Abstract
Aim
The aim of this study is to explore the association between red blood cell distribution width–to–albumin ratio (RAR) and the risk of peripheral artery disease (PAD) in patients with diabetes.
Methods
This cross-sectional study extracted the data of 1,125 participants with diabetes from the National Health and Nutrition Examination Survey database. A weighted univariable logistic regression model was used to explore variables associated with PAD. With PAD as the outcome variable, a weighted logistic regression model was established. The odds ratio (OR) and 95% confidence interval (CI) were effect size.
Results
After adjusting for covariates, the risk of PAD in patients with diabetes was observed in those with higher RAR (OR = 1.83; 95% CI: 1.06–3.15). In addition, RAR ≥3.25 was related to increased risk of PAD in patients with diabetes (OR = 2.04; 95% CI: 1.05–3.95). In people with diabetes aged ≥65, RAR was a risk factor for PAD with an OR value of 2.67 (95% CI: 1.30–5.46). RAR ≥3.25 was associated with increased risk of PAD (OR = 3.06; 95% CI: 1.15–8.11) relative to RAR <2.80. In people with diabetes who smoked, the risk of PAD was elevated in those with RAR ≥3.25 (OR = 2.85; 95% CI: 1.28–6.32). As for patients with cardiovascular disease, the risk of PAD was elevated as the increase of RAR (OR = 2.31; 95% CI: 1.05–5.10). RAR ≥3.25 was correlated with increased risk of PAD (OR = 3.75; 95% CI: 1.42–9.87). The area under the curve of RAR for the risk of PAD in patients with diabetes was 0.631 (95% CI: 0.588–0.675).
Conclusion
A higher RAR was related to increased risk of PAD in patients with diabetes. The findings might offer a reference for the management of PAD in patients with diabetes.
Keywords: red cell distribution width and albumin ratio, peripheral artery disease, diabetes, RDW (red cell distribution width), association
Introduction
Peripheral artery disease (PAD) is a direct macrovascular disorder of diabetes with an estimated prevalence at 20%–28% (1, 2). The risk of developing PAD was increased nearly 30% with each 1% increase in hemoglobin A1c (HbA1c) during the follow-up period (3). Patients with diabetes and PAD had a higher risk of lower limb amputation than patients without diabetes, and patients with PAD had a higher risk of cardiovascular disease (CVD) and mortality (4). In patients with diabetes, PAD develops early and progresses rapidly, but usually has no obvious symptoms (5). Identifying the indicators that are closely related to the risk of PAD in patients with diabetes to help identify those with high risk of PAD in these patients is necessary.
Hemogram parameters were widely reported to be associated with the risk of CVDs (6, 7). Previously, red blood cell distribution width (RDW) was reported to be associated with the severity of chronic kidney disease (CKD), macrovascular and microvascular complications, and all-cause mortality in in patients with diabetes (8, 9). In addition, albumin was also a predictor for the progression of CKD in patients with newly diagnosed type 2 diabetes (10). Recently, a new inflammatory indicator, RDW-to-albumin ratio (RAR), which combines RDW and albumin level, has been used to assess the risk of poor prognosis in some CVDs (11, 12). Another study found that, in patients with diabetes, those with higher RAR were correlated with an increased risk of developing retinopathy (13). At present, whether RAR was associated with the risk of PAD in patients with diabetes was still unclear.
This study aimed to explore the association between the RAR and the risk of PAD in patients with diabetes based on the data from the National Health and Nutrition Examination Survey (NHANES). We conducted subgroup analyses to validate the findings across different patient populations, including those stratified by age, smoking status, and the presence of CVDs.
Methods
Study design and population
This cross-sectional study extracted the data of 2,081 patients with diabetes from the NHANES database. Conducted by the Centers for Disease Control and Prevention’s National Center for Health Statistics, the NHANES performed a comprehensive monitoring of the nation’s nutrition and health status through direct physical examinations, clinical and laboratory tests, personal interviews, and related measurement procedures. The examinations are conducted in mobile examination centers that travel to various locations throughout the country, ensuring a standardized environment for the health examinations (14). In our study, diabetes was diagnosed by fasting glucose/HbA1c, physician diagnosis, or those who had anti-diabetic drug. The excluded criteria were 1) <18 years, 2) without measurement of the left or right ankle brachial pressure index (ABPI), and 3) without measurement of RDW or albumin. Finally, 1,125 participants were included.
Potential confounders and definitions
Age (<65 years or ≥65 years), gender (male or female), race (non-Hispanic White, non-Hispanic Black, or others), education [less than 9th grade, 9th to 11th grade (includes 12th grade with no diploma), high school graduate/general equivalent diploma (GED), or equivalent or some college or Associate of Arts (AA) degree/college graduate or above], poverty-to-income ratio (≤1.0, 1.0–2.0, >2.0, or unknown), marriage (never married, married, or others), physical activity [<450 metabolic equivalent of task (MET) × min/week, ≥450 MET × min/week, or unknown], smoking (yes or no), drinking (<once/week, ≥once/week, or no), hypertension (yes or no), dyslipidemia (yes or no), CVD (yes or no), diabetic retinopathy (yes or no), CKD (yes or no), family history of CVD (yes or no), body mass index (BMI) (<25 kg/m2, 25 kg/m2 to 30 kg/m2, or ≥30 kg/m2), waist circumference (cm), energy (kcal), hemoglobin (g/dL), C-reaction protein (mg/dL), anti-platelet drug (yes or no), anti-coagulants drug (yes or no), adrenal cortical steroids (yes or no), and diabetes drug (yes or no).
Physical activity was converted into energy consumption based on the questionnaire in the database. Energy consumption (MET × min) = recommended MET × exercise time of corresponding activity (min), which can be converted into weekly energy consumption, and divided into three categories including <450 MET × min/week, ≥450 MET × min/week, and unknown. BMI was grouped into normal (18.5 kg/m2 to 24.9 kg/m2), underweight (<8.5 kg/m2), overweight (25 kg/m2 to 29.9 kg/m2), and obese (≥30 kg/m2). Because there were only two people in the underweight group, the underweight group was combined with the normal group.
Main and outcome variables
The RAR was the main variable that was calculated on the basis of RAR. The continuous variable and the categorical variable of RAR were used for analysis. As a categorical variable, RAR was divided into <2.80, 2.80–2.98, 2.98–3.25, and ≥3.25 according to quarters. RDW was divided into <12.18%, 12.18%–12.56%, 12.56%–13.09%, and ≥13.09%, whereas albumin was divided into <3.95 g/dL, 3.95 g/dL to 4.19 g/dL, 4.19 g/dL to 4.39 g/dL, and ≥4.39 g/dL according to the respective quarters. PAD was the outcome, which was diagnosed on the basis of the left or right ABPI <0.9 (15).
Statistical analysis
Kolmogorov–Smirnov normality test was used for quantitative data. Normally distributed measurement data were described as mean (standard error) [mean (SE)], independent sample t-test was used for comparison between two groups, and analysis of variance was used for comparison between multiple groups. Non-normally distributed measurement data were described as median and quartiles [M (Q1, Q3)], and Kruskal–Wallis test was used for comparison among groups. The enumeration data were described as the numbers and percentages of cases [n (%)], Chi-square test was used for comparison between groups, and rank sum test was used for rank data. A weighted univariable logistic regression model was used to explore variables associated with PAD. With PAD as the outcome variable, a weighted logistic backward regression model was established. In Model I, no variable was adjusted; in Model II, age and gender were adjusted; and in Model III, age, gender, education, poverty-to-income ratio, smoking status, CVD, CKD, and anti-tuberculosis drug were adjusted. Missing values were manipulated via random forest using python miceforest package for interpolation processing ( Supplementary Table 1 ). Poverty-to-income ratio and physical activity had missing values >5%, and the missing values were classified as the unknown group. Sensitivity analysis was performed to compare the missing data before and after interpolation ( Supplementary Table 2 ). Subgroup analysis was stratified on the basis of age, CVD, and smoking status. The odds ratio (OR) and 95% confidence interval (CI) were effect size. Receiver operator characteristic curves of RAR, RDW, and albumin for the risk of PAD in patients with diabetes were plotted, and the areas under the curve (AUCs) were calculated and compared via Delong test. All statistical tests were conducted by a two-sided test with the test level α = 0.05. Python 3.9 was used for missing value processing, and SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used for model statistical analysis.
Results
Comparisons of the characteristics between the PAD group and the non-PAD group
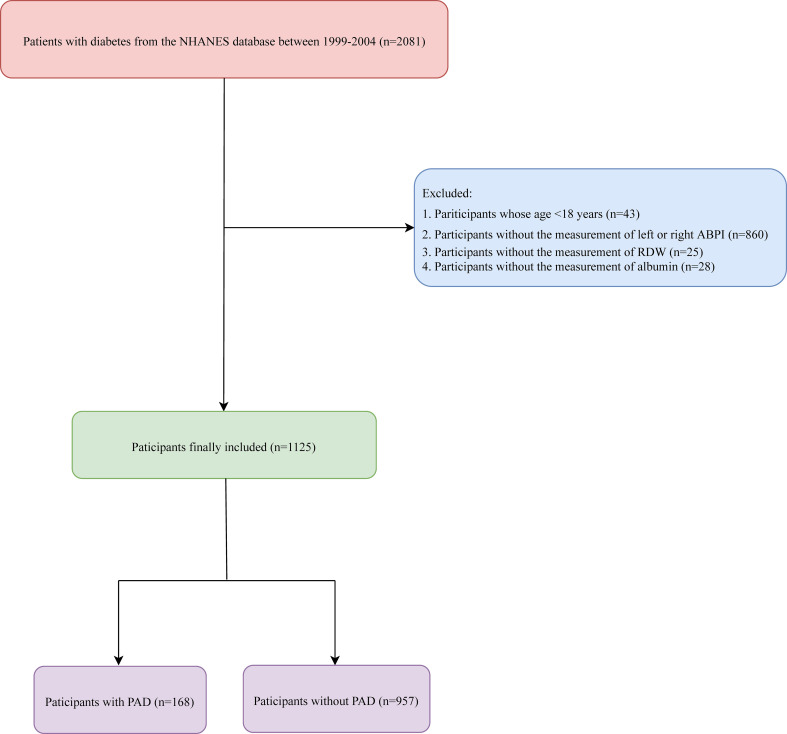
In total, the data of 2,081 patients with diabetes were retrieved from the NHANES database. Among them, participants whose age <18 years (n = 43), patients without measurement of left or right ABPI (n = 860), and those without measurement of RDW (n = 25) or albumin (n = 28) were excluded. Finally, 1,125 participants were included. The screen process is displayed in Figure 1 .
Figure 1.
The screen process of the participants.
The mean RAR in the PAD group was higher than that in the non-PAD group (3.32 vs. 3.04). The percentages of patients with different RAR levels in the PAD group were different compared with that in the non-PAD group. The mean RDW in the PAD group was higher than that in the non-PAD group (13.50% vs. 12.78%). The mean albumin in the PAD group was lower than that in the non-PAD group (4.11 g/dL vs. 4.23 g/dL). The percentages of subjects with CKD in the PAD group was higher than that in the non-PAD group (27.88% vs. 5.07%). More detailed information is presented in Table 1 .
Table 1.
Comparisons of the characteristics between the PAD group and the non-PAD group.
| Variables | Total (n = 1,125) | Non-PAD (n = 957) | PAD (n = 168) | Statistics | P |
|---|---|---|---|---|---|
| RAR, mean (SE) | 3.08 (0.01) | 3.04 (0.01) | 3.32 (0.06) | t = −4.50 | <0.001 |
| RAR groups, n (%) | χ2 = 27.97 | <0.001 | |||
| <2.80 | 246 (24.86) | 226 (26.19) | 20 (14.85) | ||
| 2.80–2.98 | 259 (24.34) | 231 (25.34) | 28 (16.80) | ||
| 2.98–3.25 | 282 (25.67) | 243 (26.19) | 39 (21.78) | ||
| ≥3.25 | 338 (25.12) | 257 (22.29) | 81 (46.56) | ||
| RDW, %, mean (SE) | 12.87 (0.04) | 12.78 (0.03) | 13.50 (0.17) | t = −4.17 | <0.001 |
| Albumin, g/dL, mean (SE) | 4.22 (0.01) | 4.23 (0.01) | 4.11 (0.03) | t = 3.52 | 0.001 |
| Age, years, mean (SE) | 60.23 (0.43) | 59.05 (0.42) | 69.14 (1.12) | t = −9.65 | <0.001 |
| Age, n (%) | χ2 = 37.33 | <0.001 | |||
| <65 | 591 (62.45) | 551 (66.81) | 40 (29.48) | ||
| ≥65 | 534 (37.55) | 406 (33.19) | 128 (70.52) | ||
| Gender, n (%) | χ2 = 0.03 | 0.870 | |||
| Male | 642 (56.89) | 542 (56.98) | 100 (56.21) | ||
| Female | 483 (43.11) | 415 (43.02) | 68 (43.79) | ||
| Race, n (%) | χ2 = 20.30 | <0.001 | |||
| Non-Hispanic White | 462 (66.88) | 385 (66.07) | 77 (73.05) | ||
| Non-Hispanic Black | 248 (12.54) | 194 (11.67) | 54 (19.15) | ||
| Others | 415 (20.57) | 378 (22.27) | 37 (7.80) | ||
| Education, n (%) | χ2 = 17.07 | <0.001 | |||
| Less than 9th grade | 282 (12.34) | 225 (10.70) | 57 (24.72) | ||
| 9th–11th grade (includes 12th grade with no diploma) | 221 (17.87) | 185 (17.81) | 36 (18.32) | ||
| High school graduate/GED or equivalent | 241 (24.99) | 205 (24.76) | 36 (26.72) | ||
| Some college or AA degree/College graduate or above | 381 (44.81) | 342 (46.74) | 39 (30.23) | ||
| Poverty-to-income ratio, n (%) | χ2 = 19.85 | <0.001 | |||
| ≤1.0 | 209 (12.83) | 166 (12.07) | 43 (18.54) | ||
| 1.0–2.0 | 326 (23.89) | 266 (21.88) | 60 (39.10) | ||
| >2.0 | 488 (53.59) | 435 (55.85) | 53 (36.53) | ||
| Unknown | 102 (9.69) | 90 (10.20) | 12 (5.82) | ||
| Marriage, n (%) | χ2 = 5.37 | 0.068 | |||
| Never married | 61 (6.78) | 56 (7.08) | 5 (4.54) | ||
| Married | 692 (64.60) | 600 (65.87) | 92 (55.02) | ||
| Others | 372 (28.61) | 301 (27.05) | 71 (40.44) | ||
| Physical activity, n (%) | χ2 = 15.21 | <0.001 | |||
| <450 MET × min/week | 235 (25.79) | 214 (26.98) | 21 (16.81) | ||
| ≥450 MET × min/week | 253 (24.80) | 223 (25.95) | 30 (16.10) | ||
| Unknown | 637 (49.42) | 520 (47.07) | 117 (67.10) | ||
| Smoking, n (%) | χ2 = 13.48 | <0.001 | |||
| No | 481 (44.12) | 435 (46.64) | 46 (25.13) | ||
| Yes | 644 (55.88) | 522 (53.36) | 122 (74.87) | ||
| Drinking, n (%) | χ2 = 5.14 | 0.077 | |||
| No | 424 (37.22) | 365 (37.91) | 59 (32.01) | ||
| <Once/week | 518 (44.43) | 432 (42.93) | 86 (55.74) | ||
| ≥Once/week | 183 (18.35) | 160 (19.16) | 23 (12.25) | ||
| Hypertension, n (%) | χ2 = 17.41 | <0.001 | |||
| No | 253 (26.32) | 240 (28.80) | 13 (7.57) | ||
| Yes | 872 (73.68) | 717 (71.20) | 155 (92.43) | ||
| Dyslipidemia, n (%) | χ2 = 1.00 | 0.317 | |||
| No | 118 (9.43) | 104 (9.76) | 14 (6.98) | ||
| Yes | 1007 (90.57) | 853 (90.24) | 154 (93.02) | ||
| CVD, n (%) | χ2 = 23.38 | <0.001 | |||
| No | 654 (59.93) | 585 (63.54) | 69 (32.68) | ||
| Yes | 471 (40.07) | 372 (36.46) | 99 (67.32) | ||
| Diabetic retinopathy, n (%) | χ2 = 0.31 | 0.576 | |||
| No | 943 (85.26) | 801 (85.51) | 142 (83.40) | ||
| Yes | 182 (14.74) | 156 (14.49) | 26 (16.60) | ||
| CKD, n (%) | χ2 = 56.06 | <0.001 | |||
| No | 1024 (92.26) | 898 (94.93) | 126 (72.12) | ||
| Yes | 101 (7.74) | 59 (5.07) | 42 (27.88) | ||
| Family history of CVD, n (%) | χ2 = 0.17 | 0.685 | |||
| No | 991 (83.68) | 840 (83.44) | 151 (85.47) | ||
| Yes | 134 (16.32) | 117 (16.56) | 17 (14.53) | ||
| Body mass index, n(%) | χ2 = 4.25 | 0.119 | |||
| <25 kg/m2 | 176 (15.13) | 143 (15.15) | 33 (15.04) | ||
| 25 kg/m2 to 30kg/m2 | 425 (33.74) | 350 (32.59) | 75 (42.45) | ||
| ≥30 kg/m2 | 524 (51.12) | 464 (52.26) | 60 (42.51) | ||
| Waist circumference, cm, mean (SE) | 107.59 (0.76) | 107.64 (0.82) | 107.23 (1.29) | t = 0.30 | 0.769 |
| Energy, kcal, mean (SE) | 1963.35 (38.14) | 1993.42 (39.82) | 1736.32 (126.16) | t = 2.03 | 0.048 |
| Hemoglobin, g/dL, mean (SE) | 14.52 (0.08) | 14.60 (0.08) | 13.94 (0.18) | t = 3.92 | <0.001 |
| C-reaction protein, mg/dL, mean (SE) | 0.61 (0.03) | 0.57 (0.03) | 0.89 (0.21) | t = −1.48 | 0.145 |
| Anti-platelet drug, n (%) | χ2 = 23.71 | <0.001 | |||
| No | 1061 (94.92) | 916 (96.54) | 145 (82.66) | ||
| Yes | 64 (5.08) | 41 (3.46) | 23 (17.34) | ||
| Anti-coagulants drug, n (%) | χ2 = 7.38 | 0.007 | |||
| No | 1088 (96.81) | 932 (97.49) | 156 (91.66) | ||
| Yes | 37 (3.19) | 25 (2.51) | 12 (8.34) | ||
| Adrenal cortical steroids, n (%) | χ2 = 0.02 | 0.888 | |||
| No | 1092 (97.08) | 928 (97.05) | 164 (97.31) | ||
| Yes | 33 (2.92) | 29 (2.95) | 4 (2.69) | ||
| Anti-tuberculosis drug, n (%) | |||||
| No | 1124 (99.98) | 956 (99.98) | 168 (100.00) | ||
| Yes | 1 (0.02) | 1 (0.02) | 0 (0.00) | ||
| Diabetes drug, n (%) | χ2 = 5.24 | 0.022 | |||
| No | 396 (38.21) | 354 (39.75) | 42 (26.60) | ||
| Yes | 729 (61.79) | 603 (60.25) | 126 (73.40) |
PAD, peripheral arterial diseases; SE, standard error; RAR, the red blood cell distribution width–to–albumin ratio; RDW, red blood cell distribution width; CVD, cardiovascular disease; CKD, chronic kidney disease; GED, general equivalent diploma; AA, Associate of Arts.
Association between the RAR and the risk of PAD in patients with diabetes
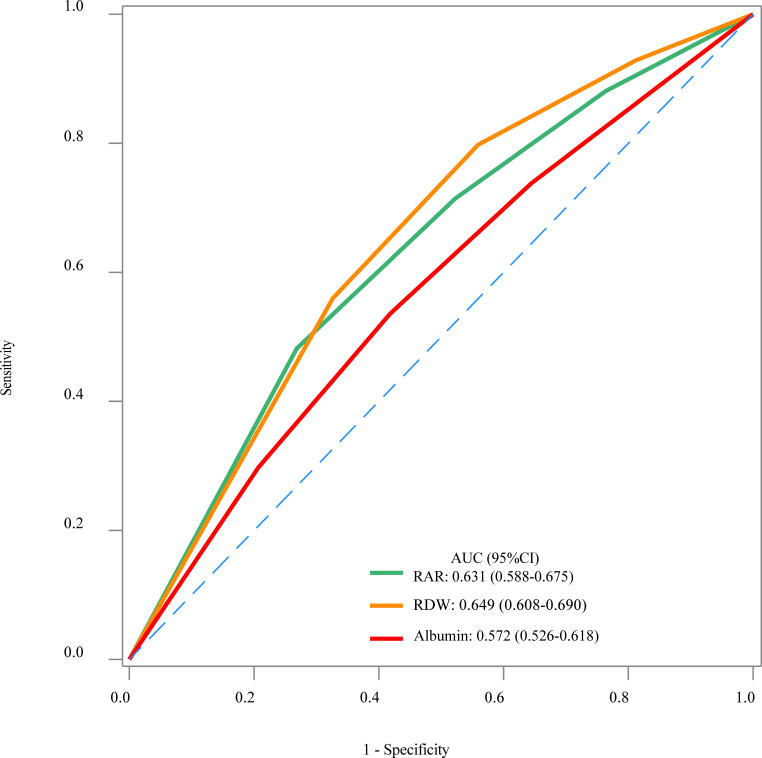
The results of weighted univariable logistic regression model revealed that age (OR = 4.82; 95% CI: 2.91–7.97), race (OR = 0.32; 95% CI: 0.17–0.60), education, poverty-to-income ratio, physical activity (OR = 2.29; 95% CI: 1.38–3.79), smoking status (OR = 2.60; 95% CI: 1.54–4.41), hypertension (OR = 4.94; 95% CI: 2.20–11.07), CVD (OR = 3.59; 95% CI: 2.08–6.21), CKD (OR = 7.23, 95% CI: 4.00–13.09), hemoglobin (OR = 0.76; 95% CI: 0.66–0.88), C-reaction protein (OR = 1.17; 95% CI: 1.04–1.32), anti-platelet drug (OR = 5.85; 95% CI: 2.60–13.15), anti-coagulants drug (OR = 3.54; 95% CI: 1.28–9.79), and diabetes drug (OR = 1.82; 95% CI: 1.06–3.13) were potential covariates associated with the risk of PAD in patients with diabetes ( Supplementary Table 3 ). Backward stepwise regression data indicated that age, gender, education, poverty-to-income ratio, smoking status, CVD, CKD, and anti-tuberculosis drug were covariates. In the crude model, increased RAR might be associated with the elevated risk of PAD in patients with diabetes. Compared with the RAR <2.80 group, RAR ≥3.25 might increase the risk of PAD in patients with diabetes. After adjusting for covariates, the risk of PAD in patients with diabetes was observed in those with higher RAR (OR = 1.83; 95% CI: 1.06–3.15). In addition, RAR ≥3.25 was related to the increased risk of PAD in patients with diabetes (OR = 2.04; 95% CI: 1.05–3.95) ( Table 2 ). No significant association between RDW and the risk of PAD in patients with diabetes was observed (P > 0.005). Moreover, the association between albumin and the risk of PAD in patients with diabetes was not statistically different (P > 0.005) ( Table 2 ). The AUC of RAR (AUC = 0.631; 95% CI: 0.588–0.675) for the risk of PAD in patients with diabetes was higher than that of albumin (AUC = 0.572; 95% CI: 0.526–0.618) ( Figure 2 , Table 3 ). No significant difference was found between the AUC of RAR and that of RDW (AUC = 0.649; 95% CI: 0.608–0.690) for the risk of PAD in patients with diabetes (P > 0.05) ( Figure 2 , Table 3 ).
Table 2.
Association between the RAR and the risk of PAD in patients with diabetes.
| Variables | Model I | Model II | Model III | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| RAR | 3.24 (1.94–5.41) | <0.001 | 2.92 (1.84–4.64) | <0.001 | 1.83 (1.06–3.15) | 0.030 |
| RAR groups | ||||||
| <2.80 | Ref | Ref | Ref | |||
| 2.80–2.98 | 1.17 (0.51–2.70) | 0.708 | 1.06 (0.47–2.42) | 0.880 | 1.03 (0.45–2.35) | 0.942 |
| 2.98–3.25 | 1.47 (0.71–3.03) | 0.293 | 1.24 (0.59–2.63) | 0.565 | 0.93 (0.45–1.94) | 0.848 |
| ≥3.25 | 3.68 (1.87–7.25) | <0.001 | 3.06 (1.52–6.14) | 0.002 | 2.04 (1.05–3.95) | 0.036 |
| RDW | 1.52 (1.22–1.91) | <0.001 | 1.43 (1.18–1.74) | <0.001 | 1.22 (0.99–1.50) | 0.059 |
| RDW | ||||||
| <12.18 | Ref | Ref | Ref | |||
| 12.18–12.56 | 1.43 (0.48–4.31) | 0.514 | 1.18 (0.36–3.85) | 0.777 | 1.00 (0.31–3.22) | 0.995 |
| 12.56–13.09 | 2.03 (0.77–5.30) | 0.146 | 1.61 (0.60–4.35) | 0.340 | 1.42 (0.53–3.80) | 0.475 |
| ≥13.09 | 4.80 (2.03–11.35) | <0.001 | 3.63 (1.48–8.86) | 0.006 | 2.19 (0.91–5.28) | 0.079 |
| Albumin | 0.34 (0.19–0.61) | <0.001 | 0.34 (0.18–0.65) | 0.002 | 0.55 (0.30–1.01) | 0.053 |
| Albumin | ||||||
| <3.95 | Ref | Ref | Ref | |||
| 3.95–4.19 | 0.87 (0.43–1.73) | 0.677 | 0.83 (0.41–1.65) | 0.578 | 1.01 (0.46–2.21) | 0.987 |
| 4.19–4.39 | 0.41 (0.22–0.76) | 0.006 | 0.44 (0.23–0.84) | 0.013 | 0.62 (0.32–1.19) | 0.146 |
| ≥4.39 | 0.47 (0.29–0.77) | 0.004 | 0.48 (0.27–0.84) | 0.012 | 0.65 (0.36–1.19) | 0.161 |
PAD, peripheral arterial diseases; RAR, the red blood cell distribution width–to–albumin ratio; RDW, red blood cell distribution width; OR, odds ratio; CI, confidence interval; Ref, reference; CVD, cardiovascular disease; CKD, chronic kidney disease.
Model I: Weighted univariable logistic regression model.
Model II: Weighted multivariable logistic regression model adjusted for age and gender.
Model III: Weighted multivariable logistic regression model adjusted for age, gender, education, poverty-to-income ratio, smoking status, CVD, CKD, and anti-tuberculosis drug.
Figure 2.
The receiver operator characteristic curves of the RAR, RDW, and albumin for the risk of PAD in patients with diabetes.
Table 3.
The AUC of the RAR for the risk of PAD in patients with diabetes.
| Variables | AUC (95% CI) | Delong test |
|---|---|---|
| RAR | 0.631 (0.588–0.675) | Ref |
| RDW | 0.649 (0.608–0.690) | 0.2937 |
| Albumin | 0.572 (0.526–0.618) | <0.001 |
PAD, peripheral arterial diseases; RAR, the red blood cell distribution width–to–albumin ratio; RDW, red blood cell distribution width; AUC, area under the curve; CI, confidence interval; Ref, reference.
Subgroup analysis of the association between the RAR and the risk of PAD in patients with diabetes
In people with diabetes aged ≥65, RAR was a risk factor for PAD with an OR value of 2.67 (95% CI: 1.30–5.46). RAR ≥3.25 was associated with the increased risk of PAD (OR = 3.06; 95% CI: 1.15–8.11) relative to RAR <2.80. No significant association between the RAR and the risk of PAD was found in patients with diabetes <65 years (P > 0.05). In people with diabetes who smoked, the risk of PAD was elevated in those with RAR ≥3.25 (OR = 2.85; 95% CI: 1.28–6.32). The association between the RAR and the risk of PAD was not statistically different in non-smoking patients with diabetes (P > 0.05). As for patients with CVD, the risk of PAD was elevated as the increase of RAR (OR = 2.31; 95% CI: 1.05–5.10). RAR ≥3.25 was correlated with the increased risk of PAD (OR = 3.75; 95% CI: 1.42–9.87). No association was identified in people with diabetes who are not complicated with CVD (P > 0.05) ( Table 4 ).
Table 4.
Subgroup analysis of the association between the RAR and the risk of PAD in patients with diabetes.
| Variables | n | OR (95% CI) | P | n | OR (95% CI) | P |
|---|---|---|---|---|---|---|
| Subgroup I: Age | Age < 65 (n = 591) | Age ≥ 65 (n = 534) | ||||
| RAR | 1.09 (0.47–2.52) | 0.840 | 2.67 (1.30–5.46) | 0.008 | ||
| RAR groups | ||||||
| <2.80 | 158 | Ref | 88 | Ref | ||
| 2.80–2.98 | 147 | 0.86 (0.19–3.88) | 0.845 | 112 | 1.41 (0.53–3.78) | 0.486 |
| 2.98–3.25 | 131 | 0.59 (0.16–2.14) | 0.416 | 151 | 1.50 (0.57–3.91) | 0.402 |
| ≥3.25 | 155 | 1.64 (0.55–4.85) | 0.366 | 183 | 3.06 (1.15–8.11) | 0.026 |
| Subgroup II: Smoking status | No (n = 481) | Yes (n = 644) | ||||
| RAR | 1.16 (0.56–2.42) | 0.680 | 2.14 (0.93–4.97) | 0.074 | ||
| RAR groups | ||||||
| <2.80 | 100 | Ref | 146 | Ref | ||
| 2.80–2.98 | 115 | 0.44 (0.09–2.08) | 0.293 | 144 | 1.40 (0.50–3.96) | 0.515 |
| 2.98–3.25 | 118 | 1.21 (0.28–5.18) | 0.794 | 164 | 0.91 (0.40–2.08) | 0.816 |
| ≥3.25 | 148 | 1.06 (0.35–3.21) | 0.917 | 190 | 2.85 (1.28–6.32) | 0.011 |
| Subgroup III: CVD | No (n = 654) | Yes (n = 471) | ||||
| RAR | 1.10 (0.42–2.88) | 0.847 | 2.31 (1.05–5.10) | 0.038 | ||
| RAR groups | ||||||
| <2.80 | 172 | Ref | 74 | Ref | ||
| 2.80–2.98 | 171 | 0.71 (0.23–2.16) | 0.539 | 88 | 1.50 (0.59–3.83) | 0.388 |
| 2.98–3.25 | 161 | 0.74 (0.26–2.08) | 0.556 | 121 | 1.24 (0.45–3.44) | 0.675 |
| ≥3.25 | 150 | 0.84 (0.23–3.01) | 0.779 | 188 | 3.75 (1.42–9.87) | 0.009 |
PAD, peripheral arterial diseases; RAR, the red blood cell distribution width–to–albumin ratio; OR, odds ratio; CI, confidence interval; Ref, reference; CVD, cardiovascular disease; CKD, chronic kidney disease.
Subgroup I: Weighted multivariable logistic regression model adjusted for gender, education, poverty-to-income ratio, smoking status, CVD, CKD, and anti-tuberculosis drug.
Subgroup II: Weighted multivariable logistic regression model adjusted for age, gender, education, poverty-to-income ratio, CVD, CKD, and anti-tuberculosis drug.
Subgroup III: Weighted multivariable logistic regression model adjusted for age, gender, education, poverty-to-income ratio, smoking status, CKD, and anti-tuberculosis drug.
Discussion
This study evaluated the association between the RAR and the risk of PAD in patients with diabetes. The results delineated that the increased RAR was correlated with the higher risk of PAD in patients with diabetes. RAR ≥3.25 was related to the increased risk of PAD in patients with diabetes compared with that in the RAR <2.80 group. Subgroup analysis revealed that RAR ≥3.25 was associated with the increased risk of PAD in people with diabetes aged ≥65 years, who smoked, and who are complicated with CVD. The findings might provide a reference for the better management of PAD in diabetes patients.
RDW was a routinely available inflammatory marker that was reported to be an independent prognostic marker in patients with PAD (16). Elevated RDW was found to be a predictor of cardiovascular outcomes in extensive aortoiliac disease (17). Sincer et al. found that patients with inadequate coronary collateral development had significantly higher RDW levels compared to patients with adequate coronary collateral development, and RDW was significantly associated with Rentrop collateral grading (18). Zalawadiya et al. indicated that the higher levels of RDW were independently associated with a higher risk of PAD and had a better predictive value for PAD than those in the American College of Cardiology/American Heart Association–defined PAD screening criteria (19). Albumin was also delineated to be associated with PAD in some studies. The increased adjusted ischemia–modified albumin levels were identified as predictors of the presence and severity of PAD (20). Ding et al. elucidated that serum albumin was associated with the risk of PAD in patients with hypersensitivity (21). As a new combined parameter, RAR was previously found to be associated with diabetes-related complications such as diabetic nephropathy and microvascular complications (22, 23). RAR was also identified to be correlated with all-cause mortality in patients with type 2 diabetes and foot ulcers (24). In the present study, RAR was found to be associated with the risk of PAD in patients with diabetes. The increased risk of PAD was observed in diabetes patients with RAR ≥3.25.
The possible mechanisms for the association of the RAR and the risk of PAD in patients with diabetes might due to the inflammatory response (25–27). Inflammation contributes to higher RDW, promoting red cell apoptosis and erythropoietin resistance and reducing erythropoietin production and bioavailability of iron (28). Oxidative stress induces increased RDW by shortening the life span of erythrocytes and increasing the migration of premature erythrocytes to the peripheral circulation (29). Serum albumin exerts anti‐inflammatory and antioxidant properties, and lower serum albumin was associated with increased risk of inflammation, the main mechanism of impaired vascular function (30). Subgroup analysis showed that RAR ≥3.25 was associated with the increased risk of PAD in people with diabetes aged ≥65 years, who smoked, and who are complicated with CVD. Age was widely accepted to be a risk factor for PAD, and people with older age were associated with the higher risk of PAD (31). Smoking status was reported to be associated with low serum albumin levels, as reported in the previous studies, and was also a risk factor for PAD, which increased oxidative stress and inflammation and induced endothelial dysfunction (32, 33). RAR seems to have the potential to provide a risk stratification in patients with diabetes.
This study evaluated the association between the RAR and the risk of PAD in patients with diabetes using multi-stage complex sampling, and the sample representativeness was good. RDW and albumin are routinely measured as part of the extensively used complete blood counts, and they would not require any additional cost, providing a simple and feasible tool for PAD risk identification in patients with diabetes. Some limitations existed in our study. Firstly, the history of diseases and other data were obtained through questionnaires, which might have recall bias. Secondly, because of the limitation of the NHANES, more detailed treatment information and other possible confounding factors were not included. Thirdly, the measurement of the left or right ABPI used for PAD diagnosis was only performed during 1999–2004; thus, the sample size of PAD was small. Further well‐designed prospective cohort studies with adequate sample size are needed to determine the causal association and to clarify the potential underlying mechanisms of the RAR and the risk of PAD in patients with diabetes.
Conclusions
The current study explores the association between the RAR and the risk of PAD in patients with diabetes and found that a higher RAR was related to the increased risk of PAD in patients with diabetes. The findings might offer a reference for the management of PAD in patients with diabetes.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics statement
The requirement of ethical approval was waived by Nanfang Hospital, Southern Medical University for the studies involving humans because the data was accessed from a publicly available database. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because retrospective nature of the study.
Author contributions
DL: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. JL: Data curation, Formal Analysis, Methodology, Writing – review & editing. JZ: Data curation, Formal Analysis, Methodology, Writing – review & editing. MH: Data curation, Formal Analysis, Methodology, Writing – review & editing. QZ: Data curation, Formal Analysis, Methodology, Writing – review & editing. QH: Data curation, Formal Analysis, Methodology, Writing – review & editing. WZ: Data curation, Formal Analysis, Methodology, Writing – review & editing. YC: Data curation, Formal Analysis, Methodology, Writing – review & editing. MZ: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Natural Science Foundation of China (82170840), Guangdong Basic and Applied Basic Research Foundation (2021A1515220098), and “Outstanding Youth Cultivation Program” of Nanfang Hospital of Southern Medical University (2021J003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1272573/full#supplementary-material
References
- 1. Viigimaa M, Sachinidis A, Toumpourleka M, Koutsampasopoulos K, Alliksoo S, Titma T. Macrovascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol (2020) 18(2):110–6. doi: 10.2174/1570161117666190405165151 [DOI] [PubMed] [Google Scholar]
- 2. Kreutzburg T, Peters F, Rieß HC, Hischke S, Marschall U, Kriston L, et al. Editor’s choice - comorbidity patterns among patients with peripheral arterial occlusive disease in Germany: A trend analysis of health insurance claims data. Eur J Vasc endovascular Surg (2020) 59(1):59–66. doi: 10.1016/j.ejvs.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 3. Achim A, Stanek A, Homorodean C, Spinu M, Onea HL, Lazăr L, et al. Approaches to peripheral artery disease in diabetes: are there any differences? Int J Environ Res Public Health (2022) 19(16). doi: 10.3390/ijerph19169801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stoberock K, Kaschwich M, Nicolay SS, Mahmoud N, Heidemann F, Rieß HC, et al. The interrelationship between diabetes mellitus and peripheral arterial disease. VASA Z fur Gefasskrankheiten (2021) 50(5):323–30. doi: 10.1024/0301-1526/a000925 [DOI] [PubMed] [Google Scholar]
- 5. Yang SL, Zhu LY, Han R, Sun LL, Li JX, Dou JT. Pathophysiology of peripheral arterial disease in diabetes mellitus. J Diabetes (2017) 9(2):133–40. doi: 10.1111/1753-0407.12474 [DOI] [PubMed] [Google Scholar]
- 6. Sincer I, Gunes Y, Mansiroglu AK, Aktas G. Differential value of eosinophil count in acute coronary syndrome among elderly patients. Aging Male (2020) 66(2):160–65. doi: 10.1590/1806-9282.66.2.160 [DOI] [PubMed] [Google Scholar]
- 7. Sincer I, Gunes Y, Mansiroglu AK, Aktas G. Differential value of eosinophil count in acute coronary syndrome among elderly patients. Aging male (2020) 23(5):958–61. doi: 10.1080/13685538.2019.1643310 [DOI] [PubMed] [Google Scholar]
- 8. Gu L, Xue S. The association between red blood cell distribution width andthe severity of diabetic chronic kidney disease. Int J Gen Med (2021) 14:8355–63. doi: 10.2147/ijgm.S332848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mo M, Huang Z, Huo D, Pan L, Xia N, Liao Y, et al. Influence of red blood cell distribution width on all-cause death in critical diabetic patients with acute kidney injury. Diabetes Metab syndrome obesity: Targets Ther (2022) 15:2301–9. doi: 10.2147/dmso.S377650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Y, Ji X, Ni W, Luo Y, Ding B, Ma J, et al. Serum albumin and albuminuria predict the progression of chronic kidney disease in patients with newly diagnosed type 2 diabetes: A retrospective study. PeerJ (2021) 9:e11735. doi: 10.7717/peerj.11735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ni Q, Wang X, Wang J, Chen P. The red blood cell distribution width-albumin ratio: A promising predictor of mortality in heart failure patients - a cohort study. Clinica chimica acta; Int J Clin Chem (2022) 527:38–46. doi: 10.1016/j.cca.2021.12.027 [DOI] [PubMed] [Google Scholar]
- 12. Weng Y, Peng Y, Xu Y, Wang L, Wu B, Xiang H, et al. The ratio of red blood cell distribution width to albumin is correlated with all-cause mortality of patients after percutaneous coronary intervention - a retrospective cohort study. Front Cardiovasc Med (2022) 9:869816. doi: 10.3389/fcvm.2022.869816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao F, Liu M, Kong L. Association between red blood cell distribution width-to-albumin ratio and diabetic retinopathy. J Clin Lab Anal (2022) 36(4):e24351. doi: 10.1002/jcla.24351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paulose-Ram R, Graber JE, Woodwell D, Ahluwalia N. The national health and nutrition examination survey (Nhanes), 2021-2022: adapting data collection in a covid-19 environment. Am J Public Health (2021) 111(12):2149–56. doi: 10.2105/ajph.2021.306517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Firnhaber JM, Powell CS. Lower extremity peripheral artery disease: diagnosis and treatment. Am Family physician (2019) 99(6):362–9. [PubMed] [Google Scholar]
- 16. Ye Z, Smith C, Kullo IJ. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am J Cardiol (2011) 107(8):1241–5. doi: 10.1016/j.amjcard.2010.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vieira-Cardoso N, Pereira-Neves A, Fragão-Marques M, Duarte-Gamas L, Domingues-Monteiro D, Vidoedo J, et al. Red blood cell distribution width as a predictor of cardiovascular outcomes in extensive aortoiliac disease. J Cardiovasc Surg (2023) 64(1):48–57. doi: 10.23736/s0021-9509.22.12210-x [DOI] [PubMed] [Google Scholar]
- 18. Sincer I, Gunes Y, Mansiroglu AK, Cosgun M, Aktas G. Association of mean platelet volume and red blood cell distribution width with coronary collateral development in stable coronary artery disease. Postepy w kardiologii interwencyjnej = Adv interventional Cardiol (2018) 14(3):263–69. doi: 10.5114/aic.2018.78329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zalawadiya SK, Veeranna V, Panaich SS, Afonso L. Red cell distribution width and risk of peripheral artery disease: analysis of national health and nutrition examination survey 1999-2004. Vasc Med (London England) (2012) 17(3):155–63. doi: 10.1177/1358863x12442443 [DOI] [PubMed] [Google Scholar]
- 20. Özsin KK, Engin M, Sanrı US, Toktaş F, Kahraman N, Huysal K, et al. Evaluation of the relationship between adjusted ischemia-modified albumin and the presence and severity of peripheral artery disease. Vascular (2022), 17085381221141473. doi: 10.1177/17085381221141473 [DOI] [PubMed] [Google Scholar]
- 21. Ding C, Wang H, Huang X, Hu L, Shi Y, Li M, et al. Association between serum albumin and peripheral arterial disease in hypertensive patients. J Clin hypertension (Greenwich Conn) (2020) 22(12):2250–7. doi: 10.1111/jch.14071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bilgin S, Kurtkulagi O, Atak Tel BM, Duman TT, Kahveci G, Khalid A, et al. Does C-reactive protein to serum albumin ratio correlate with diabetic nephropathy in patients with type 2 diabetes mellitus? The care time study. Primary Care Diabetes (2021) 15(6):1071–4. doi: 10.1016/j.pcd.2021.08.015 [DOI] [PubMed] [Google Scholar]
- 23. Kocak MZ, Aktas G, Atak BM, Duman TT, Yis OM, Erkus E, et al. Is neuregulin-4 a predictive marker of microvascular complications in type 2 diabetes mellitus? Eur J Clin Invest (2020) 50(3):e13206. doi: 10.1111/eci.13206 [DOI] [PubMed] [Google Scholar]
- 24. Hong J, Hu X, Liu W, Qian X, Jiang F, Xu Z, et al. Impact of red cell distribution width and red cell distribution width/albumin ratio on all-cause mortality in patients with type 2 diabetes and foot ulcers: A retrospective cohort study. Cardiovasc Diabetol (2022) 21(1):91. doi: 10.1186/s12933-022-01534-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Danielsson P, Truedsson L, Eriksson KF, Norgren L. Inflammatory markers and il-6 polymorphism in peripheral arterial disease with and without diabetes mellitus. Vasc Med (London England) (2005) 10(3):191–8. doi: 10.1191/1358863x05vm617oa [DOI] [PubMed] [Google Scholar]
- 26. Semba RD, Patel KV, Ferrucci L, Sun K, Roy CN, Guralnik JM, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the women’s health and aging study I. Clin Nutr (Edinburgh Scotland) (2010) 29(5):600–4. doi: 10.1016/j.clnu.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dialysis (2004) 17(6):432–7. doi: 10.1111/j.0894-0959.2004.17603.x [DOI] [PubMed] [Google Scholar]
- 28. Weiss G, Goodnough LT. Anemia of chronic disease. New Engl J Med (2005) 352(10):1011–23. doi: 10.1056/NEJMra041809 [DOI] [PubMed] [Google Scholar]
- 29. Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxidants Redox Signaling (2008) 10(11):1923–40. doi: 10.1089/ars.2008.2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kadono M, Hasegawa G, Shigeta M, Nakazawa A, Ueda M, Yamazaki M, et al. Serum albumin levels predict vascular dysfunction with paradoxical pathogenesis in healthy individuals. Atherosclerosis (2010) 209(1):266–70. doi: 10.1016/j.atherosclerosis.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 31. Aday AW, Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circ Res (2021) 128(12):1818–32. doi: 10.1161/circresaha.121.318535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shaper AG, Wannamethee SG, Whincup PH. Serum albumin and risk of stroke, coronary heart disease, and mortality: the role of cigarette smoking. J Clin Epidemiol (2004) 57(2):195–202. doi: 10.1016/j.jclinepi.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 33. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol (2004) 43(10):1731–7. doi: 10.1016/j.jacc.2003.12.047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.