Abstract
Vascular aging is an independent risk factor for age-related diseases and a specific type of organic aging. Endothelial progenitor cells (EPCs), a type of bone marrow stem cell, has been linked to vascular aging. The purpose of this study is to investigate if Ginseng-Sanqi-Chuanxiong (GSC) extract, a traditional Chinese medicine, can delay aortic aging in mice by enhancing the performance and aging of EPCs in vivo and to analyze the potential mechanisms through a d-Galactose (D-gal)-induced vascular aging model in mice. Our study revealed that GSC extracts not only enhanced the aortic structure, endothelial function, oxidative stress levels, and aging in mice, but also enhanced the proliferation, migration, adhesion, and secretion of EPCs in vivo, while reducing the expression of p53, p21, and p16. To conclude, GSC can delay vascular senescence by enhancing the function and aging of EPCs, which could be linked to a decrease in p16 and p53/p21 signaling. Consequently, utilizing GSC extracts to enhance the function and senescence of autologous EPCs may present a novel avenue for enhancing autologous stem cells in alleviating senescence.
Keywords: Ginseng-sanqi-chuanxiong, D-gal, Vascular aging, Endothelial progenitor cells, Senescence
1. Introduction
People are more susceptible to developing diseases as they age. The occurrence of tumors, neurodegenerative diseases, and cardiovascular diseases (CVD) increases annually [1]. It is estimated that by 2030, 23.6 million individuals will succumb to CVD, making it the most common cause of death among those aged 65 and above globally. Vascular aging is an independent risk factor for age-related diseases and a specific type of organic aging that affects the threshold, course, and severity of various CVD [2]. The process of vascular aging is marked by progressive alterations in vascular structure and function, primarily manifested through medial intima thickening, increased fibrosis, and decreased endothelial function [[3], [4], [5], [6]].
Endothelial progenitor cells (EPCs) act as precursors to endothelial cells, which can stimulate angiogenesis through their own processes of differentiation and secretion [7,8]. EPC therapy is an important endogenous vascular repair strategy with potential value for the treatment of diseases associated with endothelial integrity and dysfunction. One important repair mechanism for improving endothelial function is the recruitment of EPCs [9]. The circulating levels of EPCs in the bloodstream can be used to anticipate the aging of blood vessels [10]. Additionally, the aging process has demonstrated a decrease in the quantity and/or operational capabilities of EPCs in both animals and humans [11]. Hence, it is essential to investigate and enhance the performance of autologous endothelial progenitor cells for anti-aging.
The traditional Chinese medicine, Ginseng-Sanqi-Chuanxiong (GSC), is composed of Panax ginseng C. A. Mey., Panax notoginseng (Burk.) F. H. Chen, and Ligusticum chuanxiong Hort. With a ratio of 2:3:4, which has been utilized in the treatment of cardiovascular and diabetic conditions [12]. Our prior research demonstrated that GSC has the potential to enhance morphological alterations of aortas in aging mice, diminish production of reactive oxygen species in vascular tissues, alleviate stiffness of blood vessels in aging mice, diminish vascular remodeling, and postpone the onset of vascular aging [13]. Furthermore, GSCs can impede the aging of endothelial cells, stimulate the proliferation of aged endothelial cells, and enhance endothelial performance [14]. However, it is uncertain if GSCs can delay vascular aging by enhancing endothelial progenitor cell function. In this study, we developed a mouse aging model induced by D-gal to investigate the influence of GSC on aortic aging and endothelial progenitor cell function in aging mice, providing a fresh outlook on preventing and alleviating vascular aging.
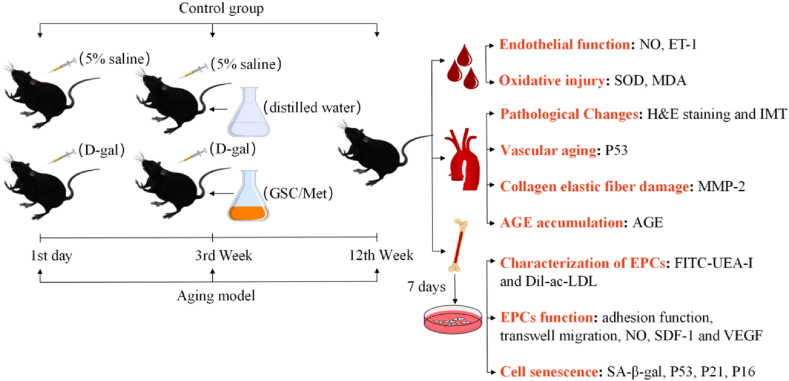
Prolonged consumption of d-galactose (D-gal) can result in excessive production of advanced glycation end products (AGE), ultimately causing oxidative stress. D-gal models can be initiated at any point in adolescence, and the outcomes of experiments can be rapidly compared to the pathological progression observed in naturally aging model animals [15,16]. Consequently, we employed D-gal to reproduce the aging model of mice in this study. This study's schematic design is shown in Fig. 1. In summary, we assessed the effectiveness of GSC in reducing vascular senescence in mice by examining the alterations in aortic histopathology and the expression of p53 [17,18]. Furthermore, the increase in AGE and matrix metalloproteinase-2 (MMP-2) causes an increase in vascular stiffness, which plays a key role in aging-induced vascular remodeling [19,20]. Moreover, oxidative stress and endothelial dysfunction exert a significant impact on the degree of vascular senescence, which can be assessed by detecting superoxide dismutase (SOD) activity, malondialdehyde (MDA), nitric oxide (NO) and endothelin-1 (ET-1) content in serum [21,22]. Additionally, to corroborate that GSC ameliorates vascular senescence along with EPCs, we obtained endothelial progenitor cells by extracting and in vitro inducing culture of bone marrow cells from mouse leg bones. We detected the proliferation, migration, adhesion and secretion of stromal cell derived factor-1 (SDF-1), NO and vascular endothelial growth factor (VEGF) of EPCs to assess the cell function [23,24]. Lastly, we investigated the variations of senescence staining, p53/p21 and p16 signaling pathways [25] of EPCs to establish clues and mechanisms that GSC has an intervening role in the senescence of EPCs and vascular aging.
Fig. 1.
The design of the experiment. The mice were treated with D-gal (180 mg/kg) for 11 weeks (s.c.). In the third week, the mice were given Methionine (Met), GSC or distilled water (oral). The blood, aortas, femurs and tibias were collected separately.
2. Materials and methods
2.1. Preparation of GSC extracts
According to our previous study [12], GSC ethanol extracts were provided by YinKeRuiSi Medical Technology Co. Ltd. (Beijing, China). Raw herbs were prepared into dry powder containing a final concentration of 4.286 g dried crude herb per g through ethanol extraction, concentration and vacuum decompression. According to the pharmacopoeia, we analyzed the main components and contents of GSC extract by chromatographic analysis: ferulic acid 1.00 mg/g, notoginsenoside R1 8.45 mg/g, ginsenoside Rg1 55.84 mg/g, ginsenoside Re 6.47 mg/g and ginsenoside Rb1 44.57 mg/g (as shown in the Supplement file-1).
2.2. Animals
Male C57/BL 6 N mice (5-week-old) were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China, SYXK2017-0033). The standard conditions of the animal facility were maintained as follows: temperature 24 ± 1 °C; humidity 45% ± 10%; 12-h light/dark cycle (Lights on 06:00–18:00). During the model construction procedure, the mice had normal daily activities without behavior limitations, optimum eating and drinking, and regular grooming. All mice were left undisturbed and acclimatized for seven days prior to the onset of studies at the Experimental Animal Center of Institute of basic theory for Chinese Medicine, China Academy of Chinese Medical Sciences (China). Animal welfare and experimental procedures strictly abided to the guidelines for ethical care of experimental animals and were approved by the Institutional Animal Research ethics committee of Institute of basic theory for Chinese Medicine, China Academy of Chinese Medical Sciences (Ethics number: 2020-011). Every endeavor was undertaken to mitigate animal distress and diminish the utilization of animals.
2.3. Pharmaceutical intervention
After seven days of adaptive feeding, the aging model was established in mice by dorsal cervical injections of D-gal (180 mg/kg/day) for 11 weeks, as reported previously [26,27]. We converted human dose to mouse dose based on clinical human equivalent dose and the animals’ surface area [28]. In summary, the mice were randomly divided into six groups (eight mice in one group), i.e., Control (5% saline + distilled water), Model (D-gal + distilled water), Met (D-gal + metformin 150 mg/kg/day), GSC-L (D-gal + GSC 477.5 mg/kg/day), GSC-M (D-gal + GSC 955 mg/kg/day), and GSC-H (D-gal + GSC 1910 mg/kg/day) groups. From the third to the eleventh week of D-gal treatment, GSC and metformin were orally administered to aging mice once daily for consecutive nine weeks. After 11 weeks, the mice were sacrificed to collect blood, aortas, femurs, and tibias.
2.4. Histological examination
C57BL/6 mouse aorta tissue was fixed in 4% neutral formalin solution. The aorta tissue was then embedded in paraffin and cut into 4μm thick slices. Finally, hematoxylin-eosin (H&E) staining was performed to observe histopathological changes of the aortas under optical microscope. Intima-media thickness (IMT) was measured by OLYMPUS OlyVIA 2.9 software (Olympus, Tokyo, Japan) with at least five visual fields per mouse and 3–5 per group for quantification.
2.5. Immunohistochemistry (IHC)
For IHC, the paraffin-embedded sections (4 μm) were rehydrated and treated with 0.01 M sodium citrate for antigen retrieval. After being blocked with 3% BSA for 30 min, the sections were incubated overnight at 4 °C in a humidified box with specific antibodies against p53 (1:3200, Proteintech Group, Wuhan, China, 60283-2Ig), MMP-2 (1:400, Proteintech Group, Wuhan, China, 10373-2-AP), AGE (1:100, Proteintech Group, Wuhan, China, 19003-1-AP). Specimens were then treated with a peroxidase conjugated goat anti-mouse and anti-rabbit secondary antibodies (1:500, Seracare Life Sciences Inc, Milford, MA, USA, 5220-0341, 5220-0336) and diaminobenzidine (Shitai Laboratory Equipment Co., Ltd, Jiangsu, China, 2005289). For each section, five fields were selected from aortas tissue and captured at × 200 magnification using microscope (Olympus, Tokyo, Japan, BX61VS). The semi-quantitative analysis was performed by ImageJ 1.52v software (National Institutes of Health, USA).
2.6. Biochemical analysis in serum
Blood was sampled from eyes of the mice. After clotting at room temperature, serum samples were obtained by centrifugation at 3500 rpm for 15 min and stored at −80 °C until further analysis. Total SOD, MDA, NO and ET-1 detection kits (Jiancheng Bioengineering Institute, Nanjing, China, A001–3, A003–1, A013-2-1, H093) were used to determine the MDA, NO and ET-1 levels and SOD activities according to manufacturer's instructions. At room temperature, the absorbance was measured at 532, 550 or 450 nm wavelengths.
2.7. Isolation and culture of EPCs
The extraction of EPCs was based on a method described in literature [29]. Briefly, the femurs and tibias were isolated and washed with PBS. The epiphysis on both sides was separated and the marrow cavity was flushed with EGM-2MV medium (LONZA, Walkersville, MD, USA, CC3202) and a 1 ml syringe. After filtration through a 75 μm cell strainer, the cells were cultured in EGM-2MV medium, and unattached cells were abandoned on the fourth day. The EGM-2MV medium was replaced every second day until the seventh day of the experiments.
2.8. Characterization of EPCs
Characterization of EPCs was performed as previously described [30,31]. Briefly, the cells were incubated with 50 μg/ml acetylated DiI Lipoprotein from human plasma (Dil-ac-LDL, Solarbio, Beijing, China, H7970) at 37 °C for 4 h. The cells were washed with PBS and fixed with paraformaldehyde, then incubated with 15 μg/ml Lectin from Ulex europaeus FITC Conjugate (FITC-UEA-1, Sigma-Aldrich, Darmstadt, Germany, L9006) at 37 °C in the dark for 3 h. The cells were then rinsed three times with PBS and visualized under a confocal microscope (Olympus, Tokyo, Japan, FV1000).
2.9. Assessment of EPCs proliferation, adhesion, migration and secretion function
2.9.1. EPC proliferation assay
EPCs proliferation was measured by CCK-8 assay [32]. Each group was seeded in 96-well plates at 10,000 cells/well. The optical density (OD) of cell adhesion was used as a control to calculate growth rate of absorbance after culturing the cells at 450 nm for 24 h. Importantly, 4–6 duplicate wells were set up in each group.
2.9.2. EPC adhesion assay
For adhesion function, EPCs were seeded in a 24-well plate at a density of 1 × 10^5/well and cultured at 37 °C for 3 h. After that, the non-adherent cells were aspirated, fixed with 5% paraformaldehyde, and incubated with 4′,6-diamidino-2-phenylindole (DAPI) at room temperature for 10 min, and then placed under a fluorescence microscope (Olympus, Tokyo, Japan, BX61VS) for imaging, counting, and photographing. Five fields of view were selected for each group.
2.9.3. EPC migration assay
Migration of EPCs was assessed by transwell migration assay [24]. After using serum-free EGM-2 basal medium to adjust each group of cells to 2 × 10^5/ml, we added 200 μl cell suspension and 600 μl EGM-2MV medium to the upper and lower chambers of transwell, cultured at 37 °C for 24 h. The transwell chambers were fixed in 4% paraformaldehyde solution and stained with crystal violet. After washing with PBS and air-drying, the cells were observed and counted under a microscope with at least five fields of view in each group.
2.9.4. EPC secretion assay
Enzyme-linked immunosorbent assay (ELISA) was used to assess the secretion of EPCs according to the manufacturer's protocol [33]. After each group of cells adhered, the medium was changed to serum-free EGM basal medium at 37 °C for 48 h. Cell supernatants were collected and assayed using NO, SDF-1 and VEGF ELISA kits (Proteintech Group, Wuhan, China, KE10049, KE10009). Each group had 4–6 duplicate holes.
2.10. Senescence-associated-β-galactosidase (SA-β-gal) staining
EPCs were stained using a Senescence Cells Histochemical Staining kit (Beyotime Institute of Biotechnology, Shanghai, China, C0602) to assess senescence [24]. Briefly, the cells were washed with PBS three times and then incubated for 18 h in SA-β-gal staining solution at 37 °C with no CO2. Then, the samples were washed again with PBS three times and cover-slipped for direct imaging and counting under a microscope.
2.11. Western blot analysis
Murine protein samples were lysed in cold lysis buffer (Solarbio, Beijing, China, R0010) and quantified by BCA protein quantitation kit (Solarbio, Beijing, China, PC0020). The proteins were denatured in sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample loading buffer for 5 min. Then, 30 μg protein sample was added to 12% SDS-PAGE gel for separation and transferred to polyvinylidene difluoride (PVDF) membrane by electrophoresis. The membranes were then blocked with 5% non-fat milk for an hour at room temperature and incubated with primary appropriate antibodies at 4 °C overnight. After washing three times with TBST, the membranes were incubated in secondary antibodies for an hour at room temperature. Enhanced chemiluminescence detection system was used to visualize the immunoreactive bands. Image was used to quantify the protein expression from at least three independent experiments. The following primary antibodies were used in Western blot analysis: p53 (1:2000, Proteintech Group, Wuhan, China, 60282-2-lg), p21 (1:2000, Proteintech Group, Wuhan, China, 28248-1-AP), p16 (1:2500, Abcam, Cambridge, UK, ab211542) and GAPDH (1:1000, Proteintech Group, Wuhan, China, 60004-1-lg).
2.12. Statistical analysis
Statistical analysis was conducted through one-way analysis of variance (ANOVA) using SPSS 20.0 software (IBM, New York, NY, USA). All data were presented as the mean ± SD, and values of p < 0.05, p < 0.01 and p < 0.001 were considered statistically significant.
3. Results
3.1. Effect of GSC on morphology of mouse aorta and IMT
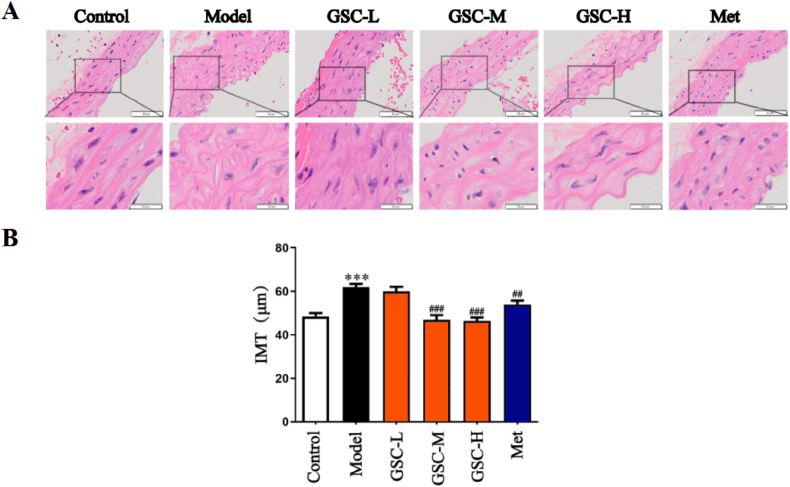
The main pathology of vascular aging is the disorganization and proliferation of the intima-media structure [34]. The pathological structure of the aorta was assessed by H&E staining. The structure of the aorta in the Model group was disordered, and the medial intima was significantly thicker than that in the Control group (M vs. C: 61.57 ± 6.14 vs. 49.55 ± 5.77 μm); the vascular structures of the Met, GSC-M, and GSC-H groups were better than those of the Model group. The group was relatively intact, with no obvious thickening of the intima (GSC-M vs. M: 46.95 ± 8.15 vs. 61.57 ± 6.14 μm; GSC-H vs. M: 46.12 ± 5.94 vs. 61.57 ± 6.14 μm; Met vs. M: 53.48 ± 7.05 vs. 61.57 ± 6.14 μm). Furthermore, the vascular structure of the GSC-L group was disordered while no difference was observed between GSC-L and Model groups in the concentrations of IMT (GSC-L vs. M: 59.02 ± 7.94 vs. 61.57 ± 6.14 μm). As shown in Fig. 2. A–B, the results suggest that GSC and Met can improve structural disorganization and mid-endothelial thickening of the aorta in aging mice.
Fig. 2.
Pathological changes of mouse aorta and comparison of IMT. (A) HE staining of aorta in each group. (B) Comparison of IMT in each group. N = 4–6, results are presented as mean ± SD, Model vs. Control, ***p < 0.001, GSC-L, GSC-M, GSC-H and Met vs. Model, ##p < 0.01, ###p < 0.001.
3.2. Effect of GSC on the vascular endothelial function
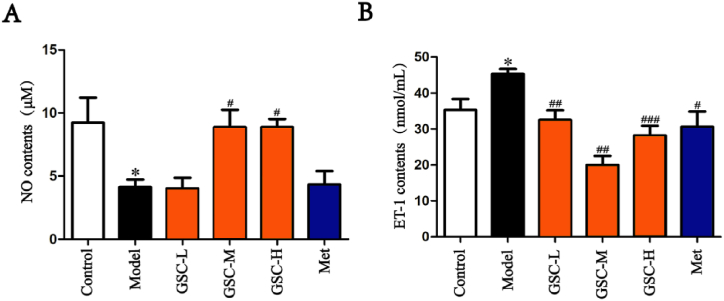
Serum levels of NO and ET-1 reflect vascular endothelial function [35,36]. We therefore assessed the vascular endothelial function by detecting NO and ET-1 in the serum. The NO level in the Model group was significantly lower than that in the Control group; and higher in the GSC-M and GSC-H groups than that in the Model group (Fig. 3. A). In addition, ET-1 level in the Model group was significantly higher than that in the Control group; while being significantly lower in the GSC-L, GSC-M, GSC-H, and Met groups than that in the Model group (Fig. 3. B). The results suggested that GSC and Met improved vascular endothelial function to varying degrees, with GSC showing a more pronounced improvement than Met.
Fig. 3.
Comparison of serum NO and ET-1 contents in mice. (A) Comparison of NO contents. (B) Comparison of ET-1 contents. N = 6–8, results are presented as mean ± SD, Model vs. Control, *p < 0.05, GSC-L, GSC-M, GSC-H and Met vs. Model, #p < 0.05, ##p < 0.01, ###p < 0.001.
3.3. Effect of GSC on oxidative stress
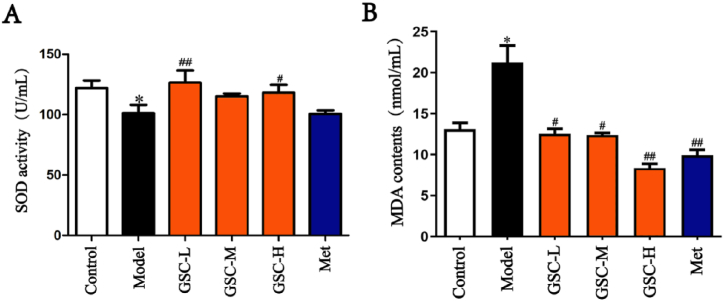
We aimed to determine whether GSC could ameliorate oxidative stress in mice. The SOD activity and MDA contents in the serum showed that the SOD activity of the Model group was lower than that of the Control group, and the SOD activity of the GSC-L and GSC-H groups was higher than that of the Model group. However, the SOD activity of GSC-M and Met groups had no significant difference compared with the Model group (Fig. 4. A). MDA content in the Model group was higher than that in the Control group; while that in the GSC-L, GSC-M, GSC-H, and Met groups were significantly lower than that in the Model group (Fig. 4. B). These results suggest that GSC and Met can improve the level of oxidative stress in mice, and the improvement of GSC was better than that of Met.
Fig. 4.
Comparison of serum SOD activity and MDA contents in mice. (A) Comparison of SOD activity. (B) Comparison of MDA contents. N = 6–8, results are presented as mean ± SD, Model vs. Control, *p < 0.05, GSC-L, GSC-M, GSC-H and Met vs. Model, #p < 0.05, ##p < 0.01.
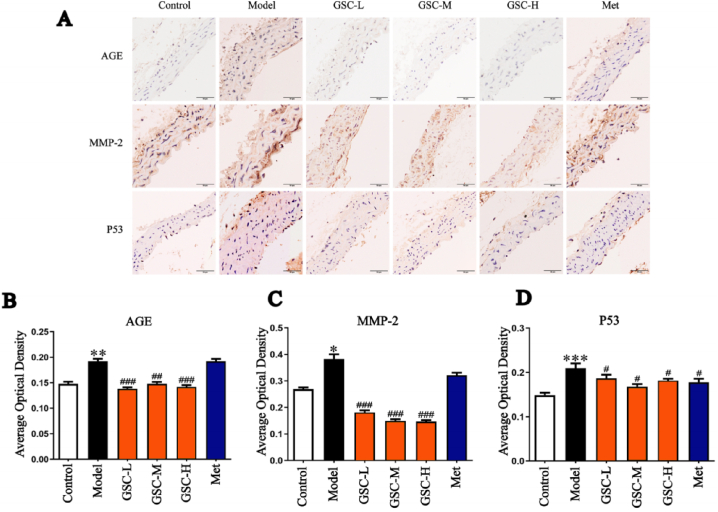
3.4. Effect of GSC on AGE, MMP-2, and p53 immunohistochemistry
To further validate the effect of GSC on aortic aging, we compared aortic vascular aging protein, collagen elastic fiber damage, and AGE accumulation by immunohistochemical of aortic p53, MMP-2, and AGE in each group. Compared with the Control group, the average optical density values of AGE, MMP-2, and p53 in the Model group were significantly higher. In addition, the average optical densities of the GSC-L, GSC-M, and GSC-H groups were significantly lower than that of the Model group (Fig. 5. A–D). However, the average optical density of AGE and MMP-2 in the Met group was not significantly different from that of the Model group (Fig. 5. A–C). These results showed that GSC and Met improved the expression of vascular aging proteins, while GSC was superior to Met with respect to the amelioration of vascular sclerosis.
Fig. 5.
Expression of AGE, MMP-2 and P53 of aortic. (A) Representative images of immunohistochemical of AGE, MMP-2 and P53, scale bars represent 50 μm. (B) Protein expression of AGE, MMP-2 and P53 quantification analysis. N = 3–4, results are presented as mean ± SD, Model vs. Control, *p < 0.05, **p < 0.01, ***p<0.001, GSC-L, GSC-M, GSC-H and Met vs. Model, #p < 0.05, ##p < 0.01, ###p < 0.001.
3.5. Effects of GSC on the proliferation, adhesion, migration and secretion of EPCs
Positive dual fluorescence staining of FITC-UEA-I and Dil-ac-LDL is a common method to identify EPCs [37]. We performed FITC-UEA-I and Dil-ac-LDL double fluorescence staining on cells after seven days of culture, which proved that the cells induced in this experiment were EPCs (Fig. 6. A). The proliferation function of EPCs was detected by CCK-8, and the 24-h proliferation rate of the Model group was significantly lower than that of the Control group. The proliferation rate of the GSC-L, GSC-M, and GSC-H groups was significantly higher than that of the Model group. However, the value-added rate of the Met group was not significantly different from that of the Model group (Fig. 6. B). Adhesion function test showed that the number of adherent cells in the Model group was significantly less than that in the Control group; the number of adherent cells in the GSC-M and GSC-H groups increased compared with the Model group. There was no significant difference in the number of adhesions between the GSC-L and Met groups compared with the Model group (Fig. 6. C–D). The transwell migration assay showed that the number of migrating cells in the Model group was significantly lower than that in the Control group, and the number of migrating cells in GSC-H was increased compared with the Model group. However, the number of migrating cells of GSC-L, GSC-M, and Met had no significant difference compared with the Model group (Fig. 6. E–F). We assessed the secretory function of cells by detecting NO, VEGF, and SDF-1 contents in the cell supernatant. NO, VEGF, and SDF-1 contents in the Model group were lower than those in the Control group; and higher in the GSC-H and Met groups than those in the Model group; however, the VEGF factor in GSC-L was not significantly higher than that in the Model group. SDF-1 content in GSC-M was not significantly different from that in the Model group, while NO and VEGF contents were higher than those in the Model group (Fig. 6. G–I). The findings indicated that GSC and Met had varying degrees of impact on the proliferation, adhesion, migration, and secretion of NO, VEGF, and SDF-1 of EPCs in vascular aging mice.
Fig. 6.
Characterization and functions of mouse bone marrow-derived EPCs. (A) Characterization of EPCs. FITC-UEA-I and Dil-ac-LDL double fluorescent staining was positive, scale bars represent 50 μm. (B) Comparison of 24H proliferation function of EPCs. 24-hour proliferation rate in each group. N = 6. (C–D) Comparison of the adhesion function of EPCs. The images and number of adherent cells in each group within 3 h. N = 6, scale bars represent 50 μm. (E–F) Comparison of the transwell migration assay of EPCs. The number of cells migrated in each group. N = 6, scale bars represent 50 μm. (G–I) Comparison of Secretory Function of EPCs. The content of NO, VEGF and SDF-1 in the supernatant of cells in each group. N = 4–6. Results are presented as mean ± SD, Model vs. Control, *p < 0.05, **p < 0.01, GSC-L, GSC-M and GSC-H vs. Model, #p < 0.05, ##p < 0.01, ###p < 0.001.
3.6. Effects of GSC on SA-β-gal staining of EPCs
We aimed to determine the senescence of EPCs in vascular aging mice and the effect of GSC intervention. SA-β-gal staining can evaluate the senescence of cells [38]. From the results, the number of blue-stained cells in the Model group was significantly more than that in the Control group. Moreover, the number of blue-stained cells of the GSC-L, GSC-M, GSC-H, and Met groups were significantly reduced compared with the Model group (Fig. 7. A–B). The results showed that GSC ameliorated the senescence of EPCs in vascular aging mice in vivo.
Fig. 7.
Comparison of SA-β-gal staining in EPCs. (A-B) The number of senescent EPCs in each group. N = 6, results are presented as mean ± SD, Model vs. Control, **p < 0.01, GSC-L, GSC-M, GSC-H and Met vs. Model, ##p < 0.01, scale bars represent 50 μm.
3.7. Effect of GSC on the expression of p53, p21, and p16 proteins in the EPCs
To explore the mechanism by which GSC ameliorates senescence in EPCs, we examined the protein expression of the cellular senescence-related pathways p53/p21 and p16. We found that the expressions of p53, p21, and p16 in the Model group were significantly higher than those in the Control group. The expressions of p53, p21, and p16 in GSC-M and GSC-H groups were significantly lower than those in the Model group, while p16 in GSC-L was significantly lower than that in the Model group, and the rest of the proteins had no statistical difference. However, the expression of p53 in the Met group was lower than that in the Model group, and there was no significant difference in other proteins (Fig. 8 A–D). These results suggest that GSC and Met may inhibit the senescence and functions of EPCs by reducing p53/p21 and p16 signaling.
Fig. 8.
Comparison of the expression of senescence proteins in EPCs. (A-D) Protein expression and quantification analysis of P53, P21 and P16. N = 3–5, results are presented as mean ± SD, Model vs. Control, *p < 0.05, **p < 0.01, GSC-L, GSC-M, GSC-H and Met vs. Model, #p < 0.05, ##p < 0.01.(The uncropped versions of Western blot images were shown in theSupplement file-2).
4. Discussion
D-gal injection into the back of the neck in this study led to a successful construction of a vascular aging mouse model. Pathological changes of the aorta, IMT, vascular endothelial function damage, and the enhancement of senescence protein expression were all verified. Additionally, it was found that GSCs can delay vascular aging by regulating the aforementioned conditions; and can inhibit oxidative stress, MMP-2 and AGE protein expression, and partially alleviate vascular aging induced by D-gal in mice. Subsequently, we successfully extracted and cultured EPCs from vascular aging mice, and identified them by FITC-UEA-I and Dil-ac-LDL double fluorescent staining. It was observed that the proliferation, adhesion, migration, and secretion of EPCs in vascular aging mice decreased, accompanied by an increase in the aging markers of EPCs. Consequently, we believe that GSCs possess the ability to delay vascular senescence induced by D-gal by reinstating the functionality and senescence of EPCs.
D-gal was first discovered by a Chinese research team that it can accelerate rodent aging by elevating MDA levels, diminishing SOD activity, and triggering oxidative stress [39]. As research progressed, the extended administration of D-gal was found to enhance AGE, advanced glycation end product receptor, aldose reductase, sorbitol dehydrogenase, telomere length shortening, telomerase activity, site amyloid senescence markers like precursor protein lyase 1, amyloid, senescence-related genes (p16, p21, p53, p19Arf, p21Cip1/Waf1), and SA-β-gal staining [18]. D-gal is frequently employed as an animal model for assessing the aging process of the heart, brain, kidney, liver, and genitals; however documented evidence of aortic vascular aging is lacking. Qiao Weili [40] created a model of aortic aging through the subcutaneous injection of D-gal into rats. Vascular aging is largely characterized by alterations in their structure and impaired endothelial function [41]. The thickening of the vessel wall in age-related structural remodeling is caused by changes in the intima and media, and an increase in IMT is evident in the aging process [34]. Endothelial dysfunction is significantly influenced by the disparity between vasodilation and contraction. The balance between expansion and vasoconstriction is mainly regulated by the synthesis and release of vasodilators (e.g., NO) and vasoconstrictors (e.g., ET-1) [35,36]. This study compared pathological changes of the aorta between the Control and Model groups, considering the thickness of IMT, the activity of SOD, the content of MDA, NO and ET-1, as well as the aortic AGE, p53 and MMP-2, as previously conducted. Results confirmed the effectiveness of this modeling.
In this study, we investigated how GSC therapy could protect vascular senescence caused by D-gal injection. We selected metformin as the positive drug, considering prior research [42,43]. Following 11 weeks of modeling and intervention, the aorta structure of the Met group was more intact than that of the Model group, and IMT notably decreased, which could lead to a decrease in ET-1 and MDA in serum, as well as a decrease in p53 expression in blood vessels. All of these findings validate the impact of metformin on protecting blood vessels and enhancing the functionality of EPCs, aligning with documented research [[44], [45], [46], [47], [48]]. Simultaneously, we discovered that GSC had the ability to diminish the levels of NO and SOD activity in the serum of mice, along with the levels of AGE and MMP-2 in blood vessels, alongside the aforementioned indicators. The findings validated the ability of GSC to enhance oxidative stress, D-gal-induced vascular senescence, and the optimal protective impact of high-dose GSC on the aorta.
The aging process necessitates the presence of endothelial cells to regulate arterial attributes, including vascular tone, vascular permeability, angiogenesis, and response to inflammation [49]. As vascular aging advances, mature endothelial cells eventually differentiate into endothelial cells with limited ability to proliferate, hindering their capacity to replace damaged endothelial cells [50]. Other cell types may be necessary for endothelial repair. Bone marrow is the source of EPCs, which are scarce in the bloodstream of adults, constituting approximately 0.01% of blood mononuclear cells [51]. The ability of EPCs to proliferate and differentiate into endothelial cells as seed cells is crucial for their involvement in angiogenesis and vascular function during adulthood [52]. Research has indicated that as people age, the amount of EPCs in both humans and animals diminishes, which could be linked to the aging process of EPCs [53]. Senescence in EPCs will result in dysfunctions like proliferation and migration [54]. This study revealed that bone marrow-derived EPCs from vascular senescent mice exhibited an increase in the senescence marker SA-β-gal, which was accompanied by a considerable decline in cell proliferation, migration, adhesion, and secretion. Consequently, we believe that improving the senescence of autologous EPCs is a significant factor in delaying vascular aging, which could be linked to the manner in which GSCs delay vascular aging. Subsequently, we conducted a comparative analysis of the EPCs' proliferation, migration, adhesion, secretion function, and cell senescence markers in every group of mice. The findings indicated that GSC had a considerable impact on the biological performance of autologous EPCs and hindered cell aging. Furthermore, in line with the vascular findings, the optimal protective impact of elevated GSC doses on EPCs was also observed, verifying that the effectiveness of GSC on blood vessels and EPCs could be influenced by dosage. Activation of the p53/p21WAF1/CIP1 and p16INK4A/pRB tumor suppressor pathways plays a central role in regulating senescence [55]. To investigate potential ways in which GSC disrupt senescence in EPCs, we analyzed the proteins p53, p21, and p16. Consistent with other findings, GSC diminished the activation of the p53/p21 and p16 pathways.
Despite this, this study has its limitations. At the outset, we solely employed three doses of GSC. Further trials of GSC should be conducted in the future to determine the most effective dosage. Moreover, further investigation is necessary to understand how GSC can delay vascular aging by enhancing the performance of EPCs. The link between animal trials and clinical results has yet to be determined, and further research, such as randomized controlled trials, is necessary to prove the efficacy of GSC in preventing vascular aging and senescence of EPCs.
In conclusion, GSC can impede vascular senescence caused by D-gal injection by prolonging the senescence of EPCs and enhancing the proliferation, migration, adhesion, and secretion capabilities of senescent EPCs. The findings imply that GSC may be a viable medication for combating vascular aging and the senescence of autologous EPCs. Additionally, it provides potential therapeutic approaches to combat aging by enhancing the functionality of its own stem cells, thus contributing to the alleviation of global aging.
Ethics statement
The animal study was reviewed and approved by the Institutional Animal Research ethics committee of Institute of basic theory for Chinese Medicine, China Academy of Chinese Medical Sciences (Ethics number: 2020−011).
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) No. 81673822; the Independent Project of China Academy of Chinese Medical Sciences, (No. ZZ2018014 and No. ZZ2019012).
CRediT authorship contribution statement
Yinan Liu: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Yiqing Liu: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Xue Wang: Data curation. Chengkui Xiu: Data curation. Yanhong Hu: Formal analysis. Jiali Wang: Formal analysis. Yan Lei: Funding acquisition, Formal analysis, Data curation, Conceptualization. Jing Yang: Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Yingying Li, Guihua Yu and Bowen Yu from China Academy of Chinese Medical Sciences, for their assistance with animal operations and guidance in statistics.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e25253.
Contributor Information
Yan Lei, Email: 13651217893@163.com.
Jing Yang, Email: yangjing@163.com.
Abbreviations
- GSC
Ginseng-Sanqi-Chuanxiong
- EPCs
endothelial progenitor cells
- D-gal
d-galactose
- IMT
Intima-media thickness
- SOD
superoxide dismutase
- MDA
Malondialdehyde
- NO
nitric oxide
- ET-1
Endothelin-1
- CVD
cardiovascular diseases
- AGE
advanced glycation end products
- Dil-ac-LDL
DiI Lipoprotein from human plasma
- FITC-UEA-1
Lectin from Ulex europaeus FITC Conjugate
- OD
optical density
- SA-β-gal
Senescence-associated-β-galactosidase
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Du S., Ling H., Guo Z., Cao Q., Song C. Roles of exosomal miRNA in vascular aging. Pharmacological Research. 2021;165 doi: 10.1016/j.phrs.2020.105278. [DOI] [PubMed] [Google Scholar]
- 2.Ni Y.-Q., Zhan J.-K., Liu Y.-S. Roles and mechanisms of MFG-E8 in vascular aging-related diseases. Ageing Research Reviews. 2020;64 doi: 10.1016/j.arr.2020.101176. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta E.G., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 4.Ibarrola J., Kim S.K., Lu Q., DuPont J.J., Creech A., Sun Z., Hill M.A., Jaffe J.D., Jaffe I.Z. Smooth muscle mineralocorticoid receptor as an epigenetic regulator of vascular ageing. Cardiovascular Research. 2022 doi: 10.1093/cvr/cvac007. cvac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui X.-Y., Zhan J.-K., Liu Y.-S. Roles and functions of antisense lncRNA in vascular aging. Ageing Research Reviews. 2021;72 doi: 10.1016/j.arr.2021.101480. [DOI] [PubMed] [Google Scholar]
- 6.Xu H., Li S., Liu Y.-S. Roles and mechanisms of DNA methylation in vascular aging and related diseases. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.699374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayraktutan U. Endothelial progenitor cells: potential novel therapeutics for ischaemic stroke. Pharmacological Research. 2019;144:181–191. doi: 10.1016/j.phrs.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Poh K.K., Lee P.S.S., Djohan A.H., Galupo M.J., Songco G.G., Yeo T.C., Tan H.C., Richards A.M., Ye L. Transplantation of endothelial progenitor cells in obese diabetic rats following myocardial infarction: role of thymosin beta-4. Cells. 2020;9:949. doi: 10.3390/cells9040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan Y., Yuan L., Oettgen P. Alterations in transcriptional responses associated with vascular aging. J Inflamm. 2009;6:16. doi: 10.1186/1476-9255-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balistreri C.R., Pisano C., Bertoldo F., Massoud R., Dolci S., Ruvolo G. Red blood cell distribution width, vascular aging biomarkers, and endothelial progenitor cells for predicting vascular aging and diagnosing/prognosing age-related degenerative arterial diseases. Rejuvenation Research. 2019;22:399–408. doi: 10.1089/rej.2018.2144. [DOI] [PubMed] [Google Scholar]
- 11.Groleau J., Dussault S., Turgeon J., Haddad P., Rivard A. Accelerated vascular aging in CuZnSOD-deficient mice: impact on EPC function and reparative neovascularization. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0023308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X., Zhang J.-Q., Xiu C.-K., Yang J., Fang J.-Y., Lei Y. Ginseng-sanqi-chuanxiong (GSC) extracts ameliorate diabetes-induced endothelial cell senescence through regulating mitophagy via the AMPK pathway. Oxidative Medicine and Cellular Longevity. 2020:1–22. doi: 10.1155/2020/7151946. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lei Y., Yang J., Zhao H. [Experimental study on extracts from ginseng, notoginseng and chuanxiong for delaying vascular aging in senescent mice] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010;30:946–951. [PubMed] [Google Scholar]
- 14.Yang J., Lei Y., Fang S.-P. [Study on acting mechanism of extracts from ginseng, notoginseng and chuanxiong for delaying the aging of endothelial cells induced by angiotensin II] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29:524–528. [PubMed] [Google Scholar]
- 15.Huang J., Hou B., Zhang S., Wang M., Lu X., Wang Q., Liu Y. The protective effect of adiponectin-transfected endothelial progenitor cells on cognitive function in D-galactose-induced aging rats. Neural Plasticity. 2020:1–10. doi: 10.1155/2020/1273198. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rusu M.E., Georgiu C., Pop A., Mocan A., Kiss B., Vostinaru O., Fizesan I., Stefan M.-G., Gheldiu A.-M., Mates L., Moldovan R., Muntean D.M., Loghin F., Vlase L., Popa D.-S. Antioxidant effects of walnut (Juglans regia L.) kernel and walnut septum extract in a D-galactose-induced aging model and in naturally aged rats. Antioxidants. 2020;9:424. doi: 10.3390/antiox9050424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hildreth K.L., Schwartz R.S., Vande Griend J., Kohrt W.M., Blatchford P.J., Moreau K.L. Effects of testosterone and progressive resistance exercise on vascular function in older men. Journal of Applied Physiology. 2018;125:1693–1701. doi: 10.1152/japplphysiol.00165.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shwe T., Pratchayasakul W., Chattipakorn N., Chattipakorn S.C. Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Experimental Gerontology. 2018;101:13–36. doi: 10.1016/j.exger.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Vlassara H. Advanced glycation end-products and atherosclerosis. Annals of Medicine. 1996;28:419–426. doi: 10.3109/07853899608999102. [DOI] [PubMed] [Google Scholar]
- 20.Diaz-Canestro C., Puspitasari Y.M., Liberale L., Guzik T.J., Flammer A.J., Bonetti N.R., Wüst P., Costantino S., Paneni F., Akhmedov A., Varga Z., Ministrini S., Beer J.H., Ruschitzka F., Hermann M., Lüscher T.F., Sudano I., Camici G.G. MMP-2 knockdown blunts age-dependent carotid stiffness by decreasing elastin degradation and augmenting eNOS activation. Cardiovascular Research. 2021 doi: 10.1093/cvr/cvab300. cvab300. [DOI] [PubMed] [Google Scholar]
- 21.Liu T.-H., Zhao L., Zhang C.-Y., Li X.-Y., Wu T.-L., Dai Y.-Y., Sheng Y.-Y., Ren Y.-L., Xue Y.-Z. Gut microbial evidence chain in high-salt diet exacerbates intestinal aging process. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.1046833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao B., Zhu L., Ye M., Lou X., Mou Q., Hu Y., Zhang H., Zhao Y. Oxidative stress and epigenetics in ocular vascular aging: an updated review. Mol Med. 2023;29:28. doi: 10.1186/s10020-023-00624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheng Z.-Q., Li Y.-F., Zheng K.-L., Lu H.-H., Xie J., Wu H., Xu B. The relationship between EPCs and VEGF165 and SDF-1 in coronary artery spasm. European Review for Medical and Pharmacological Sciences. 2018;22:2767–2777. doi: 10.26355/eurrev_201805_14974. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y.-H., Lin S.-J., Lin F.-Y., Wu T.-C., Tsao C.-R., Huang P.-H., Liu P.-L., Chen Y.-L., Chen J.-W. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide–related but not oxidative stress–mediated mechanisms. Diabetes. 2007;56:1559–1568. doi: 10.2337/db06-1103. [DOI] [PubMed] [Google Scholar]
- 25.Barnes P.J., Baker J., Donnelly L.E. Cellular senescence as a mechanism and target in chronic lung diseases. American Journal of Respiratory and Critical Care Medicine. 2019;200:556–564. doi: 10.1164/rccm.201810-1975TR. [DOI] [PubMed] [Google Scholar]
- 26.Yinhang Y., Fuliang B., Yaonan L., Yongbi Y., Qingyan Y., Dehua Z., Susu Q., Guiyou T., Liying S., Tong Z., Siming L., YunYe L., Wenfei W., Guiping R., Deshan L. Fibroblast growth factor (FGF21) protects mouse liver against D-galactose-induced oxidative stress and apoptosis via activating Nrf2 and PI3K/Akt pathways. Mol Cell Biochem. 2015;403:287–299. doi: 10.1007/s11010-015-2358-6. [DOI] [PubMed] [Google Scholar]
- 27.Miao J., Liu J., Niu J., Zhang Y., Shen W., Luo C., Liu Y., Li C., Li H., Yang P., Liu Y., Hou F.F., Zhou L. Wnt/β‐catenin/RAS signaling mediates age‐related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell. 2019;18 doi: 10.1111/acel.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S., Sun Y., Li J., Dong J., Bian Q. Preparation of herbal medicine: Er-xian decoction and Er-Xian-containing serum for in vivo and in vitro experiments. JoVE. 2017 doi: 10.3791/55654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R., Liu L., Liu H., Wu K., Liu Y., Bai L., Wang Q., Qi B., Qi B., Zhang L. Reduced NRF2 expression suppresses endothelial progenitor cell function and induces senescence during aging. Aging. 2019;11:7021–7035. doi: 10.18632/aging.102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding S., Xu J., Zhang Q., Chen F., Zhang J., Gui K., Xiong M., Li B., Ruan Z., Zhao M. OGR1 mediates the inhibitory effects of acidic environment on proliferation and angiogenesis of endothelial progenitor cells. Cell Biol Int. 2019;43:1307–1316. doi: 10.1002/cbin.11179. [DOI] [PubMed] [Google Scholar]
- 31.Wang R., Liu L., Liu H., Wu K., An J., Wang Q., Liu Y., Bai L., Qi B., Qi B., Zhang L. Nrf2 protects against diabetic dysfunction of endothelial progenitor cells via regulating cell senescence. Int J Mol Med. 2018 doi: 10.3892/ijmm.2018.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Y., Liu X.-P., Liu X.-X., Zheng Y.-F., Liu W.-F., Luo M.-L., Gao H., Zhao Y., Zou L. Role of axl in preeclamptic EPCs functions. J Huazhong Univ Sci Technolog Med Sci. 2016;36:395–401. doi: 10.1007/s11596-016-1598-3. [DOI] [PubMed] [Google Scholar]
- 33.Tamari T., Kawar-Jaraisy R., Doppelt O., Giladi B., Sabbah N., Zigdon-Giladi H. The paracrine role of endothelial cells in bone formation via CXCR4/SDF-1 pathway. Cells. 2020;9:1325. doi: 10.3390/cells9061325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu X., Wang B., Ren C., Hu J., Greenberg D.A., Chen T., Xie L., Jin K. Age-related impairment of vascular structure and functions. Aging and Disease. 2017;8:590. doi: 10.14336/AD.2017.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahluwalia A., Jones M.K., Szabo S., Tarnawski A.S. Aging impairs transcriptional regulation of vascular endothelial growth factor in human microvascular endothelial cells: implications for angiogenesis and cell survival. J Physiol Pharmacol. 2014;65:209–215. [PubMed] [Google Scholar]
- 36.Barton M., d'Uscio L.V., Shaw S., Meyer P., Moreau P., Lüscher T.F., et al. A receptor blockade prevents increased tissue endothelin-1, vascular hypertrophy, and endothelial dysfunction in salt-sensitive hypertension. Hypertension. 1998;31:499–504. doi: 10.1161/01.HYP.31.1.499. [DOI] [PubMed] [Google Scholar]
- 37.Wu L., Chen W., Chen Z., Cao J., Dai X., Chen H., Tan X., Yu M. Protocol update for late endothelial progenitor cells identified by double‐positive staining. J Cellular Molecular Medi. 2022;26:306–311. doi: 10.1111/jcmm.17079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sikora E., Arendt T., Bennett M., Narita M. Impact of cellular senescence signature on ageing research. Ageing Research Reviews. 2011;10:146–152. doi: 10.1016/j.arr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 39.He J., Jiang Y.-F., Liang L., Wang D.-J., Wei W.-X., Ji P.-P., Huang Y.-C., Song H., Lu X.-L., Zhao Y.-X. Targeting of AUF1 to vascular endothelial cells as a novel anti-aging therapy. J Geriatr Cardiol. 2017;14:515–523. doi: 10.11909/j.issn.1671-5411.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiao W., Yang W., Liu L., Shi Y. [Exogenous hydrogen sulfde reduces vascular aging in D-galactose-induced subacute aging rats] Acta Physiologica Sinica. 2014;66:276–282. doi: 10.13294/j.aps.2014.003. [DOI] [PubMed] [Google Scholar]
- 41.Azman K.F., Zakaria R. d-Galactose-induced accelerated aging model: an overview. Biogerontology. 2019;20:763–782. doi: 10.1007/s10522-019-09837-y. [DOI] [PubMed] [Google Scholar]
- 42.Bjornstad P., Schäfer M., Truong U., Cree-Green M., Pyle L., Baumgartner A., Garcia Reyes Y., Maniatis A., Nayak S., Wadwa R.P., Browne L.P., Reusch J.E.B., Nadeau K.J. Metformin improves insulin sensitivity and vascular Health in youth with type 1 diabetes mellitus: randomized controlled trial. Circulation. 2018;138:2895–2907. doi: 10.1161/CIRCULATIONAHA.118.035525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulkarni A.S., Gubbi S., Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metabolism. 2020;32:15–30. doi: 10.1016/j.cmet.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida T., Matsuura K., Goya S., Ma D., Shimada K., Kitpipatkun P., Namiki R., Uemura A., Suzuki K., Tanaka R. Metformin prevents the development of monocrotaline-induced pulmonary hypertension by decreasing serum levels of big endothelin-1. Exp Ther Med. 2020;20:149. doi: 10.3892/etm.2020.9278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sritawan N., Suwannakot K., Naewla S., Chaisawang P., Aranarochana A., Sirichoat A., Pannangrong W., Wigmore P., Welbat J.U. Effect of metformin treatment on memory and hippocampal neurogenesis decline correlated with oxidative stress induced by methotrexate in rats. Biomedicine & Pharmacotherapy. 2021;144 doi: 10.1016/j.biopha.2021.112280. [DOI] [PubMed] [Google Scholar]
- 46.Nandula S.R., Kundu N., Awal H.B., Brichacek B., Fakhri M., Aimalla N., Elzarki A., Amdur R.L., Sen S. Role of Canagliflozin on function of CD34+ve endothelial progenitor cells (EPC) in patients with type 2 diabetes. Cardiovasc Diabetol. 2021;20:44. doi: 10.1186/s12933-021-01235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen M., Fu Y., Wang X., Wu R., Su D., Zhou N., Qi Y. Metformin protects lens epithelial cells against senescence in a naturally aged mouse model. Cell Death Discov. 2022;8:8. doi: 10.1038/s41420-021-00800-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azemi A.K., Mokhtar S.S., Sharif S.E.T., Rasool A.H.G. Clinacanthus nutans attenuates atherosclerosis progression in rats with type 2 diabetes by reducing vascular oxidative stress and inflammation. Pharmaceutical Biology. 2021;59:1432–1440. doi: 10.1080/13880209.2021.1990357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laurent S. Defining vascular aging and cardiovascular risk. Journal of Hypertension. 2012;30 doi: 10.1097/HJH.0b013e328353e501. S3–S8. [DOI] [PubMed] [Google Scholar]
- 50.Yang J.-X., Pan Y.-Y., Wang X.-X., Qiu Y.-G., Mao W. Endothelial progenitor cells in age-related vascular remodeling. Cell Transplant. 2018;27:786–795. doi: 10.1177/0963689718779345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duan Y., Yu S., Xu P., Wang X., Feng X., Mao Z., Gao C. Co-immobilization of CD133 antibodies, vascular endothelial growth factors, and REDV peptide promotes capture, proliferation, and differentiation of endothelial progenitor cells. Acta Biomaterialia. 2019;96:137–148. doi: 10.1016/j.actbio.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Garikipati V.N.S., Kishore R. Adult Stem Cells. Springer; New York: 2017. Erratum to: chapter 7 endothelial progenitor cells: procedure for cell isolation and applications. New York, NY. [DOI] [PubMed] [Google Scholar]
- 53.Lee Y.-N., Wang H.-H., Su C.-H., Lee H.-I., Chou Y.-H., Hsieh C.-L., Liu W.-T., Shu K.-T., Chang K.-T., Yeh H.-I., Wu Y.-J. Deferoxamine accelerates endothelial progenitor cell senescence and compromises angiogenesis. Aging. 2021;13:21364–21384. doi: 10.18632/aging.203469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C., Wang F., Li Z., Cao Q., Huang L., Chen S. MeCP2-mediated epigenetic regulation in senescent endothelial progenitor cells. Stem Cell Res Ther. 2018;9:87. doi: 10.1186/s13287-018-0828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumari R., Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.