Abstract
Harmful algal blooms (HABs) and their consequences cause multiple devastating effects in various freshwater, brackish and marine ecosystems. However, HAB species at moderate population densities have positive ecological roles as primary producers of organic matter and food for zooplankton and fish. They also enhance benthic-pelagic coupling and participate in the biogeochemical cycles. The consequences of HABs are transported across the conventional environmental boundaries by numerous cascade effects in the food webs and beyond. Meanwhile, forecasts of bloom events are still limited, largely because of scarcity of reliable information on ecological niches of the bloom-forming algae. To fill up this knowledge gap, this study focused on dinoflagellates, a diverse group of mostly photosynthesizing protists (unicellular eukaryotes) capable of mixotrophy, since they play a key role in primary production and formation of blooms in marine and brackish waters worldwide. In this study, ecological niches of 17 abundant bloom-forming dinoflagellate species from coastal regions of the southern Baltic Sea were identified for the first time. It was hypothesized that wider ecological niches ensure more frequent dinoflagellate blooms compared to the species with narrower niches. This hypothesis was verified using the long-term (44 years) database on phytoplankton abundance and physical-chemical characteristics of the environment. It were analyzed 4534 datasets collected from 1972 to 2016. Fourteen abiotic parameters (water temperature, salinity, Secchi depth, pH, Chl a, and concentration of basic nutrients) were considered as ecological niche dimensions. The Principal Component Analysis presented the dissolved inorganic nitrogen, total nitrogen, Chl a, and temperature as principal niche dimensions of dinoflagellates. The algal bloom criteria were refined. It was for the first time proved statistically that HAB frequency of dinoflagellate species robustly correlated with the width of their ecological niches.
Keywords: Baltic sea, Brackish waters, Dinoflagellates, Ecological niche, Harmful algal blooms, Nutrients
Graphical abstract
Highlights
-
•
Ecological niches of 17 red-tide dinoflagellates were determined for the first time.
-
•
14 abiotic parameters in 4534 datasets of the 44 years-long database were analyzed.
-
•
PCA presented DIN, total N, Chl a, T as dinoflagellate principal niche dimensions.
-
•
It was proved that dinoflagellate bloom frequency depends on ecological niche width.
1. Introduction
Intricate biotic events like harmful algal blooms (HABs) affect many freshwater, brackish and marine ecosystems of the globe. Current knowledge postulates that the excreted secondary metabolites of HAB species can be toxic or potentially toxic and often lead to poisoning of aquatic organisms, causing significant harm to flora and fauna, natural ecosystems, aquaculture, fisheries, recreational business, tourism, and human health [[1], [2], [3], [4], [5]]. HABs can also contribute substantially to interconnection of chemosphere, hydrosphere, and atmosphere because HAB consequences are transported across the conventional borders of these realms by numerous destructive cascade effects in the food webs and beyond.
In general, HAB effects include but are not limited to production of toxins that are transmitted along the food chains, accumulate in filter-feeders, and are consumed further by predators and humans; accumulation of high algal biomass, which causes light attenuation leading to oxygen depletion in water column and oxygen starvation or even death of aquatic organisms in the pelagic zone and at the bottom; production of aerosol toxins that penetrate in the air and adversely affect the human respiratory system, and many others [6]. Moreover, recent studies show that HABs can contribute directly to mortality of wildlife, including not only water birds and marine mammals but also the terrestrial species, such as bats, coyotes, squirrels and others that are endangered through consumption of aquatic prey and sometimes algae or toxin-laden waters [7].
However, HABs can also have some positive ecological effects. In particular, HAB species of planktonic bloom-forming dinoflagellates are effective primary producers and food for zooplankton and fish [8,9]. They intensify benthic-pelagic coupling due to formation of cysts that accumulate at the bottom, often remaining there for many decades but re-inoculating the water column when favorable conditions are restored [[10], [11], [12], [13]]. On the global time scale, the shells of dinoflagellates, after their massive decay following bloom termination, contribute to the formation of fossil fuel [14]. Additionally, they can be used in extraction of oil products from algae conversion [15]. Besides, high primary productivity of bloom-forming algae that release large amount of oxygen into the environment during photosynthesis and cause a substantial regulatory effect on the global climate change by participating in the biogeochemical cycles that directly depend on the population density and composition of pelagic communities [8,11]. Thus, due to manifold functions, the bloom-forming algae can be considered as principal habitat-shaping actors that bind biotic processes in the total environment, including water column, sediments and bottom biotopes of marine, freshwater and brackish ecosystems, as well as terrestrial habitats and the atmosphere. Meanwhile, identification and measurement of these vital interconnections usually face certain difficulties because of high physical-chemical variability of natural aquatic ecosystems, particularly in marine coastal areas [13,16].
At the same time, HABs are especially detrimental in coastal marine ecosystems, gulfs, estuaries, and brackish semi-enclosed seas like the Baltic Sea, with densely populated coastal areas [6,11,17,18]. These phenomena can be large-scale and prolonged [19], while the criteria for bloom intensity differ for various algae and for water bodies of different types and trophic levels [1,9,13,[20], [21], [22]]. Consequently, specification of bloom criteria, and evaluation of the role of HABs and their dependence on the basic environmental characteristics can only be resolved when using long-term data on the dynamics of the bloom-forming species and their ecological preferences.
Moreover, the ecology of HAB species cannot be understood fully without comprehensive knowledge of their individual niches in the environment. Various ecological tools and approaches can be used to explain and predict species distributions along environmental gradients [[23], [24], [25], [26]]. However, it is difficult to determine which traits are most important to define an organism's ecological niche [27]. Theory supports the concept that stable coexistence of species inevitably involves important ecological distinctions expressed in differentiation of their niches [28]. Like in the earlier studies of dinoflagellates from the genus Prorocentrum [21] and of the bloom-forming cyanobacteria [22], here Grinnell's definition of an ecological niche as the habitat requirements of a certain species for survival and reproduction was accepted [29]. Additionally, the concept of Hutchinson [30], who expanded the latter definition, was considered. According to Moore [31], the space, which a species occupies within Hutchinson's hypervolume is termed the fundamental niche and is defined by the species' basic resource requirements. The fundamental niche further shrinks to the level of realized niche, which can be determined using field data [[31], [32], [33]].
This study is focused on planktonic bloom-forming dinoflagellates, a diverse group of mostly photosynthesizing protists (unicellular eukaryotes) that are capable of mixotrophy, which is a combination of phototrophy and phagotrophy (food capture, phagocytosis) [5]. These organisms play key roles in microplankton biodiversity and primary productivity [14,34], biological carbon pump and nutrients transfer [35]. They form harmful red tides in marine and brackish-water coastal regions worldwide [9,[36], [37], [38]], including the Arctic seas [39,40]. By 2021, it was shown that out of 188 known species of marine microorganisms that are toxic to humans and animals, dinoflagellates constituted more than a half, 103 species [4; http://ipt.iobis.org/hab].
This research aimed at determining ecological niches of common and abundant bloom-forming dinoflagellate species in the brackish coastal waters of the southern Baltic Sea and assessing their role in bloom formation. The research objectives were to (1) distinguish the most common dinoflagellate species, (2) rate their major ecological niche dimensions, (3) elaborate a comprehensive criterion for bloom assessment, and (4) correlate HABs frequency with the ecological niche sizes of the selected species. It was hypothesized that wider ecological niches ensure more frequent dinoflagellate blooms compared to the species with narrower niches. This hypothesis was verified using the long-term (44 years) database on the abundance of 17 dinoflagellate species and physical-chemical characteristics of the environment. Fourteen abiotic parameters were considered as niche dimensions, including water temperature and salinity above pycnocline, Secchi depth, pH, chlorophyll a (Chl a), and concentration of basic nutrients. The dinoflagellate bloom criteria were refined and the study proved statistically that bloom frequency of dinoflagellate species correlated positively with the width of their ecological niches.
2. Material and methods
2.1. Study area and the database description
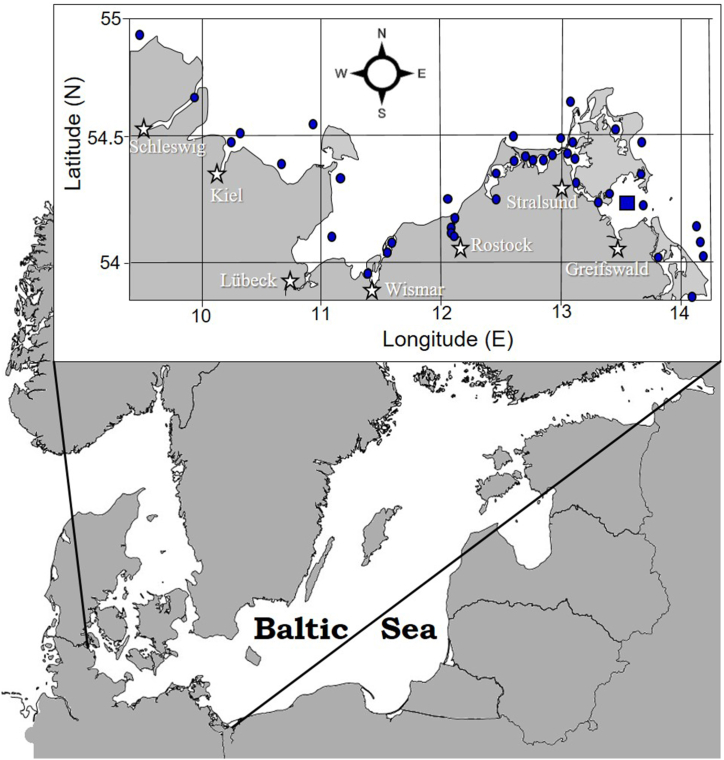
For evaluation of ecological niches of the most common bloom-forming dinoflagellates, their assemblages in the southern Baltic Sea coastal waters were investigated. This geographic area is of utmost international importance since the semi-closed brackish-water Baltic Sea is surrounded by nine bordering countries and their nearshore lands are densely populated, forming one of the most economically significant regions of the world [[41], [42], [43]]. The long-term database available at the authors’ disposal and described earlier by Sagert et al. [44] was used, which was further completed by incorporating the more recent own data [13,21]. The amended database consists of altogether 7934 datasets containing information on phytoplankton composition, abundance and biomass as well as the biotic and abiotic parameters sampled/measured at 64 stations located in the open waters, large lagoons and small riverine lagoons along the ca. 2200 km long German Baltic Sea coastline (Fig. 1). The analyzed data cover an overall sampling period of 44 years, from 1972 until 2016.
Fig. 1.
Schematic map of the study region in the southern Baltic Sea. Dots indicate the main sampling sites represented in the database (described in Refs. [13,21]). The filled square shows the location of the monitoring sampling station GB19.
Phytoplankton species identification was performed as recommended by Edler [45] and Edler et al. [46]. Changes in nomenclature since the publication of the above-mentioned main Baltic phytoplankton determination keys were verified and updated using the databases GBIF (https://www.gbif.org/) and AlgaeBase (https://www.algaebase.org/).
Since the database was compiled using various sources (monitoring programs, short- and mid-term field experiments, etc.), initially its content was not uniform. Therefore, datasets lacking any important parameters such as nutrient concentrations were excluded prior to the analysis. The removal of all sampling points/dates/datasets with incomplete information, and restriction to the samples taken between March and December (in order to exclude the periods of ice cover in January and February) resulted in 4534 datasets that were analyzed in this study.
2.2. Bloom criteria
The long-term phytoplankton database was used for selecting the most common and abundant dinoflagellate species that formed blooms in the southern Baltic coastal waters during the past four decades. To achieve the highest precision of the results and avoid possible inaccuracies due to conversion, in this study the dinoflagellate abundances were expressed and analyzed using the originally measured values of algal biovolume (BV).
For the analysis of ecological niche limits of the most abundant bloom-forming dinoflagellate species and their optimum environmental conditions in the southern Baltic coastal waters, the criterion using the statistical approach was applied. This approach followed the principles developed in the earlier study on cyanobacterial HABs [22], allowing for setting the biovolume class limits and frequency of occurrence of the selected dinoflagellate species. Specifically, dinoflagellates in the database were recorded in 2179 samples; in 59 of those samples, the overall dinoflagellate biovolume exceeded 3 mm³ L−1. Based on the previous results [22], a bloom event was defined here as a case when a species exceeded the 95 percentile of the mean dinoflagellate biovolume measured. Consequently, using the abovementioned proportions and a bloom threshold level of BV ≥ 3.0 mm3 L−1, the data were sorted into six categories according to the species' occurrence and biovolume, in order to provide a BV-class related basis for visualization of the results of multivariate statistics as well as niche dimensions. Those categories were ‘rare’ (BV < 0.044 mm3 L−1), ‘present’ (0.044 – < 0.12 mm3 L−1), ‘abundant’ (0.12 – < 0.4 mm3 L−1), ‘very abundant’ (0.4 – < 1.0 mm3 L−1), ‘plentiful’ (1.0 – < 3.0 mm3 L−1), and ‘blooming’ (≥3.0 mm3 L−1) (Supplementary Table S1). Only the species that were represented at biovolumes ≥0.4 mm3 L−1 (BV category ‘very abundant’ and higher categories, including ‘blooming’) in a certain number of datasets (≥50), which is sufficient for the statistical evaluation, were chosen for the analysis (Supplementary Table S1).
2.3. Ecological niche evaluation and other calculations
The major physical-chemical parameters, analyzed as ecological niche dimensions, were salinity1 and temperature above pycnocline, Secchi depth, pH, chlorophyll a concentration (Chl a), and concentrations of basic nutrients: NO2−, NO3−, NH4+, PO43−, SiO44−, dissolved inorganic nitrogen (DIN), total nitrogen (TN), and total phosphorus (TP). In addition, TN/TP ratio was taken as a parameter according to Tilman [47]. Only the data on abiotic parameters registered simultaneously with the presence of each dinoflagellate species in the dataset were used for niche determination.
The Gaussian logistic regression analysis [48] was carried out for detecting the optimum environmental conditions and the ecological niches of the selected most abundant bloom-forming dinoflagellate species. For this purpose, the probability of occurrence (as the species’ presence/absence at different biovolume-class densities) was calculated by means of a quadratic linear predictor adapted by minimizing the residual deviance (see chapter 2.8, pp. 26–29 in Ref. [48]).
Relative frequency of the species’ occurrence under the conditions when all parameters fell within the niche range was calculated as the ratio of the total number of samples with niche conditions fulfilled to the number of samples, in which the niche conditions were fulfilled and the species was present at the respective biovolume class.
The ecological niche width was defined as the parameter’ intensity range covering the 25–75 % quartiles of biovolume registered for each species. For the individual parameters as niche dimensions, a linear regression analysis of the correlations between bloom events, niche width, and frequency of occurrence of dinoflagellate species within certain biovolume ranges was performed using the RGP-function in the Excel Program. For this analysis, the data on species' occurrences were sorted (a) by the biovolume classes and (b) by the cumulated biovolume (i.e., adding all occurrence events with the species’ biovolume above the lower limit of the respective class).
For non-linear relations, unimodal models using a quadratic linear predictor (as described in the previous section) were analyzed following Leyer and Wesche [48].
In addition, the Principal Component Analysis (PCA) and the non-metric multi-dimensional scaling (nMDS) using a Bray–Curtis dissimilarity matrix were carried out. The similarity/dissimilarity between groups of samples was tested by one-way analysis of similarities (ANOSIM) of the normalized data. The similarity percentage analysis (SIMPER) was used to examine the contribution of each set of samples to the average dissimilarity between the groups of samples. For all statistical tests, analyses and visualization of the results the program PRIMER V6 (Primer-E Ltd, Plymouth, UK) was used.
3. Results
3.1. Bloom-forming dinoflagellates and their ecological niches
The alphabetic list of the 17 selected most common bloom-forming dinoflagellate species in the southern Baltic coastal waters includes Amphidinium crassum Lohmann, 1908; Ceratium furca (Ehrenberg) Claparède & Lachmann, 1859; Ceratium fusus (Ehrenberg) Dujardin, 1841; Ceratium lineatum (Ehrenberg) Cleve, 1899; Ceratium tripos (O.F.Müller) Nitzsch, 1817; Dinophysis acuminata Claparède & Lachmann, 1859; Dinophysis norvegica Claparède & Lachmann, 1859; Gyrodinium spirale (Bergh) Kofoid & Swezy, 1921; Kryptoperidinium triquetrum (Ehrenberg) U.Tillmann, M.Gottschling, M.Elbrächter, W.-H.Kusber & M.Hoppenrath, 2019; Prorocentrum micans Ehrenberg, 1834; Prorocentrum cordatum (Ostenfeld) J.D.Dodge, 1975; Proterythropsis vigilans Marshall, 1925; Protoperidinium bipes (Paulsen, 1904) Balech, 1974; Protoperidinium brevipes (Paulsen, 1908) Balech, 1974; Protoperidinium conicum (Gran) Balech, 1974; Protoperidinium pellucidum Bergh, 1881; and Protoperidinium steinii (Jørgensen, 1899) Balech, 1974.
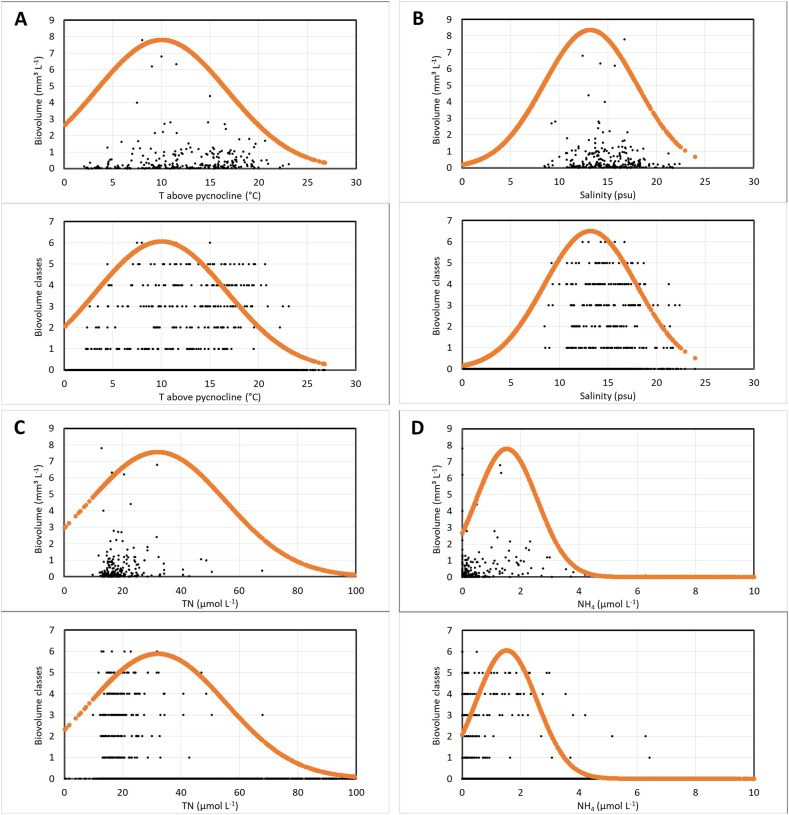
Using the Gaussian logistic regression analysis, the individual ecological niche limits of these most common dinoflagellate species were determined based on classification of the frequency of the species' occurrence and biovolume within the gradually changing environmental parameters (Supplementary Fig. S1). Conformity of the original field data on the species’ biovolume and biovolume-class distribution in the gradients of some principal environmental parameters (temperature and salinity above pycnocline, total N, and NH4+) to the Gaussian distribution model is demonstrated by the data on Ceratium tripos (Fig. 2).
Fig. 2.
Match-up of the in-situ data on the species' biovolume (upper panels of the A, B, C and D) and biovolume-class distribution (lower panels) of the dinoflagellate Ceratium tripos to the Gaussian distribution model (curve) in the gradients of environmental parameters (A – T water above pycnocline, B – salinity above pycnocline, C – total N, and D – NH4+).
The individual ecological niches of 16 (out of 17) most common dinoflagellate species and their optimum values of environmental parameters in the study region are presented in Table 1. Data for Kryptoperidinium triquetrum (syn. Heterocapsa triquetra) were not included in the table due to ambiguity of the results on temperature and PO43− niches of this species, which requires additional investigations. Specifically, with respect to temperature and ortho-phosphate concentration, K. triquetrum exhibited a split niche with two distinct optima for each parameter in different seasons (not shown; a topic of a separate study).
Table 1.
Preferable environmental conditions (Optimum) and niche limits (Niche) of the individual parameters of dominant bloom-forming dinoflagellate species in the Baltic coastal waters, calculated using the Gaussian-model approach. T – temperature above pycnocline, °C; Sal – salinity above pycnocline; TP and TN – total phosphorus and total nitrogen, μmol L−1; ntot – total number of the species' occurrences in the database; n – number of the species’ occurrences within the realized niche limits of the measured parameter. Optimum values of the parameters are highlighted in bold.
| Speciesa | Features | T (°C) | Sal | TP | TN | TN/TPb |
|---|---|---|---|---|---|---|
| Amphidinium crassum ntot = 115 | Optimum | 15.06 | 8.0 | 8.99 | 146.90 | 16.34 |
| Niche | 10.82–19.30 | 0.93–15.07 | 2.63–15.36 | 91.12–202.69 | ||
| n | 62 | 88 | 27 | 12 | ||
| Ceratium furca ntot = 36 | Optimum | 14.74 | 16.14 | 1.08 | 21.13 | 19.56 |
| Niche | 9.34–20.14 | 13.42–18.87 | 0.65–1.51 | 0–47.77 | ||
| n | 27 | 31 | 19 | 36 | ||
| Ceratium fusus ntot = 253 | Optimum | 12.76 | 16.41 | 1.09 | 29.19 | 26.78 |
| Niche | 9.22–16.30 | 10.0–22.82 | 0–2.24 | 16.92–41.47 | ||
| n | 110 | 239 | 246 | 122 | ||
| Ceratium lineatum ntot = 108 | Optimum | 9.45 | 25.45 | 1.30 | 19.22 | 14.78 |
| Niche | 5.07–13.83 | 18.44–32.46 | 0.38–2.21 | 0.87–37.57 | ||
| n | 73 | 29 | 88 | 86 | ||
| Ceratium tripos ntot = 282 | Optimum | 10.01 | 13.19 | 1.63 | 32.04 | 19.66 |
| Niche | 3.26–16.77 | 8.41–17.97 | 0.28–2.97 | 8.69–55.38 | ||
| n | 206 | 231 | 205 | 216 | ||
| Dinophysis acuminata ntot = 197 | Optimum | 12.17 | 17.08 | 2.36 | 31.38 | 13.30 |
| Niche | 3.97–20.37 | 9.92–24.24 | 0–4.87 | 12.07–50.68 | ||
| n | 169 | 185 | 197 | 159 | ||
| Dinophysis norvegica ntot = 232 | Optimum | 8.33 | 19.69 | 1.16 | 30.65 | 26.42 |
| Niche | 1.08–15.57 | 14.92–24.45 | 0.53–1.80 | 12.37–48.92 | ||
| n | 174 | 139 | 108 | 190 | ||
| Gyrodinium spirale ntot = 80 | Optimum | 9.30 | 16.90 | 0.52 | 23.30 | 44.81 |
| Niche | 5.07–13.53 | 13.26–20.53 | 0–1.45 | 11.53–35.08 | ||
| n | 48 | 50 | 75 | 61 | ||
| Prorocentrum cordatum ntot = 457 | Optimum | 21.79 | 10.67 | 2.76 | 32.66 | 11.83 |
| Niche | 12.58–31.00 | 5.56–15.78 | 0.04–5.48 | 9.74–55.59 | ||
| n | 278 | 386 | 234 | 222 | ||
| Prorocentrum micans ntot = 242 | Optimum | 18.2 | 12.07 | 0.88 | 31.38 | 35.66 |
| Niche | 12.4–24.0 | 2.70–21.44 | 0–2.32 | 21.95–40.82 | ||
| n | 139 | 222 | 234 | 70 | ||
| Proterythropsis vigilans ntot = 84 | Optimum | 11.50 | 15.91 | 0.62 | 30.67 | 49.47 |
| Niche | 3.81–19.18 | 11.16–20.67 | 0–1.26 | 14.38–46.96 | ||
| n | 70 | 73 | 80 | 72 | ||
| Protoperidinium bipes ntot = 105 | Optimum | 11.03 | 18.04 | 0.86 | 19.19 | 22.31 |
| Niche | 4.50–17.57 | 13.40–22.68 | 0.05–1.67 | 6.53–31.85 | ||
| n | 81 | 75 | 85 | 76 | ||
| Protoperidinium brevipes ntot = 86 | Optimum | 11.04 | 18.82 | 0.76 | 31.63 | 41.62 |
| Niche | 6.48–15.61 | 11.77–25.87 | 0.07–1.46 | 24.55–38.70 | ||
| n | 57 | 78 | 73 | 16 | ||
| Protoperidinium conicum ntot = 57 | Optimum | 12.40 | 23.38 | 1.29 | 24.34 | 18.87 |
| Niche | 7.77–17.04 | 19.33–27.44 | 0.82–1.75 | 11.73–36.95 | ||
| n | 35 | 17 | 16 | 45 | ||
| Protoperidinium pellucidum ntot = 165 | Optimum | 11.37 | 17.86 | 0.56 | 18.52 | 33.07 |
| Niche | 5.86–16.88 | 11.57–24.14 | 0.20–0.93 | 14.48–22.55 | ||
| n | 165 | 152 | 97 | 86 | ||
| Protoperidinium steinii ntot = 107 | Optimum | 11.83 | 15.7 | 1.10 | 19.5 | 17.73 |
| Niche | 7.28–16.38 | 12.27–19.13 | 0.49–1.71 | 15.31–23.0 | ||
| n | 84 | 84 | 63 | 53 |
Data for Kryptoperidinium triquetrum were not included in the table due to ambiguity of the results on temperature and PO43− niches that require additional analysis, which will be considered in a separate study.
The TN/TP ratio was calculated as the relation of TN optimum to TP optimum of each species.
For the majority of studied species, the n-niche values (i.e., the numbers of the species' occurrences within the individual niche limits of each measured parameter) and ntot (total number of the species' occurrences in the database) were high. The n-niche values generally exceeded 50 and often were above 200, reaching the maximum of n = 386 (and ntot = 457) for Prorocentrum cordatum (Table 1). These large numbers of the species’ occurrences in the database ensure the robustness of the calculated niche ranges.
The results demonstrated that the variation of data was significant, and the calculated optimum parameters for different species was high (Table 1). The optimum water temperature above pycnocline varied for the studied species between 8.33 °C (Dinophysis norvegica) and 21.79 °C (Prorocentrum cordatum). Optimal water salinity varied within the range 8.0–25.45, total nitrogen 18.52–146.9 μmol L−1, and total phosphorus 0.52–8.99 μmol L−1 (Table 1). The highest optimum values of the three latter parameters (salinity, TN and TP) were the characteristics of Amphidinium crassum. The calculated optimum TN/TP ratio varied from 11.83 (P. cordatum) to 49.47 (Proterythropsis vigilans).
Niche width was calculated as the difference between the highest and the lowest value of the parameter's niche limits for each species. The temperature-niche width was the largest for P. cordatum, salinity-niche width – for Prorocentrum micans; TN and TP niches were the widest for A. crassum.
3.2. Results of the Principal Component Analysis and Multidimensional Scaling
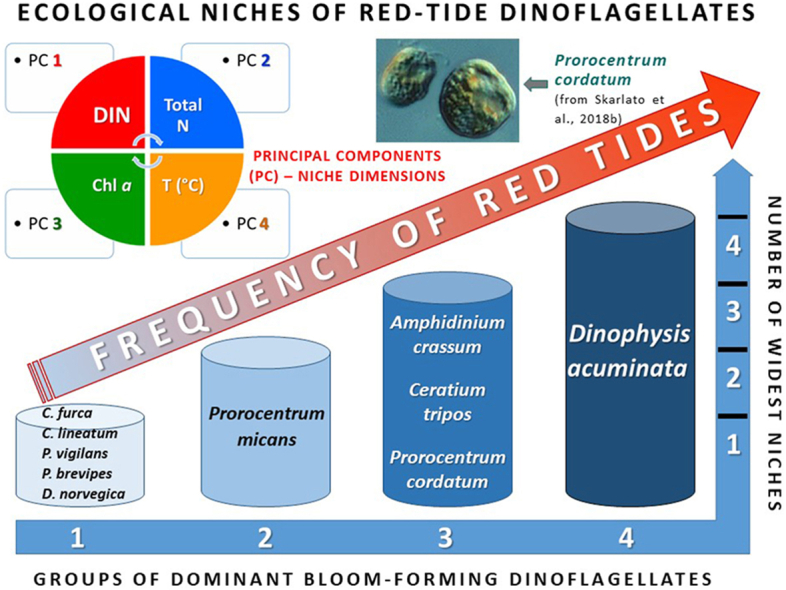
The Principal Component Analysis revealed major environmental variables that ensure ecological niches and back up successful proliferation of the dominant dinoflagellates in the Baltic coastal waters. A cumulative analysis of the combination of major bloom-forming dinoflagellate species demonstrated that Eigenvalues of the four (out of five) principal components (PCs) equaled 5.12, 1.77, 1.57 and 0.868, explaining 42.7, 14.7, 13.1 and 7.2 % of the total variability, respectively (Supplementary Table S2A). On top of the list of PCs for the whole set of studied species were total N (Eigenvector 0.413; PC1), water temperature (Eigenvector 0.515; PC2), PO43− (Eigenvector 0.455; PC3), and NO3− (Eigenvector 0.557; PC4). Altogether, these factors explained 77.7 % of the overall data variability (Supplementary Table S2A).
The PCA results for individual species are presented in the Supplementary Table S2B. In essence, DIN was the main factor (PC1) for 10 out of 14 analyzed species (71.4 %), and total N – for the other 4 species (28.6 %). These two factors together explained up to 58.7 % of the variability (Table 2). In certain species, Chl a concentration (PC2) explained up to 25.2 % of the variability, and water temperature acted simultaneously as PC3, PC4 and PC5, being responsible for up to 15.3 % of the variability for more than 71 % of all dinoflagellate species studied (Table 2).
Table 2.
The principal components and environmental variables that ensure proliferation of the dominant dinoflagellate species (see Supplementary Table S2 for more details).
| Principal component (PC) | % Variation (min–max) | Environmental variables | Number of species (%) | Species |
|---|---|---|---|---|
| PC 1 | 28.2–58.7 | DIN | 71.4 | Amphidinium crassum, Ceratium fusus, Ceratium lineatum, Ceratium tripos, Dinophysis norvegica, Prorocentrum micans, Proterythropsis vigilans, Protoperidinium bipes, Protoperidinium brevipes, Protoperidinium steinii |
| Total N | 28.6 | Dinophysis acuminata, Kryptoperidinium triquetrum, Prorocentrum cordatum, Protoperidinium pellucidum | ||
| PC 2 | 14.9–25.2 | Chl a | 21.4 | C. lineatum, P. bipes, P. steinii |
| PC 3 | 9.9–15.3 | T water | 21.4 | A. crassum, C. lineatum, P. pellucidum |
| PC 4 | 6.3–12.4 | T water | 42.9 | C. tripos, D. acuminata, P. cordatum, P. vigilans, P. bipes, P. pellucidum |
| PC 5 | 3.2–8.6 | T water | 28.6 | D. norvegica, P. cordatum, P. vigilans, P. brevipes |
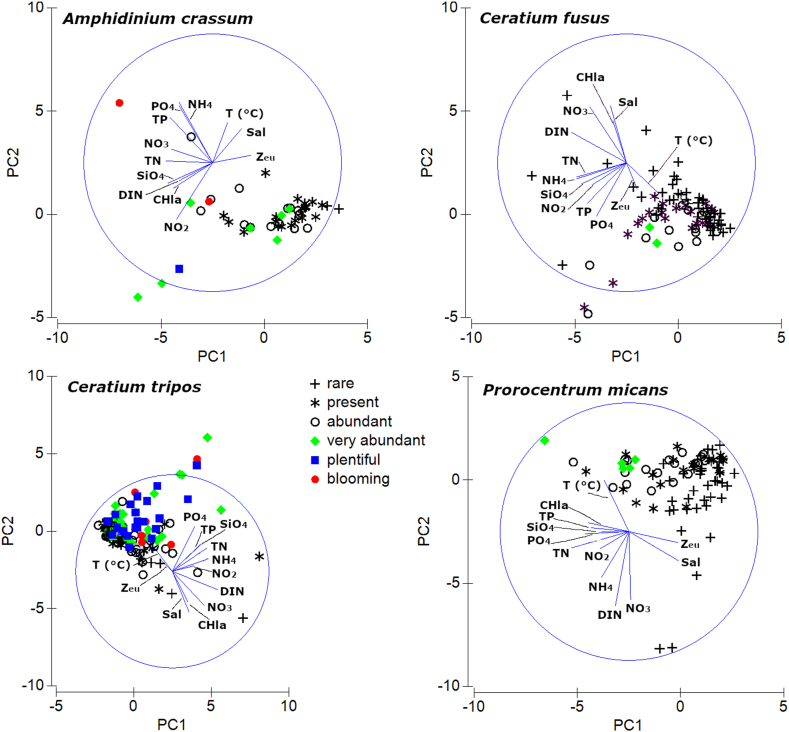
Fig. 3 illustrates the results of the PCA analysis of ecological niche dimensions of the selected dinoflagellate species: Amphidinium crassum, Ceratium fusus, C. tripos and Prorocentrum micans.
Fig. 3.
The PCA results showing contribution of main environmental variables to ecological niche dimensions of the bloom-forming dinoflagellates Amphidinium crassum, Ceratium fusus, C. tripos and Prorocentrum micans in the Baltic coastal waters: T – temperature above pycnocline (°C), Sal – salinity above pycnocline, Zeu – Secchi depth, Chla – chlorophyll a, TN – total nitrogen, DIN – dissolved inorganic nitrogen, TP – total phosphorus, pH, NO2−, NO3−, NH4+, PO43−, and SiO44− (nutrient concentrations are measured in μmol L−1).
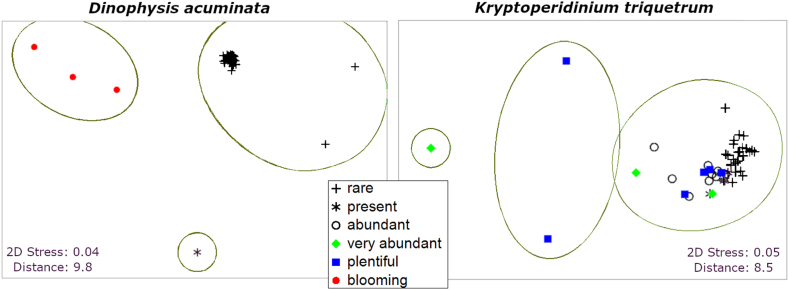
The non-metric Multidimensional Scaling (MDS) revealed the data clustering illustrated here by Dinophysis acuminata and Kryptoperidinium triquetrum (Fig. 4), as well as for the other dominant dinoflagellate species mentioned above (Supplementary Fig. S2).
Fig. 4.
The MDS plots showing the clustering of obtained results for the dinoflagellates Dinophysis acuminata and Kryptoperidinium triquetrum.
3.3. Correlations between principal niche dimensions and occurrence of dinoflagellates
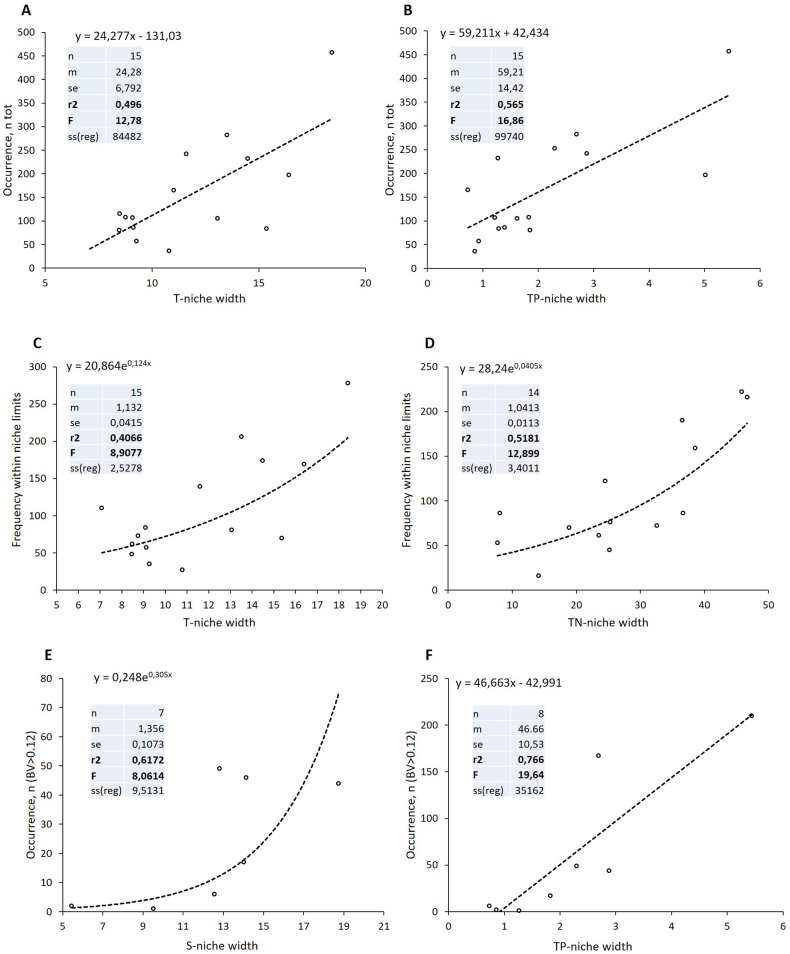
The regression analysis demonstrated strong statistically significant (p < 0.05) correlations between the overall frequency of occurrence of dinoflagellates and the sizes of their temperature niche (Fig. 5A), total P niche (Fig. 5B), and salinity niche (Supplementary Fig. S3A).
Fig. 5.
Correlations between occurrence of the bloom-forming dinoflagellates at different concentrations and the sizes of their ecological niches in the Baltic Sea. A, B: Dependence of the overall frequency of occurrence (Occurrence, ntot) of dinoflagellates on their temperature-niche width, T (A), and total phosphorus-niche width, TP (B). Calculated using all cases in the entire database when the studied species were present in the samples. C, D: Dependence of the frequency of occurrence of the dinoflagellate species within their individual niche limits and the width of the respective niches, T (C), and TN (D). E, F: Correlation of the frequency of occurrence of the dinoflagellates at high biovolumes (BV > 0.12 mm³ L−1), including blooms, and the width of their T niche (E) and TP niche (F). The inserted tables show the statistics (p < 0.05): n – number of occurrences of the dinoflagellates with the certain niche width of the measured parameter; m – slope; se – standard error; r2 – coefficient of determination (R2); F – the overall F-test; ss(reg) – the square sum of regression.
The trends shown in Fig. 5A and B were calculated using all cases available in the entire database when the studied species were present in the samples and the relevant parameters were measured (for the number of data see Table 1). The robustness of these results was supported by the sufficiently high values of the coefficient of determination (R2 0.31–0.57, p < 0.05). The significance of the calculated coefficients R2 was tested by means of the overall F-test. Only the correlations with significant overall F-test values were analyzed and described here.
On a narrower scale, i.e. using only the data on species' occurrences within the calculated niche limits, it was shown that the width of temperature niche (Fig. 5C), total N niche (Fig. 5D), and total P niche (Supplementary Fig. S3B) influenced significantly the frequencies of species' occurrences (R2 0.41, 0.52, and 0.46, respectively; p < 0.05). Correlations of other niche parameters with the dinoflagellates’ occurrences within their niche ranges were statistically insignificant (not shown).
In the case of a smaller dataset, including the algal biovolume classes ‘blooming’, ‘plentiful’, ‘very abundant’ and ‘abundant’ (in total, at BV > 0.12 mm³ L−1), the occurrence of dinoflagellates correlated positively with the width of their salinity niches (Fig. 5E) and total P niches (Fig. 5F). Importantly, these relations were highly significant and strong (R2 0.62 and 0.77, respectively; p < 0.05).
In addition, the statistically significant negative exponential relation between the TP-niche width and the TN/TP ratio was revealed based on the cumulative data for all studied species in the entire database (Supplementary Fig. S3C).
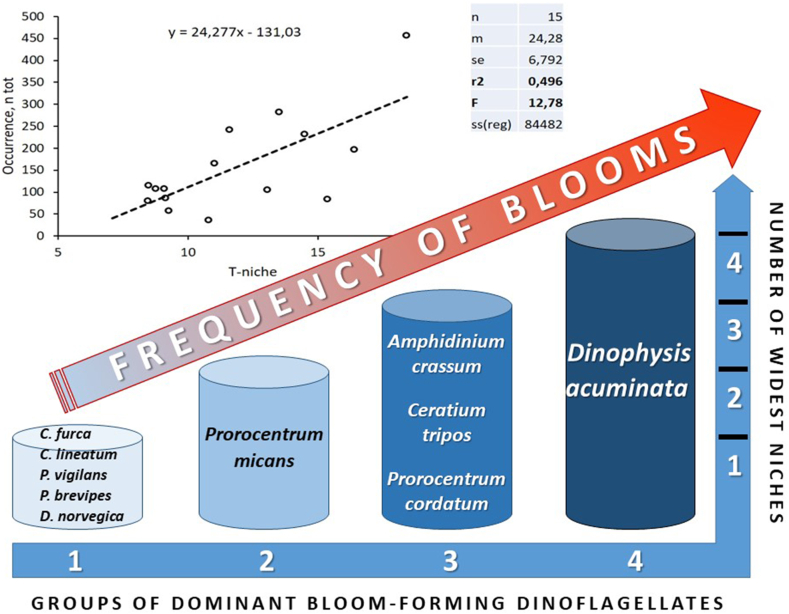
3.4. Conceptual model
Based on the obtained results, a conceptual model was suggested, which illustrates that the species’ occurrence and frequency of blooms of the most common dinoflagellates in the Baltic Sea depends on the width of their ecological niches (Fig. 6). For this, the width of a niche was calculated using the data on specific niche limits given in Table 1. Then, all studied species were classified into four groups according to the niche width of four major abiotic parameters (niche dimensions) determined in this study: temperature, salinity, total N, and total P. For each niche dimension, the top five species with the broadest niches were selected and ranked according to the width of these niches. Finally, the resulting list of the dinoflagellate species, ranked according to the number of the widest niches that they occupy in the study region, was compiled (Supplementary Table S3).
Fig. 6.
Conceptual model illustrating how occurrence and frequency of blooms of ten most common dinoflagellate species in the Baltic Sea depends on the width of their ecological niches. The species were grouped according to niche limits of major abiotic parameters determined in this study: temperature, salinity, total N, and total P. T-niche – temperature niche width. Notations as in Fig. 5.
Results showed (Fig. 6, Supplementary Table S3) that species of the first group were characterized by just one widest niche (Ceratium furca – total N, C. lineatum and Protoperidinium brevipes – salinity, Proterythropsis vigilans and Dinophysis norvegica – temperature). Prorocentrum micans belonged to the second group and possessed two widest niches: salinity and total P. The third group comprised the species that had three widest niches: Amphidinium crassum (salinity, total N and total P), Ceratium tripos and Prorocentrum cordatum (temperature, total N and total P, for both species). Dinophysis acuminata represented the fourth group of species with the highest number of widest niches: it was among the top five species according to all four analyzed niche dimensions (Fig. 6).
These results, coupled with the statistically significant correlations between the species’ occurrences at highest concentrations and the width of their individual niches, as presented in Fig. 5, allow concluding that higher number of the widest individual niches secures more frequent dinoflagellate occurrences and blooms. Specifically, the results show that the species of the groups 3 and 4 (Fig. 6) have the strongest potential for most frequent bloom events in the fluctuating Baltic coastal environment due to the widest niche limits for temperature and salinity, as well as for concentrations of total nitrogen and total phosphorus.
4. Discussion
4.1. HABs affect biogeochemical cycles and interconnect global spheres
In this study, algal blooms are considered as essential phenomena that play key roles in binding biotic processes in the total environment, including water column, sediments and bottom biotopes of marine, freshwater and brackish ecosystems, terrestrial habitats and the atmosphere. This approach, according to the authors' knowledge, has never been applied to HAB-studies previously. Only a few of these interconnecting links have been evaluated numerically, either in the field or by modeling [18]. Meanwhile, the diversity and efficient ecological roles of many nano- and microorganisms in seawaters, and particularly in transboundary environments, have long been underestimated [[49], [50], [51]]. The functioning of microplankton communities that harbor bloom events is complex because algal population densities are the consequences of a large array of species-specific cellular, molecular, and physiological traits as drivers, as well as their external triggers [5,11,23,27,52,53]. For example, high concentration of dinoflagellates during red-tide events can be linked to the excystation of algal resting stages [12] and the ecdysis, i.e. cells’ shell shedding, after stress [52,54]. Both processes enhance benthic-pelagic coupling in aquatic ecosystems and ensure future HABs: the encystment provides a reservoir of resting cysts in the sediments while the excystment acts as a seed pool for the next bloom event [55]. In addition, HABs are also the results of stochastic processes reinforced by multiple, mostly nonlinear, biotic interactions including trophic cascades [13,[56], [57], [58], [59]].
Expansion of red tides due to inflow of anthropogenic nitrogen and phosphorus into aquatic ecosystems and intensification of HABs are among the largest environmental problems that cause detrimental ecological and economic consequences worldwide [4,6,8,18,39,60]. Against this background, identifying the ecological niches of common bloom-forming microalgae and resolving their linkage to the frequency of HABs is essential for adequate assessment of biogeochemical cycles and improvement of HAB predictive tools. Currently, a number of methods to control HABs exist [55], and various models are applied to forecast HABs in general and those formed by dinoflagellates in particular [7,16,18,40,[61], [62], [63]]. Presumably in the future, the data on ecological niches of these hazardous protists and their impact on bloom frequency could be incorporated in modeling of basic processes interconnecting hydrosphere and atmosphere, to improve the forecasts of HABs and advance the assessment of ecosystems health.
4.2. Use of long-term data for refining the algal bloom criteria
At a large scale, the linkage of different realms within the biosphere can only be evaluated using long-term data. For this reason, a long-run (44 years) database with the information on phytoplankton composition, abundance and biomass as well as abiotic parameters sampled in the southern coastal waters of the Baltic Sea was analyzed [13,21,44].
The previous meta-analysis of long-term trends in the taxonomic composition, abundance and biomass of phytoplankton in the Baltic coastal waters during 30 years of research allowed registering 15 dinoflagellate blooms with the algal population density of ≥1000 cells mL−1 [13,21]. However, the bloom criteria has still remained a vague issue because a comprehensive definition of which algal concentration constitutes a bloom depends on the resolution scales, trophic status of a waterbody, toxicity of a species, and many other parameters. Previous studies showed that at a broad scale, blooms are generally defined as algal densities between ca. 103 and above 106 cells mL−1 [16,21,64]. Meanwhile, the amplitude of a bloom is influenced by its expansion range, which is difficult to evaluate unequivocally, and little quantitative capability exists to assess these metrics at regular intervals across broad geographic scales [65]. At smaller spatial scales, the criteria for bloom events differ for various algal species depending on their adverse effects in different environments [9,20,21,51]. For example, the hazardous threshold concentration of Dinophysis acuta in the Norwegian waters and off the Swedish west coast is rather low, 100 cells mL−1 [40]. The population density of P. cordatum equal to just 82 cells mL−1 in the Onega and Kandalaksha bays of the White Sea is likewise considered as a harmful bloom for these northern areas [9].
The refinement of the algal bloom criteria using the statistical approach, as described in sections 3, 2.2.1 of this study, allowed for a more comprehensive and flexible assessment of the dinoflagellate HABs. The suggested criteria can be used in different environments because they are less dependent on trophic status and geographic location of a waterbody compared to other classifications of blooms, e.g. those based on cell counts or biomass values of the blooming algae, as discussed earlier elsewhere [9,20].
4.3. Species with wider ecological niches bloom more often compared to those with narrower niches
According to Schoener [66], who analyzed the resource requirements of several species from different taxa, the principal niche axes (dimensions) usually are food, habitat use, and time. In the present study, this list was partially confirmed and expanded. Results of the PCA presented temperature, DIN, total N, and Chl a as dinoflagellates’ principal niche dimensions. It was also shown that the significance of these niche axes is species-specific, as indicated in Table 2. However, despite numerous adverse effects of HABs and their global distribution, the species-specific information on preferable environmental conditions is scarce even for the most common bloom-forming dinoflagellates.
The earlier studies revealed that the species of the genus Prorocentrum dominated the majority of dinoflagellate blooms in the Baltic coastal waters, with the prevalence of the invasive, potentially toxic, mixotrophic P. cordatum, also known by its most common synonym, P. minimum (Pavillard) J. Schiller, 1933 [9,13,21,67]. In the current study, P. cordatum was likewise represented in the highest number of datasets (448) in considerable abundances, as shown in Supplementary Table S1. Broad ecological niches and optimum values of temperature and salinity conditions for P. cordatum and its close congener, P. micans, obtained in this study, largely confirm the respective parameters determined and discussed for these two species earlier [21]. Similar and even broader temperature and salinity tolerance limits of P. cordatum in the Chesapeake Bay were presented recently [16].
Along with P. cordatum and P. micans, the other three species have most wide niche ranges in the Baltic coastal waters: Amphidinium crassum, Ceratium tripos and Dinophysis acuminata (Supplementary Table S3). Contrary to our findings, the recent parsimonious models that analyzed the effects of temperature, salinity and water stratification on A. crassum found neither clear trends nor distinct environmental effects of these parameters on its population in the Baltic Sea [60]. The published data on ecological preferences of C. tripos in the Baltic Sea is even scarcer [68,69], suggesting that the results on the 14 ecological niche dimensions and environmental optima of these bloom-forming species presented here are of particular importance.
Dinophysis acuminata crowns the list of the top-5 species with the largest number of broadest individual ecological niches among the 17 bloom-forming dinoflagellates discussed in this study. D. acuminata is the main subject of a significant number of publications because it is a toxic species, which produces dangerous diarrhetic shellfish toxins (DST) and pectenotoxins (PTX) [70]. Being released in seawater, DST and PTX are transferred through the food web, affecting in particular the early larval stages of mollusks and fish, accumulating in both wild and aquaculture sea fauna and, therefore, posing a threat to human health [40,71]. Among the ten toxin-producing Dinophysis species, D. acuminata is the main agent of lipophilic toxin accumulation in shellfish and thus – the principal public health risk and threat to mussel farms in the European Atlantic region [18].
Overall, D. acuminata can be characterized as an “Arctic-boreal-tropical species” [39]. Its environmental preferences differ geographically: in coastal Dutch waters, D. acuminata bloomed at 9.5–14 °C [72]; in Virginia estuaries, the east coast of the USA, during late winter through early spring, its blooms were registered at 4.5–20.7 °C and at salinities between 10.6 and 21.3 [39]. These temperature characteristics and particularly salinity values are well in line with our data from the Baltic Sea (3.97–20.37 °C; salinity range 9.92–24.24), thus supporting the conclusion about the wide ecological niche of D. acuminata. Other authors show that D. acuminata proliferate only in waters warmer than 8 °C, both in laboratory experiments [73] and in nature [[74], [75], [76], [77]]. The results of the PCA (as shown in Table 2) and niche calculations for D. acuminata coincide completely with those model calculations, indicating that among all environmental factors, water temperature is of highest importance for proliferation of this species [40].
4.4. Pitfalls, assumptions, and limitations: lessons learned
The major pitfalls of this study are several assumptions that, however, are mostly theoretical, while the true limitations are scarce. Moreover, the latter additionally highlight the importance and timeliness of our findings. In particular, based on the available knowledge and theories discussed above, it was assumed that HABs are the ambiguous biological events that not only cause multiple devastating effects in many freshwater, brackish and marine ecosystems, but also can have some positive ecological consequences being involved in biogeochemical cycles and playing a key part in ecosystem maintenance. Due to these numerous roles, HABs can contribute substantially to complex interconnections of the processes within the chemosphere, hydrosphere and atmosphere, since the consequences of HABs are transported across the conventional borders of these realms.
Meanwhile, direct evaluation of these intricate interconnections sometimes remains problematic, thus causing certain limitations of the research. For instance, although carefully measured and adequately calculated, the data for Kryptoperidinium triquetrum provided the ambiguous results on its certain niche dimensions that were not included in this article. In particular, with respect to temperature and ortho-phosphate concentration, K. triquetrum exhibited a split niche with two distinct optima for each parameter in different seasons. The similar phenomenon was described earlier for some other algae, for example, a mixotrophic cryptophyte Rhodomonas salina, which relies on photosynthesis in summer and on bacterivory/osmotrophy – in winter [78,79]. Therefore, the data on K. triquetrum require additional analysis, which will be considered in a separate study in the future.
Moreover, the optimum values of some abiotic parameters, given in Table 1, appear higher than the data published by other authors [60]. Two examples can be the results on the optimum temperature for A. crassum and the optimum salinity for D. acuminata that are much higher than the outcome of the previous model calculations for the Baltic Sea presented by Forsblom et al. [60]. However, like in the latter research, the optimum values obtained in the present study are the model results but not the field data; therefore, they have to be considered and used with caution. The differences between our findings and previously published data, to certain extent, can be attributed also to heterogeneity and high physical-chemical variability of shallow brackish-water coastal ecosystems [13,25,58,80]. Additionally, the considerable nonlinearity of biotic interactions in highly variable plankton communities can back up these inconsistences [40,56,58,81].
Specifically, the previous results demonstrated that the dinoflagellate bloom events were usually bound to higher physical-chemical stability in the study region, i.e. to low variability of water temperature, pH, and concentrations of total nitrogen, nitrite, nitrate and ammonium [13]. Thus, abiotic stability but not just the critical absolute values of the environmental parameters has been proven to act as a bloom trigger [13]. Moreover, a substantial diversity of nutrient substrates in natural coastal ecosystems is another source of uncertainties, which, coupled with high ecological adaptability and a variety of feeding strategies employed by dinoflagellates, most likely hinders the exposure of clear correlations between their abundance and physical-chemical features of the environment [5,13,33,52]. Yet, the topical issues of the role of abiotic variability in coastal ecosystems are discussed in more detail elsewhere [13].
In addition, the PCA results in the current study show that obviously any single parameter cannot be responsible for the entire ecological niche formation, as shown earlier for the cyanobacteria [22]. The latter conclusion abides by the definition of an ecological niche, which involves the multiple habitat requirements of a certain species for survival and reproduction [[29], [30], [31], [32], [33]].
Thus, the abovementioned pitfalls highlight the necessity of careful consideration of HABs ambiguity: their multiple grounds, complex exposures, manifold impacts, and multidirectional consequences in aquatic ecosystems that hamper modeling of algal blooms and prevent effective management of their repercussions. The detailed description of ecological niche dimensions of major bloom-forming dinoflagellates, provided in this study, can pave the road for a more precise assessment of an ecosystem state and its transformation under global environmental changes. Coupled with the future evaluation of the diatom/dinoflagellate ratio [82] and its long-term dynamics in the study region, and aimed at determination of triggers and drivers of the coastal ecosystem shifts, these results could contribute to the forthcoming predictive modeling of HAB dynamics in the Baltic Sea and beyond.
5. Conclusions
In this study, harmful algal blooms are considered as essential phenomena that play key roles in binding vital biotic processes in the total environment. The obtained results expand the knowledge of ecology of bloom-forming dinoflagellates and can contribute to refinement of prognostic modeling of HABs’ frequency, their ecological and economic consequences. The large data array (14 abiotic parameters as niche dimensions, 17 HAB species, 44 years-long database, 4534 datasets analyzed) for the first time allowed getting the statistically significant results on ecological niches of common red-tide dinoflagellates. For the majority of species analyzed in this study, the occurrences in the database were very high, often ≥200 (maximum 457), and this fact guaranteed the robustness of the calculated niche ranges. The suggested approach for defining an algal bloom allowed for a more flexible assessment of HABs in different environments compared to the existing bloom definitions. Finally, this research for the first time draws attention to broad ecological niches of HAB-forming, toxic or potentially toxic unicellular protists that ensure high frequency of their harmful blooms.
CRediT authorship contribution statement
Irena Telesh: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Funding acquisition, Conceptualization. Hendrik Schubert: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Sergei Skarlato: Writing – review & editing, Supervision, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the Russian Science Foundation (project # 22-14-00056; https://rscf.ru/project/22-14-00056/). The English language was edited by the Effective Language Tutoring Services.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e26495.
Ethics.
The study design, data presentation and writing style comply with the Cell Press Editorial Ethics Policies and meet the rigorous technical and ethical standards.
Salinity is reported using the Practical Salinity Scale approved by the Joint Panel of Oceanographic Tables and Standards, according to which salinity is defined as a pure ratio, and has no dimensions or units.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Anderson C.R., Berdalet E., Kudela R.M., Cusak C.K., et al. Scaling up from regional case studies to a global Harmful Algal Bloom observing system. Front. Mar. Sci. 2019;6:250. doi: 10.3389/fmars.2019.00250. [DOI] [Google Scholar]
- 2.Anderson D.M., Fensin E., Gobler C.J., et al. Marine harmful algal blooms (HABs) in the United States: History, current status and future trends. Harmful Algae. 2021;102 doi: 10.1016/j.hal.2021.101975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown A.R., Lilley M., Shutler J., et al. Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Rev. Aquacult. 2020;12(3):1663–1688. doi: 10.1111/raq.12403. [DOI] [Google Scholar]
- 4.Hallegraeff G.M., Anderson D.M., Belin C., et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021;2:117. doi: 10.1038/s43247-021-00178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Telesh I.V., Skarlato S.O. Harmful blooms of the potentially toxic dinoflagellates in the Baltic Sea: ecological, cellular and molecular background. Russ. J. Ecol. 2022;53(6):464–477. doi: 10.1134/S1067413622060157. [DOI] [Google Scholar]
- 6.Karlson B., Andersen P., Arneborg L., et al. Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algae. 2021;102 doi: 10.1016/j.hal.2021.101989. [DOI] [PubMed] [Google Scholar]
- 7.Rattner B.A., Wazniak C.E., Lankton J.S., et al. Review of harmful algal bloom effects on birds with implications for avian wildlife in the Chesapeake Bay region. Harmful Algae. 2022;120 doi: 10.1016/j.hal.2022.102319. [DOI] [PubMed] [Google Scholar]
- 8.Winder M., Sommer U. Phytoplankton response to a changing climate. Hydrobiologia. 2012;698:5–16. doi: 10.1007/s10750-012-1149-2. [DOI] [Google Scholar]
- 9.Khanaychenko A.N., Telesh I.V., Skarlato S.O. Bloom-forming potentially toxic dinoflagellates Prorocentrum cordatum in marine plankton food webs. Protistology. 2019;13(3):95–125. doi: 10.21685/1680-0826-2019-13-3-1. [DOI] [Google Scholar]
- 10.Bravo I., Figueroa R.I. Towards an ecological understanding of dinoflagellate cyst functions. Microorganisms. 2014;2:11–32. doi: 10.3390/microorganisms2010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skarlato S., Filatova N., Knyazev N., Berdieva M., Telesh I. Salinity stress response of the invasive dinoflagellate Prorocentrum minimum. Estuar. Coast Shelf Sci. 2018;211:199–207. doi: 10.1016/j.ecss.2017.07.007. [DOI] [Google Scholar]
- 12.Liu Y., Hu Z., Deng Y., Tang Y.Z. Evidence for production of sexual resting cysts by the toxic dinoflagellate Karenia mikimotoi in clonal cultures and marine sediments. J. Phycol. 2020;56:121–134. doi: 10.1111/jpy.12925. [DOI] [PubMed] [Google Scholar]
- 13.Telesh I., Schubert H., Skarlato S. Abiotic stability promotes dinoflagellate blooms in marine coastal ecosystems. Estuar. Coast Shelf Sci. 2021;251 doi: 10.1016/j.ecss.2021.107239. [DOI] [Google Scholar]
- 14.Okolodkov YuB. Dinoflagellata. Protists: Guide on Zoology. 2011;3:7–94. St. Petersburg: KMK. [Google Scholar]
- 15.Zhou Y., Hu C. Catalytic Thermochemical conversion of algae and Upgrading of algal oil for the production of high-Grade Liquid fuel: a review. Catalysts. 2020;10:145. doi: 10.3390/catal10020145. [DOI] [Google Scholar]
- 16.Horemans D.M.L., Friedrichs M.A.M., St-Laurent P., Hood R.R., Brown C.W. Forecasting Prorocentrum minimum blooms in the Chesapeake Bay using empirical habitat models. Front. Mar. Sci. 2023;10 doi: 10.3389/fmars.2023.1127649. [DOI] [Google Scholar]
- 17.Carstensen J., Conley D.J., Almroth-Rosell E., et al. Factors regulating the coastal nutrient filter in the Baltic Sea. Ambio. 2020;49:1194–1210. doi: 10.1007/s13280-019-01282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandes-Salvador J.A., Davidson K., Sourisseau M., et al. Current status of forecasting toxic harmful algae for the North-east Atlantic shellfish aquaculture Industry. Front. Mar. Sci. 2021;8 doi: 10.3389/fmars.2021.666583. [DOI] [Google Scholar]
- 19.Glibert P.M. Harmful algae at the complex nexus of eutrophication and climate change. Harmful Algae. 2020;91 doi: 10.1016/j.hal.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Carstensen J., Klais R., Cloern J.E. Phytoplankton blooms in estuarine and coastal waters: seasonal patterns and key species. Estuar. Coast Shelf Sci. 2015;162:98–109. doi: 10.1016/j.ecss.2015.05.005. [DOI] [Google Scholar]
- 21.Telesh I.V., Schubert H., Skarlato S.O. Ecological niche partitioning of the invasive dinoflagellate Prorocentrum minimum and its native congeners in the Baltic Sea. Harmful Algae. 2016;59:100–111. doi: 10.1016/j.hal.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Telesh I., Schubert H., Skarlato S. Ecological niches of bloom-forming cyanobacteria in brackish Baltic Sea coastal waters. Estuar. Coast Shelf Sci. 2023;295 doi: 10.1016/j.ecss.2023.108571. [DOI] [Google Scholar]
- 23.Litchman E., de Tezanos Pinto P., Klausmeier C.A., Thomas M.K., Yoshiyama K. Linking traits to species diversity and community structure in phytoplankton. Hydrobiologia. 2010;653:15–28. doi: 10.1007/s10750-010-0341-5. [DOI] [Google Scholar]
- 24.Telesh I.V., Khlebovich V.V. Principal processes within the estuarine salinity gradient: a review. Mar. Pollut. Bull. 2010;61(4–6):149–155. doi: 10.1016/j.marpolbul.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Telesh I., Schubert H., Skarlato S. Life in the salinity gradient: discovering mechanisms behind a new biodiversity pattern. Estuar. Coast Shelf Sci. 2013;135:317–327. doi: 10.1016/j.ecss.2013.10.013. [DOI] [Google Scholar]
- 26.Telesh I.V., Schubert H., Skarlato S.O. Size, seasonality, or salinity: what drives the protistan species maximum in the horohalinicum? Estuar. Coast Shelf Sci. 2015;161:102–111. doi: 10.1016/j.ecss.2015.05.003. [DOI] [Google Scholar]
- 27.Litchman E., Klausmeier C.A., Schofield O.M., Falkowski P.G. The role of functional traits and trade-offs in structuring phytoplankton communities: scaling from cellular to ecosystem level. Ecol. Lett. 2007;10:1170–1181. doi: 10.1111/j.1461-0248.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 28.Chesson P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Systemat. 2000;31:343–366. doi: 10.1146/annurev.ecolsys.31.1.343. [DOI] [Google Scholar]
- 29.Grinnell J. The niche-relationships of the California thrasher. The Auk. 1917;34(4):427–433. doi: 10.2307/4072271. [DOI] [Google Scholar]
- 30.Hutchinson G.E. Homage to Santa Rosalia or Why are there so many kinds of animals? Am. Nat. 1959;93(870):145–159. https://www.jstor.org/stable/2458768 [Google Scholar]
- 31.Moore J.C. In: Encyclopedia of Biodiversity. second ed. Levin S.A., editor. Elsevier; 2013. Diversity, taxonomic versus functional; pp. 648–656. [DOI] [Google Scholar]
- 32.Karasiewicz S., Dolédec S., Lefebvre S. Within outlying mean indexes: refining the OMI analysis for the realized niche decomposition. PeerJ. 2017;5:3364. doi: 10.7717/peerj.3364. https://peerj.com/articles/3364/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schubert H., Telesh I., Nikinmaa M., Skarlato S. In: Biological Oceanography of the Baltic Sea. Snoeijs-Leijonmalm P., Schubert H., Radziejewska T., editors. Springer Science+Business Media; Dordrecht: 2017. Physiological adaptations; pp. 255–278. [DOI] [Google Scholar]
- 34.Hällfors G. Checklist of Baltic Sea phytoplankton species (including some heterotrophic protistan groups) Baltic Sea Environ. Proc. 2004;95:1–208. [Google Scholar]
- 35.Mitra A., Flynn K.J., Burkholder J.M., et al. The role of mixotrophic protists in the biological carbon pump. Biogeosciences. 2014;11:995–1005. 105194/bg-11-995-2014. [Google Scholar]
- 36.Heil C.A., Glibert P.M., Fan C. Prorocentrum minimum (Pavillard) Schiller—a review of a harmful algal bloom species of growing worldwide importance. Harmful Algae. 2005;4:449–470. doi: 10.1016/j.hal.2004.08.003. [DOI] [Google Scholar]
- 37.Orlova T.Y., Konovalova G.V., Stonik I.V., et al. Harmful algal blooms on the eastern coast of Russia. PICES Sci. Rep. 2014;47:41–58. https://www.researchgate.net/publication/242186001 [Google Scholar]
- 38.Li M., Chen Y., Zhang F., et al. A threedimensional mixotrophic model of Karlodinium veneficum blooms for a eutrophic estuary. Harmful Algae. 2022;113 doi: 10.1016/j.hal.2022.102203. [DOI] [PubMed] [Google Scholar]
- 39.Okolodkov YuB. The global distributional patterns of toxic, bloom dinoflagellates recorded from the Eurasian Arctic. Harmful Algae. 2005;4:351–369. doi: 10.1016/j.hal.2004.06.016. [DOI] [Google Scholar]
- 40.Silva E., Counillon F., Brajard J., et al. Forecasting harmful algae blooms: application to Dinophysis acuminata in northern Norway. Harmful Algae. 2023;126 doi: 10.1016/j.hal.2023.102442. [DOI] [PubMed] [Google Scholar]
- 41.Ojaveer H., Jaanus A., MacKenzie, et al. Status of biodiversity in the Baltic Sea. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snoeijs-Leijonmalm P., Schubert H., Radziejewska T. Springer Science+Business Media Dordrecht; 2017. Biological Oceanography of the Baltic Sea; p. 683. [DOI] [Google Scholar]
- 43.Viitasalo M., Bonsdorff E. Global climate change and the Baltic Sea ecosystem: direct and indirect effects on species, communities and ecosystem functioning. Earth Syst. Dynam. 2022;13:711–747. doi: 10.5194/esd-13-711-2022. [DOI] [Google Scholar]
- 44.Sagert S., Rieling T., Eggert A., Schubert H. Development of a phytoplankton indicator system for the ecological assessment of brackish coastal waters (German Baltic Sea coast) Hydrobiologia. 2008;611(1):91–103. doi: 10.1007/s10750-008-9456-3. [DOI] [Google Scholar]
- 45.Recommendations on methods for marine biological studies in the Baltic Sea: phytoplankton and chlorophyll. Edler L., editor. The Baltic Marine Biologists Publ. 1979;5:1–38. [Google Scholar]
- 46.Edler L., Hällfors G., Niemi A. A preliminary check-list of the phytoplankton of the Baltic Sea. Acta Bot. Fennica. 1984;128:1–26. [Google Scholar]
- 47.Tilman D. Resources: a graphical-mechanistic approach to competition and predation. Am. Nat. 1980;116(3):362–393. doi: 10.1086/679066. [DOI] [Google Scholar]
- 48.Leyer I., Wesche K. Springer; Berlin/Heidelberg: 2007. Multivariate Statistik in der Ökologie.https://link.springer.com/book/10.1007/978-3-540-37706-1 [Google Scholar]
- 49.Herlemann D.P.R., Labrenz M., Jürgens K., et al. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011:1571–1579. doi: 10.1038/ismej.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herlemann D.P.R., Woelk J., Labrenz M., Jürgens K. Diversity and abundance of ‘‘Pelagibacterales’’ (SAR11) in the Baltic Sea salinity gradient. Syst. Appl. Microbiol. 2014;37:601–604. doi: 10.1016/j.syapm.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Telesh I.V., Schubert H., Skarlato S.O. Revisiting Remane's concept: evidence for high plankton diversity and a protistan species maximum in the horohalinicum of the Baltic Sea. Mar. Ecol. Prog. Ser. 2011;421:1–11. doi: 10.3354/meps08928. [DOI] [Google Scholar]
- 52.Skarlato S.O., Telesh I.V., Matantseva O.V., et al. Studies of bloom-forming dinoflagellates Prorocentrum minimum in fluctuating environment: contribution to aquatic ecology, cell biology and invasion theory. Protistology. 2018;12(3):113–157. doi: 10.21685/1680-0826-2018-12-3-1. [DOI] [Google Scholar]
- 53.Flynn K.J., Mitra A., Anestis K., et al. Mixotrophic protists and a new paradigm for marine ecology: where does plankton research go now? J. Plankton Res. 2019;41(4):375–391. doi: 10.1093/plankt/fbz026. [DOI] [Google Scholar]
- 54.Berdieva M., Pozdnyakov I., Matantseva O., et al. Actin as a cytoskeletal basis for cell architecture and a protein essential for ecdysis in Prorocentrum minimum (Dinophyceae, Prorocentrales) Phycol. Res. 2018;66(2):127–136. doi: 10.1111/pre.12214. [DOI] [Google Scholar]
- 55.Balaji-Prasath B., Wang Y., Su Y.P., Hamilton D.P., et al. Methods to control harmful algal blooms: a review. Environ. Chem. Lett. 2022;20:3133–3152. doi: 10.1007/s10311-022-01457-2. [DOI] [Google Scholar]
- 56.Medvinsky A.B., Adamovich B.V., Chakraborty A., et al. Chaos far away from the edge of chaos: a recurrence quantification analysis of plankton time series. Ecol. Complex. 2015;23:61–67. doi: 10.1016/j.ecocom.2015.07.001. [DOI] [Google Scholar]
- 57.Flynn K.J., Mitra A., Glibert P.M., Burkholder J.M. In: Global Ecology and Oceanography of Harmful Algal Blooms. Glibert P.M., Berdalet E., Burford M.A., Pitcher G.C., Zhou M., editors. Springer-Verlag; Cham: 2018. Mixotrophy in HABs: by whom, on whom, when, why and what next; pp. 113–132. [Google Scholar]
- 58.Telesh I.V., Schubert H., Joehnk K.D., et al. Chaos theory discloses triggers and drivers of plankton dynamics in stable environment. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-56851-8. https://www.nature.com/articles/s41598-019-56851-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rengefors K. In: Dinoflagellates. Rao D.V.S., editor. Nova Science Publishers, Inc.; 2020. Species assemblies and seasonal succession of dinoflagellates; pp. 63–84. [Google Scholar]
- 60.Forsblom L., Engström-Öst J., Lehtinen S., et al. Environmental variables driving species and genus level changes in annual plankton biomass. J. Plankton Res. 2019;41(6):925–938. doi: 10.1093/plankt/fbz063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flynn K.J., Mitra A. Building the “perfect beast”: modelling mixotrophic plankton. J. Plankton Res. 2009;31(9):965–992. doi: 10.1093/plankt/fbp044. [DOI] [Google Scholar]
- 62.Min J., Kim K.Y. Seasonal change and subniche dynamics of three Alexandrium species in the Korea Strait. Harmful Algae. 2023;125 doi: 10.1016/j.hal.2023.102420. [DOI] [PubMed] [Google Scholar]
- 63.Stoner O., Economou T., Torres R., et al. Quantifying Spatio-temporal risk of Harmful Algal Blooms and their impacts on bivalve shellfish mariculture using a data-driven modelling approach. Harmful Algae. 2023;121 doi: 10.1016/j.hal.2022.102363. [DOI] [PubMed] [Google Scholar]
- 64.Borowitzka M.A. In: Microalgae in Health and Disease Prevention. Levine I.A., Fleurence J., editors. Academic Press; 2018. Biology of microalgae; pp. 23–72. [DOI] [Google Scholar]
- 65.Schaeffer B.A., Urquhart E., Coffer M., et al. Satellites quantify the spatial extent of cyanobacterial blooms across the United States at multiple scales. Ecol. Indic. 2022;140 doi: 10.1016/j.ecolind.2022.108990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schoener T.W. Resource partitioning in ecological communities. Science. 1974;185(4145):27–39. doi: 10.1126/science.185.4145.27. [DOI] [PubMed] [Google Scholar]
- 67.Skarlato S.O., Telesh I.V. In: The Most Dangerous Invasive Species of Russia (TOP-100), Dgebuadze, Yu.Yu. Petrosyan V.G., Khlyap L.A., editors. KMK; Moscow: 2018. Prorocentrum minimum – dinoflagellates; pp. 227–233. [Google Scholar]
- 68.Søderberg L.M., Hansen P.J. Growth limitation due to high pH and low inorganic carbon concentrations in temperate species of the dinoflagellate genus Ceratium. Mar. Ecol. Prog. Ser. 2007;351:103–112. doi: 10.3354/meps07146. [DOI] [Google Scholar]
- 69.Tunin-Ley A., Ibañez F., Labat J.-P., et al. Phytoplankton biodiversity and NW Mediterranean Sea warming: changes in the dinoflagellate genus Ceratium in the 20th century. Mar. Ecol. Prog. Ser. 2009;375:85–99. doi: 10.3354/meps07730. [DOI] [Google Scholar]
- 70.Reguera B., Velo-Suárez L., Raine R., Park M. Harmful Dinophysis species: a review. Harmful Algae. 2012;14:87–106. doi: 10.1016/j.hal.2011.10.016. [DOI] [Google Scholar]
- 71.Velasco-Senovilla E., Díaz P.A., Nogueira E., et al. The niche of a stress-tolerant specialist, Dinophysis acuminata, in a coastal upwelling system. Harmful Algae. 2023;125 doi: 10.1016/j.hal.2023.102427. [DOI] [PubMed] [Google Scholar]
- 72.Kat M. In: Red Tides: Biology, Environmental Science, and Toxicology. Okaichi T., Anderson D.M., Nemoto T., editors. Elsevier; 1989. Toxic and non-toxic dinoflagellate blooms on the Dutch coast; pp. 73–76. [Google Scholar]
- 73.Basti L., Suzuki T., Uchida H., Kamiyama T., Nagai S. Thermal acclimation affects growth and lipophilic toxin production in a strain of cosmopolitan harmful alga Dinophysis acuminata. Harmful Algae. 2018;73:119–128. doi: 10.1016/j.hal.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 74.Hoshiai G., Suzuki T., Kamiyama T., Yamasaki M., Ichimi K. Water temperature and salinity during the occurrence of Dinophysis fortii and D. acuminata in Kesennuma Bay, northern Japan. Fisheries Sci. 2003;69:1303–1305. doi: 10.1111/j.0919-9268.2003.00760.x. [DOI] [Google Scholar]
- 75.Hattenrath-Lehmann T.K., Marcoval M.A., Mittlesdorf H., et al. Nitrogenous nutrients promote the growth and toxicity of Dinophysis acuminata during estuarine bloom events. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alves-de-Souza C., Iriarte J.L., Mardones J.I. Interannual variability of Dinophysis acuminata and Protoceratium reticulatum in a Chilean fjord: insights from the realized niche analysis. Toxins. 2019;11(1):19. doi: 10.3390/toxins11010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boivin-Rioux A., Starr M., Chassé J., et al. Harmful algae and climate change on the Canadian East Coast: exploring occurrence predictions of Dinophysis acuminata, D. norvegica, and Pseudo-nitzschia seriata. Harmful Algae. 2022;112 doi: 10.1016/j.hal.2022.102183. [DOI] [PubMed] [Google Scholar]
- 78.Hammer A., Schumann R., Schubert H. Light and temperature acclimation of Rhodomonas salina (Cryptophyceae): photosynthetic performance. Aquat. Microb. Ecol. 2002;29:287–296. doi: 10.3354/ame029287. [DOI] [Google Scholar]
- 79.Schumann R., Hammer A., Görs S., Schubert H. Winter and spring phytoplankton composition and production in a shallow eutrophic Baltic lagoon. Estuar. Coast Shelf Sci. 2005;62:169–181. doi: 10.1016/j.ecss.2004.08.015. [DOI] [Google Scholar]
- 80.Karasiewicz S., Chapelle A., Bacher C., Soudant D. Harmful algae niche responses to environmental and community variation along the French coast. Harmful Algae. 2020;93 doi: 10.1016/j.hal.2020.101785. [DOI] [PubMed] [Google Scholar]
- 81.Boeing J. Visual analysis of nonlinear dynamical systems: chaos, fractals, self-similarity and the limits of prediction. Systems. 2016;4:37. doi: 10.3390/systems4040037. [DOI] [Google Scholar]
- 82.Wasmund N., Kownacka J., Göbel J., Jaanus A., Johansen M., Jurgensone I., Lehtinen S., Powilleit M. The diatom/dinoflagellate index as an indicator of ecosystem changes in the Baltic Sea 1. Principle and handling instruction. Front. Mar. Sci. 2017;4:22. doi: 10.3389/fmars.2017.00022. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.