Abstract
Background
The development of strategies to better detect and manage patients with multiple long-term conditions requires estimates of the most prevalent condition combinations. However, standard meta-analysis tools are not well suited to synthesising heterogeneous multimorbidity data.
Methods
We developed a statistical model to synthesise data on associations between diseases and nationally representative prevalence estimates and applied the model to South Africa. Published and unpublished data were reviewed, and meta-regression analysis was conducted to assess pairwise associations between 10 conditions: arthritis, asthma, chronic obstructive pulmonary disease (COPD), depression, diabetes, HIV, hypertension, ischaemic heart disease (IHD), stroke and tuberculosis. The national prevalence of each condition in individuals aged 15 and older was then independently estimated, and these estimates were integrated with the ORs from the meta-regressions in a statistical model, to estimate the national prevalence of each condition combination.
Results
The strongest disease associations in South Africa are between COPD and asthma (OR 14.6, 95% CI 10.3 to 19.9), COPD and IHD (OR 9.2, 95% CI 8.3 to 10.2) and IHD and stroke (OR 7.2, 95% CI 5.9 to 8.4). The most prevalent condition combinations in individuals aged 15+ are hypertension and arthritis (7.6%, 95% CI 5.8% to 9.5%), hypertension and diabetes (7.5%, 95% CI 6.4% to 8.6%) and hypertension and HIV (4.8%, 95% CI 3.3% to 6.6%). The average numbers of comorbidities are greatest in the case of COPD (2.3, 95% CI 2.1 to 2.6), stroke (2.1, 95% CI 1.8 to 2.4) and IHD (1.9, 95% CI 1.6 to 2.2).
Conclusion
South Africa has high levels of HIV, hypertension, diabetes and arthritis, by international standards, and these are reflected in the most prevalent condition combinations. However, less prevalent conditions such as COPD, stroke and IHD contribute disproportionately to the multimorbidity burden, with high rates of comorbidity. This modelling approach can be used in other settings to characterise the most important disease combinations and levels of comorbidity.
Keywords: multimorbidity, multiple long-term conditions, comorbidity, non-communicable diseases, South Africa
WHAT IS ALREADY KNOWN ON THIS TOPIC
In most countries, the most commonly occurring sources of multimorbidity include hypertension, diabetes and arthritis.
South Africa has an exceptionally high prevalence of non-communicable diseases (NCDs), compared with other countries in Africa and globally.
In high-income settings, HIV tends to be positively associated with NCDs.
WHAT THIS STUDY ADDS
We present a novel approach to synthesising data on the prevalence of different disease combinations, which can be used to produce estimates with greater generalisability, precision and range than would be possible when relying on a single data source.
The most frequent disease combinations in South Africa are similar to those in high-income settings.
However, in South Africa, HIV is either not associated or negatively associated with NCDs.
The NCDs that are associated with the highest frequency of comorbidity are chronic obstructive pulmonary disease (COPD), stroke and ischaemic heart disease (IHD).
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This novel approach can be applied in other settings.
Standard OR models used in meta-analysis may be inappropriate in the context of multimorbidity research.
Although there has been much focus on HIV and tuberculosis screening in South Africa, there is a greater need to screen for NCDs and mental health.
The need to screen for comorbidities is greatest in patients with COPD, stroke and IHD.
Introduction
Multimorbidity, commonly defined as having multiple long-term conditions, is increasingly recognised as a global challenge. A recent systematic review estimated that 37% of the world’s adult population is experiencing multimorbidity, with prevalence estimates ranging between 28% in Africa and 46% in South America.1 In order to prioritise and reorient health systems, policies and clinical practice guidelines to address this, it is important to have reliable local estimates of the prevalence of different configurations of conditions, both in the population as a whole, and in patients with specific index conditions. This would assist policy-makers in identifying the most common condition combinations on which to focus interventions, and assist healthcare workers in identifying the conditions they most need to screen for and manage, in patients with a given chronic condition.
However, national prevalence levels of different condition combinations are difficult to estimate reliably and consistently. Many studies of multimorbidity are based on medical records2–4 and miss most of the burden of undiagnosed and untreated disease. Many studies are conducted in specific locations,5–7 which are not nationally representative, or are conducted only in older adults.7–10 National household surveys typically rely on self-reported measures of past diagnosis or symptoms8 9 11 and are often underpowered to estimate the prevalence of less frequent condition combinations. Although it would be ideal to synthesise data from multiple sources, in order to improve both precision and generalisability, standard meta-analytical methods are not well suited to this task, given extensive heterogeneity in disease definitions and populations studied,12 and small numbers of studies for certain condition combinations. Simple modelling techniques are required to synthesise these data more effectively.
In low-income and middle-income countries (LMICs), multimorbidity is relatively under-researched.1 12–14 Although Africa has a low reported prevalence of multimorbidity, this may be due in part to underdiagnosis of chronic conditions.1 Further, Africa has a rapidly ageing population15 and faces an associated increase in the burden of non-communicable diseases (NCDs). This is a challenge in the context of weak and verticalised healthcare systems that are ill equipped to deliver chronic care, given a historic focus on communicable diseases and maternal and child health. The burgeoning NCD challenges may be aggravated by HIV, which is thought to increase the risk of several NCDs.16–18
South Africa is an important context in which to assess multimorbidity, as it is an African country with a growing NCD burden and it has one of the highest multimorbidity prevalence levels across LMICs.11 It has the largest population of people living with HIV globally, with an adult HIV prevalence of 18.2%19 compared with 0.6% globally.20 It has an adult diabetes prevalence of 10.2%,21 compared with an average of 4.2% in sub-Saharan Africa.22 Compared with other sub-Saharan African countries, it also has high levels of hypertension23 24 and arthritis,22 25 both of which are linked to high rates of obesity.26 Tuberculosis (TB) prevalence is roughly double the African average,27 28 and although conventionally considered an acute condition, is associated with substantial long-term morbidity (including structural lung disease and chronic obstructive pulmonary disease (COPD)).29–31 South Africa is also an important setting in which to assess novel methods for data synthesis, as it has a large body of multimorbidity data. Previous reviews of South African NCD data have focused on specific conditions (diabetes,32 arthritis,25 stroke and coronary heart disease33) and NCD surveillance,34 but multimorbidity data have only been synthesised narratively.35
The objective of this study is to propose a novel statistical framework for integrating epidemiological evidence from different sources, to produce nationally representative estimates of the prevalence of different condition combinations. We apply the approach to South Africa and discuss how the results may be used to inform public health policy. This study was undertaken as part of a wider effort to reorientate primary healthcare systems,36 37 guidelines38 and health worker training39 to better manage the growing numbers of people living with multiple conditions, ensuring that we address the leading combinations of multimorbidity in our context.
Methods
We followed a three-step process to estimate the prevalence of different condition combinations in South Africa: (1) estimating the strength of association between different conditions, based on meta-analyses of previous South African studies, (2) estimating the prevalence of individual conditions, based on nationally representative data sources and (3) combining the results of steps (1) and (2) using a statistical model that estimated the national prevalence of each condition combination. The analysis was limited to conditions that fulfilled three criteria: they appeared in a recently recommended ‘core list’ of conditions to include in studies of multimorbidity12; there were nationally representative estimates of their prevalence; and there were at least four South African studies/datasets reporting on their association with other conditions. The 10 conditions that satisfied all criteria were arthritis, asthma, COPD, depression, diabetes, HIV, hypertension, ischaemic heart disease (IHD), stroke and TB (online supplemental file 1 table S1.1 provides more detail on the application of the three criteria).
bmjgh-2023-013376supp001.pdf (915.6KB, pdf)
Step 1: estimating the strength of association between conditions
We conducted a review of published South African epidemiological studies investigating multimorbidity in adults. We searched PubMed and Web of Science for articles that included the terms ‘South Africa’ and ‘multimorbidity’. Studies were also identified by examining reference lists of recent systematic reviews in South Africa of multimorbidity,35 diabetes,32 arthritis,25 TB,29 COPD,40 and stroke and coronary heart disease.33 Studies were included if they reported the prevalence of at least two of the ten listed conditions, as well as the proportions of people with any combination of two conditions, in adults or predominantly adult groups. Where multiple studies reported on the same dataset, we avoided double-counting by including only one set of estimates for each condition combination.
We augmented these published data sources with datasets for which it was possible to extract individual-level data on the prevalence of chronic conditions, provided these included at least four of the conditions of interest and did not overlap with the published data. Four datasets were identified:
The Medscheme database: Medscheme is one of the largest private medical scheme administrators in South Africa. Data have previously been analysed to assess, for example, the incidence of diabetes and mental disorders in people living with HIV.41 42 Analysis was limited to active members aged 15 and older in January 2022.
The Western Cape Provincial Health Data Centre (PHDC) database: This is a database of all people who have accessed public health facilities in the Western Cape province. All medical records within the public sector are linked through a single unique patient identifier, including data from disease registers, pharmacy data, laboratory data and other sources.43 Prevalence was calculated in individuals aged 15 and older in January 2022, who had any public health service contact over the last 5 years (2017–2022).
The 2016 Demographic and Health Survey (DHS) data: The survey results are reported elsewhere,44 but prevalence estimates for specific condition combinations have not previously been reported.
The 2003 World Health Survey (WHS): As with the 2016 DHS, overall results have been published,11 45 but no prevalence estimates for specific condition combinations have been published.
The Medscheme and PHDC datasets were analysed cross-sectionally, with the prevalence of each condition being defined in terms of the cumulative incidence of the condition.
For each included study or dataset, an unadjusted OR was calculated to represent the strength of association between each pair of reported conditions, based on 2×2 contingency tables. A random effects meta-analysis was performed to pool the ORs for each pair of conditions. In cases where three or more ORs were estimated for a given pair of conditions, a meta-regression was performed to assess the dependence of the OR on the product of the prevalence of the first condition and the prevalence of the second condition (the product represents the ‘expected prevalence’ of the combination if the conditions occurred independently). This meta-regression was performed because it was anticipated that differences in condition prevalence across studies/datasets would be an important source of heterogeneity in ORs (see online supplemental file 2). In the case of the TB-depression association, no local evidence could be obtained so we relied instead on ORs from a systematic review of studies in LMICs.46 All meta-analyses and meta-regressions were performed using STATA V.17.0 (StataCorp). Secondary analyses were conducted to assess the effect of limiting analysis to studies/data for adults aged 50 and older; due to the smaller number of datasets and greater similarity of ORs in this analysis, pooled ORs were estimated using meta-analysis rather than meta-regression. Further analyses were conducted using Medscheme data, to assess whether ORs changed substantially when controlling for age, and excluding studies of patients attending health facilities, in line with previous reviews.1 47 48
bmjgh-2023-013376supp002.pdf (576.4KB, pdf)
Step 2: estimating the prevalence of individual conditions, at a national level
Nationally representative prevalence estimates for the population aged 15 and older were obtained from a number of sources. For HIV and TB, we used estimates from locally developed mathematical models that had been calibrated to multiple nationally representative datasets (surveys, vital registration data and routine health systems data).19 49 For hypertension and diabetes, we used clinically measured estimates from the 2012 South African National Health and Nutrition Examination Survey (SANHANES).21 Diabetes was defined by a haemoglobin A1c (HbA1c)≥6.5% or a self-report of current diabetes medication, and hypertension was defined by a systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg or self-report of current blood pressure medication.21 Stroke and IHD were defined by self-report (of past stroke or past heart attack/chest pains, respectively), again as measured in the 2012 SANHANES survey.21 Arthritis was defined by self-report of a previous diagnosis, as measured in the 2003 WHS,11 after reweighting using the 2021 South African population age distribution. Asthma was also defined by self-report of a previous diagnosis, as measured in the 2016 DHS.44 COPD was defined by a cough with phlegm of at least 3 months duration, as measured in the 2016 DHS.44 Depression was defined in accordance with the Diagnostic and Statistical Manual for Mental Disorders fourth edition criteria, as measured in the 2002–2004 South African Stress and Health study with the WHO Composite International Diagnostic Interview, that is, based on self-reported symptoms of depression over the last 12 months.50 For the purpose of age-specific secondary analyses, estimates were obtained separately for the 15–49 and the 50 or older populations.
Step 3: estimating the prevalence of pairwise combinations at a national level
If the national prevalence of conditions i and j are and , respectively, and the proportion of the national population with both conditions i and j is , then the OR for the association between conditions i and j is
Because the prevalence levels and are known (from step 2) and the OR is also known (from step 1), the prevalence of the condition combination can be calculated by expressing the above equation as a quadratic in , and solving for 51 :
The prevalence of condition j, in individuals with condition i, is then .
Where meta-regression was performed in step 1, the expected OR was calculated as , where m and c are the slope and constant outputs, respectively, from the meta-regression model. Parametric bootstrapping was performed to calculate 95% CIs. For each bootstrap iteration, values of and were randomly sampled from the uncertainty ranges (assuming normally distributed values), and where the OR was estimated by simple meta-analysis, we similarly sampled from the estimated uncertainty range for . Where the OR was calculated by meta-regression, we instead sampled values of m and c from the estimated uncertainty ranges, assuming a multivariate normal distribution and accounting for the covariance between the two parameters.
The expected number of comorbidities in individuals with condition i was calculated as
The variance of was approximated as the sum of the variances of the terms, as estimated in the bootstrapping step (assuming independence). A log-linear regression model was fitted to assess the association between the expected number of comorbidities (natural log scale) and the prevalence of each condition. All data, STATA code and formulas used in bootstrapping are included in an Excel workbook (online supplemental file 3).
bmjgh-2023-013376supp003.xlsx (381.8KB, xlsx)
Results
In addition to the four data sources from which we extracted individual-level data, we identified 17 South African datasets (reported in 20 studies) from which it was possible to calculate the prevalence of multiple conditions as well as the prevalence of at least one condition combination, giving a total of 21 datasets (table 1, with further details on the search results in online supplemental file 1, section 1). Of the 21 datasets, 7 were from nationally representative household surveys, and 5 were based on patients attending public health facilities. All estimated the prevalence of chronic conditions in 1998 or later. Diabetes and hypertension were the most commonly estimated conditions (each assessed in 16 of the 21 datasets), while arthritis was the least frequently estimated condition (in four datasets). Definitions of conditions varied across studies (online supplemental file 1, table S1.1).
Table 1.
Studies included in meta-analysis
| Study | Population/survey | Years | Chronic conditions included | |||||||||
| Arthritis | Asthma | COPD | Depression | Diabetes | HIV/AIDS | Hypertension | IHD/angina | Stroke | Tuberculosis | |||
| Chang et al, Pengpid and Peltzer7 73 |
Adults aged 40+in Agincourt | 2014–15 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Corbett et al74 | Mineworkers | 2000–01 | ✓ | ✓ | ||||||||
| Ehrlich et al75 76 | People aged 15+ (DHS national survey) | 1998 | ✓ | ✓ | ✓ | |||||||
| Folb et al55 | Adults attending public clinics, Eden district | 2011 | ✓ | ✓ | ✓ | ✓ | ||||||
| Garin et al, Negin et al23 77 | Adults aged 50+ (SAGE national survey) | 2007 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Grimsrud et al50 | Adults aged 18+ (SASH national survey) | 2002–04 | ✓ | ✓ | ||||||||
| Jithoo78 | People aged 40+, Cape Town | 2005 | ✓ | ✓ | ✓ | |||||||
| Lalkhen and Mash79 | People attending public clinics in four provinces | 2010 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Middelkoop et al80 | People aged 15+, Cape Town | 2005, 2008 | ✓ | ✓ | ||||||||
| Oni et al56 | Adults aged 18+, attending public clinics in Cape Town | 2012–13 | ✓ | ✓ | ✓ | ✓ | ||||||
| Pengpid and Peltzer81 | People aged 15+ (NIDS national survey) | 2014–15 | ✓ | ✓ | ✓ | ✓ | ||||||
| Petersen et al82 | Adults aged 18+ attending public clinics, North West province | 2014–15 | ✓ | ✓ | ✓ | ✓ | ||||||
| Sewpaul et al21 | People aged 15+ (SANHANES national survey) | 2012 | ✓ | ✓ | ✓ | ✓ | ||||||
| Sharman and Bachmann83 | People aged 15+, uMkhanyakude district | 2015 | ✓ | ✓ | ✓ | ✓ | ||||||
| van Heerden et al84 | Adults aged 18+, uMgungundlovu district | 2015 | ✓ | ✓ | ✓ | ✓ | ||||||
| Weimann et al52 | People aged 15+ (NIDS national surveys) | 2008, 2012 | ✓ | ✓ | ✓ | ✓ | ||||||
| Wong et al53 | People aged 15+, uMkhanyakude district | 2018–19 | ✓ | ✓ | ✓ | ✓ | ||||||
| Unpublished | Members of Medscheme medical schemes, ages 15+ | 2022 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Unpublished | People aged 15+ attending public health facilities, Western Cape province | 2022 | ✓ | ✓ | ✓ | ✓ | ||||||
| Unpublished | People aged 15+ (DHS national survey) | 2016 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Unpublished | People aged 18+ (WHS national survey) | 2003 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
COPD, chronic obstructive pulmonary disease; DHS, Demographic and Health Survey; IHD, ischaemic heart disease; NIDS, National Income Dynamics Study; SAGE, Study on Global Ageing and Adult Health; SANHANES, South African National Health and Nutrition Examination Survey; SASH, South African Stress and Health; WHS, World Health Survey.
For 38 of the 45 possible condition combinations, there were sufficient data to perform meta-regressions. In 17 of these, there was a significant negative association between the observed OR and the ‘expected prevalence’ under conditions of independence, in line with prior expectation. Full results of the meta-analyses and meta-regressions are summarised in online supplemental file 1, tables S2-45 and figures S1-38.
Estimates of the national prevalence of individual conditions are summarised in table 2. Hypertension is the most highly prevalent condition (33.9%), followed by HIV (18.2%), arthritis (11.3%) and diabetes (10.2%). For all conditions other than HIV and depression, prevalence is higher at ages 50 and older than in the 15–49 age group, with the prevalence of hypertension reaching 70.6% in South African adults aged 50 and older.
Table 2.
National prevalence estimates for individual conditions
| Ages 15+ | Ages 15–49 | Ages 50+ | Source | |
| Arthritis | 11.3% (8.9–13.7) | 5.6% (3.7–7.5) | 28.4% (20.7–36.2) | WHS 200311* |
| Asthma | 3.5% (3.1–4.0) | 2.7% (2.2–3.1) | 5.9% (4.9–6.9) | DHS 201644* |
| COPD | 1.8% (1.5–2.2) | 1.2% (0.8–1.5) | 3.5% (2.7–4.3) | DHS 201644† |
| Depression | 4.9% (3.9–5.9%) | 4.9% (3.8–6.1) | 4.8% (2.6–6.9) | SASH 2002–450† |
| Diabetes | 10.2% (8.9–11.7) | 4.6% (3.6–5.8) | 24.7% (21.6–28.1) | SANHANES 201221‡ |
| HIV/AIDS | 18.2% (17.5–18.6) | 19.2% (18.5–19.7) | 15.2% (14.3–15.9) | Thembisa 202119§ |
| Hypertension | 33.9% (31.8–36.1) | 19.7% (17.7–21.9) | 70.6% (66.8–74.1) | SANHANES 201221‡ |
| IHD/angina | 5.6% (4.5–6.9) | 4.0% (3.0–5.4) | 9.7% (7.4–12.5) | SANHANES 201221* |
| Stroke | 2.6% (2.0–3.5) | 1.0% (0.7–1.6) | 6.7% (4.7–9.4) | SANHANES 201221* |
| Tuberculosis | 0.99% (0.95–1.04) | 0.92% (0.88–0.97) | 1.21% (1.17–1.25) | Thembisa 202149§¶ |
*Self-reported past diagnosis by a healthcare worker.
†Self-reported symptoms suggestive of the condition.
‡Laboratory/clinical diagnosis.
§Mathematical model calibrated to laboratory and survey data.
¶Representing the current prevalence of active tuberculosis, including currently treated and untreated individuals.
COPD, chronic obstructive pulmonary disease; DHS, Demographic and Health Survey; IHD, ischaemic heart disease; SANHANES, South African National Health and Nutrition Examination Survey; SASH, South African Stress and Health Survey; WHS, World Health Survey.
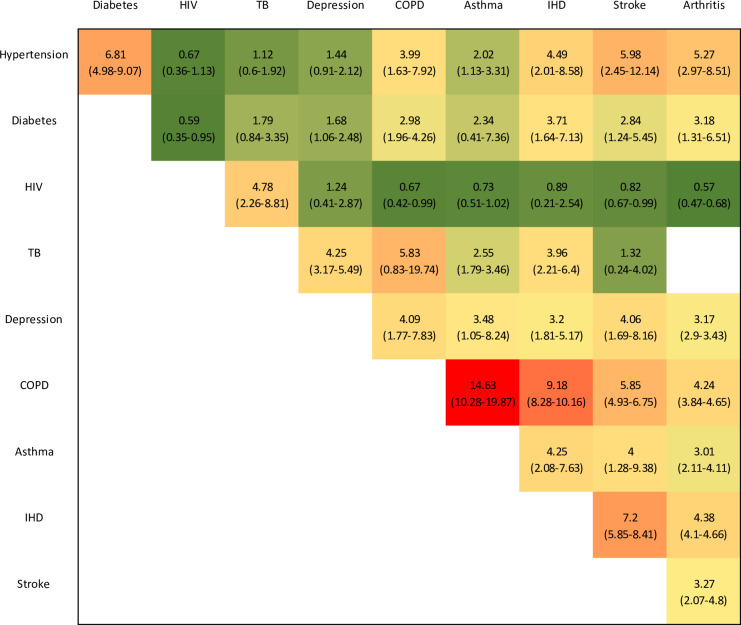
Figure 1 summarises the ORs estimated for each condition combination (where the ORs are estimated by meta-regression, the ORs and CIs are estimated using the bootstrapping procedure described previously, that is, standardising to the national prevalence levels in table 2). The strongest positive associations are estimated in the case of COPD and asthma (OR 14.6, 95% CI 10.3 to 19.9), COPD and IHD (OR 9.2, 95% CI 8.3 to 10.2) and IHD and stroke (OR 7.2, 95% CI 5.9 to 8.4). HIV is negatively associated with most other conditions, the negative associations being most significant for HIV and arthritis (OR 0.57, 95% CI 0.47 to 0.68) and HIV and diabetes (OR 0.59, 95% CI 0.35 to 0.95). In the secondary analysis in which ORs were calculated only for ages 50 and older, these were lower than those in figure 1 for almost all condition combinations (online supplemental file 1, figure S39). Controlling for age brought ORs closer to 1 for almost all condition combinations, although most ORs remained significantly different from 1 even after age adjustment (online supplemental file 1, figure S42). Excluding the five studies of patients attending health facilities led to slight increases in ORs for some disease combinations (notably those involving diabetes and hypertension) but slight decreases for others (particularly arthritis) (online supplemental file 1, figure S44).
Figure 1.
ORs representing strength of association between conditions. ORs range from less than 1 (dark green) to 15 (red). 95% CIs are in brackets. COPD, chronic obstructive pulmonary disease; IHD, ischaemic heart disease; TB, tuberculosis.
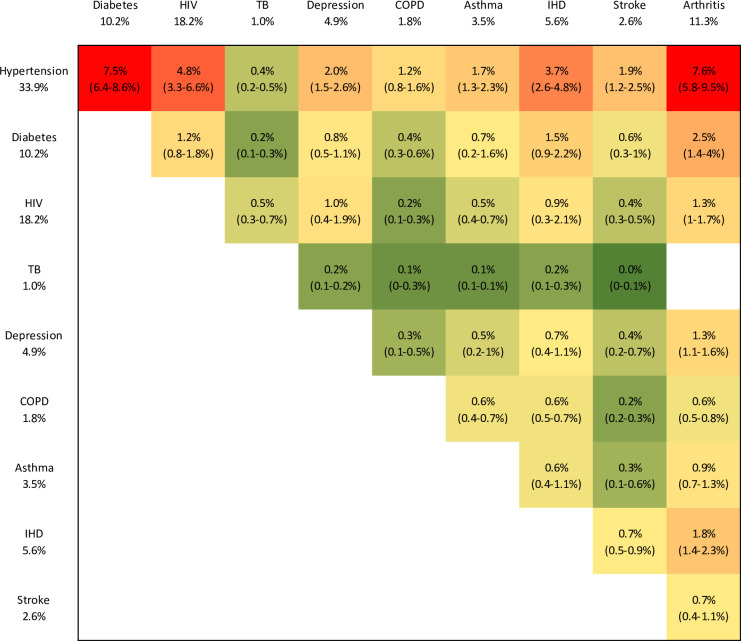
Figure 2 summarises the prevalence of each pairwise combination. The most highly prevalent condition combinations in individuals aged 15+ are hypertension and arthritis (7.6%, 95% CI 5.8% to 9.5%), hypertension and diabetes (7.5%, 95% CI 6.4% to 8.6%), hypertension and HIV (4.8%, 95% CI 3.3% to 6.6%), hypertension and IHD (3.7%, 95% CI 2.6% to 4.8%) and diabetes and arthritis (2.5%, 95% CI 1.4% to 4.0%). In secondary analyses restricted to the 50+ age groups, estimates of prevalence are consistently higher in the 50+ age group, except in the case of the HIV–depression and TB–depression disease combinations (online supplemental file 1, figure S40). The most highly prevalent disease combinations in older adults are again hypertension and arthritis (22.5%, 95% CI 15.2% to 29.7%), and hypertension and diabetes (21.4%, 95% CI 18.5% to 24.2%). Similar results were obtained when excluding studies in patients attending health facilities (online supplemental file 1, figure S45), although the prevalence of the hypertension–arthritis combination decreased and its uncertainty increased substantially (6.5%, 95% CI 2.1% to 10.5%).
Figure 2.
Prevalence of common condition combinations in South Africans aged 15 and older. Prevalence levels range from close to 0% (green) to 8% (red). 95% CIs are in brackets. The prevalence levels for individual conditions (in the row and column headings) are the same as in table 2. COPD, chronic obstructive pulmonary disease; IHD, ischaemic heart disease; TB, tuberculosis.
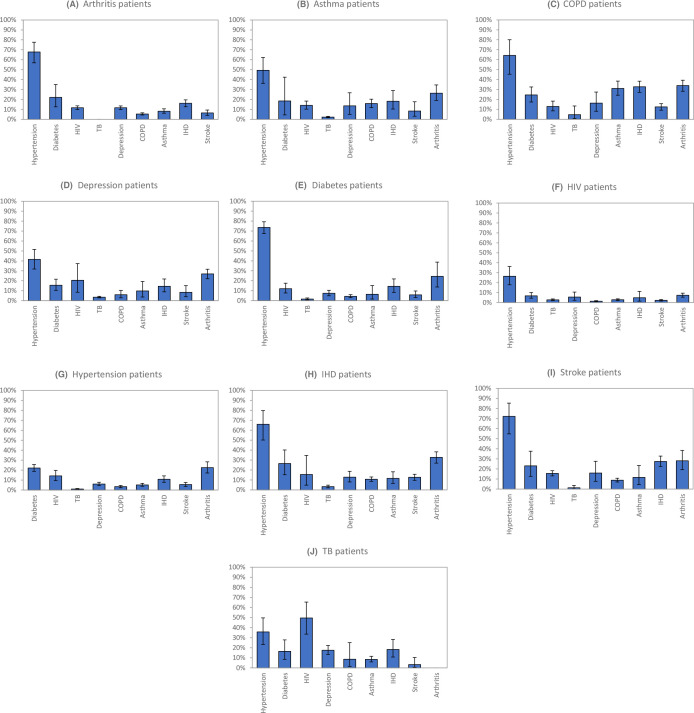
Figure 3 represents the expected prevalence of different comorbidities, for patients with each index condition. Hypertension occurs in the majority of patients with NCDs (the prevalence being as high as 74% in diabetes patients) but is less frequent in HIV and TB patients. Diabetes is particularly common in patients with IHD (26%), COPD (25%) and stroke (23%). The expected prevalence of depression is highest in people with TB (17%), COPD (16%) and stroke (16%). HIV prevalence is high in people with TB (50%) but is relatively less common in other patient groups. Arthritis is particularly common in people with COPD (34%) and IHD (33%). IHD is particularly prevalent in people with COPD (33%) and stroke (28%). Asthma is relatively uncommon, except in patients with COPD (31%), and correspondingly, the prevalence of COPD is only substantial in patients with asthma (16%). Stroke is also relatively infrequent, its prevalence being highest in patients with IHD and COPD (13%). TB is the least frequent comorbidity, its prevalence being highest in patients with COPD (5%), depression (4%) and IHD (3%).
Figure 3.
Expected prevalence of different conditions in patients with each index condition. COPD, chronic obstructive pulmonary disease; IHD, ischaemic heart disease; TB, tuberculosis.
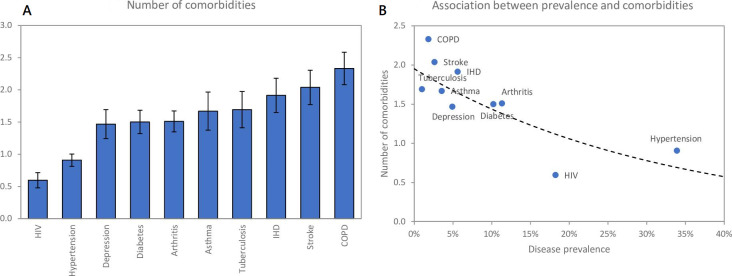
The average number of comorbidities, for patients with each index condition, is represented in figure 4A. People with COPD have the highest average number of comorbidities (2.3, 95% CI 2.1 to 2.6), followed by stroke (2.1, 95% CI 1.8 to 2.4), IHD (1.9, 95% CI 1.6 to 2.2), TB and asthma (both 1.7, 95% CI 1.4 to 2.0). Relatively low numbers of comorbidities are expected in people living with HIV (0.6, 95% CI 0.5 to 0.7) and hypertension (0.9, 95% CI 0.8 to 1.0). The average number of comorbidities is negatively associated with the prevalence of the index condition (r=−0.78), reducing by a factor of 0.74 (95% CI 0.62 to 0.88) for each 10% increase in prevalence (figure 4B).
Figure 4.
Average number of comorbidities in patients with each index condition. COPD, chronic obstructive pulmonary disease; IHD, ischaemic heart disease.
Discussion
By international standards, South Africa has very high levels of HIV, hypertension, diabetes and arthritis, and this is reflected in our estimates of the most frequent disease combinations: hypertension and arthritis, hypertension and diabetes, and hypertension and HIV. However, the strongest associations are between less prevalent conditions (COPD, asthma, IHD and stroke), and there are correspondingly high estimates of numbers of comorbidities in patients with these conditions. These latter conditions, therefore, contribute disproportionately to the multimorbidity burden in South Africa. Our results also show that there is an extremely high prevalence of most condition combinations in older adults (ages 50 and older). We also show that although HIV and TB are strongly associated, HIV is at present largely uncorrelated with the cluster of cardiometabolic and other chronic conditions. The high prevalence of these conditions is widely recognised due to the huge load they place on South African health services, but the high rates of comorbidity are less well recognised, and highlight the need for health services to consider, test for and manage multimorbidity, especially in older adults already receiving long-term care.
Previous studies on multimorbidity in South Africa have focused on characterising the overall prevalence of multimorbidity and the relative frequency of different numbers of conditions, as well as age, sex and socioeconomic differences in the prevalence of multimorbidity.7 52–54 There have been relatively few attempts to characterise the specific condition combinations that contribute most to the multimorbidity burden. Although a number of studies have reported the hypertension–diabetes disease combination to be most prevalent in the South African setting,21 52 55 56 the hypertension–arthritis combination, which we found to be most prevalent, has not previously been identified as common except in international settings.8 47 These findings have important implications for health service design. They suggest a high prevalence of single-condition burdens related to HIV and hypertension, high numbers of comorbidities in people with certain index conditions such as COPD and IHD, and substantial populations of people living with two conditions, some of which are historically recognised (eg, diabetes and hypertension, and TB and HIV35) and others less so (eg, hypertension and arthritis). These burdens require more nuanced service design for people living with chronic conditions: convenience-based fast-tracked services for those with single conditions that are well controlled57; longer consultations58 and greater multidisciplinary involvement59 for index conditions that are inherently multimorbid; and review of clinical considerations for combinations where adverse drug effects limit therapeutic options (eg, non-steroidal anti-inflammatory drugs, commonly used to treat arthritis, increase risk of cardiovascular disease60).
Our approach is novel, as it integrates evidence from multiple epidemiological data sources, including national surveys, smaller studies in specific districts, routine data from health services (both public and private) and outputs from mathematical models. In addition to achieving greater statistical precision (ie, narrower confidence intervals), this enables us to produce more generalisable estimates for a greater range of conditions, which is important given that no single data source covers all of the conditions in which we are interested. Unlike previous studies that have synthesised multimorbidity data,1 47 48 our goal is not to produce an estimate of the prevalence of multimorbidity or to systematically review multimorbidity data, but rather to produce standardised estimates of the prevalence of different condition combinations and levels of comorbidity in patients with different index conditions. Our approach builds on an OR model, which is appropriate for understanding ‘associative multimorbidity’ (ie, understanding which condition combinations are most strongly associated rather than merely most frequent).14 Although this method for estimating the joint prevalence of two conditions has previously been proposed,51 we are not aware of it having been applied in multimorbidity research.
An important insight is that it is insufficient to combine measures of association between two diseases using simple meta-analysis: in almost all cases, meta-regression modelling suggests that the strength of association is negatively related to the prevalence of the respective conditions. This negative relationship emerges due to differences between studies in the relative size of the ‘healthy’ population without any conditions: the larger the proportion of ‘healthy’ individuals in the sample, the more likely it is that different conditions will appear to cluster together (online supplemental file 2). In the extreme case where there are no ‘healthy’ individuals (eg, in studies of people attending health facilities), measures of association may even become negative. The meta-regression approach thus enables us to combine data from different study types, and to standardise the measure of association for a given expected prevalence of each condition.
Although our study includes a more comprehensive list of conditions than most previous studies of multimorbidity in South Africa, there are a number of conditions that it was not possible to include. Hyperlipidaemia, heart failure, dysrhythmias, chronic kidney disease, hypothyroidism, gastro-oesophageal reflux disease, epilepsy and bipolar disorder were all found to be relatively common in the private sector database but were excluded due to a lack of nationally representative prevalence estimates. Anaemia, although highly prevalent in South Africa,7 44 was excluded because it was considered an episodic, curable condition.61 Obesity was excluded as it was considered a risk factor rather than a disease. The high prevalence of obesity in South Africa, especially in women,26 could be important in explaining why South Africa has relatively high levels of hypertension and diabetes, as well as arthritis.62
Our analysis does not consider combinations of three or more diseases. This is a limitation of the OR model. In South Africa, the fraction of multimorbid patients who have three or more conditions has been estimated as being between 15% and 54%7 54 63 (although the higher estimates are from studies that include very common conditions that were not included in our analysis, notably anaemia and hyperlipidaemia). There would thus be value in assessing the relative frequency of these combinations of three or more diseases. However, there are challenges in defining appropriate measures of association and graphically representing three-way frequencies/associations, and an analysis of these three-way disease combinations is beyond the scope of this paper.
Another limitation of this modelling approach is that we do not explicitly adjust for differences across studies in methods used to determine the prevalence of different conditions. However, most studies use similar definitions for each condition (online supplemental file 1, table S1.1), and even where there is substantial variation, this heterogeneity would be reflected in the variance around the ORs predicted by the meta-regression model. In defining the national prevalence of certain conditions (table 2), we have relied on self-reported past diagnosis or self-reported symptoms of the condition, which may be inaccurate. However, South African studies of the prevalence of arthritis and asthma find relatively consistent estimates when comparing these two self-reported measures.44 64 In the case of IHD, the report of past diagnosis (the measure we have used) yields a slightly higher prevalence than self-report based on symptoms.65 It is therefore unlikely that these self-reported measures would substantially understate the true prevalence of the respective conditions.
Our analysis focuses only on crude measures of association between different conditions (ie, unadjusted for likely common risk factors that might explain these associations), as this is a necessary requirement of the statistical model for estimating joint distributions.51 It is therefore important not to interpret the ORs as indicating a causal relationship. The NCDs that we have considered are more prevalent at older ages than at younger ages, and age is therefore an obvious explanation for many of the positive associations between NCDs in figure 1. In contrast, HIV is more prevalent in younger adults, and this might explain the negative association seen between HIV and some NCDs, despite international evidence that HIV may increase the risk of cardiovascular disease.16 However, in our analysis of the Medscheme data, most disease associations remained significant even when controlling for age (online supplemental file 1, figure S42), indicating that age only partially explains the observed associations. Other risk factors, such as obesity and smoking, are also common to many NCDs and may also be important in explaining the observed associations. Most conditions are more prevalent in women than in men, which might partly explain why conditions appear associated in analyses that are not stratified by sex.
Respiratory conditions are particularly strongly associated with other conditions. TB is strongly positively associated with several NCDs, in line with previous reviews of associations with depression,46 diabetes,66 cardiovascular disease,67 asthma and COPD.29 Asthma and COPD are the most strongly associated conditions. In the context of private sector data, this could be partly due to patients registering for both COPD and asthma care in order to access a broader range of drugs (even if they only have one of the conditions). In the context of population-level survey data, the association could be due to survey respondents interpreting asthma and COPD as the same condition, or clinicians misdiagnosing the two conditions. Our estimates for asthma and COPD therefore need to be treated with some caution.
Although South Africa has achieved high levels of treatment coverage for HIV (69% in 202119), treatment coverage for most other chronic conditions is relatively low (eg, 8% for mental illness,68 23% for hypertension69 and 38% for diabetes70). The HIV-TB cluster has been a major focus of guideline development, policies and health systems strengthening, while other conditions have been less of a priority. For example, 95%-95%-95% targets have been set in the case of HIV (for diagnosis, treatment uptake and control, respectively),71 but for diabetes and hypertension the corresponding targets are 90%-60%-50%, and for most other conditions no targets have been set.72 Multimorbidity involving NCDs and mental illness has not received the attention it urgently needs.72
Conclusion
This study estimates a high prevalence of various condition combinations in South Africa. The need to screen for comorbidities is greatest in patients with less common conditions (such as COPD, stroke and IHD), who have relatively more comorbidities. However, for the older population, a more holistic regular screening programme might be appropriate, given the high prevalence of multimorbidity in this group. Our estimates inform interventions that focus on enhanced and integrated screening and management of conditions in primary healthcare.
Acknowledgments
We are grateful to Ronel Sewpaul (Human Sciences Research Council) for sharing unpublished information from the 2012 SANHANES survey, to Steven Dorfman for extracting data from the Medscheme database, and to Alexa Heekes for extracting data from the Western Cape provincial health data centre. We are also grateful to Macro International for providing access to the 2016 DHS data, and to the WHO for providing access to the 2003 WHO World Health Survey.
Footnotes
Handling editor: Seye Abimbola
Twitter: @LeighJohnson9
Collaborators: This study is a collaboration between researchers in high-income and middle-income countries (United Kingdom and South Africa respectively). The conception, conduct and writing of the study were led by researchers based in South Africa.
Contributors: LJ, NSL, MOB and LRF designed the study. NF and SB assisted in accessing and analysing Medscheme data. RK and AB assisted in accessing and analysing the Western Cape Provincial Health Data Centre data. LJ and RK developed the statistical methods. LJ and RAR conducted the literature review. RC, LRF, NSL and KB assisted in the management of the study, and MOB, LJ and RC managed the ethical approval. LJ drafted the first version of the manuscript. All authors contributed to the interpretation of the data and the further drafting of the discussion. All authors have read and approved the final submission. LJ accepts full responsibility for the work and conduct of the study, had access to all the data, and controlled the decision to publish.
Funding: This research was funded by the National Institute for Health and Care Research (NIHR) on Development and evaluation of a targeted, integrated, coherent and people-centred approach to the management of Multiple Long-Term Conditions (MLTC-M) in South African primary healthcare (NIHR 201816) using UK aid from the UK Government to support global health research.
Disclaimer: The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research. However, preliminary results have been shared with patient advocacy groups and stakeholders in the South African Department of Health.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Supplementary data are available online (SupplementaryFile1.pdf includes more detailed results, SupplementaryFile2.pdf explains why ORs may depend on the prevalence of the conditions of interest, and SupplementaryFile3.xlsx contains the study data and key calculations).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The analysis was approved by the University of Cape Town Human Research Ethics Committee (714/2021) and the Kings College London Research Ethics Office (LRS/DP-21/22-26780).
References
- 1.Chowdhury SR, Chandra Das D, Sunna TC, et al. Global and regional prevalence of multimorbidity in the adult population in community settings: a systematic review and meta-analysis. EClinicalMedicine 2023;57:101860. 10.1016/j.eclinm.2023.101860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 3.Cassell A, Edwards D, Harshfield A, et al. The epidemiology of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 2018;68:e245–51. 10.3399/bjgp18X695465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ornstein SM, Nietert PJ, Jenkins RG, et al. The prevalence of chronic diseases and multimorbidity in primary care practice: a PPRNet report. J Am Board Fam Med 2013;26:518–24. 10.3122/jabfm.2013.05.130012 [DOI] [PubMed] [Google Scholar]
- 5.Araujo MEA, Silva MT, Galvao TF, et al. Prevalence and patterns of multimorbidity in Amazon region of Brazil and associated determinants: a cross-sectional study. BMJ Open 2018;8:e023398. 10.1136/bmjopen-2018-023398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gummidi B, Gautam V, John O, et al. Patterns of multimorbidity among a community-based cohort in rural India. J Multimorb Comorb 2023;13:26335565221149623. 10.1177/26335565221149623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang AY, Gómez-Olivé FX, Payne C, et al. Chronic multimorbidity among older adults in rural South Africa. BMJ Glob Health 2019;4:e001386. 10.1136/bmjgh-2018-001386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islam MM, Valderas JM, Yen L, et al. Multimorbidity and comorbidity of chronic diseases among the senior Australians: prevalence and patterns. PLoS One 2014;9:e83783. 10.1371/journal.pone.0083783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Mishra GD, Dobson AJ, et al. Progression of diabetes, heart disease, and stroke multimorbidity in middle-aged women: a 20-year cohort study. PLoS Med 2018;15:e1002516. 10.1371/journal.pmed.1002516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong Y, Qin G, Xi H, et al. Prevalence, patterns of multimorbidity and associations with health care utilization among middle-aged and older people in China. BMC Public Health 2023;23:537. 10.1186/s12889-023-15412-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afshar S, Roderick PJ, Kowal P, et al. Multimorbidity and the inequalities of global ageing: a cross-sectional study of 28 countries using the world health surveys. BMC Public Health 2015;15:776. 10.1186/s12889-015-2008-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho IS-S, Azcoaga-Lorenzo A, Akbari A, et al. Examining variation in the measurement of multimorbidity in research: a systematic review of 566 studies. Lancet Public Health 2021;6:e587–97. 10.1016/S2468-2667(21)00107-9 [DOI] [PubMed] [Google Scholar]
- 13.Academy of Medical Sciences . Multimorbidity: a priority for global health research. 2018. Available: https://acmedsci.ac.uk/file-download/82222577 [Accessed 24 Aug 2019].
- 14.Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, et al. Multimorbidity patterns: a systematic review. J Clin Epidemiol 2014;67:254–66. 10.1016/j.jclinepi.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 15.United Nations Department of Economic and Social Affairs Population Division . Sub-Saharan Africa’s growing population of older persons. 2016. Available: https://www.un.org/en/development/desa/population/publications/pdf/popfacts/PopFacts_2016-1.pdf [Accessed 10 Oct 2022].
- 16.Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation 2019;140:e98–124. 10.1161/CIR.0000000000000695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutierrez J, Albuquerque ALA, Falzon L. HIV infection as vascular risk: a systematic review of the literature and meta-analysis. PLoS One 2017;12:e0176686. 10.1371/journal.pone.0176686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao SG, Galaviz KI, Gay HC, et al. Factors associated with excess myocardial infarction risk in HIV-infected adults: a systematic review and meta-analysis. J Acquir Immune Defic Syndr 2019;81:224–30. 10.1097/QAI.0000000000001996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson LF, Meyer-Rath G, Dorrington RE, et al. The effect of HIV programmes in South Africa on national HIV incidence trends, 2000–2019. J Acquir Immune Defic Syndr 2022;90:115–23. 10.1097/QAI.0000000000002927 [DOI] [PubMed] [Google Scholar]
- 20.UNAIDS . The path that ends AIDS: 2023 global AIDS update. Geneva 2023, Available: https://www.unaids.org/en/resources/documents/2023/global-aids-update-2023 [Accessed 22 Sep 2023].
- 21.Sewpaul R, Mbewu AD, Fagbamigbe AF, et al. Prevalence of multimorbidity of cardiometabolic conditions and associated risk factors in a population-based sample of South Africans: a cross-sectional study. Public Health Pract (Oxf) 2021;2:100193. 10.1016/j.puhip.2021.100193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray CJL. The global burden of disease study at 30 years. Nat Med 2022;28:2019–26. 10.1038/s41591-022-01990-1 [DOI] [PubMed] [Google Scholar]
- 23.Garin N, Koyanagi A, Chatterji S, et al. Global multimorbidity patterns: a cross-sectional, population-based, multi-country study. J Gerontol A Biol Sci Med Sci 2016;71:205–14. 10.1093/gerona/glv128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016;134:441–50. 10.1161/CIRCULATIONAHA.115.018912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Usenbo A, Kramer V, Young T, et al. Prevalence of arthritis in Africa: a systematic review and meta-analysis. PLoS One 2015;10:e0133858. 10.1371/journal.pone.0133858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NCD Risk Factor Collaboration (NCD-RisC) . Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016;387:1377–96. 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moyo S, Ismail F, Van der Walt M, et al. Prevalence of bacteriologically confirmed pulmonary tuberculosis in South Africa, 2017–19: a multistage, cluster-based, cross-sectional survey. Lancet Infect Dis 2022;22:1172–80. 10.1016/S1473-3099(22)00149-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Law I, Floyd K, African TB Prevalence Survey Group . National tuberculosis prevalence surveys in Africa, 2008–2016: an overview of results and lessons learned. Trop Med Int Health 2020;25:1308–27. 10.1111/tmi.13485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrlich RI, Adams S, Baatjies R, et al. Chronic airflow obstruction and respiratory symptoms following tuberculosis: a review of South African studies. Int J Tuberc Lung Dis 2011;15:886–91. 10.5588/ijtld.10.0526 [DOI] [PubMed] [Google Scholar]
- 30.Menzies NA, Quaife M, Allwood BW, et al. Lifetime burden of disease due to incident tuberculosis: a global reappraisal including post-tuberculosis sequelae. Lancet Glob Health 2021;9:e1679–87. 10.1016/S2214-109X(21)00367-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravimohan S, Kornfeld H, Weissman D, et al. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev 2018;27:27.:170077. 10.1183/16000617.0077-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pheiffer C, Pillay-van Wyk V, Turawa E, et al. Prevalence of type 2 diabetes in South Africa: a systematic review and meta-analysis. Int J Environ Res Public Health 2021;18:18.:5868. 10.3390/ijerph18115868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdelatif N, Peer N, Manda SO. National prevalence of coronary heart disease and stroke in South Africa from 1990–2017: a systematic review and meta-analysis. Cardiovasc J Afr 2021;32:156–60. 10.5830/CVJA-2020-045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wandai M, Aagaard-Hansen J, Day C, et al. Data sources for monitoring non-communicable diseases and their risk factors in South Africa. S Afr Med J 2017;107:331–7. 10.7196/SAMJ.2017.v107i4.11438 [DOI] [PubMed] [Google Scholar]
- 35.Roomaney RA, van Wyk B, Turawa EB, et al. Multimorbidity in South Africa: a systematic review of prevalence studies. BMJ Open 2021;11:e048676. 10.1136/bmjopen-2021-048676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Department of Health . Integrated clinical services management 2017. Available: https://knowledgehub.health.gov.za/system/files/elibdownloads/2023-04/Integrated%252520Clinical%252520Services%252520Management%252520%252520Manual%2525205th%252520June%252520FINAL.pdf [Accessed 1 Oct 2023]. [Google Scholar]
- 37.Hunter JR, Chandran TM, Asmall S, et al. The ideal clinic in South Africa: progress and challenges in implementation. In: Padarath A, Barron P, eds. South African Health Review 2017. Durban: Health Systems Trust, 2017: 111–23. [Google Scholar]
- 38.Cornick R, Picken S, Wattrus C, et al. The practical approach to care kit (PACK) guide: developing a clinical decision support tool to simplify, standardise and strengthen primary healthcare delivery. BMJ Glob Health 2018;3(Suppl 5):e000962. 10.1136/bmjgh-2018-000962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simelane ML, Georgeu-Pepper D, Ras C-J, et al. The practical approach to care kit (PACK) training programme: scaling up and sustaining support for health workers to improve primary care. BMJ Glob Health 2018;3(Suppl 5):e001124. 10.1136/bmjgh-2018-001124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adeloye D, Basquill C, Papana A, et al. An estimate of the prevalence of COPD in Africa: a systematic analysis. COPD 2015;12:71–81. 10.3109/15412555.2014.908834 [DOI] [PubMed] [Google Scholar]
- 41.Karamchand S, Leisegang R, Schomaker M, et al. Risk factors for incident diabetes in a cohort taking first-line nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Medicine (Baltimore) 2016;95:e2844. 10.1097/MD.0000000000002844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruffieux Y, Efthimiou O, Van den Heuvel LL, et al. The treatment gap for mental disorders in adults enrolled in HIV treatment programmes in South Africa: a cohort study using linked electronic health records. Epidemiol Psychiatr Sci 2021;30:e37. 10.1017/S2045796021000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boulle A, Heekes A, Tiffin N, et al. Data centre profile: the Provincial Health Data Centre of the Western Cape province, South Africa. Int J Popul Data Sci 2019;4:1143.:1143. 10.23889/ijpds.v4i2.1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Department of health, Statistics South Africa, South African Medical Research Council, ICF. South Africa Demographic and Health Survey Pretoria 2019 2016. Available: https://www.dhsprogram.com/pubs/pdf/FR337/FR337.pdf [Accessed 19 Mar 2019]. [Google Scholar]
- 45.World Health Organization . World Health Survey: Report of South Africa. 2005. Available: https://apps.who.int/healthinfo/systems/surveydata/index.php/catalog/71 [Accessed 20 Jul 2022].
- 46.Hayward SE, Deal A, Rustage K, et al. The relationship between mental health and risk of active tuberculosis: a systematic review. BMJ Open 2022;12:e048945. 10.1136/bmjopen-2021-048945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Padhi A, Wei T, et al. Community prevalence and dyad disease pattern of multimorbidity in China and India: a systematic review. BMJ Glob Health 2022;7:e008880. 10.1136/bmjgh-2022-008880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen H, Manolova G, Daskalopoulou C, et al. Prevalence of multimorbidity in community settings: a systematic review and meta-analysis of observational studies. J Comorb 2019;9:2235042X19870934. 10.1177/2235042X19870934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kubjane M, Osman M, Boulle A, et al. The impact of HIV and tuberculosis interventions on South African adult tuberculosis trends, 1990–2019: a mathematical modelling analysis. Int J Infect Dis 2022;122:811–9. 10.1016/j.ijid.2022.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grimsrud A, Stein DJ, Seedat S, et al. The association between hypertension and depression and anxiety disorders: results from a nationally-representative sample of South African adults. PLoS One 2009;4:e5552. 10.1371/journal.pone.0005552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geenens G. Copula modeling for discrete random vectors. Depend Model 2020;8:417–40. 10.1515/demo-2020-0022 [DOI] [Google Scholar]
- 52.Weimann A, Dai D, Oni T. A cross-sectional and spatial analysis of the prevalence of multimorbidity and its association with socioeconomic disadvantage in South Africa: a comparison between 2008 and 2012. Soc Sci Med 2016;163:144–56. 10.1016/j.socscimed.2016.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong EB, Olivier S, Gunda R, et al. Convergence of infectious and non-communicable disease epidemics in rural South Africa: a cross-sectional, population-based multimorbidity study. Lancet Glob Health 2021;9:e967–76. 10.1016/S2214-109X(21)00176-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roomaney RA, van Wyk B, Cois A, et al. One in five South Africans are multimorbid: an analysis of the 2016 demographic and health survey. PLoS One 2022;17:e0269081. 10.1371/journal.pone.0269081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Folb N, Timmerman V, Levitt NS, et al. Multimorbidity, control and treatment of noncommunicable diseases among primary healthcare attenders in the Western Cape, South Africa. S Afr Med J 2015;105:642–7. 10.7196/samjnew.8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oni T, Youngblood E, Boulle A, et al. Patterns of HIV, TB, and non-communicable disease multi-morbidity in peri-urban South Africa- a cross sectional study. BMC Infect Dis 2015;15:20. 10.1186/s12879-015-0750-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolton Moore C, Pry JM, Mukumbwa-Mwenechanya M, et al. A controlled study to assess the effects of a fast track (FT) service delivery model among stable HIV patients in Lusaka Zambia. PLOS Glob Public Health 2022;2:e0000108. 10.1371/journal.pgph.0000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mercer SW, Zhou Y, Humphris GM, et al. Multimorbidity and socioeconomic deprivation in primary care consultations. Ann Fam Med 2018;16:127–31. 10.1370/afm.2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shakib S, Dundon BK, Maddison J, et al. Effect of a multidisciplinary outpatient model of care on health outcomes in older patients with multimorbidity: a retrospective case control study. PLoS One 2016;11:e0161382. 10.1371/journal.pone.0161382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varga Z, Sabzwari SRA, Vargova V. Cardiovascular risk of nonsteroidal anti-inflammatory drugs: an under-recognized public health issue. Cureus 2017;9:e1144. 10.7759/cureus.1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller JL. Iron deficiency anemia: a common and curable disease. Cold Spring Harb Perspect Med 2013;3:a011866. 10.1101/cshperspect.a011866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bliddal H, Leeds AR, Christensen R. Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons - a scoping review. Obes Rev 2014;15:578–86. 10.1111/obr.12173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roomaney RA, van Wyk B, Cois A, et al. Multimorbidity patterns in a national HIV survey of South African youth and adults. Front Public Health 2022;10:862993. 10.3389/fpubh.2022.862993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arokiasamy P, Kowal P, Capistrant BD, et al. Chronic Noncommunicable diseases in 6 Low- and middle-income countries: findings from wave 1 of the World Health Organization’s Study on Global Ageing and Adult Health (SAGE). Am J Epidemiol 2017;185:414–28. 10.1093/aje/kww125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vellakkal S, Millett C, Basu S, et al. Are estimates of socioeconomic inequalities in chronic disease artefactually narrowed by self-reported measures of prevalence in low-income and middle-income countries? Findings from the WHO-SAGE survey. J Epidemiol Community Health 2015;69:218–25. 10.1136/jech-2014-204621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Rifai RH, Pearson F, Critchley JA, et al. Association between diabetes mellitus and active tuberculosis: a systematic review and meta-analysis. PLoS One 2017;12:e0187967. 10.1371/journal.pone.0187967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huaman MA, Henson D, Ticona E, et al. Tuberculosis and cardiovascular disease: linking the epidemics. Trop Dis Travel Med Vaccines 2015;1:10. 10.1186/s40794-015-0014-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Docrat S, Besada D, Cleary S, et al. Mental health system costs, resources and constraints in South Africa: a national survey. Health Policy Plan 2019;34:706–19. 10.1093/heapol/czz085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berry KM, Parker WA, Mchiza ZJ, et al. Quantifying unmet need for hypertension care in South Africa through a care cascade: evidence from the SANHANES, 2011-2012. BMJ Glob Health 2017;2:e000348. 10.1136/bmjgh-2017-000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stokes A, Berry KM, Mchiza Z, et al. Prevalence and unmet need for diabetes care across the care continuum in a national sample of South African adults: evidence from the SANHANES-1, 2011-2012. PLoS One 2017;12:e0184264. 10.1371/journal.pone.0184264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.UNAIDS . World AIDS day report 2020: prevailing against pandemics by putting people at the centre. 2020. Available: https://aidstargets2025.unaids.org/ [Accessed 2 Dec 2020].
- 72.Department of Health . National strategic plan for the prevention and control of non-communicable diseases 2022-2027. 2022. Available: https://bhekisisa.org/wp-content/uploads/2022/06/NCDs-NSP-SA-2022-2027-1.pdf [Accessed 20 Sep 2022].
- 73.Pengpid S, Peltzer K. Mental morbidity and its associations with socio-behavioural factors and chronic conditions in rural middle- and older-aged adults in South Africa. J Psychol Afr 2020;30:257–63. 10.1080/14330237.2020.1767956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corbett EL, Charalambous S, Moloi VM, et al. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am J Respir Crit Care Med 2004;170:673–9. 10.1164/rccm.200405-590OC [DOI] [PubMed] [Google Scholar]
- 75.Ehrlich RI, White N, Norman R, et al. Wheeze, asthma diagnosis and medication use: a national adult survey in a developing country. Thorax 2005;60:895–901. 10.1136/thx.2004.030932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ehrlich RI, White N, Norman R, et al. Predictors of chronic bronchitis in South African adults. Int J Tuberc Lung Dis 2004;8:369–76. [PubMed] [Google Scholar]
- 77.Negin J, Martiniuk A, Cumming RG, et al. Prevalence of HIV and chronic comorbidities among older adults. AIDS 2012;26 Suppl 1:S55–63. 10.1097/QAD.0b013e3283558459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jithoo A. Respiratory symptoms and chronic obstructive pulmonary disease: prevalence and risk factors in a predominantly low-income urban area of Cape Town, South Africa. Cape Town: University of Cape Town, 2006. [Google Scholar]
- 79.Lalkhen H, Mash R. Multimorbidity in non-communicable diseases in South African primary healthcare. S Afr Med J 2015;105:134–8. 10.7196/samj.8696 [DOI] [PubMed] [Google Scholar]
- 80.Middelkoop K, Bekker L-G, Myer L, et al. Antiretroviral program associated with reduction in untreated prevalent tuberculosis in a South African Township. Am J Respir Crit Care Med 2010;182:1080–5. 10.1164/rccm.201004-0598OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pengpid S, Peltzer K. Depression symptoms: their association with socio-demographic factors and health among adults in South Africa. Journal of Psychology in Africa 2018;28:62–5. 10.1080/14330237.2017.1375212 [DOI] [Google Scholar]
- 82.Petersen I, Rathod S, Kathree T, et al. Risk correlates for physical-mental Multimorbidities in South Africa: a cross-sectional study. Epidemiol Psychiatr Sci 2019;28:418–26. 10.1017/S2045796017000737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharman M, Bachmann M. Prevalence and health effects of communicable and non-communicable disease Comorbidity in rural Kwazulu-natal, South Africa. Trop Med Int Health 2019;24:1198–207. 10.1111/tmi.13297 [DOI] [PubMed] [Google Scholar]
- 84.van Heerden A, Barnabas RV, Norris SA, et al. High prevalence of HIV and non-communicable disease (NCD) risk factors in rural Kwazulu-natal, South Africa. J Int AIDS Soc 2017;20:20. 10.1002/jia2.25012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2023-013376supp001.pdf (915.6KB, pdf)
bmjgh-2023-013376supp002.pdf (576.4KB, pdf)
bmjgh-2023-013376supp003.xlsx (381.8KB, xlsx)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Supplementary data are available online (SupplementaryFile1.pdf includes more detailed results, SupplementaryFile2.pdf explains why ORs may depend on the prevalence of the conditions of interest, and SupplementaryFile3.xlsx contains the study data and key calculations).