Introduction
Acute kidney injury (AKI) complicates 13–18% of hospital admissions in the UK.1 While high-profile initiatives have sought to predict AKI onset using machine learning methods,2 predicting which inpatient cases progress to require renal replacement therapy (RRT) remains an unaddressed challenge. Early, reliable prediction of RRT requirement among inpatients with AKI can avoid hasty assessments of suitability, rushed counselling, delays, suboptimal bridging therapies and added costs.3 This proof-of-concept (PoC) study assessed the value of a recurrent neural network (RNN) in predicting, up to 48 hours in advance, the risk of an inpatient with AKI requiring RRT, being admitted to an intensive treatment unit (ITU), or dying.
Methods
21,225 sets of anonymised inpatient consecutive AKI episodes (stages 1–3) were identified. Associated anonymised electronic health records (EHR) of demographics, prescriptions, observations, and serum creatinine and electrolytes were extracted, generating over 25,000,000 data points. Data pipelining was constructed for compatibility with DeepMind's EHR prediction framework. Each entry was represented as a combination of six tensors (relating to feature categories, numerical feature values and feature presence flags) covering indices and values of feature changes within that specific entry. Time values were binned into 6-hour blocks. The outcomes of interest (RRT, ITU admission, death) were labelled at the appropriate 6-hour block. Data were randomly split into a training and validation set. DeepMind's RNN for predicting AKI among veterans was adapted for the outcomes of this work, and trained. Occlusion analysis was performed to assess ‘explainability’, providing insights into which data points the model most relied on for predictions. The model was tested on the validation dataset.
Results
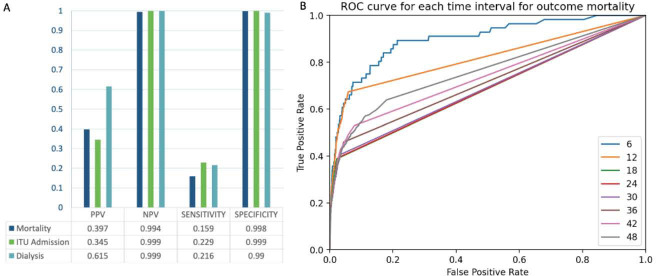
15% of AKI cases culminated in death, 4% required ITU, and 1.5% received dialysis on a kidney unit. The model was assessed for positive predictive value (PPV), negative predictive value (NPV), sensitivity, specificity, and accuracy, for each outcome. NPV, specificity and accuracy exceeded 99% (Fig 1). PPV was highest for the outcome for dialysis (62%) and sensitivity highest for ITU admission (23%). The imbalance in these values is likely accounted for by the weighting of the dataset; most patients did not reach the predefined outcomes. Occlusion analysis (assessed using cross entropy differential values relative to un-occluded baseline) highlighted systolic and diastolic blood pressure, oxygen saturations and serum sodium as having the most influence on model predictions (Fig 2).
Fig 1.
Assessment of the model. A) PPV, NPV, sensitivity and specificity for each outcome. B) ROC curve: shorter time windows lead to improved classifications.
Fig 2.
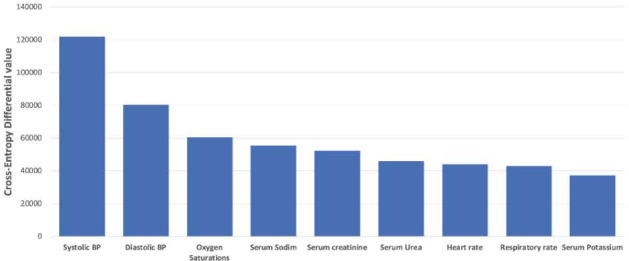
Physiological influencers.
Conclusion
This PoC model highlights a value of RNN based models in predicting AKI progression. The most powerful predictors for outcome reflect current understanding of AKI, hinting at disturbances in cardiovascular and fluid status playing being key influencers of subsequent deterioration. These findings indicate clinical relevance of the model even at this early stage PoC stage. This is promising, and further steps are being explored to improve the model, including increasing cases and variablesused for training, improving outcome labelling, and adjusting model architecture. If validated, this model may improve patient care while streamlining healthcare resource allocation.
References
- 1.Rewa O, et al. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol 2014;10:193–207. [DOI] [PubMed] [Google Scholar]
- 2.Tomašev N, et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature 2019;572(7767):116–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Health And Social Care Directorate . Quality standards and indicators briefing paper: AKI. 2014. www.nice.org.uk/guidance/qs76/documents/acute-kidney-injury-briefing-paper2. Accessed September 29, 2021.