Abstract
Objectives:
To identify a pediatric ventilator-associated condition definition for use in neonates and children by exploring whether potential ventilator-associated condition definitions identify patients with worse outcomes.
Design:
Retrospective cohort study and a matched cohort analysis.
Setting:
Pediatric, cardiac, and neonatal ICUs in five U.S. hospitals.
Patients:
Children 18 years old or younger ventilated for at least 1 day.
Interventions:
None.
Measurements and Main Results:
We evaluated the evidence of worsening oxygenation via a range of thresholds for increases in daily minimum fraction of inspired oxygen (by 0.20, 0.25, and 0.30) and daily minimum mean airway pressure (by 4, 5, 6, and 7 cm H2O). We required worsening oxygenation be sustained for at least 2 days after at least 2 days of stability. We matched patients with a ventilator-associated condition to those without and used Cox proportional hazard models with frailties to examine associations with hospital mortality, hospital and ICU length of stay, and duration of ventilation. The cohort included 8,862 children with 10,209 hospitalizations and 77,751 ventilator days. For the fraction of inspired oxygen 0.25/mean airway pressure 4 definition (i.e., increase in minimum daily fraction of inspired oxygen by 0.25 or mean airway pressure by 4), rates ranged from 2.9 to 3.2 per 1,000 ventilator days depending on ICU type; the fraction of inspired oxygen 0.30/mean airway pressure 7 definition yielded ventilator-associated condition rates of 1.1–1.3 per 1,000 ventilator days. All definitions were significantly associated with greater risk of hospital death, with hazard ratios ranging from 1.6 (95% CI, 0.7–3.4) to 6.8 (2.9–16.0), depending on thresholds and ICU type. Each definition was associated with prolonged hospitalization, time in ICU, and duration of ventilation, among survivors. The advisory board of the study proposed using the fraction of inspired oxygen 0.25/mean airway pressure 4 thresholds to identify pediatric ventilator-associated conditions in ICUs.
Conclusions:
Pediatric patients with ventilator-associated conditions are at substantially higher risk for mortality and morbidity across ICUs, regardless of thresholds used. Next steps include identification of risk factors, etiologies, and preventative measures for pediatric ventilator-associated conditions.
Keywords: hospital mortality, intensive care units, neonatal, intensive care units, pediatric, patient safety, pneumonia, ventilator-associated, ventilators, mechanical
In January 2013, the Centers for Disease Control and Prevention (CDC) replaced its national surveillance definition targeting ventilator-associated pneumonia (VAP) in adults with definitions for ventilator-associated events (VAEs) (1). The new definitions were designed to broaden the focus of surveillance to encompass additional complications of mechanical ventilation. This change acknowledged the inherent difficulty in determining whether a patient had VAP because of the subjectivity of the criteria used to define pneumonia. In response to external pressures of public reporting and payment reform, which might otherwise influence interpretation of subjective data, CDC and investigators from the Prevention Epicenters pivoted toward the use of a surveillance definition based on objective criteria (2). Among adult patients, CDC now uses three definitions that rely on objective criteria: 1) ventilator-associated conditions (VACs), which identify deterioration on the ventilator after a period of stability, 2) infection-related VACs (iVACs), which aim to identify the subset of VAC cases potentially caused by infection, and 3) possible VAP, which aims to identify the subset of iVAC cases caused by pneumonia (1).
Given ongoing efforts to enhance public reporting and to link quality measurement results to reimbursement, there exists a critical and urgent need to develop quality measures that are appropriate for use in neonatal and pediatric populations. In the absence of measures tailored for these populations, surveillance definitions intended for adults may be uncritically applied to pediatric populations. When the Centers for Medicaid and Medicare Services first implemented the hospital-acquired conditions policy in 2008, pediatric facilities were appropriately excluded from the policy change until further evaluation was possible (3). However, these definitions were subsequently adopted by other insurers without a full understanding of whether the metrics were appropriate for use in neonatal and pediatric populations. Although these efforts may be well intentioned, they can result in the inefficient use of hospital resources to collect and respond to data that clinicians feel are not reflective of true quality of care. In addition, they can lead to a lack of support by the clinical community, if clinicians believe quality improvement efforts are directed to a metric that is wrong or clinically irrelevant (4).
In September 2012, CDC convened the Pediatric and Neonatal Ventilator Associated Event Working Group, which was composed of 20 representatives from pediatric, pediatric critical care, neonatal critical care, and infectious disease societies. The group sought to explore the feasibility of developing VAE surveillance definitions and methods for use in PICU and neonatal ICU (NICU) locations. After a review of literature and an expert panel review of the proposed definition that took place over several months, the group decided that additional data were needed on outcomes to apply a definition to the pediatric population.
We sought to identify a pediatric VAC definition suitable for use in neonates and children by exploring whether potential VAC definitions for pediatric populations can discriminate patients with worse outcomes. Our goal was to support the development of an objective surveillance metric that corresponds to meaningful patient outcomes.
MATERIALS AND METHODS
Study Population
We included five U.S. hospitals caring for neonates and/or children: Beth Israel Deaconess Medical Center, Boston Children’s Hospital, Children’s Hospital of Philadelphia, Rush University Medical Center, and Primary Children’s Hospital, Intermountain Healthcare. Each hospital identified consecutive children 18 years old or younger admitted to a PICU, cardiac ICU (CICU), or NICU and ventilated for at least 1 day. We included all modes of invasive mechanical ventilation delivered through a nasotracheal or orotracheal tube or via tracheostomy, including high-frequency oscillatory or jet ventilation (HFV) and airway pressure release ventilation. We excluded ventilation episodes for patients who were only ventilated through noninvasive means and those ventilated with high-frequency percussive ventilation since mean airway pressure (MAP) measures, one of the variables for the candidate definitions, were not available with this particular modality. We also excluded invasive ventilation episodes missing ventilator setting data for at least one calendar day(s).
The study period varied for each hospital based on the timing of availability of electronic data for ventilator variables, with February 5, 2008, being the earliest start date. For all participating hospitals, the study period ended on June 30, 2013. The study periods ranged from 8 to 66 months. The hospitals each had a VAP bundle in place, and the overall elements of each were comparable across the sites (e.g., regular oral hygiene and daily assessment of extubation readiness).
We defined ventilation episodes using the CDC National Healthcare Safety Network (NHSN) definition of calendar days. The ventilation start date began on the first calendar day of invasive mechanical ventilation. Each consecutive calendar day of ventilation was considered part of the same ventilation episode (1). Resumption of invasive ventilation after at least one calendar day off mechanical ventilation constituted a new episode. Children could have multiple NHSN ventilation episodes during an ICU admission, multiple ICU admissions during the hospitalization, and multiple hospitalizations during the period of observation for each hospital. Within each ventilation episode, the occurrence of VAC, using multiple candidate definitions, was assessed.
Adapting the Adult Definition for Neonates and Children
The current adult definition identifies a VAC when, after a period of stability or improvement on the ventilator, a patient has one of the both of the following indicators of worsening oxygenation (1): the minimum daily fraction of inspired oxygen (Fio2) increases at least 0.20 over the daily minimum Fio2 in the preceding two calendar days and the increase is sustained for at least 2 days, or (2) the minimum daily positive end-expiratory pressure (PEEP) values increase at least 3 cm H2O over the daily minimum PEEP in the preceding two calendar days and the increase is sustained for at least 2 days (1).
We first explored whether the adult VAC definition had face validity among clinicians who care for neonates and children by convening four separate focus groups with clinicians from each ICU type at Boston Children’s Hospital in August 2010. These focus groups included a multidisciplinary team of physicians (neonatologists, pediatric intensivists, and cardiac intensivists), ICU nurses, and respiratory therapists. Key feedback from these focus group discussions included (1) a suggestion to use MAP rather than PEEP as a criterion since MAP more accurately reflects changes in lung compliance (which worsens with VAC, frequently along with worsening oxygenation) than PEEP, which is set by clinicians; and (2) the need to allow HFV to be included in the surveillance definition, given the frequency of its use in neonatal and pediatric populations, although it is excluded in the adult definition (1).
We presented these recommendations to our Pediatric VAC Advisory Board and the CDC Neonatal and Pediatric VAE Working Group. The Pediatric VAC Advisory Board was formed to provide guidance to our study team regarding the development of the pediatric VAC definition. The advisory board is comprised of representatives from the CDC, Institute for Healthcare Improvement, Pediatric Cardiac Intensive Care Society, Society of Critical Care Medicine, and the Vermont Oxford Network, in order to capture expertise from stakeholders in each type of ICU setting. The CDC Neonatal and Pediatric VAE Working Group includes representation from national organizations and societies that was constituted to provide CDC with guidance regarding the implementation of a VAC surveillance definition within the NHSN. Both groups affirmed the need to adapt VAC for neonatal and pediatric populations by replacing PEEP with a MAP criterion and including HFV as a ventilation mode.
To identify criteria for a pediatric VAC definition, we examined a range of different threshold changes in daily minimum MAP or Fio2, following a period of stability. Specifically, we first required stable or decreasing minimum daily MAP or Fio2 measures for at least 2 days. Evidence of VAC was then defined as an increase in minimum daily MAP of 4, 5, 6, or 7 cm H2O or an increase of Fio2 by 0.20, 0.25, or 0.30 for at least 2 days. This resulted in 12 candidate definitions: MAP increasing by 4 or Fio2 by 0.20, MAP increasing by 4 or Fio2 by 0.25, and so forth. Whether a corollary definition for infection-associated VAC for neonates and children can be developed is currently under analysis and will be reported separately.
Analyses
We estimated rates of VAC per 1,000 ventilator days, using alternative MAP- and Fio2-based definitions, separately for each ICU type. Next, we conducted a matched cohort study to assess whether VAC would discriminate patients with worse outcomes. Our primary outcome was hospital mortality, and our secondary outcomes were time to hospital discharge to home, ICU discharge, and extubation. For each candidate pediatric VAC definition, we identified subjects with a VAC event by that definition and matched up to four subjects without a VAC, without replacement (i.e., once a subject without a VAC was matched to a subject with VAC, she/he was removed from the list of potential matches), by hospital, ICU type, age ± 50% (unless the VAC subject was 8 days old or younger, in which case comparison subjects were matched within 2 days of age), gestational age in weeks (for NICU patients only; matched ± 1 wk), the presence of congenital heart disease (based on International Classification of Diseases, 9th Edition diagnostic and procedures codes, excluding patent ductus arteriosus) (5), and the number of ventilation days before VAC event plus 1 day. If a patient had greater than one VAC event during the study period for a given definition, one event was randomly chosen for the analysis (a new VAC required a 14-day gap between VAC events if they occurred within the same admission). For matched subjects, if they had more than one eligible ventilation episode, one episode was randomly chosen for inclusion in the analysis. Cox proportional hazard models with shared frailties for each matched set were used to estimate hazard ratios (HRs) and 95% CIs for primary and secondary outcomes associated with each candidate definition. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Overall pediatric VAC rates and the hazards for the primary and secondary outcomes for each candidate definition, by ICU type, were presented to our Pediatric VAC Advisory Board. Based on the results for these analyses both within and across ICU types, these stakeholders identified thresholds for use in PICU, CICU, and NICU population-based surveillance. This study was approved by the institutional review board at Harvard Pilgrim Health Care and by the review boards at each of the participating hospitals.
RESULTS
Study Population
Our study population included 8,862 children admitted to four PICUs, three CICUs, and four NICUs in five U.S. hospitals. The children in the study accounted for 10,209 unique hospital admissions associated with mechanical ventilation, and over half of these admissions occurred in children 12 months old or younger (Table 1). Eighty-eight percent of hospital admissions were associated with a single episode of ventilation. The mean and median duration of ventilation were 6.5 and 3.0 days, respectively, across all ICU types, with a range of 1–266 days.
TABLE 1.
Characteristics of Neonates and Children Ventilated for At Least 1 Day in ICUs
| Hospital Admissions (n = 10,209) (%) | Cardiac ICU Admissions (n = 3,363) (%) | Neonatal ICU Admissions (n = 2,441) (%) | PICU Admissions (n = 4,916) (%) | |
|---|---|---|---|---|
|
| ||||
| Hospitala | ||||
| 1 | 3,898 (38.2) | 2,154 (64.1) | 577 (23.6) | 1,485 (30.2) |
| 2 | 742 (7.3) | 0 | 742 (30.4) | 0 |
| 3 | 826 (8.1) | 160 (4.8) | 268 (11.0) | 485 (9.9) |
| 4 | 919 (9.0) | 0 | 854 (35.0) | 66 (1.3) |
| 5 | 3,824 (37.5) | 1,049 (31.2) | 0 | 2,880 (58.1) |
| Age at admission | ||||
| 0 to < 1 mo | 3,252 (31.9) | 833 (24.8) | 2,174 (89.1) | 297 (6.0) |
| 1 to < 3 mo | 780 (76) | 312 (9.3) | 173 (7.1) | 383 (7.8) |
| 3 to < 12 mo | 1,657 (16.2) | 882 (26.2) | 94 (3.9) | 865 (17.6) |
| 1 to < 5 yr | 2,037 (19.9) | 712 (21.2) | 0 | 1,436 (29.2) |
| 5 to < 10 yr | 980 (9.6) | 254 (76) | 0 | 758 (15.4) |
| 1 0 to 1 8 yr | 1,503 (14.7) | 370 (11.0) | 0 | 1,177 (23.9) |
| Women | 4,443 (43.5) | 1,478 (44.0) | 1,035 (42.4) | 2,162 (44.0) |
| Comorbid diagnosis (by International Classification of Diseases, 9th Edition, codes)b | ||||
| Congenital heart disease | 4,223 (41.4) | 3,059 (91.0) | 735 (30.1) | 757 (15.4) |
| Moderate immunocompromise | 288 (2.8) | 92 (2.7) | 30 (1.2) | 208 (4.2) |
| Significant immunocompromise | 777 (7.6) | 235 (7.0) | 129 (5.3) | 503 (10.2) |
| No. of eligible ventilation episodesc, per hospital admission | ||||
| 1 | 8,966 (87.8) | |||
| 2 | 924 (9.1) | |||
| 3 | 211 (2.1) | |||
| 4+ | 108 (1.1) | |||
| No. of eligible ventilation days | 77,751 | 18,367 | 29,535 | 30,092 |
| Mean/median (range) total duration of ventilation per admission, dc | 6.49/3.00 (1–266) | |||
There were 948 children with multiple hospital admissions and 189 hospital admissions with multiple ICU types.
Based on Agency for Healthcare Research and Quality Pediatric Heart Surgery Volume, Quality Indicators (5). This definition of congenital heart disease excludes patent ductus arteriosus as the sole condition.
Defined as consecutive calendar days and no missing mean airway pressure or fraction of inspired oxygen data.
Blank cells indicate not calculated (because of movement between ICUs within the same hospitalization or ventilation episode).
Pediatric VAC Rates for Candidate Definitions
As might be expected, as more stringent thresholds for Fio2 and MAP were applied, pediatric VAC rates declined across all ICU types (Fig. 1). Using the Fio2 0.20/MAP 4 definition (i.e., defined as an increase in minimum daily Fio2 by ≥ 0.20 or MAP by ≥ 4 cm H2O for at least two consecutive days, after 2 days of stable or decreasing minimum daily Fio2 or MAP measures), VAC rates ranged from 3.3 to 4.6 per 1,000 ventilator days depending on ICU type, with the CICU having a higher rate compared with the NICU and PICU. Using the Fio2 0.25/MAP 4 definition, the rates were less variable across ICU types, ranging from 2.9 to 3.2 per 1,000 ventilator days. At the other end of the spectrum, an Fio2 0.30/MAP 7 definition was associated with VAC rates of 1.1–1.3 per 1,000 ventilator days depending on ICU. In the CICU, changes in the Fio2 threshold had a greater impact on rates than MAP, and VAC rates were higher in CICUs compared with other ICU types when an Fio2 0.20 threshold was used. In NICU and PICU settings, changes to the MAP threshold had a greater impact on VAC rates than changes to the Fio2 threshold.
Figure 1.
Rates of pediatric ventilator-associated conditions (VAC) per 1,000 ventilation days for each of the 12 candidate definitions by ICU type (cardiac ICU [CICU], neonatal ICU [NICU], and PICU). The VAC definitions indicate worsening oxygenation via a range of thresholds for increases in daily minimum fraction of inspired oxygen (Fio2) (by 0.20, 0.25, and 0.30) and daily minimum mean airway pressure (MAP; by 4, 5, 6, and 7 cm H2O); worsening oxygenation was sustained for ≥ 2 days after ≥ 2 days of stability.
Hospital Mortality
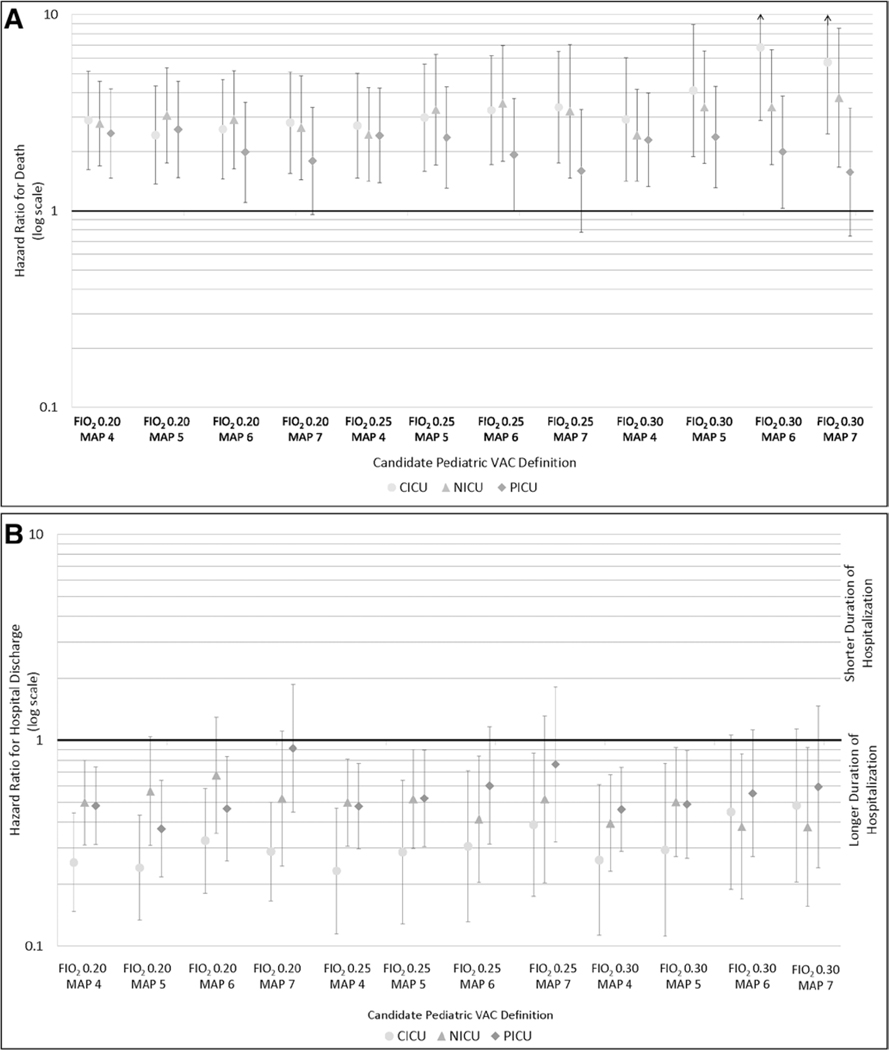
Table 2 shows the number of subjects with a VAC and matched subjects without a VAC by each candidate definition and ICU, including the percent of death during hospitalization and corresponding HRs and 95% CIs. Patients with a VAC event were at much higher risk of death in all ICU types and by all definitions compared with matched subjects without a VAC. The increased hazard of death associated with VAC ranged from an HR of 2.4 (95% CI, 1.4–4.2; Fio2 0.30/MAP 4) to 3.8 (95% CI, 1.7–8.5; Fio2 0.30/MAP 7) in the NICU, 2.4 (95% CI, 1.4–4.3; Fio2 0.20/MAP 5) to 6.8 (95% CI, 2.9–16.0; Fio2 0.30/MAP 6) in the CICU, and HR 1.6 (95% CI, 0.7–3.4; Fio2 0.30/MAP 7) to 2.6 (95% CI, 4.6–1.5; Fio2 0.20/MAP 5) in the PICU (Fig. 2A). Although the mortality results in the PICU are not statistically significant at the highest threshold of MAP, they are consistent with all other results in the analysis.
TABLE 2.
Subjects With a Pediatric Ventilator-Associated Condition, Matched Subjects Without a Ventilator-Associated Condition, and Hospital Mortality by Definition in the Neonatal ICU, Cardiac ICU, and PICU
| Pediatric VAC Definition | Subjects With a VAC (n) | Matched Subjects Without a VAC (n) | VAC Subjects Who Died (%) | Matched Subjects Who Died (%) | Hazard Ratio for Death (95% CI) | Mean (Median) Days to VAC |
|---|---|---|---|---|---|---|
|
| ||||||
| Cardiac ICU | ||||||
| Fio2 20 or MAP 4 | 65 | 217 | 41.6 | 11 | 2.9 (1.6–5.2) | 10.2 (7.0) |
| Fio2 20 or MAP 5 | 60 | 202 | 41.6 | 12.8 | 2.4 (1.4–4.3) | 11.1 (7.0) |
| Fio2 20 or MAP 6 | 58 | 196 | 43.2 | 12.8 | 2.6 (1.5–4.7) | 10.0 (7.0) |
| Fio2 20 or MAP 7 | 57 | 196 | 42.2 | 12.2 | 2.8 (1.6–5.1) | 9.8 (7.0) |
| Fio2 25 or MAP 4 | 47 | 159 | 44.6 | 14.4 | 2.7 (1.5–5.0) | 11.9 (7.0) |
| Fio2 25 or MAP 5 | 40 | 137 | 50 | 15.4 | 3.0 (1.6–5.6) | 13.6 (6.0) |
| Fio2 25 or MAP 6 | 39 | 136 | 51.2 | 14.8 | 3.3 (1.7–6.2) | 12.1 (6.0) |
| Fio2 25 or MAP 7 | 38 | 132 | 50 | 14.4 | 3.4 (1.8–6.5) | 11.6 (6.0) |
| Fio2 30 or MAP 4 | 33 | 116 | 45.4 | 14.6 | 2.9 (1.4–6.0) | 13.0 (7.0) |
| Fio2 30 or MAP 5 | 25 | 85 | 56 | 17.6 | 4.1 (1.9–8.9) | 18.0 (7.0) |
| Fio2 30 or MAP 6 | 24 | 84 | 58.4 | 13 | 6.8 (2.9–16.0) | 15.0 (7.0) |
| Fio2 30 or MAP 7 | 23 | 80 | 56.6 | 15 | 5.7 (2.5–13.2) | 14.4 (7.0) |
| Neonatal ICU | ||||||
| Fio2 20 or MAP 4 | 91 | 322 | 30.8 | 11.2 | 2.8 (1.7–4.6) | 15.1 (9.0) |
| Fio2 20 or MAP 5 | 62 | 222 | 37 | 12.2 | 3.1 (1.8–5.4) | 15.4 (9.0) |
| Fio2 20 or MAP 6 | 56 | 192 | 39.2 | 14 | 2.9 (1.6–5.2) | 18.3 (11.0) |
| Fio2 20 or MAP 7 | 46 | 155 | 41.4 | 16.2 | 2.7 (1.4–4.9) | 18.5 (11.0) |
| Fio2 25 or MAP 4 | 80 | 294 | 26.2 | 11.6 | 2.5 (1.4–4.3) | 14.2 (9.0) |
| Fio2 25 or MAP 5 | 52 | 194 | 32.6 | 11.4 | 3.3 (1.7–6.3) | 13.5 (9.0) |
| Fio2 25 or MAP 6 | 44 | 159 | 36.4 | 12 | 3.5 (1.8–7.0) | 16.1 (9.0) |
| Fio2 25 or MAP 7 | 33 | 112 | 36.4 | 13.4 | 3.2 (1.5–7.0) | 17.7 (9.0) |
| Fio2 30 or MAP 4 | 78 | 278 | 28.2 | 11.8 | 2.4 (1.4–4.2) | 15.6 (9.0) |
| Fio2 30 or MAP 5 | 50 | 179 | 34 | 11.2 | 3.4 (1.7–6.5) | 16.8 (9.0) |
| Fio2 30 or MAP 6 | 41 | 144 | 39 | 13.2 | 3.4 (1.7–6.6) | 17.6 (11.0) |
| Fio2 30 or MAP 7 | 30 | 100 | 40 | 13 | 3.8 (1.7–8.5) | 18.4 (10.0) |
| PICU | ||||||
| Fio2 20 or MAP 4 | 83 | 308 | 31.4 | 11 | 2.5 (1.5–4.2) | 9.6 (6.0) |
| Fio2 20 or MAP 5 | 62 | 230 | 35.4 | 12.6 | 2.6 (1.5–4.6) | 10.4 (6.5) |
| Fio2 20 or MAP 6 | 52 | 191 | 34.6 | 15.8 | 2.0 (1.1–3.6) | 11.2 (7.0) |
| Fio2 20 or MAP 7 | 44 | 159 | 34 | 17.6 | 1.8 (1.0–3.4) | 12.2 (9.5) |
| Fio2 25 or MAP 4 | 75 | 279 | 30.6 | 10.4 | 2.4 (1.4–4.2) | 10.2 (6.0) |
| Fio2 25 or MAP 5 | 55 | 207 | 34.6 | 13 | 2.4 (1.3–4.3) | 10.4 (7.0) |
| Fio2 25 or MAP 6 | 44 | 165 | 31.8 | 15.2 | 1.9 (1.0–3.7) | 10.6 (7.0) |
| Fio2 25 or MAP 7 | 36 | 133 | 30.6 | 18 | 1.6 (0.8–3.3) | 11.6 (9.5) |
| Fio2 30 or MAP 4 | 74 | 279 | 31 | 11.4 | 2.3 (1.3–4.0) | 10.2 (6.0) |
| Fio2 30 or MAP 5 | 54 | 204 | 35.2 | 13.2 | 2.4 (1.3–4.3) | 10.4 (6.5) |
| Fio2 30 or MAP 6 | 43 | 163 | 32.6 | 15.4 | 2.0 (1.0–3.9) | 10.6 (7.0) |
| Fio2 30 or MAP 7 | 34 | 127 | 29.4 | 17.4 | 1.6 (0.7–3.4) | 11.4 (9.0) |
VAC = ventilator-associated condition, Fio2 = fraction of inspired oxygen, MAP = mean airway pressure.
Death was defined as death during hospitalization.
Figure 2.
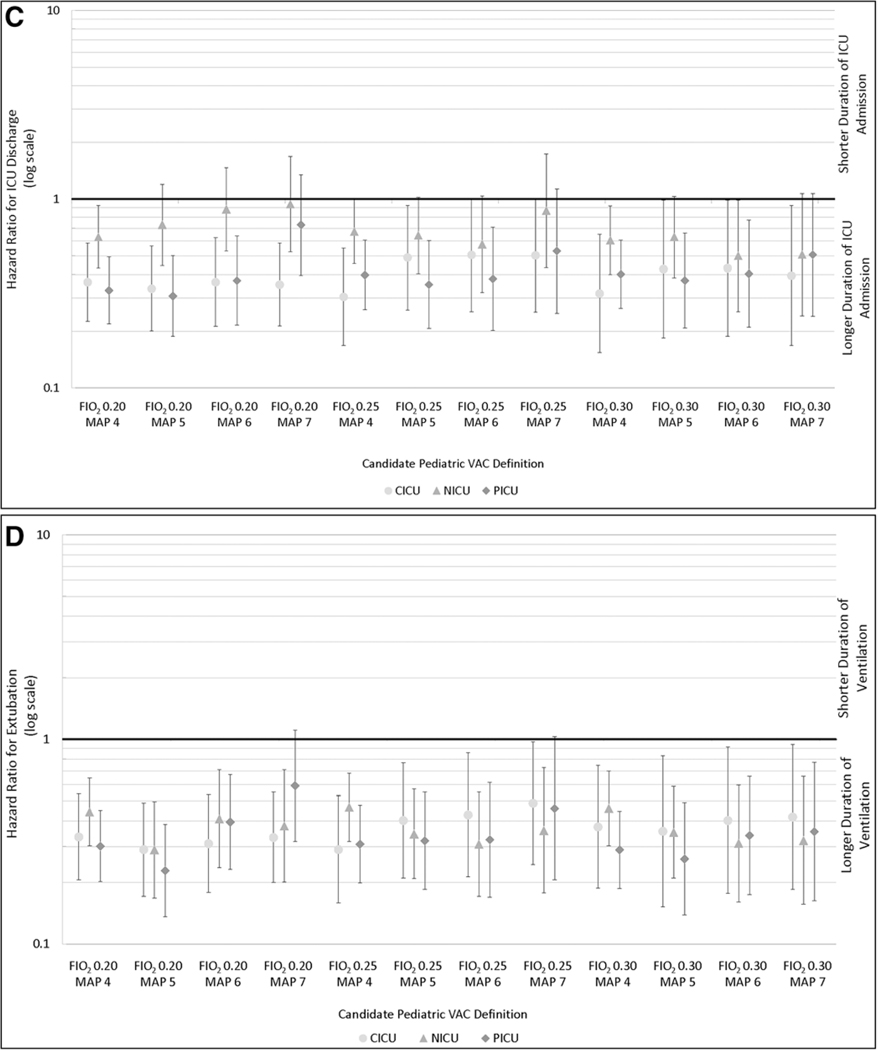
Adjusted hazard ratios and 95% CIs for hospital death (A), hospital discharge (b), ICU discharge (C), and the end of ventilation episode (D) for patients with a pediatric ventilator-associated condition (VAC) compared with their matches for each candidate VAC definition by ICU type. Analyses B-D are among survivors only. CICU = cardiac ICU, MAP = mean airway pressure, NICU = neonatal ICU.
Secondary Outcomes
To understand the impact of pediatric VAC on hospital and ICU length of stay and duration of ventilation, we conducted a survivor-only analysis (i.e., if a VAC subject or one or more of her/his matched subjects without VAC died in the hospital, that cluster was removed from this analysis). Among survivors, pediatric VAC was associated with prolonged hospitalizations (i.e., lower hazard for hospital discharge to home), ICU length of stay (i.e., lower hazard for ICU discharge), and subsequent duration of ventilation after the VAC event date or corresponding index date (i.e., lower hazard for extubation). This was true across the candidate definitions and ICUs (Fig. 2, B–D).
Choosing a Single Pediatric VAC Definition
The rates of VAC and the HRs for mortality and the secondary adverse outcomes for the candidate VAC definitions were presented to the study’s advisory board. The board recognized that the choice of optimal thresholds for defining pediatric VAC depends on the values stakeholders place on tradeoffs in prevalence and magnitude of impact on outcomes, which is related in part to the purpose of the quality measure, whether for internal quality improvement or for external public reporting or reimbursement. In addition, another criterion was that the definition should lead to a “reasonable” event rate—one that would inform possible opportunities for improvement. Finally, our advisory board also considered whether each definition was associated with meaningful differences in patient outcomes; we would consider using alternate diagnostic thresholds with better discriminatory capacity if a particular definition did not seem to be associated with worse outcomes. As anticipated, VAC rates were higher when less stringent thresholds of Fio2 and MAP were used, although greater variability was noted across ICU types with the Fio2 0.20 threshold. The highest pediatric VAC rates of 3.3–4.6 per 1,000 ventilator days in the NICU and PICU using the Fio2 0.20/MAP 4 definition were lower than reported adult VAC rates of 7–12 per 1,000 ventilator days (6–8).
All candidate definitions seemed to be associated with increased HRs for mortality, and the HRs were fairly similar. The rate of VAC by the different candidate definitions, as well as their consistency across ICU types, was also taken into account. Given the overall low rate of VAC events in our neonatal and pediatric populations, the strong association with mortality, and the need to continue to strive for improvements in patient outcomes, our advisory board recommended the Fio2 0.25/MAP 4 definition as a reasonable candidate definition for use in national surveillance, with the expectation that further information about etiology, risk factors, and degree of preventability would lead to future refinements.
DISCUSSION
Finding the right metric to address a myriad of needs—surveillance, clinical practice, cost, and reimbursement—continues to be a struggle for measure developers, measurement endorsement bodies, U.S. hospitals, and other stakeholders. The challenge is heightened when healthcare leaders are judged by their ability to produce short-term results. This challenge becomes particularly evident when metrics focused on quality improvement are tied to benchmarking and payment. In the former, quantifying an institution’s current outcomes and standing among peer institutions, as well as providing the ability to measure the effects of ongoing quality improvement efforts, help to move organizations forward. In the latter, identifying the right answer takes precedence. In seeking to define pediatric VAC in neonatal and pediatric populations, our goal is to identify potential metrics that offer opportunities for improvement, while facilitating innovations that will have a meaningful long-term impact on patient outcomes. As noted above, additional work must be conducted before this surveillance measure moves beyond a focus for improvement and is tied to payment.
In this first large-scale national study conducted in PICU, CICU, and NICU settings, we considered 12 alternative definitions for pediatric VAC. To provide the opportunities for improvement across all settings, we propose the use of the Fio2 0.25/MAP 4 definition for pediatric VAC (i.e., an increase in minimum daily Fio2 by ≥ 0.25 or MAP by ≥ 4) to identify patients at significantly increased risk for adverse outcomes. The proposed definition has two distinct advantages over the previous VAP definition. First, the criteria used (i.e., Fio2 and MAP) are clinically relevant, routinely measured, and available from institutional electronic data sources. By using quantitative, objective criteria to define pediatric VAC, we can minimize variability in measurement across facilities, unlike the VAP definition based on subjective criteria, such as chest x-ray interpretation (9). Second, pediatric VAC patients have substantially higher mortality rates and longer durations of hospitalization, ICU stay, and ventilation in all ICU types, compared with similar patients without a VAC event, thus providing validation that patients with meaningfully worse outcomes can be identified.
Because of differences in populations cared for in different ICU types, we considered PICU, CICU, and NICU VAC rates and outcomes separately in our study. Although rates were similar in PICU and NICU patients, the definitions with the Fio2 0.20 threshold were associated with higher VAC rates in CICU patients, which may be due in part to greater variability in oxygen needs in patients with cyanotic congenital heart disease and the presence of variable degrees of venoarterial shunting. This must be balanced with the population of acquired heart disease that would match the profile of PICU patients. Nonetheless, substantial morbidity and mortality were observed in patients using these thresholds in the CICU setting. As we attain a greater understanding of the risk factors for and etiologies of VAC in this subpopulation of children, alternative thresholds may be worth considering for the CICU. As anticipated, the impact of VAC on length of hospitalization and ICU stay was attenuated in the NICU setting, compared with PICU or CICU settings, because each of these outcomes is likely to be most strongly associated with gestational age. In contrast, mortality rates and duration of ventilation were similar among VAC patients in all three ICU types, which again suggests that the definition is able to discriminate between patients with worse versus better outcomes. The proposed pediatric VAC definition provides, at a minimum, an initial mechanism for tracking meaningful differences in pediatric healthcare quality across ICUs.
Pediatric VAC offers a unique opportunity to shift our cognitive framework for prevention, by expanding the overall paradigm to include both infectious and noninfectious complications. A broader definition may compel clinicians to consider a wider range of interventions based on different causal pathways. Answers remain uncertain, however, with regard to preventability of these conditions. Understanding preventability will first depend on a rigorous evaluation of risk factors associated with pediatric VAC. Studies in adults suggest that VACs are mainly attributable to pneumonia, pulmonary edema, atelectasis, and acute respiratory distress syndrome (7, 8, 10, 11). A small number of interventional studies suggest that a significant fraction is preventable (12–14). The extent to which these findings extend to neonates and children remains to be seen.
We anticipate that in a significant proportion of cases, pediatric VAC may be a marker for progression of underlying clinical disease, such as the development of bronchopulmonary dysplasia in a premature infant in the NICU or progressive heart failure despite maximal therapy in a child with congenital heart disease in the CICU. Risk adjustment for these non-modifiable factors is an important part of developing a quality metric, particularly if metrics are used for interinstitutional comparisons. Nevertheless, we also anticipate that a substantial proportion of pediatric VAC cases may be entirely preventable. Among VAC events deemed reasonably preventable (e.g., fluid overload, barotrauma, and infection), reaching for zero should be the goal given the serious prognosis associated with the development of VAC in neonates and children. As we deepen our understanding of etiologies and potentially modifiable risk factors associated with pediatric VAC, we may identify several concomitant interventions worth pursuing. For example, in the adult literature, improved fluid management (12) and the use of spontaneous awakening and spontaneous breathing trials have reduced VAC rates in adults (14). Our understanding of preventability in pediatrics will be inextricably linked to available evidence regarding prevention in pediatric populations.
There are several potential limitations for this study. First, a pediatric VAC event as we have defined it focuses on neonates and children who acutely deteriorate while mechanically ventilated and does not include complications of extubation, such as postextubation stridor, failed extubation, or unplanned extubation. We believe these additional complications are important clinical consequences of mechanical ventilation that should be monitored by institutions caring for these patients. Similarly, there is increasing use of noninvasive ventilation techniques that may also affect outcomes, although our current definition for VAC does not address these modalities of care. Second, the definition requires at least four consecutive days of ventilation, which means we may miss other types of adverse events associated with shorter ventilation. Third, our proposed definition of Fio2 0.25/MAP 4 may identify cases that do not always reflect clinically significant worsening. Although these thresholds may be imperfect, our advisory board feels that the opportunity for improvement is imperative, particularly because pediatric VAC rates are already lower than reported VAC rates in adults. Fourth, despite matching for relevant confounders available in electronic data, our HR estimates for the primary and secondary outcomes of interest may have residual confounding. If pediatric VAC is used as a national surveillance measure, the burden of routinely collecting additional confounder information (e.g., Pediatric Index of Mortality-II, Pediatric Risk of Mortality-III, or Score for Neonatal Acute Physiology Perinatal Extension risk scores) should be weighed against the relative improvement in accuracy. Additional work is currently underway to identify risk factors for pediatric VAC. Information from these studies will become critical as we seek to define preventability in neonates and children, as the proposed definition does not tell us whether these events are preventable.
CONCLUSIONS
In conclusion, we identify a candidate pediatric VAC definition—based on an increase in minimum daily Fio2 by at least 0.25 or MAP by at least 4 cm H2O for two or more days after a period of stability—for use in ventilated neonates and children. As we continue to refine our questions about appropriate targets for improvement and to identify high-risk populations that might incur the greatest benefit from our efforts, we hope to construct the foundation to identify the interventions that can best prevent VAC and optimize long-term outcomes for pediatric patients.
ACKNOWLEDGMENTS
We are grateful to our hospital colleagues who contributed to this study, including Chandini Bandaru, Sarah Clarke, Stephanie Coulombre, Sunita Dilwali, Meagan Dubosky, Kathy Jenkins, Valerie Kalinowski, Sarah Klieger, Christie Lawrence, Sheila Levins, Anne McDonald, Janusz Pasierbski, and Jean Silvestri. We also thank our Pediatric Ventilator-Associated Condition (VAC) Advisory Members, Shelley Magill, Jeffrey Burns, Jeffrey Horbar, Roger Resar, and Donald Goldmann, for their guidance and input on the development of a neonatal and pediatric VAC definition.
This research was funded by the Agency for Healthcare Research and Quality (AHRQ) R18 grant (#1R18HS021636) to Dr. Lee. Dr. Gray has received funding from the Vermont Oxford Network. Dr. Logan also receives from the National Institutes of Health (NIH) (5K08AI112506-01; not related to this grant).
Dr. Cocoros’ institution received funding from the Agency for Healthcare Research and Quality (AHRQ). Dr. Kleinman’s institution received funding from AHRQ. Dr. Priebe’s institution received funding from AHRQ. Dr. Gray received funding from the Vermont Oxford Network. His institution received funding from AHRQ. Dr. Logan received support for article research from the National Institutes of Health (NIH) and is funded for her main research by the NIH (5K08AI112506-01 - not related to manuscript). Dr. Toltzis’ institution received funding from AHRQ (through a subcontract to Harvard Pilgrim Health). Dr. Burton received funding from the AHRQ. Dr. Sims received support for article research from AHRQ. Her institution received funding from AHRQ. Dr. Harper’s institution received funding from AHRQ. Dr. Sandora received funding from the Society for Healthcare Epidemiology of America. His institution received funding from AHRQ. Dr. Klompas’ institution received funding from AHRQ and CDC. Dr. Lee served on an IOM Board, AHRQ study section, PIDS Board of directors, and as VON faculty (None are paid except for travel expenses to meetings) and received support for article research from the AHRQ. Her institution received funding from the AHRQ R18 grant.
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC). Ventilator-Associated Event (VAE) Protocol, 2013. Atlanta, Centers for Disease Control and Prevention (CDC). Available at: http://www.cdc.gov/nhsn/acutecare-hospital/vae/. Accessed February 18, 2015 [Google Scholar]
- 2.Magill SS, Klompas M, Balk R, et al. : Developing a new, national approach to surveillance for ventilator-associated events. Crit Care Med 2013; 41:2467–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Conference of State Legislatures (NCSL): Medicare Nonpayment for Medical Errors. Washington, DC, National Conference of State Legislatures (NCSL), 2008. Available at: http://www.ncsl.org/Portals/1/documents/health/MCHAC.pdf. Accessed September 25, 2015 [Google Scholar]
- 4.Halpern NA, Hale KE, Sepkowitz KA, et al. : A world without ventilator-associated pneumonia: Time to abandon surveillance and deconstruct the bundle. Crit Care Med 2012; 40:267–270 [DOI] [PubMed] [Google Scholar]
- 5.Agency for Healthcare Research and Quality (AHRQ). RACHS-1 Pediatric Heart Surgery Volume, Technical Specifications, Pediatric Quality Indicators #7 (PDI #7), Version 4.5. 2013. Available at: http://www.qualityindicators.ahrq.gov/Downloads/Modules/PDI/V45/TechSpecs/PDI%2007%20RACHS-1%20Pediatric%20Heart%20Surgery%20Volume.pdf. Accessed September 25, 2015
- 6.Klompas M, Kleinman K, Murphy MV: Descriptive epidemiology and attributable morbidity of ventilator-associated events. Infect Control Hosp Epidemiol 2014; 35:502–510 [DOI] [PubMed] [Google Scholar]
- 7.Boyer AF, Schoenberg N, Babcock H, et al. : A prospective evaluation of ventilator-associated conditions and infection-related ventilator-associated conditions. Chest 2015; 147:68–81 [DOI] [PubMed] [Google Scholar]
- 8.Klein Klouwenberg PM, van Mourik MS, Ong DS, et al. ; MARS Consortium: Electronic implementation of a novel surveillance paradigm for ventilator-associated events. Feasibility and validation. Am J Respir Crit Care Med 2014; 189:947–955 [DOI] [PubMed] [Google Scholar]
- 9.Klompas M: Complications of mechanical ventilation–the CDC’s new surveillance paradigm. N Engl J Med 2013; 368:1472–1475 [DOI] [PubMed] [Google Scholar]
- 10.Klompas M, Khan Y, Kleinman K, et al. ; CDC Prevention Epicenters Program: Multicenter evaluation of a novel surveillance paradigm for complications of mechanical ventilation. PLoS One 2011; 6:e18062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi Y, Morisawa K, Klompas M, et al. : Toward improved surveillance: The impact of ventilator-associated complications on length of stay and antibiotic use in patients in intensive care units. Clin Infect Dis 2013; 56:471–477 [DOI] [PubMed] [Google Scholar]
- 12.Mekontso Dessap A, Katsahian S, Roche-Campo F, et al. : Ventilator-associated pneumonia during weaning from mechanical ventilation: Role of fluid management. Chest 2014; 146:58–65 [DOI] [PubMed] [Google Scholar]
- 13.Muscedere J, Sinuff T, Heyland DK, et al. ; Canadian Critical Care Trials Group: The clinical impact and preventability of ventilator-associated conditions in critically ill patients who are mechanically ventilated. Chest 2013; 144:1453–1460 [DOI] [PubMed] [Google Scholar]
- 14.Klompas M, Anderson D, Trick W, et al. ; CDC Prevention Epicenters: The preventability of ventilator-associated events. The CDC Prevention Epicenters Wake Up and Breathe Collaborative. Am J Respir Crit Care Med 2015; 191:292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]