with synaptophysin (a, b), chromogranin A (c, d), and neural cell adhesion molecule (NCAM) (e, f) between the immune checkpoint inhibitor (ICI) plus chemotherapy and chemotherapy alone groups.
FIGURE 2.
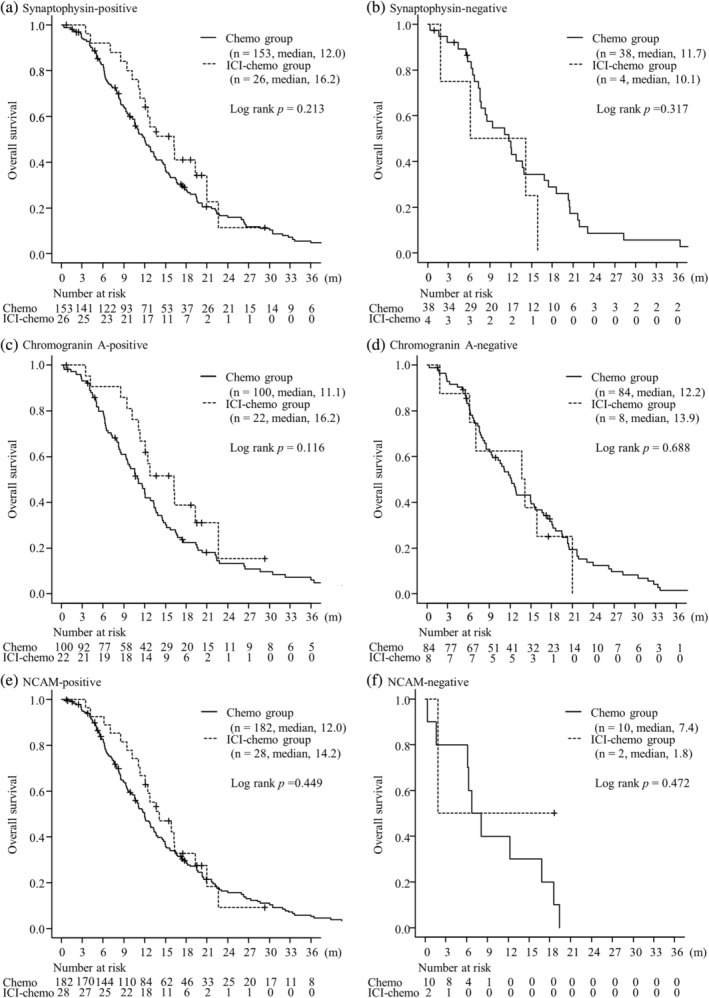
Overall survival of patients with tumors stained by synaptophysin (a, b), chromogranin A (c, d), and neural cell adhesion molecule (NCAM) (e, f) between the immune checkpoint inhibitor (ICI) plus chemotherapy group and chemotherapy alone groups.
DISCUSSION
We retrospectively analyzed the relationship between the efficacy of ICIs and the expression patterns of conventional NE markers such as synaptophysin, chromogranin A, and NCAM in patients with ED‐SCLC. PFS tended to be better in the ICI‐chemo group than in the chemo‐group in patients with any NE marker‐positive SCLC, and was statistically significant in patients with chromogranin A‐positive SCLC. However, no significant differences were observed in any of the NE marker‐negative SCLC patients between the groups. Our study suggests that the efficacy of ICIs in addition to chemotherapy may not be demonstrated in conventional NE marker‐negative SCLC.
It has recently been proposed that SCLC can be divided into four subtypes based on RNA expression: Achaete‐Scute family bHLH transcription factor 1 (ASCL1), neurogenic differentiation factor 1 (NeuroD1), POU class 2 homeobox 3 (POU2F3), and yes‐associated protein 1 (YAP1). 11 These subgroups are referred to as SCLC‐A, SCLC‐N, SCLC‐P, and SCLC‐Y, respectively. Gay et al. reported four subtypes based on the transcription factors ASCL1, NEUROD1, and POU2F3. 12 In their report, the expression of YAP1 was also observed in SCLC‐P, and subgroup classification based on the expression of YAP1 was not possible. Instead of SCLC‐Y cells, they proposed a fourth group named the inflamed subtype SCLC‐I with a low expression of ASCL1, NEUROD1, and POU2F3, which expressed genes associated with immune checkpoints and the human leukocyte antigen. This group was named the inflamed subtype SCLC‐I. They also assessed the expression of ASCL1, NEUROD1, and POU2F3 using 276 samples from patients who participated in the IMPower 133 study. The proportions with SCLC‐A, SCLC‐N, SCLC‐I, and SCLC‐P were 51%, 23%, 18%, and 7%, respectively. The HR for death tended to be better in the carboplatin and etoposide plus atezolizumab (EP + atezo) group than in the carboplatin and etoposide (EP) group in all four subgroups. However, no significant difference in OS was found between the four groups in the EP arm. In particular, the difference in MST between EP + atezo and EP was greatest in SCLC‐I, with a significantly longer OS than that of the other subgroups in the EP + atezo arm (HR: 0.566, 95% CI: 0.321–0.998). Based on these results, it is suggested that the classification of SCLC based on RNA expression, especially SCLC‐I, may be predictive of the efficacy of ICIs. Furthermore, SCLC‐P was especially poor in terms of OS relative to the other three subtypes in each arm, despite the trend towards improvement in the EP + atezo arm. Therefore, SCLC‐P may be a marker of poor prognosis. Further, the association with the classification of SCLC based on RNA expression and conventional NE markers has been reported. Gay et al. also assessed the expression levels of NE genes, such as chromogranin A and synaptophysin, in the four subgroups mentioned above, and found that SCLC‐A and SCLC‐N have significantly higher mRNA expression levels of NE genes than SCLC‐I and SCLC‐P. Baine et al. assessed the expression levels of ASCL1, NEUROD1, POU2F3, and YAP1 by immunohistochemistry using the H‐score and the relationship between these new markers and conventional NE markers, chromogranin A, synaptophysin, CD56 (NCAM), and insulinoma‐associated 1 (INSM1). 13 The ASCL1/NEUROD1 double‐negative group showed significantly lower expression of conventional NE markers than the ASCL1 or NEUROD1 dominant subtypes. Although it has been suggested that the conventional NE marker may become a surrogate for these new classifications and a predictive marker of treatment, there has been few reports on the efficacy of treatment based on conventional NE markers in SCLC. In this report, our study suggests that the efficacy of ICIs in addition to chemotherapy may be poor in conventional NE marker‐negative SCLC. A previous report showed SCLC‐P, which expresses POU2F3, tends to have worse OS than other subtype groups in patients treated with EP + atezo. 12 Other reports have shown that SCLC‐P is strongly associated with low immunohistochemical expression of conventional NE markers, such as chromogranin A, synaptophysin, and CD56. 13 , 14 , 15 Therefore, our results might be associated with a previous report that SCLC‐P had a shorter OS than the other three subtypes in the treatment of atezolizumab in addition to chemotherapy. 12
Few studies have assessed the relationship between NE marker expression and SCLC prognosis. Previous studies have assessed the association between the efficacy of cytotoxic chemotherapy alone and prognosis. However, it is unclear whether the expression of conventional NE markers is associated with prognosis. 16 , 17 Therefore, we believe that our report will be useful in the era of ICI therapy. Furthermore, transcription factors, such as ASCL1, NEUROD1, and POU2F3 are not routinely evaluated because they are difficult to measure in clinical practice. However, IHC testing for conventional NE markers, such as synaptophysin, chromogranin A, and NCAM is widely used to diagnose SCLC. 1 , 18 Therefore, our results may be more meaningful than those previously reported to assess prognosis using transcription factors in clinical practice. 12 Currently, SCLC treatments do not differ based on the expression of conventional NE markers. Based on our results showing that the efficacy of ICIs in addition to chemotherapy might be poor in conventional NE marker‐negative SCLC, personalized medicine based on the expression of conventional NE markers is needed in SCLC.
Finally, our study had several limitations. First, the sample size was small, and this study was the result of a single‐center analysis. However, there are few reports on the relationship between the expression of conventional NE markers and the efficacy of ICIs in first‐line therapy; thus, the results of this report may be considered important. This finding needs to be further validated using a larger sample size in multicenter studies. Second, we assessed each NE marker‐negative SCLC as a tumor in which all tumor cells were negative for each NE marker, and NE marker‐positive SCLC as a tumor that was slightly positive. Previous reports have assessed the expression of markers based on the percentage of positive cells, intensity of labeling, and H‐score. 12 If our study had used the H‐score, the results may have differed. However, the definition of positivity in our study was considered more stringent than that of the H‐score. Therefore, we consider our results credible. Third, all SCLC tumors in the chemo group were not stained by synaptophysin, chromogranin A, and NCAM. We did not assess the association between ICI efficacy and the number of positive results using conventional NE markers. Finally, the timing of the response assessment was decided by each physician, which might have introduced variance in the ORR and PFS. Considering this limitation, we also assessed the OS, which is a reliable endpoint.
In conclusion, our results suggest that the efficacy of ICIs in addition to chemotherapy may be poor in conventional NE marker‐negative SCLC.
AUTHOR CONTRIBUTIONS
All authors had full access to the study data and take responsibility for the integrity of the data and accuracy of the data analysis. All the authors have read and approved the submission of the manuscript.
Conceptualization: Y.I. and K.W. Data curation: Y.I. Investigation: Y.I., M.M., M.S., K.D., M.Y., H.K., K.M., and N.M. Supervision: N.M., H.K., R.K., A.O., H.K., T.N., H.M., Y.G., and T.T. Writing–original draft preparation: Y.I., K.W., and T.K. Writing—review and editing: Y.I., K.W., and T.T.
CONFLICT OF INTEREST STATEMENT
Dr Wakuda reports receiving grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants and personal fees from AstraZeneca K.K., grants from Novartis Pharma K.K., grants from AbbVie, grants from AMGEN, grant and personal fees from MSD K.K., grant and personal fees from Daiichi Sankyo Co., Ltd., grant from Dizal Pharma, personal fees from Taiho Pharmaceutical, Boehringer Ingelheim, Eli Lilly K.K, Ono Pharmaceutical, Janssen Pharmaceutical K.K., Takeda Pharmaceutical, and Nihon Kayaku, outside the submitted work. Dr Sekikawa reports receiving personal fees from Chugai Pharmaceutical Co., Ltd and Takeda Pharmaceutical, outside the submitted work. Dr Kodama reports receiving personal fees from Chugai Pharmaceutical Co., Ltd and Novartis Pharma K.K., outside the submitted work. Dr Miura reports receiving personal fees from AstraZeneca, Chugai Pharmaceutical, and Taiho Pharmaceutical, outside the submitted work. Dr Mamesaya reports receiving grants and personal fees from Boehringer Ingelheim, personal fees from Chugai Pharmaceutical Co., Ltd., personal fees from Taiho Pharmaceuticals, personal fees from MSD K.K., personal fees from AstraZeneca K.K., personal fees from Ono Pharmaceutical Co., Ltd., outside the submitted work. Dr Kobayashi reports receiving personal fees from Eli Lilly K.K., personal fees from Novartis Pharma K.K., personal fees from Taiho Pharmaceutical., personal fees from AstraZeneca K.K., personal fees from Chugai Pharmaceutical Co., Ltd., personal fees from Ono Pharmaceutical Co., LTD., personal fees from Bristol‐Myers Squibb Company, and personal fees from Daiichi Sankyo Co., outside the submitted work. Dr Ko reports receiving grants and personal fees from MSD K.K., personal fees from Taiho Pharmaceuticals, personal fees from Chugai Pharmaceutical Co., Ltd., personal fees from Eli Lilly K.K., personal fees from Ono Pharmaceutical, personal fees from Daiichi Sankyo, personal fees from Takeda, and grants and personal fees from AstraZeneca K.K., outside the submitted work. Dr Ono reports receiving personal fees from AstraZeneca K.K., personal fees from Chugai Pharmaceutical Co., Ltd., personal fees from Ono Pharmaceutical Co., Ltd., and personal fees from Indica laboratories, outside the submitted work. Dr Kenmotsu reports receiving personal fees from AMGEN, grants and personal fees from AstraZeneca K.K., personal fees from Bayer, personal fees from Boehringer Ingelheim, personal fees from Bristol‐Myers Squibb, personal fees from Chugai Pharmaceutical Co., personal fees from Daiichi‐Sankyo Co., Ltd., grants and personal fees from Novartis Pharma K.K., grants from Loxo Oncology, grants and personal fees from Ono Pharmaceutical Co, Ltd., grants and personal fees from Eli Lilly K.K., personal fees from Kyowa Hakko Kirin Co., Ltd., personal fees from Taiho Pharmaceuticals, personal fees from Takeda Pharmaceutical Co., Ltd, personal fees from Merck Biopharma Co., Ltd., personal fees from MSD K.K, personal fees from Pfizer, outside the submitted work. Dr Naito reports receiving grants from Otsuka Pharmaceutical K.K., personal fees from Ono Pharmaceutical Co., Ltd., receiving grants from Japan Agency for Medical Research and Development (AMED), and personal fees from Helsinn Healthcare SA, outside the submitted work. Dr Murakami reports receiving grants and personal fees from AstraZeneca K.K., grants and personal fees from Takeda Pharmaceutical Co., Ltd., grants and personal fees from Daiichi‐Sankyo Co., grants from AbbVie, grants from IQvia, grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants and personal fees from Taiho Pharmaceutical, grants from Bayer, personal fees from Amgen, personal fees from Ono Pharmaceutical Co., Ltd., personal fees from Bristol‐Myers Squibb Japan, personal fees from MSD K.K., personal fees from Pfizer Inc., personal fees from Novartis Pharma K.K., personal fees from Eli Lilly Japan K.K., personal fees from Eisai, personal fees from Nihon Kayaku, outside the submitted work. Dr Takahashi reports receiving grants and personal fees from AstraZeneca K.K., grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants and personal fees from Eli Lilly Japan K.K., personal fees from Ono Pharmaceutical Co., Ltd., grants and personal fees from MSD, grants and personal fees from Pfizer Japan Inc., grants and personal fees from Amgen Inc., grants from Merck Biopharma CO., LTD, grants from Janssen Pharmaceutical K.K., grants from AnHeart Therapeutics Inc., personal fees from BMS Japan, personal fees from Takeda Pharmaceuticals Co., and personal fees from Novartis You, outside the submitted work. The remaining authors have nothing to disclose.
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Iida Y, Wakuda K, Kawata T, Morita M, Sekikawa M, Doshita K, et al. Relationship between patterns of immunohistochemical conventional neuroendocrine markers and efficacy of immune check point inhibitors in patients with extensive disease small cell lung cancer. Thorac Cancer. 2024;15(6):477–485. 10.1111/1759-7714.15218
REFERENCES
- 1. van Meerbeeck JP, Fennell DA, De Ruysscher DA. Small‐cell lung cancer. Lancet. 2011;378:1741–1755. [DOI] [PubMed] [Google Scholar]
- 2. Puglisi M, Dolly S, Faria A, Myerson JS, Popat S, O'Brien ME. Treatment options for small cell lung cancer–do we have more choice? Br J Cancer. 2010;102:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First‐line atezolizumab plus chemotherapy in extensive‐stage small‐cell lung cancer. N Engl J Med. 2018;379:2220–2229. [DOI] [PubMed] [Google Scholar]
- 4. Paz‐Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum‐etoposide versus platinum‐etoposide in first‐line treatment of extensive‐stage small‐cell lung cancer (Caspian): a randomized, controlled, open‐label, phase 3 trial. Lancet. 2019;394:1929–1939. [DOI] [PubMed] [Google Scholar]
- 5. Hanna NH, Schneider BJ, Temin S, Baker S Jr, Brahmer J, Ellis PM, et al. Therapy for stage IV non‐small‐cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2020;38:1608–1632. [DOI] [PubMed] [Google Scholar]
- 6. Hanna NH, Robinson AG, Temin S, Baker S Jr, Brahmer JR, Ellis PM, et al. Therapy for stage IV non‐small‐cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2021;39:1040–1091. [DOI] [PubMed] [Google Scholar]
- 7. Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, et al. Pan‐Asian adapted clinical practice guidelines for the management of patients with metastatic non‐small‐cell lung cancer: a CSCO‐ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:171–210. [DOI] [PubMed] [Google Scholar]
- 8. Akamatsu H, Ninomiya K, Kenmotsu H, Morise M, Daga H, Goto Y, et al. The Japanese lung cancer society guideline for non‐small cell lung cancer, stage IV. Int J Clin Oncol. 2019;24:731–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. [DOI] [PubMed] [Google Scholar]
- 10. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gay CM, Stewart CA, Park EM, Diao L, Groves SM, Heeke S, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 2021;39:346–360.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baine MK, Hsieh MS, Lai WV, Egger JV, Jungbluth AA, Daneshbod Y, et al. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J Thorac Oncol. 2020;15:1823–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baine MK, Febres‐Aldana CA, Chang JC, Jungbluth AA, Sethi S, Antonescu CR, et al. POU2F3 in SCLC: Clinicopathologic and genomic analysis with a focus on its diagnostic utility in neuroendocrine‐low SCLC. J Thorac Oncol. 2022;17:1109–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Jin Y, Shen X, Zheng Q, Xue Q, Chen L, et al. POU2F3: a sensitive and specific diagnostic marker for neuroendocrine‐low/negative small cell lung cancer. Am J Surg Pathol. 2023;47:1059–1066. [DOI] [PubMed] [Google Scholar]
- 16. Gkika E, Benndorf M, Oerther B, Mohammad F, Beitinger S, Adebahr S, et al. Immunohistochemistry and radiomic features for survival prediction in small cell lung cancer. Front Oncol. 2020;10:1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sloman A, D'Amico F, Yousem SA. Immunohistochemical markers of prolonged survival in small cell carcinoma of the lung. Arch Pathol Lab Med. 1996;120:465–472. [PubMed] [Google Scholar]
- 18. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer; 2015. [DOI] [PubMed] [Google Scholar]


