Abstract
Background
The incidence of heart failure, the terminal stage of several cardiovascular diseases, is increasing owing to population growth and aging. Bidirectional crosstalk between the gut and heart plays a significant role in heart failure. This study aimed to analyze the gut-heart axis and heart failure from a bibliometric perspective.
Methods
We extracted literature regarding the gut-heart axis and heart failure from the Web of Science Core Collection database (January 1, 1993, to June 30, 2023) and conducted bibliometric and visualization analyses using Microsoft Excel, CiteSpace, VOSviewer, and the R package “bibliometrix.”
Results
The final analysis included 1646 articles with an average of 35.38 citations per article. Despite some fluctuations, the number of articles published per year has steadily increased over the past 31 years, particularly since 2018. A total of 9412 authors from 2287 institutions in 86 countries have contributed to this field. The USA and China have been the most productive countries, with the Cleveland Clinic in the USA and Charité-Universitätsmedizin Berlin in Germany being the most active institutions. The cooperation between countries/regions and institutions was relatively close. Professor Tang WHW was the most productive author in the field and the journal Shocks published the highest number of articles. "Heart failure," "gut microbiota," "trimethylamine N-oxide," and "inflammation" were the most common keywords, representing the current research hotspots. The keyword burst analysis indicated that "gut microbiota" and "short-chain fatty acids" are the current frontier research topics in this field.
Conclusion
Research on the gut-heart axis and heart failure is increasing. This bibliometric analysis indicated that the mechanisms associated with the gut-heart axis and heart failure, particularly the gut microbiota, trimethylamine N-oxide, inflammation, and short-chain fatty acids, will become hotspots and emerging trends in research in this field. These findings provide valuable insights into current research and future directions.
Keywords: Heart failure, Gut-heart axis, Gut microbiota, Bibliometric analysis, Trimethylamine N-Oxide, Inflammation, Short-chain fatty acids
1. Introduction
Heart failure (HF) is a multifaceted chronic and progressive condition in which the myocardium fails to pump sufficient blood to fulfill the body's demands for oxygen and nutrients; it represents the terminal stage of many cardiovascular diseases (CVDs) [1]. The global incidence of HF exceeds 64 million individuals [2,3], and this number is expected to increase owing to population growth, aging populations, and increased survival rates following diagnosis [4]. This large and increasing healthcare burden poses significant societal challenges [4].
The gut microbiota (GM), which consists of trillions of bacteria in the gastrointestinal tract, plays essential roles in the regulation of host immunity and maintenance of metabolic homeostasis [5] and is closely associated with human health [6]. Dysbiosis, characterized by imbalances in the composition and function of the GM, has been implicated in the pathogenesis and progression of a broad range of conditions, including gastrointestinal, inflammatory, and metabolic diseases, and CVDs [7]. Growing evidence indicates a correlation between the GM and HF [8]. This correlation refers to bidirectional crosstalk between the heart, gut, and GM known as the “gut-heart axis” [9]. In HF, this primarily refers to the hemodynamic changes caused by HF, including reduced perfusion and tissue hypoxia, triggering intestinal mucosal barrier dysfunction, leading to increased intestinal permeability. Consequently, bacteria, endotoxins, and metabolic byproducts translocate from the gut into the systemic circulation, triggering systemic inflammation [10]. Furthermore, HF is associated with GM dysbiosis and potentially the abnormal production of gut microbe-derived metabolites [9]. This imbalance in gut microbe-derived metabolites combined with gut epithelial dysfunction contributes to cardiac dysfunction, inflammation, malnutrition, and other health issues in patients with HF [9]. Owing to these effects, GM profiles may serve as potential biomarkers in patients with HF [11].
Current research in this field relies heavily on manual searches and personal experience, which lack a comprehensive and holistic approach. Bibliometrics, a literature analysis method that employs mathematical and statistical techniques, provides quantitative and qualitative evaluations of publications within a specific research field [12]. This method compares contributions from different countries/regions, institutions, authors, journals, references, and keywords, thereby identifying scientific output, research hotspots, and developing trends within a particular field [13].
In recent years, extensive research has been conducted on the gut-heart axis and HF [[14], [15], [16], [17]], suggesting innovative diagnostic and therapeutic approaches for HF [9,18]. A thorough understanding of the current research trends in this field is therefore crucial. This study presents a comprehensive exploration of the gut-heart axis in HF research from a bibliometric perspective, offering insights into the current state, future development trends, and prospects in this field.
2. Materials and methods
2.1. Data sources and search strategy
This bibliometric and visual analysis was based on a comprehensive search of the Science Citation Index Expanded and Social Sciences Citation Index databases in the Web of Science Core Collection (WoSCC) database (https://www.webofscience.com/wos/woscc/basic-search), which are the most widely used and suitable sources for bibliometric analyses [19,20], for publications related to the gut-heart axis and HF published from January 1, 1993, to June 30, 2023. Only publications defined as “articles” or “reviews” were included in the analysis; other publications such as meeting abstracts, proceedings papers, or book chapter were excluded. Publications that had not been formally published, such as early access papers, were also excluded.
In accordance with previous studies [12], we used the key terms recommended previously for the gut-heart axis and HF, such as “microbiome,” “gut,” “gut-heart axis,” and “heart failure” as search terms. The detailed search terms were set as follows: [TS = (microbiome OR microbiota OR flora OR intestinal OR dysbiosis)] AND [TS = (gut OR gastrointestinal OR intestinal OR gut-heart axis OR heart-gut axis)] AND [TS = (heart failure OR cardiac failure)] AND [LA = (English)]. The type of publication was set to “articles” and “reviews.” Bibliometric data was exported as "plain text file" documents with the record content set to “Full Record and Cited References.”
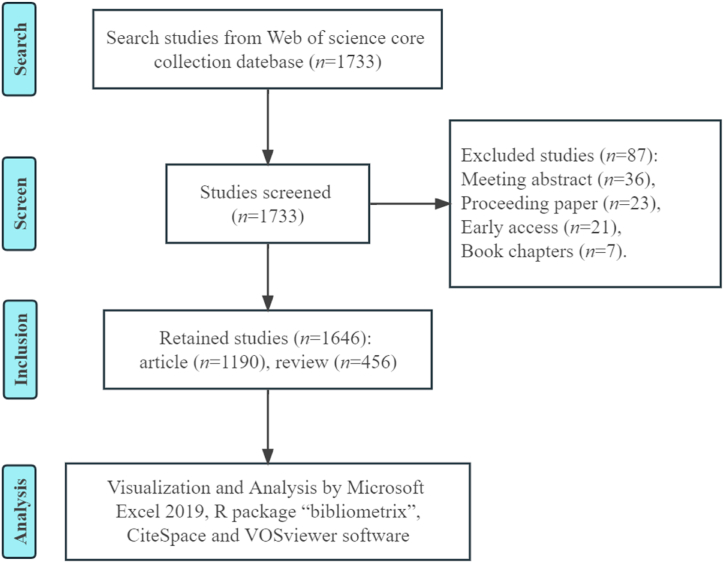
A total of 1733 records were retrieved; 87 publications were excluded due to document type or non-publication, including 36 conference abstracts, 23 proceedings papers, 21 early access papers, and 7 book chapters. Ultimately, 1646 retrieved records were analyzed. The detailed literature screening process is shown in Fig. 1.
Fig. 1.
Flowchart of the literature screening process.
2.2. Data analysis
Information from each publication, including publication year, total number of citations, country/region, institution, journal, journal impact factor (IF), authors, references, and keywords, were extracted from the WoSCC database. Microsoft Office Excel 2019, the “bibliometrix” package in R software [21], VOSviewer software version 1.6.18 [22], and CiteSpace software version 6.2. R2 [23] were used for the bibliometric and visual analyses.
Microsoft Office Excel 2019 was used to visualize the annual number of publications (NP) and number of citations (NC). A global distribution network of publications on the gut-heart axis and HF was constructed in the R package “bibliometrix” (https://www.bibliometrix.org).
VOSviewer software is widely used for bibliometric analysis and scientific mapping. It allows the visualization of collaborative relationships, co-occurrences between countries/regions, institutions, journals, co-cited journals, authors, and co-cited authors, as well as research topics in the field of the gut-heart axis and HF. A node on a map represents a country/region, institution, journal, author, or keyword and its size is positively correlated with the NP or keyword occurrences. The line thickness between nodes indicates the strength of associations, with broader lines indicating tighter cooperation; this can also be described in terms of total link strength (TLS). Different colors in the network visualization represent different clusters. In the overlay visualization, the colors are sorted from blue to yellow by the average publication year (APY).
CiteSpace is another bibliometric analysis and visualization software, developed by Professor Chaomei Chen. In our study, CiteSpace software was used to perform citation burst analysis of references and keywords. The intensity value reflects the cited frequency, and the red bar displays the years of burst.
3. Results
3.1. Publication outputs and temporal trends
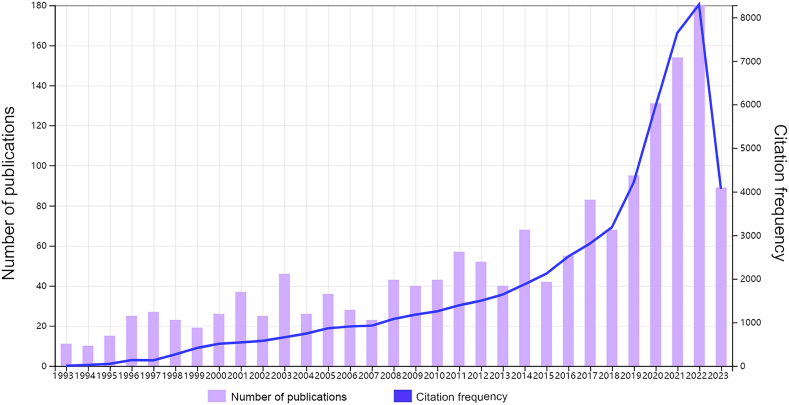
Annual publications provide an overview of developments in a particular field. From January 1993 to June 2023, a total of 1646 studies were published, including 1190 “articles” and 456 “reviews.” These studies received 58234 citations, with an average of 35.38 citations per study. Fig. 2 displays the general upward trend in publications on the gut-heart axis and HF; annual publications showed a fluctuating increase until 2018, followed by a rapid increase. Fig. 2 also indicates an increasing trend in citation frequency on the gut-heart axis and HF, again particularly since 2018. This demonstrates the growing number of scholars conducting research in this field.
Fig. 2.
Temporal distribution map of publications and citations. The number of annual publications and citations for the period from 1993 to 2023 are presented.
3.2. Country/region and institutional analysis
The 1646 publications originated from 2287 institutions across 86 countries/regions. The ten most productive countries/regions and institutions are listed in Table 1. The USA (NP: 511, 31.04%) and China (NP: 264, 16.04%) were the most productive countries, with the combined number of publications from the USA and China accounting for almost half of the total (47.08%), indicating their research strength and influence in the field of gut-heart axis and HF-related research. These countries were followed by Germany (NP: 141, 8.57%), Italy (NP = 122, 7.41%), Japan (NP: 111, 6.74%), and England (NP: 110, 6.68%).
Table 1.
Publications in the 10 most productive countries/regions.
| Rank | Country/Region | NP | Proportion | NC | TLS |
|---|---|---|---|---|---|
| 1 | USA | 511 | 31.04% | 27295 | 261 |
| 2 | China | 264 | 16.04% | 6744 | 80 |
| 3 | Germany | 141 | 8.57% | 6116 | 180 |
| 4 | Italy | 122 | 7.41% | 3729 | 130 |
| 5 | Japan | 111 | 6.74% | 2115 | 130 |
| 6 | England | 110 | 6.68% | 6570 | 183 |
| 7 | France | 65 | 3.95% | 2133 | 110 |
| 8 | Netherlands | 61 | 3.71% | 2101 | 103 |
| 9 | Canada | 56 | 3.40% | 2813 | 17 |
| 10 | Australia | 50 | 3.04% | 2407 | 44 |
NP: Number of publications; NC: Number of citations; TLS: Total link strength.
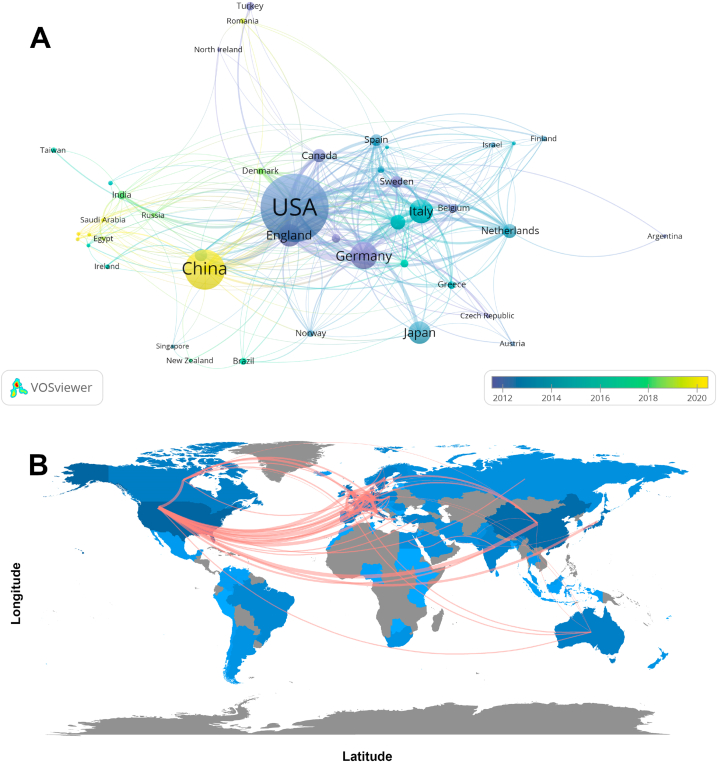
We used VOSviewer software to filter the 43 countries/regions with five or more publications and construct collaborative networks based on the number of publications and relationships within each country/region (Fig. 3A). Each circle in Fig. 3A represents a country/region; the circle size is proportional to the number of publications, and the circle color represents the APY. Fig. 3A reveals that the USA is the primary core country in this research field with the highest NP, and China has the highest APY (2020.00), indicating that although China entered this field relatively late, it has rapidly caught up in recent years. Additionally, we utilized the R package “bibliometrix” to create a geographic visualization of the international cooperation map for each country/region (Fig. 3B). The darker the blue color filling the country/region, the higher the number of publications. The line thickness between countries/regions indicates the strength of international collaborative relationships, with broader lines representing stronger cooperation. Fig. 3B reveals that the closest cooperation existsbetween the USA and China, and active cooperation between the USA, Germany, and England is also apparent.
Fig. 3.
Visualization of countries/regions involved in gut-heart axis and heart failure research. (A) Collaborative network visualization of countries/regions. (B) Geographic distribution and collaboration map of countries/regions.
The ten most productive institutions are listed in Table 2. The most prolific organizations were the Cleveland Clinic (NP: 34, 2.07%), followed by Charité-Universitätsmedizin Berlin (NP: 30, 1.829%), the University of Pittsburgh (NP: 23, 1.40%), and Harvard University (NP: 22, 1.34%). Almost all of the top ten institutions were in the USA, indicating the dominance of the USA in the field of the gut-heart axis and HF.
Table 2.
Publications in the 10 most productive institutions.
| Rank | Institution | Country | NP | Proportion | NC | TLS |
|---|---|---|---|---|---|---|
| 1 | Cleveland Clinic | USA | 34 | 2.07% | 4608 | 42 |
| 2 | Charité-Universitätsmedizin Berlin | Germany | 30 | 1.82% | 1687 | 62 |
| 3 | University of Pittsburgh | USA | 23 | 1.40% | 1964 | 30 |
| 4 | Harvard University | USA | 22 | 1.34% | 2359 | 26 |
| 5 | University of California, San Diego | USA | 15 | 0.91% | 839 | 16 |
| 6 | University of Copenhagen | Denmark | 15 | 0.91% | 468 | 32 |
| 7 | Oslo University | Norway | 14 | 0.85% | 686 | 29 |
| 8 | Columbia University | USA | 14 | 0.85% | 310 | 11 |
| 9 | University of California, Los Angeles | USA | 13 | 0.79% | 1224 | 19 |
| 10 | Stanford University | USA | 13 | 0.79% | 1021 | 26 |
NP: Number of publications; NC: Number of citations; TLS: Total link strength.
Supplementary Fig. 1 illustrates collaborations between 158 institutions with at least 5 publications each. Charité-Universitätsmedizin Berlin (TLS: 62) in Germany and the Cleveland Clinic (TLS: 42) in the USA have the strongest collaborations with other institutions. Supplementary Fig. 1A highlights the strong collaboration between institutions such as Oslo University Hospital (Oslo, Norway) and Oslo University (Oslo, Norway), and Baker IDI Heart and Diabetes Institute (Melbourne, Australia) and Monash University (Melbourne, Australia). Supplementary Fig. 1B shows the recent active involvement of Chinese institutions in gut-heart axis and HF research (highlighted in yellow), such as Southern Medical University (NP: 10, APY: 2022.00), Zhejiang University (NP: 6, APY: 2022.00), and Chinese Academy of Medical Sciences & Peking Union Medical College (NP: 7, APY: 2021.71).
3.3. Authors and Co-cited authors
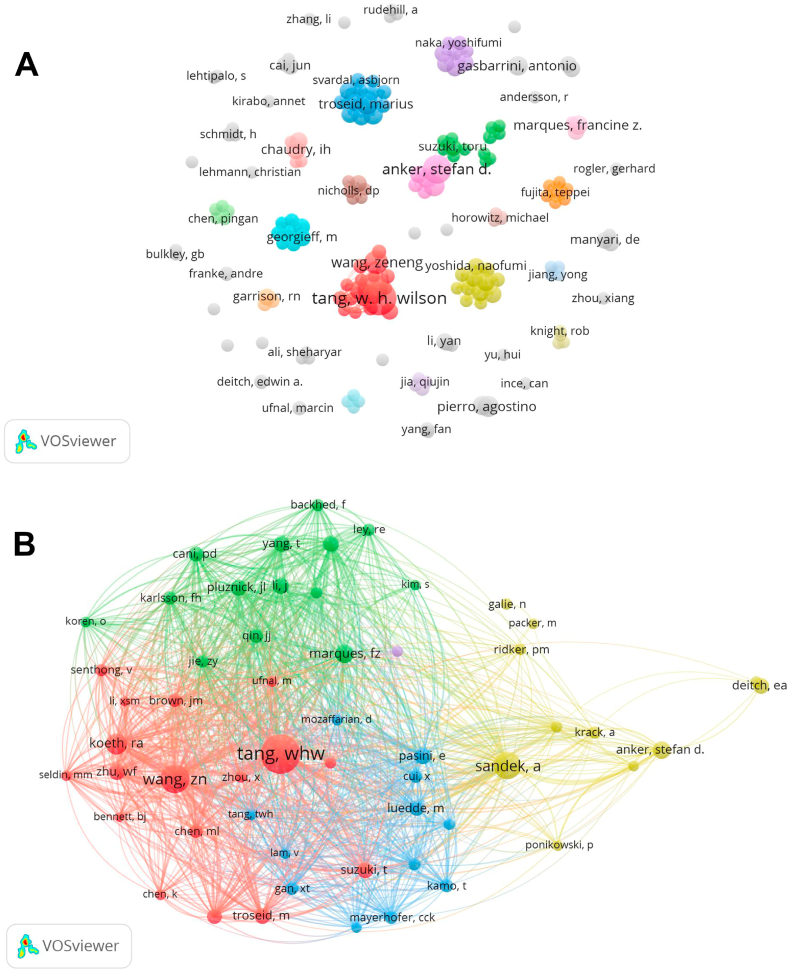
A total of 9412 authors were associated with gut-heart axis and HF research publications. Table 3 lists the ten authors with the highest number of publications and citations. Tang WHW of the Cleveland Clinic Heart and Vascular Institute had the most publications (NP: 21, NC: 3584), followed by Hazen SL (NP: 17, NC: 3425), Anker SD (NP: 15, NC: 1508), and Wang Z (NP: 10, NC: 1511). In addition, we observed close cooperation among several authors. For example, Hazen SL closely collaborated with Tang WHW and Wang Z, while Anker SD actively collaborated with Von Haehling S and Sandek A (Fig. 4A).
Table 3.
Top 10 authors and co-cited authors related to the gut-heart axis and heart failure.
| Rank | Authors | NP | NC | Cited Authors | NC |
|---|---|---|---|---|---|
| 1 | Tang WHW | 21 | 3584 | Tang WHW | 721 |
| 2 | Hazen SL | 17 | 3425 | Wang Z | 383 |
| 3 | Anker SD | 15 | 1508 | Sandek A | 341 |
| 4 | Wang Z | 10 | 1511 | Koeth RA | 203 |
| 5 | von Haehling S | 8 | 1034 | Marques FZ | 156 |
| 6 | Troseid M | 8 | 337 | Anker SD | 145 |
| 7 | Pierro A | 8 | 242 | Pasini E | 129 |
| 8 | Sandek A | 7 | 1016 | Zhu W | 128 |
| 9 | Marques FZ | 7 | 697 | Troseid M | 123 |
| 10 | Li l | 7 | 583 | Deitch EA | 122 |
NP: Number of publications; NC: Number of citations.
Fig. 4.
Visualization of authors involved in gut-heart axis and heart failure research. (A) Collaborative network visualization of authors. (B) Collaborative network visualization of co-cited authors.
Co-cited authors refer to two or more authors who are cited simultaneously in a study, indicating similarities in their research. The analysis of these authors can reveal highly influential experts in a subject area. A total of 57369 co-cited authors were identified. Three experts were co-cited more than 300 times: Tang WHW (NC: 693), Wang Z (NC: 364), and Sandek A (NC: 322) (Table 3). VOSviewer software was used to construct a collaborative network with a minimum co-citation threshold of 50 (Fig. 4B). Circles belonging to a cluster have the same color, and the five colors indicate that these authors belong to five research groups with similar interests.
3.4. Journals and Co-cited journals
Publications on the gut-heart axis and HF were published in 761 journals, of which 65 published more than 5 articles. Shocks published the most articles (NC: 23), followed by Journal of Surgical Research (NC: 21), Frontiers in Cardiovascular Medicine (NC: 21), and Critical Care Medicine (NC: 20) (Table 4). However, the ranking of journals based on the number of citations was inconsistent with the ranking based on the number of publications (Table 4). As an influential journal, Circulation Research had the highest number of citations, with 2931 citations of only 14 publications. This was followed by Critical Care Medicine with 1095 citations of 20 publications. The ten journals with the highest number of publications were all in Q1 or Q2 according to Journal Citation Reports (JCR); nine had IFs above 3.
Table 4.
Top 10 journals and co-cited journals related to the gut-heart axis and heart failure.
| Rank | Journal | NP | NC | IF (JCR 2022) | JCR Quartile | Co-cited journal | NC | IF (JCR 2022) | JCR Quartile |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Shock | 23 | 1002 | 3.1 | Q1 | Circulation | 2619 | 37.8 | Q1 |
| 2 | Journal of Surgical Research | 21 | 342 | 2.2 | Q2 | The New England Journal of medicine | 1714 | 158.5 | Q1 |
| 3 | Frontiers in Cardiovascular Medicine | 21 | 87 | 3.6 | Q2 | Journal of the American College of Cardiology | 1669 | 24.0 | Q1 |
| 4 | Critical Care Medicine | 20 | 1095 | 8.8 | Q1 | Nature | 1660 | 64.8 | Q1 |
| 5 | International Journal of Molecular Sciences | 17 | 287 | 5.6 | Q1 | Circulation Research | 1302 | 20.1 | Q1 |
| 6 | Nutrients | 17 | 245 | 5.9 | Q1 | Proceedings of the National Academy of Sciences of the United States of America | 1234 | 11.1 | Q1 |
| 7 | American Journal of Physiology-Heart and Circulatory Physiology | 15 | 330 | 4.8 | Q1 | PLoS One | 1168 | 3.7 | Q2 |
| 8 | Circulation Research | 14 | 2931 | 20.1 | Q1 | Lancet | 1153 | 168.9 | Q1 |
| 9 | Biomedicines | 14 | 76 | 4.7 | Q1 | European Heart Journal | 933 | 7.1 | Q1 |
| 10 | Intensive Care Medicine | 12 | 260 | 38.9 | Q1 | Hypertension | 930 | 8.3 | Q1 |
NP: Number of publications; NC: Number of citations; IF: Impact Factor; JCR: Journal Citation Reports.
Regarding co-cited journals, 188 of the 8099 journals were cited over 100 times, and eight had citations surpassing 1000 (Table 4). Circulation was the most co-cited journal (NC: 2619), followed by The New England Journal of Medicine (NC: 1714), Journal of the American College of Cardiology (NC: 1669), and Nature (NC: 1660), all with more than 1500 citations. Supplementary Fig. 2 shows that Circulation has an active co-citation relationship with other journals.
3.5. Co-cited references and references bursts
Co-cited references are those cited simultaneously in one or more articles, forming the knowledge base of a particular field. The frequent citations of these references indicate their wide relevance and similar research themes. In the context of the gut-heart axis and HF, a total of 80,634 co-cited references were identified. The ten most frequently cited references [[24], [25], [26], [27], [28], [29], [30], [31], [32], [33]], all of which are research articles, are presented in Table 5.
Table 5.
Top 10 co-cited references regarding the gut-heart axis and heart failure.
| Rank | Title | First author | Publication year | Journal | NC |
|---|---|---|---|---|---|
| 1 | Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease [24] | Wang Z | 2011 | Nature | 177 |
| 2 | Altered intestinal function in patients with chronic heart failure [25] | Sandek A | 2007 | Journal of the American College of Cardiology | 149 |
| 3 | Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis [26] | Koeth RA | 2013 | Nature Medicine | 144 |
| 4 | Prognostic Value of Elevated Levels of Intestinal Microbe-Generated Metabolite Trimethylamine-N-Oxide in Patients With Heart Failure Refining the Gut Hypothesis [27] | Tang WHW | 2014 | Journal of the American College of Cardiology | 142 |
| 5 | Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk [28] | Tang WHW | 2013 | The New England Journal of Medicine | 134 |
| 6 | Pathogenic Gut Flora in Patients With Chronic Heart Failure [29] | Pasini E | 2016 | JACC. Heart Failure | 122 |
| 7 | High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice [30] | Marques FZ | 2017 | Circulation | 118 |
| 8 | Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk [31] | Zhu W | 2016 | Cell | 103 |
| 9 | Heart failure is associated with depletion of core intestinal microbiota [32] | Luedde M | 2017 | European Journal of Heart Failure | 102 |
| 10 | Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis [33] | Wang Z | 2015 | Cell | 97 |
NC: Number of citations.
The article "Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease" [24], published in Nature by Wang Z et al., received the highest number of citations. Wang Z et al. conducted metabolomics research and identified, for the first time, a correlation between increased levels of specific metabolites in plasma and an increased risk of CVD [24].
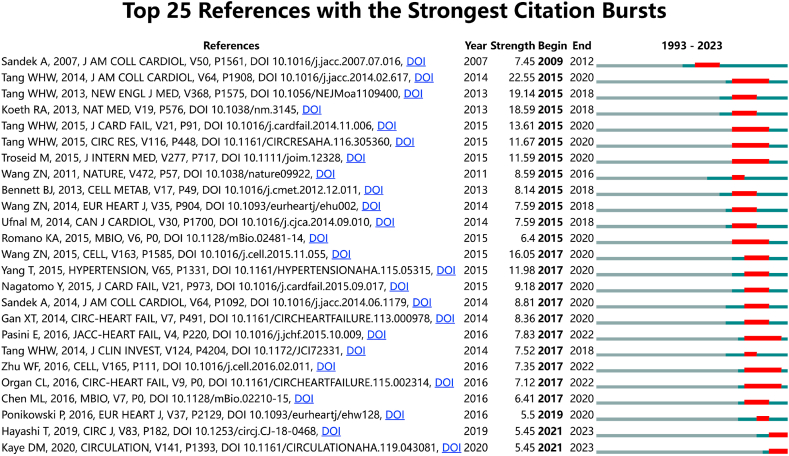
A reference burst refers to the frequent citation of a reference in a particular period of time and reflects a change in the focus of research in a given field over time [34]. Reference bursts were analyzed and visualized using CiteSpace software; the 25 references with the strongest citation bursts are shown in Fig. 5. Each bar represents a year, and the red bars represent the citation bursts occurring between 2009 and 2023. The article "Prognostic Value of Elevated Levels of Intestinal Microbe-Generated Metabolite Trimethylamine-N-Oxide in Patients With Heart Failure: Refining the Gut Hypothesis" [27] by Tang WHW et al., in the Journal of the American College of Cardiology demonstrated the strongest citation burst. This study [27], with a strength of 22.55, was the first to verify the association between elevated trimethylamine-N-oxide (TMAO) levels and poor prognosis in patients with HF, therefore significantly advancing understanding of the gut-heart axis and HF. Five of the 25 strongest citation bursts were authored by Tang WHW, demonstrating the influence of this research group.
Fig. 5.
Top 25 references with the strongest citation bursts from 1993 to 2023. Red bars indicate the length of the bursts. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.6. Keyword and keyword bursts
Keyword analysis can identify hotspots and directions in the field. Table 6 lists the 20 most frequently occurring keywords in the field of the gut-heart axis and HF. “Heart failure” was the most important term with 504 occurrences, followed by “gut microbiota” (235 occurrences), “trimethylamine N-oxide” (177 occurrences), “inflammation” (153 occurrences), “cardiovascular disease” (149 occurrences), “myocardial infarction” (115 occurrences), and “metabolism” (106 occurrences).
Table 6.
Top 20 keywords regarding the gut-heart axis and heart failure.
| Rank | Keywords | Occurrences | Rank | Keywords | Occurrences |
|---|---|---|---|---|---|
| 1 | heart failure | 504 | 11 | risk | 93 |
| 2 | gut microbiota | 235 | 12 | failure | 84 |
| 3 | trimethylamine N-oxide | 177 | 13 | hypertension | 83 |
| 4 | inflammation | 153 | 14 | dysfunction | 81 |
| 5 | cardiovascular disease | 149 | 15 | Short-chain fatty acids | 79 |
| 6 | myocardial infarction | 115 | 16 | blood pressure | 77 |
| 7 | metabolism | 106 | 17 | oxidative stress | 74 |
| 8 | diseases | 104 | 18 | chronic heart failure | 74 |
| 9 | intestinal microbiota | 97 | 19 | management | 67 |
| 10 | mortality | 97 | 20 | microbiota | 66 |
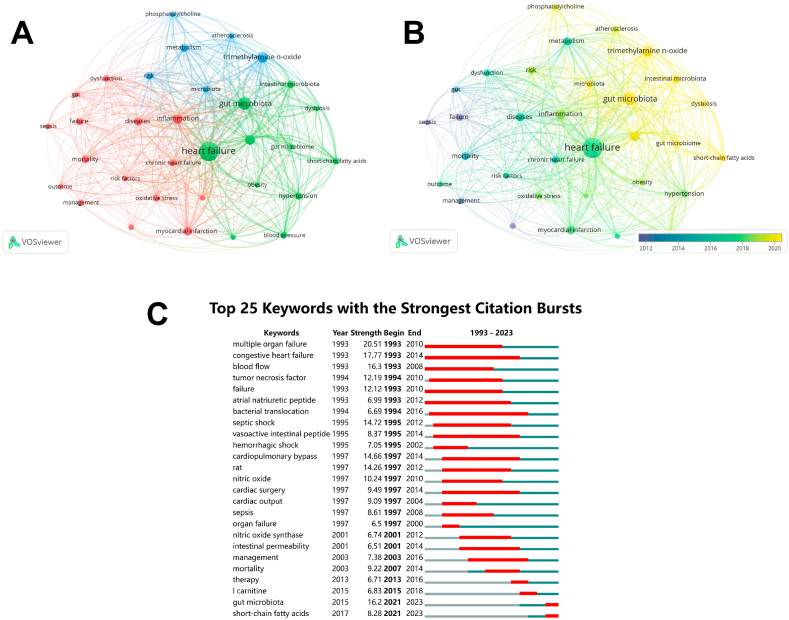
The 32 keywords with at least 50 occurrences were analyzed using VOSviewer software to identify hot topics and future directions. The analysis resulted in three clusters representing three research directions (Fig. 6A). The blue cluster includes keywords such as “trimethylamine N-oxide,” “metabolism,” risk,” “microbiota,” and “phosphatidylcholine.” The red cluster consists of keywords such as “inflammation,” “myocardial infarction,” “oxidative stress,” and “mortality.” The green cluster includes keywords such as “heart failure,” “gut microbiota,” “cardiovascular disease,” “intestinal microbiota,” and “hypertension.” In the overlay visualization (Fig. 6B), keywords are marked with different colors according to their APY. Keywords highlighted in yellow, such as “short-chain fatty acids,” “gut microbiota,” and “trimethylamine N-oxide,” are those that have become increasingly popular in recent years and may be prospective hot topics.
Fig. 6.
Analysis of keywords in gut-heart axis and heart failure research. (A) Network visualization of keywords. (B) Overlay visualization of keywords. (C) Top 25 keywords with the strongest citation bursts from 1993 to 2023.
Furthermore, the analysis of keyword bursts using CiteSpace software revealed that “gut microbiota” and “short-chain fatty acids” had the most recent bursts, suggesting their potential as future research hotspots in the field of the gut-heart axis and HF (Fig. 6C).
4. Discussion
In recent decades, there has been a dramatic increase in the prevalence of HF, which is now a major global health issue. HF is a complex syndrome with different etiologies, of which ischemic heart disease and hypertension are the most common causes [35]. Other specific risk factors include obesity, type 2 diabetes mellitus (T2DM), and valvular heart disease, all of which contribute to the acceleration of cardiovascular aging [36]. These risk factors can act independently or more commonly in combination, either directly or indirectly. Myocardial metabolism is almost exclusively aerobic, and oxygen delivery depends on arterial oxygen content and coronary blood flow. Ischemia irreversibly impairs cardiac function and alters the size and shape of the failing heart, known as ventricular remodeling. In the case of acute ischemia, for example in acute myocardial infarction, the loss of functioning cardiomyocytes leads to myocardial stunning and necrosis, followed by inflammation, hypertrophy, and fibrosis. These changes activate a neurohormonal cascade, leading to adverse left ventricular remodeling and subsequent dilation and functional impairment, which is the primary pathophysiological mechanism of HF [37]. Longstanding hypertension causes constant cardiac pressure and overload, and drives left ventricular hypertrophy, myocardial fibrosis, and diastolic dysfunction, resulting in hypertensive heart disease, including left ventricular diastolic dysfunction and dilated cardiomyopathy with HF and reduced ejection fraction, which ultimately manifests as HF [38]. Obesity and T2DM may lead to HF through myocardial infarction and left ventricular remodeling [36]. Cardiotoxicity caused by chemotherapy and other factors can also significantly contribute to the development of HF [39].
In recent years, numerous studies have demonstrated that the GM represents an important component of human physiology and metabolic homeostasis, and can directly or indirectly through derived metabolites, affect the heart and exacerbate the progression of HF [40], making the GM a new and promising therapeutic target for the treatment of HF [41]. One important aspect of this relationship is the metabolic pathways within the GM, including the production of trimethylamine (TMA)/TMAO, short-chain fatty acids (SCFAs), and secondary bile acids (BAs). These are well known pathophysiological linkages between biological processes dysregulated in HF, such as cardiac remodeling and repair capability, systemic vascular and inflammatory tone, and energy metabolism [15,42,43]. TMA, a GM-derived metabolite resulting from diets abundant in phosphatidylcholine, choline, betaine, and l-carnitine [44,45], can be converted into TMAO by hepatic flavin-containing monooxygenase 3 in the liver [46]. Circulating TMAO can activate platelet aggregation, increase foam cell formation, induce inflammatory responses, decrease reverse cholesterol transport, and accelerate endothelial dysfunction, contributing to the progression of atherosclerosis and HF [24,[47], [48], [49], [50]]. Li Z et al. [51] demonstrated that TMAO promotes myocardial hypertrophy and fibrosis through the Smad3 signaling pathway, both in vivo and in vitro. In cardiomyocytes, TMAO decreases energy metabolism and mitochondrial function by affecting pyruvate and fatty acid oxidation, which are involved in the tricarboxylic acid cycle [52]. It also negatively affects myocardial contractile function and intracellular calcium processing [53]. This pathway may be influenced by dietary factors such as foods or nutritional supplements rich in phosphatidylcholine, including red meat, eggs, milk, and certain fish [54], which may increase the risk of CVD through its metabolites, such as choline [24]. Saturated fatty acids with six or fewer carbon molecules are defined as SCFAs, and include cellulose, lignin, and pectin [55]. SCFAs can serve as a source of energy or fuel for glucose and lipid synthesis [56,57], and are also anti-inflammatory, as they bind to signaling receptors on target cells to modulate immune cell chemotaxis and cytokine release [58]. Additionally, SCFAs help reduce blood pressure and accompanying cardiac hypertrophy and myocardial fibrosis [58]. Primary BAs are synthesized in the liver through cholesterol oxidation [59], and secondary BAs, such as deoxycholate, lithocholate, and ursodeoxycholate (UDCA), are derived from primary BAs through a process that relies on the unique biosynthetic capabilities of certain microbes [60]. Early studies demonstrated that primary BAs exert direct dose-dependent negative myocardial chronotropic effects [61,62]. Secondary BAs activate sphingosine-1-phosphate receptor (S1PR) 2, which promotes apoptosis or survival signaling. In cardiac fibroblasts, S1PR plays a pivotal role in various physiological activities, including proliferation, remodeling, and differentiation. Furthermore, S1PR participates in endothelial cell responses and mediates peripheral vascular tone in both endothelial cells and smooth muscle cells [63]. UDCA, a secondary BA used in the treatment of cholestatic liver disease, has anti-inflammatory and cytoprotective properties and forms mixed micelles around lipopolysaccharide, helping to improve peripheral blood flow in patients with HF [64]. During HF, edema and impaired barrier function of the bowel wall result in translocation of GM components into the host circulation and endotoxemia, which in turn leads to increased systemic inflammation, further aggravating HF [25,29].
The association between the gut-heart axis and HF is therefore attracting increasing attention. Bibliometric analysis is a powerful tool that provides experienced researchers with systematic and visual knowledge structures while also informing novice researchers of the prevailing trends within their field of study [65]. Herein, we present a bibliometric analysis of publications on the gut-heart axis and HF published in the WoSCC database, investigating research dynamics and hotspots in the field.
4.1. General information
A bibliometric analysis using the WoSCC database revealed that 1664 articles related to the gut-heart axis and HF were published from January 1993 to June 2023, involving 9412 authors from 2287 institutions in 86 countries/regions. These articles were published in 761 different journals.
There was a general increasing trend in both the number of publications and citations, indicating the growing interest in this field. This growth aligns with previous studies in the broader field of human GM research [66], as well as the specific subfield focusing on the GM and other CVDs [12,67,68], suggesting that the field of gut-heart axis research will continue to flourish.
Visual analysis revealed that numerous nations and institutions have been actively conducting research on the gut-heart axis and HF, demonstrating the global interest in this field. Notably, the USA has contributed the highest number of publications and is home to several of the most productive institutions, including the Cleveland Clinic, Harvard University, and the University of Pittsburgh. This may in part be attributed to the second phase of the National Institutes of Health-funded Integrated Human Microbiome Program [69]. Chinese institutions have shown increasing participation in this field in recent years.
Professor Tang WHW is a renowned expert in CVD at the Cleveland Clinic, and has the greatest number of publications and citations in the field of the gut-heart axis and HF. Wang Z works alongside Professor Tang WHW and has the fourth highest number of publications and the third highest number of citations. Their team has long been committed to investigating the relationship between the gut-heart axis and CVD. They have examined the impact of the GM on heart health and its correlation with conditions such as HF, and proposed potential treatment strategies [70]. In 2011, they published a groundbreaking article [24] examining the association between the GM, CVDs, and diet. This influential article [24] revealed that three metabolites from dietary phospholipid choline, namely choline, TMAO, and betaine, can be used to predict the risk of CVDs. Further experiments confirmed that these dietary metabolites can promote the development of atherosclerosis, a major cause of CVDs. In atherosclerosis-prone mice, suppression of the intestinal microflora inhibited dietary choline-enhanced atherosclerosis. This study [24] provided new insights into the gut-dependent metabolism of dietary phospholipid choline and its role in the pathogenesis of CVDs. This study also sheds light on how nutrients are metabolized by the GM and their impact on human health, offering potential novel strategies for the prevention and treatment of atherosclerotic CVD. This article [24] is the most cited in the field, demonstrating its importance and influence. Additionally, Tang WHW et al. [27] investigated the relationship between fasting plasma TMAO levels and all-cause mortality in 720 patients with stable HF who were followed up for 5 years. This study [27] revealed that patients with HF and high TMAO levels have a higher long-term mortality risk, independent of traditional risk factors and heart-kidney indices. Notably, this study [27] has the strongest citation burst in this field.
Interestingly, critical care journals, including Shock, Critical Care Medicine, and Intensive Care Medicine, are among the highest publishing journals in the field of the gut-heart axis and HF. Of these, Shock has published more articles on the gut-heart axis and HF than any other journal and Intensive Care Medicine had the highest IF (38.9), indicating that critical care medicine may be a current focus of attention for the gut-heart axis and HF and suggesting relatively high-quality literature in this field. Recent evidence has revealed that critically ill patients exhibit altered GM, referred to as pathobiota, which is a prominent contributor to the development of clinical complications. Intestinal overgrowth of pathogenic bacteria, such as Candida, Campylobacter, Shigella, and Salmonella was observed in patients with HF and associated with disease severity [29]. In a pilot clinical trial, Saccharomyces boulardii supplementation in patients with HF improved the left ventricular fraction and reduced the left atrium diameter [71]. However, the available data remain controversial, potentially due to variations in the levels of dysbiosis observed among the participants included in these studies [71]. Of note, the Journal of Surgical Research published the second highest number of articles in the field of the gut-heart axis and HF, revealing that surgical treatment of HF may be one of the current research focuses. Despite significant therapeutic advances, HF remains a progressive disease that, in its advanced stages, is associated with considerable mortality and morbidity. For patients with advanced HF who require cardiac replacement therapy, only two options are available: left ventricular assist device (LVAD) implantation and heart transplantation (HT) [72]. However, a recent study [73] has demonstrated that microbial perturbations persist long-term following LVAD and HT, along with residual inflammation and oxidative stress. Moreover, there is an increasing recognition of the critical role of the microbiome in the metabolism of immunosuppressive drugs after HT.
Circulation, The New England Journal of Medicine, Journal of the American College of Cardiology, and Nature were the most co-cited journals, reminding scholars interested in this topic to pay particular attention to these journals. Shock, which has published the highest number of articles, and Circulation, which has the highest number of citations, have different emphases. The former primarily covers research related to critical illness, shock, and acute injury, while the latter is one of the top journals in the field of cardiovascular research, emphasizing cardiovascular epidemiology, pathophysiology, and pharmacology. Circulation, renowned for its expertise and extensive coverage in cardiology [65], has unsurprisingly received the highest number of citations in the field of the gut-heart axis and HF.
4.2. Hotspots and frontiers
Keyword analysis is a crucial step in exploring research hotspots and predicting future directions [74]. In this study, the keyword analysis identified three keyword clusters, representing three main research directions (Fig. 6A).
One cluster (green cluster) was dominated by “heart failure,” “gut microbiota,” “cardiovascular disease”, “hypertension,” and “obesity,” and mainly focused on the relationship between the GM and various cardiometabolic diseases. In healthy individuals, the GM maintains a relatively stable composition, dominated by a few phyla, including Bacteroidetes, Firmicutes, Proteobacteria, Verrucomicrobia, and Actinobacteria [75]. Alterations in the composition of the GM and gut microbial metabolism are linked to the etiology of chronic noncommunicable diseases, including gastrointestinal, cardiovascular, and metabolic illnesses, and cancer [76]. Mounting evidence indicates that the GM plays a significant role in blood pressure regulation and the pathogenesis of arterial hypertension. Clinical studies have demonstrated discernible disparities between individuals classified as prehypertensive or hypertensive and their normotensive counterparts in GM composition, particularly in Prevotella and Klebsiella, which belong to Bacteroidetes and Proteobacteria, respectively [77]. Simultaneously, clinical studies have provided evidence for the pivotal role of the GM in fat and sugar metabolism and revealed notable alterations in microbiota composition in individuals with obesity and T2DM [78]. A comparison of fecal bacteria between individuals with chronic HF and healthy individuals demonstrated that the former group exhibited greater colonization of pathogenic bacteria, specifically Shigella, Salmonella, Campylobacter, and Yersinia enterocolitica, all of which belong to Proteobacteria [79], than the latter group.
The second cluster (blue cluster) was dominated by “trimethylamine N-oxide,” “metabolism,” “risk,” “microbiota,” “phosphatidylcholine,” and “atherosclerosis” and mainly focused on the microbiota and its metabolites. The GM maintains a profound symbiotic relationship with its human host and operates akin to an endocrine organ, generating bioactive metabolites, such as SCFAs, TMA/TMAO, and bile acids. These metabolites contribute to host health through a multitude of pathways [80]. SCFAs, specifically acetate, propionate, butyrate, and malonate, are beneficial GM-derived metabolites produced by the bacterial fermentation of dietary fiber [81] and play a significant role in host immunity and cardiac repair [82]. Acetate [30] regulates hypertension and protects against atherosclerosis. Both propionate [83,84] and butyrate [85,86] have been shown to lower blood pressure, mitigate ischemia/reperfusion injury, and reduce the risk of CVD and atherosclerosis. Although TMAO has long been known, it was not until 2011 that Wang Z et al. suggested that it could be detrimental to human health [24]. Some studies have suggested that TMAO may cause pro-inflammatory responses [87] and apoptosis [88], and disrupt lipid homeostasis [89]. Multiple studies have demonstrated an association between higher plasma TMAO concentrations and an increased risk of atherothrombotic CVD [90,91], HF [41], and adverse outcomes [92].
The third cluster (red cluster) was dominated by “inflammation,” “myocardial infarction,” “oxidative stress,” “mortality,” “management,” and “gut,” and mainly focused on the etiology, pathogenesis, and management of this field. The connection between the gut-heart axis and HF, which represents the interplay between the gut, GM, and HF, refers to cardiac dysfunction resulting in chronic gut hypoperfusion and venous congestion. Subsequently, this leads to reduced nutrient absorption, increased intestinal mucosal permeability, and translocation of microbial products into the systemic circulation, inducing low-level chronic inflammation and dysregulation of the immune system, which further exacerbates HF [9,93]. Furthermore, the GM in patients with chronic HF is characterized by an overall decrease in diversity and anti-inflammatory microbes, particularly those belonging to the Firmicutes and Proteobacteria phyla [32,[94], [95], [96]]. As the influence of the GM on HF has become better understood, gut microbes and metabolites have become targets for disease prevention and treatment. Strategies include dietary interventions, prebiotic and probiotic therapies, fecal microbiota transplantation (FMT), and antibiotic interventions [41]. For example, a high-fiber diet promotes the growth of acetate-producing microbiota, resulting in reduced blood pressure and attenuated cardiac hypertrophy and fibrosis [30]. Probiotics typically encompass a range of microorganisms including bifidobacteria, yeasts, and lactic acid bacteria. A study in rats demonstrated that the administration of probiotics, specifically Lactobacillus rhamnosus GR-1, significantly improved left ventricular hypertrophy and ejection fraction in a model of acute myocardial infarction [97]. FMT is a method of treating intestinal microecological imbalance and restoring normal intestinal function by introducing bacteria or metabolites from the feces of healthy donors into diseased recipients, and is mostly used to treat Clostridium difficile infection [41]. While FMT has thus far shown minimal side effects, its effectiveness and safety in treating patients with HF remains uncertain [98]. Additionally, a study in mice showed that injecting antibiotics to eliminate intestinal bacterial translocation alleviated systemic inflammation and myocardial cell damage in a model of myocardial infarction [99].
4.3. Limitations
Our study presents a comprehensive overview of the association between the gut-heart axis and HF. However, it is important to acknowledge the limitations of this study. First, a significant inherent limitation of bibliometric analysis lies in its exclusive emphasis on quantitative metrics, which may not always accurately reflect the genuine impact of certain research work or emerging trends that have not yet garnered a substantial number of citations [55]. Second, we relied solely on the WoSCC database for literature collection, and therefore relevant studies from other databases may have been overlooked. Third, the exclusion of non-English research articles may have introduced source bias. Additionally, owing to the cut-off date of our study, we did not fully evaluate studies published in 2023. Because of the possibility of bias, our findings should be interpreted with some caution.
5. Conclusion
In conclusion, our study revealed that research on the gut-heart axis and HF is increasing steadily. Currently, 86 countries/regions worldwide are involved in this research, led by the USA, China, and Germany. The cooperation between countries/regions and institutions is relatively close. Notably, Professor Tang WHW from the Cleveland Clinic in the United States has made significant contributions to this field, being the most prolific author in terms of publications and citations. Dysbiosis of the GM and metabolic abnormalities are the primary pathways influencing the etiology and pathogenesis of HF. According to the keywords analysis, the mechanism underlying the association between the gut-heart axis and HF remains the focus of research. Moreover, modulation of the GM has the potential to be a significant target for intervention in patients with HF; this may be a promising direction for future research. This study provides researchers with new knowledge and perspectives, which may allow greater clinical application of research findings, expanding the strategies available for disease prevention, diagnosis, and treatment, and maximizing the benefits for patients with HF.
Ethical approval
Because all data used were obtained from the Web of Science database, ethical approval was not required.
Data availability statement
Data included in article/supplementary material/referenced in article. Further data will be made available on request.
Funding statement
This study was supported by the China Academy of Chinese Medical Sciences Innovation Fund (No. CI2021A00920), and the Beijing Traditional Chinese Medicine Technology Development Fund Project (No. JJ-2020-79).
CRediT authorship contribution statement
Jiahui Ouyang: Writing – original draft, Software, Methodology, Formal analysis. Lingli Zhao: Writing – original draft, Methodology, Formal analysis. Yewen Song: Writing – original draft, Visualization, Methodology, Formal analysis. Hua Qu: Writing – review & editing, Methodology, Data curation. Tianyi Du: Writing – original draft, Formal analysis, Data curation. Liu Shi: Writing – original draft, Software, Formal analysis. Zhijie Cui: Writing – original draft, Formal analysis. Zhonghui Jiang: Writing – review & editing, Visualization, Software, Methodology, Conceptualization. Zhuye Gao: Writing – review & editing, Visualization, Software, Methodology, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e25995.
Contributor Information
Zhonghui Jiang, Email: jzh2021@126.com.
Zhuye Gao, Email: zhuyegao@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kim A.H., Jang J.E., Han J. Current status on the therapeutic strategies for heart failure and diabetic cardiomyopathy. Biomed. Pharmacother. 2022;145 doi: 10.1016/j.biopha.2021.112463. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savarese G., Becher P.M., Lund L.H., Seferovic P., Rosano G.M.C., Coats A.J.S. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023;118(17):3272–3287. doi: 10.1093/cvr/cvac013. [DOI] [PubMed] [Google Scholar]
- 4.Groenewegen A., Rutten F.H., Mosterd A., Hoes A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020;22(8):1342–1356. doi: 10.1002/ejhf.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troseid M., Andersen G.O., Broch K., Hov J.R. The gut microbiome in coronary artery disease and heart failure: current knowledge and future directions. EBioMedicine. 2020;52 doi: 10.1016/j.ebiom.2020.102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman M.M., Islam F., Or-Rashid M.H., Mamun A.A., Rahaman M.S., Islam M.M., et al. The gut microbiota (microbiome) in cardiovascular disease and its therapeutic regulation. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.903570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y., Aquino-Martinez R., Hutchison E., Allayee H., Lusis A.J., Rey F.E. Role of gut microbe-derived metabolites in cardiometabolic diseases: systems based approach. Mol. Metabol. 2022;64 doi: 10.1016/j.molmet.2022.101557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao P., Zhao S., Tian J., Liu X. Significance of gut microbiota and short-chain fatty acids in heart failure. Nutrients. 2022;14(18) doi: 10.3390/nu14183758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamo T., Akazawa H., Suzuki J.I., Komuro I. Novel concept of a heart-gut Axis in the pathophysiology of heart failure. Korean Circ. J. 2017;47(5):663–669. doi: 10.4070/kcj.2017.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsiras D., Bezati S., Ventoulis I., Verras C., Parissis J., Polyzogopoulou E. Gut failure: a review of the pathophysiology and therapeutic potentials in the gut-heart Axis. J. Clin. Med. 2023;(7):12. doi: 10.3390/jcm12072567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kummen M., Mayerhofer C.C.K., Vestad B., Broch K., Awoyemi A., Storm-Larsen C., et al. Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J. Am. Coll. Cardiol. 2018;71(10):1184–1186. doi: 10.1016/j.jacc.2017.12.057. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Li D., Jia Z., Hui J., Xin Q., Zhou Q., et al. A bibliometric analysis of research on the links between gut microbiota and atherosclerosis. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.941607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu H., Zhou Y., Wang Y., Tong L., Wang F., Song S., et al. Current state and future directions of intranasal delivery route for central nervous system disorders: a scientometric and visualization analysis. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.717192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forkosh E., Ilan Y. The heart-gut axis: new target for atherosclerosis and congestive heart failure therapy. Open Heart. 2019;6(1) doi: 10.1136/openhrt-2018-000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang W.H.W., Li D.Y., Hazen S.L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 2019;16(3):137–154. doi: 10.1038/s41569-018-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yukino-Iwashita M., Nagatomo Y., Kawai A., Taruoka A., Yumita Y., Kagami K., et al. Short-chain fatty acids in gut-heart Axis: their role in the pathology of heart failure. J. Personalized Med. 2022;12(11) doi: 10.3390/jpm12111805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karmazyn M., Gan X.T. Probiotics as potential treatments to reduce myocardial remodelling and heart failure via the gut-heart axis: state-of-the-art review. Mol. Cell. Biochem. 2023 doi: 10.1007/s11010-023-04683-6. [DOI] [PubMed] [Google Scholar]
- 18.Du Z., Wang J., Lu Y., Ma X., Wen R., Lin J., et al. The cardiac protection of Baoyuan decoction via gut-heart axis metabolic pathway. Phytomedicine. 2020;79 doi: 10.1016/j.phymed.2020.153322. [DOI] [PubMed] [Google Scholar]
- 19.Ding X., Yang Z. Knowledge mapping of platform research: a visual analysis using VOSviewer and CiteSpace. Electron. Commer. Res. 2022;22(3):787–809. doi: 10.1007/s10660-020-09410-7. [DOI] [Google Scholar]
- 20.Merigo J.M., Yang J.B. A bibliometric analysis of operations research and management science. Omega-Int J Manage Sci. 2017;73:37–48. doi: 10.1016/j.omega.2016.12.004. [DOI] [Google Scholar]
- 21.Dervis H. Bibliometric analysis using bibliometrix an R package. J. Scientometric Res. 2019;8(3):156–160. doi: 10.5530/jscires.8.3.32. [DOI] [Google Scholar]
- 22.van Eck N.J., Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C.M. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006;57(3):359–377. doi: 10.1002/asi.20317. [DOI] [Google Scholar]
- 24.Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–U82. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandek A., Bauditz J., Swidsinski A., Buhner S., Weber-Eibel J., von Haehling S., et al. Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007;50(16):1561–1569. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang W.H.W., Wang Z., Fan Y., Levison B., Hazen J.E., Donahue L.M., et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure refining the gut Hypothesis. J. Am. Coll. Cardiol. 2014;64(18):1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang W.H.W., Wang Z., Levison B.S., Koeth R.A., Britt E.B., Fu X., et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasini E., Aquilani R., Testa C., Baiardi P., Angioletti S., Boschi F., et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016;4(3):220–227. doi: 10.1016/j.jchf.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Marques F.Z., Nelson E., Chu P.-Y., Horlock D., Fiedler A., Ziemann M., et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10) doi: 10.1161/CIRCULATIONAHA.116.024545. 964-+ [DOI] [PubMed] [Google Scholar]
- 31.Zhu W., Gregory J.C., Org E., Buffa J.A., Gupta N., Wang Z., et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luedde M., Winkler T., Heinsen F.-A., Ruehlemann M.C., Spehlmann M.E., Bajrovic A., et al. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail. 2017;4(3):282–290. doi: 10.1002/ehf2.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z., Roberts A.B., Buffa J.A., Levison B.S., Zhu W., Org E., et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163(7):1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldberg D., McCouch S., Kleinberg J. Constructing comparative genome maps with unresolved marker order. Pac. Symp. Biocomput. 2002:139–150. [PubMed] [Google Scholar]
- 35.Investigators G.C., Joseph P., Roy A., Lonn E., Stork S., Floras J., et al. Global variations in heart failure etiology, management, and outcomes. JAMA. 2023;329(19):1650–1661. doi: 10.1001/jama.2023.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Triposkiadis F., Xanthopoulos A., Parissis J., Butler J., Farmakis D. Pathogenesis of chronic heart failure: cardiovascular aging, risk factors, comorbidities, and disease modifiers. Heart Fail. Rev. 2022;27(1):337–344. doi: 10.1007/s10741-020-09987-z. [DOI] [PubMed] [Google Scholar]
- 37.Sutton M.G., Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 38.Messerli F.H., Rimoldi S.F., Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail. 2017;5(8):543–551. doi: 10.1016/j.jchf.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Hahn V.S., Zhang K.W., Sun L., Narayan V., Lenihan D.J., Ky B. Heart failure with targeted cancer therapies: mechanisms and cardioprotection. Circ. Res. 2021;128(10):1576–1593. doi: 10.1161/CIRCRESAHA.121.318223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lupu V.V., Adam Raileanu A., Mihai C.M., Morariu I.D., Lupu A., Starcea I.M., et al. The implication of the gut microbiome in heart failure. Cells. 2023;(8):12. doi: 10.3390/cells12081158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia Q., Li H., Zhou H., Zhang X., Zhang A., Xie Y., et al. Role and effective therapeutic target of gut microbiota in heart failure. Cardiovasc.Ther. 2019;2019 doi: 10.1155/2019/5164298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu J., Lin S., Zheng B., Cheung P.C.K. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018;58(8):1243–1249. doi: 10.1080/10408398.2016.1245650. [DOI] [PubMed] [Google Scholar]
- 43.Stanciu A.E. Cytokines in heart failure. Adv. Clin. Chem. 2019;93:63–113. doi: 10.1016/bs.acc.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Zeisel S.H., Wishnok J.S., Blusztajn J.K. Formation of methylamines from ingested choline and lecithin. J. Pharmacol. Exp. Therapeut. 1983;225(2):320–324. [PubMed] [Google Scholar]
- 45.Wang Z., Bergeron N., Levison B.S., Li X.S., Chiu S., Jia X., et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019;40(7):583–594. doi: 10.1093/eurheartj/ehy799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker J.R., Chaykin S. The biosynthesis of trimethylamine-N-oxide. J. Biol. Chem. 1962;237:1309–1313. [PubMed] [Google Scholar]
- 47.Tang W.H.W., Backhed F., Landmesser U., Hazen S.L. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019;73(16):2089–2105. doi: 10.1016/j.jacc.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu W., Buffa J.A., Wang Z., Warrier M., Schugar R., Shih D.M., et al. Flavin monooxygenase 3, the host hepatic enzyme in the metaorganismal trimethylamine N-oxide-generating pathway, modulates platelet responsiveness and thrombosis risk. J. Thromb. Haemostasis. 2018;16(9):1857–1872. doi: 10.1111/jth.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Wang Y., Ke B., Du J. TMAO: how gut microbiota contributes to heart failure. Transl. Res. 2021;228:109–125. doi: 10.1016/j.trsl.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Ma G., Pan B., Chen Y., Guo C., Zhao M., Zheng L., et al. Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 2017;(2):37. doi: 10.1038/s41374-018-0091-y. 10.1042/BSR20160244 Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y, et al. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest. (2019) 99(3):346-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z., Wu Z., Yan J., Liu H., Liu Q., Deng Y., et al. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab. Invest. 2019;99(3):346–357. doi: 10.1038/s41374-018-0091-y. [DOI] [PubMed] [Google Scholar]
- 52.Makrecka-Kuka M., Volska K., Antone U., Vilskersts R., Grinberga S., Bandere D., et al. Trimethylamine N-oxide impairs pyruvate and fatty acid oxidation in cardiac mitochondria. Toxicol. Lett. 2017;267:32–38. doi: 10.1016/j.toxlet.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 53.Savi M., Bocchi L., Bresciani L., Falco A., Quaini F., Mena P., et al. Trimethylamine-N-Oxide (TMAO)-Induced impairment of cardiomyocyte function and the protective role of urolithin B-glucuronide. Molecules. 2018;23(3) doi: 10.3390/molecules23030549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z., Hazen J., Jia X., Org E., Zhao Y., Osborn L.J., et al. The nutritional supplement L-alpha glycerylphosphorylcholine promotes atherosclerosis. Int. J. Mol. Sci. 2021;(24):22. doi: 10.3390/ijms222413477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aroeira R.I. M ARBC. Can citation metrics predict the true impact of scientific papers? FEBS J. 2020;287(12):2440–2448. doi: 10.1111/febs.15255. [DOI] [PubMed] [Google Scholar]
- 56.Amiri P., Hosseini S.A., Ghaffari S., Tutunchi H., Ghaffari S., Mosharkesh E., et al. Role of butyrate, a gut microbiota derived metabolite, in cardiovascular diseases: a comprehensive narrative review. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.837509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Panagia M., He H., Baka T., Pimentel D.R., Croteau D., Bachschmid M.M., et al. Increasing mitochondrial ATP synthesis with butyrate normalizes ADP and contractile function in metabolic heart disease. NMR Biomed. 2020;33(5) doi: 10.1002/nbm.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaye D.M., Shihata W.A., Jama H.A., Tsyganov K., Ziemann M., Kiriazis H., et al. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation. 2020;141(17):1393–1403. doi: 10.1161/CIRCULATIONAHA.119.043081. [DOI] [PubMed] [Google Scholar]
- 59.Lefebvre P., Cariou B., Lien F., Kuipers F., Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009;89(1):147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 60.Sinha S.R., Haileselassie Y., Nguyen L.P., Tropini C., Wang M., Becker L.S., et al. Dysbiosis-Induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe. 2020;27(4):659–670 e5. doi: 10.1016/j.chom.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joubert P. An in vivo investigation of the negative chronotropic effect of cholic acid in the rat. Clin. Exp. Pharmacol. Physiol. 1978;5(1):1–8. doi: 10.1111/j.1440-1681.1978.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 62.Gao J., Yuan G., Xu Z., Lan L., Xin W. Chenodeoxycholic and deoxycholic acids induced positive inotropic and negative chronotropic effects on rat heart. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021;394(4):765–773. doi: 10.1007/s00210-020-01962-7. [DOI] [PubMed] [Google Scholar]
- 63.Ruiz M., Frej C., Holmer A., Guo L.J., Tran S., Dahlback B. High-density lipoprotein-associated apolipoprotein M limits endothelial inflammation by delivering sphingosine-1-phosphate to the sphingosine-1-phosphate receptor 1. Arterioscler. Thromb. Vasc. Biol. 2017;37(1):118–129. doi: 10.1161/ATVBAHA.116.308435. [DOI] [PubMed] [Google Scholar]
- 64.Aouad K., Calmus Y., Nordlinger B., Myara A., Weill B., Poupon R. Immunosuppressive effects of endotoxins and bile acids in vivo in the rat. Eur. J. Clin. Invest. 1996;26(1):45–48. doi: 10.1046/j.1365-2362.1996.95240.x. [DOI] [PubMed] [Google Scholar]
- 65.He Z., Dai L., Zuo Y., Chen Y., Wang H., Zeng H. Hotspots and frontiers in pulmonary arterial hypertension research: a bibliometric and visualization analysis from 2011 to 2020. Bioengineered. 2022;13(6):14667–14680. doi: 10.1080/21655979.2022.2100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang Z., Liu K., Ma W., Li D., Mo T., Liu Q. The gut microbiome in human health and disease-Where are we and where are we going? A bibliometric analysis. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.1018594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheng M., Xu S., Chen W.W., Li F.Q., Zhong Y.M., Ouyang Y.X., et al. A bibliometric analysis of studies on the gut microbiota in cardiovascular disease from 2004 to 2022. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.1083995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Long D., Mao C., Zhang X., Liu Y., Shangguan X., Zou M., et al. Coronary heart disease and gut microbiota: a bibliometric and visual analysis from 2002 to 2022. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.949859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Integrative HMP (iHMP) Research Network Consortium The Integrative Human Microbiome Project: dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe. 2014;16(3):276–289. doi: 10.1016/j.chom.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang W.H., Kitai T., Hazen S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017;120(7):1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Costanza A.C., Moscavitch S.D., Faria Neto H.C., Mesquita E.T. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int. J. Cardiol. 2015;179:348–350. doi: 10.1016/j.ijcard.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 72.Yuzefpolskaya M., Bohn B., Ladanyi A., Khoruts A., Colombo P.C., Demmer R.T. Oral and gut microbiome alterations in heart failure: epidemiology, pathogenesis and response to advanced heart failure therapies. J. Heart Lung Transplant. 2023;42(3):291–300. doi: 10.1016/j.healun.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 73.Yuzefpolskaya M., Bohn B., Javaid A., Mondellini G.M., Braghieri L., Pinsino A., et al. Levels of trimethylamine N-oxide remain elevated long term after left ventricular assist device and heart transplantation and are independent from measures of inflammation and gut dysbiosis. Circ Heart Fail. 2021;14(6) doi: 10.1161/CIRCHEARTFAILURE.120.007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang F., Yang P., Chen Y., Wang R., Liu B., Wang J., et al. Bibliometric and visual analysis of fecal microbiota transplantation research from 2012 to 2021. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.1057492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Illiano P., Brambilla R., Parolini C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020;287(5):833–855. doi: 10.1111/febs.15217. [DOI] [PubMed] [Google Scholar]
- 77.Li J., Zhao F., Wang Y., Chen J., Tao J., Tian G., et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karlsson F.H., Tremaroli V., Nookaew I., Bergstrom G., Behre C.J., Fagerberg B., et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 79.Lim G.B. Heart failure: gut flora--pathogenic role in chronic heart failure. Nat. Rev. Cardiol. 2016;13(2):61. doi: 10.1038/nrcardio.2015.200. [DOI] [PubMed] [Google Scholar]
- 80.Xu H., Wang X., Feng W., Liu Q., Zhou S., Liu Q., et al. The gut microbiota and its interactions with cardiovascular disease. Microb. Biotechnol. 2020;13(3):637–656. doi: 10.1111/1751-7915.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Drapkina O.M., Yafarova A.A., Kaburova A.N., Kiselev A.R. Targeting gut microbiota as a novel strategy for prevention and treatment of hypertension, atrial fibrillation and heart failure: current knowledge and future perspectives. Biomedicines. 2022;10(8) doi: 10.3390/biomedicines10082019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang T.W.H., Chen H.C., Chen C.Y., Yen C.Y.T., Lin C.J., Prajnamitra R.P., et al. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation. 2019;139(5):647–659. doi: 10.1161/CIRCULATIONAHA.118.035235. [DOI] [PubMed] [Google Scholar]
- 83.Bartolomaeus H., Balogh A., Yakoub M., Homann S., Marko L., Hoges S., et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407–1421. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deng F., Zhang L.Q., Wu H., Chen Y., Yu W.Q., Han R.H., et al. Propionate alleviates myocardial ischemia-reperfusion injury aggravated by Angiotensin II dependent on caveolin-1/ACE2 axis through GPR41. Int. J. Biol. Sci. 2022;18(2):858–872. doi: 10.7150/ijbs.67724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang X., Huang X., Tong Y., Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391–399. doi: 10.1139/cjpp-2019-0531. [DOI] [PubMed] [Google Scholar]
- 86.Bultman S.J. Bacterial butyrate prevents atherosclerosis. Nat. Microbiol. 2018;3(12):1332–1333. doi: 10.1038/s41564-018-0299-z. [DOI] [PubMed] [Google Scholar]
- 87.Liu H., Jia K., Ren Z., Sun J., Pan L.L. PRMT5 critically mediates TMAO-induced inflammatory response in vascular smooth muscle cells. Cell Death Dis. 2022;13(4):299. doi: 10.1038/s41419-022-04719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benson T.W., Conrad K.A., Li X.S., Wang Z., Helsley R.N., Schugar R.C., et al. Gut microbiota-derived trimethylamine N-oxide contributes to abdominal aortic aneurysm through inflammatory and apoptotic mechanisms. Circulation. 2023;147(14):1079–1096. doi: 10.1161/CIRCULATIONAHA.122.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Canyelles M., Tondo M., Cedo L., Farras M., Escola-Gil J.C., Blanco-Vaca F. Trimethylamine N-oxide: a link among diet, gut microbiota, gene regulation of liver and intestine cholesterol homeostasis and hdl function. Int. J. Mol. Sci. 2018;19(10) doi: 10.3390/ijms19103228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stubbs J.R., House J.A., Ocque A.J., Zhang S., Johnson C., Kimber C., et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J. Am. Soc. Nephrol. 2016;27(1):305–313. doi: 10.1681/ASN.2014111063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dalla Via A., Gargari G., Taverniti V., Rondini G., Velardi I., Gambaro V., et al. Urinary TMAO levels are associated with the taxonomic composition of the gut microbiota and with the choline TMA-lyase gene (cutC) harbored by enterobacteriaceae. Nutrients. 2019;12(1) doi: 10.3390/nu12010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anderson K.M., Ferranti E.P., Alagha E.C., Mykityshyn E., French C.E., Reilly C.M. The heart and gut relationship: a systematic review of the evaluation of the microbiome and trimethylamine-N-oxide (TMAO) in heart failure. Heart Fail. Rev. 2022;27(6):2223–2249. doi: 10.1007/s10741-022-10254-6. [DOI] [PubMed] [Google Scholar]
- 93.Mamic P., Chaikijurajai T., Tang W.H.W. Gut microbiome - a potential mediator of pathogenesis in heart failure and its comorbidities: state-of-the-art review. J. Mol. Cell. Cardiol. 2021;152:105–117. doi: 10.1016/j.yjmcc.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kamo T., Akazawa H., Suda W., Saga-Kamo A., Shimizu Y., Yagi H., et al. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martin R., Miquel S., Chain F., Natividad J.M., Jury J., Lu J., et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015;15:67. doi: 10.1186/s12866-015-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun W., Du D., Fu T., Han Y., Li P., Ju H. Alterations of the gut microbiota in patients with severe chronic heart failure. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.813289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gan X.T., Ettinger G., Huang C.X., Burton J.P., Haist J.V., Rajapurohitam V., et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7(3):491–499. doi: 10.1161/CIRCHEARTFAILURE.113.000978. [DOI] [PubMed] [Google Scholar]
- 98.Cammarota G., Ianiro G., Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J. Clin. Gastroenterol. 2014;48(8):693–702. doi: 10.1097/MCG.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 99.Zhou X., Li J., Guo J., Geng B., Ji W., Zhao Q., et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome. 2018;6(1):66. doi: 10.1186/s40168-018-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article. Further data will be made available on request.