Abstract
Background:
Very preterm infants are at high risk for neurodevelopmental impairments. We used a child-centered approach (latent profile analysis [LPA]) to describe 2-year neurobehavioral profiles for very preterm infants based on cognitive, motor, and behavioral outcomes. We hypothesized that distinct outcome profiles would differ in the severity and co-occurrence of neurodevelopmental and behavioral impairment.
Methods:
We studied children born <33 weeks’ gestation from the Environmental influences on Child Health Outcomes (ECHO) Program with at least one neurobehavioral assessment at age 2 (Bayley Scales of Infant and Toddler Development, Child Behavior Checklist, Modified Checklist for Autism in Toddlers, cerebral palsy diagnosis). We applied LPA to identify subgroups of children with different patterns of outcomes.
Results:
In 2,036 children (52% male; 48% female), we found four distinct neurobehavioral profiles. Most children (~85%) were categorized into one of two profiles characterized by no/mild neurodevelopmental delay and a low prevalence of behavioral problems. Fewer children (~15%) fell into one of two profiles characterized by severe neurodevelopmental impairments. One profile consisted of children (5%) with co-occurring neurodevelopmental impairment and behavioral problems.
Conclusion:
Child-centered approaches provide a comprehensive, parsimonious description of neurodevelopment following preterm birth and can be useful for clinical and research purposes.
INTRODUCTION
Birth outcomes for infants born very preterm (<33 weeks gestational age [GA]) have been steadily improving over the past several decades.1,2 With more children born at early GA surviving into childhood, the extent to which they will experience clinically relevant neurodevelopmental or behavioral problems is unclear.3,4 Outcomes for children born < 33 weeks GA are variable, with some children showing few neurodevelopmental concerns and others showing significant impairment.2,5 However, prior research has tended to take a variable-centered approach, reporting rates of impairment in single domains or on single assessments as if they are independent from one another. For example, studies have reported rates of neurodevelopmental delay separately from rates of behavioral problems, although these outcomes could be experienced separately or in tandem within different subgroups of children. Some studies have created composite indices of neurodevelopmental impairment that incorporate multiple neurological domains (e.g., cognitive impairment, motor impairment, hearing loss, blindness)6 but these indices only capture degree of impairment (e.g., any vs. none or total number of impairments), rather than describing specific patterns of outcomes in children.
More recently, child-centered approaches to studying neurodevelopmental outcomes have been developed and applied to children born very preterm.7–10 These approaches aim to identify subgroups of children with similar neurodevelopmental profiles within a larger, heterogeneous sample. The benefit of child-centered approaches is that they result in comprehensive, holistic descriptions of children, including their unique combinations of cognitive, motor, and behavioral strengths and weaknesses. Unlike prior studies that describe neurodevelopmental and behavioral outcomes separately11, the holistic descriptions derived from child-centered approaches integrate multiple measures into a single outcome profile that is parsimonious and easily interpretable, two strengths that could benefit clinicians in deciding which children require closer follow-up or intervention and which kinds of services would be most beneficial for specific subgroups of children.
Current research has begun to examine neurodevelopmental profiles of infants born preterm. Overall, these studies have shown that there are distinct subgroups of children who vary both in the severity of their neurodevelopmental and/or behavioral difficulties8–10 and in the co-occurrence of these problems.7 The National Institutes of Health-funded Environmental influences on Child Health Outcomes (ECHO) Program12 offers an opportunity to examine neurodevelopmental profiles in a larger sample of children from multiple cohorts across a wide geographic region with diverse demographic and medical characteristics. The goal of the current study was to apply a child-centered approach to characterize outcomes for very preterm infants in the ECHO Program at their 2-year follow-up. We hypothesized that we would observe distinct neurobehavioral profiles that differ in the severity and co-occurrence of neurodevelopmental, motor, and behavioral strengths and difficulties.
METHODS
Study Population
The ECHO Program was launched in 2016 to investigate the influence of environmental exposures on child health and development.12–14 Of the 69 extant cohorts within ECHO (https://www.nih.gov/echo/pediatric-cohorts), three specifically recruited infants born very preterm (<33 weeks GA) and were therefore included in this analysis (Table 1).15 Infants with data for any of our outcome measures assessed at 2 years of age (corrected age) were retained in the analytic sample, resulting in a total of 2,036 children (MGA=26.1 weeks, SDGA=1.8 weeks, range=22–32 weeks; 52% male, 48% female). Within each cohort, local Institutional Review Boards reviewed and approved all data collection procedures, and participants provided written informed consent.
Table 1.
ECHO cohort description.
| Cohort | Years of enrollment | Inclusion criteria | Exclusion criteria | Sample size in current analysis |
|---|---|---|---|---|
| ECHO-NOVI | 2014–2016 | -Gestational age (GA) less than 30 weeks at birth -Parental ability to read and speak English or Spanish -Residence within 3 hours of the neonatal intensive care unit (NICU)/follow-up clinic |
-Major congenital anomalies affecting NNNS administration prior to discharge | 567 |
| NICU-HEALTH | Phase 1: 2011–2013 Phase 2: 2015-present |
-Birth weight <1500 grams or gestational age 28 through 32–6/7 weeks -Birth at Mount Sinai Hospital during the time period of study enrollment -No diagnosis of genetic or major structural congenital abnormality |
-Evidence of perinatal hypoxic ischemic encephalopathy during follow-up | 380 |
| ELGAN | 2002–2004 | -Birth prior to 28 weeks of gestation -Mother gave informed consent -Birth at one of 15 participating medical centers in Illinois, Michigan, North Carolina, Connecticut, or Massachusetts |
-Anencephaly | 1089 |
NNNS, NICU Network Neurobehavioral Scale.
Measures
Bayley Scales of Infant and Toddler Development (Bayley)
The Bayley is a widely used assessment of child developmental outcomes across motor, language, and cognitive domains.16 The motor scale captures both fine and gross motor skills, whereas the language scale captures expressive and receptive language. Because one cohort used an earlier version of this assessment (Bayley-II: n=965; Bayley-III: n=736), the Bayley outcome measure for this analysis was harmonized Bayley Mental Development Index (hMDI) scores. Briefly, data were harmonized by applying a previously published algorithm for converting Bayley-III language and cognitive composite scores into a single Bayley MDI score.17 This approach has been used before in studies of very preterm18 and extremely preterm infants.19 For a small subset of children with very low language and/or cognitive scores (n=82; ~5%), this procedure resulted in hMDI scores that were outside the plausible range; for these cases, their converted scores were set to 49, the lowest observed Bayley MDI score. With the application of the hMDI score, children from all three cohorts provided Bayley data (n=1701).
Child Behavior Checklist 1 ½-5 years (CBCL 1 ½-5)
The CBCL/1 ½-5 is a parent-report measure assessing child behavioral problems.20 Parents were asked to report on 99 child behaviors using a 3-point rating scale of 0 (“Not True), 1 (“Somewhat or Sometimes True”), or 2 (“Very True or Often True”). Sum scores are calculated for seven symptom subscales: Emotionally Reactive, Anxious/Depressed, Somatic Complaints, Withdrawn, Sleep Problems, Attention Problems, and Aggressive Behaviors. We used the seven raw subscale scores as outcomes in the analysis. Children from all three cohorts provided CBCL/1 ½-5 data (n=1390), referred to here as CBCL.
Cerebral palsy (CP)
The presence or absence of cerebral palsy (CP) at age 2 was determined using at least one of the following measures: Neonatal Research Network CP exam21, Extremely Low Gestational Age Neonates (ELGAN) neurological exam22, Gross Motor Function Classification System, and/or parent-report of a CP diagnosis. The severity and type of CP (e.g., quadriplegia, hemiplegia) was not available from all cohorts; therefore, a single dichotomous variable indicating the presence or absence of any CP was used as the final outcome variable. Children from all three cohorts provided CP data (n=1477).
Modified Checklist for Autism in Toddlers (M-CHAT)
The M-CHAT is a parent-report screening tool to identify children at high risk for autism spectrum disorder (ASD). Parents responded to 23 (M-CHAT-R23; original instrument) or 20 (M-CHAT-R/F24; revised instrument) items regarding their child’s social and language behavior. A single harmonized dichotomous variable indicating whether the child screened positive for high autism risk was used as an outcome. Children from all three cohorts provided M-CHAT data (n=1809).
Statistical Analysis
We addressed our main study aims by applying latent profile analysis (LPA) to the following outcome variables: Bayley hMDI score, CBCL subscale scores, M-CHAT positive screen (yes/no), and CP (yes/no). LPA classifies individuals into groups based on patterns of responses to several observed variables. Enumerating the optimal number of latent profiles involves estimating models with increasing numbers of latent profiles and comparing their model fit. We did this systematically by applying five rules. First, there could be no evidence of convergence problems (indicating that the model could not be estimated). Second, we preferred models with a low Bayesian Information Criterion (BIC), a numerical index of how well the model fits the data that balances model fit with parsimony. Third, we preferred models with high entropy and latent class probabilities, indicating high classification accuracy. Fourth, we conducted Lo-Mendell-Rubin (LMR) and Bootstrapped Likelihood Ratio (BLR) tests, which test whether a model with k profiles fits significantly better than a model with k-1 profiles. We preferred significant LMR and BLR test results. Fifth, we preferred models where the smallest class contained at least 5% of the sample to avoid overfitting or non-generalizable solutions. All models were run assuming equal variances for the outcome variables across the latent profiles. Missing data were handled using full information maximum likelihood. This approach allowed us to retain all participants who supplied at least one outcome variable, without the need to impute missing data. Additional information regarding data availability across cohorts is supplied in Supplemental Table 1.
Based on the best-fitting solution, we grouped children into their most likely latent profile and then described the profiles based on their mean Bayley and CBCL scores, as well as the prevalence of CP and a positive screen on the M-CHAT. We additionally compared the means and prevalence estimates across the profiles using one-way ANOVA and chi-squared tests, following up significant omnibus tests with post-hoc comparisons (i.e., Tukey tests and pairwise chi-squared tests) to determine which profiles were significantly different from one another for each outcome variable.
For additional context, we examined the proportion of children in each profile who fell in the clinical range on the Bayley and CBCL. For the Bayley, we describe the proportion of children in each profile with mild (hMDI <85) and severe (hMDI <70) cognitive delay. For the CBCL, we describe the proportion of children with borderline (T ≥65) or elevated (T ≥70) T-scores in each behavioral domain.
We conducted LPA using Mplus version 8.8. Descriptive statistics and profile comparisons were completed using SAS version 9.4.
RESULTS
Sample Description
Descriptive statistics for the children and mothers in this sample are shown in Table 2. The sample was racially (3% Asian, 24% Black, 6.5% Multiracial, <2% Native Hawaiian/Pacific Islander or American Indian/Alaskan Native, and 58% White) and ethnically diverse (15% Hispanic/Latino/a). Approximately one-third (29%) of mothers had a 4-year college degree, and the majority (76%) had a relationship partner. Demographic information additionally broken down by cohort is available in Supplemental Table 2.
Table 2.
Characteristics of study sample.
| Full sample (N=2036) |
||
|---|---|---|
| Variable | N | M (SD) or % |
| Neonatal characteristics | ||
| Gestational age at birth (weeks) | 2026 | 26.1 (1.75) |
| Birth weight (grams) | 2021 | 886.4 (279.5) |
| Small for gestational age | 1282 | 15% |
| Male sex | 2036 | 52% |
| Female sex | 2036 | 48% |
| Infant admitted to NICU | 2036 | 100% |
| Infant race | 2036 | |
| American Indian/Alaskan Native | <1% | |
| Asian | 3% | |
| Black | 24% | |
| Multiple Races | 6.5% | |
| Native Hawaiian/Pacific Islander | <1% | |
| Other Race | 5.9% | |
| Race Unknown | 1.5% | |
| White | 58% | |
| Infant ethnicity | 2036 | |
| Hispanic/Latino/a | 15% | |
| Not Hispanic/Latino/a | 85% | |
| Full sample (N=1761) |
||
| N | M (SD) or % | |
| Maternal demographics | ||
| Age at birth (years) | 1663 | 29.1 (6.6) |
| 4-year college degree | 1467 | 29% |
| Married/partnered | 1491 | 76% |
| Maternal medical problems | ||
| Gestational diabetes | 1433 | 8.0% |
| Pre-eclampsia | 1756 | 20% |
| Perinatal maternal substance use | ||
| Tobacco | 1687 | 13% |
| Alcohol | 763 | 3.5% |
| Opioid | 784 | 18% |
| Marijuana | 755 | 7.3% |
| Illicit drugs | 753 | 4.1% |
NICU, neonatal intensive care unit.
Developmental outcomes for the full sample are summarized in Table 3. The mean Bayley hMDI score was 82 (SD=20), which is below the cutoff for mild delay (<85). Approximately one-half (53%) of the sample met the criteria for mild delay, and approximately one-quarter (27%) met the criteria for severe delay. Raw CBCL subscale scores ranged from 1.53 (Withdrawn) to 8.37 (Aggressive Behaviors). Between 3% (Anxious/Depressed) and 13% (Attention Problems) of the sample had CBCL scores in the borderline elevated range (T ≥65). Additionally, 12% of the sample had a CP diagnosis and 19% screened positive on the M-CHAT.
Table 3.
Means and percentages of outcome variables in the full sample.
| Child outcomes | N | Mean | SD | Range | <1 SD (%) | <2 SD (%) |
|---|---|---|---|---|---|---|
| Bayley Scales of Infant and Toddler Development | ||||||
| Harmonized Mental Development Index (hMDI) | 1701 | 82.2 | 20.2 | 49–145 | 53% | 27% |
| N | Mean | SD | Range | T ≥65 (%) | T ≥70 (%) | |
| CBCL/1 ½−5 syndrome scales (raw scores) | ||||||
| Emotionally reactive | 1387 | 1.88 | 2.15 | 0–15 | 7.0% | 1.5% |
| Anxious/depressed | 1389 | 2.06 | 2.00 | 0–11 | 3.3% | 0.9% |
| Somatic complaints | 1389 | 1.58 | 1.95 | 0–14 | 8.1% | 3.4% |
| Withdrawn | 1389 | 1.53 | 2.01 | 0–14 | 8.9% | 5.5% |
| Sleep problems | 1390 | 2.41 | 2.61 | 0–14 | 5.3% | 3.3% |
| Attention problems | 1390 | 2.84 | 2.17 | 0–10 | 13% | 7.6% |
| Aggressive behaviors | 1372 | 8.37 | 6.20 | 0–29 | 5.0% | 1.9% |
| N | % | Range | ||||
| Diagnosis/Risk | ||||||
| Cerebral palsy diagnosis | 1477 | 12% | 0–1 | |||
| ASD, M-CHAT positive screen | 1809 | 19% | 0–1 |
ASD, autism spectrum disorder; CBCL/1 ½−5, Child Behavior Checklist for Ages 1½−5; M-CHAT, Modified Checklist for Autism in Toddlers.
LPA Analysis
We estimated LPA models with 1–6 profiles. Fit statistics are shown in Table 4. Based on our selection criteria, we determined that a 4-profile solution fit the data best. Although the BIC was lower and the BLR test was significant for the 5- and 6-profile solutions, these solutions resulted in small classes with fewer than 2% of the sample represented. Entropy and average class probabilities were very similar between the 4-, 5-, and 6-profile solutions. For this reason, we selected the 4-profile solution.
Table 4.
Model fit statistics for LPA models.
| Number profiles | Convergence problems | Lowest LLH replicated | BIC | SS Adj BIC | Entropy | Avg class probabilities | Smallest class size | LMR statistic | LMR p-value | BLR statistic | BLR p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | No | Yes | 63252 | 63195 | -- | -- | -- | -- | -- | -- | |
| 2 | No | Yes | 60428 | 60336 | 0.76 | 0.89–0.94 | N=310 (15%) | 2907 | p<.001 | 2907 | p<.001 |
| 3 | No | Yes | 59607 | 59480 | 0.67 | 0.83–0.84 | N=145 (7.1%) | 905 | p<.001 | 905 | p<.001 |
| 4 | No | Yes | 59255 | 59093 | 0.69 | 0.72–0.94 | N=112 (5.5%) | 436 | p<.001 | 436 | p<.001 |
| 5 | No | Yes | 59004 | 58807 | 0.70 | 0.70–0.97 | N=30 (1.5%) | 335 | p=.54 | 335 | p<.001 |
| 6 | No | Yes | 58887 | 58655 | 0.70 | 0.66–0.94 | N=32 (1.6%) | 201 | p=.26 | 201 | p<.001 |
Convergence problems were noted when the majority of solutions failed to converge. BIC, Bayesian Information Criterion; BLR, bootstrapped likelihood ratio test; LLH, log likelihood; LMR, Lo-Mendell-Rubin test; LPA, latent profile analysis; SS Adj BIC, sample size–adjusted Bayesian Information Criterion.
We next compared developmental outcomes by profile (Table 5; Figure 1). Profile 1 comprised almost two-thirds of the sample (60%, n=1218). Children in this profile had the highest Bayley hMDI scores in the sample (M=89, SD=17), although this was still below the average for normative populations (M=100, SD=15).16 Children in this profile had the fewest behavioral problems in the sample. Rates of CP were low (~6%), and very few children in this profile (<2%) screened positive on the M-CHAT.
Table 5.
Means and percentages of outcome variables by latent profile.
| Child outcomes | Profile 1 (60%) |
Profile 2 (24%) |
Profile 3 (5.5%) |
Profile 4 (11%) |
|
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | P-value | |
| Bayley Scales of Infant and Toddler Development | |||||
| Mental Development Index (hMDI) | 89.3 (17.0) | 79.2 (19.1) | 67.0 (16.9) | 54.1 (9.1) | <.0001 |
| CBCL/1½−5 syndrome scales (raw scores) | |||||
| Emotionally reactive | 0.77 (0.94) | 2.81 (1.55) | 6.82 (2.48) | 1.12 (1.22) | <.0001 |
| Anxious/depressed | 1.08 (1.17) | 3.00 (1.70) | 5.77 (2.07) | 1.54 (1.54) | <.0001 |
| Somatic complaints | 0.96 (1.34) | 2.00 (1.84) | 4.22 (3.18) | 1.53 (1.71) | <.0001 |
| Withdrawn | 0.48 (0.78) | 1.98 (1.63) | 5.33 (2.76) | 2.98 (2.08) | <.0001 |
| Sleep problems | 1.46+ (1.75) | 3.46 (2.66) | 6.15 (3.29) | 1.22+ (1.52) | <.0001 |
| Attention problems | 1.63 (1.39) | 4.02 (1.74) | 6.44 (1.75) | 3.00 (1.88) | <.0001 |
| Aggressive behaviors | 4.98+ (3.62) | 12.8 (4.32) | 19.9 (5.60) | 4.93+ (3.84) | <.0001 |
| Diagnosis/Risk | |||||
| Cerebral palsy diagnosis (%) | 5.8% | 9.3% | 18% | 52% | <.0001 |
| ASD, M-CHAT positive screen (%) | 1.6% | 20% | 58% | 90% | <.0001 |
P values correspond to omnibus test statistics (i.e., F test for continuous variables, chi-squared for dichotomous variables). Post-hoc pairwise comparisons were also conducted, and groups with means that were not significantly different (p>.05) are noted (+ or ^). Within each row, bolded values represent the most optimal scores (highest mean Bayley score, lowest mean CBCL/1½−5 score, lowest prevalence of CP/ASD risk), and italicized values represent the least optimal scores (lowest mean Bayley score, highest mean CBCL/1½−5 score, highest prevalence of CP/ASD risk). ASD, autism spectrum disorder; CBCL/1½−5, Child Behavior Checklist for Ages 1½−5; CP, cerebral palsy; M-CHAT, Modified Checklist for Autism in Toddlers.
Figure 1.
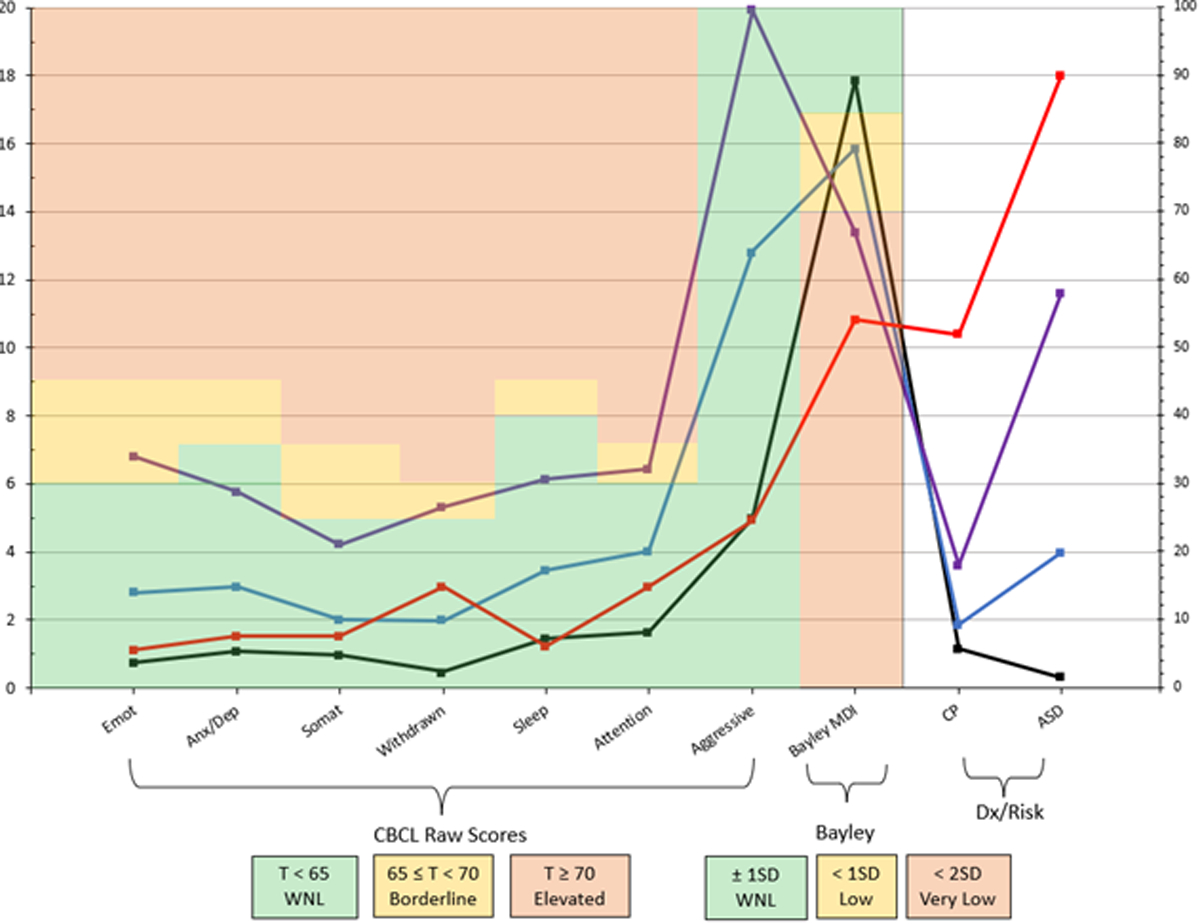
The means and percentages of outcome variables by latent profile. Profile 1 (black; 60%) had mean Bayley scores within normal limits and few behavioral problems. Profile 2 (blue; 24%) had mean Bayley scores indicative of mild impairment but few behavioral problems. Profile 3 (purple; 5%) had mean Bayley scores indicative of severe impairment and borderline elevated CBCL/1½-5 scores. Profile 4 (red; 11%) had mean Bayley scores indicative of severe impairment but few behavioral problems. CBCL/1½-5 outcomes are syndrome scale raw scores and Bayley outcome is the harmonized Mental Development Index (hMDI; normative mean=100, standard deviation=15). CP refers to a cerebral palsy diagnosis, and ASD refers to a positive screen on the M-CHAT. CBCL/1½-5 scores are plotted according to the left axis (ranging from 0–20), whereas Bayley, CP, and ASD variables are plotted according to the right axis (ranging from 0–100). Colored shading provides information about normal limits (green), borderline elevated or mild impairment (yellow), and elevated or severe impairment (red). For CBCL/1½-5, raw scores were used for model estimation, but colored shading is based on corresponding T-scores. Aggressive, aggressive problems; Anx/Dep, anxious/depressed; ASD, autism spectrum disorder; Attention, attention problems; CP, cerebral palsy; Emot, emotionally reactive; Sleep, sleep problems; Somat, somatic complaints; WNL, within normal limits.
Profile 2 contained approximately one-quarter of the sample (24%; n=491). Children in profile 2 had Bayley hMDI scores that were significantly lower than those of the children in profile 1 (M=79, SD=19), p<.001. Additionally, the mean level of behavioral problems in profile 2 was significantly higher than in profile 1 (all p<.001). Rates of CP were higher in profile 2 compared with profile 1 (9.3%, p=.02), and the children in this profile were more likely to screen positive on the M-CHAT (20%, p<.001) compared with the children in profile 1.
Profile 3 contained 5.5% of the sample (n=112). Children in profile 3 had lower Bayley hMDI scores than children in profiles 1 or 2 (M=67, SD=17), p<.001. In addition, they had the highest levels of behavioral problems compared with all other profiles (all p<.001). The rate of CP in profile 3 was higher than in profile 2 (18%, p=.02). More than half of the children in this profile screened positive on the M-CHAT (58%), which was significantly higher than in profiles 1 or 2 (all p<.001).
Finally, profile 4 contained 11% of the sample (n=215). Children in this profile had the lowest Bayley hMDI scores compared with all other profiles (M=54, SD=9.1, all p < .001). Behavioral problems were higher in profile 4 compared with profile 1 (all p ≤ .001), with the exception of two subscales (Sleep Problems, Aggressive Behaviors) that were equally as low as in profile 1 (all p>.26). More than half of the children in profile 4 had CP (52%), and nearly all of the children screened positive on the M-CHAT (90%), which were the highest rates observed across all of the profiles (all p<.001).
The prevalence of clinically relevant scores by profile are shown in Table 6. Notably, while rates of severe delay (<2 SD) on the Bayley were low in profile 1 (11%), more than half of the children in profile 3 (60%) and nearly all the children in profile 4 (91%) had scores in the severe delay range. Rates of borderline (T ≥65) and elevated (T ≥70) behavioral problems were relatively low in profiles 1, 2, and 4, but they were notably higher in profile 3, ranging from 31% (Sleep Problems) to 72% (Attention Problems) for borderline elevated scores. The two most prevalent behavioral problems in this group were attention problems (72%) and emotionally reactive problems (66%), indicating specific difficulties with externalizing problems (although 58% of children also had withdrawn symptoms in the borderline elevated range).
Table 6.
Prevalence of clinically relevant scores by latent profile.
| Child outcomes | Profile 1 (60%) |
Profile 2 (24%) |
Profile 3 (5%) |
Profile 4 (11%) |
||||
|---|---|---|---|---|---|---|---|---|
| <1 SD | <2 SD | <1 SD | <2 SD | <1 SD | <2 SD | <1 SD | <2 SD | |
| Bayley Scales of Infant and Toddler Development | ||||||||
| Mental Development Index (hMDI) | 41% | 11% | 59% | 33% | 82% | 60% | 98% | 91% |
| T ≥65 | T ≥70 | T ≥65 | T ≥70 | T ≥65 | T ≥70 | T ≥65 | T ≥70 | |
| CBCL/1½−5 syndrome scales | ||||||||
| Emotionally reactive | 0.0% | 0.0% | 6.0% | 0.0% | 66% | 20% | 0.8% | 0.0% |
| Anxious/depressed | 0.0% | 0.0% | 2.6% | 0.0% | 33% | 11% | 0.0% | 0.0% |
| Somatic complaints | 2.7% | 0.7% | 9.8% | 2.6% | 40% | 27% | 7.3% | 1.6% |
| Withdrawn | 0.1% | 0.0% | 9.1% | 2.9% | 58% | 47% | 18% | 11% |
| Sleep problems | 1.0% | 0.3% | 7.9% | 5.0% | 31% | 22% | 0.8% | 0.0% |
| Attention problems | 0.5% | 0.0% | 22% | 9.5% | 72% | 53% | 11% | 6.4% |
| Aggressive behaviors | 0.0% | 0.0% | 6.0% | 1.2% | 48% | 23% | 0.0% | 0.0% |
Values in the table correspond to the percentages of children in each profile with Bayley scores more than 1 SD or 2 SD below the standardized mean or CBCL/1½−5 scores that correspond to T scores ≥65 or ≥70. CBCL/1½−5, Child Behavior Checklist for Ages 1½−5.
Secondary Analysis
Although the goal of the current investigation was to describe outcome profiles, rather than predict membership in these profiles, we aimed to understand the extent to which children of different GAs were represented in each of the four outcome profiles. The mean GAs of children in each profile were as follows: profile 1 (MGA=26.3 weeks, SD=1.8 weeks), profile 2 (MGA=26.1 weeks, SD=1.6 weeks), profile 3 (MGA=25.9 weeks, SD=1.6 weeks), and profile 4 (MGA=25.4 weeks, SD=1.7 weeks). The GAs of children in profiles 1 and 2 were not statistically different (p=.07). Children in profile 3 had a significantly lower GA compared with children in profile 1 (p=.03) but not compared with children in profile 2 (p=.28). Children in profile 4 had the lowest GA compared with all other profiles (all p<.02). The magnitude of these differences was small (< 1 week).
DISCUSSION
The purpose of this investigation was to take a child-centered approach to characterize the neurodevelopmental profiles of children at age 2 following very preterm birth. We capitalized on data from the nationwide ECHO Program to examine these profiles in the largest sample of children born at <33 weeks studied to date. We found evidence for four distinct neurobehavioral profiles that consisted of different combinations of cognitive, motor, and behavioral proficiencies and difficulties.
The majority of children (~85%) were categorized into one of two profiles characterized by no or mild neurodevelopmental delay and a low prevalence of behavioral problems. For example, 41% of children in profile 1 had Bayley hMDI scores <85, meaning most children in this profile (59%) had Bayley scores within the normal range. Children in profile 2 were more likely to have mild neurodevelopmental delay (59%), and one-third of children in profile 2 had severe neurodevelopmental delay (Bayley hMDI <70). Rates of behavioral problems were the lowest in these two profiles, with the most prevalent concern being attention problems, which were borderline elevated in 22% of children in profile 2. Rates of CP were lowest in profile 1 (6%) and second lowest in profile 2 (9%), indicating a low prevalence of gross motor disability among these children. Very few children in profile 1 screened positive on the M-CHAT for elevated ASD risk, whereas a larger proportion (20%) screened positive in profile 2. Overall, while children in these two profiles may be at risk for mild neurodevelopmental delay and/or behavioral problems, many children (particularly in profile 1) may have no developmental concerns.
The rest of the sample (~15%) fell into one of two profiles characterized by more severe neurodevelopmental and/or behavioral difficulties. Children in profile 3 (5%) were characterized by co-occurring, severe neurodevelopmental and behavioral problems. More than half of the children in this profile (60%) had severe neurodevelopmental delay, and rates of elevated behavioral problems (particularly attentional and emotional problems) were the highest in the sample. Children in profile 4 were characterized by an even higher likelihood of severe neurodevelopmental delay, but unlike children in profile 3, they were much less likely to have behavioral problems. Nearly all children (91%) in profile 4 had Bayley hMDI scores <70, indicative of severe neurodevelopmental delay. Additionally, over half of the children in this profile had a CP diagnosis, and the majority of children screened positive for elevated ASD risk on the M-CHAT.
Several recent studies have used group-based clustering approaches to describe outcomes for preterm children, and our results share similarities and differences with this prior work. For example, we recently examined neurodevelopmental profiles at age 2 for one of the cohorts included in this ECHO-wide analysis (ECHO-NOVI; see Table 1).7 In this prior analysis, we similarly found four profiles that differed in terms of degree and combination of strengths and impairments. In ECHO-NOVI, approximately 70% of children fell into one of two profiles that were similar to profiles 1 and 2 in the current study (containing ~80% of children in the current sample). These profiles had low rates of neurodevelopmental and behavioral problems as well as low rates of CP and few positive screens on the M-CHAT. In ECHO-NOVI, 11% of the sample were categorized into a profile similar to profile 3 in this study (5% of the current sample), characterized by elevated rates of neurodevelopmental impairment accompanied by elevated behavioral problems. Finally, 16% of the ECHO-NOVI sample fell into a profile similar to profile 4 in this study (11% of the current sample), characterized by severe neurodevelopmental delay but few behavioral problems. The replication of prior ECHO-NOVI findings in this much larger, multicohort study is notable, especially given the increased range of GA (<33 weeks in the current study, <30 weeks in ECHO-NOVI) and the recruitment time frame (2002-present in the current study, 2014–2016 in ECHO-NOVI).
One of the cohorts represented in this study (ELGAN; see Table 1) previously used LPA to describe cognitive outcomes for their sample at age 10 years.8 At age 10, approximately one-third of children in this cohort had a cognitive profile with IQ and executive functioning scores in the normal range, whereas 41% had a “low-normal” profile. Smaller proportions of children were categorized into “moderately impaired” (17%) and “severely impaired” (8%) profiles. It is difficult to compare the prior ELGAN findings with the present findings because of the different ages of the children and the different assessments included (e.g., cognitive outcomes alone versus in combination with behavioral outcomes). However, the ELGAN findings of a gradient of cognitive performance ranging from normal or near normal to severe impairment are similar to those of this study. Other studies have similarly used LPA to describe developmental outcomes for children following extremely9 and moderately preterm birth10, although these studies have examined older children (age 7–8 years) and investigated outcomes within single domains (either cognitive or behavioral outcomes). Similar to the current findings and the prior findings in ECHO-NOVI7 and ELGAN8, these studies found four subgroups of children with varied levels of functioning, from few cognitive and/or behavioral problems to globally elevated and clinically relevant impairments.9,10
The gradient of impairments observed across the four profiles in this study and the differing combinations of neurodevelopmental and behavioral strengths and difficulties demonstrate the diversity of outcomes for children born at <33 weeks GA. Additionally, these results illustrate the strength of the LPA approach for characterizing outcomes in these children. LPA is particularly useful in summarizing the strengths and difficulties of children across multiple domains of functioning (i.e., cognitive, motor, and behavior). Only one of the profiles (profile 3) showed clinically relevant impairments in both neurodevelopmental and behavioral outcomes, with mean Bayley hMDI scores <2 SD below the mean and CBCL scores in the borderline elevated range for multiple subscales. This profile was distinct from profile 4, which also had mean Bayley hMDI scores <2 SD below the mean, coupled with high rates of CP and a high risk for ASD but with CBCL scores within normal limits. While children in profile 4 would likely require additional follow-up services due to cognitive and motor concerns, children in profile 3 would require follow-up for cognitive, motor, and behavioral concerns. Understanding how these profiles differ from one another, and from more neurotypical profiles, could help clinicians identify the different ‘phenotypes’ of preterm children they may see at follow-up and could aid in the development of new or improved interventions that target multiple, co-occurring neurodevelopmental difficulties in tandem.
The strengths of this study include our use of a large, multi-cohort population, the largest sample of children born < 33 weeks GA in the United States to date. The multi-cohort nature of this study, spanning a wide geographical area, allowed us to achieve greater diversity in child and family characteristics than any single study alone. This study also had several limitations. Most notably, our use of a multi-cohort study means that there was heterogeneity in how multiple outcomes were measured (e.g., multiple procedures for determining CP, Bayley-II versus Bayley-III for assessing neurodevelopment, and MCHAT-R versus MCHAT-R/F for determining autism risk). The three cohorts also differed in terms of child year of birth, gestational age criteria for enrollment, and sample size, which may have had an impact on our findings. Despite the large overall sample, sizable proportions of data were missing for each individual outcome assessed. Although this limitation did not lead to issues with model estimation, there is the possibility that children with the most severe neurodevelopmental delays were not able to be seen for follow-up or assessments were not able to be conducted. These results may therefore not represent outcomes for the most high-risk children. Additionally, we were limited in the assessments that were available and could be harmonized across the three cohorts. While we were able to harmonize Bayley-II and Bayley-III cognitive scores17, no published algorithm is available for converting Bayley motor scores across different versions of the instrument. Additionally, while we were able to take a fine-grained approach to assessing behavioral problems using the CBCL syndrome scales (rather than composite scores), we were not able to distinguish between subdomains of cognition or language (e.g., expressive versus receptive communication) on the Bayley due to the differences in instrument versions. Finally, these results may not accurately represent outcomes for all premature infants, particularly those born in the past few years, as most cohorts included in this analysis recruited infants prior to 2016. The profiles described here may also not be representative of very preterm children assessed at older ages (i.e., older than 2 years of age). Understanding the stability or changes in neurodevelopmental profiles for children across time would be an important future research direction.
In summary, the current study reports four distinct neurodevelopmental profiles of very preterm infants, ranging from few or no developmental concerns to severe impairment in one or more domains. Child-centered approaches provide a comprehensive, parsimonious description of child neurodevelopment following preterm birth and can be useful for both clinical care and research applications. These results demonstrate the importance of assessing behavioral as well as neurodevelopmental outcomes in follow-up programs in order to provide more comprehensive evaluation and intervention services. Future research should investigate which factors in the pre-, peri-, and postnatal environment are related to these profiles to better understand the risk and protective factors for very preterm children.
Supplementary Material
IMPACT.
Most research on outcomes for children born very preterm have reported rates of impairment in single domains.
Child-centered approaches describe profiles of children with unique combinations of cognitive, motor, and behavioral strengths and weaknesses.
We capitalized on data from the nationwide Environmental influences on Child Health Outcomes (ECHO) Program to examine these profiles in a large sample of children born <33 weeks gestational age.
We found four distinct neurobehavioral profiles consisting of different combinations of cognitive, motor, and behavioral characteristics.
This information could aid in the development of clinical interventions that target different profiles of children with unique developmental needs.
ACKNOWLEDGMENTS
The authors wish to thank our ECHO colleagues; the medical, nursing, and program staff; and the children and families participating in the ECHO cohorts. We also acknowledge the contribution of the following ECHO program collaborators:
ECHO Components—Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: Smith PB, Newby KL; Data Analysis Center: Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland: Jacobson LP; Research Triangle Institute, Durham, North Carolina: Catellier DJ; Person-Reported Outcomes Core: Northwestern University, Evanston, Illinois: Gershon R, Cella D.
ECHO Awardees and Cohorts— Icahn School of Medicine at Mount Sinai, New York, NY: Teitelbaum SL; Baystate Children’s Hospital, Springfield, MA: Vaidya R; Beaumont Children’s Hospital, Royal Oak, MI: Obeid R; Boston Children’s Hospital, Boston, MA: Rollins C; East Carolina University, Brody School of Medicine, Greenville, NC: Bear K; Michigan State University College of Human Medicine, East Lansing, MI: Lenski M; Tufts University School of Medicine, Boston, MA: Singh R; University of Chicago, Chicago, IL: Msall M; University of Massachusetts Chan Medical School, Worcester, MA: Frazier J o Yale School of Medicine, New Haven, CT: Montgomery A; Boston Medical Center, Boston, MA: Kuban K, Douglass L, Jara H.
FUNDING
Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of the Director, National Institutes of Health, under Award Numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 with co-funding from the Office of Behavioral and Social Science Research (PRO Core), UH3OD023320 (Aschner), UH3OD023347 (Lester), UH3OD023348 (O’Shea). Dr. Camerota was additionally supported by a career development award from the National Institute of Mental Health (NIMH), grant K01MH129510 (Camerota). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
See Acknowledgments for full listing of collaborators
COMPETING INTERESTS
The authors declare no competing interests.
CONSENT STATEMENT
This study was approved by local Institutional Review Boards, and participants gave informed consent.
DATA AVAILABILITY STATEMENT
De-identified data from the ECHO Program are available through NICHD’s Data and Specimen Hub (DASH) (https://dash.nichd.nih.gov). DASH is a centralized resource that allows researchers to access data from various studies via a controlled-access mechanism. Researchers can now request access to these data by creating a DASH account and submitting a Data Request Form. The NICHD DASH Data Access Committee will review the request and provide a response in approximately two to three weeks. Once granted access, researchers will be able to use the data for three years. See the DASH Tutorial for more detailed information on the process (https://dash.nichd.nih.gov/resource/tutorial).
REFERENCES
- 1.Glass HC, et al. Outcomes for extremely premature infants. Anesthesia and analgesia. 2015;120(6):1337–1351. doi: 10.1213/ANE.0000000000000705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vohr BR. Neurodevelopmental Outcomes of Extremely Low Birth Weight Infants <32 Weeks’ Gestation Between 1993 and 1998. PEDIATRICS. 2005;116(3):635–643. doi: 10.1542/peds.2004-2247 [DOI] [PubMed] [Google Scholar]
- 3.Cheong JLY, et al. Changing Neurodevelopment at 8 Years in Children Born Extremely Preterm Since the 1990s. Pediatrics. 2017;139(6):e20164086. doi: 10.1542/peds.2016-4086 [DOI] [PubMed] [Google Scholar]
- 4.Burnett AC, et al. Trends in Executive Functioning in Extremely Preterm Children Across 3 Birth Eras. Pediatrics. 2018;141(1):e20171958. doi: 10.1542/peds.2017-1958 [DOI] [PubMed] [Google Scholar]
- 5.Allen MC. Neurodevelopmental outcomes of preterm infants. Current opinion in neurology. 2008;21(2):123–128. doi: 10.1097/WCO.0b013e3282f88bb4 [DOI] [PubMed] [Google Scholar]
- 6.Adams-Chapman I, et al. Neurodevelopmental Impairment Among Extremely Preterm Infants in the Neonatal Research Network. Pediatrics. 2018;141(5):e20173091. doi: 10.1542/peds.2017-3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camerota M, et al. Neurodevelopmental profiles of infants born <30 weeks gestation at 2 years of age. Pediatr Res. 2022;91(6):1579–1586. doi: 10.1038/s41390-021-01871-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heeren T, et al. Cognitive functioning at the age of 10 years among children born extremely preterm: A latent profile approach. Pediatric Research. 2017;82(4):614–619. doi: 10.1038/pr.2017.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnett AC, et al. Exploring the “preterm Behavioral Phenotype” in Children Born Extremely Preterm. Journal of Developmental and Behavioral Pediatrics. 2019;40(3):200–207. doi: 10.1097/DBP.0000000000000646 [DOI] [PubMed] [Google Scholar]
- 10.Cserjesi R, et al. Patterns of functioning and predictive factors in children born moderately preterm or at term. Developmental Medicine and Child Neurology. 2012;54(8):710–715. doi: 10.1111/j.1469-8749.2012.04328.x [DOI] [PubMed] [Google Scholar]
- 11.McGowan EC, et al. Analysis of Neonatal Neurobehavior and Developmental Outcomes Among Preterm Infants. JAMA Netw Open. 2022;5(7):e2222249. doi: 10.1001/jamanetworkopen.2022.22249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillman MW, Blaisdell CJ. Environmental influences on Child Health Outcomes, a Research Program of the National Institutes of Health. Current Opinion in Pediatrics. 2018;30(2):260–262. doi: 10.1097/MOP.0000000000000600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackwell CK, Wakschlag LS, Gershon RC, Cella D. Measurement framework for the Environmental influences on Child Health Outcomes research program. Current Opinion in Pediatrics. 2018;30(2):276–284. doi: 10.1097/MOP.0000000000000606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson LP, Lau B, Catellier D, Parker CB. An Environmental influences on Child Health Outcomes viewpoint of data analysis centers for collaborative study designs. Current Opinion in Pediatrics. 2018;30(2):269–275. doi: 10.1097/MOP.0000000000000602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Shea TM, et al. Environmental influences on child health outcomes: cohorts of individuals born very preterm. Pediatric Research. Published online August 10, 2022. doi: 10.1038/s41390-022-02230-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayley N Bayley Scales of Infant Development. Harcourt Assessment; 2006. [Google Scholar]
- 17.Moore T, Johnson S, Haider S, Hennessy E, Marlow N. Relationship between Test Scores Using the Second and Third Editions of the Bayley Scales in Extremely Preterm Children. The Journal of Pediatrics. 2012;160(4):553–558. doi: 10.1016/j.jpeds.2011.09.047 [DOI] [PubMed] [Google Scholar]
- 18.Lin YC, et al. Postnatal Serum Total Thyroxine of Very Preterm Infants and Long-Term Neurodevelopmental Outcome. Nutrients. 2021;13(4):1055. doi: 10.3390/nu13041055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plomgaard AM, et al. No neurodevelopmental benefit of cerebral oximetry in the first randomised trial (SafeBoosC II) in preterm infants during the first days of life. Acta Paediatr. 2019;108(2):275–281. doi: 10.1111/apa.14463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Achenbach T, Rescorla L. Manual for the ASEBA Preschool Forms & Profiles.; 2000.
- 21.Palisano R, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental Medicine & Child Neurology. 2008;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- 22.Kuban KCK, et al. An Algorithm for Identifying and Classifying Cerebral Palsy in Young Children. The Journal of Pediatrics. 2008;153(4):466–472.e1. doi: 10.1016/j.jpeds.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robins DL, Fein D, Barton ML, Green JA. The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord. 2001;31(2):131–144. doi: 10.1023/a:1010738829569 [DOI] [PubMed] [Google Scholar]
- 24.Robins DL, et al. Validation of the Modified Checklist for Autism in Toddlers, Revised With Follow-up (M-CHAT-R/F). Pediatrics. 2014;133(1):37–45. doi: 10.1542/peds.2013-1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data from the ECHO Program are available through NICHD’s Data and Specimen Hub (DASH) (https://dash.nichd.nih.gov). DASH is a centralized resource that allows researchers to access data from various studies via a controlled-access mechanism. Researchers can now request access to these data by creating a DASH account and submitting a Data Request Form. The NICHD DASH Data Access Committee will review the request and provide a response in approximately two to three weeks. Once granted access, researchers will be able to use the data for three years. See the DASH Tutorial for more detailed information on the process (https://dash.nichd.nih.gov/resource/tutorial).


