Abstract
T-cell antigen receptor (TCR) engagement activates multiple protein tyrosine kinases (PTKs), including the Src family member, Lck, and the Syk-related PTK, ZAP-70. Studies in ZAP-70-deficient humans have demonstrated that ZAP-70 plays crucial roles in T-cell activation and development. However, progress toward a detailed understanding of the regulation and function of ZAP-70 during TCR signaling has been hampered by the lack of a suitable T-cell model for biochemical and genetic analyses. In this report, we describe the isolation and phenotypic characterization of a Syk- and ZAP-70-negative somatic mutant derived from the Jurkat T-cell line. The P116 cell line displays severe defects in TCR-induced signaling functions, including protein tyrosine phosphorylation, intracellular Ca2+ mobilization, and interleukin-2 promoter-driven transcription. These signaling defects were fully reversed by reintroduction of catalytically active versions of either Syk or ZAP-70 into the P116 cells. However, in contrast to ZAP-70 expression, Syk expression triggered a significant degree of cellular activation in the absence of TCR ligation. Transfection experiments with ZAP-70–Syk chimeric proteins indicated that both the amino-terminal regulatory regions and the carboxy-terminal catalytic domains of Syk and ZAP-70 contribute to the distinctive functional properties of these PTKs. These studies underscore the crucial role of ZAP-70 in TCR signaling and offer a powerful genetic model for further analyses of ZAP-70 regulation and function in T cells.
Ligation of the T-cell antigen receptor (TCR) triggers a cascade of intracellular signals that culminate in cytokine gene expression, proliferation, and the execution of T-cell effector functions. Signal transmission from the TCR is mediated by the sequential activation of two families of protein tyrosine kinases (PTKs) (6, 30). Members of the Src family, Lck and FynT, initiate this process by phosphorylating tyrosine residues in the cytoplasmic domains of the CD3 and ζ subunits (30, 53). These tyrosines are embedded within conserved signaling modules termed immunoreceptor tyrosine-based activation motifs (ITAMs) (28, 38, 44). The phosphorylated ITAMs serve as docking sites for the recruitment of a Syk family PTK, most commonly ZAP-70, to the activated receptor complex (10, 26, 53, 58). The clustered Src and Syk family PTKs subsequently phosphorylate a series of cytoplasmic substrates, including PLC-γ1, Vav, Slp-76, and p36. However, the specific contribution of each receptor-associated PTK to the phosphorylation of downstream substrates remains largely undefined.
Genetic studies involving T-cell somatic mutants and gene-targeted mice have dramatically underscored the critical roles of Lck and FynT in TCR signaling. Targeted disruption of the Lck and FynT genes in mice causes defects in T-cell development and TCR responsiveness to ligand stimulation (1, 25, 39, 49, 54). Lck-negative somatic mutants derived from the human leukemic T-cell line, Jurkat, or the murine cytotoxic T-cell line, CTLL-2, also display severe defects in TCR signaling (31, 50).
The importance of ZAP-70 in T-cell activation and development is highlighted by the identification of a familial form of severe combined immunodeficiency caused by loss of function mutations in both ZAP-70 alleles (2, 12, 19, 23). These patients lack mature CD8+ T cells, and the low numbers of CD4+ T cells that do exit the thymus are nonresponsive to stimulation by TCR ligands. Mice rendered nullizygous at the ZAP-70 gene locus display an even more severe phenotype characterized by the complete absence of CD4+ and CD8+ T cells (40). Although these studies indicate that ZAP-70 is critical for signaling through the TCR, the actual contributions of this PTK to the tyrosine phosphorylation of downstream substrates are poorly understood. Several proteins, including SLP-76, α-tubulin, and the human erythrocyte band 3 protein, have been identified as in vitro substrates for ZAP-70 (29, 59), and it is generally believed that ZAP-70 directly phosphorylates specific target proteins in activated T cells (8). In addition, ZAP-70 itself undergoes phosphorylation on multiple tyrosine residues in response to TCR stimulation. These phosphotyrosines may create binding sites for SH2 domain-containing proteins, perhaps facilitating their phosphorylation by the colocalized Src family PTKs, Lck and FynT (41).
The role of Syk in signal transduction through the TCR is less well defined. Although Syk is expressed in developing αβ T cells, thymocyte maturation is not grossly impaired in Syk−/− mice, suggesting that the loss of this PTK is effectively compensated for by the coexpressed ZAP-70 (13, 52). The functional overlap between Syk and ZAP-70 is further illustrated by the ability of either PTK to restore B-cell antigen receptor signaling in a Syk−/− B-cell line (36). However, accumulating data suggest that Syk and ZAP-70 exhibit significant differences in their mechanisms of activation by receptor-mediated stimuli (34). Whereas binding of Syk to a phosphorylated ITAM is sufficient to activate the Syk catalytic domain (15, 45, 48), ZAP-70 requires an additional stimulatory input from Lck, which phosphorylates ZAP-70 at its regulatory Y493 residue (9, 29, 35, 41, 57). These observations were recently extended with the finding that Syk, but not ZAP-70, is capable of reversing the TCR signaling deficits observed in both Lck-negative and CD45-negative Jurkat T cells (14).
Studies aimed toward understanding the regulation and function of ZAP-70 and Syk in T cells would be greatly facilitated by the availability of a genetically tractable T-cell model that is deficient in expression of both PTKs. In this study, we provide the initial description of a ZAP-70- and Syk-deficient somatic mutant derived from the Jurkat E6 T-cell line. The P116 subclone displays severe defects in TCR signaling functions that are corrected by reintroduction of wild-type but not catalytically inactive ZAP-70. The TCR signaling defects observed in P116 cells were also reversed by expression of Syk; however, the activation requirements for Syk and ZAP-70 were notably different in P116 cells. Transfection studies with ZAP-70–Syk chimeric proteins revealed that both the amino-terminal regulatory and the carboxy-terminal catalytic domains of these PTKs contributed to their distinctive functional activities in P116 cells. The P116 cell line therefore represents a useful model in which the roles of Syk family PTKs in lymphocyte signaling can be dissected.
MATERIALS AND METHODS
Reagents and cell lines.
The human CD3-ɛ-specific monoclonal antibody OKT3 (55) and the monoclonal antibody (MAb) 9E10 (21), which recognizes a peptide from the Myc polypeptide, were purified from murine ascites by chromatography over a protein G-agarose affinity column. Monoclonal antiphosphotyrosine antibody 4G10 was obtained from Upstate Biotechnology, Inc. (Lake Placid, N.Y.). The rabbit polyclonal anti-PLC-γ1 and anti-ζ antisera have been described previously (42, 51). The sheep polyclonal anti-SLP-76 antiserum was provided by Gary Koretzky (University of Iowa). The ZAP-70-specific antiserum was prepared by immunization of rabbits with a keyhole limpet hemocyanin-coupled peptide corresponding to residues 326 to 341 of human ZAP-70.
The human leukemic T-cell line Jurkat, subclone E6, and all mutant cell lines derived from this subclone were maintained in standard growth medium (RPMI 1640 medium supplemented with 5% fetal bovine serum, 5% bovine calf serum, 10 mM HEPES [pH 7.4], 2 mM l-glutamine, and 50 μM β-mercaptoethanol). Cells were maintained at culture densities below 5 × 105 cells per ml. J.CaM1 cells and K562 erythroleukemia cells were obtained from American Type Culture Collection (Rockville, Md.). The B-cell lymphoma Tf.wild (5) was kindly provided by David McKean.
Plasmids.
The wild-type ZAP-70 expression vector was prepared by amplification of the full-length open reading frame of ZAP-70 by reverse transcriptase PCR, with Jurkat cell-derived mRNA as the template. The cDNA was sequenced to verify the fidelity of the PCR and was cloned into the EcoRI-NotI restriction sites in the mammalian expression vector, pcDNA3 (Invitrogen), to yield the pcDNA3-ZAP plasmid. The 5′ terminus of the ZAP-70 open reading frame was modified to encode the Myc epitope tag recognized by MAb 9E10. The resulting expression vector was designated pcDNA3-mZAP. The cDNA encoding a catalytically inactive ZAP mutant (Lys369 → Arg) was similarly tagged with the Myc epitope coding sequence and was cloned into the pSX plasmid (57). The cDNA insert was excised from this plasmid with BamHI and subcloned into the BamHI site of pBJneo to generate the pBJ-mZAPKD vector used in the present study.
Porcine Syk was excised from a CDM12 vector encoding a CD16:7:PSyk fusion protein (kindly provided by Brian Seed, Massachusetts General Hospital, Boston, Mass.) by digestion with MluI and NotI. The restriction fragment was ligated into pcDNA3 at the EcoRV and NotI sites. To generate the pcDNA3-mSyk plasmid, the 5′ terminus of the Syk cDNA was modified by PCR to append the Myc epitope tag to the encoded Syk protein.
ZAP-70–Syk chimeric constructs were prepared in the pcDNA3 expression vector. First, silent mutations were introduced into pcDNA3-mZAP and pcDNA3-mSyk with the Transformer kit (Clontech) and the following primers: CTCAAGGACAAGAAGCTTTTCCTGAAGCGC (for ZAP-70) and CTGGGAAGCTGTCCATCCCCG, GACCGGAAGCTTCTGACCCTGGAG (for Syk). These mutations generate a unique HindIII restriction site in the nucleotides encoding amino acids located at the junction of the linker regions and catalytic domains of Syk (residues 368 to 369) and ZAP-70 (residues 329 to 330). Restriction digests with EcoRI-HindIII or HindIII-NotI were performed, and the resulting fragments were ligated into the EcoRI-NotI restriction sites of pcDNA3 to create pcDNA3-mZAPSyk and pcDNA3-mSykZAP.
The bacterial expression plasmid cdb3 T7-7 (56), which encodes the cytoplasmic domain of human band 3 (cdb3), was kindly provided by Philip Low (Purdue University, West Lafayette, Ind.). A HindIII-NotI fragment containing the cdb3 coding sequence was ligated into the bacterial expression vector, pET-28a (Novagen), which fuses an in-frame six-histidine (His6) tag to the amino terminus of cdb3. Recombinant His6-cdb3 was expressed in Escherichia coli and was purified over a nickel-chelating resin according to the manufacturer’s instructions.
Derivation of ZAP-70-negative Jurkat somatic mutant.
Jurkat cells were suspended at 5 × 105 cells per ml in standard growth medium containing 5 μg of ICR-191 (Sigma) per ml. After 6 h at 37°C, the mutagenized cells were washed three times with phosphate-buffered saline and resuspended at a cell density of 2 × 105 cells per ml in standard growth medium. The cells were cultured for 2 weeks to allow recovery from the mutagenesis procedure, and then selection for Ca2+ signaling mutants was initiated. The cells were loaded with the Ca2+ indicator dye, indo-1 (see below), and placed on-line in a FACStar Plus cell sorter (Becton-Dickinson). Intracellular Ca2+ mobilization was stimulated with 100 μM pervanadate. After approximately 90 s at 37°C, cells that failed to respond to the stimulus were collected by cell sorting. Preliminary experiments demonstrated that virtually 100% of unmutagenized Jurkat cells displayed a clear increase in their 405- to 495-nm fluorescence emission ratios (a direct reflection of the ratio of Ca2+-bound to -unbound indo-1) after exposure to pervanadate for 90 s. The sorted cells were diluted into standard growth medium, and cell numbers were expanded in culture, at which time the selection procedure was repeated. After four cycles, the sorted cell population contained greater than 50% pervanadate-nonresponsive cells. The cells were stained with fluorescein isothiocyanate-conjugated anti-CD3 (OKT3) MAb (Becton Dickinson), and TCR-positive cells were plated into 96-well plates at 1 cell per well with an automated cell depositing unit on a FACS Vantage cell sorter.
Intracellular free Ca2+ measurements.
Jurkat cells were harvested from growth medium, and 15 × 106 cells were resuspended in 1.5 ml of solution no. 1 (Hanks balanced salt solution supplemented with 5 mM dextrose and buffered to pH 7.0 with 10 mM HEPES). Indo-1-acetoxymethylester (Calbiochem) was dissolved in dimethylsulfoxide and added to the cell suspension to a final concentration of 5 μM. After 30 min at 37°C, an equal volume of solution no. 2 (Hanks balanced salt solution containing 5 mM dextrose and buffered at pH 7.4 with 10 mM HEPES) was added and cells were incubated for an additional 30 min. The indo-1 loaded cells were washed with 10 volumes of solution no. 3 (solution no. 2 supplemented with 0.05% bovine serum albumin [BSA]) and resuspended in solution no. 3 at a density of 1 × 106 cells/ml. Samples were incubated for 5 min at 37°C, and pervanadate was added to a final concentration of 100 μM. The pervanadate stock solution was prepared by mixing 2.3 μl of 30% H2O2 (Sigma) with 1 ml of 20 mM Na3VO4 and allowing the mixture to react for 5 min at room temperature. Stimulus-induced changes in intracellular Ca2+ concentration ([Ca2+]i) in the cell population were determined by monitoring the fluorescence emission ratio at 405 to 495 nm as described above.
Northern analysis.
Total cellular RNA was isolated with RNAzol B (Cinna/Biotecx Laboratories, Inc.). The RNA (30 μg) was electrophoresed through a 1% agarose gel containing 6% formaldehyde and transferred to a nitrocellulose membrane. The membrane was sequentially probed with a XhoI-ScaI fragment from the ZAP-70 cDNA and a PstI fragment from pIBI30-GADPH (provided by Selina Shen-Kiang, Mt. Sinai School of Medicine, New York, N.Y.) which hybridizes to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. The DNA probes were radiolabeled with [α-32P]CTP with random oligonucleotide primers (Oligolabelling kit, Pharmacia).
Immunoblot analysis.
For ZAP-70 or Myc epitope tag immunoblots, 106 Jurkat cells were lysed in 30 μl of buffer A (20 mM Tris, 40 mM NaCl, 50 mM NaF, 30 mM sodium pyrophosphate, 5 mM EDTA [pH 7.4] supplemented with 1% Triton X-100, 10 μg of leupeptin per ml, 5 μg of pepstatin per ml, and 5 μg of aprotinin per ml). Postnuclear supernatants were mixed with 10 μl of 4× sodium dodecyl sulfate (SDS) sample buffer (32) and boiled for 5 min. Proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) through a 10% gel and electrophoretically transferred to an Immobilon-P membrane (Millipore). The membrane was blocked overnight in Tris-buffered saline containing 0.2% Tween 20 (TBST) supplemented with 2% BSA. Membranes were probed for 1 h with ZAP-70 antiserum (1:500 in TBST) or 9E10 MAb (1 μg/ml in TBST containing 0.1% BSA). The 9E10 MAb immunoblots were incubated for 1 h with rabbit anti-mouse immunoglobulin G (IgG) (RaMIg) (Pierce). Immunoreactive proteins were detected with horseradish peroxidase-coupled protein A followed by the enhanced chemiluminescence reagent (Amersham).
For antiphosphotyrosine immunoblots, cells were harvested from standard growth medium and resuspended in ice-cold solution no. 2 at a density of 5 × 106 cells per ml. The solution contained either no antibody or 1 μg of OKT3 antibodies per ml. After 20 min on ice, the cells were pelleted and resuspended in either 50 μl of prewarmed solution no. 2 containing 10 μg of RaMIg per ml or 50 μl of 100 μM pervanadate. The cells were stimulated for 2 min at 37°C and then lysed with an equal volume of 2× lysis buffer (20 mM Tris [pH 7.4], 50 mM β-glycerophosphate, 60 mM NaF, 40 mM sodium pyrophosphate, 2 mM EDTA, 2 mM sodium orthovanadate, 2% Triton X-100, and protease inhibitors). The cleared extracts were mixed with 20 μl of 4× SDS sample buffer and heated at 100°C. After separation by SDS-PAGE, the proteins were transferred to an Immobilon-P membrane. The membranes were blocked overnight in TBST containing 2% BSA and then were probed for 1 h with 100 ng of antiphosphotyrosine antibody per ml. Membranes were washed in TBST and incubated for 1 h with 1 μg of RaMIg per ml. Washed membranes were then incubated with horseradish peroxidase-conjugated protein A, and proteins were detected by enhanced chemiluminescence.
Tyrosine phosphorylation of PLC-γ1, SLP-76, and ζ was examined with a sequential immunoprecipitation-immunoblotting procedure. Cells were harvested from growth medium, washed in phosphate-buffered saline, and suspended in solution no. 2 at a density of 2.5 × 107 cells per ml. Aliquots (200 μl) were stimulated for various times with 100 μM pervanadate or 1 μg of OKT3 per ml at 37°C. Reactions were terminated with 800 μl of buffer A. After 5 min on ice, insoluble material was cleared by centrifugation and the extracts were mixed with 15 μl of packed protein A-Sepharose and 4 μl of anti-PLC-γ1 or 10 μl of anti-ζ antiserum for 1 h at 4°C. SLP-76 immunoprecipitations were done with 2 μl of sheep polyclonal anti-SLP-76 antiserum and 15 μl of protein G-Sepharose. The immunoprecipitates were washed three times in lysis buffer A and boiled in 30 μl of 2× SDS sample buffer. The solubilized proteins were separated by SDS-PAGE, transferred to Immobilon-P, and probed with antiphosphotyrosine antibodies. The blots were subsequently stripped and reprobed with the corresponding PLC-γ1-, ζ-, or SLP-76-specific antibodies.
Transfections.
Jurkat subclones were harvested from growth medium and resuspended at 4 × 107 cells per ml in fresh growth medium. For transient transfections, pcDNA3-based ZAP-70 or Syk expression plasmids were cotransfected along with the appropriate reporter plasmid. Aliquots of the cell suspension (107 cells) were placed in 0.4-cm-path-length cuvettes and, unless indicated otherwise, were mixed with 30 μg of total DNA in a final volume of 300 μl. The cells were electroporated with a BTX model T 820 square-wave electroporator (San Diego, Calif.) set at 300 V (pulse duration, 10 ms). For stable expression, P116 cells were electroporated with pcDNA3-ZAP or pBJ-mZAPKD cDNAs, and after 24 h, the cells were selected in standard growth medium supplemented with 2 mg of G418 per ml. The drug-resistant bulk population was cloned by limiting dilution, and individual subclones were screened for expression of wild-type or kinase-inactive ZAP-70 protein by immunoblot analysis of whole-cell lysates.
ZAP-70 kinase assays.
K562 cells (107/sample) were electroporated with 40 μg of the indicated expression plasmids as described above. The transfected cells were diluted to a density of 5 × 105 per ml in culture medium. After 24 h, the cells were washed with phosphate-buffered saline and lysed in ZAP-lysis buffer (25 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM sodium orthovanadate, 5 mM EDTA, 1% Brij-96, and protease inhibitors). The cleared extracts were mixed with 10 μg of 9E10 MAb and 15 μl of protein A-Sepharose precoated with 5 μg of RaMIg. After 1 h at 4°C, the immunoprecipitates were washed once in ZAP-lysis buffer, once in high-salt buffer (100 mM Tris-HCl [pH 7.4], 0.5 M LiCl, and 0.1% Brij-96), and twice in Z-kinase buffer (20 mM Tris [pH 7.4], 10 mM MgCl2, 10 mM MnCl2). Immune complex kinase reactions were initiated with 25 μl of Z-kinase buffer containing 10 μM ATP, 20 μCi of [γ-32P]ATP, and 2 μg of His6-cdb3. The samples were incubated for 5 min at 22°C, and the reactions were terminated with 10 μl of 4× SDS sample buffer. The samples were heated for 5 min at 100°C, and the solubilized proteins were separated by SDS-PAGE and transferred to Immobilon-P. Radiolabeled proteins were detected by autoradiography, and incorporation of 32P into His6-cdb3 was quantitated with an AMBIS phosphorimager system.
Cellular infections with recombinant vaccinia viruses.
Recombinant vaccinia viruses encoding ZAP-70 and Syk were kind gifts from A. M. Scharenberg and J. P. Kinet (Harvard Medical School, Boston, Mass.). Infections with wild-type and recombinant vaccinia viruses were performed as previously described (3). Jurkat subclones were infected with vaccinia viruses at a multiplicity of infection of 20:1 and were used in experiments at 3 h postinfection.
Luciferase assays.
Cells were transfected with 10 μg of a pT81Luc-based reporter plasmid containing 2 kb of nucleotide sequence from the human interleukin-2 (IL-2) promoter (pIL2-Luc) or three tandem copies of the nuclear factor of activated T cells (NFAT) DNA binding site (pNFAT-Luc) (kind gifts of David McKean, Mayo Clinic). At 6 to 18 h posttransfection, the cells were stimulated for an additional 6 to 12 h with 20 ng of tetradecanoyl-o-phorbol-13-acetate (TPA) per ml plus 2 μM ionomycin (with 20 ng of TPA per ml and 1 μg of OKT3 antibody per ml) or with 1 μg of OKT3 antibody per ml alone. Cells were lysed, and luciferase activity was determined with a commercial kit (Promega) and a Berthold Lumat luminometer.
RESULTS
Isolation of a ZAP-70-negative Jurkat subclone.
In an effort to generate new genetic model systems for studies of TCR signaling, we implemented a screening strategy designed to isolate Jurkat T-cell-derived somatic mutants bearing defects in downstream components of the pathway leading to the mobilization of intracellular Ca2+. Wild-type Jurkat E6 cells were mutagenized with the frameshift mutagen, ICR-191, and the resulting bulk population was repetitively selected for cells that failed to increase [Ca2+]i in response to pervanadate. The protocol was similar to that employed by Goldsmith and Weiss (24) except that the stimulus employed in the present study was pervanadate, a powerful inducer of PTK-dependent activation responses in T cells. The choice of pervanadate as the stimulus stemmed from the earlier observation that this agent triggered both protein tyrosine phosphorylation and [Ca2+]i increases in TCR-negative or CD45-negative Jurkat cells (46). Hence, the pervanadate-based selection procedure should facilitate the isolation of mutant cells bearing loss of function mutations in more distal elements of the Ca2+ response pathway in these cells.
The selected Jurkat subclones were rescreened for their abilities to mount a [Ca2+]i increase in response to anti-CD3 antibody (OKT3) or pervanadate stimulation. One of these clones, designated P116, was nonresponsive to antibody-mediated TCR cross-linkage and exhibited a delayed response to pervanadate in comparison to wild-type Jurkat cells (Fig. 1). FACS analysis of OKT3 antibody-stained P116 cells indicated that TCR expression on these cells was identical to that found on wild-type Jurkat cells (data not shown). In contrast to the P116 subclone, the Lck-negative Jurkat subclone, J.CaM1, failed to mobilize Ca2+ in response to either anti-CD3 antibodies or pervanadate. This phenotypic difference suggested that the signaling defects displayed by P116 cells were not explained by the loss of Lck. Indeed, immunoblot analysis of whole-cell lysates indicated that P116 cells contained wild-type levels of Lck as well as FynT and PLC-γ1 (data not shown).
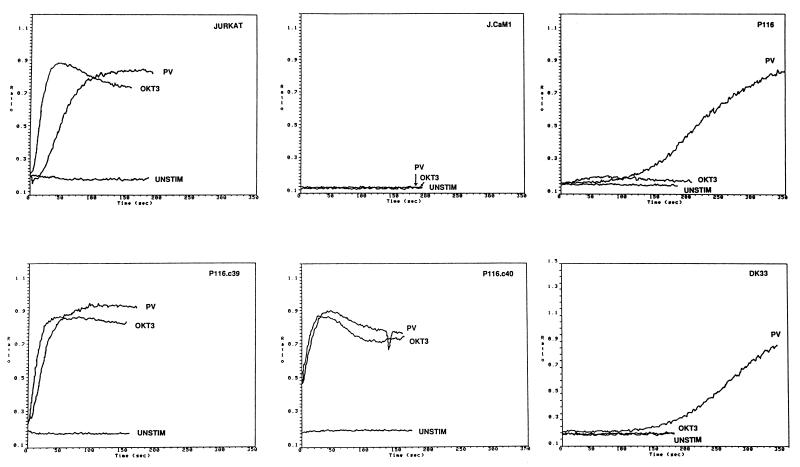
FIG. 1.
Stimulus-induced calcium mobilization in wild-type and mutant Jurkat cell lines. Jurkat subclones were loaded with indo-1 and stimulated with 1 μg of anti-CD3 antibody (OKT3) per ml or 0.1 mM pervanadate (PV). The ratio of the fluorescence emission of the Ca2+-bound to -free form of indo-1 is plotted as a function of time after addition of the stimulus. The cells tested in each panel are as follows: JURKAT, wild-type Jurkat cells; J.CaM1, Lck-negative Jurkat subclone; P116, ZAP-70-negative Jurkat subclone; P116.C39 and P116.C40, ZAP-70-transfected P116 subclones; DK33, P116 subclone transfected with a catalytically inactive ZAP-70 mutant.
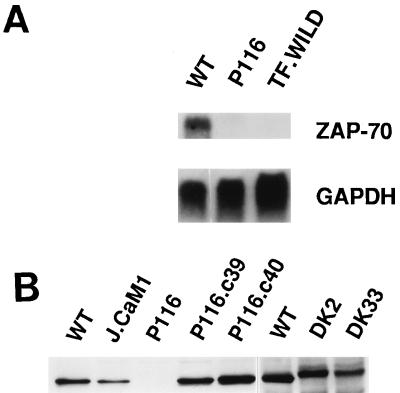
Further analyses of candidate signaling enzymes engaged during TCR stimulation revealed that the P116 subclone lacked ZAP-70 protein and mRNA (Fig. 2). To confirm that loss of ZAP-70 was causally related to the TCR signaling defect, P116 cells were stably transfected with a wild-type ZAP-70 expression vector. Two clones, P116.c39 and P116.c40, were selected for further analysis based on their expression of ZAP-70 at levels approximating those found in the parental Jurkat cell line (Fig. 2B). Two additional P116 subclones (DK2 and DK33) that stably expressed a catalytically inactive version of ZAP-70 (Lys369 → Arg) were derived in a similar fashion. Reintroduction of ZAP-70 into P116 cells restored the Ca2+ mobilization response to anti-CD3 antibody-mediated TCR cross-linkage and eliminated the time lag for the pervanadate-induced [Ca2+]i increase (Fig. 1). Neither response was reconstituted in the DK2 and DK33 transfectants, indicating that the PTK activity of ZAP-70 was essential for coupling the TCR to the Ca2+-mobilizing machinery and for the rapid [Ca2+]i increase triggered by pervanadate.
FIG. 2.
ZAP-70 expression in Jurkat-derived subclones. (A) Northern blot analysis. Total cellular RNA (30 μg) from Jurkat cells (wild type [WT]), P116 cells, or TF.wild B-lymphoma cells was separated electrophoretically and blotted onto a nitrocellulose membrane. The Northern blot was sequentially hybridized with 32P-labeled DNA probes for ZAP-70 and GAPDH. (B) Immunoblot analysis. Detergent-soluble proteins from wild-type Jurkat or the indicated Jurkat subclones (see Fig. 1 legend for description of subclones) were separated by SDS-PAGE and immunoblotted with ZAP-70-specific antibodies. The decreased electrophoretic mobility of the kinase-inactive ZAP-70 mutant expressed in DK2 and DK33 cells is due to the presence of the amino-terminal Myc epitope tag.
ZAP-70 reconstitutes TCR-dependent phosphorylation events in P116 cells.
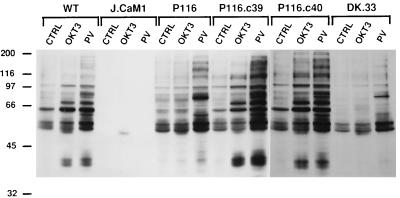
To determine the role of ZAP-70 in TCR-dependent protein tyrosine phosphorylation, ZAP-70-positive and -negative Jurkat subclones were stimulated with anti-CD3 antibodies or pervanadate and detergent-soluble proteins were immunoblotted with antiphosphotyrosine antibodies. TCR stimulation triggered rapid increases in the tyrosine phosphorylation of several proteins in wild-type Jurkat cells (Fig. 3). As reported previously (46), pervanadate stimulation provoked a more dramatic increase in overall protein tyrosine phosphorylation in these cells. In contrast, the ZAP-70-negative P116 cells showed no consistent increase in protein tyrosine phosphorylation in response to anti-CD3 antibodies. Interestingly, the P116 cells displayed a robust phosphorylation response to the pharmacologic stimulus, pervanadate. The latter characteristic clearly distinguished the phenotype of P116 cells from that of Lck-negative J.CaM1 cells, as the latter cells were refractory to either anti-CD3 antibody or pervanadate stimulation. The basal level of protein tyrosine phosphorylation in unstimulated J.CaM1 cells was also strikingly lower than that observed in either wild-type Jurkat or P116 cells. The protein tyrosine phosphorylation defects in P116 cells were reversed in the ZAP-70-transfected P116.c39 and P116.c40 cells but not in the DK.33 and DK.2 subclones (Fig. 3 and data not shown), which express a catalytically inactive form of ZAP-70. Thus, correction of the defective signaling phenotype exhibited by the P116 cells is dependent on the PTK activity of ZAP-70.
FIG. 3.
Stimulus-induced protein tyrosine phosphorylation in Jurkat-derived subclones. Wild-type (WT) Jurkat cells or Jurkat-derived subclones were stimulated for 2 min with medium only (CTRL), 1 μg of anti-CD3 (OKT3) antibody per ml cross-linked with 10 μg of RaMIg per ml, or 0.1 mM pervanadate (PV). Detergent-soluble proteins were separated by SDS-PAGE, followed by immunoblotting with antiphosphotyrosine MAb. The numbers on the left indicate molecular mass in kilodaltons.
An important target substrate for TCR-linked PTK activities is PLC-γ1 (43, 47, 61). To determine the role of ZAP-70 in the stimulus-dependent tyrosine phosphorylation of PLC-γ1, we stimulated wild-type Jurkat, J.CaM1, or P116 cells with anti-CD3 antibodies or pervanadate and immunoprecipitated PLC-γ1 from cell extracts prior to antiphosphotyrosine immunoblotting (Fig. 4A). Both stimuli provoked clear increases in the tyrosine phosphorylation of PLC-γ1 in wild-type Jurkat cells. In contrast, J.CaM1 and P116 cells showed no increase in PLC-γ1 tyrosine phosphorylation after a 2-min stimulation with either anti-CD3 antibodies or pervanadate, indicating that both Lck and ZAP-70 are necessary for rapid modification of PLC-γ1 induced by these agents. As expected, the PLC-γ1 phosphorylation defect was fully reversed in the ZAP-70-transfected P116.c39 and P116.c40 cells, confirming that the signaling abnormalities in P116 cells are explained by the loss of ZAP-70.
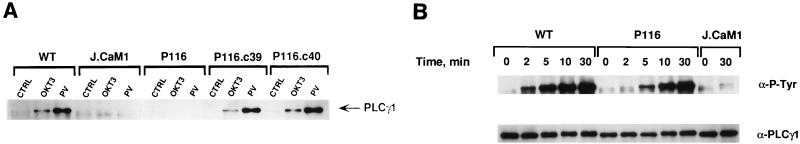
FIG. 4.
PLC-γ1 phosphorylation in Jurkat-derived subclones. (A) Phosphorylation of PLC-γ1 in Jurkat subclones. Wild-type (WT) Jurkat cells and the indicated Jurkat subclones were stimulated for 2 min with either anti-CD3 antibody (OKT3) or pervanadate (PV), and cellular extracts were immunoprecipitated with anti-PLC-γ1 antibodies. The immunoprecipitated proteins were separated by SDS-PAGE and immunoblotted with antiphosphotyrosine MAb. (B) Time course of pervanadate-induced tyrosine phosphorylation of PLC-γ1. The indicated Jurkat cell lines were stimulated for the various times with PV. The cells were lysed, and PLC-γ1 immunoprecipitates were immunoblotted with antiphosphotyrosine antibodies as described in panel A.
The inability of pervanadate to trigger an early increase in PLC-γ1 phosphorylation in P116 cells was inconsistent with the initial observation (Fig. 1) that pervanadate exposure generated a delayed but significant increase in [Ca2+]i in these cells. This apparent discrepancy was resolved by a comparison of the time courses of pervanadate-induced PLC-γ1 phosphorylation in wild-type Jurkat and P116 cells (Fig. 4B). In wild-type Jurkat cells, pervanadate stimulated a dramatic increase in the tyrosine phosphorylation of PLC-γ1 within 2 min, and a maximal phosphorylation response was observed after 10 min of stimulation. The time course of PLC-γ1 tyrosine phosphorylation was clearly delayed in P116 cells. The slower rate of PLC-γ1 phosphorylation in these cells correlated with the abnormally slow rise in [Ca2+]i induced by pervanadate. In contrast, J.CaM1 cells displayed only a slight increase in PLC-γ1 phosphorylation after 30 min of exposure to pervanadate, again paralleling the nonresponsiveness of these cells to pervanadate in the Ca2+ mobilization assay. Taken together, these results indicate that ZAP-70 is an essential component of the pathway that links TCR ligation to PLC-γ1 tyrosine phosphorylation and that the requirement for ZAP-70 can be at least partially circumvented by the pharmacologic stimulus, pervanadate.
Effect of ZAP-70 deficiency on TCR-dependent transcriptional activation.
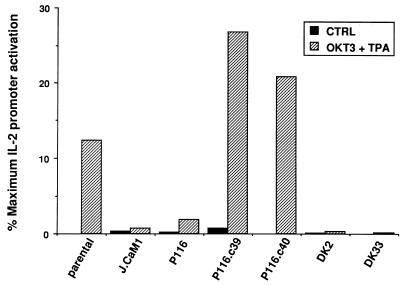
The proximal signaling events initiated by TCR engagement, in conjunction with costimulatory signals supplied by CD28 ligation or phorbol esters, ultimately regulate the transcription of a complex set of target genes, including those encoding IL-2 and other cytokines (16, 17). To define the role of ZAP-70 in TCR-dependent IL-2 gene transcription, wild-type and mutant Jurkat cell lines were transiently transfected with the pIL2-Luc reporter plasmid, which contains the 5′ enhancer region of the IL-2 gene linked to a luciferase reporter. The transfected cells were stimulated with anti-CD3 antibodies (OKT3) plus TPA, and luciferase activities were normalized to the activities measured in the same population of cells after exposure to ionomycin plus TPA (Fig. 5). Compared to wild-type Jurkat cells, ZAP-70-negative P116 cells exhibited a dramatic defect in TCR-dependent IL-2 promoter activation. As expected, the Lck-negative J.CaM1 cells were virtually nonresponsive to the stimulus provided by anti-CD3 antibodies in these transcriptional activation assays. The defective transcriptional response of P116 cells was corrected by stable expression of wild-type ZAP-70 (clones P116.c39 and P116.c40), but not by expression of a catalytically inactive ZAP-70 mutant (clones DK2 and DK33). Thus, the deficient expression of ZAP-70 in P116 cells effectively disrupts the coupling mechanism between TCR stimulation and IL-2 gene transcription in these cells.
FIG. 5.
IL-2 promoter-driven transcription in Jurkat subclones. Wild-type Jurkat cells or the indicated Jurkat subclones were transiently transfected with 10 μg of pIL2-Luc reporter plasmid. After 12 h, transfected cell populations were divided into three equivalent samples. The samples were stimulated for 12 h with medium only, 1 μg of anti-CD3 antibodies (OKT3) per ml plus 20 ng of TPA per ml or 2 μM ionomycin plus 20 ng of TPA per ml. Luciferase activities were plotted as a percentage of the maximal activity induced by ionomycin plus TPA in each cell population. The results shown are representative of seven independent trials. The P116.c39 and P116.c40 cell lines are stable transfectants expressing wild-type ZAP-70, and the DK2 and DK33 cell lines are stably transfected with a catalytically inactive ZAP-70 mutant.
Reconstitution of early signaling events by Syk.
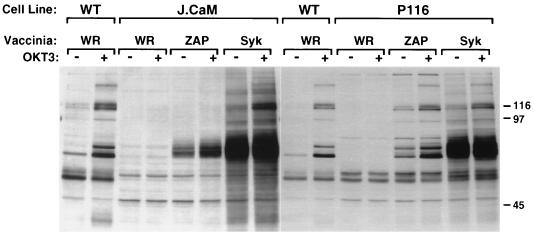
In preliminary studies, we confirmed a previous report (22) that Jurkat E6 cells fail to express Syk and that the Jurkat E6-derived subclone, P116, is similarly negative for expression of this PTK (data not shown). The P116 cell line therefore offers a suitable host for comparative studies of the signaling functions of ZAP-70 and Syk in T cells. Unfortunately, repeated efforts to isolate stable Syk-transfected P116 subclones proved unsuccessful, suggesting that long-term expression of Syk might not be tolerated by these cells (see Discussion). To circumvent this problem, we adopted a vaccinia virus-based expression approach, which permitted an evaluation of the impact of Syk on early TCR signaling events in P116 cells (Fig. 6). Wild-type Jurkat, P116, and J.CaM1 cells were infected with recombinant vaccinia viruses encoding either ZAP-70 or Syk, and the cells were stimulated with anti-CD3 antibodies at 3 h postinfection. Introduction of either Syk or ZAP-70 increased both basal and anti-CD3 antibody-stimulated protein tyrosine phosphorylation in P116 cells. The increases in baseline tyrosine phosphorylation induced by Syk and ZAP-70 may be the result of the high level of overexpression achieved during vaccinia virus infection. In contrast, Syk, but not ZAP-70, augmented both basal and TCR-stimulated protein tyrosine phosphorylation in J.CaM1 cells. The latter finding is consistent with the recent report that only Syk is capable of reversing the TCR signaling defects in both J.CaM1 cells and CD45-deficient J45.01 cells (14).
FIG. 6.
Whole-cell protein tyrosine phosphorylation in Jurkat subclones infected with Syk- or ZAP-encoding vaccinia virus. Wild-type (WT) Jurkat, J.CaM1, or P116 cells were infected with control vaccinia virus strain WR or with recombinant vaccinia virus encoding ZAP-70 or Syk. Three hours after infection, cells were stimulated for 1 min with medium only or with anti-CD3 antibody cross-linked with goat anti-mouse IgG. Detergent-soluble proteins were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine MAb.
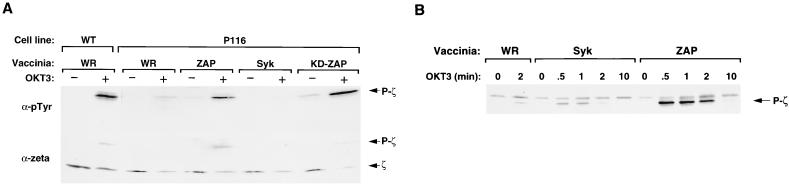
An important triggering event for the TCR-linked signaling cascade is the tyrosine phosphorylation of ITAMs in the CD3 and ζ subunits of the receptor itself. Previous studies suggested that the binding of the ZAP-70 SH2 domains to the phospho-ITAMs of the ζ subunit protected the phosphotyrosines from dephosphorylation by CD45 and other protein tyrosine phosphatases (8). This model can be tested directly in the ZAP-70- and Syk-deficient P116 cell line. Stimulation of wild-type Jurkat cells with anti-CD3 antibodies led to a dramatic increase in the tyrosine phosphorylation of the TCR ζ subunit (Fig. 7A). This response was markedly attenuated in P116 cells. Interestingly, the defect in ζ subunit phosphorylation was reversed by expression of either wild-type or catalytically inactive ZAP-70, indicating that the PTK activity of ZAP-70 is not required for this response. On the other hand, expression of catalytically active Syk failed to enhance the tyrosine phosphorylation of ζ subunits in anti-CD3 antibody-stimulated P116 cells. A time course analysis confirmed that ZAP-70, but not Syk, supported a rapid and transient increase in ζ subunit phosphorylation in response to TCR ligation (Fig. 7B). These results indicate that ZAP-70 and Syk differ significantly with respect to their abilities to induce and/or maintain the phosphorylation of the ζ ITAMs during TCR stimulation.
FIG. 7.
TCR ζ subunit phosphorylation in P116 cells expressing ZAP-70 or Syk. (A) Cells were infected with nonrecombinant (WR) or recombinant vaccinia viruses and stimulated with OKT3 as described in the legend for Fig. 6. The samples labeled KD-ZAP were from cells infected with vaccinia virus encoding a catalytically inactive ZAP-70 mutant (Lys349 → Arg). At 3 h postinfection, the cells were stimulated for 1 min with medium only or with OKT3 cross-linked with goat anti-mouse IgG. Detergent-soluble proteins were immunoprecipitated with ζ-specific antibodies. The immunoprecipitated proteins were separated by SDS-PAGE and immunoblotted with antiphosphotyrosine (upper panel) and then stripped and reblotted with anti-ζ antibodies (lower panel). (B) Time course of ζ subunit phosphorylation in ZAP-70 versus Syk-expressing P116 cells.
Syk reconstitution of TCR-dependent gene transcription.
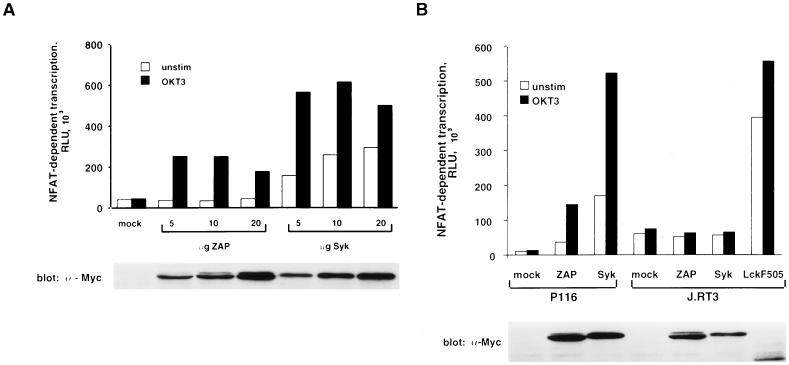
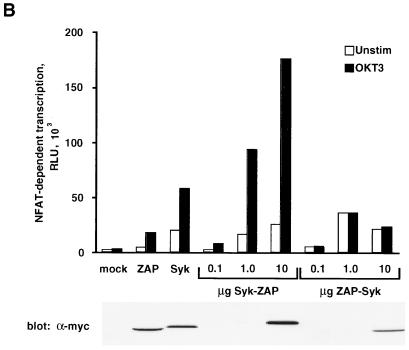
To further characterize the ability of Syk to reconstitute TCR signaling functions in P116 cells, transient transfection experiments were performed with pNFAT-Luc as a readout for transcriptional activation. In contrast to pIL2-Luc, the pNFAT-Luc reporter plasmid is transcriptionally activated by TCR cross-linkage alone, eliminating the potentially confounding effects of TPA from these assays (27, 63). P116 cells were cotransfected with the pNFAT-Luc reporter construct together with various amounts of plasmid DNA encoding epitope-tagged versions of ZAP-70 or Syk. In the absence of either PTK, anti-CD3 antibody stimulation failed to activate NFAT-dependent transcription in P116 cells, and this defect was effectively reversed by cotransfection of the cells with the ZAP-70 expression plasmid (Fig. 8A). Transient expression of Syk in P116 cells also reconstituted the transcriptional response to antibody-mediated TCR cross-linkage. However, unlike ZAP-70, Syk provoked a significant elevation in luciferase activity in unstimulated P116 cells, indicating that Syk-mediated NFAT activation was partially independent of TCR ligation. The difference in baseline luciferase activities observed in Syk- and ZAP-70-transfected P116 cells was not explained by the relative overexpression of Syk protein, as the two PTKs were expressed at comparable levels in these cells (Fig. 8A). Transfection of P116 cells with lower amounts (0.1 to 1 μg of plasmid DNA) of the Syk or ZAP-70 expression vectors resulted in a progressive reduction in anti-CD3 antibody-stimulated luciferase activity, indicating that lower amounts of either PTK could limit TCR signaling in P116 cells (62). Nonetheless, Syk expression, even at levels below those detectable with the tag-specific antibody, consistently elevated the basal level of reporter gene transcription in these cells.
FIG. 8.
Reconstitution of gene transcription in P116 cells expressing ZAP-70 or Syk. (A) Effect of Syk versus ZAP-70 on NFAT-driven gene transcription. P116 cells were transfected with 10 μg of pNFAT-Luc in the presence of empty vector (mock) or the indicated amounts of pcDNA3-mZAP or pcDNA3-mSyk. After transfection, equivalent samples of each cell population were stimulated for 6 h with medium only (unstim) or with 1 μg of anti-CD3 antibody (OKT3) per ml. Cellular extracts were assayed for luciferase activity and for expression of epitope-tagged proteins by immunoblotting with 9E10 MAb. The results shown are representative of four separate trials. (B) TCR-dependent effects of Syk and ZAP-70 on NFAT-driven gene transcription. P116 and TCR-negative J.RT3 cells were transfected with 10 μg of pNFAT-Luc and 10 μg of empty vector or the indicated plasmid. Samples were processed as in panel A. The results shown are representative of three independent experiments.
The constitutive increase in NFAT-dependent transcription induced by Syk might be the outcome of a signal amplification mechanism initiated by a low level of ITAM tyrosine phosphorylation in unstimulated P116 cells. Alternatively, Syk may simply be an autonomously active PTK when it is ectopically expressed in these cells. To distinguish between these possibilities, the TCR-negative Jurkat subclone, J.RT3, was transiently transfected with either Syk or ZAP-70 expression plasmids together with the pNFAT-Luc reporter (Fig. 8B). In the J.RT3 cell line, neither Syk nor ZAP-70 increased the basal level of reporter gene transcription, whereas expression of a constitutively active Lck mutant (Lck F505) markedly elevated luciferase activity. These results argue that the signaling functions of both Syk and ZAP-70 are dependent on the expression of TCR complexes, and presumably phosphorylated ITAMs, in the plasma membrane.
Reconstitution of TCR signaling functions by ZAP-70–Syk chimeric proteins.
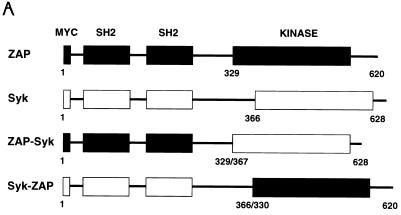
The results described above suggested that the signaling functions of Syk and ZAP-70 may be differentially regulated in P116 cells. Several potential regulatory domains have been identified in Syk and ZAP-70, including two tandem SH2 domains, a linker region containing putative tyrosine phosphorylation sites, and a carboxy-terminal catalytic domain. To further understand the structural basis for the functional differences between Syk and ZAP-70, we transiently transfected P116 cells with expression vectors encoding Myc epitope-tagged ZAP-70–Syk chimeric proteins (Fig. 9A). These chimeric constructs contained the tandem SH2 domains and linker region of one Syk family member fused to the catalytic region and carboxy terminus of the other family member.
FIG. 9.
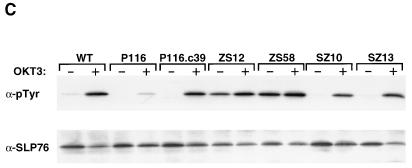
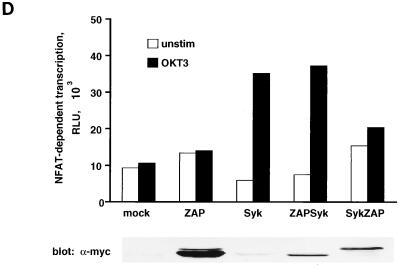
Effect of ZAP-70–Syk chimeric proteins on NFAT-dependent transcription. (A) Structures of ZAP-70–Syk chimeric proteins. The numbers below each construct indicate amino acid residue numbers from the full-length ZAP-70 and Syk proteins. (B) Reconstitution of TCR signaling functions with ZAP-70–Syk chimeric proteins. P116 cells were transfected with pNFAT-Luc along with 10 μg of either empty vector (mock), pcDNA3-mZAP, pcDNA3-mSyk, or the indicated amounts of pcDNA3-mZAPSyk or pcDNA3-mSykZAP. The cells were stimulated, and luciferase assays were done as described in Fig. 8A. (C) Effects of ZAP-70–Syk chimeric PTKs on SLP-76 tyrosine phosphorylation. P116 cells were stably transfected with wild-type ZAP-70 (P116.c39) or with Syk-ZAP (clones SZ-10 and -13) or ZAP-Syk (clones ZS-12 and -58) expression vectors. The indicated clones were selected based on expression of the transfected PTK at levels approximating those found in the parental Jurkat cell line (62). The cells were stimulated for 2 min with anti-CD3 antibodies (OKT3), and detergent-soluble proteins were immunoprecipitated with anti-SLP-76 antibodies. After separation by SDS-PAGE, the immunoprecipitated proteins were immunoblotted with antiphosphotyrosine antibodies (upper panel). The blot was stripped and reprobed with anti-SLP-76 antibodies (lower panel). The apparent decrease in band intensity in stimulated sample lanes is due to the reduced immunoreactivity of the antibodies with the tyrosine-phosphorylated forms of SLP-76 (62). (D) Role of Lck in NFAT activation by ZAP-70–Syk chimeric proteins. J.CaM1 cells were transfected with 10 μg of pNFAT-Luc along with 40 μg of empty vector (mock) or with the indicated expression plasmid. Stimulations and luciferase assays were performed as described in the legend for Fig. 8. The results in panels B and D are each representative of three separate trials.
The functional activities of the Syk-ZAP and ZAP-Syk chimeric proteins were assessed with NFAT-dependent luciferase expression as a measure of cellular activation (Fig. 9B). The two chimeric proteins had strikingly different effects on the signaling phenotype of the P116 host cell line. In comparison to full-length ZAP-70, the Syk-ZAP chimera rendered NFAT-dependent transcription superinducible by TCR stimulation, suggesting that the N-terminal regulatory domain of Syk enhances the ability of the ZAP-70 catalytic domain to transmit activating signals from the activated receptor complex. In contrast, P116 cells transiently expressing the ZAP-Syk chimera showed elevated basal, but no ligand-inducible, NFAT activity. Thus, the regulatory region of ZAP-70 cooperates poorly with the Syk catalytic domain in terms of coupling TCR ligation to NFAT-dependent transcription in P116 cells.
The differential activating effects of the ZAP-70–Syk chimeric proteins were confirmed in biochemical experiments with cells that stably expressed these PTKs. In these studies, we examined the impact of ZAP-Syk versus Syk-ZAP expression on basal and anti-CD3-antibody-stimulated tyrosine phosphorylation of SLP-76, a putative substrate for Syk family PTKs in T cells (59). As expected, nontransfected P116 cells displayed a dramatic defect in TCR-dependent SLP-76 phosphorylation, and this abnormality was reversed by stable expression of wild-type ZAP-70 (Fig. 9C). A similar correction of the SLP-76 phosphorylation defect was observed in cells expressing the Syk-ZAP chimera, consistent with the ability of this chimera to restore ligand-dependent NFAT activation in transiently transfected P116 cells (Fig. 9B). In contrast, SLP-76 was constitutively phosphorylated on tyrosine residues in the ZAP-Syk-expressing P116 cells, and this phosphorylation was not further increased by anti-CD3 antibody stimulation. Thus, these results substantiate the conclusion that the ZAP-Syk chimera carries out ligand-independent signaling functions in P116 cells.
Introduction of Syk-ZAP or ZAP-Syk into Lck-negative J.CaM1 cells yielded strikingly different results (Fig. 9D). In this case, ZAP-Syk reconstituted anti-CD3 antibody-dependent NFAT activation, whereas the converse Syk-ZAP protein was almost completely nonfunctional in these cells. Thus, the presence of the Syk catalytic domain appears to be essential for the restoration of TCR signaling in J.CaM1 cells.
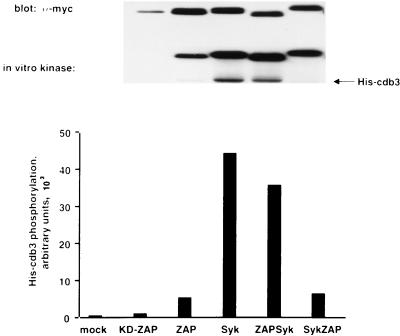
The catalytic activities of Syk-ZAP and ZAP-Syk were determined in immune complex kinase assays with cdb3 as the substrate (Fig. 10). These assays showed that the PTK activity of Syk-ZAP was similar to that of full-length ZAP-70, while ZAP-Syk was a significantly more active PTK whose activity resembled that of full-length Syk. The different in vitro kinase activities displayed by the two chimeric proteins were therefore intrinsic properties of the ZAP-70 and Syk catalytic domains.
FIG. 10.
Catalytic activities of ZAP-70–Syk chimeric proteins. K562 cells were transfected with the indicated expression plasmids. Cellular extracts were immunoprecipitated with the tag-specific 9E10 MAb. Immune complex kinase reactions were done with [γ-32P]ATP and 2 μg of His-tagged cdb3 as the substrate. Radiolabeled proteins were detected by autoradiography (middle panel), and the incorporation of 32P into His-cdb3 was quantitated with an AMBIS phosphorimager (bottom panel). The immunoprecipitated protein tyrosine kinases were detected by immunoblotting with 9E10 MAb (upper panel).
DISCUSSION
Using a Ca2+ mobilization-based selection strategy, we have isolated a ZAP-70-negative somatic mutant from the Jurkat E6 T-cell line. The P116 subclone expresses no detectable ZAP-70 mRNA or protein and, like the parental Jurkat E6 clone from which it was derived (22), also fails to express Syk. As predicted from studies of T cells from ZAP-70-deficient humans, P116 cells display global defects in TCR signaling functions, including the induction of protein tyrosine phosphorylation, Ca2+ release from intracellular stores, and IL-2 promoter-driven transcription. These signaling defects were corrected by reintroduction of either ZAP-70 or Syk into the mutant cell line. However, our results indicate that these two closely related PTKs differ significantly in terms of their mechanisms of activation in T cells.
The severe defects in TCR signaling in ZAP-70-negative P116 cells are reminiscent of those found in the Lck-deficient J.CaM1 cell line (50). However, comparative studies with pervanadate as the stimulus suggest that the loss of Lck leads to a more fundamental impairment of the downstream signaling machinery than does the loss of ZAP-70. Whereas J.CaM1 cells are virtually nonresponsive to pervanadate, P116 cells mounted a significant but delayed activation response to this agent, which is capable of activating Jurkat cells in a TCR-independent fashion (46). Notably, exposure of P116 cells to pervanadate led to the tyrosine phosphorylation and catalytic activation of PLC-γ1, a pivotal event in the initiation of IL-2 gene transcription (7, 16). The ability of the ZAP-70-deficient cells to respond to pervanadate indicates that these cells retain the PTK activity needed to set in motion the TCR-linked signaling cascade; however, this cascade cannot be engaged effectively by the TCR in the absence of ZAP-70.
The absence of both ZAP-70 and Syk in P116 cells offered a unique opportunity to compare the regulation and functions of these PTKs in the context of both the native TCR complex and the Src family member, Lck. Introduction of either ZAP-70 or Syk reversed the TCR signaling defects in P116 cells; however, the Syk-expressing cells were partially activated even in the absence of TCR ligands. The relaxed ligand dependence of Syk-mediated signaling suggests that the activation threshold for Syk is significantly lower than that of ZAP-70 in P116 cells. This notion is consistent with previous observations that Syk aggregation or binding of Syk to phospho-ITAMs is sufficient to activate this PTK, whereas ZAP-70 requires an additional stimulatory input supplied by Lck, which phosphorylates ZAP-70 at its activating Y493 residue (4, 9, 41). The unique ability of Syk to restore TCR signaling functions in Lck-deficient J.CaM1 cells is fully consistent with the conclusion that Syk can be activated in an Lck-independent fashion (14) (Fig. 9C).
The most straightforward explanation for the above findings is that the Syk catalytic domain carries an intrinsically higher level of PTK activity than does that of ZAP-70. Indeed, the specific activity of Syk was approximately 100-fold higher than that of ZAP-70 when the two PTKs were expressed in COS-1 cells (37). These investigators used chimeric ZAP-70–Syk proteins to show that the higher catalytic activity was an intrinsic property of the Syk catalytic domain. We have drawn a similar conclusion from immune complex kinase assays done with ZAP-70–Syk chimeric proteins expressed in Jurkat-derived P116 cells (Fig. 10). However, our results further indicate that both the catalytic domains and N-terminal regulatory regions of ZAP-70 and Syk contribute to the distinctive behaviors of these PTKs in P116 cells.
In spite of its higher intrinsic catalytic activity, Syk does not activate Jurkat cells in a completely autonomous fashion. The constitutive signaling activity of Syk in Jurkat cells was dependent on the coexpression of Lck and a cell surface TCR complex. A reasonable scenario is that a baseline level of ITAM phosphorylation, mediated by Lck or FynT, triggers the recruitment of a few Syk molecules to the plasma membrane, where they undergo phosphorylation and activation by Lck. This process sets up a feed-forward amplification mechanism leading to further ITAM phosphorylation and Syk activation (via transphosphorylation by other activated Syk molecules) that eventually surpasses the threshold needed to trigger the signaling cascade normally controlled by TCR ligation. A similar Src family kinase-initiated activation loop has been proposed to explain the robust activation of Syk observed after relatively weak stimulation of Fc receptors on mast cells (20).
The constitutive activation of the TCR-linked signaling cascade by Syk may explain the apparent toxicity of long-term Syk expression in P116 cells. Repeated attempts to derive stable P116 transfectants expressing Syk met with failure, whereas P116 subclones that stably express ZAP-70 were generated readily under identical conditions of transfection and drug selection (2). The cytotoxic effect of Syk may be due to the stimulation of Fas-dependent apoptosis in the transfected cell population. P116 cells exhibit a profound defect in the induction of Fas ligand expression in response to anti-CD3 antibody stimulation (18). This abnormality is corrected by the reexpression of ZAP-70, indicating that this PTK is required for the delivery of TCR-mediated signals leading to the transcription of the Fas ligand gene. Ectopically expressed Syk should also restore TCR-dependent Fas ligand expression in P116 cells; however, the propensity of Syk to become active in the absence of TCR engagement may result in constitutive expression of Fas ligand and, in turn, increased susceptibility to Fas-mediated apoptosis. A survival advantage for Syk-negative Jurkat cells may explain the spontaneous outgrowth of Jurkat P116 cells bearing loss of function mutations in both Syk alleles (22). A physiologic correlate of this phenomenon may occur during normal T-cell development. Syk is expressed in double-positive and single-positive thymocytes; however, expression of this PTK declines precipitously in postthymic T cells (11). The loss of Syk as T cells enter the periphery may reflect the need to raise the threshold for initiation of signal output from the TCR in order to avoid the deleterious consequences (cell death or immune hyperreactivity) of inappropriate receptor activation.
To circumvent the problems associated with long-term Syk expression, we used the vaccinia virus expression system to generate ZAP-70- or Syk-expressing P116 cells for analyses of the early biochemical events induced by TCR stimulation. Expression of either PTK corrected the global defects in TCR-dependent protein tyrosine phosphorylation in P116 cells. However, we found a striking difference in the tyrosine phosphorylation of the TCR-ζ subunit in ZAP-70- versus Syk-expressing P116 cells. In nontransfected P116 cells, anti-CD3 antibodies stimulated only a minor increase in ζ tyrosine phosphorylation, and this defect was fully reversed by expression of either catalytically active or inactive ZAP-70. These results suggest that binding of the SH2 domains of ZAP-70 to the ζ phospho-ITAMs protects these motifs from dephosphorylation by CD45 and other protein tyrosine phosphatases (8). In contrast to the restorative effect of ZAP-70, Syk failed to reconstitute anti-CD3 antibody-stimulated ζ tyrosine phosphorylation in P116 cells. This observation is not restricted to the Jurkat model, as we have recently obtained identical results with a Syk-expressing T-cell line derived from a ZAP-70-deficient human patient (62).
The inability of Syk to support ζ subunit phosphorylation suggests that the interaction of Syk with the ζ phospho-ITAMs differs fundamentally from that of ZAP-70. One possibility is that, unlike ZAP-70, Syk preferentially interacts with phosphorylated CD3-ɛ, -δ, or -γ ITAMs rather than with those residing in the ζ cytoplasmic domain. Alternatively, Syk may bind to the ζ phospho-ITAMs but fail to protect them from dephosphorylation due to rapid dissociation of the activated form of this PTK from the TCR complex. A recent study by Keshvara et al. (33) identified a tyrosine residue (Tyr130 in murine Syk), located in the inter-SH2 domain region, whose phosphorylation provokes both activation of the Syk catalytic domain and dissociation of the activated PTK from the B-cell antigen receptor complex. Interestingly, the inter-SH2 domain region of ZAP-70 contains a tyrosine residue (Tyr126) homologous to the regulatory Tyr130 of Syk. Although Tyr126 has been identified as an in vitro phosphorylation site in ZAP-70 (60), phosphorylation of this residue has not been observed after isolation of ZAP-70 from activated T cells. Perhaps a relatively low stoichiometry of phosphorylation at Tyr126 promotes a persistent interaction between ZAP-70 and the ζ phospho-ITAMs and thereby more effectively protects these phospho-ITAMs from attack by protein tyrosine phosphatases.
To further understand the structural basis for the functional differences between Syk and ZAP-70, we expressed ZAP-70–Syk chimeric proteins in either P116 cells or J.CaM1 cells. Unexpectedly, the signaling characteristics of these chimeric PTKs were radically different in ZAP-70- versus Lck-deficient Jurkat cells. In P116 cells, introduction of the Syk-ZAP chimera rendered NFAT activation superinducible by TCR ligation. The maximal level of reporter gene expression induced by anti-CD3 antibody stimulation of the Syk-ZAP-expressing P116 cells was approximately 100-fold greater than that conferred by reexpression of full-length ZAP-70 in these cells. On the other hand, the converse ZAP-Syk chimera constitutively activated NFAT, and, in stably-transfected P116 cells, rendered the tyrosine phosphorylation of SLP-76 independent of TCR ligation. The results obtained with the Syk-ZAP chimera suggest that the SH2 domains of Syk couple more productively to either the CD3 or ζ phospho-ITAMs than do the SH2 domains of ZAP-70. In the case of the opposite ZAP-Syk chimera, the more active Syk kinase domain may drive signaling in the presence of a low-level ITAM phosphorylation in unstimulated P116 cells. However, fusion of the ZAP-70 SH2 domains and hinge region to the Syk catalytic domain apparently compromises the ability of this chimera to respond further to the stimulus provided by antibody-mediated TCR cross-linkage.
The strikingly disparate results obtained when the chimeric ZAP-70–Syk constructs were expressed in Lck-deficient J.CaM1 cells underscore the functional importance of the interplay between Syk family PTKs and Lck during T-cell activation. As recently reported with full-length Syk (14), the ZAP-Syk chimera reconstituted the coupling pathway between TCR stimulation and the NFAT activation in J.CaM1 cells. In contrast, transient expression of the converse Syk-ZAP chimera caused only a minor restoration of TCR signaling functions in these cells. Thus, the functional consequences of expressing the two chimeric proteins in J.CaM1 cells were essentially the mirror image of those obtained in P116 cells. The results obtained with J.CaM1 cells suggest that the capacity to function in an Lck-negative intracellular milieu maps to the catalytic domain of Syk. On the other hand, when Lck is not limiting (e.g., in P116 cells) the SH2 domains of Syk appear to mediate an increased level of signaling in response to TCR ligation. Thus, both the amino-terminal regulatory regions and the carboxy-terminal catalytic domains of Syk and ZAP-70 contribute to the distinctive functional characteristics of these PTKs in T cells.
In summary, the ZAP-70-deficient P116 cell line represents a genetically manipulatable model in which the structural and biochemical parameters that govern both the redundant and unique functions of Syk and ZAP-70 in T lymphocytes can be explored. This cell line is a powerful addition to the previously described battery of Jurkat T-cell somatic mutants for studies of the mechanisms of signal initiation and propagation through the TCR. From the therapeutic viewpoint, P116 cells should prove useful in efforts to develop ZAP-70- or Syk-targeted immunosuppressive agents for use in transplantation and in the treatment of autoimmune diseases.
ACKNOWLEDGMENTS
We thank David McKean for providing the luciferase reporter gene constructs, Andrew M. Scharenberg and Jean-Pierre Kinet for supplying recombinant vaccinia virus, and Gary Koretzky for the gift of SLP-76-specific antiserum. We also express our appreciation to Amnon Altman and Larry Karnitz for many helpful discussions pertaining to this study.
This work was supported by NIH grants GM47286 (R.T.A.) and CA47752 (P.J.L.) and by the Mayo Foundation. W.Z. is a Leukemia Society of America Fellow, and R.T.A. is a Leukemia Society of America Scholar.
REFERENCES
- 1.Appleby M W, Gross J A, Cooke M P, Levin S D, Qian X, Perlmutter R M. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992;70:751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- 2.Arpaia E, Shahar M, Dadi H, Cohen A, Roifman C M. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking Zap-70 kinase. Cell. 1994;76:947–958. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 3.Binstadt B A, Brumbaugh K M, Dick C J, Scharenberg A M, Williams B L, Colonna M, Lanier L L, Kinet J-P, Abraham R T, Leibson P J. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity. 1996;5:629–638. doi: 10.1016/s1074-7613(00)80276-9. [DOI] [PubMed] [Google Scholar]
- 4.Bu J-Y, Shaw A S, Chan A C. Analysis of the interaction of ZAP-70 and Syk protein-tyrosine kinases with the T-cell antigen receptor by plasmon resonance. Proc Natl Acad Sci USA. 1995;92:5106–5110. doi: 10.1073/pnas.92.11.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buerstedde J M, Pease L R, Bell M P, Nilson A E. Identification of an immunodominant region on the I-A beta chain using site-directed mutagenesis and DNA-mediated gene transfer. J Exp Med. 1988;167:473–487. doi: 10.1084/jem.167.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkhardt A L, Stealey B, Rowley R B, Mahajan S, Prendergast M, Fargnoli J, Bolen J B. Temporal regulation of non-transmembrane protein tyrosine kinase enzyme activity following T cell antigen receptor engagement. J Biol Chem. 1994;269:23642–23647. [PubMed] [Google Scholar]
- 7.Cantrell D. T cell antigen receptor signal transduction pathway. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 8.Chan A C, Desai D M, Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immunol. 1994;12:555–592. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- 9.Chan A C, Dalton M, Johnson R, Kong G-H, Wang T, Thoma R, Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan A C, Iwashima M, Turck C W, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR ζ chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 11.Chan A C, van Oers N S C, Tran A, Turka L, Law C-L, Ryan J C, Clark E A, Weiss A. Differential expression of ZAP-70 and Syk protein tyrosine kinases, and the role of this family of protein tyrosine kinases in TCR signaling. J Immunol. 1994;152:4758–4766. [PubMed] [Google Scholar]
- 12.Chan A C, Kadlecek T A, Elder M E, Filipovich A H, Kuo W-L, Iwashima M, Parslow T G, Weiss A. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599–1601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- 13.Cheng A M, Rowley B, Pao W, Hayday A, Bolen J B, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 14.Chu D H, Spits H, Peyron J-F, Rowley R B, Bolen J B, Weiss A. The Syk protein kinase can function independently of CD45 or Lck in T cell antigen receptor signaling. EMBO J. 1996;15:6251–6261. [PMC free article] [PubMed] [Google Scholar]
- 15.Couture C, Baier G, Altman A, Mustelin T. p56lck-independent activation and tyrosine phosphorylation of p72syk by T-cell antigen receptor/CD3 stimulation. Proc Natl Acad Sci USA. 1994;91:5301–5305. doi: 10.1073/pnas.91.12.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabtree G R, Clipstone N A. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 17.Durand D B, Shaw J-P, Bush M R, Replogle R E, Belagaje R, Crabtree G R. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol. 1988;8:1715–1724. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eischen C M, Williams B L, Zhang W, Samelson L E, Lynch D H, Abraham R T, Leibson P J. ZAP-70 tyrosine kinase is required for the up-regulation of Fas ligand in activation-induced T cell apoptosis. J Immunol. 1997;159:1135–1139. [PubMed] [Google Scholar]
- 19.Elder M E, Lin D, Clever J, Chan A C, Hope T J, Weiss A, Parslow T G. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264:1596–1599. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 20.El-Hillal O, Kurosaki T, Yamamura H, Kinet J-P, Scharenberg A M. Syk kinase activation by a src kinase-initiated activation loop phosphorylation chain reaction. Proc Natl Acad Sci USA. 1997;94:1919–1924. doi: 10.1073/pnas.94.5.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evan G I, Lewis G K, Ramsay G, Bishop J M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fargnoli J, Burkhardt A L, Laverty M, Kut S A, van Oers N S C, Weiss A, Bolen J B. Syk mutation in Jurkat E6-derived clones results in lack of p72syk expression. J Biol Chem. 1995;270:26533–26537. doi: 10.1074/jbc.270.44.26533. [DOI] [PubMed] [Google Scholar]
- 23.Gelfand E W, Weinberg K, Mazer B D, Kadlecek T A, Weiss A. Absence of ZAP-70 prevents signaling through the antigen receptor on peripheral blood T cells but not on thymocytes. J Exp Med. 1995;182:1057–1065. doi: 10.1084/jem.182.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldsmith M A, Weiss A. Isolation and characterization of a T-lymphocyte somatic mutant with altered signal transduction by the antigen receptor. Proc Natl Acad Sci USA. 1987;84:6879–6883. doi: 10.1073/pnas.84.19.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groves T, Smiley P, Cooke M P, Forbush K, Perlmutter R M, Guidos C J. Fyn can partially substitute for Lck in T lymphocyte development. Immunity. 1996;5:417–428. doi: 10.1016/s1074-7613(00)80498-7. [DOI] [PubMed] [Google Scholar]
- 26.Hatada M H, Lu X, Laird E R, Green J, Morgenstern J P, Lou M, Marr C S, Phillips T B, Ram M K, Theriault K, Zoller M J, Karas J L. Molecular basis for interaction of the protein tyrosine kinase ZAP-70 with the T-cell receptor. Nature. 1995;377:32–38. doi: 10.1038/377032a0. [DOI] [PubMed] [Google Scholar]
- 27.Hivroz-Burgaud C, Clipstone N A, Cantrell D A. Signaling requirements for the expression of the transactivating factor NF-AT in human T lymphocytes. J Immunol. 1991;21:2811–2819. doi: 10.1002/eji.1830211124. [DOI] [PubMed] [Google Scholar]
- 28.Irving B A, Chan A C, Weiss A. Functional characterization of a signal transducing motif present in the T cell antigen receptor ζ chain. J Exp Med. 1993;177:1093–1103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isakov N, Wange R L, Watts J D, Aebersold R, Samelson L E. Purification and characterization of human ZAP-70 protein-tyrosine kinase from a baculovirus expression system. J Biol Chem. 1996;271:15753–15761. doi: 10.1074/jbc.271.26.15753. [DOI] [PubMed] [Google Scholar]
- 30.Iwashima M, Irving B A, van Oers N S C, Chan A C, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 31.Karnitz L, Sutor S L, Torigoe T, Reed J C, Bell M P, McKean D J, Leibson P J, Abraham R T. Effects of p56lck deficiency on the growth and cytolytic effector function of an interleukin-2-dependent cytotoxic T-cell line. Mol Cell Biol. 1992;12:4521–4530. doi: 10.1128/mcb.12.10.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karnitz L M, Burns L A, Sutor S L, Blenis J, Abraham R T. Interleukin-2 triggers a novel phosphatidylinositol 3-kinase-dependent MEK activation pathway. Mol Cell Biol. 1995;15:3049–3057. doi: 10.1128/mcb.15.6.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keshvara L M, Isaacson C, Harrison M L, Geahlen R L. Syk activation and dissociation from the B-cell antigen receptor is mediated by phosphorylation of tyrosine 130. J Biol Chem. 1997;272:10377–10381. doi: 10.1074/jbc.272.16.10377. [DOI] [PubMed] [Google Scholar]
- 34.Kolanus W, Romeo C, Seed B. T cell activation by clustered tyrosine kinases. Cell. 1993;74:171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- 35.Kong G, Dalton M, Wardenburg J B, Straus D, Kurosaki T, Chan A C. Distinct tyrosine phosphorylation sites in ZAP-70 mediate activation and negative regulation of antigen receptor function. Mol Cell Biol. 1996;16:5026–5035. doi: 10.1128/mcb.16.9.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kong G-H, Bu J-Y, Kurosaki T, Shaw A S, Chan A C. Reconstitution of Syk function by the ZAP-70 protein tyrosine kinase. Immunity. 1995;2:485–492. doi: 10.1016/1074-7613(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 37.Latour S, Chow L M L, Veillette A. Differential intrinsic enzymatic activity of Syk and ZAP-70 protein-tyrosine kinases. J Biol Chem. 1996;271:22782–22790. doi: 10.1074/jbc.271.37.22782. [DOI] [PubMed] [Google Scholar]
- 38.Letourneur F, Klausner R D. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 epsilon. Science. 1992;255:79–82. doi: 10.1126/science.1532456. [DOI] [PubMed] [Google Scholar]
- 39.Molina T J, Kishihara K, Siderovski D P, van Ewijek W, Narendram A, Timms E, Wakeham A, Paige C J, Hartmann K-U, Veillette A, Davison B, Mak T W. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 40.Negishi I, Motoyama N, Nakayama K-I, Nakayama K, Senju S, Hatakeyama S, Zhang Q, Chan A C, Loh D Y. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 41.Neumeister E N, Zhu Y, Richard S, Terhorst C, Chan A C, Shaw A S. Binding of ZAP-70 to phosphorylated T-cell receptor ζ and η enhances its autophosphorylation and generates specific binding sites for SH2 domain-containing proteins. Mol Cell Biol. 1995;15:3171–3178. doi: 10.1128/mcb.15.6.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orloff D G, Frank S J, Robey F A, Weissman A M, Klausner R D. Biochemical characterization of the eta chain of the T-cell receptor. A unique subunit related to zeta. J Biol Chem. 1989;264:14812–14817. [PubMed] [Google Scholar]
- 43.Park J, Rho H W, Rhee S G. CD3 stimulation causes phosphorylation of phospholipase C-γ1 on serine and tyrosine residues in a human T-cell line. Proc Natl Acad Sci USA. 1991;88:5453–5456. doi: 10.1073/pnas.88.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 45.Rowley R B, Burkhardt A L, Chao H G, Matsueda G R, Bolen J B. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Ig alpha/Ig beta immunoreceptor tyrosine activation motif binding and autophosphorylation. J Biol Chem. 1995;270:11590–11594. doi: 10.1074/jbc.270.19.11590. [DOI] [PubMed] [Google Scholar]
- 46.Secrist J P, Burns L A, Karnitz L, Koretzky G A, Abraham R T. Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J Biol Chem. 1993;268:5886–5893. [PubMed] [Google Scholar]
- 47.Secrist J P, Karnitz L, Abraham R T. T-cell antigen receptor ligation induces tyrosine phosphorylation of phospholipase C-γ1. J Biol Chem. 1991;255:12135–12139. [PubMed] [Google Scholar]
- 48.Shiue L, Zoller J J, Brugge J S. Syk is activated by phosphotyrosine-containing peptides representing the tyrosine-based activation motifs of the high affinity receptor for IgE. J Biol Chem. 1995;270:10498–10502. doi: 10.1074/jbc.270.18.10498. [DOI] [PubMed] [Google Scholar]
- 49.Stein P L, Lee H M, Rich S, Soriano P. p59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- 50.Straus D B, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 51.Ting A T, Karnitz L M, Schoon R A, Abraham R T, Leibson P J. Fcγ receptor activation induces the tyrosine phosphorylation of both phospholipase C (PLC)-γ1 and PLC-γ2 in natural killer cells. J Exp Med. 1992;176:1751–1755. doi: 10.1084/jem.176.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turner M, Mee P J, Costello P S, Williams O, Price A A, Duddy L P, Furlong M T, Geahlen R L, Tybulewicz V L J. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 53.van Oers N S, Killeen N, Weiss A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J Exp Med. 1996;183:1053–1062. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Oers N S C, Lowin-Kropf B, Finlay D, Connolly K, Weiss A. αβ T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- 55.Van Wauwe J P, De Mey R R, Goossens J G. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980;124:2708–2713. [PubMed] [Google Scholar]
- 56.Wang C C, Badylak J A, Lux S E, Moriyama R, Dixon J E, Low P S. Expression, purification, and characterization of the functional dimeric cytoplasmic domain of human erythrocyte band 3 in Escherichia coli. Protein Sci. 1992;1:1206–1214. doi: 10.1002/pro.5560010913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wange R L, Guitian R, Isakov N, Watts J D, Aebersold R, Samelson L E. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J Biol Chem. 1995;270:18730–18733. doi: 10.1074/jbc.270.32.18730. [DOI] [PubMed] [Google Scholar]
- 58.Wange R L, Malek S N, Desiderio S, Samelson L E. Tandem SH2 domains of ZAP-70 bind to T-cell antigen receptor ζ and CD3ɛ from activated Jurkat T cells. J Biol Chem. 1993;268:19797–19801. [PubMed] [Google Scholar]
- 59.Wardenburg J B, Fu C, Jackman J K, Flotow H, Wilkinson S E, Williams D H, Johnson R, Kong G, Chan A C, Findell P R. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J Biol Chem. 1996;271:19641–19644. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 60.Watts J D, Affolter M, Krebs D L, Wange R L, Samelson L E, Aebersold R. Identification by electrospray ionization mass spectrometry of the sites of tyrosine phosphorylation induced in activated Jurkat T cells on the protein tyrosine kinase ZAP-70. J Biol Chem. 1994;269:29520–29529. [PubMed] [Google Scholar]
- 61.Weiss A, Koretzky G, Schatzman R C, Kadlecek T. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-γ1. Proc Natl Acad Sci USA. 1991;88:5484–5488. doi: 10.1073/pnas.88.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams, B. L., and R. T. Abraham. Unpublished observations.
- 63.Woodrow M, Clipstone N A, Cantrell D. p21ras and calcineurin synergize to regulate the nuclear factor of activated T cells. J Exp Med. 1993;178:1517–1522. doi: 10.1084/jem.178.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]