Abstract
Considering Salmonella transmission occurs through several routes in integrated broiler operations, control of nontyphoidal Salmonella in commercial farms is essential. This study aimed to compare the distribution of persistent Salmonella serovars in environments and dead chickens between 5 major integrated broiler operations in Korea. The prevalence of Salmonella-positive farms in dust prior to placement by operations was 0 to 25%, but the prevalence in dust and feces at the time of depletion was increased to 16.7 to 41.7% and 16.7 to 66.7%, respectively. Moreover, the prevalence of farms with Salmonella in chickens that died within 1 week old and at 4 to 5 weeks old ranged from 8.3 to 58.3% and 16.7 to 41.7%, respectively. The prevalence of Salmonella enterica serovar Infantis-positive farms in dust prior to placement and in chickens that died within 1 week old was 5.2 and 3.4%, respectively, but the prevalence in dust and feces at the time of depletion and in chickens that died at 4 to 5 weeks old was significantly increased to 27.6, 41.4, and 20.7%, respectively (P < 0.05). Interestingly, the plasmid of emerging S. Infantis (pESI) was only identified in S. Infantis, and the prevalence of multidrug-resistance was significantly higher in pESI-positive S. Infantis (99.2%) than in pESI-negative S. Infantis (6.7%) (P < 0.05). The distribution of pulsotypes between pESI-positive and pESI-negative S. Infantis were varied, but a majority of S. Infantis were clustered only 2 pulsotypes. Moreover, pESI-positive S. Infantis harbored more virulence factors than pESI-negative S. Infantis. This study is the first report on characteristics of S. Infantis carrying the pESI plasmid in commercial broiler farms in Korea.
Key words: Salmonella Infantis, pESI plasmid, broiler, emerging pathogen
INTRODUCTION
Nontyphoidal Salmonella are widely dispersed in broad spectrum of environments, including gastrointestinal tracts of domestic and wild mammals and birds, and Salmonella infection in humans may be occur by contact with animals or the environments (Eng et al., 2015). Annually, Salmonella causes approximately 200 million to over 1 billion infections worldwide (Chlebicz and Śliżewska, 2018; Castro-Vargas et al., 2020), and in Korea, 136 cases of human salmonellosis were also reported from 2018 to 2022, affecting 7,400 patients (MFDS, 2023).
In particular, many researchers reported that poultry products, including eggs and poultry meat, are the primary sources of Salmonella for human (Hoelzer et al., 2011; Antunes et al., 2016). The widespread distribution of Salmonella in broiler production stage has been continuously reported in Korea (Choi et al., 2014; Ha et al., 2018; Wei et al., 2021), and the European Union (EU) also reported that 70% of all Salmonella isolated from foods and animals was from broiler sector (EFSA, 2022). The introduction and dissemination of Salmonella in broiler flocks can occur both vertically by infected breeder chickens and horizontally by infected flocks, contaminated feed and water, wild birds, or other biosecurity violations (Davies and Wales, 2010; Cargnel et al., 2023). In particular, Salmonella-positive commercial broilers are a major role in the dissemination of Salmonella to the slaughterhouse, which directly causes Salmonella food poisoning in human, so reducing Salmonella in broiler farms is essential for food safety (Van Immerseel et al., 2009; Rivera-Pérez et al., 2014).
Although more than 2,600 Salmonella enterica serovars have been identified (Eng et al., 2015), the United States (US) and EU reported that the most important serovars associated with poultry and human infections are S. Enteritidis, S. Typhimurium, S. Typhimurium monophasic variant, S. Infantis, S. Newport, and S. Derby (CDC; 2018; Collins et al., 2022; EFSA, 2022). In particular, S. Infantis is a poultry-adapted Salmonella enterica serovar that is increasingly being reported in broilers and frequently identified in human salmonellosis cases worldwide (Vlaanderen et al., 2019; Newton et al., 2020; EFSA, 2022; Kahn et al., 2022; Alvarez et al., 2023; Montone et al., 2023). Moreover, multidrug-resistant (MDR) S. Infantis carrying the plasmid of emerging S. Infantis (pESI) or pESI-like plasmid associated with antimicrobial resistance, biofilm formation, and resistance to disinfectant has been continuously reported in Europe, US, Russia, and Japan (Yokoyama et al., 2015; Bogomazova et al., 2020; Mughini-Gras et al., 2021; Srednik et al., 2023). Many researchers reported that the pESI plasmid and pESI-like plasmid could confer an advantage of the rapid spread of S. Infantis in a poultry (Aviv et al., 2014, 2016; Franco et al., 2015; Alba et al., 2020; McMillan et al., 2022).
The broiler supply chain is operated as vertically integrated system in many countries (Cesari et al., 2017; Dotas et al., 2021; Solano‐Blanco et al., 2023), and in Korea, integrated broiler operations account for approximately 96.5% of broiler meat production (KAPE, 2022). Considering Salmonella transmission through a variety of routes, including infected breeder chickens and poor biosecurity practices on commercial farms, there may be difference in the prevalence of Salmonella between integrated broiler operations. Therefore, the objective of this study was to compare the distribution of persistent Salmonella serovars in environments and dead chickens between 5 major integrated broiler operations in Korea and provide the genetic and phenotypic characterization of the most common serovar, S. Infantis.
MATERIALS AND METHODS
Sample Collection
According to the standards set by the National Poultry Improvement Plan (NPIP) (USDA, 2019), environment dusts were collected from the houses prior to placement and at depletion of birds, and feces were only collected from the houses at depletion of birds from 58 commercial farms of 5 major integrated broiler operations in 2022. Briefly, approximately 10 g of dust samples were obtained by swabbing 15 different spots per house using sterile surgical gauze moistened with buffered peptone water (BPW; Difco, Sparks, MD). Additionally, approximately 10 g of feces was sampled from 15 different locations per house using sterile shoe covers. All samples were placed into a sterile bag and transported to the laboratory under 4°C conditions. Moreover, chickens that died within 1 week old and at 4 to 5 weeks old per house of each farm were also transported to the laboratory under 4°C conditions. The liver and spleen samples were collected by necropsy, that is, before sampling, the liver and spleen surfaces were decontaminated using a hot sterile spatula, and incised using scalpel blade, after which sterile cotton-tipped swabs were inserted.
Isolation and Identification of Salmonella spp.
The isolation of Salmonella spp. from dust, feces, and organs were performed according to the standards set by the NPIP (USDA, 2019). Briefly, each 10 g of dust and feces samples were inoculated into 100 mL of BPW. After incubation at 37°C ± 2°C for 20 to 24 h, 0.1 mL and 1 mL pre-enriched BPW was inoculated into 10 mL Rappaport Vassiliadis broth (RV broth; Difco) and 10 mL tetrathionate broth (TT broth; Difco), respectively. The RV broth and TT broth were incubated at 42°C for 20 to 24 h and streaked onto Xylose Lysine Tergitol 4 agar (XLT4 agar; Difco). The liver and spleen swabs were inoculated into 10 mL TT broth, incubated at 37°C ± 2°C for 20 to 24 h and streaked onto XLT4 agar. At least 5 presumptive Salmonella colonies were selected from each XLT4 agar and confirmed by identifying the invA gene using polymerase chain reaction (PCR), as described previously (Rahn et al., 1992). Confirmed colonies were serotyped by agglutination tests using O and H antisera (Difco) according to the Kauffmann and White scheme (Grimont and Weill, 2007). A maximum of 3 houses per farm were sampled; however, if the isolates from the same farm at the same time showed the same serovars and antimicrobial susceptibility patterns, only 1 isolate was randomly selected.
Antimicrobial Susceptibility Testing
According to the guidelines of the Clinical and Laboratory Standards Institute guidelines (CLSI, 2023), all the S. Infantis isolates were investigated for antimicrobial resistance by the disk diffusion test using the following antimicrobial disks (BD Biosciences, Sparks, MD): ampicillin (AM, 10 µg), amoxicillin–clavulanate (AMC, 20/10 µg), cefazoline (CZ, 30 µg), cefoxitin (FOX, 30 µg), cefotaxime (CTX, 30 µg), cefepime (FEP, 30 µg), chloramphenicol (C, 30 µg), ciprofloxacin (CIP, 5 µg), gentamicin (GM, 10 µg), imipenem (IPM, 10 µg), nalidixic acid (NA, 30 µg), tetracycline (TE, 30 µg), and trimethoprim–sulfamethoxazole (SXT, 1.25/23.75 µg). Multidrug-resistance (MDR) was defined as resistance to at least 1 agent of 3 or more antimicrobial classes, as described previously (Magiorakos et al., 2012).
Detection of the pESI Plasmid and Virulence Genes
The presence of 3 target genes (repA, ipf, and K88-like) for the pESI plasmid and different virulence genes (cdtB, iron, lpfC, msgA, orgA, pagC, pefA, prgH, sefC, sifA, sipB, sitC, sopB, sopE, spaN, spiA, spvB, stn, tcfA, and tolC) was confirmed by PCR using the primers listed in Table 1. Positive results for all 3 target genes were considered indicative of the pESI plasmid, as described previously (McMillan et al., 2023).
Table 1.
Primers used in this study.
| Target | Sequence (5′→3′) | Size (bp) | References |
|---|---|---|---|
| Identification | |||
| invA | F: ATGCCCGGTAAACAGATGAG | 282 | Rahn et al., (1992) |
| R: CGACAAGACCATCACCAATG | |||
| repA | F: AAGGCGATGGAGCAACTCAG | 158 | McMillan et al., (2023) |
| R: AAGGCGATGGAGCAACTCAG | |||
| ipf | F: ACTGGTATGCTGTCCCTTGC | 177 | McMillan et al., (2023) |
| R: TGCTGCAGTCTTGGCAGTAG | |||
| K88-like | F: TGTATTCCACCCGGATTACTGC | 145 | McMillan et al., (2023) |
| R: GGCATTTCTCCCGGAATGAGG | |||
| Virulence factors | |||
| spvB | F: CTATCAGCCCCGCACGGAGAGCAGTTTTTA | 717 | Skyberg et al., (2006) |
| R: GGAGGAGGCGGTGGCGGTGGCATCATA | |||
| spiA | F: CCAGGGGTCGTTAGTGTATTGCGTGAGATG | 550 | Skyberg et al., (2006) |
| R: CGCGTAACAAAGAACCCGTAGTGATGGATT | |||
| cdtB | F: ACAACTGTCGCATCTCGCCCCGTCATT | 268 | Skyberg et al., (2006) |
| R: CAATTTGCGTGGGTTCTGTAGGTGCGAGT | |||
| msgA | F: GCCAGGCGCACGCGAAATCATCC | 189 | Skyberg et al., (2006) |
| R: GCGACCAGCCACATATCAGCCTCTTCAAAC | |||
| prgH | F: GCCCGAGCAGCCTGAGAAGTTAGAAA | 756 | Skyberg et al., (2006) |
| R: TGAAATGAGCGCCCCTTGAGCCAGTC | |||
| spaN | F: AAAAGCCGTGGAATCCGTTAGTGAAGT | 504 | Skyberg et al., (2006) |
| R: CAGCGCTGGGGATTACCGTTTTG | |||
| orgA | F: TTTTTGGCAATGCATCAGGGAACA | 255 | Skyberg et al., (2006) |
| R: GGCGAAAGCGGGGACGGTATT | |||
| tolC | F: TACCCAGGCGCAAAAAGAGGCTATC | 161 | Skyberg et al., (2006) |
| R: CCGCGTTATCCAGGTTGTTGC | |||
| sitC | F: CAGTATATGCTCAACGCGATGTGGGTCTCC | 768 | Skyberg et al., (2006) |
| R: CGGGGCGAAAATAAAGGCTGTGATGAAC | |||
| lpfC | F: GCCCCGCCTGAAGCCTGTGTTGC | 641 | Skyberg et al., (2006) |
| R: AGGTCGCCGCTGTTTGAGGTTGGATA | |||
| sifA | F: TTTGCCGAACGCGCCCCCACACG | 449 | Skyberg et al., (2006) |
| R: GTTGCCTTTTCTTGCGCTTTCCACCCATCT | |||
| sopB | F: CGGACCGGCCAGCAACAAAACAAGAAGAAG | 220 | Skyberg et al., (2006) |
| R: TAGTGATGCCCGTTATGCGTGAGTGTATT | |||
| iroN | F: ACTGGCACGGCTCGCTGTCGCTCTAT | 1205 | Skyberg et al., (2006) |
| R: CGCTTTACCGCCGTTCTGCCACTGC | |||
| pagC | F: CGCCTTTTCCGTGGGGTATGC | 454 | Skyberg et al., (2006) |
| R: GAAGCCGTTTATTTTTGTAGAGGAGATGTT | |||
| sipB | F: GGACGCCGCCCGGGAAAAACTCTC | 454 | Skyberg et al., (2006) |
| R: ACACTCCCGTCGCCGCCTTCACAA | |||
| stn | F: ATTGAGCGCTTTAATCTCCT | 543 | Choudhury et al. (2016) |
| R: GCTGTTGAATCTGTACCTGA | |||
| sopE | F: GGTAGGGCAGTATTAACCAG | 254 | Choudhury et al. (2016) |
| R: TTTATCTCCCTAGGTAGCCC | |||
| pefA | F: GCCAAAGTACTGGTTGAAAG | 185 | Choudhury et al. (2016) |
| R: TATTTGTAAGCCACTGCGAA | |||
| sefC | F: GGCAGGTCCAAAACTATACA | 609 | Choudhury et al. (2016) |
| R: GCGATAACGAAACACCATTT | |||
| tcfA | F: TCGCTATGTTTGCATGTGGT | 335 | Suez et al., (2013) |
| R: TTCAGGAACAGCCTCGAAGT |
Pulsed-Field Gel Electrophoresis (PFGE)
PFGE was performed by digesting the genomic DNA using the XbaI enzyme (Takara Bio Inc., Shiga, Japan) according to a standard protocol of the Centers for Disease Control and Prevention (CDC, USA), using a CHEF-MAPPER apparatus (Bio-Rad Laboratories, Hercules, CA), as described previously (Ribot et al., 2006). PFGE profiles were analyzed using the Bionumerics Software (Applied Maths, Sint-Martens-Laem, Belgium). Relatedness was calculated using the unweighted pair-group method with arithmetic averages (UPGMA) algorithm based on the Dice similarity index. Isolates that exhibited coefficient of similarity ≥90% were considered genetically closely related (Dionisi et al., 2011).
Statistical Analysis
Statistical analysis was performed using the Pearson's chi-square and Fisher's exact test with Bonferroni correction in IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY). Differences were considered significant at P < 0.05.
RESULTS
Prevalence and Distribution of Serovars of Salmonella spp.
The distribution of Salmonella spp. isolated from 58 commercial farms of 5 integrated broiler operations is shown in Table 2. The prevalence and distribution of serovars in Salmonella-positive farms showed the differences between environments prior to placement and environments at the time of depletion, and between chickens that died within 1 week old and chickens that died at 4 to 5 weeks old. In environmental samples, the prevalence in both dust (16.7–41.7%) and feces (16.7–66.7%) at the time of depletion was higher than in dust prior to placement (0–25.0%) by operations. Although 5 Salmonella serovars, S. Bareilly, S. Enteritidis, S. Infantis, S. Montevideo, and S. Senftenberg were confirmed, the prevalence of S. Infantis-positive farms in dust prior to placement was only 5.2%, but the prevalence in dust and feces at the time of depletion was rapidly increased to 27.6 and 41.4%, respectively (P < 0.05).
Table 2.
Distribution of Salmonella spp. isolated from 58 commercial farms of 5 integrated broiler operations.
| Operation (No. of farms) | Serovar | No. (%) of Salmonella-positive farms |
||||
|---|---|---|---|---|---|---|
| Environments |
Dead chickens |
|||||
| Prior to placement |
At the time of depletion |
Within 1 week old | At 4–5 weeks old | |||
| Dust | Dust | Feces | ||||
| A (n = 12) | S. Infantis | 0 (0.0) | 2 (16.7) | 2 (16.7) | 0 (0.0) | 2 (16.7) |
| S. Thompson | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 0 (0.0) | |
| Total | 0 (0.0) | 2 (16.7) | 2 (16.7) | 1 (8.3) | 2 (16.7) | |
| B (n = 12) | S. Agona | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 0 (0.0) |
| S. Infantis | 1 (8.3) | 3 (25.0) | 5 (41.7) | 1 (8.3) | 2 (16.7) | |
| S. Montevideo | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 0 (0.0) | |
| S. Senftenberg | 0 (0.0) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 0 (0.0) | |
| Total | 1 (8.3) | 3 (25.0) | 6 (50.0) | 3 (25.0) | 2 (16.7) | |
| C (n = 12) | S. Bareilly | 1 (8.3) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 1 (8.3) |
| S. Enteritidis | 0 (0.0) | 0 (0.0) | 2 (16.7) | 3 (25.0) | 0 (0.0) | |
| S. Infantis | 1 (8.3) | 2 (16.7) | 6 (50.0) | 1 (8.3) | 1 (8.3) | |
| S. Senftenberg | 1 (8.3) | 2 (16.7) | 0 (0.0) | 2 (16.7) | 0 (0.0) | |
| S. Thompson | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (16.7) | 1 (8.3) | |
| Total | 3 (25.0) | 4 (33.3) | 8 (66.7) | 7 (58.3) | 3 (25.0) | |
| D (n = 10) | S. Enteritidis | 0 (0.0) | 0 (0.0) | 1 (10.0) | 3 (30.0) | 1 (10.0) |
| S. Infantis | 0 (0.0) | 4 (40.0) | 4 (40.0) | 0 (0.0) | 3 (30.0) | |
| Total | 0 (0.0)B | 4 (40.0)A, B | 5 (50.0)A | 3 (30.0) | 4 (40.0) | |
| E (n = 12) | S. Agona | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 0 (0.0) |
| S. Enteritidis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (8.3) | |
| S. Infantis | 1 (8.3)B | 5 (41.7)A, B | 7 (58.3)A | 0 (0.0)D | 4 (33.3)C | |
| S. Montevideo | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| S. Senftenberg | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 2 (16.7) | |
| Total | 2 (16.7) | 5 (41.7) | 7 (58.3) | 2 (16.7) | 5 (41.7) | |
| Total (n = 58) | S. Agona | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.4) | 0 (0.0) |
| S. Bareilly | 1 (1.7) | 0 (0.0) | 0 (0.0) | 1 (1.7) | 1 (1.7) | |
| S. Enteritidis | 0 (0.0) | 0 (0.0) | 3 (5.2) | 6 (10.3) | 2 (3.4) | |
| S. Infantis | 3 (5.2)B | 16 (27.6)A | 24 (41.4)A | 2 (3.4)D | 12 (20.7)C | |
| S. Montevideo | 1 (1.7) | 0 (0.0) | 0 (0.0) | 1 (1.7) | 0 (0.0) | |
| S. Senftenberg | 1 (1.7) | 2 (3.4) | 1 (1.7) | 3 (5.2) | 2 (3.4) | |
| S. Thompson | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (5.2) | 1 (1.7) | |
| Total | 6 (10.3)B | 18 (31.0)A | 28 (48.3)A | 16 (27.6) | 16 (27.6) | |
Values with different subscript letters (A, B) represent significant differences between environments by operations, while subscript letters (C, D) represent significant differences between dead chickens (P < 0.05).
Moreover, the prevalence of farms with Salmonella in chickens that died within 1 week old and at 4 to 5 weeks old ranged from 8.3 to 58.3% and 16.7 to 41.7% by operations, respectively. The Salmonella serovars isolated from dead chickens were identified as S. Agona, and S. Thompson including 5 Salmonella spp. isolated from environments. Interestingly, S. Infantis was only found in each one (8.3%) farm of operations B and C in chickens that died within 1 week old, but it was confirmed in all 5 integrated operations (8.3–33.3%) in chickens that died at 4 to 5 weeks old. Moreover, S. Enteritidis at chickens that died within 1 week old was also confirmed in 3 (25.0%) farms and 3 (30.0%) farms by operation C and D, respectively, but at chickens that died at 4 to 5 weeks old, the prevalence of positive farms decreased to 0 and 10.0%, respectively.
Distribution of Salmonella spp. Carrying the pESI Plasmid
Distribution of Salmonella spp. carrying the pESI plasmid in 58 commercial farms of 5 integrated broiler operations is presented in Table 3. Interestingly, the pESI plasmid was only detected in S. Infantis, and was absent in all other Salmonella spp., S. Agona, S. Bareilly, S. Enteritidis, S. Montevideo, S. Senftenberg, and S. Thompson. In particular, the prevalence of farms with pESI-positive S. Infantis and pESI-negative S. Infantis in both dust prior to placement and chickens that died within 1 week old showed no significantly differences; however, in environments at the time of depletion and in chickens that died 4 to 5 weeks old, the prevalence of farms with pESI-positive S. Infantis was significantly higher than that of those with pESI-negative S. Infantis (P < 0.05).
Table 3.
Distribution of Salmonella spp. carrying the pESI plasmid in 58 commercial broiler farms of 5 integrated broiler operations.
| Environments |
Dead chickens |
||||
|---|---|---|---|---|---|
| Prior to placement |
At the time of depletion |
||||
| Dust | Dust | Feces | Within 1 week old | At 4–5 weeks old | |
| No. of S. Infantis-positive farms | 3 | 16 | 24 | 2 | 12 |
| No. (%) of pESI- positive | 1 (33.3) | 14 (87.5)A | 18 (75.0)A | 1 (50.0) | 12 (100.0)A |
| No. (%) of pESI-negative | 2 (66.7) | 2 (12.5)B | 6 (25.0)B | 1 (50.0) | 0 (0.0)B |
| No. of Salmonella spp.-positive farms1 | 3 | 2 | 4 | 15 | 6 |
| No. (%) of pESI-positive | 0 (0.0)D | 0 (0.0)D | 0 (0.0)D | 0 (0.0)D | 0 (0.0)D |
| No. (%) of pESI-negative | 3 (100.0)C | 2 (100.0)C | 4 (100.0)C | 15 (100.0)C | 6 (100.0)C |
No. of farms with confirmed Salmonella spp. (S. Agona, S. Bareilly, S. Enteritidis, S. Montevideo, S. Senftenberg, and S. Thompson) except for S. Infantis.
Values with different subscript letters (A, B or C, D) represent significant differences between pESI-positive and pESI-negative farms, respectively (P < 0.05).
Antimicrobial Resistance of S. Infantis
The characteristics of antimicrobial resistance of 136 S. Infantis isolated in this study are shown in Table 4. Although total number of isolates was significantly difference between dust prior to placement (n = 3) and dust (n = 40) and feces (n = 61) at the time of depletion, and between chickens that died within 1 week old (n = 2) and at 4 to 5 weeks old (n = 30), resistance against most antimicrobials tested, except for NA, showed higher in isolates from environments at the time of depletion and chickens that died at 4 to 5 weeks old. In particular, the resistance to AM, C, CZ, CTX, SXT, and TE showed significantly higher in isolates from dust (70.0–95.0%) and feces (60.7–85.2%) at the time of depletion and chickens that died at 4 to 5 weeks old (76.7–100%) than dust prior to placement (33.3%) and chickens that died within 1 week old (0–50.0%), respectively (P < 0.05). Interestingly, pESI-positive S. Infantis showed the significantly higher resistance to AM, C, CZ, CTX, GM, NA, SXT, and TE than pESI-negative S. Infantis (P < 0.05). Moreover, the distribution of MDR isolates was also significantly higher in pESI-positive S. Infantis (99.2%) than in pESI-negative S. Infantis (6.7%) (P < 0.05).
Table 4.
Antimicrobial resistance of 136 S. Infantis isolated from 58 commercial farms of 5 integrated broiler operations.
| Antimicrobial agents | No. of antimicrobial resistant S. Infantis isolates (%) |
||||||
|---|---|---|---|---|---|---|---|
| Environments |
Dead chickens |
Total |
|||||
| Prior to placement |
At the time of depletion |
Within 1 week old (n = 2) | At 4–5 weeks old (n = 30) | pESI-negative (n = 15) | pESI-positive (n = 121) | ||
| Dust (n = 3)1 | Dust (n = 40) | Feces (n=61) | |||||
| Amoxicillin-clavulanate | 0 (0.0) | 5 (12.5) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (5.0) |
| Ampicillin | 1 (33.3)b | 38 (95.0)a | 52 (85.2)a,b | 1 (50.0)d | 29 (96.7)c | 2 (13.3)B | 119 (98.3)A |
| Cefazolin | 1 (33.3)b | 38 (95.0)a | 50 (82.0)a,b | 1 (50.0)d | 29 (96.7)c | 0 (0.0)B | 119 (98.3)A |
| Cefepime | 0 (0.0) | 2 (5.0) | 2 (3.3) | 0 (0.0) | 4 (13.3) | 0 (0.0) | 8 (6.6) |
| Cefotaxime | 1 (33.3)b | 37 (92.5)a | 50 (82.0)a,b | 1 (50.0)d | 29 (96.7)c | 0 (0.0)B | 118 (97.5)A |
| Cefoxitin | 0 (0.0) | 7 (17.5) | 11 (18.0) | 0 (0.0) | 4 (13.3) | 1 (6.7) | 21 (17.4) |
| Chloramphenicol | 1 (33.3)b | 36 (90.0)a | 50 (82.0)a,b | 1 (50.0)d | 30 (100.0)c | 2 (13.3)B | 116 (95.9)A |
| Ciprofloxacin | 0 (0.0) | 3 (7.5) | 2 (3.3) | 0 (0.0) | 1 (3.3) | 0 (0.0) | 6 (5.0) |
| Gentamicin | 1 (33.3) | 24 (60.0) | 29 (47.5) | 1 (50.0) | 15 (50.0) | 0 (0.0)B | 70 (57.9)A |
| Imipenem | 0 (0.0) | 1 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
| Nalidixic acid | 3 (100.0) | 38 (95.0) | 56 (91.8) | 2 (100.0) | 30 (100.0) | 9 (60.0)B | 120 (99.2)A |
| Tetracycline | 1 (33.3)b | 37 (92.5)a | 48 (78.7)a,b | 1 (50.0)d | 30 (100.0)c | 0 (0.0)B | 117 (96.7)A |
| Trimethoprim/sulfamethoxazole | 1 (33.3) | 28 (70.0) | 37 (60.7) | 0 (0.0)d | 23 (76.7)c | 0 (0.0)B | 89 (73.6)A |
| MDR | 1 (33.3)b | 38 (95.0)a | 51 (83.6)a,b | 1 (50.0)d | 30 (100.0)c | 1 (6.7)B | 120 (99.2)A |
n = No. of S. Infantis isolated from 5 integrated broiler operations.
Values with different superscript letters (a, b or c, d) represent significant differences between environments or dead chickens, respectively, while subscript letters (A, B) represent significant differences between pESI-positive and pESI-negative S. Infantis (P < 0.05). Multidrug-resistance (MDR) was defined as resistance to 3 or more antimicrobial classes.
PFGE Analysis and Characteristics of S. Infantis
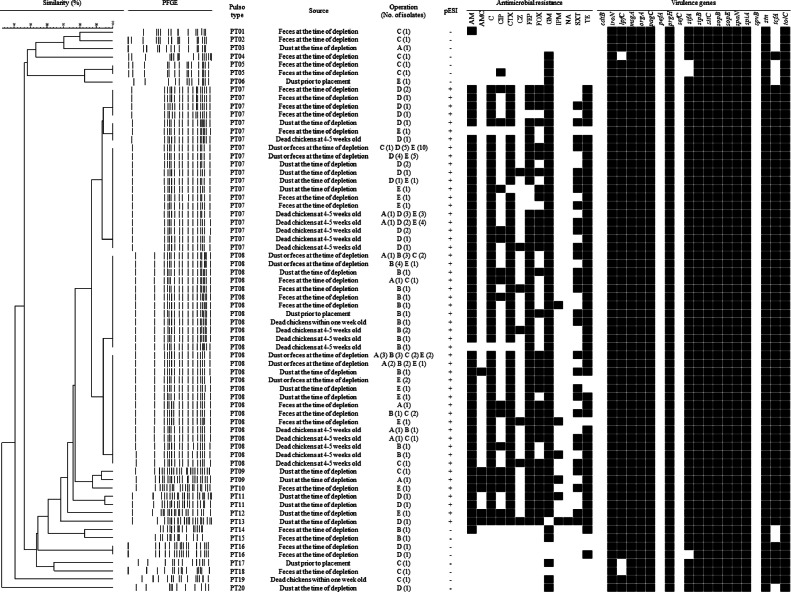
The epidemiological genetic relationship and molecular characterization of 136 S. Infantis isolates are shown in Figure 1. S. Infantis were divided into 20 pulsotypes. Interestingly, 15 pESI-negative S. Infantis were identified as PT01 to PT06 and PT14 to PT20. Otherwise, 121 pESI-positive S. Infantis were identified as PT07 to PT13. In particular, 59 (43.4%) and 55 (40.4%) among 136 isolates were identified as PT07 and PT08, respectively, which were most prevalent types. Additionally, among the 20 virulence genes tested, 13 genes (iroN, msgA, orgA, pagC, prgH, sipB, sitC, sopB, sopE, span, spiA, stn, and tolC) were detected in all S. Infantis regardless of the presence of the pESI plasmid. Also, 3 genes (lpfC, sifA, and tcfA) were identified in all 121 pESI-positive S. Infantis, but in 12 (80.0%), 12 (80.0%), and 6 (40.0%) among 15 pESI-negative S. Infantis, respectively. The cdtB, pefA, sefC, and spvB genes were not detected in any of the S. Infantis isolates.
Figure 1.
Dendrogram showing genetic relationships among 136 S. Infantis isolated from 58 commercial farms of 5 integrated broiler operations characterized by PFGE profiles. S. Infantis isolates showing similarities of < 90% in PFGE were considered to be unrelated. Abbreviations: AM, ampicillin; AMC, amoxicillin-clavulanate; C, chloramphenicol; CIP, ciprofloxacin; CTX, cefotaxime; CZ, cefazolin; FEP, cefepime; FOX, cefoxitin; GM, gentamicin; IPM, imipenem; NA, nalidixic acid, SXT, trimethoprim/sulfamethoxazole; TE, tetracycline.
DISCUSSION
Although Salmonella are primary transmitted to commercial chickens by infecting the ovaries, their presence in settled dust in poultry facilities is also a major cause of Salmonella colonization of chickens through airborne dust during the rearing period (Chinivasagam et al., 2009; Liu et al., 2022; Pal et al., 2022). Rose et al. (1999) and Volkova et al. (2010) have already reported that Salmonella contamination of the previous flock in the grow-out house and the presence of Salmonella in house prior to placement of the flock influence Salmonella prevalence until the end of next flock rearing. In addition, Janning et al. (1994) also reported that Salmonella can survive several weeks even in dry environments, and Wales et al. (2007) reported that the level of environmental contamination of Salmonella increases significantly over time. Namely, environmental Salmonella can be settled in the cecum via oral route, and finally it is re-transmitted to flocks though feces (Wang et al., 2023). Moreover, continuous fecal shedding from infected broilers can be contributor to increase of the level of environmental contamination (Van Immerseel et al., 2004; Marin and Lainez, 2009). In this study, the prevalence of Salmonella-positive farms in dust at the time of depletion was rapidly increased to 16.7 to 41.7% than in dust prior to placement (0–25%) by operations. Therefore, the presence of Salmonella in broiler flock was found to be strongly influenced during the rearing period by dust that were already contaminated prior to placement. Moreover, the prevalence of Salmonella-positive farms in feces at the time of depletion was also high, ranging from 16.7 to 66.7%.
In this study, the prevalence of farms with Salmonella in chickens that died within 1 week old ranged from 0 to 58.3% by operations. Gantois et al. (2009) reported that direct vertical transmission from parent to progeny can occur when breeder chicken with infected reproductive organs lay internally contaminated eggs. In particular, S. Enteritidis, which have high potential for vertical transmission, can lead to serious disease and death in broiler chicks (Suzuki, 1994; Liu et al., 2022). Interestingly, in operations C and D, although S. Enteritidis was not present in dust prior to placement, the prevalence of farms with confirmed S. Enteritidis in chickens that died within 1 week old was 25 and 30%, respectively, therefore, infection to day old chicks via vertical transmission from breeders is strongly suspected. In Korea, all flock found to be infected with S. Gallinarum and S. Pullorum, which are host-adapted Salmonella, have to be only completely slaughtered according to quarantine management instruction for breeder farm and hatchery by the Ministry of Agriculture, Food and Rural Affairs (MAFRA, 2022). Hence, strategies for S. Enteritidis in breeders should also be strengthened by regulatory controls that are available if needed.
But, many Salmonella serovars can exhibit pseudo-vertical transmission, where microbes are initially present on the outside surface of the egg and subsequently penetrate in the shell membranes (Berrang et al., 1999; Cox et al., 2000). Oastler et al. (2022) reported Salmonella contamination tends to increase in hatcheries along the workflow from egg areas through to setters. In this study, the presence of Salmonella in dead chickens might have been caused by contamination at the hatchery; hence, more intensive and strict management should be implemented in hatcheries to prevent the introduction of infected chicks into broiler farms.
Interestingly, in this study, S. Infantis was the most identified of all 5 operations. Namely, S. Infantis in dust prior to placement and in chickens that died within 1 week old was only confirmed in 3 and 2 among 5 operations, but S. Infantis in dust at the time of depletion and in chickens that died at 4 to 5 weeks old was confirmed in all 5 operations. Moreover, the prevalence of S. Infantis-positive farms in dust prior to placement and in chickens that died within 1 week old was only 5.2 and 3.4% among 58 farms, respectively, but the prevalence in dust at the time of depletion and in chickens that died at 4 to 5 weeks old increased rapidly to 27.6 and 20.7%, respectively. In particular, the prevalence of S. Infantis-positive farms in feces at the time of depletion was significantly high (41.4%). In Korea, although previous studies revealed that Salmonella serovars frequently isolated at broiler farms were S. Hadar and S. Montevideo (Choi et al., 2014; Ha et al., 2018; Shang et al., 2018), our finding are in accordance with those of other reports which reported that S. Infantis is the primary serovar in broilers in Europe, United States, Ecuador and Japan (Vinueza-Burgos et al., 2016; Duc et al., 2019; McMillan et al., 2022; EFSA, 2022). Generally, S. Infantis is known to be less invasive than other serovars (Berndt et al., 2007); however, recent studies demonstrated its high ability to colonize the caeca and actively adhere to host cells (Aviv et al., 2019; Drauch et al., 2021). Drauch et al. (2020) also reported that S. Infantis is more resistant to disinfectants and was able to persist on farms despite cleaning and disinfection. Therefore, if strongly cleaning and disinfection procedures are not carried out, S. Infantis can easily spread between flocks and persist longer in environments including feces.
Moreover, several researchers reported that one of the most significant virulence factors of S. Infantis is the acquisition of a conjugative megaplasmid, called pESI or pESI-like plasmid, which provides the bacteria with various genetic properties (Aviv et al., 2014, 2016; Franco et al., 2015; Srednik et al., 2023). In this study, interestingly, the presence of the pESI plasmid was only detected in S. Infantis, and was absent in all other Salmonella spp.. Moreover, the prevalence of farms with pESI-positive S. Infantis showed significantly higher in environments at the time of depletion and chickens that died 4 to 5 weeks old compared to farms with pESI-negative S. Infantis. Aviv et al. (2014) reported that acquisition of the pESI plasmid played an important role in efficient dissemination and successful spread of emergent S. Infantis, which replaced the local S. Infantis population in the short time of only 2-3 yr in Israel. Moreover, Papić et al. (2022) also reported that in Slovenia, pESI-negative S. Infantis isolated from broiler farms has been completely replaced by pESI-positive S. Infantis since 2010. Therefore, the introduction of S. Infantis carrying the pESI plasmid can lead to the rapid spread of this serovar in broiler farms despite the short rearing period of commercial broilers, as demonstrated in this study. Moreover, Cohen et al. (2022) reported that the pESI plasmid could transfer from S. Infantis to other Salmonella serovars via conjugation. Although, in this study, the presence of the pESI plasmid was only identified in S. Infantis, Cohen et al. (2022) and Santos et al. (2022) reported the presence of the pESI and pESI-like plasmid in S. Agona, S. Muenchen, S. Schwarzengrund, and S. Senftenberg. Therefore, constant surveillance is necessary to determine if the pESI plasmid is present or emerges in other Salmonella serovars in broiler farms.
The pESI plasmid and pESI-like plasmid are also closely associated with the MDR phenotype (Aviv et al., 2014; Franco et al., 2015; Bogomazova et al., 2020; Lee et al., 2021; Papić et al., 2022; Alvarez et al., 2023). In this study, the prevalence of MDR was significantly higher in pESI-positive S. Infantis (99.2%) than that of in pESI-negative S. Infantis (6.7%). In particular, all pESI-negative S. Infantis except for one isolate showed susceptibility to cephalosporin, but the prevalence of pESI-positive S. Infantis resistant to CTX representing third-generation cephalosporin was 97.5%. Third-generation cephalosporins are one of the treatment options for severe human salmonellosis cases (WHO, 2023), and several researchers reported that the pESI-like plasmid harboring the extended-spectrum beta-lactamase genes, a major cause of third-generation cephalosporin resistance, were observed in S. Infantis isolated from broilers and humans (Franco et al., 2015; Lee et al., 2021; Pietsch et al., 2021). Therefore, these results indicate that MDR S. Infantis carrying the pESI plasmid is the most widespread in the country, and further studies regarding the epidemiology and control of this serovar are required to prevent transmission from poultry to humans through the food chain.
In this study, although all S. Infantis were divided into 20 pulsotypes, the distribution of pulsotypes between pESI-positive and pESI-negative S. Infantis were clearly different. Moreover, the major pulsotypes of pESI-positive S. Infantis were PT07 (43.4%) and PT08 (40.4%). These results are consistent with other studies, which showed high genetic homogeneity among the analyzed S. Infantis isolates (Vinueza-Burgos et al., 2016; Pate et al., 2019). Alba et al. (2020) also reported that pESI-positive S. Infantis, which is widespread in Europe, were high genetically homogeneous as in this study.
Furthermore, in this study, all pESI-positive and pESI-negative S. Infantis harbored 13 virulence genes (iroN, msgA, orgA, pagC, prgH, sipB, sitC, sopB, sopE, spaN, spiA, stn, and tolC), simultaneously. These virulence genes, which are associated with host recognition and invasion, intracellular survival, filamentous structure formation, iron metabolism, and enterotoxin, are known to be frequently found in Salmonella serovars including S. Infantis (Choudhury et al., 2016; Kim and Lee, 2017; Amini et al., 2018; Karacan Sever and Akan, 2019). However, 2 genes (lpfC and sifA), which plays a role in biofilm formation and bacterial replication (Beuzón et al., 2000; Ledeboer et al., 2006), were detected in all pESI-positive S. Infantis, but in 12 (80.0%) and 12 (80.0%) of the 15 pESI-negative S. Infantis, respectively. Moreover, tcfA, which is related with intestinal colonization of S. Infantis (Azriel et al., 2017), was also detected in 121 (100.0%) pESI-positive S. Infantis, but in 6 (40.0%) pESI-negative S. Infantis. These results are consistent with other reports that S. Infantis carrying the pESI and pESI-like plasmid have increased virulence factors, which provide significant advantages in host infection and persistence on environments (Aviv et al., 2014; Franco et al., 2015; Srednik et al., 2023). But, in this study, cdtB, which is typhoid-associated virulence gene (Haghjoo and Galán, 2004), and sefA, spvB, and pefA, which are closely associated with specific serovars, such as S. Enteritidis and S. Typhimurium (Karasova et al., 2009; Amini et al., 2018; Borges et al., 2019), were not detected. To the best of our knowledge, this study is the first report on emergence of S. Infantis carrying the pESI plasmid from commercial broiler farms in Korea. Therefore, this study could provide valuable information for strategies to control Salmonella in the broiler industry in Korea.
ACKNOWLEDGMENTS
This work was supported by the Animal and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural affairs, Republic of Korea (Grant Number Z-1543061-2021-23-02).
DISCLOSURES
The authors declare that they have no competing interest.
REFERENCES
- Alba P., Leekitcharoenphon P., Carfora V., Amoruso R., Cordaro G., Di Matteo P., Ianzano A., Iurescia M., Diaconu E.L., E.-E.-A. N. Study Group. Pedersen S.K., Guerra B., Hendriksen R.S., Franco A., Battisti A. Molecular epidemiology of Salmonella infantis in Europe: insights into the success of the bacterial host and its parasitic pESI-like megaplasmid. Microb. Genom. 2020;6:1–12. doi: 10.1099/mgen.0.000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez D.M., Barrón-Montenegro R., Conejeros J., Rivera D., Undurraga E.A., Moreno-Switt A.I. A review of the global emergence of multidrug-resistant Salmonella enterica subsp. enterica serovar infantis. Int. J. Food Microbiol. 2023;403 doi: 10.1016/j.ijfoodmicro.2023.110297. [DOI] [PubMed] [Google Scholar]
- Amini K., Salehi T.Z., Nikbakht G., Ranjbar R., Amini J., Ashrafganjooei S.B. Molecular detection of invA and spv virulence genes in Salmonella enteritidis isolated from human and animals in Iran. African J. Microb. Res. 2018;4:2202–2210. [Google Scholar]
- Antunes P., Mourão J., Campos J., Peixe L. Salmonellosis: the role of poultry meat. Clin. Microbiol. Infect. 2016;22:110–121. doi: 10.1016/j.cmi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Aviv G., Cornelius A., Davidovich M., Cohen H., Suwandi A., Galeev A., Steck N., Azriel S., Rokney A., Valinsky L., Rahav G., Grassl G.A., Gal-Mor O. Differences in the expression of SPI-1 genes pathogenicity and epidemiology between the emerging Salmonella enterica serovar infantis and the model Salmonella enterica serovar typhimurium. J. Infect. Dis. 2019;220:1071–1081. doi: 10.1093/infdis/jiz235. [DOI] [PubMed] [Google Scholar]
- Aviv G., Rahav G., Gal-Mor O. Horizontal transfer of the Salmonella enterica serovar infantis resistance and virulence plasmid pESI to the gut microbiota of warm-blooded hosts. MBio. 2016;7:1–12. doi: 10.1128/mBio.01395-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv G., Tsyba K., Steck N., Salmon-Divon M., Cornelius A., Rahav G., Grassl G.A., Gal-Mor O. A unique megaplasmid contributes to stress tolerance and pathogenicity of an emergent Salmonella enterica serovar infantis strain. Environ Microbiol. 2014;16:977–994. doi: 10.1111/1462-2920.12351. [DOI] [PubMed] [Google Scholar]
- Azriel S., Goren A., Shomer I., Aviv G., Rahav G., Gal-Mor O. The Typhi colonization factor (Tcf) is encoded by multiple non-typhoidal Salmonella serovars but exhibits a varying expression profile and interchanging contribution to intestinal colonization. Virulence. 2017;8:1791–1807. doi: 10.1080/21505594.2017.1380766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A., Wilhelm A., Jugert C., Pieper J., Sachse K., Methner U. Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect. Immun. 2007;75:5993–6007. doi: 10.1128/IAI.00695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrang M.E., Cox N.A., Frank J.F., Buhr R.J. Bacterial penetration of the eggshell and shell membranes of the chicken hatching egg: a review. J. Appl. Poult. Res. 1999;8:499–504. [Google Scholar]
- Beuzón C.R., Méresse S., Unsworth K.E., Ruíz-Albert J., Garvis S., Waterman S.R., Ryder T.A., Boucrot E., Holden D.W. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000;19:3235–3249. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogomazova A.N., Gordeeva V.D., Krylova E.V., Soltynskaya I.V., Davydova E.E., Ivanova O.E., Komarov A.A. Mega-plasmid found worldwide confers multiple antimicrobial resistance in Salmonella infantis of broiler origin in Russia. Int. J. Food Microbiol. 2020;319 doi: 10.1016/j.ijfoodmicro.2019.108497. [DOI] [PubMed] [Google Scholar]
- Borges K.A., Furian T.Q., Souza S.N., Salle C.T.P., Moraes H.L.S., Nascimento V.P. Antimicrobial resistance and molecular characterization of Salmonella Enterica serotypes isolated from poultry sources in Brazil. Braz. J. Poult. Sci. 2019;21:001–008. [Google Scholar]
- Cargnel M., Filippitzi M., Van Cauteren D., Mattheus W., Botteldoorn N., Cambier L., Welby S. Assessing evidence of a potential Salmonella transmission across the poultry food chain. Zoonoses Public Health. 2023;70:22–45. doi: 10.1111/zph.12998. [DOI] [PubMed] [Google Scholar]
- Castro-Vargas R.E., Herrera-Sánchez M.P., Rodríguez-Hernández R., Rondón-Barragán I.S. Antibiotic resistance in Salmonella spp. isolated from poultry: a global overview. Vet. World. 2020;13:2070–2084. doi: 10.14202/vetworld.2020.2070-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) CDC; Atlanta, GA: 2018. National Enteric Diseases Surveillance: Salmonella Annual Report 2016. [Google Scholar]
- Cesari V., Zucali M., Sandrucci A., Tamburini A., Bava L., Toschi I. Environmental impact assessment of an Italian vertically integrated broiler system through a life cycle approach. J. Clean. Prod. 2017;143:904–911. [Google Scholar]
- Chinivasagam H.N., Tran T., Maddock L., Gale A., Blackall P.J. Mechanically Ventilated broiler sheds: a possible source of aerosolized Salmonella, Campylobacter, and Escherichia coli. Appl. Environ. Microbiol. 2009;75:7417–7425. doi: 10.1128/AEM.01380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebicz A., Śliżewska K. Campylobacteriosis, Salmonellosis, Yersiniosis, and Listeriosis as zoonotic foodborne diseases: a review. Int. J. Environ. Res. Public Health. 2018;15:1–28. doi: 10.3390/ijerph15050863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.-W., Ha J.-S., Kim B.-Y., Lee D.-H., Park J.-K., Youn H.-N., Hong Y.-H., Lee S.-B., Lee J.-B., Park S.-Y., Choi I.-S., Song C.-S. Prevalence and characterization of Salmonella species in entire steps of a single integrated broiler supply chain in Korea. Poult. Sci. 2014;93:1251–1257. doi: 10.3382/ps.2013-03558. [DOI] [PubMed] [Google Scholar]
- Choudhury M., Borah P., Sarma H.K., Barkalita L.M., Deka N.K., Hussain I., Hussain M.I. Multiplex-PCR assay for detection of some major virulence genes of Salmonella enterica serovars from diverse sources. Curr. Sci. 2016;111:1252–1258. [Google Scholar]
- Clinical and laboratory Standards Institute (CLSI) 33rd ed. CLSI; Wayne, PA: 2023. Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- Cohen E., Kriger O., Amit S., Davidovich M., Rahav G., Gal-Mor O. The emergence of a multidrug resistant Salmonella Muenchen in Israel is associated with horizontal acquisition of the epidemic pESI plasmid. Clin. Microbiol. Infect. 2022;28:1499.e7–1499.e14. doi: 10.1016/j.cmi.2022.05.029. [DOI] [PubMed] [Google Scholar]
- Collins J.P., Shah H.J., Weller D.L., Ray L.C., Smith K., McGuire S., Trevejo R.T., Jervis R.H., Vugia D.J., Rissman T., Garman K.N., Lathrop S., LaClair B., Boyle M.M., Harris S., Kufel J.Z., Tauxe R.V., Bruce B.B., Ros E.B., Griffin P.M., Payne D.C. Preliminary incidence and trends of infections caused by pathogens transmitted commonly through food - foodborne diseases active surveillance network, 10 U.S. Sites, 2016-2021. MMWR Morb Mortal Wkly Rep. 2022;1:1260–1264. doi: 10.15585/mmwr.mm7140a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox N.A., Berrang M.E., Cason J.A. Salmonella penetration of egg shells and proliferation in broiler hatching eggs–a review. Poult. Sci. 2000;79:1571–1574. doi: 10.1093/ps/79.11.1571. [DOI] [PubMed] [Google Scholar]
- Davies R.H., Wales A.D. Investigations into Salmonella contamination in poultry feedmills in the United Kingdom. J. Appl. Microbiol. 2010;109:1430–1440. doi: 10.1111/j.1365-2672.2010.04767.x. [DOI] [PubMed] [Google Scholar]
- Dionisi A.M., Lucarelli C., Benedetti I., Owczarek S., Luzzi I. Molecular characterisation of multidrug-resistant Salmonella enterica serotype infantis from humans, animals and the environment in Italy. Int. J. Antimicrob. Agents. 2011;38:384–389. doi: 10.1016/j.ijantimicag.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Dos Santos A.M.P., Panzenhagen P., Ferrari R.G., Conte-Junior C.A. Large-scale genomic analysis reveals the pESI-like megaplasmid presence in Salmonella Agona, Muenchen, Schwarzengrund, and Senftenberg. Food Microbiol. 2022;108 doi: 10.1016/j.fm.2022.104112. [DOI] [PubMed] [Google Scholar]
- Dotas V., Gourdouvelis D., Hatzizisis L., Kaimakamis I., Mitsopoulos I., Symeon G. Typology, structural characterization and sustainability of integrated broiler farming system in Epirus, Greece. Sustainability. 2021;13:13084. [Google Scholar]
- Drauch V., Ibesich C., Vogl C., Hess M., Hess C. In-vitro testing of bacteriostatic and bactericidal efficacy of commercial disinfectants against Salmonella infantis reveals substantial differences between products and bacterial strains. Int. J. Food Microbiol. 2020;328 doi: 10.1016/j.ijfoodmicro.2020.108660. [DOI] [PubMed] [Google Scholar]
- Drauch V., Kornschober C., Palmieri N., Hess M., Hess C. Infection dynamics of Salmonella infantis strains displaying different genetic backgrounds – with or without pESI-like plasmid – vary considerably. Emerg. Microbes Infect. 2021;10:1471–1480. doi: 10.1080/22221751.2021.1951124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc V.M., Nakamoto Y., Fujiwara A., Toyofuku H., Obi T., Chuma T. Prevalence of Salmonella in broiler chickens in Kagoshima, Japan in 2009 to 2012 and the relationship between serovars changing and antimicrobial resistance. BMC Vet. Res. 2019;15:108. doi: 10.1186/s12917-019-1836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng S.-K., Pusparajah P., Mutalib N.-S.A.b, Ser H.-L., Chan K.-G., Lee L.-H. Salmonella: a review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015;8:284–293. [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC) The European union one health 2021 Zoonoses report. EFSA J. 2022;20:e07666. doi: 10.2903/j.efsa.2022.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A., Leekitcharoenphon P., Feltrin F., Alba P., Cordaro G., Iurescia M., Tolli R., D'Incau M., Staffolani M., Di Giannatale E., Hendriksen R.S., Battisti A. Emergence of a clonal lineage of multidrug-resistant ESBL-producing Salmonella infantis transmitted from broilers and broiler meat to humans in Italy between 2011 and 2014. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantois I., Ducatelle R., Pasmans F., Haesebrouck F., Gast R., Humphrey T.J., Van Immerseel F. Mechanisms of egg contamination by Salmonella Enteritidis. FEMS Microbiol. Rev. 2009;33:718–738. doi: 10.1111/j.1574-6976.2008.00161.x. [DOI] [PubMed] [Google Scholar]
- Grimont P.A., Weill F.-X. Institut Pasteur; Paris, France: 2007. Antigenic Formulae of the Salmonella Serovars. WHO Collaborating Center for Reference and Research on Salmonella. [Google Scholar]
- Ha J.S., Seo K.W., Bin Kim Y., Kang M.S., Song C.-S., Lee Y.J. Prevalence and characterization of Salmonella in two integrated broiler operations in Korea. Ir. Vet. J. 2018;71:3. doi: 10.1186/s13620-018-0114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghjoo E., Galán J.E. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl. Acad. Sci. 2004;101:4614–4619. doi: 10.1073/pnas.0400932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzer K., Switt A.M., Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet. Res. 2011;42:34. doi: 10.1186/1297-9716-42-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janning B., in't Veld P.H., Notermans S., Krämer J. Resistance of bacterial strains to dry conditions: use of anhydrous silica gel in a desiccation model system. J. Appl. Bacteriol. 1994;77:319–324. doi: 10.1111/j.1365-2672.1994.tb03080.x. [DOI] [PubMed] [Google Scholar]
- Karacan Sever N., Akan M. Molecular analysis of virulence genes of Salmonella infantis isolated from chickens and turkeys. Microb. Pathog. 2019;126:199–204. doi: 10.1016/j.micpath.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Karasova D., Havlickova H., Sisak F., Rychlik I. Deletion of sodCI and spvBC in Salmonella enterica serovar enteritidis reduced its virulence to the natural virulence of serovars agona, hadar and infantis for mice but not for chickens early after infection. Vet. Microbiol. 2009;139:304–309. doi: 10.1016/j.vetmic.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Khan A.S., Pierneef R.E., Gonzalez-Escalona N., Maguire M., Li C., Tyson G.H., Ayers S., Georges K., Abebe W., Adesiyun A.A. Molecular characterization of Salmonella detected along the broiler production chain in Trinidad and Tobago. Microorganisms. 2022;10:570. doi: 10.3390/microorganisms10030570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Lee Y.J. Molecular characterization of antimicrobial resistant non-typhoidal Salmonella from poultry industries in Korea. Ir. Vet. J. 2017;70:20. doi: 10.1186/s13620-017-0095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korea Institute for Animal Products Quality Evaluation (KAPE) KAPE; Bundang: 2022. Research on the Actual Conditions of Livestock Distribution System. [Google Scholar]
- Ledeboer N.A., Frye J.G., McClelland M., Jones B.D. Salmonella enterica serovar typhimurium requires the Lpf, Pef, and Tafi Fimbriae for biofilm formation on HEp-2 tissue culture cells and chicken intestinal epithelium. Infect. Immun. 2006;74:3156–3169. doi: 10.1128/IAI.01428-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.W.Y., Mattock J., Greig D.R., Langridge G.C., Baker D., Bloomfield S., Mather A.E., Wain J.R., Edwards A.M., Hartman H., Dallman T.J., Chattaway M.A., Nair S. Characterization of a pESI-like plasmid and analysis of multidrug-resistant Salmonella enterica infantis isolates in England and Wales. Microb. Genom. 2021;7 doi: 10.1099/mgen.0.000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Zhang X., Ding X., Bin P., Zhu G. The vertical transmission of Salmonella enteritidis in a one-health context. One Heal. 2022;16 doi: 10.1016/j.onehlt.2022.100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiorakos A.-P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., Paterson D.L., Rice L.B., Stelling J., Struelens M.J., Vatopoulos A., Weber J.T., Monnet D.L. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- Marin C., Lainez M. Salmonella detection in feces during broiler rearing and after live transport to the slaughterhouse. Poult. Sci. 2009;88:1999–2005. doi: 10.3382/ps.2009-00040. [DOI] [PubMed] [Google Scholar]
- McMillan E.A., Hiott L.M., Carrico J.A., Machado M.P., Pouseele H., Jackson C.R., Frye J.G. Polymerase chain reaction for the in vitro detection of the pESI plasmid associated with the globally circulating Salmonella infantis outbreak strain. Lett. Appl. Microbiol. 2023;76:1–8. doi: 10.1093/lambio/ovad088. [DOI] [PubMed] [Google Scholar]
- McMillan E.A., Weinroth M.D., Frye J.G. Increased Prevalence of Salmonella infantis isolated from raw chicken and turkey products in the United States is due to a single clonal lineage carrying the pESI Plasmid. Microorganisms. 2022;10:1478. doi: 10.3390/microorganisms10071478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Agriculture, Food and Rural Affairs (MAFRA) MARFA; Sejong: 2022. Quarantine Management Instruction for Breeder farm and Hatchery. [Google Scholar]
- Ministry of Food and Drug Safety (MFDS). 2023. MFDS. Accessed Dec. 2023. https://www.foodsafetykorea.go.kr/portal/healthyfoodlife/foodPoisoningStat.do?menu_no=519&menu_grp=MENU_GRP02.
- Montone A.M.I., Cutarelli A., Peruzy M.F., Tela I.L.a, Brunetti R., Pirofalo M.G., Folliero V., Balestrieri A., Murru N., Capuano F. Antimicrobial resistance and genomic characterization of Salmonella infantis from different sources. Int J Mol Sci. 2023;24:5492. doi: 10.3390/ijms24065492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughini-Gras L., van Hoek A.H.A.M., Cuperus T., Dam-Deisz C., van Overbeek W., van den Beld M., Wit B., Rapallini M., Wullings B., Franz E., van der Giessen J., Dierikx C., Opsteegh M. Prevalence, risk factors and genetic traits of Salmonella infantis in Dutch broiler flocks. Vet. Microbiol. 2021;258 doi: 10.1016/j.vetmic.2021.109120. [DOI] [PubMed] [Google Scholar]
- Newton K., Gosling B., Rabie A., Davies R. Field investigations of multidrug-resistant Salmonella infantis epidemic strain incursions into broiler flocks in England and Wales. Avian Pathol. 2020;49:631–641. doi: 10.1080/03079457.2020.1809634. [DOI] [PubMed] [Google Scholar]
- Oastler C.E., Nichols C., Newton K., Cawthraw S., Gosling R.J., Martelli F., Wales A.D., Davies R.H. Observations on the distribution and control of Salmonella in commercial broiler hatcheries in Great Britain. Zoonoses Public Health. 2022;69:487–498. doi: 10.1111/zph.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A., Jackson A.P., Urrutia A., Macklin K.S., Price S.B., Buhr R.J., Bourassa D.V. Research note: bacterial composition of settled dust during grow out of broiler chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papić B., Kušar D., Mićunović J., Pirš M., Ocepek M., Avberšek J. Clonal Spread of pESI-positive multidrug-resistant ST32 Salmonella enterica serovar infantis isolates among broilers and humans in Slovenia. Microbiol. Spectr. 2022;10 doi: 10.1128/spectrum.02481-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate M., Mičunovič J., Golob M., Vestby L.K., Ocepek M. Salmonella infantis in broiler flocks in Slovenia: the prevalence of multidrug resistant strains with high genetic homogeneity and low biofilm-forming ability. Biomed Res. Int. 2019;2019:1–13. doi: 10.1155/2019/4981463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietsch M., Simon S., Meinen A., Trost E., Banerji S., Pfeifer Y., Flieger A. Third generation cephalosporin resistance in clinical non-typhoidal Salmonella enterica in Germany and emergence of blaCTX-M-harbouring pESI plasmids. Microb. Genomics. 2021;7 doi: 10.1099/mgen.0.000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn K., De Grandis S.A., Clarke R.C., McEwen S.A., Galán J.E., Ginocchio C., Curtiss R., Gyles C.L. Amplification of an invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol. Cell. Probes. 1992;6:271–279. doi: 10.1016/0890-8508(92)90002-f. [DOI] [PubMed] [Google Scholar]
- Ribot E.M., Fair M.A., Gautom R., Cameron D.N., Hunter S.B., Swaminathan B., Barrett T.J. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 2006;3:59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]
- Rivera-Pérez W., Barquero-Calvo E., Zamora-Sanabria R. Salmonella contamination risk points in broiler carcasses during slaughter line processing. J. Food Prot. 2014;77:2031–2034. doi: 10.4315/0362-028X.JFP-14-052. [DOI] [PubMed] [Google Scholar]
- Rose N., Beaudeau F., Drouin P., Toux J.., Rose V., Colin P. Risk factors for Salmonella enterica subsp. enterica contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med. 1999;39:265–277. doi: 10.1016/s0167-5877(99)00002-1. [DOI] [PubMed] [Google Scholar]
- Shang K., Wei B., Kang M. Distribution and dissemination of antimicrobial-resistant Salmonella in broiler farms with or without enrofloxacin use. BMC Vet. Res. 2018;14:257. doi: 10.1186/s12917-018-1590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyberg J.A., Logue C.M., Nolan L.K. Virulence genotyping of Salmonella spp. with multiplex PCR. Avian Dis. 2006;50:77–81. doi: 10.1637/7417.1. [DOI] [PubMed] [Google Scholar]
- Solano-Blanco A.L., González J.E., Gómez-Rueda L.O., Vargas-Sánchez J.J., Medaglia A.L. Integrated planning decisions in the broiler chicken supply chain. Int. Trans. Oper. Res. 2023;30:1931–1954. [Google Scholar]
- Srednik M.E., Morningstar-Shaw B.R., Hicks J.A., Tong C., Mackie T.A., Schlater L.K. Whole-genome sequencing and phylogenetic analysis capture the emergence of a multi-drug resistant Salmonella enterica serovar infantis clone from diagnostic animal samples in the United States. Front. Microbiol. 2023;14:1–12. doi: 10.3389/fmicb.2023.1166908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suez J., Porwollik S., Dagan A., Marzel A., Schorr Y.I., Desai P.T., Agmon V., McClelland M., Rahav G., Gal-Mor O. Virulence gene profiling and pathogenicity characterization of non-typhoidal Salmonella accounted for invasive disease in humans. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S. Pathogenicity of Salmonella enteritidis in poultry. Int. J. Food Microbiol. 1994;21:89–105. doi: 10.1016/0168-1605(94)90203-8. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture (USDA) USDA; Washington DC: 2019. National Poultry Improvement Plan Program Standards. [Google Scholar]
- Van Immerseel F., Meulemans L., De Buck J., Pasmans F., Velge P., Bottreau E., Haesebrouck F., Ducatelle R. Bacteria – host interactions of Salmonella Paratyphi B dT+ in poultry. Epidemiol. Infect. 2004;132:239–243. doi: 10.1017/s0950268803001687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel F., De Zutter L., Houf K., Pasmans F., Haesebrouck F., Ducatelle R. Strategies to control Salmonella in the broiler production chain. Worlds. Poult. Sci. J. 2009;65:367–392. [Google Scholar]
- Vinueza-Burgos C., Cevallos M., Ron-Garrido L., Bertrand S., De Zutter L. Prevalence and diversity of Salmonella serotypes in ecuadorian broilers at slaughter age. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaanderen F., M. Uiterwijk, T. Cuperus, I. Keur, M. De Rosa, H. Rozendaal, M. G. J. Koene, H. Schreurs, R. Nijsse, M. Nielen, I. Friesema, W. Van Pelt, E. Franz, L. Hogerwerf, M. Opsteegh, and C. B. Maassen. 2019. Zoönosen. RIVM ed. RIVM, Bilthoven.
- Volkova V.V., Bailey R.H., Rybolt M.L., Dazo-Galarneau K., Hubbard S.A., Magee D., Byrd J.A., Wills R.W. Inter-relationships of Salmonella status of flock and grow-out environment at sequential segments in broiler production and processing. Zoonoses Public Health. 2010;57:463–475. doi: 10.1111/j.1863-2378.2009.01263.x. [DOI] [PubMed] [Google Scholar]
- Wales A., Breslin M., Carter B., Sayers R., Davies R. A longitudinal study of environmental Salmonella contamination in caged and free-range layer flocks. Avian Pathol. 2007;36:187–197. doi: 10.1080/03079450701338755. [DOI] [PubMed] [Google Scholar]
- Wang J., Vaddu S., Bhumanapalli S., Mishra A., Applegate T., Singh M., Thippareddi H. A systematic review and meta-analysis of the sources of Salmonella in poultry production (pre-harvest) and their relative contributions to the microbial risk of poultry meat. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B., Shang K., Cha S.Y., Zhang J.F., Jang H.K., Kang M. Clonal dissemination of Salmonella enterica serovar albany with concurrent resistance to ampicillin, chloramphenicol, streptomycin, sulfisoxazole, tetracycline, and nalidixic acid in broiler chicken in Korea. Poult Sci. 2021;100 doi: 10.1016/j.psj.2021.101141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) WHO; Generva: 2023. WHO Model List of Essential Medicines for Children, 23rd list. [Google Scholar]
- Yokoyama E., Ando N., Ohta T., Kanada A., Shiwa Y., Ishige T., Murakami K., Kikuchi T., Murakami S. A novel subpopulation of Salmonella enterica serovar infantis strains isolated from broiler chicken organs other than the gastrointestinal tract. Vet. Microbiol. 2015;175:312–318. doi: 10.1016/j.vetmic.2014.11.024. [DOI] [PubMed] [Google Scholar]