Abstract
Objectives
To assess 52-week safety and efficacy of bimekizumab in patients with active psoriatic arthritis (PsA) and prior inadequate response/intolerance to tumour necrosis factor inhibitors.
Methods
Patients completing the 16-week phase III double-blind, placebo-controlled BE COMPLETE (NCT03896581) study entered the open-label extension, BE VITAL (NCT04009499). All patients in BE VITAL received 160 mg bimekizumab every 4 weeks. Safety and efficacy are reported to week 52.
Results
A total of 347/400 (86.8%) patients completed week 52. To week 52, the exposure-adjusted incidence rate/100 patient-years for ≥1 treatment-emergent adverse event (TEAE) was 126.0, and was 7.0 for serious TEAEs. The most frequent TEAEs were SARS-CoV-2 (COVID-19), oral candidiasis, nasopharyngitis and urinary tract infection. All fungal infections were mild or moderate in severity and localised; two patients discontinued the study due to oral candidiasis. No cases of active tuberculosis, uveitis or inflammatory bowel disease were reported. One sudden death occurred. Sustained efficacy was observed with bimekizumab from week 16 to 52 across clinical and patient-reported outcomes. At week 52, 51.7% bimekizumab-randomised and 40.6% placebo/bimekizumab patients (receiving bimekizumab from week 16 to 52) had ≥50% improvement in the American College of Rheumatology criteria. Complete skin clearance (Psoriasis Area and Severity Index 100) was achieved by 65.9% bimekizumab and 60.2% placebo/bimekizumab patients at week 52. Minimal disease activity was achieved by 47.2% bimekizumab and 33.1% placebo/bimekizumab patients at week 52.
Conclusions
Bimekizumab demonstrated a safety profile consistent with previous reports; no new safety signals were identified. Sustained efficacy was observed from week 16 to 52.
Keywords: arthritis, psoriatic; tumor necrosis factor inhibitors; biological therapy
Video Abstract.
Disclaimer: this video summarises a scientific article published by BMJ Publishing Group Limited (BMJ). The content of this video has not been peer-reviewed and does not constitute medical advice. Any opinions expressed are solely those of the contributors. Viewers should be aware that professionals in the field may have different opinions. BMJ does not endorse any opinions expressed or recommendations discussed. Viewers should not use the content of the video as the basis for any medical treatment. BMJ disclaims all liability and responsibility arising from any reliance placed on the content.
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Psoriatic arthritis (PsA) is a chronic, immune-mediated inflammatory disease that can negatively impact patient quality of life.
Patients who experience inadequate response or intolerance to tumour necrosis factor inhibitors (TNFi-IR) are of clinical interest, particularly as second-line biologic treatments typically demonstrate limited efficacy.
WHAT THIS STUDY ADDS
BE COMPLETE and its open-label extension, BE VITAL, demonstrated that bimekizumab was well tolerated in patients with PsA and TNFi-IR up to 52 weeks; the overall safety profile was consistent with that observed in prior studies, including incidence of Candida infections.
Treatment with bimekizumab in patients with active PsA and TNFi-IR resulted in clinically meaningful improvements in efficacy outcomes compared with placebo at week 16, which were sustained to week 52.
In patients initially randomised to placebo, switching to bimekizumab resulted in improvements in efficacy from week 16 to 52.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These results demonstrate that bimekizumab was a well-tolerated and effective treatment option up to 1 year in patients with active PsA and TNFi-IR, a subgroup of clinical interest.
The ongoing BE VITAL open-label extension will continue to assess the long-term safety and efficacy of bimekizumab treatment.
These results, alongside other published reports, provide evidence for the tolerability and efficacy of bimekizumab across the domains of disease and patient populations with PsA.
Introduction
Psoriatic arthritis (PsA) is a chronic, immune-mediated inflammatory disease that presents across multiple domains including peripheral and axial joint inflammation, skin and nail psoriasis, dactylitis and enthesitis.1 2 The combination of musculoskeletal and skin manifestations profoundly impacts patient function and quality of life.3 International treatment recommendations for PsA focus on the achievement of an optimal state of disease outcome across all domains of PsA.4 5
Although treatment recommendations do not preferentially start with tumour necrosis factor inhibitors (TNFi),6 they are widely used as a first-line treatment option,4 particularly following the emergence of and greater access to biosimilars. As a result, the population of patients who experience inadequate response or intolerance to TNFi (TNFi-IR) is of clinical interest, particularly as second-line biologic treatments typically demonstrate reduced efficacy in this population as compared with biologic disease-modifying antirheumatic drug (bDMARD)-naïve patients.7 8
The interleukin (IL)-17 cytokine superfamily is a key mediator in the pathogenesis of spondyloarthritis.9–11 Of the six IL-17 isoforms, IL-17A and IL-17F share approximately 50% sequence homology and overlap in their pro-inflammatory function.12 Studies suggesting a shift in pro-inflammatory function from IL-17A to IL-17F over time in psoriatic disease indicate that dual neutralisation of both isoforms may be necessary to prevent secondary loss of response.13
Bimekizumab is a humanised monoclonal IgG1 antibody that selectively and potently inhibits both IL-17A and IL-17F and is more effective at suppressing pro-inflammatory cytokines in vitro compared with inhibition of IL-17A or IL-17F alone.12 The phase III BE OPTIMAL and BE COMPLETE studies demonstrated the efficacy and tolerability of subcutaneous bimekizumab in bDMARD-naïve patients, and in patients with TNFi-IR, respectively.14 15 To week 16, bimekizumab demonstrated similar efficacy and tolerability irrespective of patients’ prior biologic use. Efficacy and safety has also been reported up to 52 weeks in bDMARD-naïve patients.14–16
Long-term tolerability and sustained efficacy are of importance to patients with prior TNFi-IR as they often do not achieve optimal disease outcomes and may lose response over time. As such, new therapies that can address these areas are of benefit to this population. Here, we present 52-week data of bimekizumab in patients with PsA and prior TNFi-IR, a population of clinical interest. Data are reported for patients who completed week 16 of BE COMPLETE and entered the ongoing, phase III, open-label extension (OLE) study, BE VITAL.
Methods
Study design and participants
This manuscript reports data from the BE COMPLETE study and the BE VITAL OLE up to 1 year (52 weeks) of treatment. Patients from both BE OPTIMAL and BE COMPLETE studies could enter the BE VITAL OLE and receive subcutaneous bimekizumab 160 mg every 4 weeks (Q4W) for up to 140 weeks14 15; data reported here are only for the patients with prior TNFi-IR who were randomised at week 0 of the BE COMPLETE study (online supplemental figure 1).
rmdopen-2023-003855supp001.pdf (2MB, pdf)
Full methodological details of the BE COMPLETE study are reported in the primary manuscript.14 BE COMPLETE was a 16-week, phase III, multicentre, randomised, double-blind, placebo-controlled study of patients with active PsA and prior TNFi-IR, conducted at 92 sites in Asia, Eastern Europe, Western Europe and North America. The study included a 2–5-week screening period, a 16-week double-blind, placebo-controlled treatment period and a 20-week safety follow-up period.
Patients completing week 16 of BE COMPLETE, meeting eligibility criteria and providing separate informed consent were eligible for enrolment into the BE VITAL OLE study. For any patients who discontinued early from the BE VITAL OLE for any reason, a safety follow-up visit was conducted 20 weeks after the last dose of bimekizumab.
Patients
Full inclusion and exclusion criteria were reported previously.14 Eligible patients had a documented diagnosis of adult-onset PsA meeting the Classification Criteria for PsA17 for ≥6 months prior to screening. Patients had active PsA with a baseline tender joint count (TJC) ≥3 (of 68), swollen joint count (SJC) ≥3 (of 66) and ≥1 active psoriatic lesion and/or a documented history of psoriasis. An inadequate response (defined as lack of efficacy after at least 3 months of therapy at an approved dose) or intolerance to one or two TNFi for either PsA or psoriasis, as assessed by the investigator, was required; patients with current or previous exposure to any other biologics were excluded. The washout period for all TNFi was 3 months, except for etanercept which had a washout period of 28 days. Patients with a history of inflammatory bowel disease (IBD) and no symptoms of active disease at screening were eligible. Concomitant conventional synthetic DMARDs (including methotrexate) were permitted during BE COMPLETE as per the criteria outlined in the protocol.14 The dose, dosing schedule and administration route should have remained stable until week 16.
Randomisation and masking
At baseline (week 0) of BE COMPLETE, patients were randomised 2:1 to receive either subcutaneous bimekizumab 160 mg or placebo Q4W. Patients were stratified by region and prior TNFi exposure (inadequate response to one or two prior TNFi, or intolerance to TNFi). Patients completing week 16 of BE COMPLETE and electing to enrol in the BE VITAL OLE received open-label subcutaneous bimekizumab 160 mg Q4W. All study timepoints are hereafter reported relative to baseline (week 0) of the BE COMPLETE randomised controlled study.
Study procedures and outcomes
During BE COMPLETE, efficacy and safety assessments were made at baseline (week 0) and Q4W to week 16. In the BE VITAL OLE, safety assessments were made at weeks 20, 24 and 28, then every 12 weeks to week 52. Efficacy was assessed at week 28, after which patients could self-administer bimekizumab and were required to attend study visits every 12 weeks thereafter if trained at the study site. Patients not self-administering bimekizumab could return to the study site every 4 weeks for administration of bimekizumab; no assessments were conducted at interim visits. Details of the primary, key secondary and additional outcomes assessed during BE COMPLETE are reported in the primary manuscript.14
The primary objective of the BE VITAL OLE was to evaluate the long-term safety and tolerability of subcutaneous bimekizumab, as assessed by the incidence of treatment-emergent adverse events (TEAEs) and serious TEAEs. All TEAEs were classified using the Medical Dictionary for Regulatory Activities V.19.0. Prespecified safety topics of interest included serious, opportunistic (defined as infections caused by uncommon pathogens or unusually severe infections caused by common pathogens) and fungal infections, tuberculosis (TB), neutropenia, hypersensitivity, suicidal ideation and behaviour, depression, major adverse cardiovascular events (MACE), liver function test changes and enzyme elevations, malignancies and IBD. Suicidal ideation and behaviour, MACE and IBD events were adjudicated by an external adjudication committee. A secondary safety variable was the incidence of TEAEs leading to withdrawal from study treatment.
Assessment of efficacy was a prespecified secondary objective of the OLE. Clinical efficacy outcomes assessed up to 52 weeks included ≥20%/50%/70% improvement in American College of Rheumatology response criteria (ACR20/50/70),18 ≥75%/90%/100% improvement in Psoriasis Area and Severity Index (PASI75/90/100, in patients with psoriasis involving ≥3% body surface area (BSA) at baseline)19 and ACR50+PASI100 (in patients with psoriasis affecting ≥3% BSA at baseline). Achievement of minimal and very low disease activity (MDA/VLDA) was also assessed up to 52 weeks. MDA/VLDA were achieved if 5/7 or 7/7 of the following criteria were fulfilled, respectively: TJC ≤1, SJC ≤1, either PASI ≤1 or BSA ≤3%, patient’s pain visual analogue scale (VAS) ≤15, Patient’s Global Assessment of PsA (PGA-PsA) ≤20 (VAS), Health Assessment Questionnaire-Disability Index (HAQ-DI)20 ≤0.5 and tender entheseal points ≤1 (measured with the Leeds Enthesitis Index (LEI)).21 Additional clinical efficacy outcomes assessed up to 52 weeks included SJC, TJC, Physician’s Global Assessment of PsA, high-sensitivity C-reactive protein, Disease Activity Index for Psoriatic Arthritis (DAPSA) states,22 resolution of enthesitis (in patients with enthesitis at baseline; LEI or Spondyloarthritis Research Consortium of Canada (SPARCC) >0) and resolution of dactylitis (in patients with dactylitis at baseline; Leeds Dactylitis Index (LDI) >0).23 Psoriatic Arthritis Disease Activity Score (PASDAS) states and resolution of nail psoriasis (in patients with nail psoriasis at baseline; modified Nail Psoriasis Severity Index (mNAPSI) >0) were also assessed up to 52 weeks.
Patient-reported outcome measures reported included HAQ-DI, Patient’s Assessment of Arthritis Pain (PtAAP), PGA-PsA, Psoriatic Arthritis Impact of Disease-12 (PsAID-12),24 Short-Form 36-item Health Survey Physical Component Summary (SF-36 PCS) and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue).25 SF-36 PCS, PsAID-12 and FACIT-Fatigue were only evaluated up to week 40.
Statistical analysis
The safety set consisted of all randomised study participants who received ≥1 dose of bimekizumab.14 Exposure-adjusted incidence rates (EAIR)/100 patient-years (PY) are reported for the safety sets, where available.
Statistical powering, sample size determination and details of hierarchical testing are reported in the BE COMPLETE primary publication.14 Analysis of the BE VITAL OLE was conducted relative to baseline (week 0) of BE COMPLETE, as per the EULAR guidance for reporting clinical trial extension data.26 Missing data for binary outcomes were imputed using non-responder imputation (NRI); any patients that were not enrolled in the BE VITAL OLE were considered as non-responders. P values are only reported for binary outcomes at week 16 and were generated using logistic regression with treatment, region and previous TNFi use (inadequate response to one or two previous TNFi, or intolerance to TNFi) as factors. Continuous outcomes are reported using multiple imputation (MI) for missing data based on the number of patients randomised at week 0 of BE COMPLETE. All analyses were done with SAS, V.9.3 or higher.
Results
Patient disposition and baseline characteristics
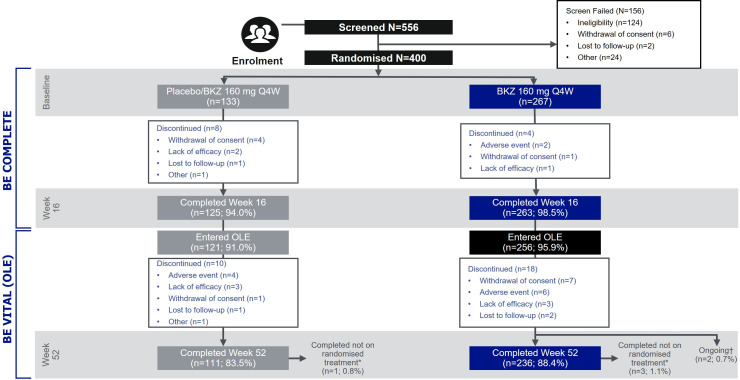
A total of 556 patients were screened for enrolment into BE COMPLETE and 400 were randomised, 267 to subcutaneous bimekizumab 160 mg Q4W and 133 to placebo. Overall, 388 (97.0%) completed week 16 (263 (98.5%) bimekizumab-randomised patients; 125 (94.0%) placebo-randomised patients; figure 1). Of all patients randomised at baseline, 377 (94.3%) (256 (95.9%) bimekizumab-randomised patients; 121 (91.0%) placebo-randomised patients) entered the BE VITAL OLE (figure 1).
Figure 1.
Patient disposition. *Patients who withdrew from the study treatment or deviated from assigned randomised treatment but returned for all scheduled visits up to and including week 52. †Two patients classified as ongoing as they did not have a visit for week 52 but no formal discontinuation reason was reported. BKZ, bimekizumab; OLE, open-label extension; Q4W, every 4 weeks.
In total, 360 (90.0%) and 347 (86.8%) patients remained in the study at weeks 40 and 52, respectively (week 40: 246 (92.1%) bimekizumab, 114 (85.7%) placebo/bimekizumab; week 52: 236 (88.4%) bimekizumab; 111 (83.5%) placebo/bimekizumab). As per the protocol, patients withdrawn from study treatment could return for all remaining study visits; of the patients that remained in the study at weeks 40 and 52, 4 (1.0%) were not on randomised treatment (3 (1.1%) bimekizumab-treated patients; 1 (0.8%) placebo/bimekizumab-treated patient). The most common reasons for discontinuation from the BE VITAL OLE were TEAEs (6 (2.2%) bimekizumab-treated patients; 4 (3.0%) placebo/bimekizumab-treated patients), withdrawal of consent (7 (2.6%) bimekizumab-treated patients; 1 (0.8%) placebo/bimekizumab-treated patient) and lack of efficacy (3 (1.1%) bimekizumab-treated patients; 3 (2.3%) placebo/bimekizumab-treated patients; figure 1).
The baseline patient demographics and disease characteristics, which have been previously reported,14 are presented in online supplemental table 1. The study population had a mean age of 50.5 (SD 12.5) years, with 190 (47.5%) being male. The mean time since PsA diagnosis was 9.5 (SD 9.3) years. 307 (76.8%) had inadequate response to one TNFi, 44 (11.0%) had inadequate response to two TNFi and 49 (12.3%) had intolerance to TNFi. 264 (66.0%) patients had psoriasis with BSA ≥3%; within this subgroup, the proportion of patients that had inadequate response to one or two TNFi, or intolerance to TNFi was comparable to the overall population. Prior TNFi included adalimumab, etanercept, golimumab, infliximab and certolizumab pegol. Additionally, mean TJC of 68 joints was 18.7 (SD 13.8), with a mean SJC of 66 joints of 9.9 (7.7). In the safety set, excluding one placebo-randomised patient, 47 (11.8%), 23 (5.8%) and 24 (6.0%) patients had a history of or ongoing cardiac disorders, diabetes mellitus or type 2 diabetes mellitus, respectively.
Safety
During the double-blind period of BE COMPLETE, 108 patients (40.4%) experienced a TEAE on bimekizumab; numerically higher than the 44 patients (33.3%) reporting a TEAE on placebo. Up to week 52, ≥1 TEAE was reported by 243 of 388 patients while receiving bimekizumab (62.6%; EAIR/100 PY: 126.0), including placebo-randomised patients who switched to bimekizumab at week 16 (68 of 121 (56.2%; EAIR/100 PY: 127.7)). To week 16, serious TEAEs were reported for five patients (1.9%) randomised to bimekizumab, with no events reported for placebo patients. Over 52 weeks, 23 patients (5.9%; EAIR/100 PY: 7.0) reported a serious TEAE while on bimekizumab, and there were 16 (4.1%; EAIR/100 PY: 4.8) study discontinuations due to TEAEs. One death (the cause of which could not be ascertained as no autopsy was performed and no death certificate was available), deemed unrelated to study treatment, occurred in a male bimekizumab-treated patient aged 54 years with a history of hypertension, aortic regurgitation and ECG changes reflective of coronary artery disease (table 1). The EAIR/100 PY for TEAEs in bimekizumab-treated patients from week 0 to 16 (167.2/100 PY) was greater than that for the week 16–52 period (125.4/100 PY; table 1).
Table 1.
Safety to week 16 and week 52
| n (%) (EAIR)* | Weeks 0–16 (double-blind period) | Weeks 16–52 (open-label period) | Weeks 0–52 (overall study period) | ||
| Placebo n=132 (PYAR: 42.5) | BKZ 160 mg Q4W n=267 (PYAR: 87.1) | Placebo/BKZ 160 mg Q4W† n=121 (PYAR: 80.3) | BKZ 160 mg Q4W n=267 (PYAR: 259.5) | BKZ 160 mg Q4W total† n=388 (PYAR: 339.8) | |
| Any TEAE | 44 (33.3) | 108 (40.4) | 68 (56.2) (127.7) | 175 (65.5) (125.4) | 243 (62.6) (126.0) |
| Severe TEAEs | 0 | 5 (1.9) | 3 (2.5) | 14 (5.2) | 17 (4.4) |
| Study discontinuation due to TEAEs | 0 | 2 (0.7) | 6 (5.0) (7.6) | 10 (3.7) (3.9) | 16 (4.1) (4.8) |
| Drug-related TEAEs | 4 (3.0) | 35 (13.1) | 21 (17.4) | 66 (24.7) | 87 (22.4) |
| Serious TEAEs | 0 | 5 (1.9) | 8 (6.6) (10.2) | 15 (5.6) (6.0) | 23 (5.9) (7.0) |
| Deaths | 0 | 0 | 1 (0.8)‡ | 0 | 1 (0.3)‡ |
| Most frequent adverse events§ | |||||
| SARS-CoV-2 (COVID-19) | 6 (4.5) | 5 (1.9) | 7 (5.8) (8.9) | 21 (7.9) (8.4) | 28 (7.2) (8.5) |
| Oral candidiasis | 0 | 7 (2.6) | 7 (5.8) (9.0) | 17 (6.4) (6.8) | 24 (6.2) (7.3) |
| Nasopharyngitis | 1 (0.8) | 10 (3.7) | 4 (3.3) (5.0) | 19 (7.1) (7.7) | 23 (5.9) (7.0) |
| Urinary tract infection | 3 (2.3) | 5 (1.9) | 4 (3.3) (5.1) | 19 (7.1) (7.7) | 23 (5.9) (7.0) |
| Infections | |||||
| Serious | 0 | 2 (0.7) | 3 (2.5) (3.8) | 4 (1.5) (1.6) | 7 (1.8) (2.1) |
| Opportunistic | 0 | 0 | 2 (1.7) (2.5)¶ | 0 | 2 (0.5) (0.6)¶ |
| Active TB | 0 | 0 | 0 | 0 | 0 |
| Uveitis | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 0 | 4 (1.5)** | 0 | 5 (1.9) (2.0)†† | 5 (1.3) (1.5)††‡‡ |
| Hypersensitivity | 1 (0.8) | 7 (2.6) | 4 (3.3) (5.1) | 15 (5.6) (6.0) | 19 (4.9) (5.8) |
| Injection site reactions | 0 | 3 (1.1) | 0 | 6 (2.2) (2.4) | 6 (1.5) (1.8) |
| Adjudicated suicidal ideation and behaviour | 0 | 0 | 0 | 0 | 0 |
| Liver function test changes/enzyme elevations | |||||
| ALT >3×ULN | 0 | 2 (0.7) | 0 | 8 (3.0) | 8 (2.1) |
| AST or ALT >3×ULN | 0 | 4 (1.5) | 2 (1.7) | 10 (3.7) | 12 (3.1) |
| Adjudicated MACE | 0 | 0 | 2 (1.7) (2.5)§§ | 0 | 2 (0.5) (0.6)§§ |
| Adjudicated IBD | 0 | 0 | 0 | 0 | 0 |
| Non-melanoma skin cancer | 1 (0.8) | 0 | 0 | 0 | 0 |
| Basal cell carcinoma | 1 (0.8) | 0 | 0 | 0 | 0 |
| Malignancies excluding non-melanoma skin cancer | 0 | 0 | 1 (0.8) (1.3) | 2 (0.7) (0.8) | 3 (0.8) (0.9) |
| Endometrial cancer stage I | 0 | 0 | 0 | 1 (0.4) (0.4) | 1 (0.3) (0.3) |
| Gastric cancer recurrent | 0 | 0 | 0 | 1 (0.4) (0.4)¶¶ | 1 (0.3) (0.3)¶¶ |
| Prostate cancer | 0 | 0 | 1 (0.8) (1.3) | 0 | 1 (0.3) (0.3) |
Safety set (all randomised subjects who received at least one dose of the study drug); one placebo-randomised patient withdrew from the study before receiving study drug.
*EAIRs are reported for treatment duration >16 weeks, where available.
†Includes patients who switched from placebo to BKZ and only includes TEAEs occurring while receiving BKZ.
‡Sudden death in a patient aged 54 years with a history of hypertension, aortic regurgitation, ECG changes reflective of coronary artery disease; no further information available.
§Most frequent adverse events are those occurring in ≥5% of patients in any study arm.
¶Two oesophageal candidiasis.
**Three neutropenia; one neutrophil count decreased.
††Four neutropenia; one neutrophil count decreased.
‡‡Four neutropenia events were grade 3; one neutropenia event was grade 2.
§§One sudden death; one cerebral haemorrhage.
¶¶A male patient aged 56 years with history of primary gastric cancer; permanently discontinued from the study.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BKZ, bimekizumab; EAIR, exposure-adjusted incident rate; IBD, inflammatory bowel disease; MACE, major adverse cardiovascular event; PYAR, patient‑years at risk; Q4W, every 4 weeks; TB, tuberculosis; TEAE, treatment-emergent adverse event; ULN, upper limit of normal.
The most frequent TEAEs reported to week 52 in bimekizumab-treated patients were SARS-CoV-2 (COVID-19) infections (7.2%; EAIR/100 PY: 8.5), oral candidiasis (6.2%; EAIR/100 PY: 7.3), nasopharyngitis (5.9%; EAIR/100 PY: 7.0) and urinary tract infection (5.9%; EAIR/100 PY: 7.0).
Of all patients receiving bimekizumab, seven (1.8%; EAIR/100 PY: 2.1) had serious infections (one case each of infective bursitis, postoperative wound infection, bronchitis, pneumonia, upper respiratory tract infection, pyelonephritis acute and pyelonephritis), two of which (bronchitis and pneumonia) occurred during the placebo-controlled period. Two cases of oesophageal candidiasis were reported (0.5%; EAIR/100 PY: 0.6); both were classified as opportunistic infections.
There were few cases of neutropenia (five patients (1.3%); EAIR/100 PY: 1.5), none required discontinuation or were associated with serious infections. Of hypersensitivity events (19 patients (4.9%); EAIR/100 PY: 5.8), 8 (2.1%) were dermatitis and eczema events. No hypersensitivity events led to study discontinuation and no serious hypersensitivity events were reported. Injection site reactions occurred in six patients (1.5%; EAIR/100 PY: 1.8). Twelve patients (3.1%) reported elevations of the liver enzymes alanine aminotransferase or aspartate aminotransferase >3 times the upper limit of normal. During the BE VITAL OLE (from week 16), two patients (0.5%; EAIR/100 PY: 0.6) had an event adjudicated as MACE while receiving bimekizumab: one sudden death (detailed above) and one cerebral haemorrhage (in a male patient aged 51 years with no history of cardiovascular risk factors noted; death did not occur), deemed unrelated to study treatment. No cases of active TB, uveitis, adjudicated IBD or adjudicated suicidal ideation and behaviour were reported.
In the total bimekizumab group, three (0.8%; EAIR/100 PY: 0.9) reported cases of malignancies were observed after week 16 (excluding non-melanoma skin cancer), consisting of one (0.3%; EAIR/100 PY: 0.3) case each of endometrial cancer (stage I), recurrent gastric cancer and prostate cancer.
A total of 37 patients (9.5%; EAIR/100 PY: 11.6) that received bimekizumab during the 52-week period (patients randomised to bimekizumab at baseline plus patients who switched to bimekizumab at week 16) experienced a fungal infection, with no reported systemic fungal infections. Of these 37 patients, 25 (6.4%; EAIR/100 PY: 7.7) experienced a Candida infection, the majority of which were oral candidiasis (24 (6.2%); EAIR/100 PY: 7.3), and 12 patients (3.1%; EAIR/100 PY: 3.6) experienced a fungal infection not elsewhere classified (NEC). Two cases of Candida infections (both oral candidiasis) resulted in study discontinuation (both bimekizumab-randomised patients). There were no serious Candida infections, with all cases being mild or moderate in severity. Five bimekizumab-treated patients (1.3%) had recurrent Candida infections; of these, four patients (1.0%) had one recurrent infection and one patient (0.3%) had four recurrent infections. The proportion of bimekizumab-randomised patients with recurrent candidiasis to week 16 was comparable to that from weeks 16 to 52. A further breakdown of fungal infections can be found in online supplemental table 2 and online supplemental figure 2.
Efficacy
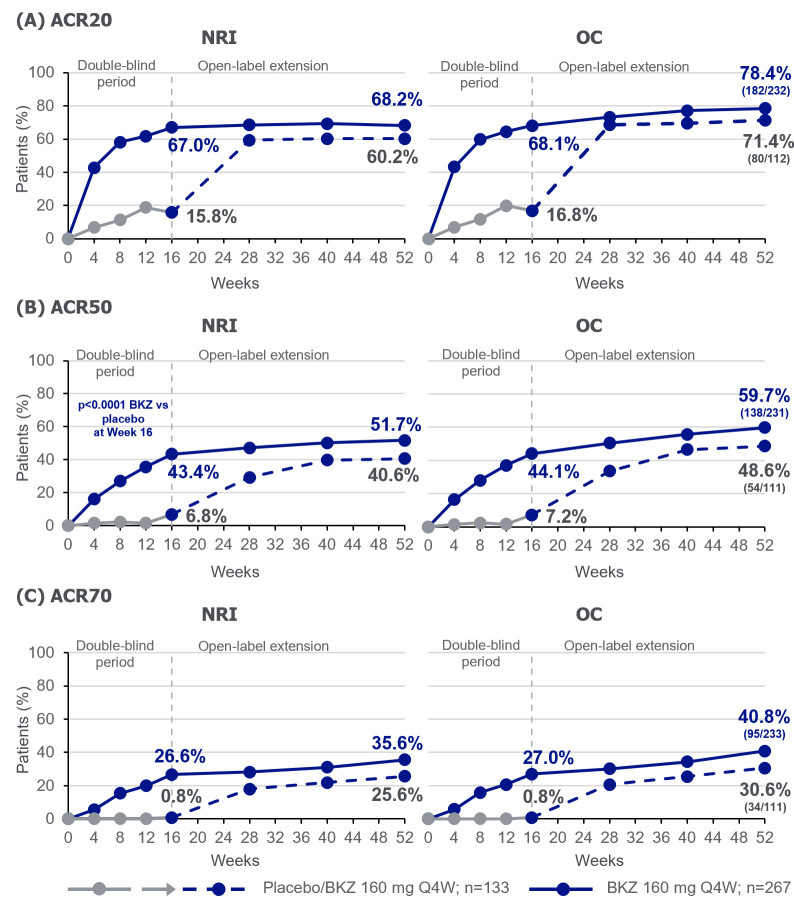
Bimekizumab was associated with sustained responses through week 52 of treatment (figures 2–4). Patients originally randomised to bimekizumab showed an ACR50 response of 43.4% at week 16 that was sustained to 51.7% at week 52, by NRI analysis (table 2, figure 2B). In patients who were initially randomised to placebo, a rapid improvement, 12 weeks after switching to bimekizumab, was observed; ACR50 response improved from 6.8% at week 16 to 40.6% at week 52 (NRI; table 2, figure 2B). ACR20 and ACR70 responses were also sustained to week 52 for bimekizumab-treated patients (ACR20: 68.2%; ACR70: 35.6%; table 2, figure 2A,C). For placebo-randomised patients, ACR20 and ACR70 responses improved from week 16 to 52 (ACR20: 15.8%–60.2%; ACR70: 0.8%–25.6%; table 2, figure 2A,C), following the switch to bimekizumab. Observed ACR responses, for patients remaining in the study at week 52, were higher than those using NRI (ACR20: 78.4% bimekizumab, 71.4% placebo/bimekizumab; ACR50: 59.7% bimekizumab, 48.6% placebo/bimekizumab; ACR70: 40.8% bimekizumab, 30.6% placebo/bimekizumab; table 2, figure 2). Additionally, improvements observed in all of the individual ACR components were sustained from week 16 through week 52 for patients randomised to bimekizumab (online supplemental figure 3).
Figure 2.
ACR Responders to Week 52. Randomised set. For binary variables, p values were calculated using a logistic regression model with treatment, prior TNFi exposure, and region as stratification factors. ACR: American College of Rheumatology; BKZ: bimekizumab; NRI: non-responder imputation; OC: observed case; Q4W: every 4 weeks; TNFi: tumour necrosis factor inhibitor.
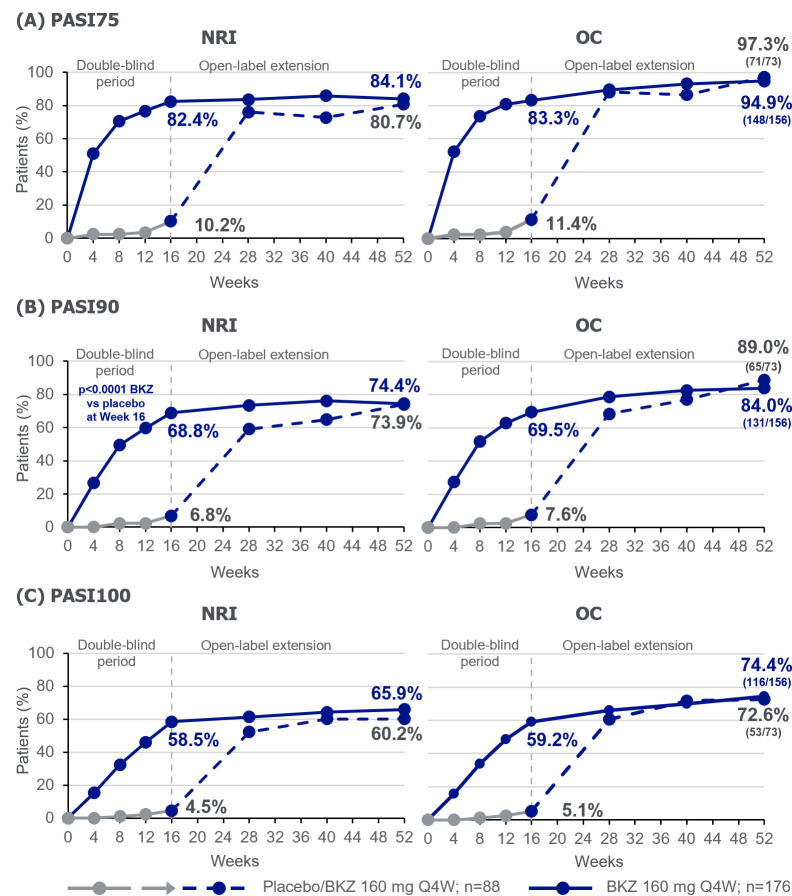
Figure 3.
PASI responders to week 52. Randomised set, in patients with psoriasis affecting ≥3% BSA at baseline. For binary variables, p values were calculated using a logistic regression model with treatment, prior TNFi exposure and region as stratification factors. BKZ, bimekizumab; BSA, body surface area; NRI, non-responder imputation; OC, observed case; PASI, Psoriasis Area and Severity Index; Q4W, every 4 weeks; TNFi, tumour necrosis factor inhibitor.
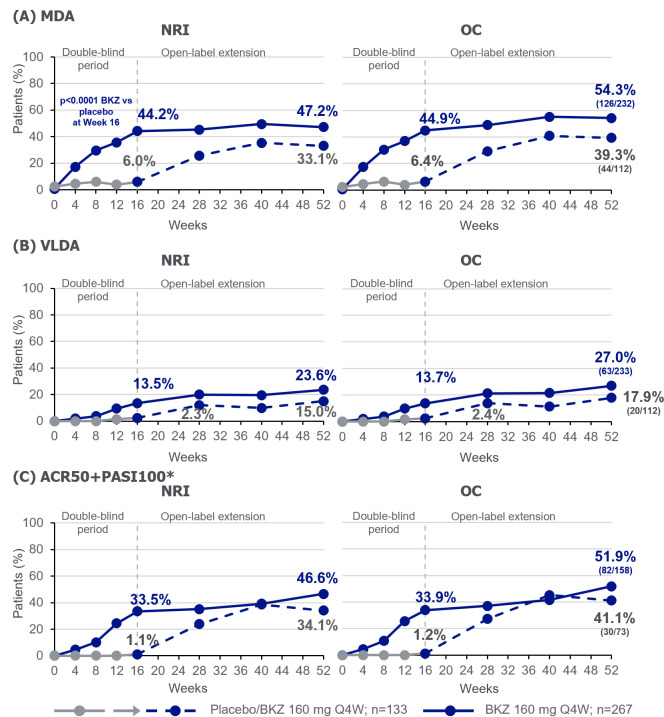
Figure 4.
Additional efficacy outcomes to week 52. Randomised set.*In patients with psoriasis affecting ≥3% BSA at baseline, PBO n=88; BKZ n=176. For binary variables, p values were calculated using a logistic regression model with treatment, prior TNFi exposure and region as stratification factors. ACR, American College of Rheumatology; BKZ, bimekizumab; BSA, body surface area; MDA, minimal disease activity; NRI, non-responder imputation; OC, observed case; PASI, Psoriasis Area and Severity Index; PBO, Placebo; Q4W, every 4 weeks; TNFi, tumour necrosis factor inhibitor; VLDA, very low disease activity.
Table 2.
Efficacy outcomes at week 16 and week 52
| Endpoint | Placebo (weeks 0–16) n=133 |
BKZ 160 mg Q4W (weeks 16–52)¶¶ n=133 |
BKZ 160 mg Q4W n=267 |
|||||
| Week 16 | Week 52 | Week 16 | Week 52 | |||||
| NRI %* |
OC % (n/N) |
NRI %* |
OC % (n/N) |
NRI %* |
OC % (n/N) |
NRI %* |
OC % (n/N) |
|
| ACR20 response | 15.8 | 16.8 (21/125) | 60.2 | 71.4 (80/112) | 67.0 | 68.1 (179/263) | 68.2 | 78.4 (182/232) |
| ACR50 response† | 6.8 | 7.2 (9/125) | 40.6 | 48.6 (54/111) | 43.4 | 44.1 (116/263) | 51.7 | 59.7 (138/231) |
| ACR70 response | 0.8 | 0.8 (1/125) | 25.6 | 30.6 (34/111) | 26.6 | 27.0 (71/263) | 35.6 | 40.8 (95/233) |
| PASI75 response‡ | 10.2 (9/88) | 11.4 (9/79) | 80.7 (71/88) | 97.3 (71/73) | 82.4 (145/176) | 83.3 (145/174) | 84.1 (148/176) | 94.9 (148/156) |
| PASI90 response‡ | 6.8 (6/88) | 7.6 (6/79) | 73.9 (65/88) | 89.0 (65/73) | 68.8 (121/176) | 69.5 (121/174) | 74.4 (131/176) | 84.0 (131/156) |
| PASI100 response‡ | 4.5 (4/88) | 5.1 (4/79) | 60.2 (53/88) | 72.6 (53/73) | 58.5 (103/176) | 59.2 (103/174) | 65.9 (116/176) | 74.4 (116/156) |
| MDA response | 6.0 | 6.4 (8/125) | 33.1 | 39.3 (44/112) | 44.2 | 44.9 (118/263) | 47.2 | 54.3 (126/232) |
| VLDA response | 2.3 | 2.4 (3/125) | 15.0 | 17.9 (20/112) | 13.5 | 13.7 (36/263) | 23.6 | 27.0 (63/233) |
| ACR50+PASI100 response‡ | 1.1 (1/88) | 1.3 (1/79) | 34.1 (30/88) | 41.7 (30/72) | 33.5 (59/176) | 33.9 (59/174) | 46.6 (82/176) | 52.9 (82/155) |
| Complete resolution of enthesitis (LEI)§ | 22.2 (8/36) | 23.5 (8/34) | 58.3 (21/36) | 72.4 (21/29) | 49.1 (52/106) | 50.0 (52/104) | 56.6 (60/106) | 68.2 (60/88) |
| Complete resolution of enthesitis (SPARCC)¶ | 23.5 (12/51) | 25.0 (12/48) | 52.9 (27/51) | 65.9 (27/41) | 45.9 (56/122) | 47.1 (56/119) | 52.5 (64/122) | 62.7 (64/102) |
| Complete resolution of dactylitis** | 42.9 (6/14) | 42.9 (6/14) | 85.7 (12/14) | 92.3 (12/13) | 70.6 (24/34) | 72.7 (24/33) | 85.3 (29/34) | 93.5 (29/31) |
| Complete resolution of nail psoriasis†† | 14.5 (12/83) | 15.4 (12/78) | 61.4 (51/83) | 68.0 (51/75) | 45.9 (73/159) | 46.5 (73/157) | 67.3 (107/159) | 74.3 (107/144) |
| HAQ-DI MCID response‡‡ | 21.8 (24/110) | 23.1 (24/104) | 50.0 (55/110) | 59.8 (55/92) | 56.3 (130/231) | 57.3 (130/227) | 55.0 (127/231) | 62.9 (127/202) |
| MI, mean (SE) | MI, mean (SE) | |||||||
| HAQ-DI change from baseline | −0.07 (0.04) | −0.35 (0.06) | −0.38 (0.03) | −0.39 (0.03) | ||||
| PtAAP score change from baseline | −4.5 (2.1) | −29.5 (2.7) | −27.7 (1.7) | −32.2 (1.8) | ||||
| Week 16 | Week 40§§ | Week 16 | Week 40§§ | |||||
| SF-36 PCS score change from baseline | 1.4 (0.7) | 7.3 (0.9) | 7.3 (0.5) | 8.4 (0.6) | ||||
| PsAID-12 total score change from baseline | −0.3 (0.2) | −2.2 (0.2) | −2.2 (0.1) | −2.5 (0.1) | ||||
| FACIT-Fatigue score change from baseline | 0.0 (0.7) | 4.4 (0.8) | 5.4 (0.6) | 6.0 (0.6) | ||||
Randomised set. Previously reported data through week 16 included for reference.14
*n/N reported for subgroups.
†ACR50 at week 16 was the primary end point of BE COMPLETE.
‡In patients with psoriasis affecting ≥3% BSA at baseline.
§Patients with enthesitis at baseline defined by LEI >0.
¶Patients with enthesitis at baseline defined by SPARCC >0.
**Patients with dactylitis at baseline defined by LDI >0.
††Patients with nail psoriasis at baseline (mNAPSI score >0).
‡‡Patients who had a HAQ-DI decrease from baseline of ≥0.35 in patients with HAQ-DI ≥0.35 at baseline.
§§Data not collected at week 52.
¶¶Patients randomised to PBO at baseline who switched to bimekizumab at week 16.
ACR, American College of Rheumatology; BKZ, bimekizumab; BSA, body surface area; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; LDI, Leeds Dactylitis Index; LEI, Leeds Enthesitis Index; MCID, minimum clinically important difference; MDA, minimal disease activity; MI, multiple imputation; mNAPSI, modified Nail Psoriasis Severity Index; NRI, non-responder imputation; OC, observed case; PASI, Psoriasis Area and Severity Index; PsAID-12, Psoriatic Arthritis Impact of Disease-12; PtAAP, Patient’s Assessment of Arthritis Pain; Q4W, every 4 weeks; SE, standard error; SF-36 PCS, Short-Form 36-item Health Survey Physical Component Summary; SPARCC, Spondyloarthritis Research Consortium of Canada; VLDA, very low disease activity.
The proportion of bimekizumab-randomised patients with ≥3% BSA affected by psoriasis at baseline that achieved complete skin clearance (PASI100; NRI) was sustained from week 16 (58.5%) to week 52 (65.9%; table 2, figure 3C). At week 16, 4.5% of placebo-randomised patients achieved PASI100. After switching to bimekizumab at week 16, and receiving 36 weeks of treatment, 60.2% of these patients achieved PASI100 at week 52 (NRI; table 2, figure 3C). PASI90 was achieved by 74.4% of bimekizumab-randomised patients, and 73.9% placebo/bimekizumab patients at week 52 (table 2, figure 3B). Observed data for patients remaining in the study at week 52 were higher than those using NRI (PASI90: 84.0% bimekizumab, 89.0% placebo/bimekizumab; PASI100: 74.4% bimekizumab, 72.6% placebo/bimekizumab; table 2, figure 3). PASI75 response rates are shown in table 2 and figure 3A.
The proportion of bimekizumab-randomised patients achieving the MDA and VLDA composite measures of disease activity were 44.2% and 13.5%, respectively, at week 16; responses were sustained to week 52 with 47.2% and 23.6% achieving these outcomes (NRI; table 2, figure 4A, B). Of placebo-randomised patients, 6.0% and 2.3% achieved MDA and VLDA at week 16, respectively. At week 52, after switching to bimekizumab, 33.1% and 15.0% achieved MDA and VLDA, respectively (NRI; table 2, figure 4A, B). For the composite ACR50+PASI100 measure, in patients with ≥3% BSA affected by psoriasis at baseline, 33.5% of bimekizumab-randomised patients achieved response at week 16, sustained to week 52 (46.6%). Of the patients randomised to placebo with baseline psoriasis, 1.1% achieved ACR50+PASI100 at week 16, which improved to 34.1% at week 52, following the switch to bimekizumab (NRI; table 2, figure 4C). Additional assessment of disease states, using DAPSA and PASDAS, are shown in online supplemental figure 4 at weeks 16 and 52 (week 40 for PASDAS).
Assessment of other core PsA disease domains, including resolution of enthesitis (LEI; SPARCC), dactylitis (LDI) and nail psoriasis (mNAPSI) showed improvement with bimekizumab treatment at week 16, and were sustained to week 52 (NRI; table 2). At week 52, proportions of patients with resolution of enthesitis were 56.6% (60/106) for LEI and 52.5% (64/122) for SPARCC. 85.3% (29/34) and 67.3% (107/159) had resolution of dactylitis and nail psoriasis, respectively. At week 52, placebo-randomised patients who switched to bimekizumab had resolution of enthesitis (LEI: 58.3% (21/36); SPARCC: 52.9% (27/51)), dactylitis (85.7% (12/14)) and nail psoriasis (61.4% (51/83)) in similar proportions to patients who started on active treatment (NRI; table 2).
Improvements in patient-reported physical functioning, as well as in the key symptoms of pain and fatigue, were also sustained from week 16 to week 52 for patients randomised to bimekizumab. At week 52, 55.0% of patients (with baseline HAQ-DI score ≥0.35) achieved HAQ-DI minimum clinically important difference, while the mean (SE) change from baseline (CfB) in HAQ-DI and PtAAP were −0.39 (0.03) and −32.2 (1.8), respectively. At week 40, the mean (SE) CfB in FACIT-Fatigue was 6.0 (0.6) (MI; table 2). The CfB in PsAID-12 total score at week 16 (−2.2 (0.1)) was sustained to week 40 (CfB: −2.5 (0.1); MI; table 2).
Discussion
BE COMPLETE and its OLE, BE VITAL, demonstrated that bimekizumab was well tolerated in patients with PsA and TNFi-IR up to 52 weeks; the safety profile was consistent with that observed in prior studies.14–16 27 28 Results from the BE COMPLETE study demonstrated that bimekizumab was superior to placebo at improving the signs and symptoms of PsA, across a range of core domains including joints, skin, enthesitis, dactylitis and nails at week 16 in patients with TNFi-IR.14 Here, we show sustained efficacy for bimekizumab-treated patients through to week 52, alongside improvements in efficacy from week 16 to week 52 in patients initially randomised to placebo, following the switch to bimekizumab.
As previously reported for the double-blind period,14 patients randomised to bimekizumab experienced numerically greater TEAEs, serious TEAEs and other AEs of interest than those on placebo; however, overall rates were low and consistent with prior studies.14–16 27 28 During the 52-week period, occurrence of serious TEAEs and discontinuations were low in bimekizumab-treated patients. As expected, given the role of IL-17 in antifungal mucosal immunity,29 dual inhibition of IL-17A and IL-17F with bimekizumab indeed led to an increased incidence of Candida infections, as has been documented previously.14 15 27 28 All Candida infections in bimekizumab-treated patients were of mild or moderate severity and no cases were systemic. In the majority of cases, Candida infections resolved after treatment with either standard topical or oral antifungal treatments and did not lead to treatment or study discontinuation. Recurrent infections were reported in few patients and, of those with recurrent candidiasis, four had a single recurrence and one had four recurrent infections. Additionally, EAIRs of Candida infections were similar between weeks 0–16 and weeks 0–52 for bimekizumab-treated patients, suggesting that incidence did not increase with increasing exposure to bimekizumab.
With the increased incidence of fungal infections reported with bimekizumab across indications, it will be important to monitor and report the occurrence of fungal infections from long-term trials and real-world evidence. Guidance on the identification and management of fungal infections, including across different sites, will be required for clinical decision making.
Patients receiving bimekizumab from week 0 demonstrated sustained improvements in clinical outcomes from week 16 to 52 across core PsA disease domains, including joint and skin outcomes, such as ACR50, PASI100 and resolution of enthesitis, dactylitis and nail psoriasis. Responses to composite outcomes, assessing multiple domains in a single measure, were also sustained to week 52. Furthermore, assessments from the patient perspective, evaluating the key symptoms of pain and fatigue, as well as health-related quality of life were also improved and sustained.
In those patients randomised to placebo, rapid improvements in the core PsA disease domains were observed at week 28, 12 weeks after the switch to bimekizumab, and were sustained to week 52. While improvements in health-related quality of life, including physical function and patient-reported pain and fatigue were observed in the switcher population after up to 36 weeks of bimekizumab treatment, they did not meet the same level of those patients initially randomised to bimekizumab. Compared with bimekizumab-randomised patients, those randomised to placebo were without bDMARD treatment for an additional 16 weeks following an initial washout period of up to 3 months, per the study protocol; therefore, one possible explanation of these results is that the longer washout period may have resulted in worse symptoms, including potential progression of structural changes, by week 16. As such, placebo/bimekizumab patients may not have achieved similar levels of response as bimekizumab with only 36 weeks of treatment. This is demonstrated in the analysis of the components of the ACR response (online supplemental figure 3), where physician-rated outcomes improved to a similar extent in both groups; however, differences were observed in patient-reported outcomes such as pain and physical function. Long-term studies, including 2-year data from the BE VITAL OLE study, will therefore be required to determine whether longer exposure to bimekizumab may lead to greater improvements in these outcomes.
As with the previously reported results to week 16 of BE COMPLETE, the results reported here for patients with PsA and TNFi-IR were of a similar magnitude to those reported in BE OPTIMAL for bDMARD-naïve patients with PsA.14–16 From these findings, it can be seen that bimekizumab treatment from week 0 resulted in clinically meaningful improvements to week 52 in a patient population that typically experiences reduced efficacy with second-line biologic treatments . The results reported are therefore of importance to patients with PsA and TNFi-IR who, traditionally, do not achieve long-term optimal disease control, with alternative therapies displaying lower efficacy and shorter drug survival when compared with bDMARD-naïve patients.7 8
Our current understanding of the evolution of PsA pathobiology is limited. Recent in vitro studies, using healthy donor human peripheral blood mononuclear cells and dermal fibroblasts and synoviocytes from patients with PsA evaluating the role of IL-17F in IL-17-mediated inflammation, suggest a shift over time to a more predominant role for IL-17F in PsA pathogenesis.12 13 As such, concurrent inhibition of both IL-17A and IL-17F may be necessary for sustained efficacy over time.
A limitation of the present study is the lack of a placebo comparator beyond week 16, after which all patients received open-label subcutaneous bimekizumab. Additionally, assessments of radiographic outcomes were not conducted in this population, but have been reported for bDMARD-naïve patients with active PsA treated with bimekizumab in the BE OPTIMAL study.16 Despite this, BE COMPLETE and the BE VITAL OLE assessed efficacy across a broad range of outcomes, including the core domains of PsA, as well as physical function and patient-reported pain and fatigue, which are important to patients and significant to their burden of disease.30 31 The BE VITAL OLE will continue to assess the long-term efficacy and safety of bimekizumab treatment.
A further limitation is that the patient population of this study may not be reflective of the wider, more heterogeneous clinical population in terms of age, skin and musculoskeletal manifestations, as well as comorbidities; therefore, the results presented may differ to those observed in a real-world clinical setting. Additionally, drug survival may be different in the trial population and within a trial setting than that which is seen in real-world clinical practice.
In conclusion, dual inhibition of IL-17A and IL-17F with bimekizumab in patients with active PsA and TNFi-IR resulted in clinically meaningful improvements in efficacy outcomes compared with placebo at week 16, which were sustained to week 52. Bimekizumab was well-tolerated and the safety profile was consistent with that reported previously.
Acknowledgments
The authors thank the patients, the investigators and their teams who took part in this study. The authors also acknowledge Heather Edens, PhD, UCB Pharma, Smyrna, Georgia, USA, for publication coordination and editorial assistance, Nadine Goldammer, PhD, UCB Pharma, Monheim, Germany, for her work as clinical programme delivery lead for the bimekizumab PsA programme and David Morgan, PhD, Costello Medical, Manchester, UK, for medical writing and editorial assistance based on the authors’ input and direction.
Footnotes
Twitter: @drlauracoates
Contributors: Substantial contributions to study conception and design: LCC, RL, IBMcI, PJM, CTR, YT, AA, FB, DDG, LG, A-MO, ABG, RBW, BI, RB, VS, JC, JFM; substantial contributions to analysis and interpretation of the data: LCC, RL, IBMcI, PJM, CTR, YT, AA, FB, DDG, LG, A-MO, ABG, RBW, BI, RB, VS, JC, JFM; drafting the article or revising it critically for important intellectual content: LCC, RL, IBMcI, PJM, CTR, YT, AA, FB, DDG, LG, A-MO, ABG, RBW, BI, RB, VS, JC, JFM; final approval of the version of the article to be published: LCC, RL, IBMcI, PJM, CTR, YT, AA, FB, DDG, LG, A-MO, ABG, RBW, BI, RB, VS, JC, JFM; manuscript guarantor: BI.
Funding: This study was sponsored by UCB Pharma. Support for third-party writing assistance for this article, provided by David Morgan, PhD, Costello Medical, Manchester, UK, was funded by UCB Pharma in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). RBW is supported by the NIHR Manchester Biomedical Centre.
Competing interests: LCC: Grants/research support from AbbVie, Amgen, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer and UCB Pharma; consultant for AbbVie, Amgen, BMS, Boehringer Ingelheim, Celgene, Domain, Eli Lilly, Galapagos, Gilead, Janssen, Moonlake Pharma, Novartis, Pfizer and UCB Pharma; speaking fees from AbbVie, Amgen, Biogen, Celgene, Eli Lilly, Galapagos, Gilead, GSK, Janssen, medac, Novartis, Pfizer and UCB Pharma. Associate Editor of RMD Open. RL: Consultancy fees from AbbVie, AstraZeneca, BMS, Eli Lilly, Novartis, Pfizer and UCB Pharma; research grants from AbbVie, Pfizer, Novartis, and UCB Pharma; owner of Rheumatology Consultancy BV, an AMS company under Dutch law. On the Editorial Board of RMD Open. IBM: Consulting fees and honoraria from AbbVie, AstraZeneca, BMS, Boehringer Ingelheim, Cabaletta, Causeway Therapeutics, Celgene, Eli Lilly, Evelo, Janssen, Moonlake Pharma, Novartis and UCB Pharma; research support from BMS, Boehringer Ingelheim, Celgene, Janssen, Novartis and UCB Pharma. On the Editorial Board of RMD Open. PJM: Research grants from AbbVie, Amgen, BMS, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Sun Pharma and UCB Pharma; consultancy fees from AbbVie, Acelyrin, Aclaris, Amgen, BMS, Boehringer Ingelheim, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Moonlake Pharma, Novartis, Pfizer, Sun Pharma and UCB Pharma; speakers’ bureau for AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer and UCB Pharma. CTR: Research for AbbVie; consultant for AbbVie, Amgen, Eli Lilly, Gilead, Janssen, Novartis, Pfizer and UCB Pharma. YT: Speaking fees and/or honoraria from AbbVie, AstraZeneca, BMS, Boehringer Ingelheim, Chugai, Eisai, Eli Lilly, Gilead, GSK, Pfizer, Taiho and Taisho; received grants from Chugai, Eisai, Mitsubishi-Tanabe and Taisho. On the Editorial Board of RMD Open. AA: Honoraria and/or research grants from AbbVie, Amgen, BMS, Boehringer Ingelheim, Eisai, Eli Lilly, Janssen, Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe Pharma, Novartis, Pfizer, Sun Pharma, Taiho Pharma, Torii Pharmaceutical Co. and UCB Pharma. FB: Consultant and/or speaker and/or investigator for AbbVie, Affibody, Amgen, BMS, Boehringer Ingelheim, Celgene, Chugai, Eli Lilly, Genzyme, GSK, Janssen, MoonLake Pharma, MSD, Novartis, Pfizer, Roche, Sandoz and Sanofi. DDG: Consultant and/or received grant support from AbbVie, Amgen, BMS, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer and UCB Pharma. LG: Grants from AbbVie, Biogen, Eli Lilly, Novartis, Sandoz and UCB Pharma; personal fees from AbbVie, Amgen, BMS, Celltrion, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, Sandoz and UCB Pharma. AMO: Research grants to Johns Hopkins University from AbbVie, Amgen, and Janssen; consulting fees from BMS, Janssen, Sanofi and UCB Pharma. ABG: Received research/educational grants from AnaptypsBio, BMS, Highlights Therapeutics, Janssen, Moonlake Immunotherapeutics AG, Novartis and UCB Pharma, (all paid to Mount Sinai School of Medicine); Received honoraria as an advisory board member and consultant for Amgen, AnaptypsBio, Avotres Therapeutics, BMS, Boehringer Ingelheim, Dice Therapeutics, Eli Lilly, Highlights Therapeutics, Janssen, Novartis, Sanofi, UCB and Xbiotech. RBW: Consulting fees from AbbVie, Almirall, Amgen, Arena, Astellas, Avillion, Biogen, BMS, Boehringer Ingelheim, Celgene, Eli Lilly, GSK, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi and UCB Pharma; research grants to his institution from AbbVie, Almirall, Janssen, LEO Pharma, Novartis and UCB Pharma; honoraria from Astellas, DICE Therapeutics, GSK and Union Therapeutics. BI: Shareholder of AbbVie, GSK and UCB Pharma; employee of UCB Pharma. RB and JC: Employees and shareholders of UCB Pharma. VS: Employee of UCB Pharma. JFM: Consultant and/or investigator for AbbVie, Amgen, Biogen, BMS, Dermavant, Eli Lilly, Janssen, LEO Pharma, Pfizer, Novartis, Regeneron, Sanofi, Sun Pharma and UCB Pharma.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data from this manuscript may be requested by qualified researchers 6 months after product approval in the USA and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymised individual patient data and redacted study documents, which may include raw datasets, analysis-ready datasets, study protocols, blank case report forms, annotated case report forms, statistical analysis plans, dataset specifications and clinical study reports. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password-protected portal.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The BE COMPLETE trial and its OLE study, BE VITAL, were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidance for Good Clinical Practice. Ethics approval was obtained from the relevant institutional review boards at participating sites. All patients provided written informed consent in accordance with local requirements, with additional written informed consent required for enrolment in the BE VITAL OLE study.
References
- 1. Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med (Lond) 2017;17:65–70. 10.7861/clinmedicine.17-1-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet 2018;391:2273–84. 10.1016/S0140-6736(18)30830-4 [DOI] [PubMed] [Google Scholar]
- 3. Salaffi F, Carotti M, Gasparini S, et al. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes 2009;7:25. 10.1186/1477-7525-7-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019;71:5–32. 10.1002/art.40726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coates LC, Soriano ER, Corp N, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol 2022;18:465–79. 10.1038/s41584-022-00798-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fagerli KM, Lie E, van der Heijde D, et al. Switching between TNF inhibitors in psoriatic arthritis: data from the NOR-DMARD study. Ann Rheum Dis 2013;72:1840–4. 10.1136/annrheumdis-2012-203018 [DOI] [PubMed] [Google Scholar]
- 8. Xie Y, Liu Y. Does previous use of tumour necrosis inhibitors change the therapeutic effect of interleukin (IL)-17 or IL-12/23 inhibitors on psoriasis and psoriatic arthritis? Results of a systematic review. Clin Exp Dermatol 2022;47:1627–35. 10.1111/ced.15237 [DOI] [PubMed] [Google Scholar]
- 9. Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol 2018;55:379–90. 10.1007/s12016-018-8702-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sieper J, Poddubnyy D, Miossec P. The IL-23-IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat Rev Rheumatol 2019;15:747–57. 10.1038/s41584-019-0294-7 [DOI] [PubMed] [Google Scholar]
- 11. Torgutalp M, Poddubnyy D. IL-17 inhibition in axial spondyloarthritis: current and future perspectives. Expert Opin Biol Ther 2019;19:631–41. 10.1080/14712598.2019.1605352 [DOI] [PubMed] [Google Scholar]
- 12. Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis 2018;77:523–32. 10.1136/annrheumdis-2017-212127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cole S, Manghera A, Burns L, et al. Differential regulation of IL-17A and IL-17F via STAT5 contributes to psoriatic disease. J Allergy Clin Immunol 2023;152:783–98. 10.1016/j.jaci.2023.03.035 [DOI] [PubMed] [Google Scholar]
- 14. Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet 2023;401:38–48. 10.1016/S0140-6736(22)02303-0 [DOI] [PubMed] [Google Scholar]
- 15. McInnes IB, Asahina A, Coates LC, et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). The Lancet 2023;401:25–37. 10.1016/S0140-6736(22)02302-9 [DOI] [PubMed] [Google Scholar]
- 16. Ritchlin CT, Coates LC, McInnes IB, et al. Bimekizumab treatment in biologic DMARD-naïve patients with active psoriatic arthritis: 52-week efficacy and safety results from the phase III, randomised, placebo-controlled, active reference BE OPTIMAL study. Ann Rheum Dis 2023;82:1404–14. 10.1136/ard-2023-224431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. 10.1002/art.21972 [DOI] [PubMed] [Google Scholar]
- 18. Mease PJ, Antoni CE, Gladman DD, et al. Psoriatic arthritis assessment tools in clinical trials. Ann Rheum Dis 2005;64:ii49–54. 10.1136/ard.2004.034165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fredriksson T, Pettersson U. Severe psoriasis - oral therapy with a new retinoid. Dermatologica 1978;157:238–44. 10.1159/000250839 [DOI] [PubMed] [Google Scholar]
- 20. Fries JF, Spitz P, Kraines RG, et al. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. 10.1002/art.1780230202 [DOI] [PubMed] [Google Scholar]
- 21. Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. 10.1136/ard.2008.102053 [DOI] [PubMed] [Google Scholar]
- 22. Schoels M, Aletaha D, Funovits J, et al. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 2010;69:1441–7. 10.1136/ard.2009.122259 [DOI] [PubMed] [Google Scholar]
- 23. Helliwell PS, Firth J, Ibrahim GH, et al. Development of an assessment tool for Dactylitis in patients with Psoriatic arthritis. J Rheumatol 2005;32:1745–50. Available: https://www.jrheum.org/content/32/9/1745.long [PubMed] [Google Scholar]
- 24. Gossec L, de Wit M, Kiltz U, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis 2014;73:1012–9. 10.1136/annrheumdis-2014-205207 [DOI] [PubMed] [Google Scholar]
- 25. Cella D, Wilson H, Shalhoub H, et al. Content validity and Psychometric evaluation of Functional Assessment of Chronic Illness Therapy-Fatigue in patients with psoriatic arthritis. J Patient Rep Outcomes 2019;3:30. 10.1186/s41687-019-0115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buch MH, Silva-Fernandez L, Carmona L, et al. Development of EULAR recommendations for the reporting of clinical trial extension studies in rheumatology. Ann Rheum Dis 2015;74:963–9. 10.1136/annrheumdis-2013-204948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coates LC, McInnes IB, Merola JF, et al. Safety and efficacy of bimekizumab in patients with active psoriatic arthritis: three-year results from a phase IIb randomized controlled trial and its open-label extension study. Arthritis Rheumatol 2022;74:1959–70. 10.1002/art.42280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ritchlin CT, Kavanaugh A, Merola JF, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet 2020;395:427–40. 10.1016/S0140-6736(19)33161-7 [DOI] [PubMed] [Google Scholar]
- 29. Iwakura Y, Ishigame H, Saijo S, et al. Functional specialization of interleukin-17 family members. Immunity 2011;34:149–62. 10.1016/j.immuni.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 30. Ogdie A, Michaud K, Nowak M, et al. Patient’s experience of psoriatic arthritis: a conceptual model based on qualitative interviews. RMD Open 2020;6:e001321. 10.1136/rmdopen-2020-001321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tillett W, Dures E, Hewlett S, et al. A multicenter nominal group study to rank outcomes important to patients, and their representation in existing composite outcome measures for psoriatic arthritis. J Rheumatol 2017;44:1445–52. 10.3899/jrheum.161459 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003855supp001.pdf (2MB, pdf)
Data Availability Statement
Data are available on reasonable request. Data from this manuscript may be requested by qualified researchers 6 months after product approval in the USA and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymised individual patient data and redacted study documents, which may include raw datasets, analysis-ready datasets, study protocols, blank case report forms, annotated case report forms, statistical analysis plans, dataset specifications and clinical study reports. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password-protected portal.