Abstract
Objective
This study aimed to explore the long-term outcome of unilateral moyamoya disease and predict the clinical and genetic factors associated with contralateral progression in unilateral moyamoya disease.
Methods
We retrospectively recruited unilateral moyamoya disease patients with available genetic data who underwent encephaloduroarteriosynangiosis (EDAS) surgery at our institution from January 2009 to November 2017. Long-term follow-up data, including clinical outcomes, angiographic features, and genetic information, were analyzed.
Results
A total of 83 unilateral moyamoya disease patients with available genetic data were enrolled in our study. The mean duration of clinical follow-up was 7.9 ± 2.0 years. Among all patients, 19 patients demonstrated contralateral progression to bilateral disease. Heterozygous Ring Finger Protein 213 p.R4810K mutations occurred significantly more frequently in unilateral moyamoya disease patients with contralateral progression. Furthermore, patients with contralateral progression typically demonstrated an earlier age of onset than those with non-progressing unilateral moyamoya disease. In the contralateral progression group, posterior circulation involvement was observed in 11 (11/19, 57.9%) patients compared to 12 (12/64, 18.8%) in the non-contralateral progression group (P = 0.001). The time to peak of cerebral perfusion and neurological status showed significant postoperative improvement.
Conclusion
Long-term follow-up revealed that the EDAS procedure might provide benefits for unilateral moyamoya disease patients. Ring Finger Protein 213 p.R4810K mutations, younger age, and posterior circulation involvement might predict the contralateral progression of unilateral moyamoya disease.
1. Introduction
Moyamoya disease (MMD) is a chronic nonatherosclerotic cerebrovascular disease characterized by stenosis and occlusion of the terminal internal carotid artery (ICA), with/without proximal middle cerebral artery (MCA), anterior cerebral artery (ACA), accompanied by abnormal collateral vessel networks at the base of the brain [1,2]. “Moyamoya” refers to the characteristic angiographic findings of tiny vessels resembling a “puff of smoke”, regardless of unknown reason of its etiology. According to the Guidelines for Diagnosis and Treatment of Moyamoya Disease, as established by the Research Committee on Moyamoya Disease in Japan, the appearance of bilateral ICA steno-occlusive lesions with abnormal vascular networks can be considered moyamoya disease [3]. However, unilateral moyamoya disease also occurs as a special subtype classified as “probable” moyamoya disease or an early stage of definitive moyamoya disease progression [3,4]. Whether bilateral MMD represents a continuum of unilateral moyamoya disease remains controversial, and the factors predicting contralateral progression remain unclear [[4], [5], [6], [7], [8], [9], [10], [11]].
Our previous study revealed the p.R4810K (c.14429G > A) variant of ring finger protein 213 (RNF213) as a susceptible gene for bilateral MMD [12]. Additionally, MMD patients with RNF213 p.R4810K mutation exhibited more severe symptoms and faster disease progression than those without [[13], [14], [15]]. Furthermore, our clinical experience with unilateral MMD has revealed that some patients develop bilateral lesions over time [16]. While the role of RNF213 p.R4810K mutations in this process has not yet been fully elucidated. Therefore, investigating the clinical characteristics, surgical outcomes, and risk factors associated with the contralateral progression of unilateral moyamoya disease, including an assessment of RNF213 p.R4810K mutation status, could provide valuable information about the mechanisms underlying unilateral MMD progression.
2. Methods
2.1. Patient selection
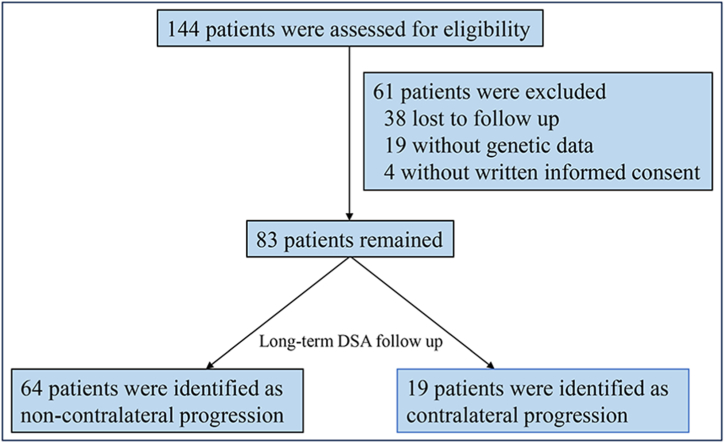
We prospectively recruited patients with unilateral MMD who underwent encephaloduroarteriosynangiosis (EDAS) procedure at the Fifth Medical Center of Chinese PLA General Hospital. The diagnosis was made according to the 2012 guidelines for unilateral MMD [3]. The inclusion criteria were as follows: 1) patients who were diagnosed with unilateral MMD at the time of the initial manifestation or on admission according to the guidelines: the diagnostic criteria comprise evidence of stenosis or occlusion affecting the terminal internal carotid arteries unilaterally, accompanied by the abnormal basal collateral vessels visualized on digital subtraction angiography (DSA) or magnetic resonance angiography (MRA); 2) patients who underwent EDAS treatment; 3) patients who received DSA detection at least twice; and 4) patients who received peripheral vein blood collection and underwent genotyping on admission or during follow-up. The exclusion criteria were as follows: 1) patients with abnormalities in the contralateral ICA; 2) patients with acute infarction or acute hemorrhage within three months. Finally, sixty-one patients without complete clinical follow-up and gene data were excluded and 83 patients were enrolled in this study. The flow diagram of enrollment is shown in Fig. 1. Approval for this study was obtained from the Research Ethics Board, and all subjects provided written informed consent to participate. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki (1964) for research involving human subjects.
Fig. 1.
The flow diagram of enrollment.
2.2. Clinical data collection
The clinical characteristics included sex, initial age, lesion side (anatomical and hemodynamic differences between bilateral ICA may influence contralateral progression tendency), initial symptoms, neurological status using the modified Rankin Scale score (mRS), primary diseases (hypertension, hyperlipidemia, diabetes mellitus), posterior circulation involvement (PCI), and family history. Initial symptoms were classified into asymptomatic, dizziness, headache, transient ischemic attack (TIA), infarction, and hemorrhage. Dynamic susceptibility contrast-magnetic resonance imaging (DSC-MRI) examination was used with key metrics, including time to peak (TTP, the time at which the contrast level reaches its peak) and the mean relative cerebral blood volume (rCBV) to evaluate the patients’ hemodynamic status.
2.3. Angiographic follow-up
Angiographic examinations were conducted before surgery for all patients, and DSA follow-up was required for each patient ≥6 months after surgery. MMD process on angiography was graded according to the Suzuki approach: stage I, stenosis at the bifurcation of the terminal ICA and no other abnormalities on DSA; stage II, stenosis at the bifurcation of the terminal ICA with the initial appearance of moyamoya vessels at the base of the brain and no extracranial–intracranial (EC–IC) circulation; stage III, moyamoya vessels become more prominent and develop into a mass; stage IV, ICA occluded to the level of the posterior communicating artery and moyamoya vessels are diminished, accompanied by indiscernible MCA or ACA with EC–IC circulation; stage V, ICA occluded to a lower level, moyamoya vessels are reduced, and EC–IC circulation is richer; and stage VI, the siphon segment of the ICA and the moyamoya vessels disappears completely, and the cerebral anterior territory supply completely depends on the external carotid or vertebral artery [1]. The progression of contralateral ICA was defined as the appearance of steno-occlusion lesions in the contralateral initially unimpaired ICA, with or without the presence of moyamoya vessels. PCI refers to posterior circulation involvement and indicates a lesion in the posterior cerebral artery (PCA), according to the Mugikura PCA lesion-grading system [17]. All angiography images were reviewed blindly by two experienced readers (Xiang-Yang Bao and Qian-Nan Wang) and any disagreement was resolved by consensus.
2.4. Genotyping
Peripheral vein blood (10 mL) was collected from the recruited patients for assaying, and genomic DNA was extracted using a Blood Genetic DNA Mini Kit (CWBIO, Beijing, China). p.R4810K genotyping was conducted using Taqman TM Probe (TaqmanTM SNP Genotyping Assays; Applied Biosystems, Foster City, CA, USA) and a QuantStudioTM 6 Flex Real-Time PCR System (Applied Biosystems, Foster City, USA) as described in our previous study [12].
2.5. Surgical treatment
EDAS surgery is performed for patients with clinically significant symptoms impairing daily living or evidence of substantially compromised cerebral perfusion based on disease severity. The superficial temporal artery is typically utilized as the donor vessel in EDAS, while the occipital artery can be selected in posterior revascularization procedures. The key surgical steps include isolating the donor vessel with an attached galeal flap, suturing this to the dural opening over the ischemic territory, and preserving burr holes at the edges of the craniectomy to maintain vessel integrity intraoperatively [18,19].
2.6. Evaluation of postsurgical long-term clinical outcome follow-up
All patients underwent the EDAS procedures on the basis of their clinical symptoms, DSA evaluation findings, and hemodynamic impairment in the initially impaired unilateral or later progressive contralateral hemispheres. Neovascularization derived from bypass grafts on DSA was described by Matsushima et al. as follows: A, the neovascular network occupies over two-thirds of the MCA distribution area; B, the neovascular network occupies between one-third and two-thirds of the MCA distribution area; C, the neovascular network occupies less than one-third of the MCA distribution area, or no neovascularization is present [20]. Meanwhile, clinical follow-ups were also required for each patient 6 months or longer after surgery. We evaluated long-term clinical outcomes by assessing neurological status improvement, cerebral hemodynamic status, and stroke recurrence, including cerebral infarction and hemorrhage.
2.7. Statistical analysis
All analyses were performed with the use of SPSS version 25.0 (IBM Corporation, Armonk, NY, USA). Categorical variables were analyzed using the chi-squared test (including Fisher's exact test), and continuous variables (the non-continuous or ordinal variables) were compared using the paired-samples Wilcoxon signed rank test or the Mann–Whitney U test (analysis of variance). Cox proportional hazards model were used to estimate the contralateral progression risk factors. To assess autocorrelation and collinearity in the regression analysis, Durbin-Watson and multicollinearity diagnostic tests were performed. The data were considered significant when P < 0.05.
3. Results
3.1. Patient characteristics and clinical data
A total of 83 patients with unilateral MMD were enrolled in our study between January 2009 and November 2017. Among all patients, 45 (54.2%) were male, and 38 (45.8%) were female, with a slight male preponderance (1.18:1); the average age at initial symptom onset was 33.9 ± 14.7 (range 3–60) with a mean duration of 7.9 ± 2.0 years (range 2.0–13.9 years) of clinical follow-up. Thirteen (15.7%) patients were under 18 years of age, and 11 patients (13.3%) had a family history of MMD. Twenty-seven (32.5%) patients had a hypertension history, 20 (24.1%) had diabetes, and 26 (31.3%) had hyperlipidemia. Among the 83 patients with unilateral MMD, the initial symptom frequency distributions were as follows: 2 (2.4%) presented as asymptomatic, 9 (10.8%) presented with dizziness, 16 (19.3%) presented with headache, 28 (33.7%) presented with TIA, 14 (16.9%) presented with infarction and 14 (16.9%) presented with hemorrhage. Sixty-five patients (78.3%) had the GG (wild-type) genotype, and 18 (21.7%) had the GA (heterozygous) genotype. (Table 1).
Table 1.
Baseline patient characteristics.
| Patient characteristics | Number of patients (n = 83) |
|---|---|
| Mean age ± SD, years | 34.3 ± 14.4 |
| Female | 38 (45.8%) |
| Left-side lesions | 44 (53.0%) |
| PCI | 23 (27.7%) |
| Stroke risk factors | |
| Hypertension | 27 (32.5%) |
| Diabetes mellitus | 20 (24.1%) |
| Hyperlipidemia | 26 (31.3%) |
| Initial symptom | |
| Asymptomatic | 2 (2.4%) |
| Dizziness | 9 (10.8%) |
| Headache | 16 (19.3%) |
| TIA | 28 (33.7%) |
| Infarction | 14 (16.9%) |
| Hemorrhage | 14 (16.9%) |
| Suzuki angiographic stage | |
| 1 | 0 (0.0%) |
| 2 | 19 (22.9%) |
| 3 | 4 (4.8%) |
| 4 | 31 (37.3%) |
| 5 | 20 (24.1%) |
| 6 | 9 (10.8%) |
| RNF213 p.R4810K Mutation | |
| GG | 65 (78.3%) |
| GA | 18 (21.7%) |
| Family History | 11 (13.3%) |
PCI: Posterior circulation involvement; TIA: Transient ischemic attack; GG: wild type (GG) of the c.14576G > A variant; GA: the heterozygote of the c.14576G > A variant.
3.2. Angiographic findings
Angiography was required for all enrolled patients preoperatively and during follow-up including staging of the initially impaired internal carotid artery over time, progression of contralateral normal internal carotid artery, and involvement of the posterior circulation vertebrobasilar system. Initial left hemisphere vasculopathy in the anterior circulation was found in 44 (53.0%) patients, and the initial Suzuki stages of the impaired side of unilateral MMD patients were as follows: stage II, 19 patients (22.9%); stage III, 4 (4.8%); stage IV, 31 (37.3%); stage V, 20 (24.1%); and stage VI, 9 (10.8%). PCI lesions were found in 23 (27.7%) patients (Table 1). Among the 23 hemispheres with PCI lesions, 11 were left-impaired, eight were right-impaired, and four were bilateral impaired. During a mean follow-up duration of 7.9 ± 2.0 years (range 2.0–13.9 years), contralateral progression was found in 19 (22.9%) patients. The mean time to contralateral progression was 4.1 ± 2.1 years (range 0.8–8.0 years). Among the 19 patients with contralateral progression, 10 developed Suzuki stage I in the contralateral hemisphere, four developed stage II, three developed stage IV, one developed stage V, and one developed stage VI. Of the 64 patients without contralateral progression, 20 demonstrated worsening Suzuki stages at the initial impaired ICA over time, and 44 remained unchanged (P < 0.001). Only one patient with no lesion in the posterior circulation demonstrated PCI progression.
3.3. Discrepancies between contralateral progression and non-contralateral progression and risk factors of contralateral progression
Patients were divided into contralateral progression and non-contralateral progression groups to identify predictive factors associated with contralateral progression. Patients with contralateral progression were younger than those with non-contralateral progression (19/83, 22.9% vs. 64/83, 77.1%; mean ages 37.0 ± 12.7 vs. 25.1 ± 16.2 years, respectively; P = 0.007). Additionally, PCI (12/64, 18.8% vs. 11/19, 57.9%, P = 0.001) and the RNF213 c.14576G > A variant (9/19, 47.5% vs. 9/64, 14.1%, P = 0.002) were more common in the contralateral progression group. No differences between the two groups were observed in the other clinical data, such as sex, lesion side, initial symptoms, clinical history, and family history (Table 2).
Table 2.
Comparison of the baseline characteristics between the non-contralateral progression (NCP) and contralateral progression (CP) groups.
| Patient characteristics | NCP (n = 64) | CP (n = 19) | P value |
|---|---|---|---|
| Mean age ± SD, years | 37.0 ± 12.7 | 25.1 ± 16.2 | 0.007 |
| Female | 28 (43.8%) | 10 (52.6%) | 0.495 |
| Left-side lesion | 34 (53.1%) | 10 (52.6%) | 0.970 |
| PCI | 12 (18.8%) | 11 (57.9%) | 0.001 |
| Stroke risk factors | |||
| Hypertension | 21 (32.8%) | 6 (31.6%) | 0.920 |
| Diabetes mellitus | 15 (23.4%) | 5 (26.3%) | 0.797 |
| Hyperlipidemia | 22 (34.3%) | 4 (21.1%) | 0.272 |
| Initial symptom | 0.562 | ||
| Asymptomatic | 2 (3.1%) | 0 (0) | |
| Dizziness | 8 (12.5%) | 1 (5.3%) | |
| Headache | 10 (15.6%) | 6 (31.6%) | |
| TIA | 23 (35.9%) | 5 (26.3%) | |
| Infarction | 10 (15.6%) | 4 (21.1%) | |
| Hemorrhage | 11 (17.2%) | 3 (15.8%) | |
| Suzuki angiographic stage | 0.686 | ||
| 1 | 0 (0) | 0 (0) | |
| 2 | 13 (20.3%) | 6 (31.6%) | |
| 3 | 4 (6.3%) | 0 (0) | |
| 4 | 25 (39.1%) | 6 (31.6%) | |
| 5 | 15 (23.4%) | 5 (26.3%) | |
| 6 | 7 (10.9%) | 2 (10.5%) | |
| RNF213 p.R4810K Mutation | 0.002 | ||
| GG | 55 (85.9%) | 10 (52.6%) | |
| GA | 9 (14.1%) | 9 (47.5%) | |
| Family History | 9 (14.1%) | 2 (10.5%) | 0.690 |
NCP: non-contralateral progression; CP: contralateral progression; PCI: Posterior circulation involvement; TIA: Transient ischemic attack; GG: wild type (GG) of the c.14576G > A variant; GA: the heterozygote of the c.14576G > A variant.
Durbin-Watson (D-W = 2.273) and multicollinearity (variance inflation factor range 1.060–1.728) tests, indicating no significant autocorrelation or collinearity that could undermine the regression model's predictive ability. Multivariate cox regression analyses also revealed that the initial age (HR = 0.960, 95% CI, [0.930–0.992], P = 0.014) of contralateral progression patients was lower than that of non-contralateral progression patients, and the frequencies of PCI (HR = 3.329, 95% CI, [1.217–9.109], P = 0.019) and the homozygous c.14576G > A variant in RNF213 p.R4810K (HR = 3.690, 95% CI, [1.432–9.513], P = 0.007) were higher, indicating that lower age, PCI, and RNF213 p.R4810K mutation are risk factors of contralateral progression in unilateral MMD. Sex, lesion side, clinical history, and family history showed no significant differences (Table 3).
Table 3.
Univariate and multivariate cox regression analyses of risk factors for contralateral progression.
| Characteristics | Univariate cox regression analyses |
Multivariate cox regression analyses |
||||||
|---|---|---|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | HR | 95% CI | |||
| Initial Age | 0.000 | 0.945 | 0.916 | 0.975 | 0.014 | 0.960 | 0.930 | 0.992 |
| Sex | 0.510 | 0.738 | 0.299 | 1.820 | ||||
| Symptoms | 0.742 | 1.060 | 0.750 | 1.499 | ||||
| Side | 0.915 | 0.952 | 0.386 | 2.349 | ||||
| PCI | 0.001 | 4.856 | 1.927 | 12.241 | 0.019 | 3.329 | 1.217 | 9.109 |
| Suzuki stage | 0.791 | 0.955 | 0.678 | 1.345 | ||||
| Hypertension | 0.853 | 0.913 | 0.347 | 2.404 | ||||
| Diabetes mellitus | 0.803 | 1.139 | 0.410 | 3.166 | ||||
| Hyperlipemia | 0.273 | 0.539 | 0.179 | 1.627 | ||||
| RNF213 p.R4810K Mutation | 0.001 | 4.381 | 1.773 | 10.824 | 0.007 | 3.690 | 1.432 | 9.513 |
| Family History | 0.457 | 0.568 | 0.128 | 2.523 | ||||
HR: hazard ratios; PCI: Posterior circulation involvement.
3.4. Long-term clinical outcome follow-up
Overall, 83 unilateral EDAS procedures were performed on the study participants, and an additional 23 (11 in the contralateral hemisphere and 12 in the occipital lobe) during follow-up. The patients’ neurological status improved significantly; the preoperative versus postoperative mRS scores were 72 vs. 78 (score 0); 7 vs. 2 (score 1); 1 vs. 1 (score 2); 2 vs. 2 (score 3); and 1 vs. 0 (score 4) (paired samples Wilcoxon signed rank test, P = 0.033). Only 1 (1.2%) hemorrhagic MMD patient experienced a worsened neurological status after surgery with a later occurrence of ischemic stroke; the Suzuki stage of the unilateral side changed from stage II to VI in this patient, and the contralateral side developed to stage VI in 6 years. The postoperative Matsushima grades of the unilateral side were as follows: eight (9.6%) patients were grade A, 71 (85.5%) were grade B, and four (4.8%) were grade C. Among 83 patients, only 25 had complete preoperative and follow-up TTP and rCBV data. Cerebral TTP showed significant improvement postoperatively (paired samples Wilcoxon signed rank test, P = 0.002); however, no improvements were observed in rCBV (P = 0.382).
4. Discussion
MMD is a chronic, progressive cerebral vascular disease with definitive bilateral lesion in ICA. The MMD diagnosis guidelines define unilateral steno-occlusive and moyamoya changes with stenosis of the contralateral ICA in children as definitive MMD. In contrast, unilateral changes without contralateral stenosis in children and unilateral changes with or without contralateral stenosis in adults are classified as unilateral (probable) MMD [3]. Whether unilateral MMD can be classified as definitive MMD remains complicated [[4], [5], [6], [7], [8], [9], [10], [11],21] Our long-term investigation of unilateral MMD progression incorporating genetics provides pivotal insights into this rare angiopathy of unknown etiology and enables optimized management, with the longest mean follow-up duration to date among unilateral MMD studies. The best management of unilateral MMD remains unclear and identifying unilateral MMD patients at risk for contralateral progression would provide significant clinical benefit. Consistent with previous studies on bilateral MMD, the patients with unilateral MMD in our study might benefited from EDAS, with 79 (95.2%) reaching Matsushima stages A and B and significantly improved neurological status. We excluded abnormalities in the contralateral ICA as such contralateral changes likely represent early definitive bilateral MMD, confounding analysis of progression from true unilateral disease.
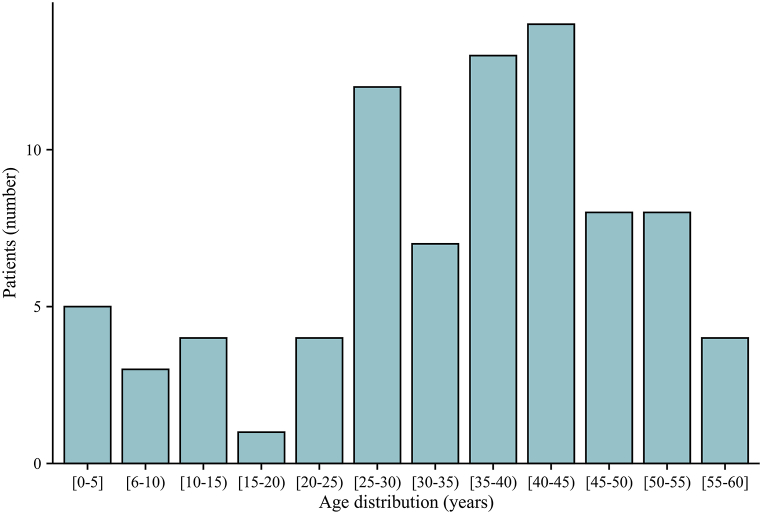
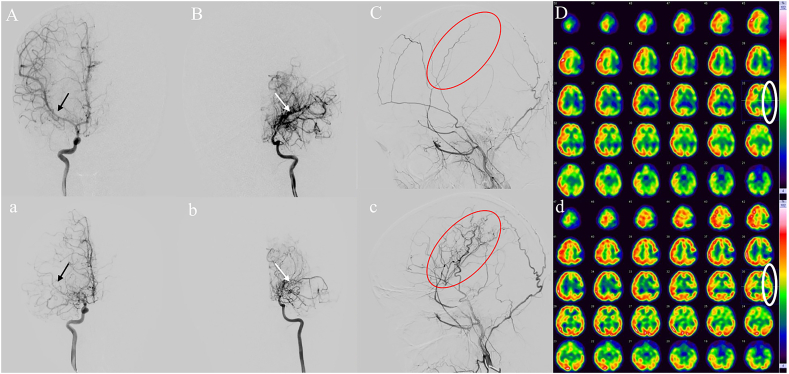
During a mean follow-up duration of 7.9 ± 2.0 years (range 2.0–13.9 years), 19/83 (22.9%) patients in our study experienced contralateral progression to definitive bilateral MMD. The frequency was higher than that in other studies in China but consistent with the results of previous Japanese and North American studies (8.3%–30%) [4,9,11,21]. This discrepancy could be attributable to the longer follow-up periods (7.9 ± 2.0 years); Kuroda and his team also reported that contralateral progression mainly occurs 7–8 years after the initial diagnosis [22]. Contralateral lesions developing in unilateral MMD patients indicate that unilateral MMD cannot be entirely eliminated from definitive MMD. The course of the progression of the contralateral lesion in unilateral MMD also enables us to better understand the pathophysiology of MMD. Family history occurred in 11 (13.3%) patients in our study which was higher than our previous epidemiological survey study of definitive MMD in China but consistent with the findings of other national-wide studies [6,10,12,23,24]. Consistent with a Japanese national survey of unilateral MMD, our analysis also showed two peaks in the age distribution pattern [6]. However, there was a discrepancy in the second peak (patients in the fourth decade in our study, but those in the fifth decade in the Japanese study, Fig. 2), which aligned with our Chinese MMD cohort study findings [23]. Several previous studies have demonstrated that contralateral progression mainly occurs in pediatric patients, and our cox regression analyses also confirmed that younger age was a predictive factor for contralateral progression [7,9]. In addition, Smith and Scott confirmed that children under 7 years of age had a more rapid progression compared to the older patients (0.9 years versus 3.1 years to progression) [4]. Therefore, it is necessary for pediatric patients with unilateral MMD to take more frequent clinical follow-up, and timely disease management is indispensable (a typical pediatric unilateral case with rapid contralateral progression is shown in Fig. 3A–D, a-d).
Fig. 2.
Age distribution of unilateral moyamoya disease patients.
Fig. 3.
A pediatric unilateral MMD patient. The right internal carotid artery (RICA) was normal (A, black arrow), the Suzuki stage of the left internal carotid artery (LICA) was IV (B, white arrow) on initial admission in 2016, and the left external carotid artery (LECA, red circle) was pre-operative (C). Emission computed tomography (ECT) showed hypoperfusion in the left hemisphere (D) pre-operatively. The RICA was impaired with occlusion of the middle cerebral artery (MCA) and stenosis of the anterior cerebral artery (ACA) (a, black arrow) and attenuation of moyamoya vessels in LICA (b, white arrow) in 2019, post-operative LECA (c, red circle) showed excellent compensatory collaterals and postoperative ECT (d, white circle) showed improvement of perfusion in the left hemisphere postoperatively compared to preoperative status (D, white circle).
Previous studies have identified the homozygous c.14576G > A variant in RNF213 as the first pathogenic gene associated with MMD [12,25,26]. This RNF213 p.R4810K mutation was present in 17 (20.5%) patients in our study, lower than the incidence observed in Japanese and South Korean studies but in accordance with the trend in our definitive MMD cohort study [9,10,12]. However, patients in the contralateral progression group showed a significantly higher rate of RNF213 p.R4810K mutation than those in the non-contralateral progression group (9/19 (47.5%) vs. 9/64 (14.1%)) (P = 0.002). Therefore, patients with RNF213 p.R4810K mutation had a significantly higher propensity to progress to contralateral lesions than those without. The multivariate cox regression analyses also showed that RNF213 p.R4810K mutation was a predictive factor of contralateral progression. Our previous studies have found that MMD patients with RNF213 p.R4810K mutation have an accelerated disease progression and unfavourable prognosis [12,13,15]. This finding may attribute to the more severe disease and more extensive vascular involvement in MMD patients with the RNF213 p.R4810K mutation, predisposing to contralateral progression [13,27,28]. Thus, the unilateral presentation likely represents an early phase of disease process which may developed to bilateral moyamoya disease in patients with RNF213 p.R4810K mutation. Clinicians should closely monitor unilateral moyamoya patients carrying the RNF213 mutation, with more frequent follow-up to enable early detection and intervention. Previous studies on unilateral MMD have identified several risk factors for contralateral progression, such as sex, age at diagnosis, contralateral abnormalities on initial angiography, infarction at onset, RNF213 p.R4810K mutation, and other lifestyle factors [6,7,[9], [10], [11],17,21]. Our study further revealed that younger age and RNF213 p.R4810K mutation were predictive factors for contralateral progression. Interestingly, PCI occurred more frequently in the contralateral progression group than in the non-contralateral progression group and was another predictive factor for contralateral progression. The PCA system is usually affected in both definitive MMD and unilateral MMD, and previous studies have revealed that MMD with PCI has a predisposition to have a more severe steno-occlusive lesions [8,17,29]. Indeed, posterior compensatory circulation, such as leptomeningeal collaterals from the PCA, plays a crucial role in feeding the anterior circulation when the ICA is impaired and the blood supply in the anterior territory is insufficient. In other words, when MMD patients have posterior circulation impairment, there is less cerebral blood compensation to the anterior circulation and more severe perfusion in the anterior and posterior circulation. Mugikura et al. reported that the lesions of the PCA system might be attributable to thrombosis via the posterior communicating artery from the ipsilateral ICA [30]. However, among our study cohort of the 23 patients with PCI lesions, five were pediatric patients, and no abnormalities were found in the prothrombotic state. Therefore, posterior circulation involvement indicates that MMD is a systemic cerebrovascular disease involving both anterior and posterior circulation, and the PCI phenomenon or PCI phenotype in MMD signifies a broader lesion in the intracranial vasculature, thus affecting the contralateral vessels.
Intriguingly, we found that 23 patients had unilateral MMD with PCI lesions in our study. Fourteen were (60.9%) ipsilateral, and nine (39.1%) were heterolateral. Posterior circulation impairment in MMD is not rare in clinical practice. Mugikura et al. reported that more severe ICA seems to involve the ipsilateral PCA system [28]. However, Hishikawa et al. found that both less and more advanced ICA lesion could complicate ipsilateral PCI in pediatric patients [31]. Regrettably, current unilateral MMD diagnostic criteria overlook posterior circulation involvement; while ipsilateral PCA lesions with unilateral ICA impairment may constitute pure unilateral disease, classifying heterolateral or bilateral PCA changes poses challenges. Therefore, incorporating posterior cerebral artery injury into the definition and diagnostic criteria of moyamoya diseases would be conducive to a more precise classification and personalized treatment of each patient.
5. Limitation
This study had several limitations. First, the sample size was relatively small since only patients with available genetic data were selected and there may be a selection bias given the retrospective design of this study. Thus, more cases are needed to confirm the results. Second, our study population was a surgical cohort; those undergoing pharmacotherapy were excluded due to the limited number resulting from a lack of second DSA follow-ups, and the effect of treatment methods on contralateral progression remains unknown. Third, although the mean time to contralateral progression has been calculated, the precise time node of contralateral progression has not yet to be fully elucidated. Fourth, although some unilateral MMD patients progressed to bilateral MMD and we identified several factors predictive of contralateral progression, the intricate relationship between unilateral and definitive MMD has not been elucidated. Therefore, studies on the etiology and pathogenesis of MMD and unilateral MMD remain indispensable.
Given that the progression of contralateral stenosis in unilateral MMD is common, our study indicated that a portion of unilateral MMD represented early-stage of definitive MMD. RNF213 p.R4810K mutations, younger age, and posterior circulation involvement may be predictors of contralateral progression in unilateral MMD patients, and more frequent follow-up is necessary.
Ethics approval statement
The study was approved by the Research Ethics Board at the Fifth Medical Center of Chinese PLA General Hospital (ky-2020-9-22) in September 2020.
Funding statement
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 82171280 & 82201451).
Digital subtraction angiography statement
Written informed consent was obtained on behalf of participant in this study from a parent or guardian.
Data availability statement
With the permission of the corresponding authors and their affiliated institutions, all data utilized for the analysis and the pertinent documentation can be obtained following ethical clearance upon request for justifiable intentions of replicating techniques and findings.
CRediT authorship contribution statement
Xiao-Peng Wang: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation. Zheng-Xing Zou: Writing – review & editing, Writing – original draft, Data curation. Xiang-Yang Bao: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Qian-Nan Wang: Writing – review & editing, Writing – original draft, Formal analysis, Conceptualization. Bin Ren: Writing – review & editing, Writing – original draft, Data curation. Dan Yu: Methodology, Data curation. Qian Zhang: Methodology, Investigation, Data curation. Jia-Qi Liu: Methodology, Investigation, Data curation. Fang-Bin Hao: Methodology, Data curation. Gan Gao: Investigation, Data curation. Qing-Bao Guo: Data curation. He-Guan Fu: Data curation. Jing-Jie Li: Methodology, Data curation. Min-Jie Wang: Methodology, Data curation. Si-Meng Liu: Methodology, Data curation. Lian Duan: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Lian Duan reports article publishing charges was provided by National Natural Science Foundation of China. Qian-Nan Wang reports article publishing charges was provided by National Natural Science Foundation of China. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Suzuki J., Takaku A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch. Neurol. 1969;20(3):288–299. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- 2.Choi J.U., Kim D.S., Kim E.Y., Lee K.C. Natural history of moyamoya disease: comparison of activity of daily living in surgery and non surgery groups. Clin. Neurol. Neurosurg. 1997;99(Suppl 2):S11–S18. doi: 10.1016/s0303-8467(97)00033-4. [DOI] [PubMed] [Google Scholar]
- 3.Research committee on the pathology and treatment of spontaneous occlusion of the circle of willis; health labour sciences research grant for research on measures for infractable diseases. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of willis) Neurol. Med.-Chir. 2012;52(5):245–266. doi: 10.2176/nmc.52.245. [DOI] [PubMed] [Google Scholar]
- 4.Smith E.R., Scott R.M. Progression of disease in unilateral moyamoya syndrome. Neurosurg. Focus. 2008;24(2):E17. doi: 10.3171/FOC/2008/24/2/E17. [DOI] [PubMed] [Google Scholar]
- 5.Houkin K., Abe H., Yoshimoto T., Takahashi A. Is "unilateral" moyamoya disease different from moyamoya disease? J. Neurosurg. 1996;85(5):772–776. doi: 10.3171/jns.1996.85.5.0772. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi K., Horie N., Izumo T., Nagata I. A nationwide survey on unilateral moyamoya disease in Japan. Clin. Neurol. Neurosurg. 2014;124:1–5. doi: 10.1016/j.clineuro.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Yeon J.Y., Shin H.J., Kong D.S., et al. The prediction of contralateral progression in children and adolescents with unilateral moyamoya disease. Stroke. 2011;42(10):2973–2976. doi: 10.1161/STROKEAHA.111.622522. [DOI] [PubMed] [Google Scholar]
- 8.Matsushima T., Fukui M., Fujii K., et al. Two pediatric cases with occlusions of the ipsilateral internal carotid and posterior cerebral arteries associated with moyamoya vessels: "unilateral" moyamoya disease. Surg. Neurol. 1990;33(4):276–280. doi: 10.1016/0090-3019(90)90048-t. [DOI] [PubMed] [Google Scholar]
- 9.Mineharu Y., Takagi Y., Koizumi A., et al. Genetic and nongenetic factors for contralateral progression of unilateral moyamoya disease: the first report from the SUPRA Japan Study Group [published correction appears in J Neurosurg. 2021 Oct 29;136(4):1207] J. Neurosurg. 2021;136(4):1005–1014. doi: 10.3171/2021.3.JNS203913. Published 2021 Sep. 10. [DOI] [PubMed] [Google Scholar]
- 10.Ok T., Jung Y.H., Kim J., et al. RNF213 R4810K variant in suspected unilateral moyamoya disease predicts contralateral progression. J. Am. Heart Assoc. 2022;11(15) doi: 10.1161/JAHA.122.025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Church E.W., Bell-Stephens T.E., Bigder M.G., Gummidipundi S., Han S.S., Steinberg G.K. Clinical course of unilateral moyamoya disease. Neurosurgery. 2020;87(6):1262–1268. doi: 10.1093/neuros/nyaa284. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Zhang Z., Wei L., et al. Predictive role of heterozygous p.R4810K of RNF213 in the phenotype of Chinese moyamoya disease. Neurology. 2020;94(7):e678–e686. doi: 10.1212/WNL.0000000000008901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao X.Y., Fan Y.N., Wang Q.N., et al. The potential mechanism behind native and therapeutic collaterals in moyamoya. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.861184. Published 2022 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan L., Wei L., Tian Y., et al. Novel susceptibility loci for moyamoya disease revealed by a genome-wide association study. Stroke. 2018;49(1):11–18. doi: 10.1161/STROKEAHA.117.017430. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q.N., Yang R.M., Zou Z.X., et al. Predictors of neoangiogenesis after indirect revascularisation in moyamoya disease: a 10-year follow-up study. J. Neurol. Neurosurg. Psychiatry. 2021;92(12):1361–1362. doi: 10.1136/jnnp-2020-325401. [DOI] [PubMed] [Google Scholar]
- 16.Bao X.Y., Wang Q.N., Wang X.P., et al. Recognition of the effect of indirect revascularization for moyamoya disease: the balance between the stage progression and neoangiogenesis. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.861187. Published 2022 May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mugikura S., Takahashi S., Higano S., et al. The relationship between cerebral infarction and angiographic characteristics in childhood moyamoya disease. AJNR Am J Neuroradiol. 1999;20(2):336–343. [PMC free article] [PubMed] [Google Scholar]
- 18.Bao X.Y., Duan L., Li D.S., et al. Clinical features, surgical treatment and long-term outcome in adult patients with Moyamoya disease in China. Cerebrovasc. Dis. 2012;34(4):305–313. doi: 10.1159/000343225. [DOI] [PubMed] [Google Scholar]
- 19.Bao X.Y., Duan L., Yang W.Z., et al. Clinical features, surgical treatment, and long-term outcome in pediatric patients with moyamoya disease in China. Cerebrovasc. Dis. 2015;39(2):75–81. doi: 10.1159/000369524. [DOI] [PubMed] [Google Scholar]
- 20.Matsushima T., Inoue T., Suzuki S.O., Fujii K., Fukui M., Hasuo K. Surgical treatment of moyamoya disease in pediatric patients--comparison between the results of indirect and direct revascularization procedures. Neurosurgery. 1992;31(3):401–405. doi: 10.1227/00006123-199209000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q., Wang R., Liu Y., et al. Clinical features and long-term outcomes of unilateral moyamoya disease. World Neurosurg. 2016;96:474–482. doi: 10.1016/j.wneu.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda S., Ishikawa T., Houkin K., Nanba R., Hokari M., Iwasaki Y. Incidence and clinical features of disease progression in adult moyamoya disease. Stroke. 2005;36(10):2148–2153. doi: 10.1161/01.STR.0000182256.32489.99. [DOI] [PubMed] [Google Scholar]
- 23.Duan L., Bao X.Y., Yang W.Z., et al. Moyamoya disease in China: its clinical features and outcomes. Stroke. 2012;43(1):56–60. doi: 10.1161/STROKEAHA.111.621300. [DOI] [PubMed] [Google Scholar]
- 24.Ahn H.S., Kazmi S.Z., Kang T., et al. Familial risk for moyamoya disease among first-degree relatives, based on a population-based aggregation study in korea. Stroke. 2020;51(9):2752–2760. doi: 10.1161/STROKEAHA.120.029251. [DOI] [PubMed] [Google Scholar]
- 25.Kamada F., Aoki Y., Narisawa A., et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J. Hum. Genet. 2011;56(1):34–40. doi: 10.1038/jhg.2010.132. [DOI] [PubMed] [Google Scholar]
- 26.Liu W., Morito D., Takashima S., et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q., Liu Y., Zhang D., et al. RNF213 as the major susceptibility gene for Chinese patients with moyamoya disease and its clinical relevance. J. Neurosurg. 2017;126(4):1106–1113. doi: 10.3171/2016.2.JNS152173. [DOI] [PubMed] [Google Scholar]
- 28.Miyatake S., Miyake N., Touho H., et al. Homozygous c.14576G>A variant of RNF213 predicts early-onset and severe form of moyamoya disease. Neurology. 2012;78(11):803–810. doi: 10.1212/WNL.0b013e318249f71f. [DOI] [PubMed] [Google Scholar]
- 29.Mugikura S., Takahashi S., Higano S., Shirane R., Sakurai Y., Yamada S. Predominant involvement of ipsilateral anterior and posterior circulations in moyamoya disease. Stroke. 2002;33(6):1497–1500. doi: 10.1161/01.str.0000016828.62708.21. [DOI] [PubMed] [Google Scholar]
- 30.Mugikura S., Higano S., Fujimura M., Shimizu H., Takahashi S. Unilateral moyamoya syndrome involving the ipsilateral anterior and posterior circulation associated with paroxysmal nocturnal hemoglobinuria. Jpn. J. Radiol. 2010;28(3):243–246. doi: 10.1007/s11604-009-0412-6. [DOI] [PubMed] [Google Scholar]
- 31.Hishikawa T., Tokunaga K., Sugiu K., Date I. Assessment of the difference in posterior circulation involvement between pediatric and adult patients with moyamoya disease. J. Neurosurg. 2013;119(4):961–965. doi: 10.3171/2013.6.JNS122099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
With the permission of the corresponding authors and their affiliated institutions, all data utilized for the analysis and the pertinent documentation can be obtained following ethical clearance upon request for justifiable intentions of replicating techniques and findings.