Abstract
Colorectal cancer is the third most common cancer worldwide and there has been a concerning increase in the incidence rate of colorectal cancer among individuals under the age of 50. This study compared the survival outcome between early-onset and late-onset metastatic colorectal cancer to find the differences and identify their prognostic factors. We obtained patient data from SEER database. Survival outcome was estimated using Kaplan–Meier survival curves and compared using the log-rank test. Univariate and multivariate analyses were conducted utilizing COX models to identify their independent prognostic factors. A total of 10,036 early-onset metastatic colorectal (EOCRC) cancer patients and 56,225 late-onset metastatic colorectal cancer (LOCRC) patients between 2010 and 2019 were included in this study. EOCRC has more survival benefits than LOCRC. Tumor primary location (p < 0.001), the location of metastasis (p < 0.001) and treatment modalities (p < 0.001) affect the survival outcomes between these two groups of patients. Female patients had better survival outcomes in EOCRC group (p < 0.001), but no difference was found in LOCRC group (p = 0.57). In conclusion, our study demonstrated that EOCRC patients have longer survival time than LOCRC patients. The sex differences in survival of metastatic colorectal cancer patients are associated with patients’ age. These findings contribute to a better understanding of the differences between metastatic EOCRC and LOCRC, and can help inform the development of more precise treatment guidelines to improve prognosis.
Keywords: Early-onset colorectal cancer, Metastatic cancer, Survival
Subject terms: Cancer, Gastrointestinal cancer, Metastasis
Introduction
Colorectal cancer is the third most common cancer worldwide and the second leading cause of cancer death in the US1,2. While the overall incidence rate of colorectal cancer is declining due to the emphasis on prevention, there has been a concerning increase in the incidence rate of colorectal cancer among individuals under the age of 50 since the 1990s3. This subset of patients is referred to as early-onset colorectal cancer4. In certain countries, the rate of early-onset colorectal cancer is growing at an alarming rate of 2–3%. It is projected that by 2030, early-onset colorectal cancer will surpass other cancers to become the most common cancer among individuals aged 20–49 in the United States5,6.
Not only the proportion of early-onset colorectal cancer patients is increasing, but early-onset colorectal cancer has more aggressive nature and higher likelihood of metastasis compared to late-onset colorectal cancer7–9. Metastasis significantly impacts patient prognosis10, making it crucial for researchers to focus on patients with metastatic early-onset colorectal cancer. However, there is controversy on the prognosis of metastatic early-onset colorectal cancer. While some multicenter studies suggest no difference in prognosis between metastatic early-onset colorectal cancer and late-onset colorectal cancer11,12, other studies indicate a disparity between the two13,14. Therefore, further research is necessary to explore this direction, and multiple data sources are required to validate these findings.
In this study, we retrieved the colorectal cancer population from the SEER database to make comparisons between early-onset and late-onset metastatic colorectal cancer and figure out the possible prognostic factors.
Methods
Data sources
The Surveillance, Epidemiology, and End Results (SEER) is a public cancer database created by the National Cancer Institute (NCI), which includes 18 population-based tumor registries covering approximately 28% of the Americans15. The data in this study were obtained from the SEER Program SEER*Stat software released in November 2021 (version8.4.0). According to the definition, a total of 66,261 patients of CRC registered between 2010 and 2019 were screened and divided into early-onset colorectal cancer (EOCRC) (N = 10,036) and late-onset colorectal cancer (LOCRC) (N = 56,225). EOCRC was defined as CRC arising in adults 20–49 years of age, while LOCRC was defined as CRC arising in adults 50–75 years of age16.
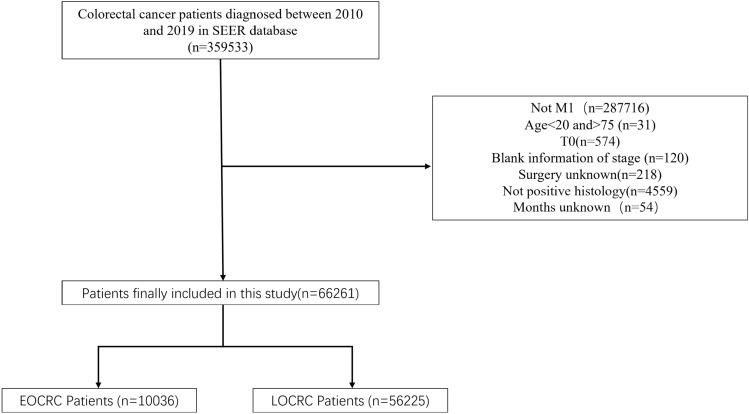
Patient selection criteria were as follows: (1) colorectal cancer patients diagnosed between 2010 and 2019; (2) exclusion of patients not in M1 stage; (3) exclusion of patients younger than 20 years old or older than 75 years old; (4) exclusion of patients in T0 stage; (5) exclusion of patients with blank information of stage; (6) exclusion of patients whose surgery information is unknown; (7) exclusion of patients whose histology is not positive (8). exclusion of patients with unknown survival months. A flow chart of the study population selection was displayed in Fig. 1.
Figure 1.
Flow chart illustrating patients selection.
Data collection
The following variables were selected for this study: year of diagnosis, gender, race, primary site, histological type, T stage, N stage, grade, surgery, radiotherapy, chemotherapy, CEA, marital status and metastatic sites.
Statistical analysis
All statistical analyses were implemented with R (version 4.1.2) software. Statistical significance was set at p < 0.05. Baseline characteristics according to EOCRC and LOCRC groups were compared using test for categorical variables. OS and CSS were calculated based on the Kaplan–Meier curves and compared between EOCRC versus LOCRC patients using the log-rank χ2test17. Overall survival (OS) were the primary endpoints of this study. The overall survival (OS) refers to the time passed from the time of the initial diagnosis of CRC to the time of death from any cause. Univariate and multivariate analyses were conducted utilizing COX models to reveal the independent prognostic factors for EOCRC and LOCRC18. Hazard ratios (HRs) and 95% confidence interval (CI) calculations were based on COX models.
Results
Baseline characteristics
In this study, 359,533 patients diagnosed with colorectal cancer between 2010 and 2019 were extracted from the SEER database, of which 66,261 met our screening criteria for further study. Among them, there were 10,036 patients with early-onset colorectal cancer and 56,225 patients with late-onset colorectal cancer (Table 1).
Table 1.
Patient characteristics in the study.
| Variable | EOCRC | LOCRC | p |
|---|---|---|---|
| Gender | 0.019 | ||
| Male | 5368 (53.5%) | 30,791 (54.8%) | |
| Female | 4668 (46.5%) | 25,434 (45.2%) | |
| Year of diagnosis | 0.020 | ||
| 2010 | 897 (8.94%) | 5340 (9.50%) | |
| 2011 | 925 (9.22%) | 5419 (9.64%) | |
| 2012 | 904 (9.01%) | 5366 (9.54%) | |
| 2013 | 988 (9.84%) | 5343 (9.50%) | |
| 2014 | 982 (9.78%) | 5698 (10.1%) | |
| 2015 | 976 (9.72%) | 5608 (9.97%) | |
| 2016 | 1067 (10.6%) | 5768 (10.3%) | |
| 2017 | 1122 (11.2%) | 5725 (10.2%) | |
| 2018 | 1065 (10.6%) | 5905 (10.5%) | |
| 2019 | 1110 (11.1%) | 6053 (10.8%) | |
| Race | < 0.001 | ||
| White | 7351 (73.2%) | 43,306 (77.0%) | |
| Black | 1486 (14.8%) | 7718 (13.7%) | |
| American Indian | 119 (1.19%) | 491 (0.87%) | |
| Asian | 1028 (10.2%) | 4556 (8.10%) | |
| Unknown | 52 (0.52%) | 154 (0.27%) | |
| Primary site | < 0.001 | ||
| Right-sided | 2177 (21.7%) | 19,307 (34.3%) | |
| Left-sided | 3204 (31.9%) | 13,827 (24.6%) | |
| Rectum | 2405 (24.0%) | 10,479 (18.6%) | |
| Others | 2250 (22.4%) | 12,612 (22.4%) | |
| Histological type | < 0.001 | ||
| Adenocarcinoma | 9535 (95.0%) | 52,685 (93.7%) | |
| Others | 501 (4.99%) | 3540 (6.30%) | |
| T | < 0.001 | ||
| T1 | 697 (6.94%) | 4307 (7.66%) | |
| T2 | 245 (2.44%) | 1199 (2.13%) | |
| T3 | 3180 (31.7%) | 16,160 (28.7%) | |
| T4 | 3132 (31.2%) | 15,816 (28.1%) | |
| Unknown | 2782 (27.7%) | 18,743 (33.3%) | |
| N | < 0.001 | ||
| N0 | 2715 (27.1%) | 18,135 (32.3%) | |
| N1 | 3080 (30.7%) | 15,429 (27.4%) | |
| N2 | 2553 (25.4%) | 11,924 (21.2%) | |
| Unknown | 1688 (16.8%) | 10,737 (19.1%) | |
| Grade | < 0.001 | ||
| 1/2 | 5674 (56.5%) | 29,314 (52.1%) | |
| 3/4 | 2245 (22.4%) | 12,357 (22.0%) | |
| Unknown | 2117 (21.1%) | 14,554 (25.9%) | |
| Surgery | < 0.001 | ||
| No | 4367 (43.5%) | 28,521 (50.7%) | |
| Yes | 5669 (56.5%) | 27,704 (49.3%) | |
| Radiation | < 0.001 | ||
| No | 8339 (83.1%) | 49,786 (88.5%) | |
| Yes | 1697 (16.9%) | 6439 (11.5%) | |
| Chemotherapy | 0.000 | ||
| No | 1486 (14.8%) | 20,939 (37.2%) | |
| Yes | 8550 (85.2%) | 35,286 (62.8%) | |
| CEA | < 0.001 | ||
| Negative/Borderline | 1503 (15.0%) | 6891 (12.3%) | |
| Positive | 5907 (58.9%) | 31,239 (55.6%) | |
| Unknown | 2626 (26.2%) | 18,095 (32.2%) | |
| Marital status | 0.000 | ||
| Married | 5236 (52.2%) | 27,871 (49.6%) | |
| Single/unmarried | 3309 (33.0%) | 10,412 (18.5%) | |
| Divorced/widowed/separated | 1019 (10.2%) | 15,154 (27.0%) | |
| Unknown | 472 (4.70%) | 2788 (4.96%) | |
| Bone metastases | 0.002 | ||
| No | 9216 (91.8%) | 51,121 (90.9%) | |
| Yes | 568 (5.66%) | 3343 (5.95%) | |
| Unknown | 252 (2.51%) | 1761 (3.13%) | |
| Brain metastases | < 0.001 | ||
| No | 9675 (96.4%) | 53,580 (95.3%) | |
| Yes | 102 (1.02%) | 753 (1.34%) | |
| Unknown | 259 (2.58%) | 1892 (3.37%) | |
| Liver metastases | 0.011 | ||
| No | 2934 (29.2%) | 15,972 (28.4%) | |
| Yes | 6972 (69.5%) | 39,324 (69.9%) | |
| Unknown | 130 (1.30%) | 929 (1.65%) | |
| Lung metastases | < 0.001 | ||
| No | 7657 (76.3%) | 40,667 (72.3%) | |
| Yes | 2102 (20.9%) | 13,735 (24.4%) | |
| Unknown | 277 (2.76%) | 1823 (3.24%) |
Comparison of overall survival between EOCRC and LOCRC
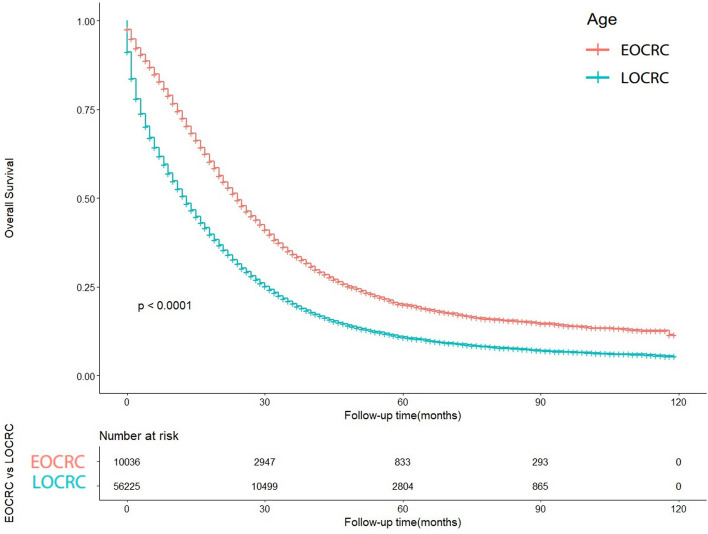
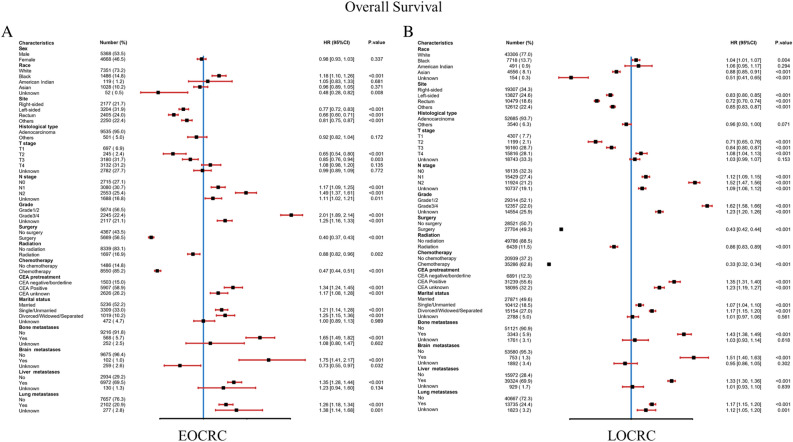
The OS of early-onset colorectal cancer (EOCRC) and late-onset colorectal cancer (LOCRC) was calculated using the Kaplan–Meier curves. The results indicated significant differences in OS between EOCRC and LOCRC (p < 0.0001) (Fig. 2). The median OS for EOCRC was 18 months (95% CI 8–33 months), while for LOCRC it was 10 months (95% CI 3–24 months). A univariable Cox proportional hazards regression analysis was performed in the EOCRC population (Table 2), considering all baseline information related to patient's OS, such as gender, primary site, race, histology, T stage, N stage, grade, surgery, radiation, chemotherapy, CEA, marital status, bone metastases, brain metastases, liver metastases, and lung metastases. These variables were also included in the subsequent multivariable Cox proportional hazards regression analysis. In the multivariate analysis, primary site, race, histology, T stage, N stage, grade, surgery, radiation, chemotherapy, CEA, marital status, bone metastases, brain metastases, liver metastases, and lung metastases were identified as independent risk or protective factors. When performing univariable Cox proportional hazards regression analysis in the LOCRC population (Table 3), primary site, race, histology, T stage, N stage, grade, surgery, radiation, chemotherapy, CEA, marital status, bone metastases, brain metastases, liver metastases, and lung metastases were related to OS. These factors were adjusted in subsequent multivariate analyses. primary site, race, histology, T stage, N stage, grade, surgery, radiation, chemotherapy, CEA, marital status, bone metastases, brain metastases, liver metastases, and lung metastases have been confirmed to be independent risk or protective factors. Forest plots of multivariate Cox regression analysis for OS in early-onset colorectal cancer (EOCRC) and late-onset colorectal cancer (LOCRC) were also conducted (Fig. 3).
Figure 2.
Kaplan–Meier-curves for overall survival in EOCRC patients and LOCRC patients. Life tables for patients at risk are given below the plot.
Table 2.
Univariable and multivariate Cox regression analysis based on all variables for overall survival of EOCRC patients.
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| Gender | ||||
| Male | [Reference] | [Reference] | ||
| Female | 0.902(0.859–0.947) | < 0.001 | 0.976(0.929–1.026) | 0.337 |
| Race | ||||
| White | [Reference] | [Reference] | ||
| Black | 1.260(1.178–1.347) | < 0.001 | 1.176(1.098–1.260) | < 0.001 |
| American Indian | 0.935(0.741–1.180) | 0.571 | 1.050(0.831–1.327) | 0.681 |
| Asian | 0.981(0.904–1.065) | 0.648 | 0.963(0.886–1.046) | 0.371 |
| Unknown | 0.538(0.312–0.927) | < 0.05 | 0.4759(0.276–0.821) | < 0.01 |
| Primary site | ||||
| Right | [Reference] | [Reference] | ||
| Left | 0.728(0.681–0.778) | < 0.001 | 0.773(0.722–0.826) | < 0.001 |
| Rectum | 0.736(0.686–0.791) | < 0.001 | 0.656(0.604–0.712) | < 0.001 |
| Others | 0.749(0.697–0.805) | < 0.001 | 0.808(0.750–0.871) | < 0.001 |
| Histological type | ||||
| Adenocarcinoma | [Reference] | [Reference] | ||
| Others | 1.362(1.225–1.513) | < 0.001 | 0.924(0.824–1.035) | 0.172 |
| T | ||||
| T1 | [Reference] | [Reference] | ||
| T2 | 0.463(0.381–0.561) | < 0.001 | 0.655(0.537–0.798) | < 0.001 |
| T3 | 0.548(0.497–0.604) | < 0.001 | 0.850(0.765–0.950) | < 0.01 |
| T4 | 0.753(0.684–0.828) | < 0.001 | 1.083(0.976–1.202) | 0.135 |
| Unknown | 1.144(1.039–1.260) | < 0.01 | 0.986(0.893–1.088) | 0.772 |
| N | ||||
| N0 | [Reference] | [Reference] | ||
| N1 | 0.965(0.905–1.029) | 0.274 | 1.166(1.090–1.248) | < 0.001 |
| N2 | 1.031(0.964–1.102) | 0.379 | 1.485(1.374–1.605) | < 0.001 |
| Unknown | 1.622(1.499–1.754) | < 0.001 | 1.113(1.025–1.209) | < 0.05 |
| Grade | ||||
| 1/2 | [Reference] | [Reference] | ||
| 3/4 | 1.954(1.843–2.072) | < 0.001 | 2.013(1.893–2.141) | < 0.001 |
| Unknown | 1.882(1.769–2.003) | < 0.001 | 1.246(1.164–1.334) | < 0.001 |
| Surgery | ||||
| No | [Reference] | [Reference] | ||
| Yes | 0.416(0.395–0.437) | < 0.001 | 0.396(0.368–0.425) | < 0.001 |
| Radiation | ||||
| No | [Reference] | [Reference] | ||
| Yes | 0.800(0.748–0.855) | < 0.001 | 0.885(0.819–0.956) | < 0.01 |
| Chemotherapy | ||||
| No | [Reference] | [Reference] | ||
| Yes | 0.487(0.456–0.519) | < 0.001 | 0.475(0.443–0.509) | < 0.001 |
| CEA | ||||
| Negative/Borderline | [Reference] | [Reference] | ||
| Positive | 1.603(1.485–1.730) | < 0.001 | 1.345(1.244–1.454) | < 0.001 |
| Unknown | 1.451(1.335–1.578) | < 0.001 | 1.174(1.077–1.280) | < 0.001 |
| Marital status | ||||
| Married | [Reference] | [Reference] | ||
| Single/Unmarried | 1.291(1.223–1.363) | < 0.001 | 1.208(1.142–1.277) | < 0.001 |
| Divorced/Widowed/Separated | 1.251(1.154–1.357) | < 0.001 | 1.253(1.154–1.360) | < 0.001 |
| Unknown | 1.098(0.977–1.234) | 0.116 | 1.001(0.890–1.126) | 0.989 |
| Bone metastases | ||||
| No | [Reference] | [Reference] | ||
| Yes | 2.338(2.125–2.572) | < 0.001 | 1.647(1.489–1.822) | < 0.001 |
| Unknown | 1.351(1.172–1.559) | < 0.001 | 1.085(0.799–1.472) | 0.602 |
| Brain metastases | ||||
| No | [Reference] | [Reference] | ||
| Yes | 2.289(1.855–2.826) | < 0.001 | 1.746(1.405–2.171) | < 0.001 |
| Unknown | 1.261(1.094–1.454) | < 0.01 | 0.731(0.549–0.973) | < 0.05 |
| Liver metastases | ||||
| No | [Reference] | [Reference] | ||
| Yes | 1.325(1.254–1.401) | < 0.001 | 1.353(1.276–1.436) | < 0.001 |
| Unknown | 1.443(1.175–1.773) | < 0.001 | 1.226(0.939–1.601) | 0.134 |
| Lung metastases | ||||
| No | [Reference] | [Reference] | ||
| Yes | 1.495(1.410–1.585) | < 0.001 | 1.259(1.184–1.339) | < 0.001 |
| Unknown | 1.502(1.309–1.723) | < 0.001 | 1.381(1.136–1.679) | < 0.01 |
Table 3.
Univariable and multivariate Cox regression analysis based on all variables for overall survival of LOCRC patients.
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| Gender | ||||
| Male | [Reference] | [Reference] | ||
| Female | 1.006(0.987–1.025) | 0.526 | ||
| Race | ||||
| White | [Reference] | [Reference] | ||
| Black | 1.100(1.0707–1.130) | < 0.001 | 1.041(1.013–1.070) | < 0.01 |
| American Indian | 1.064(0.963–1.176) | 0.225 | 1.055(0.955–1.166) | 0.294 |
| Asian | 0.902(0.871–0.935) | < 0.001 | 0.879(0.848–0.911) | < 0.001 |
| Unknown | 0.696(0.551–0.880) | < 0.01 | 0.513(0.405–0.648) | < 0.001 |
| Primary site | ||||
| Right | [Reference] | [Reference] | ||
| Left | 0.789(0.770–0.809) | < 0.001 | 0.826(0.805–0.847) | < 0.001 |
| Rectum | 0.773(0.753–0.795) | < 0.001 | 0.719(0.698–0.742) | < 0.001 |
| Others | 0.914(0.891–0.937) | < 0.001 | 0.852(0.829–0.874) | < 0.001 |
| Histological type | ||||
| Adenocarcinoma | [Reference] | [Reference] | ||
| Others | 1.357(1.307–1.409) | < 0.001 | 0.964(0.927–1.003) | 0.071 |
| T | ||||
| T1 | [Reference] | [Reference] | ||
| T2 | 0.485(0.449–0.524) | < 0.001 | 0.707(0.653–0.765) | < 0.001 |
| T3 | 0.565(0.544–0.586) | < 0.001 | 0.838(0.804–0.874) | < 0.001 |
| T4 | 0.776(0.747–0.805) | < 0.001 | 1.080(1.036–1.125) | < 0.001 |
| Unknown | 1.203(1.160–1.247) | < 0.001 | 1.028(0.990–1.066) | 0.153 |
| N | ||||
| N0 | [Reference] | [Reference] | ||
| N1 | 0.831(0.811–0.852) | < 0.001 | 1.121(1.092–1.151) | < 0.001 |
| N2 | 0.945(0.921–0.970) | < 0.001 | 1.516(1.470–1.564) | < 0.001 |
| Unknown | 1.505(1.464–1.547) | < 0.001 | 1.088(1.057–1.120) | < 0.001 |
| Grade | ||||
| 1/2 | [Reference] | [Reference] | ||
| 3/4 | 1.569(1.533–1.607) | < 0.001 | 1.615(1.576–1.655) | < 0.001 |
| Unknown | 1.807(1.766–1.848) | < 0.001 | 1.228(1.197–1.259) | < 0.001 |
| Surgery | ||||
| No | [Reference] | [Reference] | ||
| Yes | 0.472(0.463–0.482) | < 0.001 | 0.430(0.417–0.443) | < 0.001 |
| Radiation | ||||
| No | [Reference] | [Reference] | ||
| Yes | 0.700(0.679–0.722) | < 0.001 | 0.860(0.831–0.890) | < 0.001 |
| Chemotherapy | ||||
| No | [Reference] | [Reference] | ||
| Yes | 0.349(0.342–0.356) | < 0.001 | 0.330(0.324–0.337) | < 0.001 |
| CEA | ||||
| Negative/Borderline | [Reference] | [Reference] | ||
| Positive | 1.491(1.445–1.538) | < 0.001 | 1.354(1.311–1.398) | < 0.001 |
| Unknown | 1.561(1.510–1.614) | < 0.001 | 1.229(1.188–1.271) | < 0.001 |
| Marital status | ||||
| Married | [Reference] | [Reference] | ||
| Single/Unmarried | 1.200(1.169–1.231) | < 0.001 | 1.072(1.044–1.100) | < 0.001 |
| Divorced/Widowed/Separated | 1.376(1.346–1.407) | < 0.001 | 1.173(1.147–1.200) | < 0.001 |
| Unknown | 1.179(1.128–1.231) | < 0.001 | 1.013(0.969–1.059) | 0.561 |
| Bone metastases | ||||
| No | [reference] | [reference] | ||
| Yes | 0.564(0.543–0.585) | < 0.001 | 1.432(1.377–1.489) | < 0.001 |
| Unknown | 0.842(0.792–0.895) | < 0.001 | 1.027(0.926–1.138) | 0.618 |
| Brain metastases | ||||
| No | [Reference] | [Reference] | ||
| Yes | 1.947(1.803–2.101) | < 0.001 | 1.511(1.396–1.635) | < 0.001 |
| Unknown | 1.473(1.403–1.546) | < 0.001 | 0.950(0.862–1.047) | 0.302 |
| Liver metastases | ||||
| No | [Reference] | [Reference] | ||
| Yes | 1.246(1.219–1.272) | < 0.001 | 1.333(1.304–1.364) | < 0.001 |
| Unknown | 1.428(1.330–1.534) | < 0.001 | 1.009(0.926–1.099) | 0.839 |
| Lung metastases | ||||
| No | [Reference] | [Reference] | ||
| Yes | 1.300(1.272–1.328) | < 0.001 | 1.175(1.149–1.202) | < 0.001 |
| Unknown | 1.564(1.488–1.644) | < 0.001 | 1.125(1.050–1.205) | < 0.001 |
Figure 3.
Forest plot of multivariate Cox regression analysis for OS in early-onset colorectal cancer chemotherapy (EOCRC) and late-onset colorectal cancer (LOCRC).
Survival outcomes between EOCRC and LOCRC patients stratified by primary site, the location of metastasis
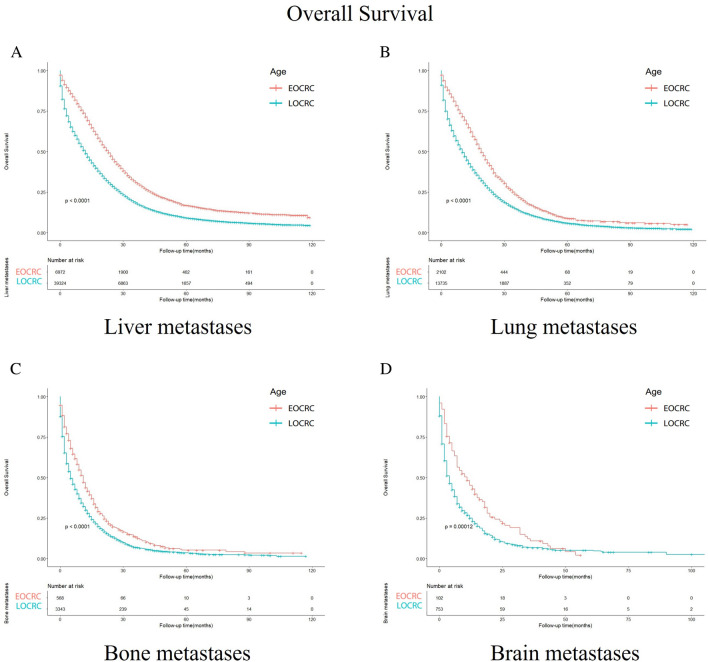
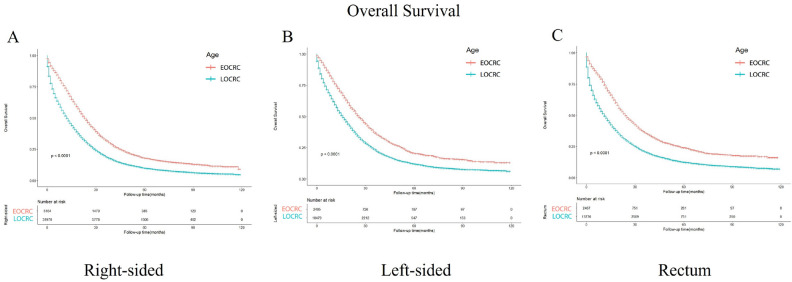
In the subgroups divided according to the metastasis site (Fig. 4), the OS of EOCRC patients with liver, lung, brain and bone metastases is better than that of LOCRC patients (p < 0.001, p < 0.001, p < 0.001, and p < 0.001). In terms of cancer primary location, the survival outcomes of all the subgroups show that EOCRC patients’ OS is better than LOCRC patients’ OS (p < 0.001) (Fig. 5).
Figure 4.
Kaplan–Meier-curves for overall survival in EOCRC and LOCRC patients with different metastasis sites. Life tables for patients at risk are given below each plot. (A) Liver metastases. (B) Lung metastases. (C) Bone metastases. (D) Brain metastases.
Figure 5.
Kaplan–Meier-curves for overall survival in EOCRC and LOCRC patients with different tumor primary sites. Life tables for patients at risk are given below each plot. (A) Right-sided. (B) Left-sided. (C) Rectum.
Survival outcomes between EOCRC and LOCRC patients stratified by treatment modalities
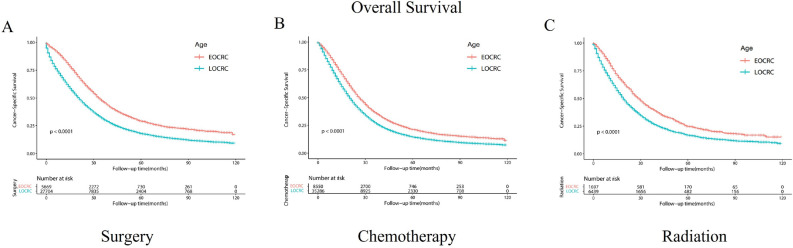
Subsequently, we conducted a comparison of the overall survival among patients diagnosed with EOCRC and LOCRC who underwent various treatment modalities. The findings indicated that within the subgroups of surgery, chemotherapy, and radiotherapy, the overall survival was notably higher among EOCRC patients in comparison to those with LOCRC (p < 0.0001) (Fig. 6).
Figure 6.
Kaplan–Meier-curves for overall survival in EOCRC and LOCRC patients with different treatment modalities. Life tables for patients at risk are given below each plot. (A) Surgery. (B) Chemotherapy. (C) Radiation.
Survival outcomes between male and female patients stratified by age
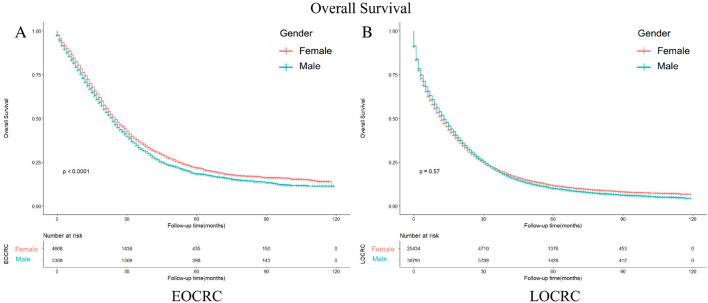
In univariate analysis, we observed differences in OS based on gender for early-onset colorectal cancer, but no such differences for late-onset colorectal cancer (Tables 2,3). We utilized Kaplan–Meier curves to do further analysis. In gender-divided subgroups, the OS shows differences between male EOCRC patients and female EOCRC patients (p < 0.001) (Fig. 7A). When performing analysis on male and female LOCRC patients, these differences disappear (p = 0.57) (Fig. 7B).
Figure 7.
Kaplan–Meier-curves for overall survival in male and female patients with different age. Life tables for patients at risk are given below each plot. (A) EOCRC patients. (B) LOCRC patients.
Discussion
In the United States, there has been a decline in colorectal cancer incidence and mortality rates due to increased colorectal cancer screening rates19. However, there is a concerning increase in early-onset colorectal cancer, which is also being observed globally20. This rise may be attributed to changes in eating habits associated with global economic development, such as increased consumption of sugary drinks, red meat, and processed meat products. Additionally, factors such as obesity, sedentary lifestyles, alcohol consumption, and the increased incidence of inflammatory bowel disease may contribute to this trend21–24. Despite these observations, our understanding of early-onset colorectal cancer remains limited, and further research is necessary to gain insights into this disease. Successful implementation of primary or secondary prevention strategies for early-onset colorectal cancer could have a significant impact on life expectancy in the future. Metastasis is the leading cause of mortality in colorectal cancer and significantly affects the prognosis of patients25–29. Distal organ metastasis is also a characteristic of young-onset cases. Therefore, we compared metastatic early-onset colorectal cancer with metastatic late-onset colorectal cancer to identify differences. Previous studies have explored the influence of various prognostic factors on patients with stage IV colorectal cancer28. Some demographic and clinicopathological variables have been identified as independent prognostic factors, including age at diagnosis, tumor location and size, and histological differentiation. However, due to the limited scale of these studies11,12, they do not fully capture the prognosis of stage IV colorectal cancer patients nor allow for a comprehensive comparison between early-onset and late-onset cases. This hinders our ability to provide more precise treatment for colorectal cancer patients based on age.
Comparing the survival of EOCRC and LOCRC, we found that the survival rate of EOCRC is approximately 8 months higher than that of LOCRC in metastatic colorectal cancer. Our research project has a larger sample size (66,261 vs. 254) compared to other studies11. Baseline data indicate that white individuals have a lower rate of early-onset colorectal cancer, which may be attributed to the quality of medical care they receive30. Younger patients showed a higher prevalence of mucus histology, consistent with another study31. The proportion of T3/4 patients in early-onset colorectal cancer patients is significantly higher than in late-onset colorectal cancer patients, indicating that the tumor size and extent are greater in early-onset cases. Similarly, N stage reveals that early-onset colorectal cancer patients have higher N1 or N2 ratios, indicating a significantly greater degree of lymph node involvement compared to late-onset cases. Despite these differences, early-onset colorectal cancer still has a better prognosis. This may be attributed to differences in treatment options, as early-onset cases often receive more aggressive treatment including surgery, radiotherapy, and chemotherapy32,33. Although some studies suggest that more aggressive treatment for early-onset colorectal cancer does not result in improved survival34–36, our results support the need for such treatment in younger patients with colorectal cancer.
In the subgroup analysis based on the location of metastasis, we examined the four most common metastatic organs: liver, lung, brain, and bone. Among these four metastases, the survival rate of early-onset colorectal cancer differed significantly from that of late-onset colorectal cancer. The prognosis for early-onset colorectal cancer was better than that for late-onset colorectal cancer (p < 0.001). Regarding specific metastases, liver metastasis was the most frequent, followed by lung metastasis, bone metastasis, and finally brain metastasis, which was the least common. These findings align with previous studies that indicate the liver as the most common site of metastatic disease in colorectal cancer patients25. In terms of location-related prognosis, for early-onset colorectal cancer, liver metastasis had the most favorable prognosis, with a median survival time of 17 months (95% CI 7–31 months), followed by lung metastasis at 15 months (95% CI 6–26 months), brain metastasis at 9.5 months (95% CI 7–31 months 3.25–18.75), and bone metastasis with the worst prognosis at 9 months (95% CI 7–31 months 3.75–17). In late-onset colorectal cancer, liver metastasis still had the best prognosis, with a median survival time of 9 months (95% CI 2–23 months). Lung metastasis had the second-best prognosis, with a median survival time of 8 months (95% CI 2–20 months), followed by bone metastasis at 5 months (95% CI 1–13 months), and brain metastasis with the worst prognosis at 3 months (95% CI 1–11 months). Previous studies have indicated that the 5-year survival rate of colorectal cancer (CRC) patients with brain metastases is lower than that of patients with liver metastases37. The different prognosis between early-onset colorectal cancer and late-onset colorectal cancer suggests the need for different treatment options based on age, which could help improve patient survival rates.
In subgroup analysis based on tumor primary location, our study found that early-onset colorectal cancer is associated with better survival compared to late-onset colorectal cancer, regardless of whether the primary location is on the left side, right side, or rectum. Among these three locations, the left side exhibits the highest survival rate, followed by the rectum, while the right side shows the lowest survival rate. These findings align with numerous previous studies38, as the right colon is generally linked to worse survival due to higher BRAF mutations and higher microsatellite instability39–42. Furthermore, aside from location-related differences in molecular biology, some studies suggest that different locations in the colon, including the rectum, have distinct embryonic origins43. Additionally, alterations in microbiota, stool composition, enzymes, and metabolites along the intestine may also contribute to variations in the colon44,45. The reasons behind the differences in survival rates among the left side, right side or the rectum, are multifactorial. Clinical studies have also demonstrated that patients with colorectal cancer benefit more from first-line treatment if the primary site is on the left side, while patients with the primary site on the right experience minimal effects. Our study suggests that for patients with metastatic colorectal cancer, the preference for the left-side colon over the right-side colon remains consistent in both early-onset and late-onset cases, with early-onset colorectal cancer having a more favorable prognosis compared to late-onset colorectal cancer.
We conducted a multivariable Cox proportional hazards regression analysis for both early-onset and late-onset colorectal cancer to assess overall survival (OS). For early-onset colorectal cancer, significant prognostic factors for OS include primary site, race, histology, T stage, N stage, grade, surgery, radiation, chemotherapy, CEA, marital status, bone metastases, brain metastases, liver metastases, and lung metastases. Similarly, for late-onset colorectal cancer, the significant prognostic factors for OS align with those for early-onset colorectal cancer.
However, in univariate analysis, we observed differences in OS based on gender for early-onset colorectal cancer, but no such differences for late-onset colorectal cancer. We suspect that the survival difference between men and women in early-onset colorectal cancer may be influenced by age. To investigate this further, we performed a Kaplan–Meier curves using age as a reference and found that among patients with early-onset colorectal cancer, female patients had a significantly better prognosis than male patients, whereas this difference in prognosis between men and women disappeared in patients with late-onset colorectal cancer. Previous studies have consistently found that male sex is an independent risk factor for overall survival (OS) in colorectal cancer. This association may be attributed to differences in men's lifestyle, such as higher levels of financial and mental stress, as well as higher rates of smoking and alcohol consumption46. Furthermore, some studies suggest that the disparity in colorectal cancer incidence between genders could be linked to male-specific genes found on the Y chromosome47. However, despite these explanations, it remains challenging to fully elucidate the age-related differences observed between men and women. Therefore, we hypothesize that the inconsistent age-related prognosis of metastatic early-onset colorectal cancer in men and women could be influenced by sex hormones. Notably, women experience menopause around the age of 50, which coincides with this age-related difference. Estrogen, a crucial sex hormone48,49, exerts various biological effects and has been shown in previous studies to inhibit colorectal cancer by altering the ratio of ERα to ERβ50. Additionally, estrogen may exert a protective role by influencing MSI status and gene methylation51. Moreover, clinical investigations have demonstrated that estrogen replacement therapy in postmenopausal women can reduce the risk of colorectal cancer52. Based on these findings, we propose the implementation of gender-specific prevention and treatment strategies for early-onset colorectal cancer.
The strengths of this article are as follows: Firstly, this is the first article to compare metastatic early-onset colorectal cancer with late-onset colorectal cancer. We conducted a detailed and comprehensive analysis to better understand the differences between these two types of colorectal cancer. Secondly, our study has a larger sample size compared to other articles that have studied early-onset colorectal cancer. This helps to eliminate some of the biases that can arise from small sample sizes. Moreover, we first discover the sex differences between EOCRC and LOCRC, which is very meaningful for precision medicine based on sex. Lastly, our findings serve as a valuable supplement and confirmation of previous studies.
However, it is important to acknowledge the limitations of our study. Firstly, due to the nature of the SEER database, our study can only establish observational correlations and cannot analyze causal relationships like MR studies. Secondly, some important factors may be neglected due to unavailable data in the SEER database, such as chemotherapy regimen, targeted therapy, bisphosphonates usage, etc. Therefore, RCT studies should be designed to further investigate and confirm our conclusions in the future.
Conclusion
Metastatic early-onset colorectal cancer patients have longer survival time than late-onset colorectal cancer patients. Tumor primary location, the location of metastasis and treatment modalities affect the survival outcomes between these two groups of patients. The sex differences in survival of metastatic colorectal cancer patients are associated with patients’ age. These findings contribute to a better understanding of the differences between metastatic early-onset colorectal cancer and late-onset colorectal cancer, and can help inform the development of more precise treatment guidelines to improve prognosis.
Acknowledgements
We thank the staff at the SEER database. This study was supported by the Key R&D Project of Shaanxi Province (No. 2020GXLH-Z-001), the Scientific Development Funding of the First Affiliated Hospital of Xi'an Jiaotong University (No. 2022QN-17) and the Natural Science Basic Research Program of Shaanxi Province (No. 2023-JC-QN-0879).
Author contributions
L.K.: conceptualization. R.B.Y.: data curation. Y.Y.: formal analysis. L.Y.: supervision. L.K.: writing—original draft. All authors contributed to the article and approved the submitted version.
Data availability
Informed patient consent was not required for data obtained from SEER, as cancer is a publicly reportable disease in every state in the USA. The datasets generated and analyzed during the current study are available in SEER database. [https://seer.cancer.gov/].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Bingyi Ren and Yichen Yang.
References
- 1.Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023;73(3):233–254. doi: 10.3322/caac.21772. [DOI] [PubMed] [Google Scholar]
- 2.Sinicrope FA. Increasing incidence of early-onset colorectal cancer. N. Engl. J. Med. 2022;386(16):1547–1558. doi: 10.1056/NEJMra2200869. [DOI] [PubMed] [Google Scholar]
- 3.Giannakis M, Ng K. A common cancer at an uncommon age. Science. 2023;379(6637):1088–1090. doi: 10.1126/science.ade7114. [DOI] [PubMed] [Google Scholar]
- 4.Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol. Oncol. 2019;13(2):109–131. doi: 10.1002/1878-0261.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akimoto N, Ugai T, Zhong R, et al. Rising incidence of early-onset colorectal cancer—A call to action. Nat. Rev. Clin. Oncol. 2021;18(4):230–243. doi: 10.1038/s41571-020-00445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung RY, Tsoi KKF, Kyaw MH, Lui AR, Lai FTT, Sung JJ. A population-based age-period-cohort study of colorectal cancer incidence comparing Asia against the West. Cancer Epidemiol. 2019;59:29–36. doi: 10.1016/j.canep.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Uy GB, Kaw LL, Punzalan CK, et al. Clinical and molecular biologic characteristics of early-onset versus late-onset colorectal carcinoma in Filipinos. World J. Surg. 2004;28(2):117–123. doi: 10.1007/s00268-003-7281-4. [DOI] [PubMed] [Google Scholar]
- 8.Silla IO, Rueda D, Rodríguez Y, García JL, de la Cruz VF, Perea J. Early-onset colorectal cancer: A separate subset of colorectal cancer. World J. Gastroenterol. 2014;20(46):17288–17296. doi: 10.3748/wjg.v20.i46.17288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dozois EJ, Boardman LA, Suwanthanma W, et al. Young-onset colorectal cancer in patients with no known genetic predisposition: Can we increase early recognition and improve outcome? Medicine (Baltimore) 2008;87(5):259–263. doi: 10.1097/MD.0b013e3181881354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding X, Yang X, Wu D, et al. Nomogram predicting the cancer-specific survival of early-onset colorectal cancer patients with synchronous liver metastasis: A population-based study. Int. J. Colorectal Dis. 2022;37(6):1309–1319. doi: 10.1007/s00384-022-04175-x. [DOI] [PubMed] [Google Scholar]
- 11.Cercek A, Chatila WK, Yaeger R, et al. A comprehensive comparison of early-onset and average-onset colorectal cancers. J. Natl. Cancer Inst. 2021;113(12):1683–1692. doi: 10.1093/jnci/djab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipsyc-Sharf M, Zhang S, Ou FS, et al. Survival in young-onset metastatic colorectal cancer: Findings from cancer and Leukemia Group B (Alliance)/SWOG 80405. J. Natl. Cancer Inst. 2022;114(3):427–435. doi: 10.1093/jnci/djab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer. 2019;125(12):2002–2010. doi: 10.1002/cncr.31994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Heide DM, Turaga KK, Chan CHF, Sherman SK. Mismatch repair status correlates with survival in young adults with metastatic colorectal cancer. J. Surg. Res. 2021;266:104–112. doi: 10.1016/j.jss.2021.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cronin KA, Ries LA, Edwards BK. The surveillance, epidemiology, and end results (SEER) Program of the National Cancer Institute. Cancer. 2014;120(23):3755–3757. doi: 10.1002/cncr.29049. [DOI] [PubMed] [Google Scholar]
- 16.Petrick JL, Barber LE, Warren Andersen S, Florio AA, Palmer JR, Rosenberg L. Racial disparities and sex differences in early- and late-onset colorectal cancer incidence, 2001–2018. Front. Oncol. 2021;11:734998. doi: 10.3389/fonc.2021.734998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966;50(3):163–170. [PubMed] [Google Scholar]
- 18.Prentice RL, Zhao S. Regression models and multivariate life tables. J. Am. Stat. Assoc. 2021;116(535):1330–1345. doi: 10.1080/01621459.2020.1713792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welch HG, Robertson DJ. Colorectal cancer on the decline-why screening can't explain it all. N. Engl. J. Med. 2016;374(17):1605–1607. doi: 10.1056/NEJMp1600448. [DOI] [PubMed] [Google Scholar]
- 20.Cheng X, Li Y, Chen D, Xu X, Liu F, Zhao F. Nomogram predicting the survival of young-onset patients with colorectal cancer liver metastases. Diagnostics (Basel) 2022 doi: 10.3390/diagnostics12061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nebbia M, Yassin NA, Spinelli A. Colorectal cancer in inflammatory bowel disease. Clin. Colon Rectal Surg. 2020;33(5):305–317. doi: 10.1055/s-0040-1713748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89–103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marley AR, Nan H. Epidemiology of colorectal cancer. Int. J. Mol. Epidemiol. Genet. 2016;7(3):105–114. [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy N, Moreno V, Hughes DJ, et al. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol. Aspects Med. 2019;69:2–9. doi: 10.1016/j.mam.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Jiao S, Guan X, Niu H, et al. The tumor characteristics, treatment strategy, and prognosis in colorectal cancer patients with synchronous liver metastasis in China and the USA. Int. J. Colorectal Dis. 2023;38(1):140. doi: 10.1007/s00384-023-04398-6. [DOI] [PubMed] [Google Scholar]
- 26.van Steenbergen LN, Elferink MAG, Krijnen P, et al. Improved survival of colon cancer due to improved treatment and detection: A nationwide population-based study in The Netherlands 1989–2006. Ann. Oncol. 2010;21(11):2206–2212. doi: 10.1093/annonc/mdq227. [DOI] [PubMed] [Google Scholar]
- 27.Elferink MA, van Steenbergen LN, Krijnen P, et al. Marked improvements in survival of patients with rectal cancer in the Netherlands following changes in therapy, 1989–2006. Eur. J. Cancer. 2010;46(8):1421–1429. doi: 10.1016/j.ejca.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 28.van der Pool AE, Damhuis RA, Ijzermans JN, et al. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: A population-based series. Colorectal Dis. 2012;14(1):56–61. doi: 10.1111/j.1463-1318.2010.02539.x. [DOI] [PubMed] [Google Scholar]
- 29.van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, de Wilt JH. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin. Exp. Metastasis. 2015;32(5):457–465. doi: 10.1007/s10585-015-9719-0. [DOI] [PubMed] [Google Scholar]
- 30.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer and race: Understanding the differences in outcomes between African Americans and whites. Med. Clin. N. Am. 2005;89(4):771–793. doi: 10.1016/j.mcna.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Holowatyj AN, Maude AS, Musa HS, et al. Patterns of early-onset colorectal cancer among Nigerians and African Americans. JCO Glob. Oncol. 2020;6:1647–1655. doi: 10.1200/go.20.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng E, Blackburn HN, Ng K, et al. Analysis of survival among adults with early-onset colorectal cancer in the national cancer database. JAMA Netw. Open. 2021;4(6):e2112539. doi: 10.1001/jamanetworkopen.2021.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnett-Hartman AN, Powers JD, Chubak J, et al. Treatment patterns and survival differ between early-onset and late-onset colorectal cancer patients: The patient outcomes to advance learning network. Cancer Causes Control. 2019;30(7):747–755. doi: 10.1007/s10552-019-01181-3. [DOI] [PubMed] [Google Scholar]
- 34.Swartjes H, Brouwer NPM, de Nes LCF, et al. Incidence, treatment and relative survival of early-onset colorectal cancer in the Netherlands since 1989. Eur. J. Cancer. 2022;166:134–144. doi: 10.1016/j.ejca.2022.01.029. [DOI] [PubMed] [Google Scholar]
- 35.Raimondi A, Randon G, Prisciandaro M, et al. Early onset metastatic colorectal cancer in patients receiving panitumumab-based upfront strategy: Overall and sex-specific outcomes in the Valentino trial. Int. J. Cancer. 2022;151(10):1760–1769. doi: 10.1002/ijc.34156. [DOI] [PubMed] [Google Scholar]
- 36.Fontana E, Meyers J, Sobrero A, et al. Early-onset colorectal adenocarcinoma in the IDEA database: Treatment adherence, toxicities, and outcomes with 3 and 6 months of adjuvant fluoropyrimidine and oxaliplatin. J. Clin. Oncol. 2021;39(36):4009–4019. doi: 10.1200/jco.21.02008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, Xu Y, Xu G, et al. Nomogram for predicting overall survival in colorectal cancer with distant metastasis. BMC Gastroenterol. 2021;21(1):103. doi: 10.1186/s12876-021-01692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lv MY, Chen XJ, Chen JG, et al. Nomogram for predicting overall survival time of patients with stage IV colorectal cancer. Gastroenterol. Rep. (Oxf). 2022;10:goac072. doi: 10.1093/gastro/goac072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ueda K, Yamada T, Ohta R, et al. BRAF V600E mutations in right-side colon cancer: Heterogeneity detected by liquid biopsy. Eur. J. Surg. Oncol. 2022;48(6):1375–1383. doi: 10.1016/j.ejso.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Tapia Rico G, Price T, Tebbutt N, et al. Right or left primary site of colorectal cancer: Outcomes from the molecular analysis of the AGITG MAX trial. Clin. Colorectal Cancer. 2019;18(2):141–148. doi: 10.1016/j.clcc.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Lee MS, Menter DG, Kopetz S. Right versus left colon cancer biology: Integrating the consensus molecular subtypes. J. Natl. Compr. Cancer Netw. 2017;15(3):411–419. doi: 10.6004/jnccn.2017.0038. [DOI] [PubMed] [Google Scholar]
- 42.Papagiorgis PC, Zizi AE, Tseleni S, Oikonomakis IN, Nikiteas NI. The pattern of epidermal growth factor receptor variation with disease progression and aggressiveness in colorectal cancer depends on tumor location. Oncol. Lett. 2012;3(5):1129–1135. doi: 10.3892/ol.2012.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koliaraki V, Pallangyo CK, Greten FR, Kollias G. Mesenchymal cells in colon cancer. Gastroenterology. 2017;152(5):964–979. doi: 10.1053/j.gastro.2016.11.049. [DOI] [PubMed] [Google Scholar]
- 44.Xi Y, Yuefen P, Wei W, et al. Analysis of prognosis, genome, microbiome, and microbial metabolome in different sites of colorectal cancer. J. Transl. Med. 2019;17(1):353. doi: 10.1186/s12967-019-2102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong M, Xiong Y, Ye Z, et al. Microbial community profiling distinguishes left-sided and right-sided colon cancer. Front. Cell Infect. Microbiol. 2020;10:498502. doi: 10.3389/fcimb.2020.498502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White A, Ironmonger L, Steele RJC, Ormiston-Smith N, Crawford C, Seims A. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer. 2018;18(1):906. doi: 10.1186/s12885-018-4786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, Lan Z, Liao W, et al. Histone demethylase KDM5D upregulation drives sex differences in colon cancer. Nature. 2023;619(7970):632–639. doi: 10.1038/s41586-023-06254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng L, Li J, Han Y, et al. PES1 promotes breast cancer by differentially regulating ERα and ERβ. J. Clin. Investig. 2012;122(8):2857–2870. doi: 10.1172/jci62676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin JH, Zhang SM, Rexrode KM, et al. Association between sex hormones and colorectal cancer risk in men and women. Clin. Gastroenterol. Hepatol. 2013;11(4):419–424.e1. doi: 10.1016/j.cgh.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu T, Zhao M, Peng L, et al. WFDC3 inhibits tumor metastasis by promoting the ERβ-mediated transcriptional repression of TGFBR1 in colorectal cancer. Cell Death Dis. 2023;14(7):425. doi: 10.1038/s41419-023-05956-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slattery ML, Potter JD, Curtin K, et al. Estrogens reduce and withdrawal of estrogens increase risk of microsatellite instability-positive colon cancer. Cancer Res. 2001;61(1):126–130. [PubMed] [Google Scholar]
- 52.Spolverato G, Fassan M, Capelli G, et al. IMMUNOREACT 5: Female patients with rectal cancer have better immune editing mechanisms than male patients—A cohort study. Int. J. Surg. 2023;109(3):323–332. doi: 10.1097/js9.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Informed patient consent was not required for data obtained from SEER, as cancer is a publicly reportable disease in every state in the USA. The datasets generated and analyzed during the current study are available in SEER database. [https://seer.cancer.gov/].