Cancer vaccines have been pursued for over a century in an attempt to harness the specificity and many resistance potentials of the immune system (1). Recent advances in immunology, including the importance of antigen-presenting dendritic cells (DCs) in initiating T cell immunity against pathogens and tumors, have provided new guidelines for immunotherapy. Simultaneously over the last decade, tumor-specific mAbs have emerged as effective and specific immunotherapeutics against human cancers (2). In this issue of PNAS, Groh et al. (3) bring the fields of DC biology and mAbs together with an approach to target a wide range of tumors to DCs. If successfully translated to the clinic, their mAb approach may prove powerful for boosting tumor immunity.
An Ab molecule is made up of two regions, the Fab fragment and the Fc portion. The Fab fragment forms the antigen-binding site, whereas the Fc domain allows the Abs to recruit cells of the immune system by engaging their Fc receptors. Groh et al. (3) use mAbs that bind to MHC class I-related chains A and B (MICA and MICB) on tumor cells. MICA and MICB are overexpressed on a broad range of epithelial tumors. Shed MICA and MICB molecules may contribute to immune evasion by ligating the NKG2D molecules on lymphocytes and inhibiting lymphocyte function (3, 4). In their experiments, Groh et al. use the Fab portion of the anti-MICA Ab for tumor cell recognition and the Fc region of this Ab to engage Fc receptors on DCs and promote the induction of cell-mediated immunity.
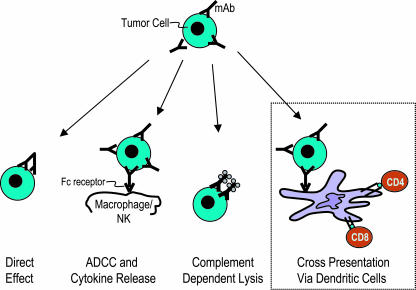
Although the Fab region of a mAb mediates specific binding to antigenic determinants on the tumor cell, the Fc portion can be critical for efficacy, at least in the case of some Abs. Studies from the laboratory of Ravetch and colleagues (5) have shown that Fcγ receptor-mediated mechanisms are required for antitumor effects of two commonly used mAbs, Rituximab (anti-CD20) and Trastuzumab (anti-HER2/ErbB2), in mice (5). For example, engagement of Fcγ receptors can lead to activation of macrophages or natural killer cells, leading to Ab-dependent cellular cytotoxicity against tumor cells. Antitumor mAbs can also mediate their effects by initiating inflammation and lysis via complement (6). Thus, the Fab and Fc portions of antitumor mAbs can, in principle, recruit several distinct antitumor mechanisms (Fig. 1).
Fig. 1.
mAbs can recruit several mechanisms for anti-tumor effects. These include direct effects of the Fab region on tumor cells; the binding of the Fc region of the mAb to Fcγ receptors on effector cells, leading to Ab-dependent cellular cytotoxicity (ADCC) and the release of cytokines; Fc-mediated complement activation leading to tumor cell lysis; and, as emphasized by Groh et al. (3), enhanced crosspresentation of antigens from tumor cells by DCs. The latter can lead to the generation of tumor-specific CD4 and CD8+ T cell immunity, maturation of the DCs, and, we propose, more durable antitumor resistance.
However, Fc-mediated cytotoxicity and Fab-mediated direct effects on tumor cells do not provide a fully satisfactory mechanism for the durable responses to mAbs observed in some patients, because it is unlikely that every tumor cell is eliminated during therapy (2). The clinical observation that repeated administration of mAbs leads to more durable responses suggests a vaccination effect (2, 7); i.e., do these Abs also elicit adaptive immunity with memory?
In most tissue cells, only endogenous or newly synthesized antigens are processed and presented to CD8+ killer T cells in the context of MHC type I molecules. In contrast, DCs are specialized to acquire antigens such as antigen-Ab complexes and tumor cells and “crosspresent” these to CD8+ T cells, without the need for new synthesis of antigen in the DCs (8). Targeting model antigens to Fcγ receptors on murine DCs leads to enhanced crosspresentation of antigens like ovalbumin and a chemical hapten, trinitrophenyl (9-11). We wondered whether this crosspresentation might help to explain the durable effects observed with some anti-tumor Abs. In fact, when we delivered myeloma cell lines and primary myeloma cells to DCs using tumor cells opsonized with anti-syndecan-1 Ab, we observed enhanced presentation of tumor antigens and the induction of CD8 and CD4 T cell immunity (12, 13). Groh et al. (3) discover a fascinating extension of this approach by targeting MICA and MICB, which, as mentioned, seem to be up-regulated on a spectrum of tumor cells, perhaps to evade lysis by different types of killer cells. Groh et al. show that loading human DCs with anti-MICA Ab-coated breast, melanoma, or ovarian tumors efficiently promoted the generation of antitumor CD4+ and CD8+ T cell responses. The elicited responses were of substantially greater breadth and magnitude than those elicited by using DCs charged with preprocessed tumor peptides or with apoptotic tumor cells without opsonization. These data provide a platform for tumor antigen discovery, DC vaccination, and adoptive T cell therapy.
The finding of enhanced generation of T cell immunity after uptake of opsonized tumor in this and other studies suggests a role for DCs in recruitment of T cell immunity via antitumor mAbs (3, 12, 14). Such responses would be desirable, because they provide a mechanism for long-term protection and immunologic memory. This may also provide a mechanism for the observations of more durable clinical responses with repeated exposure to Rituximab in lymphoma (7).
Abs have an additional potential in that of mediating processing and presentation of cell-associated antigens. This potential relates to the induction of DC differentiation or maturation. This is the process whereby DCs acquire many immune-enhancing properties, such as the expression of costimulatory molecules and the production of cytokines like IL-12 and interferons needed for T cell immunity. The Fcγ receptor system is a balance between activating and inhibitory receptors (15). Changes in the balance of these receptors can alter the activation or antigen-presenting function of DCs (16, 17). Recent studies have shown that selective blockade of inhibitory Fcγ receptors on human DCs leads to induction of DC maturation and enhanced generation of anti-tumor immunity (17). Polymorphisms of activating and inhibitory Fcγ receptors may therefore impact DC activation and generation of T cell immunity in vivo (18). Optimizing the targeting of anti-tumor mAbs to Fcγ receptors on DCs, so that the DCs both present antigens and mature appropriately to stimulate immunity, may hold the key to improving the efficacy and durability of mAb therapy of cancer.
There remains a clear potential for T cells to recognize tumor cells presented by dendritic cells.
The findings of Groh et al. (3) also deal with another critical issue in cancer immunology, that of immune tolerance to tumors. It is often assumed that tumors will behave like self tissues and tolerize the immune system to many of their potential antigens. This may well pertain in some cases, but, surprisingly, there remains a clear potential for T cells from patients to recognize tumor cells presented by DCs. We noted this in a study of T cells from patients with multiple myeloma (13). In this tumor, even T cells from the tumor bed in patients with clinically progressive tumors can be expanded to elicit tumor-reactive killer T cells. Likewise, Groh et al. show that patients with ovarian and other cancers are able to efficiently expand autologous tumor-specific T cells using DCs loaded with Ab-coated tumor.
One important advantage of Ab-mediated targeting of tumor antigens to DCs is the ability to simultaneously elicit T cell immunity against multiple antigens derived from tumor cells, thereby reducing the potential for immune escape. Adoptive transfer of antigen-loaded DCs is being actively pursued for immune therapy of cancer (19). Targeting the Fcγ receptor on DCs with anti-tumor mAbs may provide a broad platform to improve the immune efficacy of these vaccines, which needs to be tested in the clinic. In our view, improving the strength and potency of the elicited immune response is an important first step toward effective vaccination and understanding the mechanisms of immune escape in vivo. The research of Groh et al. (3) emphasizes the urgent need for more human studies to bring advances in immunologic sciences to the interface of tumor and patient.
Acknowledgments
We thank Ralph M. Steinman, M.D., for critical reading of the commentary and Judy Adams for help with the figure. We are supported in part by funds from the National Institutes of Health, the Damon Runyon Cancer Research Fund, the American Society of Clinical Oncology, the Fund to Cure Myeloma, the Dana Foundation, and the Irma T. Hirschl Foundation.
See companion article on page 6461.
References
- 1.Blattman, J. N. & Greenberg, P. D. (2004) Science 305, 200-205. [DOI] [PubMed] [Google Scholar]
- 2.Levy, R. (2000) Semin. Hematol. 37, 43-46. [DOI] [PubMed] [Google Scholar]
- 3.Groh, V., Li, Y. Q., Cioca, D., Hunder, N. N., Wang, W., Riddell, S. R., Yee, C. & Spies, T. (2005) Proc. Natl. Acad. Sci. USA 102, 6461-6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groh, V., Wu, J., Yee, C. & Spies, T. (2002) Nature 419, 734-738. [DOI] [PubMed] [Google Scholar]
- 5.Clynes, R. A., Towers, T. L., Presta, L. G. & Ravetch, J. V. (2000) Nat. Med. 6, 443-446. [DOI] [PubMed] [Google Scholar]
- 6.Lin, M. Z., Teitell, M. A. & Schiller, G. J. (2005) Clin. Cancer Res. 11, 129-138. [PubMed] [Google Scholar]
- 7.Davis, T. A., Grillo-Lopez, A. J., White, C. A., McLaughlin, P., Czuczman, M. S., Link, B. K., Maloney, D. G., Weaver, R. L., Rosenberg, J. & Levy, R. (2000) J. Clin. Oncol. 18, 3135-3143. [DOI] [PubMed] [Google Scholar]
- 8.Mellman, I. & Steinman, R. M. (2001) Cell 106, 255-258. [DOI] [PubMed] [Google Scholar]
- 9.Regnault, A., Lankar, D., Lacabanne, V., Rodriguez, A., Thery, C., Rescigno, M., Saito, T., Verbeek, S., Bonnerot, C., Ricciardi-Castagnoli, P. & Amigorena, S. (1999) J. Exp. Med. 189, 371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akiyama, K., Ebihara, S., Yada, A., Matsumara, K., Aiba, S., Nukiwa, T. & Takai, T. (2003) J. Immunol. 170, 1641-1648. [DOI] [PubMed] [Google Scholar]
- 11.Rafiq, K., Bergtold, A. & Clynes, R. (2002) J. Clin. Invest. 110, 71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhodapkar, K. M., Krasovsky, J., Williamson, B. & Dhodapkar, M. V. (2002) J. Exp. Med. 195, 125-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhodapkar, M. V., Krasovsky, J. & Olson, K. (2002) Proc. Natl. Acad. Sci. USA 99, 13009-13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amigorena, S. (2002) J. Exp. Med. 195, F1-F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravetch, J. V. & Bolland, S. (2001) Annu. Rev. Immunol. 19, 275-290. [DOI] [PubMed] [Google Scholar]
- 16.Kalergis, A. M. & Ravetch, J. V. (2002) J. Exp. Med. 195, 1653-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhodapkar, K. M., Kaufman, J. L., Ehlers, M., Banerjee, D. K., Bonvini, E., Koenig, S., Steinman, R. M., Ravetch, J. V. & Dhodapkar, M. V. (2005) Proc. Natl. Acad. Sci. USA 102, 2910-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng, W. K. & Levy, R. (2003) J. Clin. Oncol. 21, 3940-3947. [DOI] [PubMed] [Google Scholar]
- 19.Steinman, R. M. & Dhodapkar, M. (2001) Int. J. Cancer 94, 459-473. [DOI] [PubMed] [Google Scholar]