Abstract
Background
The majority of randomized controlled trials (RCTs) investigating venous thromboembolism (VTE) prophylaxis in patients with cancer involve commercial sponsorship. Commercial sponsorship overcomes feasibility limitations inherent in RCTs, such as recruitment and funding, but has attracted scrutiny for its potential for bias.
Objectives
In RCTs of VTE prophylaxis in patients with cancer, how do trial characteristics compare between commercially sponsored RCTs and noncommercially sponsored RCTs?
Methods
Medline, Embase, and Cochrane Central Register of Controlled Trials were searched for RCTs that investigated at least 1 pharmacologic intervention for VTE prophylaxis in adult patients with cancer. Screening and data extraction were conducted by independent reviewers. Outcomes included trial characteristics, reporting of favorable outcomes, protocol-manuscript discrepancies, and appraisal of spin. Outcomes were compared using the independent t-test, Mann–Whitney U-test, Pearson chi-squared test, and Fisher’s exact test. Logistic regression was performed to identify factors associated with possible bias.
Results
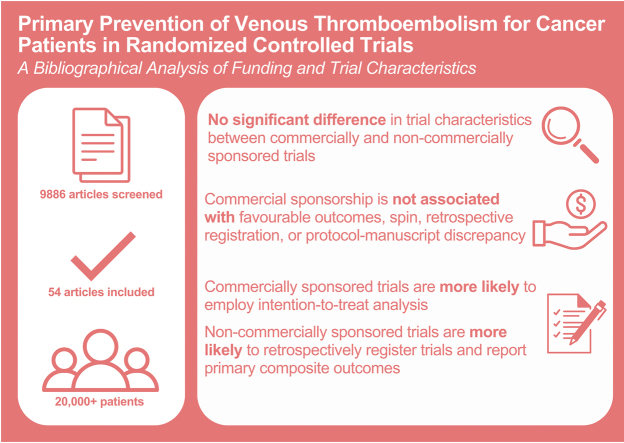
Of the 54 trials analyzed, 34 (63%) reported commercial sponsorship. Commercial sponsorship was not associated with the reporting of favorable outcomes, presence of spin, retrospective registration, or protocol-manuscript discrepancy. Spin was most prevalent in the abstract conclusions (9 out of 17 [53.3%]) and manuscript conclusions (8 out of 17 [46.7%]).
Commercially sponsored trials had a higher rate of intention-to-treat analysis. Noncommercially sponsored trials were more likely to report retrospective registration of trial protocol and the use of composite primary outcomes.
Conclusion
There were few significant differences between trial characteristics, suggesting that the evidence from commercially sponsored trials investigating VTE prophylaxis in patients with cancer is unlikely to be subject to bias attributable to commercial sponsorship.
Keywords: bias∗, cancer, funding source, neoplasms∗, primary prevention∗, randomized controlled trials∗, sponsorship, trial characteristics, venous thromboembolism∗, VTE prophylaxis
Graphical abstract
Essentials
-
•
Many venous thromboembolism randomized controlled trials (RCTs) are commercially sponsored.
-
•
The funding source can affect the characteristics of RCTs of venous thromboembolism prophylaxis in patients with cancer.
-
•
Commercial and noncommercially sponsored RCTs are comparable in trial characteristics and outcome.
-
•
Commercially sponsored RCTs in this field are unlikely to be subject to bias.
1. Introduction
Randomized control trials (RCTs) play an important role in informing evidence-based practice in thromboprophylaxis. A high-quality RCT requires significant resources to ensure methodological rigor and to address challenges with loss to follow-up and limited sample size [1]. However, the source of trial funding may influence the reporting and interpretation of study findings through the participation of the sponsor in the study design, data collection and analysis, and manuscript writing [2]. Recent evidence has found that commercially sponsored trials may be at greater likelihood of reporting favorable outcomes, employing a noninferiority design, and being published in a higher-impact journal [[3], [4], [5], [6], [7], [8]]. Commercially sponsored groups were also found to be more likely to influence the design, methods, and reporting of RCTs through paid consultancy fees and honorariums [5,9]. On the other hand, some studies have found that selective reporting of outcome also occurs frequently in government-funded trials [10]. Others have found no significant differences in study outcomes between commercially sponsored and noncommercially sponsored trials [11,12].
Early RCTs investigated the efficacy of anticoagulants, such as unfractionated heparin, vitamin K antagonists, and warfarin, as methods of primary prophylaxis [[13], [14], [15], [16]]. Later trials established low-molecular-weight heparins (LMWHs) as the gold standard for venous thromboembolism (VTE) prophylaxis [[13], [14], [15], [16], [17], [18]]. Currently, clinical guidelines still recommend LMWHs for primary VTE prophylaxis for patients with ambulatory cancer with a low risk of bleeding, although there is a lack of strong evidence to support these recommendations [19,20]. RCTs investigating primary thromboprophylaxis are needed to improve the quality of evidence and better define clinical practice guidelines. More recently, direct oral anticoagulants have become a subject of interest in cancer-associated thromboprophylaxis research [[21], [22], [23], [24]]. It has been suggested that certain direct oral anticoagulants (apixaban and rivaroxaban) may be appropriate primary prophylactic agents in patients with cancer.
Spin, defined as “the manipulation of scientific language that skews the interpretation of results,” threatens the accurate interpretation of trial results and application by clinicians [25,26]. Authors may consciously or subconsciously use spin in their interpretation of the results to suggest favorable outcomes despite statistical nonsignificance. The relationship between spin and trial funding sources has been previously investigated in different medical fields. Commercially sponsored trials in cardiovascular research were more likely to employ spin to convey negative results as treatment equivalence and to emphasize the significance of secondary outcomes despite nonsignificant findings for the primary outcome [7,27,28]. Similarly, positive associations between commercial sponsorship and spin have been observed in plastic surgery and emergency medicine research [29,30]. However, spin was not associated with commercial sponsorship in fields such as bariatric surgery, obstetrics, and psychology [[31], [32], [33]]. Studies in oncology also found that commercially sponsored trials were more likely to use robust research methods and that the absence of commercial sponsorship was associated with higher spin prevalence [12,34].
Considering the risk of VTE in patients with cancer and the number of RCTs conducted on thromboprophylaxis, a systematic review is required to assess the role of trial funding sources on the current evidence base for VTE prophylaxis. This study investigated publication characteristics, sample size and follow-up, primary outcomes, trial results, and spin in commercially and noncommercially funded VTE prophylaxis trials for patients with cancer.
2. Methods
2.1. Study design and registration
The study protocol was registered in the International Prospective Register of Systematic Reviews (CRD42022319290). The study was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline; the checklist is found in Supplementary Table S1. This study did not require ethics approval as all data was publicly available.
2.2. Search strategy
MEDLINE, Embase, and Cochrane Central Register of Controlled Trials were searched with the aid of a medical librarian at McMaster University from inception to December 2021. Forward and backward citation searching was also conducted using Google Scholar and the reference lists of the included articles to identify additional citations that were not captured in the initial search. All citations were imported into EndNote X9 (Clarviate Analytics) to remove duplicates. The detailed search strategy is found in Supplementary Table S2.
2.3. Study selection, data extraction, and outcomes
Two reviewers (J.K. and L.Z.) independently conducted title, abstract, and full-text screening using the Rayyan software for systematic reviews (Qatar Computing Research Institute). Eligible studies were full-text articles in English that assessed pharmacologic or nonpharmacologic primary prophylaxis of VTE in patients with cancer (defined as the composite of pulmonary embolism and deep vein thrombosis). The only included study types were RCTs, randomized cross-over trials, and post hoc analyses of RCTs. Exclusion criteria included articles investigating secondary VTE prophylaxis, prophylaxis for non-VTE indications, or studies with pediatric populations.
Four reviewers conducted data extraction independently (A.H., C.L., J.K., and L.Z.). A fifth reviewer (P.Y.L.) resolved any conflicts. Characteristics extracted included trial sponsorship, the name of trial sponsors, cancer type of the study sample, primary prophylactic agent, control type, single-center or multicenter study, geographic locations of participating centers, primary outcome, bleeding definition if applicable, primary analysis sample (intention-to-treat [ITT], as-treated, or per-protocol), primary analysis comparison type (superiority, equivalence, or noninferiority), number of screened patients, sample size, statistical power, treatment effect size estimation, number of events for the intervention and control groups, number of loss to follow-up, the P value of the primary outcome, and spin. Corresponding authors were contacted via email for missing information.
Our primary outcome was funding source. Our secondary outcomes included reporting of a favorable outcome, spin (as previously defined by Boutron et al. [25]), sample size, number of events, multicenter vs single-center, retrospective registration, trial design (superiority, noninferiority, equivalence), usage of ITT analysis, employment of composite primary outcome, estimated treatment effect, power, loss to follow-up, and discrepancy of primary outcome between publication and registered protocol.
2.4. Outcome definitions
2.4.1. Commercial vs noncommercial
RCTs were considered commercially sponsored if they were initiated by commercial sources or by an investigator and reported being sponsored by a commercial source in the study methods. The provision of drugs alone was not considered as commercial sponsorship. Studies that were government, hospital, or investigator-initiated and either unfunded or received sponsorship from a government or hospital source were considered noncommercially funded. Authors were contacted if the paper did not mention funding sources. Studies in which the author(s) reported personal conflicts of interest but did not report direct funding of the study were considered noncommercial.
2.4.2. Classification of favorability
Studies were considered favorable if at least 1 primary outcome reached statistical significance that favored the experimental intervention. Superiority trials are designed to test whether the experimental intervention is better than the control. Noninferiority trials test whether the experimental intervention is not worse than the control. Equivalence trials test whether the efficacy of the experimental intervention is equal to the control.
2.4.3. The discrepancy between trial registration and the published article
The final reporting of the primary outcome in the RCTs was compared with their registered protocols. A discrepancy was defined as a deviation in the primary outcome in the manuscript and protocol. A discrepancy was identified when 1) a primary outcome in the protocol was reported as a secondary outcome in the manuscript, 2) a new primary outcome was reported in the manuscript that was not defined a priori in the protocol, 3) a prespecified primary outcome in the trial registration was not reported in the final manuscript, and 4) discrepancies were found in the length of follow-up.
2.4.4. Primary outcome identification
To identify the primary outcomes, we looked for explicit mention of primary outcomes in the abstract of included manuscripts. If not present, we referred to the methodology first, then to statistical tables or calculations. If the primary outcome was not stated or was uncertain, the paper was excluded.
2.4.5. Appraisal of spin
Spin is defined as the “manipulation of language that distorts the interpretation of objective findings” [27]. As studies that find statistical significance would not demonstrate spin, studies that identified a primary outcome with P < .05 were not assessed for spin.
Nguyen et al.’s [27] adaptation of Boutron et al.’s [25] spin framework (Table 1) was used to evaluate spin in studies that did not report a statistically significant primary outcome. To summarize the strategy of the spin framework, if the authors modified their analysis to focus on statistically significant secondary results in the form of within-group comparisons, the paper was assigned spin strategy 1. If the authors interpreted the nonsignificant results to rule out an adverse event or show treatment equivalence, the paper was assigned spin strategy 2. If the authors emphasized the benefits of treatment with or without acknowledging the statistically nonsignificant outcomes, the paper was assigned spin strategy 3. If the paper did not fit into strategy 1, 2, or 3, it was categorized as spin strategy 4.
Table 1.
Spin criteria by Nguyen et al. [27].
| Assessment | Criteria |
|---|---|
| Spin strategies | |
| Strategy 1 | Authors pivoted on statistically significant secondary results in the form of focus on within-group comparison. |
| Strategy 2 | Authors interpreted statistically nonsignificant results of the primary outcomes to show treatment equivalence or to rule out an adverse event. |
| Strategy 3 | Authors emphasized the beneficial effect of the treatment with or without acknowledging the statistically nonsignificant primary outcome. |
| Strategy 4 | Other/undefined/multiple strategies. |
| Overall grade | |
| Grade A | Spin in the title or abstract conclusions. |
| Grade B | Spin elsewhere in the abstract or in the conclusion. |
| Grade C | Spin not in the abstract or conclusion. |
In terms of the level of spin, low spin suggests an acknowledgment of nonsignificant outcomes. However, uncertainty in the framing often portrays equivalence or similar efficacy, and there is a recommendation for further research. Moderate spin was present if the conclusions claimed equivalence and used uncertain language. High spin was present if the papers did not acknowledge statistical nonsignificance, focused on subgroups, and gave no recommendations for further research. For the location of spin, grade A was given if the spin was present in the title or abstract conclusions, grade B if the spin was present elsewhere in the abstract or conclusion, and grade C if the spin was present elsewhere other than the abstract and conclusion. Articles were reviewed by C.L. and A.H. independently and in duplicate. Given that the interpretation of spin results can be subjective, interrater reliability for spin was ensured through a pilot analysis. J.K. and L.Z. conducted a secondary analysis, resolving discrepancies by referring to the example spin statements in the Supplementary Material by Boutron et al. [25]. Any remaining disagreements were addressed by P.Y.L.
2.5. Quality assessment
Quality assessment was completed by the reviewers (C.L., A.H., L.Z., and J.K.) using the Joanna Briggs Institute’s (JBI) Critical appraisal tools. The JBI checklist includes the assessment of the appropriateness of study design, study population, outcomes, and statistical analysis. Questions were evaluated using 4 options: yes, no, unclear, or not applicable. The reviewers met to discuss the results of their appraisal, and any outstanding disagreements were resolved by a fifth reviewer (P.Y.L.). The JBI Critical Appraisal checklist for RCTs is in Supplementary Table S3.
2.6. Statistical analysis
Categorical variables were extrapolated as counts and continuous variables as numbers, decimals, or percentages. Continuous variables were compared using the independent t-test should the sample be normally distributed or the total sample size was no less than 30; otherwise, the Mann–Whitney U-test was performed. The Shapiro–Wilk normality test determined the normality of the distribution of the sample. Categorical variables were compared using the Pearson chi-squared test and Fisher’s exact test. Fisher’s exact test was prioritized over the Pearson chi-squared test for any categories with frequencies < 5.
Logistic regression was used to identify factors associated with 1) favorable outcomes, 2) presence of spin, 3) discrepancy between registered protocol and reported outcome, and 4) retrospective trial registration. The covariates included trial sponsorship, presence of composite outcome, sample size, number of participants lost to follow-up, year of publication, type of trial (multicenter or single-center), duration in days, primary pharmacologic agent, and usage of ITT analysis. These covariates were selected a priori based on clinical judgment and previous literature. Other than primary pharmacologic agent, all of these covariates were also critical confounders analyzed in a similar analysis by Gaudino et al. [7] in the field of cardiovascular interventions.
Independent variable selection for the logistic regression model was achieved using the univariate analysis screening approach, given that there were 9 covariates of interest and inclusion of too many independent variables in the multivariate analysis may result in decreased generalizability [35]. A univariate analysis was first conducted for each covariate-dependent variable pair, and multivariate logistic regression included covariates significantly associated with the dependent variable in univariate analysis. The findings are presented in terms of the exponential value of the regression coefficient (Exp [β]) with its corresponding 95% CI. A P < .05 was selected to indicate statistical significance. All analyses were performed with R 4.1.2 (R Foundation for Statistical Computing).
3. Results
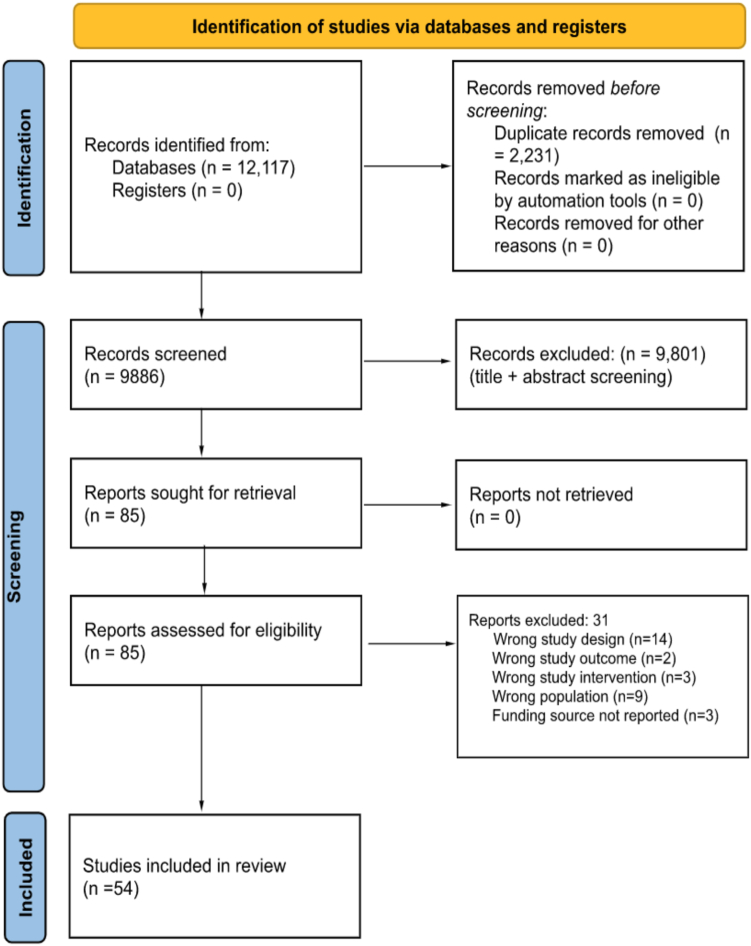
The literature search retrieved 9886 articles after the removal of duplicates (Figure). Of 127 full-text articles screened for inclusion, a total of 54 articles were included for qualitative and quantitative analysis. Of the 54 studies, 34 (63%) were commercially sponsored, and 20 (37%) were noncommercially supported (Table 2). Most trials used a superiority design (n = 48, 88.9%).
Figure.
Identification of studies via databases and registers. Source: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71 [36].
Table 2.
Summary of the characteristics of trials analyzed.
| Trial characteristic | Commercially sponsored | Noncommercially sponsored | P value |
|---|---|---|---|
| Number of trials, n (%) | 34 (63) | 20 (37) | |
| Sample size, median (IQR) | 342 (413) | 235 (337) | .17 |
| Total number of events, median (IQR) | 20 (34.25) | 14 (23) | .36 |
| Proportion of multicenter trials, n (%) | 25 (73.5) | 12 (60) | .21 |
| Retrospective registration of trial, n (%) | 7 (20.6) | 13 (65) | .007a |
| Superiority design, n (%) | 30 (88.2) | 18 (90) | .79 |
| Equivalence design, n (%) | 0 (0) | 2 (10) | .07 |
| Noninferiority design, n (%) | 4 (11.8) | 1 (5) | .38 |
| Intention-to-treat analysis, n (%) | 25 (73.5) | 6 (30) | .0006a |
| Use of composite primary outcome, n (%) | 20 (58.8) | 18 (90) | .048a |
| Estimated treatment effect, median (IQR) | 0.34 (0.44) | 0.18 (0.425) | .72 |
| Power, median (IQR) | 0.8 (0.08) | 0.8 (0.003) | .72 |
| Number of screened patients, median (IQR) | 372 (523) | 301 (334) | .20 |
| Percentage of screened patients included in sample size, median (IQR) | 97.6 (17.1) | 93.6 (20.9) | .26 |
| Sample size lost to follow-up, median (IQR) | 6.5 (9) | 2.5 (7.5) | .11 |
| Duration of follow-up, median (IQR)(days) | 60 (168) | 16 (23) | .07 |
| Favorable outcomes | 10/32 (31.2%) | 7/21 (33%) | .87 |
| Discrepancy in the primary outcome between the published manuscript and the registered protocol | 6/12 (50%) | 13/16 (81.2%) | .08 |
| Number of trials evaluated for spin | 22/33 (66.7%) | 14/21 (66.7%) | 1 |
| Spin present | 15/21 (71.4%) | 9/14 (64.3%) | .66 |
Characteristics of commercially and noncommercially sponsored trials were compared using the Mann–Whitney U-test for continuous variables and the Pearson chi-squared test for categorical variables or the Fisher’s exact test where appropriate.
Significant P < .05.
In comparison to commercially sponsored trials, noncommercially sponsored studies were more likely to report retrospective registration of the trial protocol (13 of 20 [65%] vs 7 of 34 [20.6%]; P = .007) and to define their primary outcome as a composite measure (18 of 20 [90%] vs 20 of 34 [58.8%]; P = .048). Commercially sponsored trials were more likely to use ITT analysis than noncommercially funded trials (25 of 34 [73.5%] vs 6 of 20 [30%]; P = .0006).
Most studies reported 80% power, and no significant difference was found in the estimated treatment effect size between commercially and noncommercially sponsored groups. There were no significant differences in the number of screened patients, sample size, sample size lost to follow-up, or duration of follow-up. The proportion of the total number of events and multicentered trials was similar between both groups.
3.1. Spin analysis of commercially sponsored trials
Of the 21 commercially sponsored trials assessed for spin, 15 trials (71.4%) were identified to have spin present. Spin was identified 30 times in total, with 16 occurrences in the abstract (53.3%) and 14 occurrences in the manuscript (46.7%).
3.2. Spin analysis of noncommercially sponsored trials
Of the 14 noncommercially sponsored trials assessed for spin, 9 trials (64.3%) were found to have used at least 1 spin strategy. Spin was identified 17 times in total, with 9 occurrences in the abstract (52.9%) and 8 in the manuscript (47.1%).
3.3. Overall spin analysis
Spin was most prevalent in the conclusion section of the abstract and manuscript. Spin was least likely to be found in the results section of the abstract and manuscript, with no spin identified in the results of either group. Strategy 3 was the most used method of spin in both groups, “emphasizing the beneficial effect of treatment with or without acknowledging the statistically nonsignificant primary outcome” (Table 3). Strategy 3 comprised 12 out of 30 (40%) spin strategies identified in commercially sponsored trials and 9 out of 17 (52.9%) spin strategies identified in noncommercially sponsored trials.
Table 3.
Spin strategy and location.
| Strategy | Commercially sponsored with spin (n = 15) |
Total | |||||
|---|---|---|---|---|---|---|---|
| Abstract |
Manuscript |
||||||
| Title | Results | Conclusion | Results | Discussion | Conclusion | ||
| Strategy 1 | |||||||
| Strategy 2 | 5 | 5 | 10 | ||||
| Strategy 3 | 1 | 6 | 1 | 4 | 12 | ||
| Strategy 4 | 4 | 1 | 3 | 8 | |||
| Total | 30 | ||||||
| Strategy | Noncommercially sponsored with spin (n = 9) |
||||||
|---|---|---|---|---|---|---|---|
| Abstract |
Manuscript |
||||||
| Title | Results | Conclusion | Results | Discussion | Conclusion | ||
| Strategy 1 | 2 | 1 | 3 | ||||
| Strategy 2 | 2 | 1 | 1 | 4 | |||
| Strategy 3 | 5 | 1 | 3 | 9 | |||
| Strategy 4 | 1 | 1 | |||||
| Total | 17 | ||||||
Strategy 1: authors pivoted on statistically significant secondary results in the form of focus on within-group comparisons; Strategy 2: authors interpreted statistically nonsignificant results of the primary outcomes to show treatment equivalence or to rule out an adverse event; Strategy 3: authors emphasized the beneficial effect of the treatment with or without acknowledging the statistically nonsignificant primary outcome; Strategy 4: other/undefined/multiple strategies.
3.4. Regression analysis
Commercial sponsorship was not associated with the reporting of favorable outcomes, the presence of spin, retrospective registration, or discrepancy between the protocol and reported outcomes (Table 4). Other independent variables in the regression (composite outcomes, sample size, loss to follow-up, year of publication, duration of follow-up, primary agent being LMWH, and ITT) also resulted in P values > .05. This result suggests no significant association of the aforementioned independent variables with the reporting of favorable outcomes, presence of spin, discrepancy between protocol and outcome, and retrospective registration.
Table 4.
Results of univariate regression analysis.
| Independent variable | Favorable outcomes | Spin present | Discrepancy between protocol and reported outcomes | Retrospective registration |
|---|---|---|---|---|
| Commercial sponsorship (SE) | -0.095 (0.60)a | 0.33 (0.7379)a | -1.47 (0.86)a | -0.49 (1.03)a |
| OR (95% CI) | 0.91 (0.28, 3.02) | 1.39 (0.32, 6.00) | 0.23 (0.04, 1.18) | 0.62 (0.08, 5.63) |
| Composite outcome (SE) | 0.49 (0.67)a | 0.71 (0.75)a | 1.45 (0.92)a | 1.50 (0.97)a |
| OR (95% CI) | 2.02 (0.45, 9.12) | 2.02 (0.45, 9.12) | 4.27 (0.72, 28.91) | 4.50 (0.73, 37.66) |
| Sample size (SE) | 0.00062 (0.00060)a | -0.0018 (0.0013)a | -0.00022 (0.00061)a | -0.00045 (0.00074)a |
| OR (95% CI) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) |
| LTFU (SE) | -0.030 (0.040)a | 0.085 (0.090)a | -20.93 (15091.4)a | 0.0028 (0.035)a |
| OR (95% CI) | 0.97 (0.88, 1.04) | 1.09 (0.98, 1.41) | 0 (0, ∞) | 1.00 (0.92, 1.08) |
| Year of publication (SE) | 0.078 (0.048)a | -0.050 (0.052)a | 0.032 (0.082)a | -0.079 (0.099)a |
| OR (95% CI) | 1.08 (0.99, 1.20) | 0.95 (0.85, 1.05) | 1.03 (0.87, 1.22) | 0.92 (0.76, 1.12) |
| Multicenter trial (SE) | -0.98 (0.62)a | -0.81 (0.89)a | -0.22 (0.96)a | -0.69 (0.83)a |
| OR (95% CI) | 0.38 (0.11, 1.16) | 0.44 (0.06, 2.27) | 0.80 (0.10, 4.85) | 0.50 (0.09, 2.61) |
| Duration of follow-up (SE) | 0.00016 (0.00044)a | -0.00035(0.0019)a | 0.0014 (0.0020)a | 0.00065 (0.00068)a |
| OR (95% CI) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.01) | 1.00 (1.00, 1.00) |
| Primary LMWH (SE) | 0.37 (1.2)a | 1.15 (1.49)a | 0.22 (1.31)a | 0.24 (1.32)a |
| OR (95% CI) | 1.44 (0.16, 30.81) | 3.17 (0.11, 88.86) | 1.25 (0.05, 15.66) | 1.27 (0.10, 30.47) |
| Use of ITT (SE) | -0.33 (0.59)a | 1.07 (0.75)a | -1.57 (0.93)a | 0.14 (0.74)a |
| OR (95% CI) | 0.72 (0.22, 2.32) | 2.91 (0.67, 13.49) | 0.21 (0.03, 1.13) | 1.15 (0.27, 5.07) |
ITT, intention-to-treat; LMWH, low-molecular-weight heparin; LTFU, loss to follow-up; OR, odds ratio.
Nonsignificant P > .05.
4. Discussion
VTE prophylaxis outcomes of patients with cancer in commercially sponsored RCTs were found to not differ significantly from noncommercially sponsored trials in sample size, trial design, power, estimated treatment effect size, number of patients screened, and number of patients lost to follow-up. Commercially sponsored trials were more likely to employ an ITT analysis. Noncommercially sponsored trials were associated with a higher likelihood of reporting composite primary outcomes and retrospective trial registration. Regression analysis revealed that favorable outcomes, the presence of spin, retrospective registration, or discrepancy between the protocol and reported outcomes were not associated with commercial sponsorship.
This analysis found that commercially and noncommercially sponsored papers did not differ significantly in their likelihood of reporting favorable outcomes. However, the current narrative is that the clinical merit of commercially sponsored trials warrants skepticism, as these trials have the financial capacity to employ techniques and methodologies that may help them achieve clinical significance [4,5,7]. A cross-sectional survey and meta-analysis found that commercially sponsored trials had higher odds of reporting favorable outcomes (odds ratio = 1.92) than their noncommercial counterparts (odds ratio = 1.32) [5]. The present study, however, suggests that commercially sponsored trials impart less bias than previously thought and demonstrates that commercial sponsorship in VTE prophylaxis trials is equally well-powered and methodologically robust as their noncommercial counterparts. Both commercially and noncommercially sponsored RCTs were found to not differ significantly in the presence of spin. A potential explanation for this could be the role of journals in reducing reporting bias by outlining presubmission standards and requirements [37]. Once submitted, the journal’s peer review process may also identify spin as reviewers check for overgeneralizations, inappropriate causal language, and inconsistency in the reporting of study results across the paper [38]. This points to the importance of mitigating spin and reporting bias among all publication stakeholders, including authors, peer reviewers, and editors [38].
One of the few significant differences found in this paper was the greater likelihood of composite endpoints in noncommercially sponsored studies than in commercially sponsored studies. Noncommercially sponsored trials may be motivated to employ composite endpoints as they “increase statistical efficiency, decrease in sample-size requirements, shorter trial duration, and decreased cost,” which may help the study overcome the inherent financial limitations [39]. Additionally, it is possible that the lower rate of composite endpoints observed in commercially funded trials in this study could reflect drug licensing requirements, as regulatory agencies such as the Food and Drug Administration discourage composite endpoint usage and require each component of a composite score to be individually reported [40]. The reporting of composite endpoints, such as adverse events, has been criticized for skewing in favor of statistically significant results as they act as a catch-all endpoint, leading to statistically significant results [41]. This can potentially cause an overestimation of treatment effects, particularly with respect to low-frequency but high-clinical-importance events, such as death [41].
Noninferiority design, though warranted in specific circumstances, requires less of a treatment effect to claim significance and thus can convey favorable results even when findings are inconclusive [42]. Literature shows that the coexistence of industry funding with noninferiority designs almost always obtains desirable or favorable outcomes [43]. A study found that 96.5% (55 out of 57) of commercially sponsored trials with a noninferiority design concluded favorable results compared to a superiority design, where only half of the commercially sponsored trials reached significance [43]. Our results suggest that commercially sponsored trials in VTE prophylaxis are less likely to employ noninferiority than noncommercially sponsored trials despite the appeal to reach a beneficial treatment effect. This analysis shifts the narrative to suggest that commercially sponsored trials are comparable in clinical merit and trustworthiness to noncommercially sponsored trials.
To our knowledge, the present study is one of the first in hematology to demonstrate a significant association between likelihood of retrospective trial registration and noncommercial sponsorship. Papers that register trials retrospectively may be at a greater risk of selective reporting bias as they may fail to report all outcomes or report only significant outcomes. This present study is consistent with the literature in finding that noncommercially sponsored studies were significantly more likely to retrospectively register protocol than commercially sponsored studies [44,45]. The present study also found that commercially sponsored trials were more likely to employ an ITT analysis than noncommercially sponsored trials. Inclusion of an ITT analysis is a hallmark of a high-quality study, as it preserves the balance of prognostic factors afforded by the original randomization [40]. We can speculate that commercially sponsored studies may be more inclined to prospectively register their protocols and employ an ITT analysis to demonstrate integrity in methodology, knowing the scrutiny they are under for risk of bias.
The primary strengths of the present study include the usage of validated tools for spin that enable comparisons to prior literature. Another strength of the study is the large pool of evidence, including 54 articles (encompassing data for over 20,000 patients) from 9886 screened articles. However, we are unable to comment on whether this review reached statistical power as this was not a meta-analysis. Our study findings have limitations. First, the assessment of spin is a process that may be prone to subjectivity as the interpretation of spin strategies is highly dependent on context and perspective. While there is no purely objective procedure that exists for the assessment of spin, our study attempted to reduce interrater variability by limiting the number of reviewers for data extraction. Additionally, reviewers were not able to be blinded to the funding source. This may have introduced bias in data collection. Second, we cannot infer causality from the associations found in this study, as conclusions that were influenced by the presence of confounding factors may exist. For instance, it is possible that funding sources were misclassified, as not-for-profit sponsors may be supported by private for-profit entities that indirectly bias study findings [2]. It is also possible that factors that were not investigated in this study could have influenced our results, such as researcher ties to industry sponsorship [9]. Third, the RCTs included in this study may not be fully representative of all trials in thrombosis research, as studies that do not reach completion or are terminated early may not be published due to lag bias. Of the published RCTs included in this study, several RCTs included in this study were also at high risk of bias. Fourth, the results of this study are focused on primary prophylaxis for VTE in patients with cancer and may not be generalizable to other populations. Lastly, this paper was not able to collect information on patient ethnicity and race, though data from various continents were represented in the included studies.
5. Conclusions
Most RCTs investigating VTE prophylaxis in patients with cancer involve commercial sponsorship. Few significant differences between commercially and noncommercially sponsored trials were found in the study characteristics, including sample size, duration of follow-up, reporting of favorable outcomes, presence of spin, and protocol-manuscript discrepancy, among others. Our study suggests that commercially sponsored VTE prophylaxis research in patients with cancer is unlikely to be subject to bias attributable to commercial sponsorship.
Acknowledgments
Funding
No funding was involved in this systematic review.
Ethics statement
No ethics approval was required for this systematic review.
Author contributions
J.K. and L.Z. conducted title, abstract, and full-text screening, collected and extracted data from studies for investigation, conducted data analysis and created the tables, wrote the original draft, implemented edits, and formulated supplementary materials. A.H. and C.L. performed data collection and conducted critical appraisal of included manuscripts. P.Y.L., K.Z., A.L., A.E., and M.A.C. conceptualized the project and coordinated the manuscript draft editing and review. K.Z., A.L., A.E., and P.Y.L. conducted the search strategy and developed the methodology. P.Y.L. and K.Z. undertook project administration roles to coordinate research team responsibilities. M.A.C. undertook a supervision role for oversight and leadership responsibilities of the research team.
Relationship disclosure
In the last 36 months, M.A.C. has received personal funding or has been on advisory boards for Astra Zeneca, Hemostasis Reference Laboratories, Syneos Health, and Eversana; has prepared educational materials and/or presented talks for Bayer, Pfizer, and CSL Behring; has participated in various medicolegal activities related to thrombosis, anticoagulant drugs, or other aspects of hematological practice; has worked with multiple for-profit and not-for-profit entities such as Up To Date and medical communication companies; and holds the Leo Pharma Chair in Thromboembolism, endowed at McMaster University. All other authors declare no competing interests.
Data availability
All contributory authors agree that data from the entirety of this manuscript is made publicly available on online medical databases and published articles. In the interest of transparent practices, the registration for this systematic review is available on the International Prospective Register of Systematic Reviews (CRD42022319290), and open data are available on Open Science Framework (https://osf.io/7pn2s/?view_only=3249eca3951248c7b75050f1935e75f6). For questions on the open data or supplement, contact the corresponding author.
Footnotes
Handling Editor: Dr Michael Makris
Lucy Zhao and Jayhan Kherani contributed equally to this work and are co-first authors.
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2024.102315
Supplementary material
References
- 1.Hariton E., Locascio J.J. Randomised controlled trials – the gold standard for effectiveness research. BJOG. 2018;125:1716. doi: 10.1111/1471-0528.15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hakoum M.B., Jouni N., Abou-Jaoude E.A., Hasbani D.J., Abou-Jaoude E.A., Lopes L.C., et al. Characteristics of funding of clinical trials: cross-sectional survey and proposed guidance. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-015997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lundh A., Lexchin J., Mintzes B., Schroll J.B., Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2017;2017:MR000033. doi: 10.1002/14651858.MR000033.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaphe J., Edman R., Knishkowy B., Herman J. The association between funding by commercial interests and study outcome in randomized controlled drug trials. Fam Pract. 2001;18:565–568. doi: 10.1093/fampra/18.6.565. [DOI] [PubMed] [Google Scholar]
- 5.Falk Delgado A., Falk Delgado A. The association of funding source on effect size in randomized controlled trials: 2013–2015 – a cross-sectional survey and meta-analysis. Trials. 2017;18:125. doi: 10.1186/s13063-017-1872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridker P.M., Torres J. Reported outcomes in major cardiovascular clinical trials funded by for-profit and not-for-profit organizations: 2000-2005. JAMA. 2006;295:2270–2274. doi: 10.1001/jama.295.19.2270. [DOI] [PubMed] [Google Scholar]
- 7.Gaudino M., Hameed I., Rahouma M., Khan F.M., Tam D.Y., Biondi-Zoccai G., et al. Characteristics of contemporary randomized clinical trials and their association with the trial funding source in invasive cardiovascular interventions. JAMA Intern Med. 2020;180:993–1001. doi: 10.1001/jamainternmed.2020.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Als-Nielsen B., Chen W., Gluud C., Kjaergard L.L. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events? JAMA. 2003;290:921–928. doi: 10.1001/jama.290.7.921. [DOI] [PubMed] [Google Scholar]
- 9.Ahn R., Woodbridge A., Abraham A., Saba S., Korenstein D., Madden E., et al. Financial ties of principal investigators and randomized controlled trial outcomes: cross sectional study. BMJ. 2017;356:i6770. doi: 10.1136/bmj.i6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan A.W., Krleža-Jerić K., Schmid I., Altman D.G. Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research. CMAJ. 2004;171:735–740. doi: 10.1503/cmaj.1041086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser K.A., Cofield S.S., Fontaine K.R., Glasser S.P., Thabane L., Chu R., et al. Is funding source related to study reporting quality in obesity or nutrition randomized control trials (RCTs) in top-tier medical journals? Int J Obes (Lond) 2012;36:977–981. doi: 10.1038/ijo.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linker A., Yang A., Roper N., Whitaker E., Korenstein D. Impact of industry collaboration on randomised controlled trials in oncology. Eur J Cancer. 2017;72:71–77. doi: 10.1016/j.ejca.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baykal C., Al A., Demirtaş E., Ayhan A. Comparison of enoxaparin and standard heparin in gynaecologic oncologic surgery: a randomised prospective double-blind clinical study. Eur J Gynaecol Oncol. 2001;22:127–130. [PubMed] [Google Scholar]
- 14.Larocca A., Cavallo F., Bringhen S., Di Raimondo F., Falanga A., Evangelista A., et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood. 2012;119:933–939. doi: 10.1182/blood-2011-03-344333. quiz 1093. [DOI] [PubMed] [Google Scholar]
- 15.Haas S.K., Freund M., Heigener D., Heilmann L., Kemkes-Matthes B., von Tempelhoff G.F., et al. Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin Appl Thromb Hemost. 2012;18:159–165. doi: 10.1177/1076029611433769. [DOI] [PubMed] [Google Scholar]
- 16.Merli G.J., Groce J.B. Pharmacological and clinical differences between low-molecular-weight heparins: implications for prescribing practice and therapeutic interchange. P T. 2010;35:95–105. [PMC free article] [PubMed] [Google Scholar]
- 17.Kakkar A.K., Levine M.N., Kadziola Z., Lemoine N.R., Low V., Patel H.K., et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS) J Clin Oncol. 2004;22:1944. doi: 10.1200/JCO.2004.10.002. 8. [DOI] [PubMed] [Google Scholar]
- 18.Constantini S., Kanner A., Friedman A., Shoshan Y., Israel Z., Ashkenazi E., et al. Safety of perioperative minidose heparin in patients undergoing brain tumor surgery: a prospective, randomized, double-blind study. J Neurosurg. 2001;94:918–921. doi: 10.3171/jns.2001.94.6.0918. [DOI] [PubMed] [Google Scholar]
- 19.Lyman G.H., Carrier M., Ay C., Di Nisio M., Hicks L.K., Khorana A.A., et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5:927–974. doi: 10.1182/bloodadvances.2020003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farge D., Frere C., Connors J.M., Khorana A.A., Kakkar A., Ay C., et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 2022;23:e334–e347. doi: 10.1016/S1470-2045(22)00160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S., Xu W., Song S., Wang X. Efficacy of self-heating calf sleeves for preventing deep vein thrombosis in lung cancer patients who undergo video-assisted thoracoscopic surgery lobectomy. Ann Palliat Med. 2020;9:2693–2698. doi: 10.21037/apm-20-1165. [DOI] [PubMed] [Google Scholar]
- 22.Khorana A.A., Soff G.A., Kakkar A.K., Vadhan-Raj S., Riess H., Wun T., et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. 2019;380:720–728. doi: 10.1056/NEJMoa1814630. [DOI] [PubMed] [Google Scholar]
- 23.Carrier M., Abou-Nassar K., Mallick R., Tagalakis V., Shivakumar S., Schattner A., et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711–719. doi: 10.1056/NEJMoa1814468. [DOI] [PubMed] [Google Scholar]
- 24.Guntupalli S.R., Brennecke A., Behbakht K., Tayebnejad A., Breed C.A., Babayan L.M., et al. Safety and efficacy of apixaban vs enoxaparin for preventing postoperative venous thromboembolism in women undergoing surgery for gynecologic malignant neoplasm. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boutron I., Dutton S., Ravaud P., Altman D.G. Reporting and interpretation of randomized controlled trials with statistically nonsignificant results for primary outcomes. JAMA. 2010;303:2058–2064. doi: 10.1001/jama.2010.651. [DOI] [PubMed] [Google Scholar]
- 26.Woodbridge A., Abraham A., Ahn R., Saba S., Korenstein D., Madden E., et al. A cross-sectional analysis of spin in randomized controlled trials. J Gen Intern Med. 2018;33:247–248. doi: 10.1007/s11606-017-4252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen J., Li A., Tam D.Y., Forbes T.L. Analysis of spin in vascular surgery randomized controlled trials with nonsignificant outcomes. J Vasc Surg. 2022;75:1074–1080.e17. doi: 10.1016/j.jvs.2021.09.051. [DOI] [PubMed] [Google Scholar]
- 28.Roberts W.B., Cooper C.M., Khattab M., Neff P., Wildes D., Wayant C., et al. Evaluation of “Spin” in the abstracts of randomized controlled trial reports in cardiology. J Osteopath Med. 2020;120:732–739. doi: 10.7556/jaoa.2020.133. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds-Vaughn V., Riddle J., Brown J., Schiesel M., Wayant C., Vassar M. Evaluation of spin in the abstracts of emergency medicine randomized controlled trials. Ann Emerg Med. 2020;75:423–431. doi: 10.1016/j.annemergmed.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Yuan M., Wu J., Li A., Gallo L., Chin B., Murphy J., et al. “Spin” in plastic surgery randomized controlled trials with statistically nonsignificant primary outcomes: a systematic review. Plast Reconstr Surg. 2023;151:506e–519e. doi: 10.1097/PRS.0000000000009937. [DOI] [PubMed] [Google Scholar]
- 31.Rassy N., Rives-Lange C., Carette C., Barsamian C., Moszkowicz D., Thereaux J., et al. Spin occurs in bariatric surgery randomized controlled trials with a statistically nonsignificant primary outcome: a systematic review. J Clin Epidemiol. 2021;139:87–95. doi: 10.1016/j.jclinepi.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Chow R., Huang E., Fu S., Kim E., Li S., Sodhi J., et al. Spin in randomized controlled trials in obstetrics and gynecology: a systematic review. Womens Health Rep. 2022;3:795–802. doi: 10.1089/whr.2021.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jellison S., Roberts W., Bowers A., Combs T., Beaman J., Wayant C., et al. Evaluation of spin in abstracts of papers in psychiatry and psychology journals. BMJ Evid Based Med. 2020;25:178–181. doi: 10.1136/bmjebm-2019-111176. [DOI] [PubMed] [Google Scholar]
- 34.Ito C., Hashimoto A., Uemura K., Oba K. Misleading reporting (spin) in noninferiority randomized clinical trials in oncology with statistically not significant results: a systematic review. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.35765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoltzfus J.C. Logistic regression: a brief primer. Acad Emerg Med. 2011;18:1099–1104. doi: 10.1111/j.1553-2712.2011.01185.x. [DOI] [PubMed] [Google Scholar]
- 36.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayo-Wilson E., Phillips M.L., Connor A.E., Vander Ley K.J., Naaman K., Helfand M. Peer review reduces spin in PCORI research reports. Res Integr Peer Rev. 2021;6:16. doi: 10.1186/s41073-021-00119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu K., Grundy Q., Bero L. ‘Spin’ in published biomedical literature: a methodological systematic review. PLoS Biol. 2017;15 doi: 10.1371/journal.pbio.2002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCoy C.E. Understanding the use of composite endpoints in clinical trials. West J Emerg Med. 2018;19:631–634. doi: 10.5811/westjem.2018.4.38383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.US Department of Health and Human Services, Food and Drug Administration Multiple endpoints in clinical trials guidance for industry. 2017. https://www.fda.gov/files/drugs/published/Multiple-Endpoints-in-Clinical-Trials-Guidance-for-Industry.pdf ; 2017 [accessed December 4, 2023].
- 41.Cordoba G., Schwartz L., Woloshin S., Bae H., Gøtzsche P.C. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ. 2010;341:c3920. doi: 10.1136/bmj.c3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Head S.J., Kaul S., Bogers A.J.J.C., Kappetein A.P. Non-inferiority study design: lessons to be learned from cardiovascular trials. Eur Heart J. 2012;33:1318–1324. doi: 10.1093/eurheartj/ehs099. [DOI] [PubMed] [Google Scholar]
- 43.Flacco M.E., Manzoli L., Boccia S., Capasso L., Aleksovska K., Rosso A., et al. Head-to-head randomized trials are mostly industry sponsored and almost always favor the industry sponsor. J Clin Epidemiol. 2015;68:811–820. doi: 10.1016/j.jclinepi.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 44.Braakhekke M., Scholten I., Mol F., Limpens J., Mol B.W., van der Veen F. Selective outcome reporting and sponsorship in randomized controlled trials in IVF and ICSI. Hum Reprod. 2017;32:2117–2122. doi: 10.1093/humrep/dex273. [DOI] [PubMed] [Google Scholar]
- 45.Haslberger M., Gestrich S., Strech D. Reporting of retrospective registration in clinical trial publications: a cross-sectional study of German trials. BMJ Open. 2023;13 doi: 10.1136/bmjopen-2022-069553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All contributory authors agree that data from the entirety of this manuscript is made publicly available on online medical databases and published articles. In the interest of transparent practices, the registration for this systematic review is available on the International Prospective Register of Systematic Reviews (CRD42022319290), and open data are available on Open Science Framework (https://osf.io/7pn2s/?view_only=3249eca3951248c7b75050f1935e75f6). For questions on the open data or supplement, contact the corresponding author.