Abstract
Previously, we defined a novel class of ligands for the human progesterone receptor (PR) which function as mixed agonists. These compounds induce a conformational change upon binding the receptor that is different from those induced by agonists and antagonists. This establishes a correlation between the structure of a ligand-receptor complex and its transcriptional activity. In an attempt to define the cellular components which distinguish between different ligand-induced PR conformations, we have determined, by using a mammalian two-hybrid assay, that the nuclear receptor corepressor (NCoR) and the silencing mediator for retinoid and thyroid hormone receptor (SMRT) differentially associate with PR depending upon the class of ligand bound to the receptor. Specifically, we observed that the corepressors preferentially associate with antagonist-occupied PR and that overexpression of these corepressors suppresses the partial agonist activity of antagonist-occupied PR. Binding studies performed in vitro, however, reveal that recombinant SMRT can interact with PR in a manner which is not influenced by the nature of the bound ligand. Thus, the inability of SMRT or NCoR to interact with agonist-activated PR when assayed in vivo may relate more to the increased affinity of PR for coactivators, with a subsequent displacement of corepressors, than to an inherent low affinity for the corepressor proteins. Previous work from other groups has shown that 8-bromo-cyclic AMP (8-bromo-cAMP) can convert the PR antagonist RU486 into an agonist and, additionally, can potentiate the transcriptional activity of agonist-bound PR. In this study, we show that exogenous expression of NCoR or SMRT suppresses all 8-bromo-cAMP-mediated potentiation of PR transcriptional activity. Further analysis revealed that 8-bromo-cAMP addition decreases the association of NCoR and SMRT with PR. Thus, we propose that 8-bromo-cAMP-mediated potentiation of PR transcriptional activity is due, at least in part, to a disruption of the interaction between PR and the corepressors NCoR and SMRT. Cumulatively, these results suggest that NCoR and SMRT expression may play a pivotal role in PR pharmacology.
The progesterone receptor is a ligand-activated transcription factor and a member of the nuclear receptor superfamily (15). In the absence of ligand, the receptor exists in a transcriptionally inactive state associated with heat shock proteins and other cellular chaperones (36). Upon binding ligand, the receptor undergoes a conformational change and dissociates from the heat shock proteins, allowing the receptor to dimerize and bind to progesterone-responsive elements (PRE) within regulatory regions of target genes (23). Agonist-bound progesterone receptor (PR) is believed to activate transcription by directly interacting with the general transcription machinery (20) and/or by associating with coactivators such as TIF2 (40), SRC-1 (29), and CBP (35), which act as bridging factors between the receptor and the general transcription machinery. Interestingly, upon binding an antagonist, PR undergoes a conformational change which is different from that induced upon binding agonists (1). This unique conformational change is accompanied by the displacement of the heat shock proteins, dimerization, and a subsequent interaction of the receptor with its target DNA. However, under most circumstances, antagonist-bound receptor remains transcriptionally inactive. The inability of antagonist-occupied receptor to activate transcription is hypothesized to be a consequence of its inability to associate with coactivators (29, 40) and possibly its enhanced ability to recruit a corepressor(s) (39, 43). Thus, a model has been proposed in which agonists and antagonists, by inducing different conformational changes within PR, affect the receptor’s ability to activate transcription.
In addition to agonists and antagonists, we have recently identified a new class of PR ligands which function as mixed agonists (41). As with estrogen receptor (ER) mixed agonists, the relative agonist, or antagonist, activity of PR mixed agonists is influenced by the cell and promoter context (7, 38). The likely mechanistic basis for the unique activities of the PR mixed agonists was revealed when it was shown by limited protease digestion analysis that these ligands induce a conformational change within PR which is different from that induced by either agonists or antagonists (41). These studies firmly established a link between the overall structure of the PR-ligand complex and its biological activity.
It is likely, however, that other factors in addition to alterations in receptor structure may also influence PR transcriptional activity. Specifically, it has been shown that the antagonist RU486 can function as a partial agonist in the presence of 8-bromo-cyclic AMP (8-bromo-cAMP) (6, 32). This activity of 8-Bromo-cAMP is not restricted to antagonists, since 8-bromo-cAMP will also potentiate the activity of agonist-bound PR (6, 14, 32). The mechanism by which 8-bromo-cAMP potentiates PR activity has not yet been determined. It is known that PR is a phosphoprotein which becomes hyperphosphorylated upon binding ligand and DNA; therefore, it was originally considered that 8-bromo-cAMP-mediated potentiation of PR transcriptional activity may be due to receptor phosphorylation. However, more recent studies have shown that the net phosphorylation of PR does not change in response to 8-bromo-cAMP (5, 32). Another hypothesis which has been proposed to explain the effects of 8-bromo-cAMP is that this agent stimulates the phosphorylation of a PR coactivator, enhancing its affinity for the receptor and/or a component of the general transcription machinery (6, 14). Confirmation of this hypothesis awaits the identification of a PR coactivator which becomes phosphorylated in response to 8-bromo-cAMP.
Previously, it was considered that the inhibitory activity of PR antagonists was due simply to the competition between agonists and antagonists for binding to the receptor. However, there is increasing evidence that suggests that PR antagonist activity results from an active process which involves the recruitment of transcriptional corepressors (39, 43). Recently, Jackson et al. (21) reported the cloning of the human homolog of the nuclear receptor corepressor (NCoR) by using the hinge and hormone binding domain of PR bound by the antagonist RU486 in a yeast two-hybrid screen. This was particularly interesting since NCoR (17) and the silencing mediator for retinoid and thyroid hormone receptor (SMRT) (9) are two closely related proteins (10) which had previously been shown to function as corepressors, allowing unliganded thyroid hormone (TR) and retinoid receptors to repress target gene transcription. The importance of these proteins for steroid hormone action was confirmed when it was demonstrated that overexpression of NCoR and SMRT represses the partial agonist activity of both tamoxifen-bound ER and RU486-bound PR, suggesting that these corepressors may also function as corepressors for the steroid receptors (21, 34).
Based on these findings, we hypothesized that the different transcriptional activities induced by the three classes of PR ligands may result from differential interactions with the corepressors NCoR and SMRT as a direct result of the unique receptor conformational changes which these ligands induce upon binding (41). To test this hypothesis, we looked at the ability of the two corepressors NCoR and SMRT to associate with PR when bound by the three classes of ligands and assessed the effect of this association on receptor transcriptional activity. We found that the corepressors show the strongest association with antagonist-bound PR and weaker associations with mixed-agonist- and agonist-occupied PR. Furthermore, we show that these differences are reflected in the relative effect which overexpression of NCoR or SMRT has on the biological activity of the three classes of PR ligand. Additionally, in this study, we show that overexpression of either NCoR or SMRT prevents the 8-bromo-cAMP-mediated potentiation of PR transcriptional activity regardless of the ligand bound. We further demonstrate that 8-bromo-cAMP decreases the interaction between PR and the corepressors, leading us to believe that 8-bromo-cAMP potentiation of PR transcriptional activity is due, at least in part, to a decrease in the ability of the receptor to interact with corepressors. These results suggest that corepressor expression may play a pivotal role in determining receptor transcriptional activity and may modulate the effects of alternate signaling pathways on this activity.
MATERIALS AND METHODS
Plasmids and biochemicals.
The plasmid pRS-hPR-VP16 was a gift from D. X. Wen (Ligand Pharmaceuticals, San Diego, Calif.). The mammalian two-hybrid plasmids pCMX-VP-F-hTRβ and GAL4N-RIP13ΔN4 were provided by D. D. Moore (Baylor College of Medicine, Houston, Tex.). pCMX-GAL-C-SMRT, pGEX2TA-C′C′SMRT, and pCMX-C′SMRT were provided by J. D. Chen (University of Massachusetts, Worcester, Mass.). The control, vector pVP16, was purchased from Clontech (Palo Alto, Calif.). The pCMX-VP16 plasmid was constructed by removing the TR ligand binding domain from the pCMX-VP-L-hTRβ construct provided by D. D. Moore. The TR ligand binding domain was removed by digesting pCMX-VP-L-hTRβ with Asp718 and BamHI. The 5xGAL4-TATA-luciferase (LUC) reporter was a gift from X. F. Wang (Duke University, Durham, N.C.). pCMX-NCoR and pCMX-SMRT were provided by M. G. Rosenfeld (University of California, San Diego) and R. M. Evans (Salk Institute, San Diego, Calif.), respectively. The pRSV-891 plasmid was a gift from M. J. Tsai (Baylor College of Medicine).
8-Bromo-cAMP was purchased from Boehringer Mannheim (Indianapolis, Ind.) R5020 was purchased from Dupont (Boston, Mass.). T3 was purchased from Sigma (St. Louis, Mo.). RU486 was a gift from Ligand Pharmaceuticals. RTI-3021-020 (RTI-020) was a gift from C. E. Cook (Research Triangle Institute, Durham, N.C.) (41). ZK98299 was a gift from Schering Pharmaceuticals (Berlin, Germany).
Mammalian transfections and luciferase assays.
HeLa and HepG2 cells were maintained in modified Eagle’s medium containing 10% fetal calf serum. The cells were plated in 24-well plates 24 h prior to transfection with lipofectin as described previously (28). For Fig. 1 and 6, the following amounts of DNA, totaling 3 μg of DNA per triplicate, were transfected: 500 ng of 5xGAL4-TATA-LUC, 50 ng of pCMV-β-Gal, 1,000 ng of either GAL4N-RIP13ΔN4 or pCMX-GAL-C′SMRT, and either 1,000 ng of pCMX-GAL-C-VP-F-hTRβ or 763 ng of pCMX-VP16. Equimolar amounts of cytomegalovirus (CMV)-derived expression plasmids were used for each transfection. For the experiments shown in Fig. 3 and 5, we used 1,000 ng of PRE-TK-LUC, 500 ng of SV40-PRB, 20 ng of CMV-β-Gal, and either 500 ng of pCMX-NCoR or 288 ng of Rev-TUP1 (CMV vector encoding TUP1 in the reverse orientation used for equimolar balancing of CMV) (for Fig. 3A and 5A) or 500 ng of pCMX-SMRT or 266 ng of Rev-TUP1 (for Fig. 3B and 5B). For the experiments shown in Fig. 4, HeLa cells were transfected with 1,000 ng of PRE-TK-LUC, 500 ng of pRSV-891, 20 ng of CMV-β-Gal, and either 500 ng of pCMV-NCoR, 500 ng of pCMX-SMRT, or 288 ng of Rev-TUP1. In all transfections, the total amount of DNA was adjusted to 3 μg per triplicate by using pBSII-KS+. After a 3-h incubation with the DNA-lipofectin mixture, the cells were washed and incubated with modified Eagle’s medium supplemented with 10% fetal calf serum and the appropriate ligand and/or 8-bromo-cAMP for either 24 (Fig. 1 and 6) or 48 (Fig. 3 to 5) h. Luciferase and β-galactosidase assays were performed as described previously (28).
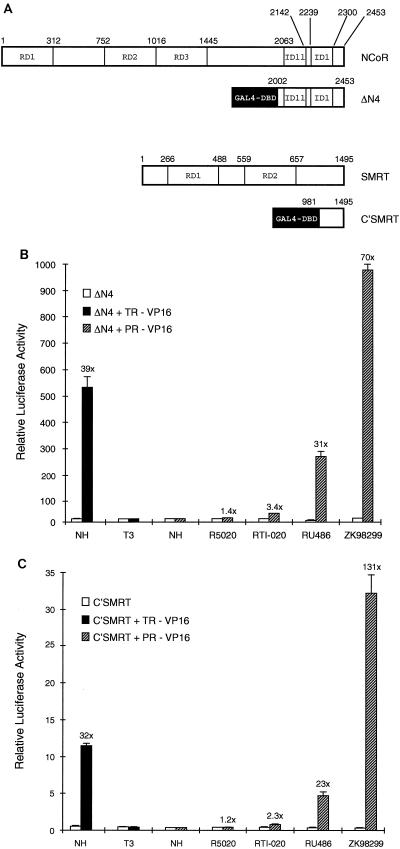
FIG. 1.
Mammalian two-hybrid analysis reveals that PR and the corepressors NCoR and SMRT interact in a ligand-dependent manner. (A) Schematic indicating the regions of NCoR (ΔN4) and SMRT (C′SMRT) which were fused to the GAL4 DNA binding domain (GAL4-DBD) for use in mammalian two-hybrid analysis. The repressive domains (RD) and interaction domains (ID) previously identified for NCoR and SMRT are indicated. (B and C) HepG2 cells were transiently transfected as indicated in Materials and Methods with a luciferase reporter plasmid containing five GAL4-responsive elements (5xGAL4-TAT-LUC), the CMV-β-Gal expression vector to control for transfection efficiency, and ΔN4 (B) or C′SMRT (C) with either an empty expression vector containing the VP16 activation domain (ΔN4), the PR-VP16 expression vector, or the TR-VP16 expression vector. After transfection, the cells were incubated with either no hormone (NH) or the designated ligands (100 nM but 10 nM for T3) for 24 h and subsequently harvested for luciferase and β-galactosidase assays. The data are presented as a normalized response, representing the absolute luciferase activity corrected for transfection efficiency by normalizing against the β-galactosidase activity. The data from a representative experiment are shown. Each data point represents the average of triplicate determinations (+ standard error of the mean).
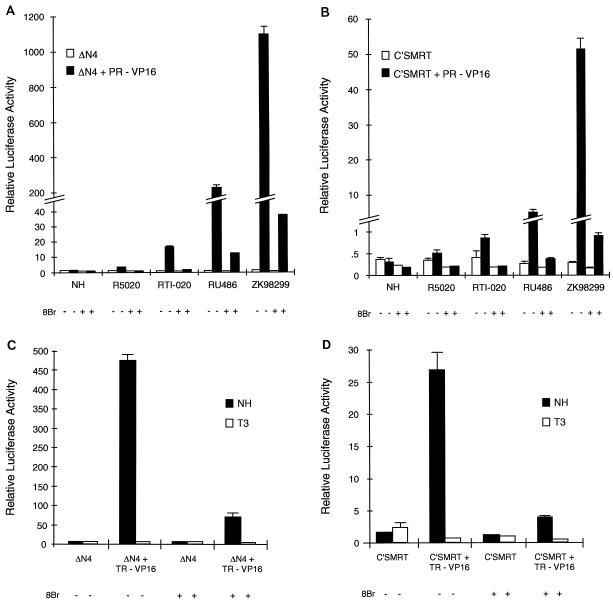
FIG. 6.
Mammalian two-hybrid analysis reveals that 8-bromo-cAMP reduces the interaction between PR and the corepressors. (A and B) HepG2 cells were transiently transfected as described previously with either ΔN4 (A) or C′SMRT (B) in the absence or presence of PR-VP16. (C and D) Cells were transfected with an expression vector for either ΔN4 (C) or C′SMRT (D) in the presence or absence of TR-VP16 as indicated. After transfection, the cells were incubated with either no hormone (NH) or the designated ligand (100 nM, but 10 nM for T3) in the presence (+) or absence (−) of 1 mM 8-bromo-cAMP for 48 h, and subsequently assayed for luciferase and β-galactosidase activities. The data from a representative experiment are shown.
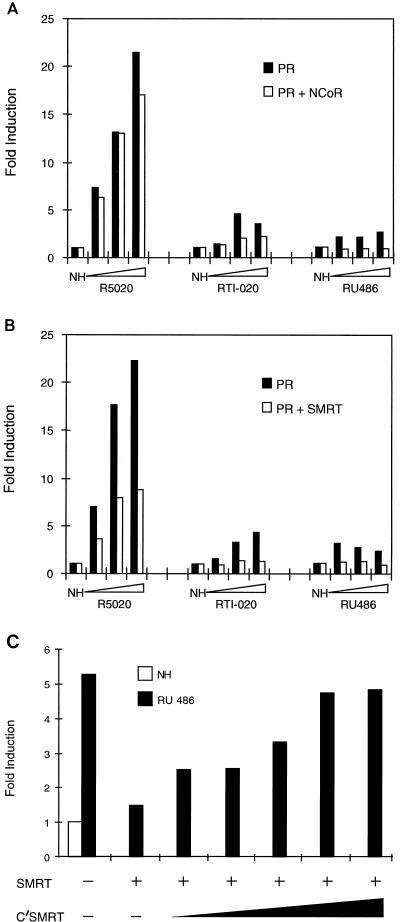
FIG. 3.
Overexpression of NCoR or SMRT decreases PR transcriptional activity in a ligand-dependent manner. (A and B) HeLa cells were transiently transfected as indicated in Materials and Methods with the luciferase reporter plasmid PRE-TK-LUC, a CMV-β-Gal expression vector (CMV-β-Gal) to control for transfection efficiency, and a PR expression vector either alone or with an expression vector for either NCoR (A) or SMRT (B). After transfection, the cells were incubated with either no hormone (NH) or designated ligand R5020 (10 pM to 1 nM), RTI-020 (10 pM to 1 nM), or RU486 (1 to 100 nM) for 48 h and subsequently harvested for luciferase and β-galactosidase assays. Luciferase data was normalized to the β-galactosidase activity and the data are represented as the fold induction over the response in the absence of hormone. The data presented are representative of multiple independent experiments. (C) HeLa cells were transfected with an expression vector for either PR alone, PR and SMRT, or PR with increasing amounts of C′SMRT expression vector as indicated. After transfection, the cells were incubated with either no hormone (NH) or 10−7 M RU486. The C′SMRT protein contains the major receptor-interacting domains but not the repression domains.
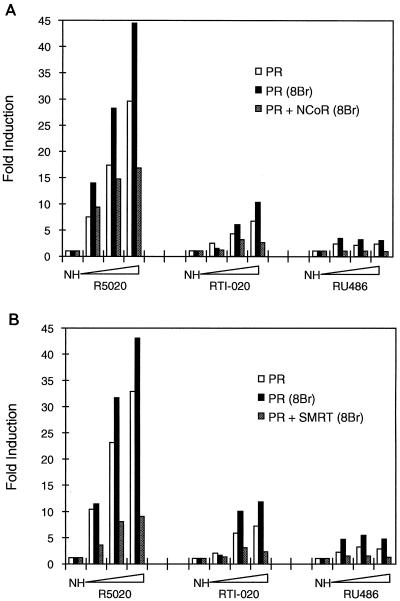
FIG. 5.
Corepressor overexpression prevents 8-bromo-cAMP-mediated potentiation of PR transcriptional activity. HeLa cells were transiently transfected as indicated previously with expression vectors for PR alone or for PR along with NCoR (A) or SMRT (B). After transfection, the cells were incubated in the absence or presence of 8-bromo-cAMP (8Br) (1 mM) with either no hormone (NH) or designated ligand R5020 (10 pM to 1 nM), RTI-020 (10 pM to 1 nM), or RU486 (1 to 100 nM) for 48 h and subsequently harvested for luciferase and β-galactosidase assays. Luciferase data were normalized to the β-galactosidase activity, and the data are represented as the fold induction over the response in the absence of hormone. The data presented are representative of multiple independent experiments.
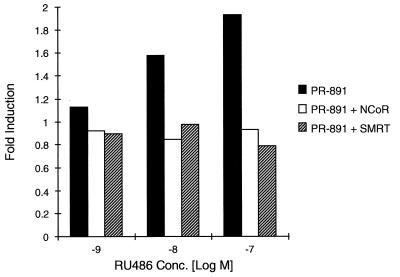
FIG. 4.
NCoR or SMRT overexpression prevents RU486 agonist activity on the PR-891 mutant. HeLa cells were transiently transfected with the PRE-TK-LUC reporter plasmid, the CMV-β-Gal expression vector, and the PR-891 expression plasmid either alone or with the NCoR or SMRT expression plasmid as described in Materials and Methods. After transfection, the cells were incubated as indicated with RU486 for 48 h and subsequently assayed for luciferase and β-galactosidase activities. The data from a representative experiment are shown.
In vitro interaction studies.
[35S]methionine-labeled PR was synthesized by using a coupled in vitro transcription and translation system in accordance with the manufacturer’s protocol (Promega, Madison, Wis.). The resultant labeled protein was incubated for 24 h at 4°C in the presence of glutathione S-transferase (GST)-Sepharose or GST-C′SMRT-Sepharose in NETN buffer (50 mM NaCl, 20 mM Tris [pH 8.0], 1 mM EDTA, 0.5% Nonidet P-40). Following incubation, the beads were washed in NETN buffer containing 100 mM NaCl, and bound proteins were eluted in sample buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The recombinant GST-C′SMRT used in this experiment was produced in Escherichia coli. Specifically, the E. coli strain BL21 was transformed with pGEX2TA-C-SMRT and grown to an A600 of 2.0, and IPTG (isopropyl-β-d-thiogalactopyranoside) was added. Following a 2-h incubation, the cells were harvested and lysed by sonication and incubated with glutathione-Sepharose beads (Pharmacia Biotech, Uppsala, Sweden) in phosphate-buffered saline containing 1% Triton X-100. The beads were subsequently washed and resuspended in phosphate-buffered saline and used for in vitro interaction studies.
Western immunoblot analysis.
Western immunoblot analysis was performed on nuclear extracts isolated from HeLa cells transiently transfected with 500 ng of SV40-PRB and either 500 ng of pCMX-NCoR or 288 ng of Rev-TUP1. Transiently transfected cells were incubated with the agonist R5020 (10 nM) for 24 h prior to nuclear extraction. Nuclear extracts were prepared as described by Schreiber et al. (33). Briefly, cells were resuspended in buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 5 μg of leupeptin per ml) and incubated on ice for 15 min. Subsequently, Nonidet P-40 was added to a final concentration of 0.6%, and the cells were vortexed and microcentrifuged. The supernatant was removed, and 50 μl of buffer C (20 mM HEPES [pH 7.9], 25% glycerol, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 5 μg of leupeptin per ml) was added to the nuclear pellet, which was then shaken vigorously for 5 min at 4°C. The nuclear extract was then centrifuged for 5 min, and the supernatant was saved. Equal amounts of total nuclear protein (21 μg) were denatured in sodium dodecyl sulfate sample buffer and loaded on a 7.5% polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane and probed with polyclonal rabbit antiserum generated against His-tagged hPR (A form). Immunocomplexes were detected by enhanced chemiluminescence as described in the manufacturer’s instructions (Amersham, Arlington Heights, Ill.).
RESULTS
The PR interacts with the corepressors NCoR and SMRT in a ligand-dependent manner.
Previously, we have shown that PR ligands can be grouped into one of three distinct classes: agonists, mixed agonists, and antagonists (41). Each ligand class induces a unique receptor conformation upon binding, and we hypothesized that these conformations ultimately influence the transcriptional activity of the receptor. One possible way in which receptor conformation may affect transcriptional activity is by allowing differential association with corepressors. We were therefore interested in assaying the ability of the corepressors NCoR and SMRT to associate with PR in the presence of the different classes of ligands. To test for an association between these proteins, we utilized a mammalian two-hybrid system. In this system, we used a construct expressing full-length PR containing the heterologous VP16 activation domain inserted into the amino terminus of the receptor and constructs expressing either the carboxyl terminus of NCoR (ΔN4) or the carboxyl terminus of SMRT (C′SMRT) fused to the GAL4 DNA binding domain (Fig. 1A). Interaction between PR and the corepressors was assessed by measuring the ability of the PR-VP16 fusion to activate transcription from a GAL4-responsive reporter plasmid. As a control, we also assayed the interaction between full-length TR-VP16 and these corepressors. The results of this analysis are shown in Fig. 1B and C. As expected, TR interacts with both ΔN4 and C′SMRT in the absence of hormone, and this interaction is inhibited upon the addition of T3 (Fig. 1B and C). Conversely, NCoR and SMRT do not interact with PR in the absence of hormone under these conditions. In the presence of the agonist R5020, however, PR interacts weakly with both ΔN4 (Fig. 1B) and C′SMRT (Fig. 1C). A more robust interaction is observed between PR and ΔN4 or C′SMRT in the presence of the mixed agonist RTI-020, as shown by the 3.4-fold (Fig. 1B) and 2.3-fold (Fig. 1C) inductions over the luciferase activities in the absence of PR-VP16, respectively. The strongest interaction between PR and the corepressors, however, occurs in the presence of PR antagonists. Specifically, RU486-activated PR-VP16 permits a 31-fold induction of transcription when assayed with ΔN4 and a 23-fold induction when C′SMRT is used. Most notable, however, are the interactions observed between PR-VP16 and the corepressors in the presence of ZK98299, where a 70-fold induction in the presence of ΔN4 (Fig. 1B) and 131-fold induction in the presence of C′SMRT (Fig. 1C) are observed. Interestingly, these interactions, in the presence of ZK98299, are stronger than the control interactions of ΔN4 and C′SMRT with TR-VP16 in the absence of hormone. These results indicate that, in the presence of antagonists, PR interacts with NCoR and SMRT and that under the conditions of this assay, these interactions are as strong as the interactions between the corepressors and TR. Cumulatively, these results suggest that the ability of PR to interact with the corepressors correlates with the transcriptional activity of the receptor such that ligands which facilitate the strongest interaction of PR with NCoR or SMRT are those which exhibit the greatest antagonist activity when assayed in a conventional PR transcription assay.
The mammalian two-hybrid data suggested two alternative possibilities: (i) in the presence of antagonists, PR had a higher affinity for the corepressors than receptors occupied by agonists, or (ii) the affinity of the corepressors for PR was unaffected by the nature of the bound ligand, but in the presence of agonists, coactivators were recruited to the receptor, an event which prevented corepressor binding. To distinguish between these possibilities, we examined the ability of PR to interact with the SMRT in vitro, where direct associations could be determined. The results of this analysis are shown in Fig. 2. In this experiment, the ability of 35S-labeled PR to interact with either bacterially expressed GST alone or a GST-C′SMRT fusion protein was assessed. Interestingly, a specific, robust interaction between PR and C′SMRT was observed; however, the magnitude of this interaction was unaffected by the nature of the bound ligand. The reciprocal experiment, in which the ability of 35S-labeled C′SMRT to interact with a GST-PR fusion protein was assessed, yielded similar results (data not shown). These data indicate that SMRT can interact directly with PR, an activity which, under the conditions of this assay, is unaffected by hormone. This is in agreement with previous studies that have shown that ER can interact directly with C′SMRT in a ligand-independent manner (34). Thus, it appears that the ability of corepressors to preferentially interact with antagonist-activated PR may be related to the inability of this complex to recruit coactivators rather than to the possession of an inherently higher affinity for NCoR and SMRT.
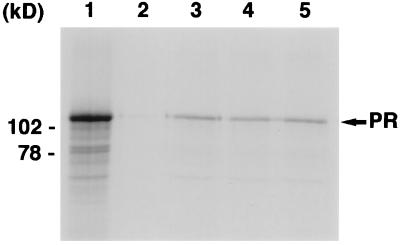
FIG. 2.
In vitro binding analysis reveals that the interaction of PR with SMRT occurs in a ligand-independent manner. Recombinant GST-C′SMRT or GST alone was produced in bacteria and immobilized on gluthathione-Sepharose beads. Full-length PR was produced by in vitro translation and labeled with [35S]methionine. The integrity of this protein was assessed by running an aliquot on a denaturing gel (lane 1, 10% of input labeled receptor). Labeled PR was then incubated either with equimolar amounts of GST alone (lane 2) or with GST-C′SMRT either in the absence of added ligand (lane 3), in the presence of progesterone (lane 4), or in the presence of RU486 (lane 5).
Overexpression of NCoR or SMRT decreases PR mixed-agonist activity and RU486 partial-agonist activity.
NCoR and SMRT were originally identified based upon their abilities to associate with unliganded TR (17) and retinoic X receptor (RXR) (9), respectively. Unlike the steroid receptors, TR and RXR can bind to DNA in the absence of hormone and repress transcription below basal levels (3, 4, 8, 12). Overexpression of NCoR and SMRT was subsequently shown to further increase the repressive activity of TR in the absence of hormone (37, 44). Based on these results, and the ability of NCoR and SMRT to associate with PR, we hypothesized that overexpression of the corepressors would decrease the transcriptional activity of PR and that the greatest effects would be observed on antagonist-bound PR. In order to analyze the effect of corepressor overexpression on PR activity, HeLa cells were transiently transfected with a luciferase reporter plasmid containing PRE inserted into the thymidine kinase (TK) promoter (PRE-TK-LUC), and transcriptional activity was assessed following cotransfection of a PR expression plasmid alone or together with an expression plasmid for either NCoR or SMRT. Surprisingly, we found that overexpression of either NCoR or SMRT increased PR transcriptional activity in the absence of hormone and at low concentrations of the agonist R5020 (data not shown). Similar results have also been observed by other laboratories (21, 37). Western analysis of nuclear extracts from transiently transfected HeLa cells shows increased levels of PR upon cotransfection of NCoR as compared to the levels of receptor when PR is transfected alone (data not shown). This increase in receptor level was ligand independent, and similar results were obtained when PR and SMRT expression plasmids were cotransfected (data not shown). Additionally, it has previously been reported that NCoR overexpression results in increased basal activity from the 3xDR1-TKLUC reporter vector (37). We believe, therefore, that the paradoxical increase in PR transcriptional activity upon the cotransfection of either corepressor is due to increased receptor levels and/or increased basal activity from the PRE-TK-LUC reporter. Thus, to account for these confounding influences, we elected to present the data as the fold induction over the response for the no-hormone control. In this manner, we were able to show that overexpression of NCoR has no effect on PR at low concentrations of the agonist R5020; however, it decreases the activity of agonist-bound receptor by 21% at the highest concentration of ligand tested (Fig. 3A). Exogenously expressed SMRT (Fig. 3B) has a stronger affect on agonist-bound PR and is able to decrease the overall activity of R5020-bound PR (PR/R5020) by approximately 54%. In the presence of the mixed agonist RTI-020, which functions as a weak agonist in this cell line, exogenous NCoR decreases receptor activity by 49% and SMRT overexpression decreases PR/RTI-020 activity to basal levels.
It has previously been reported that RU486 exhibits partial-agonist activity in HeLa cells when assayed on the TK promoter containing a single PRE (25). We observed similar results in our system and consequently wanted to determine if the strong association between antagonist-bound PR and the corepressors would decrease the RU486 partial-agonist activity. Interestingly, the partial-agonist activity of RU486 is totally suppressed by overexpression of either NCoR or SMRT (Fig. 3A and B). Thus, in addition to the heterodimeric nuclear receptors, NCoR and SMRT can function as corepressors of PR transcriptional activity. In addition, it is likely that the same activity of the repressor proteins is required for function on both classes of receptors since we have been able to show that the repressive domains required for regulation of TR are also required for PR regulation. This was demonstrated by showing that a construct containing the receptor interaction domains (C′SMRT) but lacking the repressive domains could function as a dominant negative suppressor of SMRT activity when assayed on RU486-activated PR (Fig. 3C). These data also suggest that the interaction between PR and the corepressors is direct. Collectively, these data suggest that NCoR and SMRT function as PR corepressors and that the degree to which exogenous expression of either corepressor decreases PR-ligand complex transcriptional activity is relative to the degree of association between the PR-ligand and corepressors as measured by two-hybrid analysis (Fig. 1B and C).
NCoR and SMRT repress the agonist activity of RU486 on the carboxyl terminus-truncated receptor PR-891.
It has previously been shown that truncation of the most carboxyl 44 amino acids of PR allows the antagonist RU486 to function as an agonist (39). In addition, it has been suggested that the PR carboxyl terminus functions as a repressive domain in the presence of antagonists by recruiting a corepressor (43). We were therefore interested in addressing the possibility that NCoR or SMRT may be involved in regulating the activity of this functional domain. To address this possibility, HeLa cells were transiently transfected with the PRE-TK-LUC reporter and expression vectors for either the truncated receptor alone or the truncated receptor together with either NCoR or SMRT. As expected, PR-891 shows increased transcriptional activity in response to increasing concentrations of RU486 (Fig. 4). However, upon cotransfection of either NCoR or SMRT, this response to RU486 is completely prevented. These data suggest that another protein, distinct from NCoR and SMRT, is responsible for the C-terminal repressor function of the PR C-terminal tail (39, 43).
Corepressor overexpression prevents 8-bromo-cAMP-mediated potentiation of PR transcriptional activity.
It has previously been shown that the protein kinase A activator 8-bromo-cAMP can convert class II antagonists, like RU486, into partial agonists (6, 32). Additionally, these studies have also shown that addition of 8-bromo-cAMP can potentiate the activity of agonist-bound PR. Since corepressor overexpression is able to prevent the partial-agonist activity of RU486 in HeLa cells (Fig. 3A and B), we were interested in determining if exogenous NCoR or SMRT could also repress the RU486 partial-agonist activity induced by 8-bromo-cAMP. Therefore, we transiently transfected HeLa cells with the PRE-TK-LUC reporter plasmid and expression vectors for either the receptor alone or the receptor in combination with either NCoR or SMRT and assayed the transcriptional activity of the receptor in the presence or absence of 8-bromo-cAMP. As anticipated, 8-bromo-cAMP potentiates the activity of R5020-bound PR and increases the partial-agonist activity of RU486 (Fig. 5). Surprisingly, the 8-bromo-cAMP-mediated potentiation of the transcriptional activity of agonist-bound receptor is completely suppressed by the coexpression of NCoR (Fig. 5A). Similarly, exogenous SMRT prevents the 8-bromo-cAMP-mediated potentiation of R5020 transcriptional activity (Fig. 5B). The addition of 8-bromo-cAMP also potentiates the activity of the PR mixed agonist RTI-020. As with the agonist-bound receptor, this potentiation is blocked by the cotransfection of either corepressor (Fig. 5). The partial-agonist activity of RU486 in the presence and absence of 8-bromo-cAMP is completely prevented by overexpression of NCoR (Fig. 5A) and SMRT (Fig. 5B). From these results, we conclude that overexpression of either corepressor can inhibit both the 8-bromo-cAMP-induced partial-agonist activity of RU486 and the 8-bromo-cAMP-mediated potentiation of the transcriptional activity of agonist- and mixed-agonist-bound PR.
8-bromo-cAMP reduces the association between the corepressors and PR.
The ability of exogenous NCoR or SMRT to prevent 8-bromo-cAMP-mediated potentiation of the transcriptional activity of PR bound by any ligand suggests that events regulated by 8-bromo-cAMP may lead to a decrease in the association between PR and the corepressors. To test this hypothesis, we analyzed the interaction between PR and the corepressors in the presence of 8-bromo-cAMP by using the mammalian two-hybrid system. The results shown in Fig. 6 indicate that PR-VP16 does not interact with either ΔN4 or C′SMRT in the absence of hormone (Fig. 6). The weak association between PR and ΔN4 (Fig. 6A) or C′SMRT (Fig. 6B) in the presence of the agonist R5020 is completely inhibited in the presence of 8-bromo-cAMP. As expected, PR, when bound by the mixed agonist RTI-020, shows a stronger interaction with ΔN4 and C′SMRT than when it is bound by the agonist R5020. However, as in the presence of the agonist, 8-bromo-cAMP also prevents the association between C′SMRT (Fig. 6B) and PR in the presence of the mixed agonist RTI-020 and decreases the association between ΔN4 and RTI-020-bound PR by 90% (Fig. 6A). Upon the addition of 8-bromo-cAMP, which turns RU486 into a partial agonist, the interactions between PR and C′SMRT and between PR and ΔN4 are similarly decreased, by 90 and 93%, respectively. While 8-bromo-cAMP also reduces the interaction between PR and the corepressors in the presence of the class I antagonist ZK98299, PR and ΔN4 still maintain a relatively strong interaction that is threefold stronger than the interaction between PR/RU486 and ΔN4 in the presence of 8-bromo-cAMP (Fig. 6A).
To see if the 8-bromo-cAMP reduction in the interaction between the corepressors and PR is restricted to PR, we assayed the effect of 8-bromo-cAMP on the interaction between the TR and NCoR or SMRT. Interestingly, 8-bromo-cAMP also reduces the interaction between TR and the corepressors; however, the residual interaction is still stronger than the interaction between TR and the corepressors in the presence of its cognate ligand, T3 (Fig. 6C and D). As assayed by the mammalian two-hybrid analysis, the ability of 8-bromo-cAMP to reduce the interaction of NCoR and SMRT with the nuclear receptors is not a general effect of 8-bromo-cAMP, since its addition is unable to disrupt the association between chimeric proteins of p53 fused to the GAL4 DNA binding domain and the simian virus 40 large T antigen fused to the VP16-activation domain (data not shown). In addition, the transcriptional activity of a GAL4-VP16 fusion protein was unaffected by 8-bromo-cAMP, ruling out the possibility that 8-bromo-cAMP disrupts VP16 transcriptional activity (data not shown). These data show, therefore, that 8-bromo-cAMP specifically decreases the association between the corepressors and the nuclear receptors, a result which may explain its ability to alter PR pharmacology.
DISCUSSION
The classical theory of steroid receptor activation proposes that the receptor exists in either one of two states, an active transcriptional state induced upon binding hormone or a latent state in the absence of hormone (23). With the discovery of antagonists, it was proposed that these compounds functioned by competing with the hormone for receptor binding and returned the receptor to the latent state. However, this model has not stood the test of time, and it is now apparent that antagonists actively convert the receptor to a transcriptionally inactive state. This hypothesis has been solidified by the observation that PR agonists and antagonists drive the receptor into unique conformational states, each of which is distinct from the conformational state of the apo-receptor (1). We have recently identified a third class of PR ligands known as mixed agonists. The discovery of this new clan of ligands suggests an additional level of complexity in the pharmacology of steroid hormone receptors (41). Depending on the cell and promoter context, these ligands can function as either agonists or antagonists. The molecular basis for the activity of these ligands was revealed when it was shown that mixed agonists induce a unique receptor conformation upon binding that is different from those conformations induced by either agonists or antagonists (41). Identification of the PR mixed agonists suggests that like the ER (24), PR can exist in different conformational states, each of which exhibits a different degree of transcriptional activity. It has been hypothesized that these ligand-induced conformational changes in the receptor regulate the interaction of the receptor with coactivators (31, 42). We hypothesized that in addition to differential coactivator association, conformational changes could also influence corepressor association. As with coactivator binding, interactions with corepressors would also be expected to alter the transcriptional activity of the receptor. We were interested, therefore, in analyzing the ability of the corepressors NCoR and SMRT to associate with PR and to determine if this interaction was affected by the nature of ligand bound to the receptor. We found that NCoR and SMRT do interact with liganded PR directly in vitro. However, when we analyzed these interactions by using the two-hybrid assay in mammalian cells, we observed that they were differentially affected by the class of ligand bound. Agonists permit a minimal interaction with the corepressor, while antagonists allow the strongest association. Mixed agonists, which function as weak agonists or antagonists, depending on the cell and promoter context (41), induce an interaction of intermediate strength. These results suggest that the transcriptional activity of PR-ligand complexes correlates with the ability of these complexes to associate with the corepressors NCoR and SMRT. The discovery that NCoR and SMRT associate most strongly with antagonist-bound PR raises the issue of the physiological relevance of these interactions. A remote but possible explanation is that there exists in some target cell a naturally occurring PR antagonist whose activity is mimicked by synthetic antagonists and that this requires a corepressor for activity. A more likely explanation, however, is that PR inherently has a high affinity for SMRT or NCoR in the absence or presence of hormone and that the conformational change induced by agonists increases the affinity of the receptors for coactivators, an event which is incompatible with PR-corepressor interactions. Regardless of the physiological relevance of the interaction between PR and the corepressors, however, there is clearly a pharmacological relevance. In this regard, one of the most interesting findings was the observation that NCoR and SMRT differentially associate with PR bound to the class I (ZK98299) and class II (RU486) antagonists. ZK98299 induces a stronger association of PR with the corepressors than RU486 does. The original subclassification of these PR antagonists was based on the observation that unlike class II antagonists, class I antagonists, such as ZK98299, do not allow PR to bind DNA (22). Although this is a biochemical classification, the distinct nature of these ligands is also reflected in vivo. Specifically, it has been noted that under some circumstances, type II but not type I antagonists can exhibit partial-agonist activity. Consequently, it was hypothesized that the pure-antagonist activity of ZK98299 is due to its inability to allow PR to bind DNA (6, 32). However, our unpublished results, and those of other laboratories (13), suggest that PR/ZK98299 does in fact bind DNA. We propose, therefore, that the ability of ZK98299 to function as a pure antagonist is due to its inability to recruit required coactivator proteins. Thus, by default, a strong association of PR with the corepressors NCoR and SMRT is permitted. Interestingly, it was recently demonstrated that ZK98299 induces a unique receptor conformation which is different from the conformation induced by class II antagonists (2). This finding further supports our hypothesis that different ligand-induced conformational changes within the receptor influence its association with cellular corepressors.
The ability of the corepressors to differentially interact with PR depending on the class of ligand occupying the receptor may explain not only how different classes of ligands exhibit different transcriptional activities but also how these ligands manifest different activities in different cellular contexts. The ability of mixed agonists to function as weak agonists in some contexts and antagonists in others has been hypothesized to result from differential coactivator availability (34). While coactivator expression and availability undoubtedly play a role in receptor transcriptional activity, we propose that corepressor availability also affects receptor activity. In support of this theory, we have shown that overexpression of either NCoR or SMRT results in a decrease in receptor activity. Not surprisingly, therefore, the greatest effect of corepressor overexpression is observed on antagonist-bound receptor, as shown by the ability of NCoR or SMRT overexpression to suppress all RU486 partial-agonist activity. This suggests that RU486 exhibits partial-agonist activity in those contexts in which corepressor availability has somehow been diminished. Interestingly, SMRT overexpression can also completely suppress the weak-agonist activity of the mixed agonist RTI-020. This suggests that mixed agonists function as weak agonists in those contexts in which corepressors are not available at a sufficient level to associate with the receptor, thus facilitating an increase in transcriptional activity.
Interaction of the receptor with the corepressors may be influenced not only by the availability of the corepressors but also by mutations within the receptor. Previously, it was shown that a mutation which results in truncation of PR by 42 amino acids at the carboxyl terminus results in a receptor which can no longer bind progesterone and allows RU486 to function as an agonist (39). We have further shown that both mixed agonists and antagonists function as agonists on this truncated receptor (41). Microinjection of peptides encoding the 42 amino acids of the carboxyl terminus allows RU486 to function as an agonist on full-length PR, suggesting that the carboxyl terminus functions as a repressive domain in the presence of antagonists by recruiting a corepressor(s) (43). Overexpression of either NCoR or SMRT inhibits the ability of RU486 to function as an agonist on the truncated receptor PR-891, suggesting possibly that truncation of the receptor has decreased the association between PR and the corepressors, resulting in increased transcriptional activity. Therefore, mutations within the receptor may alter the degree of interaction with corepressors. While NCoR and SMRT may not prove to be the carboxyl-terminal repressor(s) responsible for RU486 antagonist activity, these results clearly show that the relative expression of either corepressor can directly affect the transcriptional activity of the receptor.
PR association with the corepressors is also influenced by other signaling pathways. Previously, several laboratories have observed that 8-bromo-cAMP converts RU486 into a partial agonist and potentiates the activity of agonist-bound receptor (6, 32). Although at the beginning of this project we had not intended to dissect the mechanism by which 8-bromo-cAMP modulates PR transcriptional activity, the results obtained were informative in this regard. In the course of analyzing the ability of NCoR and SMRT overexpression to repress RU486 partial-agonist activity in the presence of 8-bromo-cAMP, we found that corepressor overexpression prevented not only 8-bromo-cAMP-induced RU486 partial-agonist activity but also the potentiation of PR/R5020 and PR/RTI-020 transcriptional activities. Significantly, however, further analysis revealed that 8-bromo-cAMP drastically reduces the association of PR with NCoR and SMRT. We hypothesize, therefore, that 8-bromo-cAMP potentiation of PR transcriptional activity is due to a loss of association with NCoR and SMRT.
Interestingly, PR is not the only receptor which has been shown to be affected by the protein kinase A (PKA) pathway. The transcriptional activities of ER glucocorticoid receptor (GR) (27, 30, 45) and retinoic acid receptor (RAR) (18) have also been shown to be stimulated by the PKA pathway. It has recently been shown by other laboratories that ER (34), GR (21), and RAR (9, 17) also interact with either NCoR or SMRT, suggesting that PKA potentiation of the transcriptional activity of these nuclear receptors may also result from a loss of its abilities to associate with NCoR and SMRT. These results could have important clinical implications. One case in point is the treatment of ER-positive breast cancers with the ER mixed agonist 4-hydroxy tamoxifen (tamoxifen). Unfortunately, for reasons not yet identified, the majority of women fail tamoxifen treatment within 5 years, at which time tamoxifen may begin to function as an agonist in the breast. It has been shown in some contexts in the MCF-7 breast cancer cell line that activation of the PKA pathway can result in the conversion of tamoxifen from an antagonist to an agonist (16, 19). It has also been shown that cAMP levels are higher in breast cancer tissue than in normal breast tissue (11, 26). Thus, resistance to the antagonist activities of tamoxifen could arise from cellular PKA-mediated changes in ER-corepressor interactions. Interestingly, the PR class I antagonist ZK98299 is unaffected by the PKA pathway, an activity which we believe is related to its ability to drive the receptor into a conformation which is incompatible with coactivator association. Similarly, ER bound by the pure antagonist ICI 164,384 is also unaffected by the PKA pathway (16). It will therefore be interesting to see how much of the pure-antagonist activity of ICI 164,384 is related to its abilities to interact with corepressors. These results suggest that the analysis of the interaction of NCoR and SMRT with ER may prove helpful in understanding the molecular basis for tamoxifen resistance.
Based on our data, we propose several working models (Fig. 7) that may explain how 8-bromo-cAMP decreases the association between PR and a corepressor and how these interactions influence PR pharmacology. It is unlikely that a direct effect of phosphorylation on PR is involved in regulating corepressor association since none of the known phosphorylation sites within PR are affected by 8-bromo-cAMP (42a). Thus, in developing these models, we have not considered a direct effect of PKA-mediated phosphorylation on PR. We believe instead that the available data could be explained by a series of three related models, none of which are mutually exclusive. In the first model, 8-bromo-cAMP addition leads to corepressor phosphorylation, which in turn causes the corepressor to dissociate from the receptor. In this model, potentiation of transcriptional activity would result from the loss of the repressive activity of the corepressor. In the second model, a third protein, such as a coactivator (X), is the target of the phosphorylation. This protein subsequently associates with the receptor and blocks corepressor association either through direct competition or by way of a conformational change in the receptor induced upon binding this additional protein (X). This model suggests that 8-bromo-cAMP potentiation can result not only as a consequence of corepressor dissociation but also from the association of PR with a new coactivator. This model is supported by the observation that a direct, ligand-independent, interaction of PR and C′SMRT was observed in vitro. In the third model, phosphorylation of an unidentified protein (Y) facilitates the dissociation of the corepressor from the receptor. Again, loss of association with the corepressor would result in an increase in PR transcriptional activity. We are currently in the process of investigating which of these models best explains 8-bromo-cAMP potentiation of PR transcriptional activity.
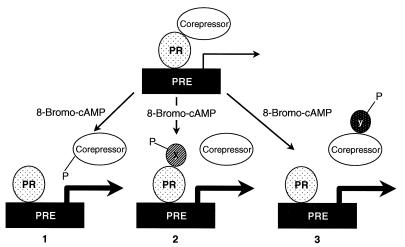
FIG. 7.
Possible mechanisms by which 8-bromo-cAMP decreases PR-corepressor association. The association of PR with both NCoR and SMRT is decreased upon the addition of 8-bromo-cAMP. This decreased association between PR and the corepressors is accompanied by an increase in receptor transcriptional activity. We propose three possible models by which 8-bromo-cAMP reduces the association between these proteins. In model 1, 8-bromo-cAMP activates a pathway which leads to the phosphorylation of the corepressor, causing it to dissociate. Simple loss of the corepressor would result in an increase in transcriptional activity. In model 2, phosphorylation of an unidentified factor X would result in association of that factor with PR, causing the dissociation of the corepressor. In this model, the increased transcriptional activity may result from both the loss of association of PR with the corepressor and the association of PR with factor X. In model 3, factor Y becomes phosphorylated by an 8-bromo-cAMP-induced signaling pathway. The corepressor has a higher affinity for the phosphorylated factor Y than for PR and is therefore titrated away from the receptor.
ACKNOWLEDGMENTS
We thank J. D. Chen, R. M. Evans, D. D. Moore, M. G. Rosenfeld, X. F. Wang, and D. X. Wen for providing plasmids. We also thank Z. Nawaz for providing reagents and assistance with the in vitro interaction studies and for sharing unpublished data.
B. L. Wagner is supported by an Advanced Predoctoral Fellowship from the PhRMA Foundation. This work was supported by NIH grant DK50494 (to D.P.M.).
REFERENCES
- 1.Allan G F, Leng X, Tsai S-T, Weigel N L, Edwards D P, Tsai M-J, O’Malley B W. Hormone and antihormone induce distinct conformational changes which are central to steroid receptor activation. J Biol Chem. 1992;267:19513–19520. [PubMed] [Google Scholar]
- 2.Allan G F, Lombardi E, Haynes-Johnson D, Palmer S, Kiddoe M, Kraft P, Campen C, Rybczynski P, Combs D W, Phillips A. Induction of a novel conformation in the progesterone receptor by ZK299 involves a defined region of the carboxyl-terminal tail. Mol Endocrinol. 1996;10:1206–1213. doi: 10.1210/mend.10.10.9121488. [DOI] [PubMed] [Google Scholar]
- 3.Baniahmad A, Köhne A C, Renkawitz R. A transferable silencing domain is present in the thyroid hormone receptor, in the v-erbA oncogene product and in the retinoic acid receptor. EMBO J. 1992;11:1015–1023. doi: 10.1002/j.1460-2075.1992.tb05140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baniahmad A, Steiner C, Kohne A C, Renkawitz R. Molecular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. EMBO J. 1990;61:505–514. doi: 10.1016/0092-8674(90)90532-j. [DOI] [PubMed] [Google Scholar]
- 5.Beck C A, Weigel N L, Edwards D P. Effects of hormone and cellular modulators of protein phosphorylation on transcriptional activity, DNA binding, and phosphorylation of human progesterone receptors. Mol Endocrinol. 1992;6:607–620. doi: 10.1210/mend.6.4.1316549. [DOI] [PubMed] [Google Scholar]
- 6.Beck C A, Weigel N L, Moyer M L, Nordeen S K, Edwards D P. The progesterone antagonist RU486 acquires agonist activity upon stimulation of cAMP signaling pathways. Proc Natl Acad Sci USA. 1993;90:4441–4445. doi: 10.1073/pnas.90.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry M, Metzgar D, Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990;9:2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brent G A, Dunn M K, Harney J W, Gulick T, Larsen P R, Moore D D. Thyroid hormone aporeceptor represses T3-inducible promoters and blocks activity of the retinoic acid receptor. New Biol. 1989;1:329–336. [PubMed] [Google Scholar]
- 9.Chen J D, Evans R M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 10.Chen J D, Umesono K, Evans R M. SMRT isoforms mediate repression and anti-repression of nuclear receptor heterodimers. Proc Natl Acad Sci USA. 1996;93:7567–7571. doi: 10.1073/pnas.93.15.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen L A, Chan P. Intracellular cAMP levels in normal rat mammary gland and adenocarcinoma: in vivo vs. in vitro. Life Sci. 1974;16:107–115. doi: 10.1016/0024-3205(75)90213-1. [DOI] [PubMed] [Google Scholar]
- 12.Damm K, Thompson C C, Evans R M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989;339:593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- 13.Delabre K, Guiochon-Mantel A, Milgrom E. In vivo evidence against the existence of antiprogestins disrupting receptor binding to DNA. Proc Natl Acad Sci USA. 1993;90:4421–4425. doi: 10.1073/pnas.90.10.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denner L A, Weigel N L, Maxwell B L, Schrader W T, O’Malley B W. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990;250:1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- 15.Evans R M. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimoto N, Katzenellenbogen B S. Alteration in the agonist/antagonist balance of antiestrogens by activation of protein kinase A signaling pathways in breast cancer cells: anitestrogen selectivity and promoter dependence. Mol Endocrinol. 1994;8:296–304. doi: 10.1210/mend.8.3.7517003. [DOI] [PubMed] [Google Scholar]
- 17.Horlein A J, Naar A M, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass C K, Rosenfeld M G. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–403. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 18.Huggenvik J I, Collard M W, Kim Y-W, Sharma R P. Modification of the retinoic acid signaling pathway by the catalytic subunit of protein kinase-a. Mol Endocrinol. 1993;7:543–550. doi: 10.1210/mend.7.4.8388997. [DOI] [PubMed] [Google Scholar]
- 19.Ince B A, Montano M M, Katzenellenbogen B S. Activation of transcriptionally inactive human estrogen receptors by cyclic adenosine 3′,5′-monophosphate and ligands including antiestrogens. Mol Endocrinol. 1994;8:1397–1406. doi: 10.1210/mend.8.10.7531820. [DOI] [PubMed] [Google Scholar]
- 20.Ing N, Beekman J, Tsai S, Tsai M-J, O’Malley B. Members of the steroid hormone receptor superfamily interact with TFIIB (S300-II) J Biol Chem. 1992;267:17617–17623. [PubMed] [Google Scholar]
- 21.Jackson T A, Richer J K, Bain D L, Takimoto G S, Tung L, Horwitz K B. The partial agonist activity of antagonist-occupied steroid receptors is controlled by a novel hinge domain-binding coactivator L7/SPA and the corepressors N-CoR and SMRT. Mol Endocrinol. 1997;11:693–705. doi: 10.1210/mend.11.6.0004. [DOI] [PubMed] [Google Scholar]
- 22.Klein-Hitpass L, Cato A C B, Henderson D, Ryffel G U. Two types of antiprogestins identified by their differential activation in transcriptionally active extracts from T47D cells. Nucleic Acids Res. 1991;19:1227–1234. doi: 10.1093/nar/19.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonnell D P. Unraveling the human progesterone receptor signal transduction pathway: insights into antiprogestin action. Trends Endocrinol Metab. 1995;6:133–138. doi: 10.1016/1043-2760(95)00065-p. [DOI] [PubMed] [Google Scholar]
- 24.McDonnell D P, Clemm D L, Herman T, Goldman M E, Pike J W. Analysis of estrogen receptor function in vitro reveals three distinct classes of antiestrogens. Mol Endocrinol. 1995;9:659–669. doi: 10.1210/mend.9.6.8592512. [DOI] [PubMed] [Google Scholar]
- 25.Meyer M E, Pornon A, Ji J, Bocquel M T, Chambon P, Gronemeyer H. Agonist and antagonist properties of RU486 on the functions of the human progesterone receptor. EMBO J. 1990;9:3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minton J P, Wisenbaugh T, Matthews R. Elevated cyclic AMP levels in human breast-cancer. J Natl Cancer Inst. 1974;53:283. doi: 10.1093/jnci/53.1.283. [DOI] [PubMed] [Google Scholar]
- 27.Nordeen S, Bona B, Moyer M. Latent agonist activity of the steroid antagonist, RU486, is unmasked in cells treated with activators of protein kinase A. Mol Endocrinol. 1993;7:731–742. doi: 10.1210/mend.7.6.8395651. [DOI] [PubMed] [Google Scholar]
- 28.Norris J, Fan D, Aleman C, Marks J R, Futreal A, Wiseman R W, Iglehart J D, Deininger P L, McDonnell D P. Identification of a new subclass of alu DNA repeats which can function as estrogen receptor-dependent transcriptional enhancers. J Biol Chem. 1995;270:22777–22782. doi: 10.1074/jbc.270.39.22777. [DOI] [PubMed] [Google Scholar]
- 29.Onate S A, Tsai S, Tsai M-J, O’Malley B W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 30.Rangarajan P N, Umesono K, Evans R M. Modulation of glucocorticoid receptor function by protein kinase A. Mol Endocrinol. 1992;6:1451–1457. doi: 10.1210/mend.6.9.1435789. [DOI] [PubMed] [Google Scholar]
- 31.Renaud J-P, Rochel N, Ruff M, Vivat V, Chambon P, Gronemeyer H, Moras D. Crystal structure of the RAR-gamma ligand-binding domain bound to all-trans retinoic acid. Nature. 1995;378:681–689. doi: 10.1038/378681a0. [DOI] [PubMed] [Google Scholar]
- 32.Sartorius C A, Tung L, Takimoto G S, Horwitz K B. Antagonist-occupied human progesterone receptors bound to DNA are functionally switched to transcriptional agonists by cAMP. J Biol Chem. 1993;268:9262–9266. [PubMed] [Google Scholar]
- 33.Schreiber E, Matthias P, Muller M M, Schaffner W. Rapid detection of octamer binding proteins with ’mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith C L, Nawaz Z, O’Malley B W. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997;11:657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- 35.Smith C L, Onate S A, Tsai M-J, O’Malley B W. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc Natl Acad Sci USA. 1996;93:8884–8888. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith D F, Stensgard B A, Welch W J, Toft D O. Assembly of progesterone receptor with heat shock proteins and receptor activation are ATP mediated events. J Biol Chem. 1992;267:1350–1356. [PubMed] [Google Scholar]
- 37.Soderstrom M, Vo A, Heinzel T, Lavinsky R M, Yang W-M, Seto E, Peterson D A, Rosenfeld M G, Glass C K. Differential effects of nuclear receptor corepressor (N-CoR) expression levels on retinoic acid receptor-mediated repression support the existence of dynamically regulated corepressor complexes. Mol Endocrinol. 1997;11:682–692. doi: 10.1210/mend.11.6.0018. [DOI] [PubMed] [Google Scholar]
- 38.Tzukerman M T, Esty A, Santiso-Mere D, Danielian P, Parker M G, Stein R B, Pike J W, McDonnell D P. Human estrogen receptor transcriptional capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol Endocrinol. 1994;8:21–30. doi: 10.1210/mend.8.1.8152428. [DOI] [PubMed] [Google Scholar]
- 39.Vegeto E, Allan G F, Schrader W T, Tsai M-J, McDonnell D P, O’Malley B W. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell. 1992;69:703–713. doi: 10.1016/0092-8674(92)90234-4. [DOI] [PubMed] [Google Scholar]
- 40.Voegel J J, Heine M J S, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner B L, Pollio G, Leonhardt S, Wani M C, Lee D Y-W, Imhof M O, Edwards D P, Cook C E, McDonnell D P. 16α-substituted anologs of the antiprogestin RU486 induce a unique conformation in the human progesterone receptor resulting in mixed agonist activity. Proc Natl Acad Sci USA. 1996;93:8739–8744. doi: 10.1073/pnas.93.16.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner R L, Apriletti J W, McGrath M E, West B L, Baxter J D, Fletterick R J. A structural role for hormone in the thyroid hormone receptor. Nature. 1995;378:690–697. doi: 10.1038/378690a0. [DOI] [PubMed] [Google Scholar]
- 42a.Weigel, N. L. Unpublished data.
- 43.Xu J, Nawaz Z, Tsai S Y, Tsai M-J, O’Malley B W. The extreme C terminus of progesterone receptor contains a transcriptional repressor domain that functions through a putative corepressor. Proc Natl Acad Sci USA. 1996;93:12195–12199. doi: 10.1073/pnas.93.22.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zamir I, Zhang J, Lazar M A. Stoichiometric and steric principles governing repression by nuclear hormone receptors. Genes Dev. 1997;11:835–846. doi: 10.1101/gad.11.7.835. [DOI] [PubMed] [Google Scholar]
- 45.Zhang S, Danielson M. Selective effects of 8-br-cAMP on agonists and antagonists of the glucocorticoid receptor. Endocrine. 1995;3:5–12. doi: 10.1007/BF02917442. [DOI] [PubMed] [Google Scholar]