Abstract
Hepatocyte growth factor/scatter factor (HGF/SF) is a potent mitogen, motogen, and morphogen for epithelial cells expressing its tyrosine kinase receptor, the c-met proto-oncogene product, and is required for normal development in the mouse. Inappropriate stimulation of Met signal transduction induces aberrant morphogenesis and oncogenesis in mice and has been implicated in human cancer. NK1 is a naturally occurring HGF/SF splice variant composed of only the amino terminus and first kringle domain. While the biological activities of NK1 have been controversial, in vitro data suggest that it may have therapeutic value as an HGF/SF antagonist. Here, we directly test this hypothesis in vivo by expressing mouse NK1 in transgenic mice and comparing the consequent effects with those observed for mice carrying an HGF/SF transgene. Despite robust expression, NK1 did not behave as an HGF/SF antagonist in vivo. Instead, NK1-transgenic mice displayed most of the phenotypic characteristics associated with HGF/SF-transgenic mice, including enlarged livers, ectopic skeletal-muscle formation, progressive renal disease, aberrant pigment cell localization, precocious mammary lobuloalveolar development, and the appearance of mammary, hepatocellular, and melanocytic tumors. And like HGF/SF-transgenic livers, NK1 livers had higher levels of tyrosine-phosphorylated complexes associated with Met, suggesting that the mechanistic basis for the effects of NK1 overexpression in vivo was autocrine activation of Met. We conclude that NK1 acts in vivo as a partial agonist. As such, the efficacy of NK1 as a therapeutic HGF/SF antagonist must be seriously questioned.
Hepatocyte growth factor/scatter factor (HGF/SF) is a multifunctional cytokine important in diverse biological processes such as cell growth, motility, and morphogenesis (reviewed in references 11, 16, 39, and 42). HGF/SF was initially identified as a powerful stimulator of hepatocyte proliferation but is now known to act as a mitogen for many epithelial cell types, including renal (9) and mammary (20) epithelia. HGF/SF is also a potent inducer of cell motility in epithelial cells, myoblasts, and melanoblasts (2, 7, 35). In addition, HGF/SF has morphogenic activity, promoting the development of tubular structures in kidney (40) and mammary (20) epithelia. In vivo studies have demonstrated the critical role of HGF/SF and its tyrosine kinase receptor Met in the development of multiple organ systems. Mice with targeted disruptions of either the HGF/SF or the c-met gene show impaired liver, placenta, and muscle development and die in utero (2, 28, 38). Overexpression in transgenic mice reveals the essential role of HGF/SF in regulating the development of skeletal muscle and the neural crest (35). These activities and the fact that HGF/SF is produced by mesenchymal cells and exerts its effects on epithelial cells expressing Met suggests that HGF/SF acts as a paracrine factor in mesenchymal cell-epithelial cell interactions in vivo (31, 36).
In addition to its pleiotropic activities in normal cells, the HGF/SF-Met signaling pathway has also been implicated in cancer. The establishment of an autocrine loop by coexpression of HGF/SF and Met in cultured cells results in neoplastic transformation (1, 23, 24, 25). A number of tumors, including melanomas, hepatomas, and carcinomas of the breast, exhibit inappropriate expression of Met (5, 6, 19, 22, 24, 25, 37, 41). HGF/SF overexpression in transgenic mice results in neoplasms of the liver, mammary gland, skeletal muscle, and melanocytes (27, 33). Recently, missense mutations in the tyrosine kinase domain of the c-met gene were found in the germ lines of affected members of families with hereditary papillary renal carcinoma; these mutations are likely to lead to the constitutive activation of Met (29). Paradoxically, HGF/SF can serve as a cytostatic factor for certain carcinoma cells (23, 32).
In humans, HGF/SF mRNA can undergo alternative splicing to form truncated isoforms. While full-length HGF/SF is a heterodimeric protein of 90 kDa that includes four kringle domains, the smallest of these HGF/SF variants, NK1, consists only of the HGF/SF amino terminus through the first kringle domain with the addition of two amino acid residues followed by a termination codon (Fig. 1) (4, 15). The biological activities of NK1 are not fully understood. NK1 was initially characterized as an HGF/SF antagonist, since it was able to compete with full-length HGF/SF for binding to Met but lacked intrinsic mitogenic activity in primary rat hepatocyte cultures (15). Thus, NK1 was proposed as a potential therapeutic agent for tumors where HGF/SF or Met is expressed (3, 15). Recent in vitro studies have shown, however, that there are important differences in NK1 activity that depend on the cell types used. While NK1 can act as an HGF/SF antagonist on primary hepatocytes (15), it behaves as a partial agonist on mink lung epithelial cells (30), human mammary epithelial cells (4), and Chinese hamster ovary cells (26). The discrepancy may be explained by differences in the glycosaminoglycan compositions of the cells (13, 26, 30), but this issue remains largely unresolved. While the activities of NK1 in vitro remain controversial, the properties of NK1 in vivo are completely unknown.
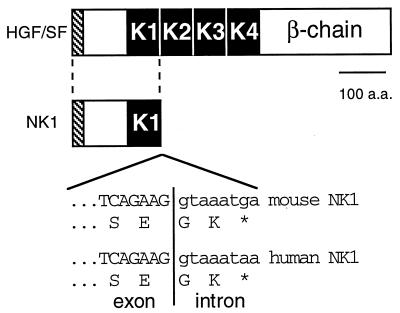
FIG. 1.
Characterization of the cDNA for mouse NK1. A schematic (top) comparison of mouse HGF/SF and the NK1 splicing variant is shown. The hatched boxes represent the signal peptide regions. The black boxes labeled K1 through K4 represent kringle domains. Below the schematic is a sequence comparison of mouse and human HGF/SF exon-intron junctions and the modified translation producing the NK1 alternative splice variants. The position of the exon-intron junction for the murine NK1 is based on the mouse HGF/SF cDNA sequence (14) and the human NK1 sequence (4). a.a., amino acids. ∗, translational stop codon.
We report here the isolation of the cDNA for the mouse homolog of NK1 and the development of a transgenic-mouse system to explore the physiological activities of NK1 in the whole animal. We used the same transgene expression system as in our previously described HGF/SF-transgenic mice (35) so that we could directly compare the in vivo activities of NK1 and HGF/SF. In HGF/SF-transgenic mice, broad expression of the transgene leads to enlarged livers and the stimulation of hepatocellular proliferation (27), the appearance of ectopic muscle and melanocytes in the central nervous system (35), the development of a progressive renal disease (34), aberrant migration of melanocytes (35), precocious mammary lobuloalveolar development (33), and susceptibility to a diverse range of tumor types (27, 33). We show here that mice overexpressing NK1 reveal a phenotype that is remarkably similar to that of HGF/SF-transgenic mice. We conclude that NK1 behaves in vivo as a partial agonist of HGF/SF. Therefore, the efficacy of NK1 as an HGF/SF antagonist in therapeutic applications must be more thoroughly evaluated.
MATERIALS AND METHODS
cDNA library screen.
A mouse fibroblast cDNA library (pCV27-NIH3T3) was screened by hybridization with human HGF/SF cDNA to identify murine HGF/SF as well as variant murine HGF/SF transcripts. A 1.2-kb cDNA that corresponded to the 5′ coding region of HGF/SF and that was structurally homologous to the human HGF/SF variant isoform NK1 was isolated, and its nucleotide sequence was determined.
Transgenic-mouse production.
The mouse NK1 cDNA was cloned into the transgene expression construct used previously for HGF/SF-transgenic mice (35). This construct contained the mouse metallothionein (MT) gene-1 promoter, the human growth hormone polyadenylation signal, and flanking mouse MT gene locus control regions (21) (Fig. 2A). A 19-kb ClaI/SstII fragment of the MT-NK1 transgene construct (LA741) was microinjected into single-cell mouse embryos (FVB/N inbred genetic background) as described previously (12). Transgenic mice were identified by genomic blot analysis of tail DNA with NK1 cDNA as the probe (Fig. 2A) or by PCR with transgene-specific primers. The primers used were 5′-TCGTCCTATCCGAGCCAGTCGT-3′, which was specific to the MT promoter region, and 5′-CTGAGGAATGTCACAGACTTCGTA-3′, which annealed in the NK1 cDNA sequence. Two lines were established, MN21 and MN10, each with three to five copies of the transgene. We focused on the line with the highest level of expression, MN21, for detailed analysis. All animal work was performed in accordance with National Institutes of Health animal care guidelines.
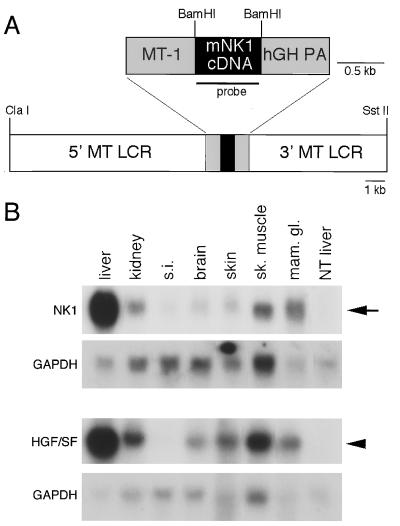
FIG. 2.
Structure and expression of the MT-NK1 transgene. (A) Schematic representation of the 19-kb ClaI/SstII DNA fragment used to generate NK1-transgenic mice. The expression construct included the mouse NK1 cDNA (black box), the MT-1 promoter and human growth hormone gene polyadenylation site (hGH PA), and the 5′ and 3′ flanking sequences containing the locus control regions (LCR) of the mouse MT gene (white boxes). The region used as a probe for the Northern analysis whose results are shown in panel B is underlined. (B) Comparison of the expression levels of the MT-NK1 and MT-HGF/SF transgenes. The arrow indicates the position of the 0.8-kb MT-NK1 transgene transcript in NK1 line MN21 mice; the arrowhead shows the position of the 2.4-kb HGF/SF transgene transcript in HGF/SF-transgenic line MH19 mice (35). Filters were stripped and rehybridized to a probe for GAPDH to control for differences in loading and transfer of RNAs. s.i., small intestine; sk. muscle, skeletal muscle; mam. gl., mammary gland.
RNA and protein analysis.
Selected tissues were removed from euthanized mice and frozen on dry ice. Total RNA was isolated by homogenization of frozen tissue in TRIzol (Life Technologies, Inc., Gaithersburg, Md.) according to the manufacturer’s protocol. Expression of the MT-NK1 and MT-HGF/SF transgenes was measured by Northern analysis. Fifteen micrograms of total RNA was electrophoresed on a 1% agarose-formaldehyde gel, transferred to a Zeta-probe GT (Bio-Rad) membrane, and hybridized to a 32P-radiolabeled 0.7-kb BamHI fragment of the NK1 cDNA (Fig. 2A) at 65°C in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Blots were washed in 1× SSC at 65°C and subjected to autoradiography. Blots were stripped and rehybridized to 32P-radiolabeled mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) DNA to control for sample loading differences. Serum HGF/SF levels were determined by a two-site enzyme-linked immunosorbent assay (ELISA) kit (Institute of Immunology, Tokyo, Japan) as previously described (35). The solid-phase antibody in this ELISA kit recognizes an epitope in the α chain of HGF/SF that is not present in NK1; thus, this assay is specific for endogenous mouse HGF/SF in NK1-transgenic mice.
Immunohistochemistry.
Tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned. Muscle tissue in the spinal cord was identified by incubating tissue sections with a mouse α-actin monoclonal antibody (Dako) at a 1:100 dilution. Melanomas were positively identified by immunohistochemistry with a mouse monoclonal antibody to the melanoma-specific marker HMB-45 (Dako) at a 1:150 dilution. All other tissues were stained with hematoxylin and eosin.
Hepatocyte proliferation levels.
Hepatocyte proliferation levels were measured by calculating the percentages of hepatocyte nuclei that incorporated the S-phase marker, bromodeoxyuridine (BrdU). Five-week-old NK1-transgenic mice and nontransgenic littermates were injected with 0.1 mg of BrdU per g of body weight 1 h before sacrifice. Livers were removed, fixed in 70% ethanol, sectioned, and stained with a monoclonal antibody to BrdU (Dako) at a 1:2,000 dilution. Seventy-five microscope fields (magnification, ×400) representing at least 13,000 hepatocyte nuclei were assessed for each of four animals in the NK1-transgenic group and each of four in the nontransgenic group. The level of hepatocellular proliferation in each group was expressed as the number of positively staining hepatocytes divided by the total number of hepatocytes in each field, multiplied by 100 (means ± standard deviations were recorded). A two-tailed Student’s t test was used to test differences between the means.
Met phosphotyrosine analysis.
Analysis of phosphotyrosine associated with Met was performed as previously described (27). Briefly, 300 mg of transgenic or nontransgenic liver was minced, homogenized, and solubilized at 10 mg/ml (wet weight) in immunoprecipitation buffer. Equivalent amounts of cleared lysates were incubated with 5 μg of antiphosphotyrosine monoclonal antibody (Upstate Biotechnology Inc., Lake Placid, N.Y.), control rabbit antibody, or anti-Met antibody (Santa Cruz Biotechnology Inc., Santa Cruz, Calif.). Immunocomplexes were fractionated on sodium dodecyl sulfate–8% polyacrylamide gels and electrophoretically transferred to Immobilon P membranes. After blocking, membranes were incubated with anti-Met antibody and the cross-reactive species were visualized by incubation with anti-rabbit antibody conjugated to horseradish peroxidase and enhanced chemiluminescence (kit from Amersham, Arlington Heights, Ill.).
Nucleotide sequence accession number.
The 1.2-kb mouse cDNA sequence was deposited in the GenBank/EMBL data bank with the accession no. AF042856.
RESULTS
Cloning and sequencing of the mouse homolog of human NK1.
The in vivo activity of NK1 was explored by first isolating the mouse homolog of human NK1 (hNK1). A mouse fibroblast cDNA library was screened with a human HGF/SF probe, and cDNA clones of several sizes were isolated, including HGF/SF (6- and 4.4-kb transcripts) and two variant HGF/SF transcripts (2.2 and 1.2 kb). DNA sequence analysis revealed that the 1.2-kb transcript contained a truncated HGF/SF transcript whose coding region included the 5′ amino terminus and first kringle domain as well as 79 bp of 3′ untranslated sequence not found in the full-length mouse HGF/SF mRNA. The deduced protein was 211 amino acids in length and was identical to the corresponding domains of mouse HGF/SF, with the exception of its two carboxyl-terminal amino acid residues (Fig. 1). These two residues were identical to those at the carboxyl terminus of hNK1 (3, 4). Like hNK1, this mouse variant appears to be the product of alternative splicing of the HGF/SF transcript. The unique 79 bp of 3′ untranslated sequence was identical to the intron sequence of the mouse HGF/SF gene downstream of the K1 domain and had a poly(A) signal 15 bp upstream from the poly(A) tail (data not shown). We conclude that this 1.2-kb cDNA encodes the mouse homolog of hNK1 and apparently arises from alternative splicing of the HGF/SF transcript by a mechanism analogous to that of hNK1.
Targeted expression of the mouse NK1 cDNA in transgenic mice.
In order to study the activities of mouse NK1 in vivo, the cDNA was placed under the transcriptional control of the mouse MT gene promoter and the flanking MT gene locus control regions (21) (Fig. 2A) and injected into single-cell mouse zygotes, from which transgenic mouse lines were then established. We used the same expression construct as in our previously described HGF/SF-transgenic mice (35) to target expression of the MT-NK1 transgene to the epithelial cell types used with the MT-HGF/SF transgene, thus allowing us to directly compare the in vivo activities of NK1 and HGF/SF.
Despite the lethal effects of homozygous disruption of either the HGF/SF or the c-met gene in mouse embryos (2, 28, 38) and the apparent inhibition of HGF/SF by NK1 in cell culture (4, 15, 30), mice overexpressing NK1 appeared normal at birth. In addition, these mice showed no unusual mortality and both sexes were fertile. High levels of expression of the MT-NK1 transgene were detected in many adult tissues. Levels of transgene RNA in the liver and mammary gland were comparable to those seen in HGF/SF-transgenic mice (35), while somewhat smaller amounts were observed in the kidney, skeletal muscle, brain, and skin (Fig. 2B).
Effects of NK1 on growth and development of multiple organs in transgenic mice.
NK1 overexpression resulted in phenotypic characteristics that, although often less severe, were strikingly similar to those associated with HGF/SF overexpression. These attributes included effects on tissue homeostasis, development, and susceptibility to tumors. NK1-transgenic mice had livers that were significantly larger than those of nontransgenic littermates, ranging from 1.2- to 1.5-fold greater by 5 weeks of age (Table 1). The increase in liver mass in NK1 mice was associated with significantly higher levels of hepatocyte proliferation, as determined by immunohistochemical detection of incorporated BrdU, a marker for proliferating cells. The labeling index in 5-week-old NK1 mice was 3.7-fold higher than in nontransgenic littermates (1.57% ± 1.31% in NK1 mice versus 0.42% ± 0.12% in nontransgenic mice; P < 0.001). In addition, by 2 months of age, NK1 mouse livers showed centrilobular hypertrophy of hepatocytes (Fig. 3B), which was not seen in nontransgenic mice (Fig. 3A).
TABLE 1.
Liver weight as a percentage of body weight for NK1-transgenic mice
| Age (mo) | % Liver weight (no. of animals)a
|
||
|---|---|---|---|
| FVB/N control | NK1 line MN21 | NK1 line MN10 | |
| 1–2 | 5.46 ± 0.54 (5) | 7.87 ± 0.74 (6)b | 6.70 ± 0.71 (2) |
| 2–5 | 5.24 ± 0.79 (10) | 6.98 ± 1.13 (9)b | NDc |
| >5 | 5.06 ± 0.50 (4) | 7.35 ± 1.91 (2) | 7.46 ± 1.37 (8)b |
Liver weight as a percentage of body weight. Values are means ± standard deviations.
P < 0.001 versus FVB/N control (two-tailed Student’s t test).
ND, not done.
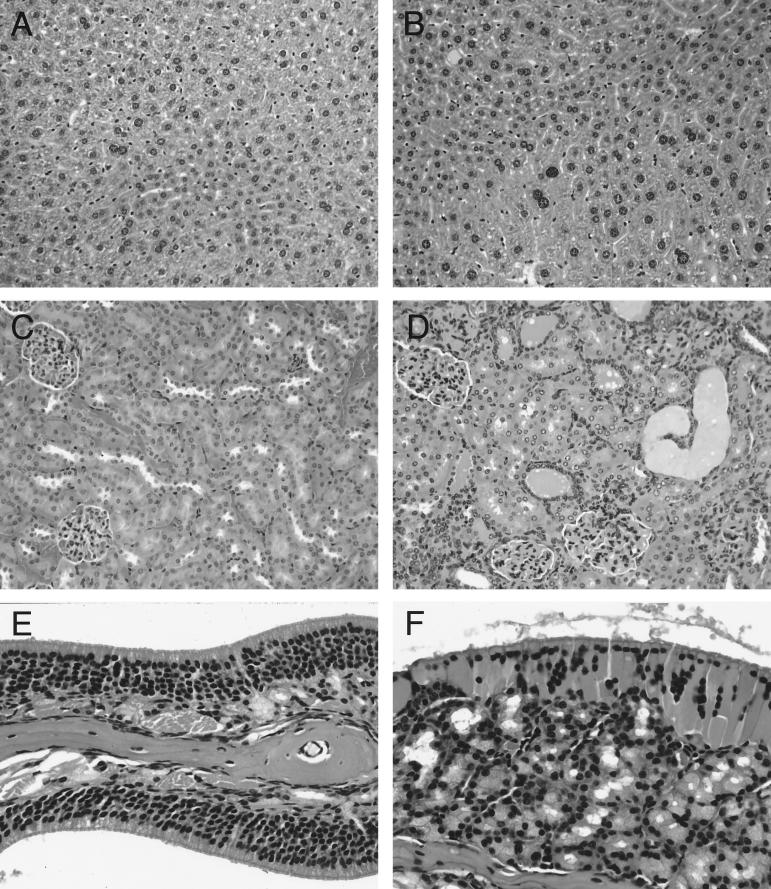
FIG. 3.
Histopathological effects of NK1 overexpression on liver (A and B), kidney (C and D), and olfactory mucosum (E and F). NK1 liver (B) displays centrilobular hepatocellular hypertrophy not present in nontransgenic FVB/N liver (A). Renal disease in NK1 kidney (D) is characterized by tubular hyperplasia and tubular dilatation with proteinaceous casts. Control nontransgenic kidney is shown in panel C. The olfactory mucosum of NK1 mice (F) is disorganized and shows degeneration of the olfactory epithelium and hyperplasia of the olfactory glands, compared to control mice (E). All tissues were stained with hematoxylin and eosin. Magnifications, ×200 (A to D) and ×400 (E and F).
We analyzed other organ systems in which HGF/SF overexpression disrupted tissue homeostasis. With regard to the kidney, NK1-transgenic mice were susceptible to tubular epithelial hyperplasia and microcystic dilatation with proteinaceous casts (Fig. 3C and D) accompanied by focal sclerotic glomeruli with increased mesangial matrix, a renal pathology similar to that observed in HGF/SF-transgenic mice (34). And like the nasal turbinates of HGF/SF mice (33), those of NK1-transgenic mice demonstrated disorganization and degeneration of the olfactory mucosum, hyperplasia of the olfactory glands, and depletion of the olfactory nerves (Fig. 3E and F).
HGF/SF is critical as a paracrine regulator of mesenchymal cell–epithelial cell interactions during development (31, 36). Expression of the MT-HGF/SF transgene in vivo results in a number of developmental abnormalities, one of the more dramatic of which is the formation of ectopic skeletal muscle around the spinal cord (35). Striated muscle also formed around the spinal cords and dorsal and ventral nerve roots of all NK1-transgenic mice over the age of 3 months. The presence of ectopic muscle in the spinal cord was confirmed by staining with an antibody to the muscle marker α-actin (Fig. 4A and B). We also saw one case of ectopic muscle formation in the lung (data not shown). And like the mammary glands in HGF/SF-transgenic females, the mammary glands of NK1 virgin females showed precocious alveolar development and hyperplasia (Fig. 4C and D).
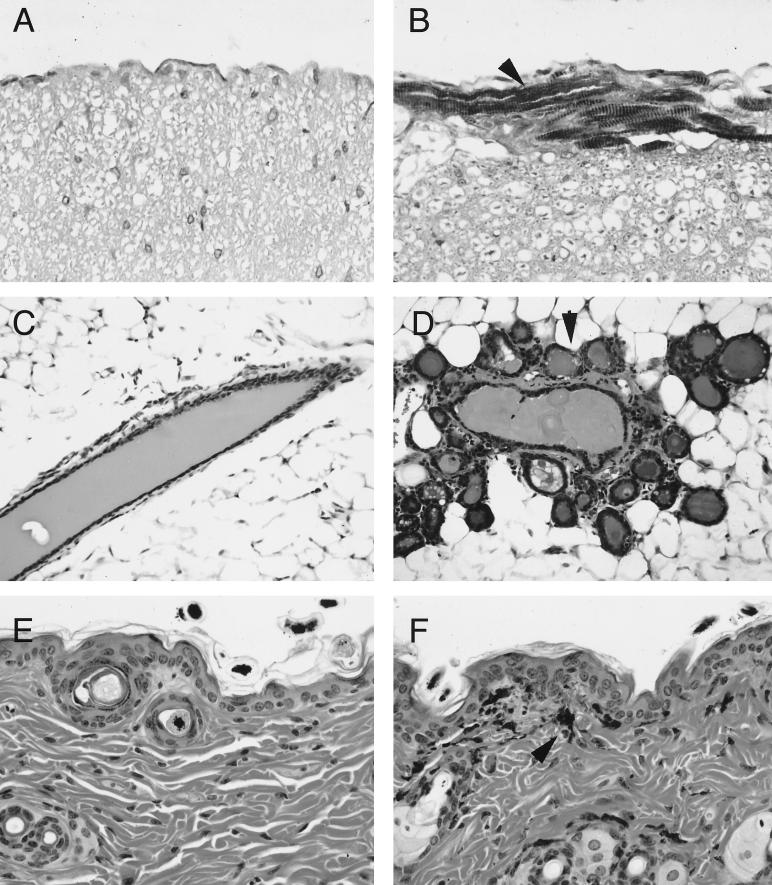
FIG. 4.
Developmental effects of MT-NK1 transgene expression. Immunohistochemical analysis of nontransgenic (A) and transgenic (B) spinal cords with α-actin antibody reveals presence of striated muscle (arrowhead) only in NK1 mice. NK1 mammary glands (D) show precocious development of lobuloalveolar structures (arrowhead), in contrast to the normal ducts of nontransgenic glands (C). Aberrant localization of pigment cells (arrowhead) in the dermis and epidermis of adult NK1 skin (F), compared to nontransgenic skin (E). Tissues in panels C to F were stained with hematoxylin and eosin. Magnifications, ×400 (A, B, E, and F) and ×200 (C and D).
NK1-transgenic mice also showed inappropriate localization of pigment cells. Ten-day-old transgenic offspring of albino NK1-transgenic and pigmented C57BL/6 parental mice exhibited a patterned hyperpigmentation of the skin on the paws, nose, ears, and tail. Cross sections of the skin revealed the presence of pigment cells throughout the dermis and epidermis (Fig. 4F), in contrast to nontransgenic skin sections, where these cells were localized mainly in the hair shafts (Fig. 4E). We also observed the aberrant localization of pigment cells in the lymph nodes and spinal cords of NK1 mice (data not shown), in addition to the skin.
NK1 expression in transgenic mice associated with susceptibility to diverse tumor types.
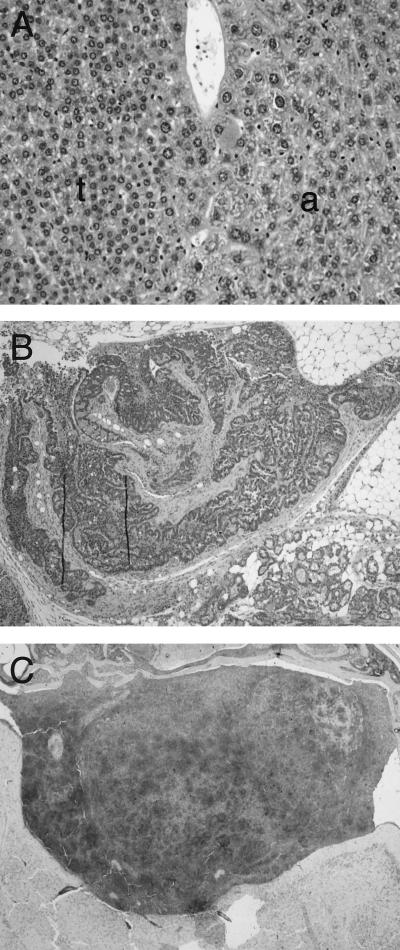
The HGF/SF-Met signaling pathway has been implicated in oncogenesis (1, 16, 23, 24, 37). Diverse tumor types arise in HGF/SF-transgenic mice, especially in cell types that show developmental abnormalities, such as melanocytes and mammary epithelium and muscle cells (33). A wide variety of tumor types also arose in older NK1-transgenic mice. By 21 months of age, at least 26% (5 of 19) of NK1-transgenic mice developed one or more neoplasms, including tumors of the liver (Fig. 5A), mammary gland (Fig. 5B), melanocytes (Fig. 5C), pancreas, glandular stomach, and Harderian gland. The incidences of these tumor types are extremely low in wild-type FVB/N mice (reference 33 and unpublished results).
FIG. 5.
Diverse tumor types in NK1-transgenic mice. (A) Hepatocellular adenoma (t) and adjacent normal liver (a) in an 18-month-old NK1 male. (B) Adenocarcinoma of the mammary gland in a 21-month-old virgin NK1 female. (C) Melanoma in the brain of the male used for panel A. Dark areas show positive immunohistochemical staining for the melanocyte marker HMB-45. Tissues in panels A and B were stained with hematoxylin and eosin. Magnifications, ×50 (A and B) and ×25 (C).
NK1 stimulates Met activation in vivo.
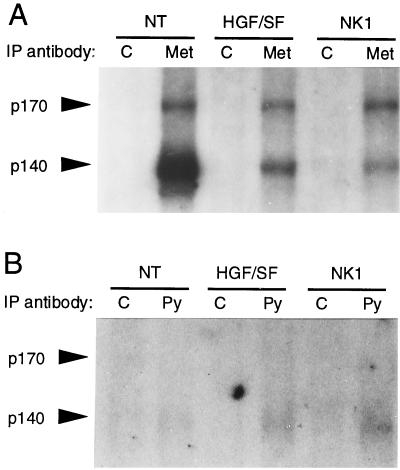
The biochemical mechanism by which the MT-HGF/SF transgene exerts its phenotypic effects consists of increased phosphorylation of Met and its signaling substrates (27). To determine whether the in vivo consequences of MT-NK1 transgene expression were also associated with Met activation, we compared the phosphotyrosine contents of Met immunoprecipitates in liver extracts from NK1-transgenic mice, HGF/SF-transgenic mice, and nontransgenic mice. Immunoprecipitation with either an anti-Met antibody or an antiphosphotyrosine monoclonal antibody was performed on liver extracts, followed by immunoblotting with the anti-Met antibody. We consistently observed at least twofold-higher levels of antiphosphotyrosine-immunoprecipitable p140 Met in both NK1- and HGF/SF-transgenic livers, compared to levels in nontransgenic livers (Fig. 6B). The increase in activated Met levels is even greater when it is taken into account that the total levels of p140 Met in the NK1- and HGF/SF-transgenic livers were lower than in nontransgenic livers (Fig. 6A) (27). We conclude that NK1, like HGF/SF, stimulates Met activation in vivo and potentially downregulates p140 Met.
FIG. 6.
Met receptor levels in NK1- and HGF/SF-transgenic livers. (A) Total levels of Met receptor in transgenic livers. (B) Levels of Met associated with tyrosine-phosphorylated complexes in transgenic livers. Liver lysates from nontransgenic (NT) and NK1- and HGF/SF-transgenic mice were immunoprecipitated (IP) with control antibody (C), anti-Met (Met), or antiphosphotyrosine (Py), subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to Immobilon P membranes, and immunoblotted with anti-Met. Arrowheads indicate positions of p170 and p140 Met.
NK1 overexpression does not affect endogenous HGF/SF expression.
One possible explanation for the phenotypic similarities between NK1- and HGF/SF-transgenic mice was that expression of the MT-NK1 transgene increased the levels of endogenous HGF/SF mRNA or protein through some compensatory mechanism. To investigate this possibility, we first compared the levels of endogenous HGF/SF mRNA in NK1-transgenic and nontransgenic mice. No significant difference was found in either the liver or the kidney by Northern blot analysis (data not shown). This was consistent with previous observations that indicate that there is no significant upregulation of the endogenous-HGF/SF level in HGF/SF-transgenic mice (35). We also compared levels of endogenous HGF/SF in sera from NK1-transgenic and nontransgenic mice. An ELISA that recognizes an epitope at the carboxyl terminus of the α chain was used; thus, only endogenous full-length HGF/SF protein, not NK1, is detected. Levels of HGF/SF in sera from NK1-transgenic mice were the same as those in nontransgenic animals (3.2 versus 3.4 ng/ml) and lower than levels found in HGF/SF-transgenic mice (7.5 ng/ml). We conclude from the Northern blot and ELISA data that there is no significant increase in endogenous HGF/SF mRNA or protein that could account for the phenotypic similarities between the NK1- and HGF/SF-transgenic mice.
DISCUSSION
The original characterization of NK1 as an antagonist for HGF/SF binding and Met activation suggested that NK1 could have therapeutic value in the growth inhibition of certain tumors (3, 15). Recently, however, NK1 was found to have partial agonist properties that were dependent on the glycosaminoglycan composition of the culture conditions (4, 30). While the biological properties of NK1 in vitro remain controversial, there is virtually nothing known about its activity in vivo.
We have cloned the cDNA for the mouse homolog of human NK1 and tested its activity in a transgenic-mouse model system. When inappropriately expressed in transgenic mice, NK1 was found to have effects on tissue homeostasis, development, and tumor susceptibility similar to those associated with overexpression of full-length HGF/SF (summarized in Table 2). For example, the effects of NK1 overexpression in the transgenic liver were similar to those associated with HGF/SF overexpression: enlarged livers, centrilobular hypertrophy, and increased hepatocellular-proliferation rates (27, 35). NK1-transgenic mice develop a renal disease nearly identical to the prominent tubular cystic disease and progressive glomerulosclerosis observed in HGF/SF-transgenic mice (34). The phenotypic effects in the olfactory mucosa of NK1-transgenic mice mirror the marked degeneration of the olfactory epithelium and nerve bundles and hyperplasia of the olfactory glands seen in HGF/SF-transgenic mice (33). The developmental effects of NK1 expression mimic the defects seen in HGF/SF transgenic mice, such as ectopic muscle formation in the spinal cord (35), precocious lobuloalveolar development in the mammary glands (33), and aberrant migration of melanoblasts that results in the appearance of ectopic melanocytes in the skin, lymph nodes, and central nervous system (35). The diverse tumor types that appear in NK1 mice are also prominent features of HGF/SF-transgenic mice, which have a high incidence of liver and mammary gland tumors and melanomas (27, 33). And, as with HGF/SF mice (27), the phenotypic characteristics of NK1 overexpression in vivo are associated with chronic activation of Met; we observed increased levels of tyrosine-phosphorylated complexes associated with Met in NK1-transgenic liver, at levels similar to those in HGF/SF liver. This suggests that Met is stimulated in an autocrine manner in the liver and provides a mechanistic basis for the effects of NK1 overexpression in vivo. Thus, the remarkable similarities in the phenotypes and in the associated effects on Met activation strongly indicate that NK1 acts as an HGF/SF agonist in vivo.
TABLE 2.
Phenotypic comparison of NK1- and HGF/SF-transgenic mice
| Mouse type | Increase in liver mass (fold) | Elevation in hepatocyte proliferation (fold) | Severity of:
|
Incidence of:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renal disease
|
Olfactory mucosal degeneration | Ectopic skeletal muscle | Mammary hyperplasia | Patterned hyperpigmentation | Tumors
|
Met activation in liver | ||||||
| Glomerulosclerosis | Tubular hyperplasia | Mammary adenocarcinoma | Melanoma | Liver adenoma | ||||||||
| NK1 | 1.4 | 3.7 | Moderate | Moderate | Mild | Moderate | Moderate | Moderate | Present | Present | Present | Present |
| HGF/SFa | 2.0b | 11c | Severe | Severe | Moderate | Moderate | Severe | Severe | High | High | Present | Present |
Our finding that NK1 behaves as a partial agonist of HGF/SF in vivo is remarkable in light of the structural differences between the two proteins. Mature HGF/SF is a heterodimeric protein consisting of a 60-kDa α chain containing four kringle domains and a 30-kDa β chain with a serine protease-like domain (11, 16, 39, 42). NK1, on the other hand, is a protein of 22-kDa and consists only of the amino terminus and first kringle domain of the α chain. This suggests that the amino terminus and first kringle domain of HGF/SF are sufficient for relevant levels of Met binding, activation, and intracellular signaling in vivo, despite the lower affinity of NK1 for Met in vitro (4, 15, 30).
Yet despite the strong similarity in phenotypes, many of the effects of NK1 overexpression were not as severe as those exhibited with HGF/SF overexpression (Table 2). For example, the nearly 1.5-fold increase in NK1 liver size was not as dramatic as the two- to threefold increase seen in HGF/SF mice (27); even in older animals, NK1 livers never reached the size of HGF/SF livers. The increase in hepatocellular proliferation levels was more pronounced in HGF/SF-transgenic mice than in NK1-transgenic mice. Ectopic muscle formation in the spinal cord was not as extensive in NK1 mice as in HGF/SF-transgenic mice; we saw none of the evidence for hind-limb paralysis in NK1 mice that has been seen in about 5% of HGF/SF-transgenic mice (35). While NK1-transgenic mice developed the progressive renal disease characteristic of HGF/SF-transgenic mice, it was rarely lethal, as it is in some lines of HGF/SF mice, where up to a quarter of the mice die from kidney failure (34). The tumor-promoting capacity of NK1 appears to be lower too. Even though NK1 mice develop many of the same types of tumors, such as mammary adenocarcinomas, liver tumors, and melanomas, the incidences are lower than those observed for HGF/SF mice (27, 33).
The differences in phenotypic severity observed between NK1- and HGF/SF-transgenic mice may be due to the biochemical properties of NK1 that restrict its effects to those of a partial agonist. Compared to HGF/SF, NK1 is diminished in Met binding activity (4, 15, 30) and, even under conditions where similar levels of Met phosphorylation are achieved, NK1 is less efficient in stimulating DNA synthesis (4). The possibility also remains that subtle differences in transgene expression account for the quantitative phenotypic differences observed between NK1- and HGF/SF-transgenic mice, especially in tissues such as kidney, brain, skin, and skeletal muscle.
The agonistic activity we observed for NK1 is consistent with the in vitro findings of Schwall et al (30) and Cioce et al (4), in which NK1 was converted from an HGF/SF antagonist to a partial agonist by changing the glycosaminoglycan composition of the cell culture conditions. Our finding that NK1 has agonistic properties when overexpressed in transgenic mice suggests that their in vitro conditions more closely mimicked the in vivo milieu than was the case in earlier studies in which NK1 was shown to be an HGF/SF antagonist (15).
Another naturally occurring HGF/SF splice variant in humans, NK2, is composed of the amino terminus and first two kringle domains of HGF/SF (3, 18). Like NK1, NK2 is generated by alternative splicing and has been shown to act as either an antagonist or a partial agonist of HGF/SF, depending on cell culture conditions (30). Little is known about the physiological roles of either isoform. NK1 and NK2 are expressed in normal and transformed fibroblast cell lines (3, 4, 8, 10) and normal placenta (18). Now that we have established that NK1 can act as a partial agonist in vivo, it will be interesting to see how NK2 behaves in the context of the whole animal.
The utility of NK1 as a therapeutic antagonist of HGF/SF is directly challenged by the in vivo data presented in this report. However, NK1 may still have pharmacological applications as a molecule that is significantly smaller than HGF/SF yet retains many of its biological activities. It has been proposed that there are situations where HGF/SF can have therapeutic value. For example, in acute renal failure induced by nephrotoxic compounds or by renal ischemia, HGF/SF administration enhanced the regeneration of renal tubules and the restoration of kidney functions (17). Although our data on the in vivo consequences of NK1 and HGF/SF overexpression demonstrate that the clear risks in high chronic doses of this growth factor potentially restrict therapeutic approaches such as gene therapy, limited and subchronic dosages of NK1 or HGF/SF may still have therapeutic value.
ACKNOWLEDGMENTS
We thank Jeff Rubin, Hiromi Sakata, and Ralph Schwall for helpful discussions, Miriam Anver for assistance with histopathology, Andrew Chan and Stuart Aaronson for providing the human HGF/SF clone used to isolate the murine NK1, and Paul Kriebel and Mary May for technical assistance. We also thank Steve Neal and Ricardo Dreyfuss for photography.
REFERENCES
- 1.Bellusci S, Moens G, Gaudino G, Comoglio P, Nakamura T, Thierey J P, Jouanneau J. Creation of an hepatocyte growth factor/scatter factor autocrine loop in carcinoma cells induces invasive properties associated with increased tumorigenicity. Oncogene. 1994;9:1091–1099. [PubMed] [Google Scholar]
- 2.Bladt T, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 3.Chan A M-L, Rubin J S, Bottaro D P, Hirschfield D W, Chedid M, Aaronson S A. Identification of a competitive HGF antagonist encoded by an alternative transcript. Science. 1991;254:1382–1385. doi: 10.1126/science.1720571. [DOI] [PubMed] [Google Scholar]
- 4.Cioce V, Csaky K G, Chan A M-L, Bottaro D P, Taylor W G, Jensen R, Aaronson S A, Rubin J S. Hepatocyte growth factor (HGF)/NK1 is a naturally occurring HGF/scatter factor variant with partial agonist/antagonist activity. J Biol Chem. 1996;271:13110–13115. doi: 10.1074/jbc.271.22.13110. [DOI] [PubMed] [Google Scholar]
- 5.Di Renzo M F, Poulsom R, Olivero M, Comoglio P M, Lemoine N R. Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res. 1995;55:1129–1138. [PubMed] [Google Scholar]
- 6.Ferracini R, Olivero M, Di Renzo M F, Martano M, De Giovanni C, Nanni P, Basso G, Scotlandi K, Lollini P-L, Comoglio P M. Retrogenic expression of the Met proto-oncogene correlates with the invasive phenotype of human rhabdomyosarcomas. Oncogene. 1996;11:1697–1705. [PubMed] [Google Scholar]
- 7.Halaban R, Rubin J S, Funasaka Y, Cobb M, Boulton T, Faletto D, Rosen E, Chan A, Yoko K, White W, Cook C, Moellmann G. Met and hepatocyte growth factor/scatter factor signal transduction in normal melanocytes and melanoma cells. Oncogene. 1992;7:2195–2206. [PubMed] [Google Scholar]
- 8.Hartmann G, Naldini L, Weidner K M, Sachs M, Vigna E, Comoglio P M, Birchmeier W. A functional domain in the heavy chain of scatter factor/hepatocyte growth factor binds the c-Met receptor and induces cell dissociation but no mitogenesis. Proc Natl Acad Sci USA. 1992;89:11574–11578. doi: 10.1073/pnas.89.23.11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igawa T, Kanda S, Kanetake H, Saitoh Y, Ichihara A, Tomita Y, Nakamura T. Hepatocyte growth factor is a potent mitogen for cultured rabbit renal tubular epithelial cells. Biochem Biophys Res Commun. 1991;174:831–838. doi: 10.1016/0006-291x(91)91493-v. [DOI] [PubMed] [Google Scholar]
- 10.Itakura Y, Yamamoto T, Matsumoto K, Nakamura T. Autocrine stimulation of motility in SBC-5 human lung carcinoma cells by a two-kringle variant of HGF. Cancer Lett. 1994;83:235–243. doi: 10.1016/0304-3835(94)90325-5. [DOI] [PubMed] [Google Scholar]
- 11.Jeffers M, Rong S, Vande Woude G F. Hepatocyte growth factor/scatter factor Met signaling in tumorigenicity and invasion/metastasis. J Mol Med. 1996;74:505–513. doi: 10.1007/BF00204976. [DOI] [PubMed] [Google Scholar]
- 12.Jhappan C, Stahle C, Harkins R N, Fausto N, Smith G H, Merlino G T. TGFa overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990;61:1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- 13.Lamszus K, Joseph A, Jin L, Yao Y, Chowdhury S, Fuchs A, Polvrini P J, Goldberg I D, Rosen E M. Scatter factor binds to thrombospondin and other extracellular matrix components. Am J Pathol. 1996;149:805–819. [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Michalopoulos G K, Zarnegar R. Molecular cloning and characterization of cDNA encoding mouse hepatocyte growth factor. Biochim Biophys Acta. 1993;1216:299–303. doi: 10.1016/0167-4781(93)90159-b. [DOI] [PubMed] [Google Scholar]
- 15.Lokker N A, Godowski P J. Generation and characterization of a competitive antagonist of human hepatocyte growth factor, HGF/NK1. J Biol Chem. 1993;268:17145–17150. [PubMed] [Google Scholar]
- 16.Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119:591–600. doi: 10.1093/oxfordjournals.jbchem.a021283. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto K, Nakamura T. Hepatocyte growth factor. In: Goligorsky M, editor. Acute renal failure: new concepts and therapeutic strategies. New York, N.Y: Churchill Livingstone; 1995. pp. 450–474. [Google Scholar]
- 18.Miyazawa K, Kitamura A, Naka D, Kitamura N. An alternatively processed mRNA generated from human hepatocyte growth factor gene. Eur J Biochem. 1991;197:15–22. doi: 10.1111/j.1432-1033.1991.tb15876.x. [DOI] [PubMed] [Google Scholar]
- 19.Natali P G, Nicotra M R, Di Renzo M F, Prat M, Bigotti A, Cavaliere R, Comoglio P M. Expression of the c-met/HGF receptor in human melanocytic neoplasms: demonstration of the relationship to malignant melanoma tumour progression. Br J Cancer. 1993;68:746–750. doi: 10.1038/bjc.1993.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niranjan B, Buluwela L, Yant J, Perusinghe N, Atherton A, Phippard D, Dale T, Gusterson B, Kamalati T. HGF/SF: a potent cytokine for mammary growth, morphogenesis and development. Development. 1995;121:2897–2908. doi: 10.1242/dev.121.9.2897. [DOI] [PubMed] [Google Scholar]
- 21.Palmiter R D, Sandgren E P, Koeller D M, Brinster R L. Distal regulatory elements from the mouse metallothionein locus stimulate gene expression in transgenic mice. Mol Cell Biol. 1993;13:5266–5275. doi: 10.1128/mcb.13.9.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prat M, Narsimhan R P, Crepaldi T, Nicotra M R, Natali P G, Comoglio P M. The receptor encoded by the human c-MET oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int J Cancer. 1991;49:323–328. doi: 10.1002/ijc.2910490302. [DOI] [PubMed] [Google Scholar]
- 23.Rahimi N, Tremblay E, McAdam L, Park M, Schwall R, Elliott B. Identification of a hepatocyte growth factor autocrine loop in a murine mammary carcinoma. Cell Growth Differ. 1996;7:263–270. [PubMed] [Google Scholar]
- 24.Rong S, Donehower L A, Hansen M F, Strong L, Tainsky M, Jeffers M, Resau J H, Hudson E, Tsarfaty I, Vande Woude G F. Met proto-oncogene product is overexpressed in tumors of p53-deficient mice and tumors of Li-Fraumeni patients. Cancer Res. 1995;55:1963–1970. [PubMed] [Google Scholar]
- 25.Rong S, Jeffers M, Resau J H, Tsarfaty I, Oskarsson M, Vande Woude G F. Met expression and sarcoma tumorigenicity. Cancer Res. 1993;53:5355–5360. [PubMed] [Google Scholar]
- 26.Sakata H, Stahl S J, Taylor W G, Rosenberg J M, Sakaguchi K, Wingfield P T, Rubin J S. Heparin binding and oligomerization of hepatocyte growth factor/scatter factor isoforms. J Biol Chem. 1997;272:9457–9463. doi: 10.1074/jbc.272.14.9457. [DOI] [PubMed] [Google Scholar]
- 27.Sakata H, Takayama H, Sharp R, Rubin J S, Merlino G, LaRochelle W J. Hepatocyte growth factor/scatter factor overexpression induces growth, abnormal development, and tumor formation in transgenic mouse livers. Cell Growth Differ. 1996;7:1513–1523. [PubMed] [Google Scholar]
- 28.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt L, Duh F-M, Chen F, Kishida T, Glenn G, Choyke P, Scherer S W, Zhuang Z, Lubensky I, Dean M, Allikmets R, Chidambaram A, Bergerheim U R, Feltis J T, Casadevall C, Zamarron A, Bernues M, Richard S, Lips C J M, Walther M M, Tsui L-C, Geil L, Orcutt M L, Stackhouse T, Lipan J, Slife L, Brauch H, Decker J, Niehans G, Hughson M D, Moch H, Storkel S, Lerman M I, Linehan W M, Zbar B. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 30.Schwall R H, Chang L Y, Godowski P J, Kahn D W, Hillan K J, Bauer K D, Zioncheck T F. Heparin induces dimerization and confers proliferative activity onto the hepatocyte growth factor antagonists NK1 and NK2. J Cell Biol. 1996;133:709–718. doi: 10.1083/jcb.133.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonnenberg E, Meyer D, Weidner K M, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993;123:223–235. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tajima H, Matsumoto K, Nakamura T. Hepatocyte growth factor has potent anti-proliferative activity in various tumor cell lines. FEBS Lett. 1991;291:229–232. doi: 10.1016/0014-5793(91)81291-f. [DOI] [PubMed] [Google Scholar]
- 33.Takayama H, LaRochelle W J, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson S A, Merlino G. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci USA. 1997;94:701–706. doi: 10.1073/pnas.94.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takayama H, LaRochelle W J, Sabnis S G, Otsuka T, Merlino G. Renal tubular hyperplasia, polycystic disease and glomerulosclerosis in transgenic mice overexpressing hepatocyte growth factor/scatter factor. Lab Investig. 1997;77:131–138. [PubMed] [Google Scholar]
- 35.Takayama H, LaRochelle W J, Anver M, Bockman D E, Merlino G. Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc Natl Acad Sci USA. 1996;93:5866–5871. doi: 10.1073/pnas.93.12.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsarfaty I, Rong S, Resau J H, Rulong S, da Silva P P, Vande Woude G F. The Met proto-oncogene mesenchymal to epithelial cell conversion. Science. 1994;263:98–101. doi: 10.1126/science.7505952. [DOI] [PubMed] [Google Scholar]
- 37.Tuck A B, Park M, Sterns E E, Boag A, Elliott B E. Coexpression of hepatocyte growth factor and receptor (Met) in human breast carcinoma. Am J Pathol. 1996;148:225–232. [PMC free article] [PubMed] [Google Scholar]
- 38.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 39.Vigna E, Naldini L, Tamagnone L, Longati P, Bardelli A, Maina F, Ponzetto C, Comoglio P M. Hepatocyte growth factor and its receptor, the tyrosine kinase encoded by the c-MET proto-oncogene. Cell Mol Biol (Noisy-le-Grand) 1994;40:597–604. [PubMed] [Google Scholar]
- 40.Woolf A S, Kolatsi-Joannou M, Hardman P, Andermarcher E, Moorby C, Fine L G, Jat P S, Noble M D, Gherardi E. Roles of hepatocyte growth factor/scatter factor and the Met receptor in the early development of the metanephros. J Cell Biol. 1995;128:171–184. doi: 10.1083/jcb.128.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamashita J, Ogawa M, Yamashita S, Nomura K, Kuramoto M, Saishoji T, Shin S. Immunoreactive hepatocyte growth factor is a strong and independent predictor of recurrence and survival in human breast cancer. Cancer Res. 1994;54:1630–1633. [PubMed] [Google Scholar]
- 42.Zarnegar R, Michalopoulos G K. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol. 1995;129:1177–1180. doi: 10.1083/jcb.129.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]