Abstract
Objective.
A spectrum of chronic kidney disease (CKD) and end-stage renal disease (ESRD) may occur in antineutrophil cytoplasmic antibody–associated vasculitis (AAV). The longitudinal trajectory of renal function in AAV is poorly understood.
Methods.
Patients with ≥2 creatinine measurements, including at baseline (±30 days of treatment initiation), were included from the Mass General Brigham AAV Cohort. We calculated estimated glomerular filtration rate (eGFR). We incorporated longitudinal changes in eGFR into a group-based trajectory model to identify patients with similar patterns of change in renal function. The chi-square test and the Kruskal-Wallis test were used to evaluate differences between groups in categorical variables and non-normally distributed continuous variables, respectively.
Results.
In 255 AAV patients, we identified 4 renal trajectory groups: rapid decline (n = 20), impaired (n = 82), preserved (n = 129), and recovery (n = 24). The rapid decline and impaired groups had greater baseline comorbidity (P = 0.01) and lower prevasculitis eGFR (P = 0.02). Clinically significant CKD (eGFR <60 ml/minute/1.73 m2) persisted over 5 years in >75% of the impaired group, compared to <40% of patients in the preserved group (P < 0.001). ESRD occurred most frequently in the rapid decline (100%), followed by the impaired and preserved groups (7% each). Baseline AAV renal involvement was present prior to 95% of ESRD. However, ESRD etiology varied, with 90% of rapid-onset ESRD attributed to vasculitis, versus 17–44% in impaired or preserved groups (P = 0.001).
Conclusion.
We identified 4 longitudinal patterns of renal function after AAV diagnosis. Our findings highlight the burden of CKD in AAV and provide a framework for future research into personalized care in this vulnerable population.
INTRODUCTION
Renal involvement is common in antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV), affecting more than half of patients with AAV (1). Renal manifestations of AAV span a range of severity, including microscopic hematuria and proteinuria, to transient acute kidney injury, to rapidly progressive glomerulonephritis and end-stage renal disease (ESRD). While ESRD is among the most consequential manifestations of AAV for the approximately 20% of patients reaching this outcome, AAV patients likely experience a spectrum of chronic kidney disease (CKD) with or without ESRD. However, the longitudinal trajectory of renal function following a diagnosis of AAV has not been well characterized in patients with diverse presenting features of AAV (2). Filling this knowledge gap will facilitate efforts to study factors driving renal function patterns after diagnosis, personalize care, and prevent progressive renal disease.
Previous analyses have indicated that not all ESRD in AAV occurs precipitously at the onset of disease. Lionaki et al found that, in a cohort of 136 AAV patients who reached the outcome of ESRD, 43% of the events occurred in patients with no clinical evidence of active vasculitic renal disease at the time of ESRD onset (3). There has been little work using longitudinal data to characterize and differentiate slow progressors from those who experience rapid renal function deterioration early in their disease course. While several studies have developed algorithms to identify AAV patients at high risk of ESRD, most require biopsy data, use only 1 renal function timepoint, do not differentiate slow and rapid ESRD progress, and are derived from cohorts defined by renal involvement, which may introduce selection biases when identifying risk factors (4,5). Given these gaps in knowledge, we aimed to use a large cohort of AAV patients with diverse disease manifestations, followed over time, to assess whether distinct patterns of renal function change could be identified using trajectory analysis, an agnostic approach (6).
PATIENTS AND METHODS
Study population.
We included patients from the Mass General Brigham AAV cohort, a longitudinal inception cohort including patients treated between 2002 and 2017; this cohort has been previously described in detail and is defined by the use of both a validated algorithm and manual chart review for identification of cases (7,8). Inclusion in this study required that a patient have a baseline creatinine value recorded, i.e., a serum creatinine available within ±30 days of the onset of AAV-directed therapy, plus at least 1 subsequent creatinine measurement. The date of initiation of AAV-directed therapy was the index date. The study was approved by the Mass General Brigham Institutional Review Board, protocol number 2016P000633.
Data collection.
We extracted data from both structured sources (laboratory values, demographic data, including self-identified race and ethnicity, and medication data) and unstructured sources (clinical notes and chart review) in the electronic medical record. The extraction of variables, including Birmingham Vasculitis Activity Score/granulomatosis with polyangiitis (GPA) score (9), clinical phenotypes, and dates of treatment, has been previously described (7). Follow-up for outcomes began at the index date and was truncated 10 years after treatment initiation. For development of trajectory models, the estimated glomerular filtration rate (eGFR) was evaluated up to monthly for 1 year prior to and 2 years after treatment initiation. If >1 creatinine measurement was available in any month, including the baseline month, the mean of all available measurements in that month was recorded as the monthly value. eGFR measurements after the date of ESRD were recorded as 0 to avoid false signals from fluctuations during dialysis or improvement in renal function after transplantation. See Supplementary Methods in Supplementary Appendix A, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25100, for further details regarding data collection for comorbidities and AAV-related treatments.
We calculated eGFR using CKD-epidemiology, without the use of a race multiplier (10). We assessed renal function at baseline, a timepoint we defined as the creatinine closest to the index date (between −30 and + 30 days). In the subset of patients who had such data available, we also assessed pretreatment creatinine prior to vasculitis treatment (between −365 and −30 days relative to the index date). Biopsy results were classified according to the schema of Berden et al based on the original interpretation available in the electronic health record (11).
Renal involvement by AAV was defined according to Birmingham Vasculitis Activity Score/GPA score classification (9). Renal treatment resistance was defined by the absence of remission within 6 months of treatment initiation. Renal remission was defined by stabilization or improvement of the creatinine level with the absence of hematuria for at least 1 month. In patients who did not have a repeat urinalysis available within 6 months of initiation of treatment, stabilization or improvement of creatinine was considered sufficient evidence of remission. This definition is similar to the one previously described by Lionaki et al (3). ESRD was defined as 1) a need for dialysis for >60 days, 2) dialysis until death if the patient died between 14 and 60 days of follow-up, or 3) renal transplant, as identified by chart review and US Renal Data System records (12).
Statistical analysis.
Derivation of trajectory groups.
We used semiparametric, group-based mixture modeling (GBTM) (PROC TRAJ in SAS), to identify groups with similar longitudinal change in renal function, defined as the percentage of baseline eGFR for 1 year prior to and 2 years after treatment initiation (the index date) (6,13). This approach sorts each patient’s longitudinal set of measurements (in this case, change in renal function) into clusters and estimates distinct trajectories based on the clusters. A strength of this approach is that renal function assessments do not have to be available at the same time or at a prescribed frequency.
When selecting a measurement type to evaluate over time as a primary input in the GBTM model, we chose to use the percent change in eGFR rather than absolute change in eGFR or eGFR itself. Percent change was calculated relative to the baseline eGFR measurement (within 30 days of AAV treatment initiation). We selected the percent change metric to avoid biasing the GBTM models overly strongly toward the baseline level of renal function. Due to non-normality of the distribution of percent change in eGFR, we applied a Yeo-Johnson transformation to normalize the variable (14).
We performed model selection according to an accepted iterative procedure; details of the models evaluated in the selection process are reported in Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25100 (15,16). We expected to ultimately evaluate models with ≥3 groups, as we anticipated ≥2 trajectories with decreasing renal function over time (3), plus a subset with stable renal function, and minor or no renal involvement, and likely some patients with improvement in an initial renal function insult. When selecting the degree of polynomial to test in our models, we anticipated that there may be up to 2 inflection points in renal trajectories, reflecting the initial renal insult and subsequent stabilization at very low eGFR (i.e., ESRD) or improved renal function (patients with renal recovery); thus, we evaluated up to the third-order polynomial. We did not evaluate models with >5 groups due to decreasing size of trajectory groups (i.e., <5% of the overall cohort). Our final model was selected from all possible candidate models containing up to 5 groups and up to the third-order polynomial, using statistical validity (Bayesian information criterion and the estimate of the log Bayes factor), group size criteria (>5% of the sample) and face validity based on the authors’ clinical expertise. Code for the final model and additional details of model development are included in the Supplementary Methods in Supplementary Appendix A, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25100. After deciding on a model through this process, we labeled each trajectory (e.g., rapid decline, impaired, preserved, recovery) based on the apparent renal function trend in each group. Similar approaches have been published previously (17–19).
Statistical methods for group comparisons.
Measures of central tendency are reported as mean ± SD or median (25th–75th percentile with interquartile range [IQR]). Between-group differences were tested using the chi-square or Fisher’s exact test for categorical data, Kruskal-Wallis test for continuous variables, or analysis of variance for normally distributed continuous variables, and were evaluated between all 4 trajectory groups unless otherwise specified. We used the nonparametric method of the log-rank test to evaluate differences in time to the composite outcome of ESRD or death between groups; we did not perform proportional hazards testing due to violation of the proportional hazards assumption.
Due to the small sample size of the more severe renal dysfunction trajectories (rapid decline and impaired), we collapsed these trajectories into 1 group and compared it with any other group (i.e., preserved/recovery group) for regression analyses. We performed logistic regression to evaluate the relation of hypertension and diabetes mellitus (baseline characteristics a priori known to be associated with kidney disease) to the risk of group membership (coded binarily as rapid decline/impaired versus preserved/recovery). In the logistic regression model we adjusted for age, sex, and ANCA type. Statistical significance was defined as a 2-tailed P value less than 0.05. SAS software, version 9.4 was used for all statistical analysis. Patients were not directly involved in the design of this research.
RESULTS
Of the 484 patients in the overall Mass General Brigham AAV cohort, 255 were included in the final analysis, as they had a baseline creatinine value within 30 days of the index date and at least 1 additional creatinine measurement. The median number of monthly renal function measurements was 9 (IQR 5–15), and the median number of measurements was numerically similar across trajectory groups (P = 0.08) (Table 1). The majority of patients were female (60%) and the mean ± SD age at treatment initiation was 61 ± 17 years (Table 1). Comorbid baseline diabetes mellitus and hypertension at baseline were present in 41 patients (16%) and 122 patients (48%), respectively.
Table 1.
Characteristics of patients at diagnosis stratified by renal function trajectory*
| Characteristic | Overall | Rapid decline | Impaired | Preserved | Recovery | P |
|---|---|---|---|---|---|---|
| No. (%) of cohort | 255 (100) | 20 (8) | 82 (32) | 129 (51) | 24 (9) | – |
| Posterior probability of group membership, median (IQR) | – | 1.0 (1.0–1.0) | 0.99 (0.98–1.0) | 0.99 (0.92–1.0) | 0.99 (0.99–1.0) | – |
| No. of visits (36 months of follow-up), median (IQR) | 9 (5–15) | 7 (4–10) | 10 (7–16) | 8 (6–15) | 8 (4–15) | 0.08 |
| Demographic data | ||||||
| Age at diagnosis, mean ± SD | 61 ± 17 | 62 ± 17 | 64 ± 16 | 59 ± 17 | 63 ± 19 | 0.21 |
| Female | 153 (60) | 11 (55) | 53 (65) | 78 (60) | 11 (46) | 0.40 |
| Race/ethnicity | ||||||
| White | 213 (87) | 18 (95) | 70 (86) | 103 (84) | 22 (92) | |
| Black | 6 (2) | 0 (0) | 3 (4) | 2 (2) | 1 (4) | – |
| Hispanic | 9 (4) | 0 (0) | 2 (2) | 7 (6) | 0 (0) | – |
| Asian | 4 (2) | 1 (5) | 0 (0) | 3 (2) | 0 (0) | – |
| Other | 5 (2) | 0 (0) | 1 (1) | 3 (2) | 1 (4) | – |
| Not recorded | 9 (4) | 0 (0) | 5 (6) | 4 (3) | 0 (0) | – |
| Clinical characteristics at baseline | ||||||
| ANCA type | 0.91 | |||||
| MPO | 182 (71) | 15 (75) | 60 (73) | 91 (71) | 16 (67) | – |
| PR3 | 73 (29) | 5 (25) | 22 (27) | 38 (29) | 8 (33) | – |
| BVAS/GPA, median (IQR) | 4 (4–6) | 5 (4–7) | 4 (4–6) | 4 (3–6) | 6 (4.5–7) | <0.001 |
| Renal† | 175 (69) | 20 (100) | 68 (83) | 63 (49) | 24 (100) | <0.001 |
| Mucosal/ocular† | 24 (9) | 0 (0) | 3 (4) | 20 (16) | 1 (4) | 0.01 |
| Pulmonary† | 106 (42) | 11 (55) | 26 (32) | 63 (49) | 6 (25) | 0.02 |
| Neurologic† | 25 (10) | 2 (10) | 6 (7) | 16 (12) | 1 (4) | 0.49 |
| Biopsy category | ||||||
| No.‡ | 66 | 9 | 31 | 17 | 9 | |
| Crescentic | 16 (24) | 2 (22) | 8 (26) | 3 (18) | 3 (19) | – |
| Focal | 10 (15) | 0 (0) | 7 (23) | 3 (18) | 0 (0) | – |
| Mixed | 14 (21) | 2 (22) | 6 (19) | 3 (18) | 3 (33) | – |
| Sclerotic | 20 (30) | 4 (44) | 8 (26) | 6 (35) | 2 (22) | – |
| Normal or other | 6 (9) | 1 (11) | 2 (7) | 2 (12) | 1 (11) | – |
| Comorbidities | ||||||
| CCI, median (IQR) | 4 (2–6) | 4.5 (3.5–6) | 4 (3–6) | 3 (1–7) | 4 (2.5–5) | 0.01 |
| Baseline diabetes mellitus | 41 (16) | 3 (15) | 17 (21) | 19 (15) | 2 (8) | 0.46 |
| Baseline hypertension§ | 122 (48) | 12 (60) | 43 (52) | 54 (42) | 13 (54) | 0.25 |
| Baseline renal function | ||||||
| Pretreatment eGFR (−365 to −30 days), median (IQR)¶ | 65 (34–81) | 48 (37–88) | 44 (25–72) | 71 (50–81) | 75 (41 −88) | 0.02 |
| Baseline eGFR (±30 days), median (IQR))¶ | 40 (17–80) | 7 (6–9) | 25 (17–36) | 78 (51–92) | 10 (7–16) | <0.001 |
| Induction treatment | ||||||
| CYC-based | 100 (39) | 8 (40) | 34 (41) | 47 (36) | 12 (50) | – |
| RTX-based | 121 (47) | 11 (55) | 40 (49) | 58 (45) | 12 (50) | – |
| Other | 34 (13) | 1 (5) | 8 (10) | 24 (19) | 1 (4) | – |
| Plasma exchange# | 67 (26) | 15 (75) | 25 (30) | 13 (10) | 14 (58) | <0.001 |
| Maintenance treatment | ||||||
| RTX** | 113 (44) | 6 (30) | 39 (48) | 59 (46) | 9 (38) | 0.07 |
| Non-RTX immunosuppression | 116 (45) | 7 (35) | 38 (46) | 62 (48) | 9 (38) | 0.18 |
| None | 33 (13) | 6 (30) | 10 (12) | 15 (12) | 2 (8) | 0.02 |
| Lost to follow-up or deceased before maintenance period | 20 (8) | 3 (15) | 5 (6) | 7 (5) | 5 (21) | 0.02 |
Values are the number (%) unless indicated otherwise. P values shown reflect analysis of variance (for normally distributed continuous variables), Kruskal-Wallis (for other continuous variables), and chi-square or Fisher’s test (for categorical variables) results across the 4 groups. Column sums are >100% in some cases for maintenance treatment because patients could be categorized as receiving both non-rituximab (RTX) maintenance immunosuppression and maintenance RTX. Data in this table are not from the United States Renal Data System. ANCA = antineutrophil cytoplasmic antibody; BVAS = Birmingham Vasculitis Activity Score; CCI = Charlson Comorbidity Index; CYC = cyclophosphamide; eGFR = estimated glomerular filtration rate (ml/minute/1.73 m2); GPA = granulomatosis with polyangiitis; IQR = interquartile range; MPO = myeloperoxidase; PR3 = proteinase 3.
Organ system involvement by BVAS/GPA score.
Total number with biopsy in each group. Columns may add up to smaller numbers due to nonclassifiable or normal biopsies.
Includes n = 3 total patients with comorbid hypertension of unknown onset time.
Relative to the index date (date of treatment initiation); n = 143 for pretreatment eGFR, n = 255 for baseline eGFR.
Plasma exchange was not mutually exclusive with other treatment regimens.
Patients who received short, “bridging” courses of cyclophosphamide with initiation of RTX were categorized as receiving primarily RTX-based induction treatment (n = 67 total, including n = 6 rapid decline, n = 24 impaired, n = 31 stable, and n = 6 recovery.) Maintenance treatment column sums may exceed 100% because patients could be classified as using both RTX and non-RTX maintenance immunosuppression.
Among those with pretreatment (−365 to −30 days of the index date) creatinine measurements available (n = 143), the median pretreatment eGFR was 65 ml/minute/1.73 m2 (IQR 34–81), with 48% of patients having an eGFR of 60 ml/minute/1.73 m2 or less. The median baseline (±30 days of the index date) eGFR among the entire cohort was 40 ml/minute/1.73 m2 (IQR 17–80).
Describing trajectory groups.
We identified 4 trajectory groups of renal function using group-based trajectory modeling. Based on the trajectory of renal function in each group, we refer to these as rapid decline (n = 20 [8%]), impaired (n = 82 [32%]), preserved (n = 129 [51%]), and recovery (n = 24 [9%]) (Figure 1).
Figure 1.
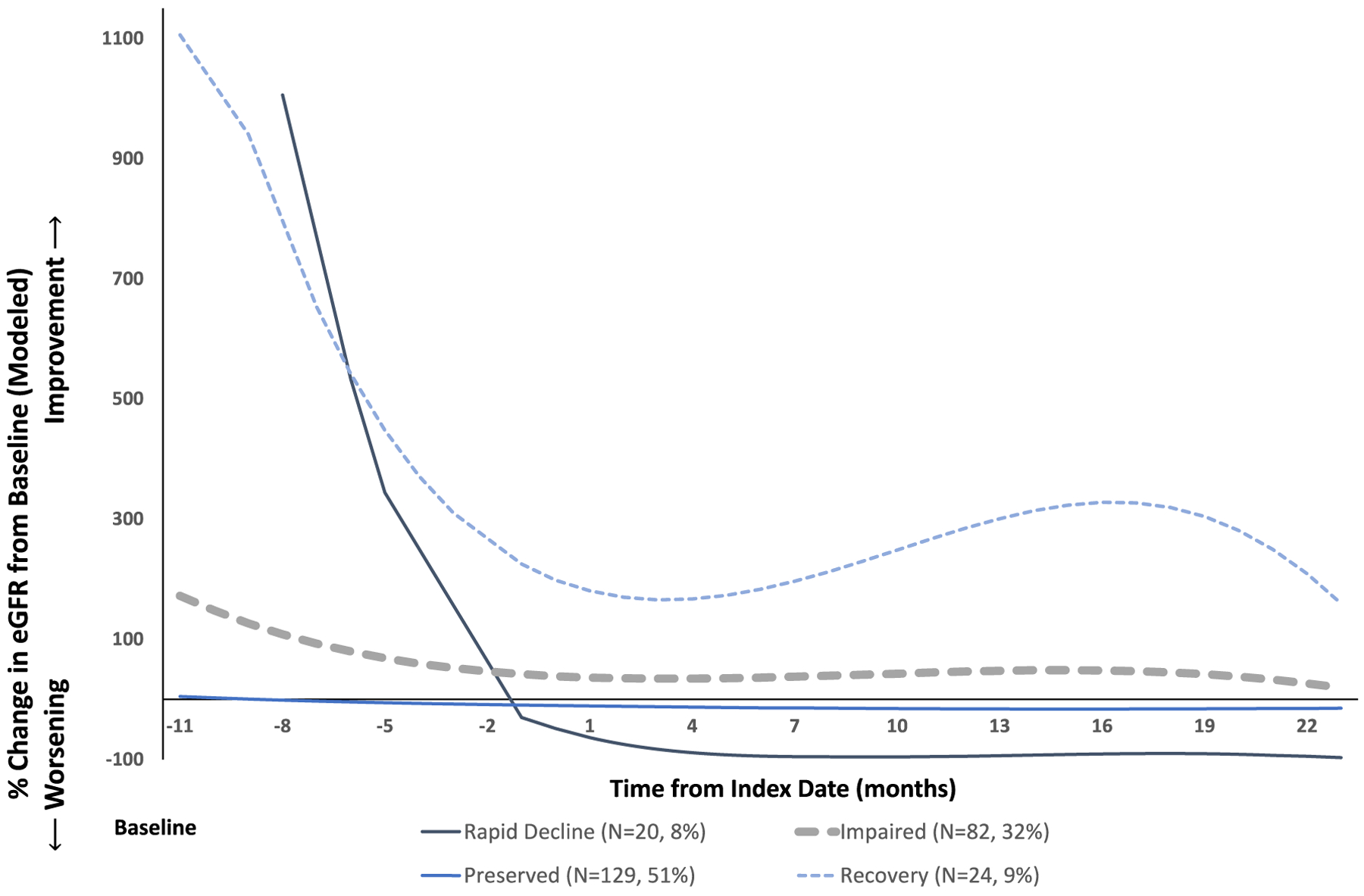
Renal function trajectories in 4 groups identified by group-based trajectory modeling. This chart displays the model estimated renal function measurement at each monthly timepoint within a given trajectory group. eGFR = estimated glomerular filtration rate.
Differences in renal function between groups were observable at baseline. Among the entire cohort, the rapid decline group had the lowest baseline eGFR with a median of 7 ml/minute/1.73 m2 (IQR 6–9) compared to the impaired (median 25 ml/minute/1.73 m2 [IQR 17–36]), preserved (median 78 ml/minute/1.73 m2 [IQR 51–92]), and recovery groups (median 10 ml/minute/1.73 m2 [IQR 7–16]). Because pretreatment renal function measurements were relatively sparse in this data set, there is significant fluctuation, with apparent large increases and decreases in mean eGFR in the smaller trajectory groups (rapid decline and recovery) prior to treatment in the raw within-group averages over time as shown in Supplementary Figure 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25100. These pretreatment fluctuations are less likely to be clinically meaningful than patterns of renal function after treatment initiation; the modeled trajectories, which emphasize the trends that are best supported by our models, are shown in Figure 1.
The trajectory of renal disease after treatment initiation varied between groups (Figures 1 and 2 and Table 2). The rapid decline group was characterized by the rapid development of ESRD. In the impaired group, the eGFR decreased from a pretreatment (−30 to −365 days prior to treatment initiation) median of 44 to 25 ml/minute/1.73 m2 at initiation of treatment (among those with pretreatment eGFR available [n = 49 of 82]). This initial insult observed at the time of diagnosis in the impaired group appeared to slightly improve over follow-up at a group level; however, renal function in this group remained substantially impaired (5-year median eGFR 48 ml/minute/1.73 m2). The impaired group had a lower median eGFR at 2 and 5 years of follow-up compared to the preserved and recovery groups; lower median eGFR was reflected in a greater burden of CKD in the impaired group over time, with >75% of patients in the impaired group remaining with CKD stage 3 or greater at years 1, 2, and 5 (Table 2 and Figure 2). These patterns of kidney disease severity over time were also observed in a sensitivity analysis including only patients who had 5-year follow-up renal function data available (n = 160) (see Supplementary Figure 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25100).
Figure 2.
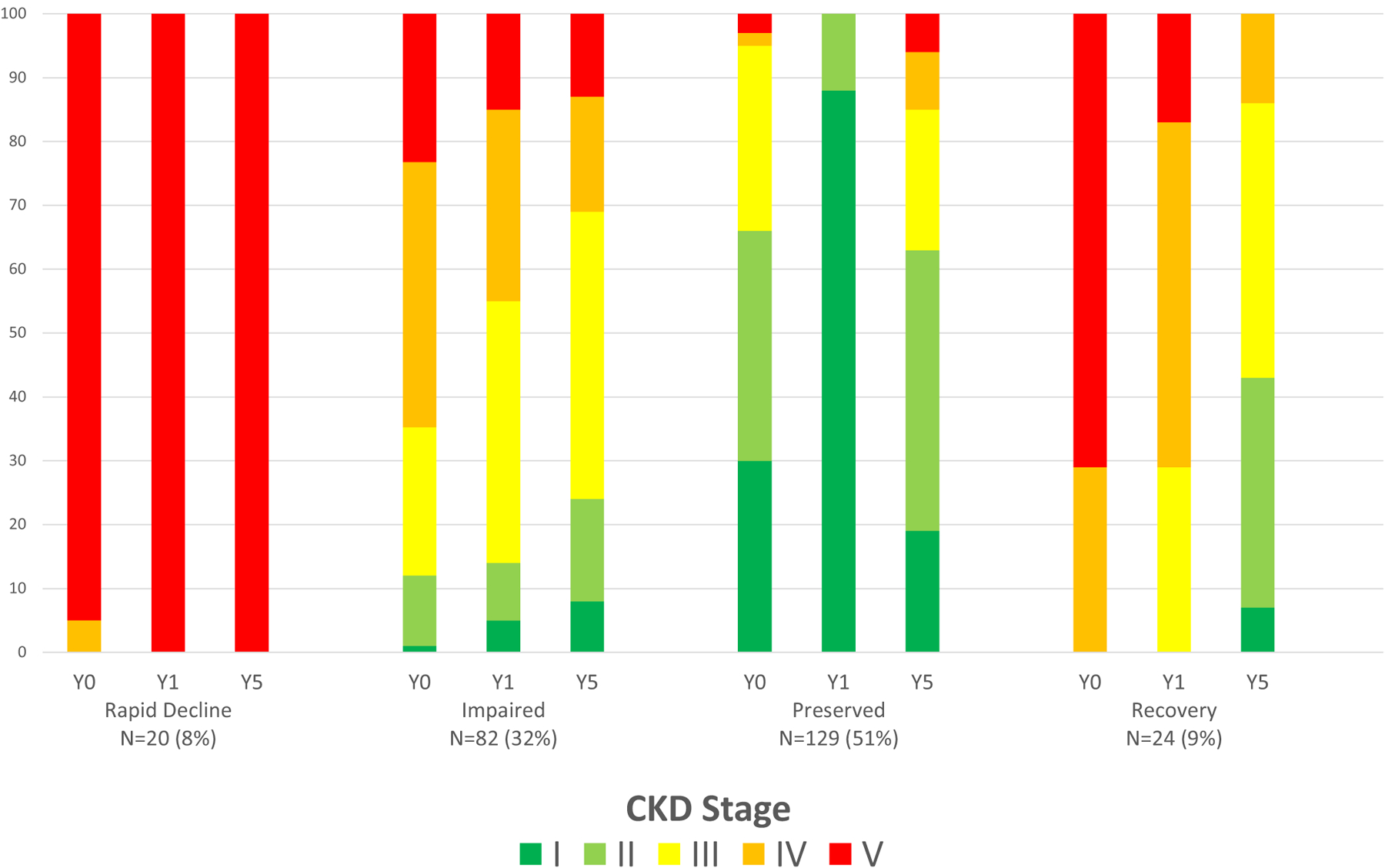
Progression of renal disease as represented by chronic kidney disease (CKD) stage over time among trajectory groups. The horizontal axis depicts the year of assessment of renal function on the top line and the trajectory group on the second line. The CKD stage is depicted in colors ranging from stage 1 (green, estimated glomerular filtration rate [eGFR] >90) to stage 5 (red, eGFR <15). Data in this figure are not from the United States Renal Data System. Y0 = baseline renal function within 30 days of initiation of therapy; Y1 = CKD stage at 1 year of follow-up, averaged over 1 year; Y5 = CKD stage at 5 years of follow-up, averaged over 1 year.
Table 2.
Clinical outcomes, longitudinal renal function, and mortality stratified by trajectory group*
| Outcome | Overall | Rapid decline | Stable impaired | Stable preserved | Recovery | P |
|---|---|---|---|---|---|---|
| No. (%) of cohort | 255 (100) | 20 (8) | 82 (32) | 129 (51) | 24 (9) | – |
| Treatment resistance, no. (%) | ||||||
| Yes | 51 (20) | 17 (85) | 15 (18) | 15 (12) | 4 (17) | – |
| No | 107 (42) | 1 (5) | 49 (60) | 39 (30) | 18 (75) | – |
| Insufficient data | 19 (7) | 2 (10) | 8 (10) | 7 (5) | 2 (8) | – |
| No renal involvement | 78 (31) | 0 (0) | 10 (12) | 68 (53) | 0 (0) | – |
| Renal outcomes | ||||||
| Any dialysis, no. (%) | 52 (20) | 20 (100) | 14 (17) | 13 (10) | 5 (21) | <0.001 |
| Transplant, no. (%) | 8 (3) | 4 (20) | 1 (1) | 3 (2) | 0 (0) | <0.001 |
| Permanent ESRD, no. (%)† | 36 (14) | 20 (100) | 6 (7) | 9 (7) | 1 (4) | <0.001 |
| Active vasculitis as cause of ESRD, no. (%)‡ | 23 (62) | 18 (90) | 1 (17) | 4 (44) | 0 (0) | 0.001 |
| Time to ESRD‡ | 0.34 (0.02–4.7) | 0.02 (0.004–0.15) | 4.2 (1.1–6.8) | 4.7 (2.5–5.6) | 10 (10–10) | – |
| eGFR at 1 year | 43 (23–76) | 2 (1–5) | 32 (22–46) | 71 (44–89) | 24 (18–36) | <0.001 |
| eGFR at 2 years | 53 (31–77) | 0 (0–0) | 41 (26–58) | 69 (45–86) | 48 (29–61) | <0.001 |
| eGFR at 5 years | 54 (29–77) | 0 (0–0) | 48 (26–60) | 69 (43–84) | 58 (36–71) | <0.001 |
| CKD 3+ at 1 year, no. (%) | 162 (64) | 20 (100) | 71 (87) | 47 (36) | 24 (100) | <0.001 |
| CKD 3+ at 2 years, no. (%) | 117 (58) | 12 (100) | 54 (79) | 39 (36) | 12 (75) | <0.001 |
| CKD 3+ at 5 years, no. (%) | 89 (56) | 10 (100) | 39 (76) | 32 (38) | 8 (57) | <0.001 |
| Mortality | ||||||
| Death, no. (%) | 70 (27) | 8 (40) | 27 (33) | 29 (22) | 6 (25) | 0.21 |
| Follow-up time to ESRD, death, or censorship | 6.5 (3.9–9.8) | 0.2 (0.004–0.15) | 6.4 (4.0–9.8) | 7.3 (5.0–10) | 7.4 (5.1–10) | <0.001 |
| Follow-up time to death or censorship | 7.3 (4.7–10) | 6.8 (1.8–10) | 6.6 (4.0–10) | 7.4 (5.5–10) | 7.4 (5.1–10) | – |
Values are the median (interquartile range) unless indicated otherwise. End-stage renal disease (ESRD) was defined as 1) a need for dialysis for >60 days, 2) dialysis until death if the patient died between 14 and 60 days of follow-up, or 3) renal transplant, as identified by chart review and US Renal Data System records. Because temporary dialysis was included in the count of patients with dialysis, more patients received dialysis than experienced ESRD by this definition. Data in this table are not from the United States Renal Data System. CKD = chronic kidney disease; CKD 3+ = CKD stage 3 or higher, i.e., estimated glomerular filtration rate (eGFR) <60 ml/minute/1.73 m2.
ESRD newly occurring during follow-up, i.e., no earlier than 1 year prior to initiation of treatment for vasculitis.
Among those with ESRD.
The renal recovery group showed an initial decrement in renal function that dramatically improved; unlike the impaired group, the recovery group frequently had resolution of clinically significant CKD (stage 3+) (Figure 2). The recovery group had a baseline median eGFR of 10 ml/minute/1.73 m2 (IQR 7–16) which improved to 24 ml/minute/1.73 m2 (IQR 18–35) by 1 year and 58 ml/minute/1.73 m2 (IQR 33–71) by 5 years of follow-up. The preserved group had little change in renal function during follow-up. ESRD was uncommon in the recovery and preserved groups (1 [4%] and 9 [7%], respectively).
Baseline features associated with trajectory group membership.
Age, sex, and race were not statistically different between groups (Table 1). However, the baseline comorbidity burden, as measured by the Charlson Comorbidity Index, was greater in the rapid decline and impaired groups (4.5 [IQR 3.5–6] and 4 [IQR 3–6], respectively) compared to the preserved group (3 [IQR 1–7]); P = 0.01). The preserved renal function group had the lowest proportion, with baseline AAV-associated renal involvement (n = 63 [49%] in preserved compared to n = 20 [100%] in rapid decline, n = 68 [83%] in impaired, and n = 24 [100%] in recovery). The distribution of myeloperoxidase- versus proteinase 3–ANCA type was similar across groups (P = 0.91).
Renal biopsies were uncommon (n = 66 [26%]) in this cohort. Histopathologic categorization among rapid decline patients (number with biopsy = 9 of 20) was more often sclerotic (n = 4 [44%]), compared to the impaired group (number with biopsy = 31 of 82; sclerotic categorization observed in 8 patients [26%]). Despite this trend, no statistically significant differences were observed across groups (P = 0.86).
We observed differences in the proportion of subjects in each trajectory with hypertension, a key driver of CKD in the general population. A history of hypertension at baseline was most common among patients in the rapid decline group (n = 12 [60%]), followed by patients in the impaired and recovery groups (n = 43 [52%] and n = 13 [54%], respectively). Hypertension was less common in the preserved group, although these differences did not meet statistical significance (n = 54 [42%]; P = 0.25 for difference across all 4 groups) (Table 1). There was no strong association observed between a history of diabetes mellitus at baseline and trajectory group (P = 0.46). After adjustment for age, sex, and ANCA type, neither baseline hypertension (adjusted odds ratio [OR] 1.1 [95% confidence interval (95% CI) 0.6–2.1]) nor diabetes mellitus (adjusted OR 1.3 [95% CI 0.6–2.8]) was associated with the outcome of membership in a composite renal dysfunction group (collapsed rapid decline and impaired groups together). In the subgroup of patients with CKD stage 3 or 4 at baseline, differences in comorbidity burden were less striking between the impaired and preserved groups, suggesting that other factors may drive renal function trajectories in this subgroup (see Supplementary Table 2 and Supplementary Results in Supplementary Appendix A, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25100).
Use of AAV-specific treatments across trajectory groups.
Cyclophosphamide- and rituximab-based induction regimens were used at similar rates between groups, with the exception that non-rituximab, non-cyclophosphamide–based induction regimens (steroids alone or conventional disease-modifying antirheumatic drugs) were used more frequently in the preserved group (n = 24 [19%] versus 4–10% in all other groups). Plasma exchange was used more frequently among patients with either rapid decline or renal recovery, which was expected given the indication for consideration of this treatment among individuals with severe renal injury (n = 15 [75%] rapid decline and n = 14 [58%] recovery, versus impaired n = 25 [30%] and preserved n = 13 [10%]; P < 0.001). Rituximab and non-rituximab maintenance medications were used at statistically similar rates across groups overall (P = 0.07 and P = 0.18, respectively); however, they were somewhat less frequently prescribed to patients in the rapid decline group.
Outcomes of ESRD and mortality across trajectory groups.
Over a mean follow-up of 73 months, 36 patients (14%) experienced ESRD (2.3 ESRD events per 100 person-years) (Table 2). Of the 36 patients with ESRD, 2 did not have baseline renal involvement by AAV; ESRD was attributed to an unrelated immune complex glomerulonephritis in 1 case and a preexisting condition dating back to childhood in another case. The majority of ESRD occurred in the rapid decline group (n = 20 [56% of all ESRD events]), followed by the preserved group (n = 9 [25%]) and the impaired group (n = 6 [17%]). Among those patients who experienced ESRD in the rapid decline group and impaired groups, ESRD occurred at a median of 0.02 years (IQR 0.004–0.2) after the index date compared to 4.2 years (IQR 1.1–6.8) in the impaired group. The composite outcome of ESRD or death occurred earliest in the rapid decline group, followed by the impaired group and then the preserved and recovery groups (log-rank P < 0.001). Renal treatment resistance was more common in the rapid decline than the impaired group (n = 17 [85%] versus n = 15 [18%]; P < 0.001).
Differences in the etiology of ESRD were observed across trajectory groups. Active vasculitis was thought to be the cause of ESRD in 90% of patients categorized as rapid decline (n = 18 of 20), compared to 17% of patients categorized as impaired (n = 1 of 6), 44% of patients in the preserved group (n = 4 of 9) and no patients in the recovery group (n = 0 of 1; P = 0.001 for comparison across 4 groups).
Over a mean follow-up of 81 months, 70 patients (27%) died (4.1 deaths per 100 person-years) (Table 2). Although the small size of some trajectory groups limits conclusions, a greater proportion of deaths was observed in the groups characterized by worse renal function (rapid decline: n = 8 [40%], impaired: n = 27 [33%], preserved: n = 29 [22%], recovery: n = 6 [25%]).
DISCUSSION
We used an agnostic methodologic approach to identify 4 patterns of longitudinal renal function in an incident cohort of AAV patients with diverse manifestations. Each trajectory group was characterized by distinct courses with respect to renal function, such that patients with persistent renal dysfunction generally follow 1 of 2 clinical courses: precipitous decline to ESRD, or chronic impaired renal function, during which many patients are left with advanced CKD and a portion develop ESRD. Nearly 70% of patients with ESRD reach that endpoint from these 2 CKD trajectories, which are distinguished from other groups by demographic and clinical features. Our findings provide an innovative approach to conceptualize the impact of a new diagnosis of AAV on renal function and highlight groups that would benefit from personalized, multidisciplinary approaches to care. These trajectories serve as a framework within which strategies can be developed, tested, and implemented to further improve AAV outcomes, especially for CKD.
Patients in the rapid decline group had quick, nearly universal onset of ESRD with very severe renal impairment at treatment initiation. Given that the eGFR prior to diagnosis in the rapid decline group was lower than that observed in the recovery group (at 48 ml/minute/1.73 m2 compared to >70), they may have been affected by a more indolent, subclinical progression of vasculitic renal damage prior to diagnosis. Alternatively, patients in the rapid decline group may have had preexisting kidney disease due to hypertension and other comorbidities that predisposed them to rapid, irreversible renal deterioration with AAV onset. These possibilities highlight the potential impact of delays in AAV diagnosis as well as the uncertainties regarding whether ideal treatment for patients presenting with AAV renal involvement might vary based on their prediagnosis renal function, if available (20,21). A strength of our study in contrast to others was the availability, in a subset of patients, of prediagnosis measures of renal function.
Of particular interest regarding renal function trajectories are the characteristics that distinguish the impaired group from preserved and recovery groups, given the differences between groups in the etiology of ESRD occurring months to years after AAV diagnosis. Previous studies have suggested that a subset of AAV patients develop late-onset ESRD in the absence of clinically evident active AAV; these patients likely reflect the impaired trajectory phenotype, as illustrated in our study (3). The impaired group was older than the preserved and recovery groups and had higher rates of general comorbidity compared to the preserved group; renal involvement by AAV and hypertension was more common in the impaired group than the preserved group. These observations highlight high-risk patients who might benefit from a personalized approach to care based on their age, comorbidity burden, and renal function trajectory. Indeed, even by 3 months, there were striking differences in the trajectory of renal function among those classified in the impaired versus recovery groups, highlighting the implications of persistent renal dysfunction at this time point.
Our findings provide important empirical evidence in an incident AAV cohort, followed from diagnosis, that both comorbidities and the history of prior renal involvement by AAV are influential factors contributing to CKD and progression to ESRD months to years after diagnosis. Additional studies are needed to evaluate whether longitudinal assessment of renal biomarkers beyond serum creatinine and urinalysis, such as soluble CD163, CD25 and others, may identify patients who stand to benefit from modified or intensified immunosuppression as opposed to more aggressive ESRD risk factor modification (22,23). This type of personalized care for patients at risk for renal impairment may improve outcomes by helping to prevent or reduce the progression of CKD in AAV. Identifying and studying these trajectories is increasingly important as survival improves for patients with AAV and management strategies evolve (24–27). The need to identify and study is especially true in the face of the ongoing COVID-19 pandemic, as providers and patients weigh individualized decisions regarding the risks of decreasing or holding immunosuppression. Robust data to inform renal risk stratification in these scenarios would be of significant clinical utility.
Previous work has established several risk models for the outcome of ESRD in AAV, incorporating biopsy features, age, ANCA serotype, induction therapy type, and initial renal function (4,28–30). This study adds to the existing literature via an unbiased approach to describe the longitudinal arc of renal function and CKD in AAV in more nuanced terms than prior ESRD-focused work in this space. Our work quantitatively confirms the existence and frequency of a phenotype with largely stable but clinically significant renal function impairment. Additionally, we performed these analyses in an AAV cohort with diverse manifestations and have provided detailed examination of the clinical features of patients exhibiting these trajectories, which lends greater generalizability to our work.
Our study has certain limitations. First and most importantly, we believe that the current investigation provides important preliminary evidence that there are clinically distinct renal phenotypes or trajectories that exist in AAV; however, given our small sample size, the single health care system in which our study was conducted, and the need for validation in other cohorts, based on this study alone we cannot apply the concept of trajectory group membership to individual patients in clinical practice. Additional studies will be necessary to validate these observed trajectories in other cohorts. Second, attributing causality of renal outcomes or trajectory to the treatments received is not possible, due to the likely role of confounding by indication. However, most treatment strategies were distributed similarly across trajectory groups. Third, renal biopsies were not obtained in all patients, limiting our ability to associate specific renal biopsy features with trajectory membership. However, biopsies are uncommonly obtained in the context of positive ANCA tests with a consistent clinical context, as previously observed in our health care system (12). Fourth, our definition of renal treatment resistance may be biased by persistent hematuria in patients with renal damage despite lack of ongoing active vasculitis; however, the definition of treatment resistance reflects a similar definition used in another study assessing progression to ESRD in AAV. Finally, small sample sizes in some of our trajectory groups limit the assessment of statistically significant differences between groups and may increase the likelihood of Type 2 errors, especially for heterogeneous outcomes, such as treatment or AAV-related organ involvement.
In this AAV cohort, we identified 4 distinct patterns of change in renal function. The increased baseline comorbidity and risk of clinically significant CKD in the rapid decline and impaired trajectories underscores the importance of personalized, multidisciplinary care for AAV patients; further investigation of tailored strategies to preserve renal function is warranted. Our findings provide a framework for future research into next steps to improve renal outcomes for this vulnerable population.
Supplementary Material
SIGNIFICANCE & INNOVATIONS.
Chronic kidney disease is a common and potentially devastating complication of antineutrophil cytoplasmic antibody–associated vasculitis (AAV) when it culminates in end-stage renal disease (ESRD). Attention has focused on identification of risk factors for ESRD, but less is known about the spectrum of longitudinal changes in renal function in AAV following diagnosis.
We present an innovative trajectory analysis of longitudinal renal function data that identifies 4 renal trajectory groups, including rapidly declining renal function, impaired renal function, preserved renal function, and renal recovery. The rapid decline and impaired groups had a greater burden of clinically significant kidney disease as well as overall comorbidity compared to the groups with preserved function or recovery.
Our findings provide an approach that may be leveraged in future studies to inform how we might develop, test, and implement strategies that personalize care for patients with AAV. Such personalized approaches may help prevent or slow the progression of chronic kidney disease in this vulnerable population.
Acknowledgments
Supported by the Massachusetts General Hospital Department of Medicine Scholars Research Funding and by the Rheumatology Research Foundation (Resident Research Award). Dr. Wallace’s work was supported by the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (K23-AR-073334 and R03-AR-078938) and by the Rheumatology Research Foundation (K Supplement).
Footnotes
The data reported here have been supplied by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Author disclosures and a graphical abstract can be found online at https://onlinelibrary.wiley.com/doi/10.1002/acr.25100.
REFERENCES
- 1.Binda V, Moroni G, Messa P. ANCA-associated vasculitis with renal involvement. J Nephrol 2018;31:197–208. [DOI] [PubMed] [Google Scholar]
- 2.Sagmeister MS, Grigorescu M, Schonermarck U. Kidney transplantation in ANCA-associated vasculitis. J Nephrol 2019;32:919–26. [DOI] [PubMed] [Google Scholar]
- 3.Lionaki S, Hogan SL, Jennette CE, et al. The clinical course of ANCA small-vessel vasculitis on chronic dialysis. Kidney Int 2009;76: 644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brix SR, Noriega M, Tennstedt P, et al. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int 2018;94:1177–88. [DOI] [PubMed] [Google Scholar]
- 5.Menez S, Hruskova Z, Scott J, et al. Predictors of renal outcomes in sclerotic class anti-neutrophil cytoplasmic antibody glomerulonephritis. Am J Nephrol 2018;48:465–71. [DOI] [PubMed] [Google Scholar]
- 6.Nagin DS, Jones BL, Passos VL, et al. Group-based multi-trajectory modeling. Stat Methods Med Res 2018;27:2015–23. [DOI] [PubMed] [Google Scholar]
- 7.Wallace ZS, Fu X, Harkness T, et al. All-cause and cause-specific mortality in ANCA-associated vasculitis: overall and according to ANCA type. Rheumatology (Oxford) 2020;59:2308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watts R, Lane S, Hanslik T, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007;66:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukhtyar C, Lee R, Brown D, et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 2009;68:1827–32. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Int Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berden AE, Ferrario F, Hagen EC, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 2010; 21:1628–36. [DOI] [PubMed] [Google Scholar]
- 12.Cook CE, Fu X, Zhang Y, et al. Validation of antineutrophil cytoplasmic antibody-associated vasculitis as the cause of end-stage renal disease in the US Renal Data System. ACR Open Rheumatol 2022:4; 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res 2007;35:542–71. [Google Scholar]
- 14.Yeo IK, Johnson RA. A new family of power transformations to improve normality or symmetry. Biometrika 2000;87:954–9. [Google Scholar]
- 15.Nguena Nguefack HL, Page MG, Katz J, et al. Trajectory modelling techniques useful to epidemiological research: a comparative narrative review of approaches. Clin Epidemiol 2020;12:1205–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andruff H, Carraro N, Thompson A, et al. Latent class growth modelling: a tutorial. Tutorials Quant Meth Psychol 2009;5:11–24. [Google Scholar]
- 17.Hanberg JS, Akgun KM, Hsieh E, et al. Incidence and presentation of sarcoidosis with and without HIV infection. Open Forum Infect Dis 2020;7:ofaa441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall BD, Tate JP, McGinnis KA, et al. Long-term alcohol use patterns and HIV disease severity. AIDS 2017;31:1313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boucquemont J, Loubere L, Metzger M, et al. Identifying subgroups of renal function trajectories. Nephrol Dial Transplant. 2017;32 Suppl 2:ii185–93. [DOI] [PubMed] [Google Scholar]
- 20.Taimen K, Mustonen A, Pirila L. The delay and costs of diagnosing systemic vasculitis in a tertiary-level clinic. Rheumatol Ther 2021;8: 233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sreih AG, Cronin K, Shaw DG, et al. Diagnostic delays in vasculitis and factors associated with time to diagnosis. Orphanet J Rare Dis 2021; 16:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dekkema GJ, Abdulahad WH, Bijma T, et al. Urinary and serum soluble CD25 complements urinary soluble CD163 to detect active renal anti-neutrophil cytoplasmic autoantibody-associated vasculitis: a cohort study. Nephrol Dial Transplant 2019;34:234–42. [DOI] [PubMed] [Google Scholar]
- 23.Moran SM, Scott J, Clarkson MR, et al. The clinical application of urine soluble CD163 in ANCA-associated vasculitis. J Am Soc Nephrol 2021;32:2920–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace ZS, Miloslavsky EM. Management of ANCA associated vasculitis. BMJ 2020;368:m421. [DOI] [PubMed] [Google Scholar]
- 25.Wallace ZS, Lu N, Miloslavsky E, et al. Nationwide trends in hospitalizations and in-hospital mortality in granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res (Hoboken) 2017;69:915–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace ZS, Lu N, Unizony S, et al. Improved survival in granulomatosis with polyangiitis: a general population-based study. Semin Arthritis Rheum 2016;45:483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holle JU, Gross WL, Latza U, et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum 2011;63:257–66. [DOI] [PubMed] [Google Scholar]
- 28.De Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, et al. Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: a prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol 2006;17:2264–74. [DOI] [PubMed] [Google Scholar]
- 29.Hauer HA, Bajema IM, Van Houwelingen HC, et al. Determinants of outcome in ANCA-associated glomerulonephritis: a prospective clinico-histopathological analysis of 96 patients. Kidney Int 2002;62: 1732–42. [DOI] [PubMed] [Google Scholar]
- 30.Lee T, Gasim A, Derebail VK, et al. Predictors of treatment outcomes in ANCA-associated vasculitis with severe kidney failure. Clin J Am Soc Nephrol 2014;9:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


