Abstract
Exposure of mammalian cells to adverse stimuli triggers the expression of numerous stress response genes, many of which are presumed to enhance cell survival. In this study, we examined the mechanisms contributing to the induction of p21Waf1 by stress and its influence on the survival of cells subjected to short-wavelength UVC irradiation. UVC was found to elevate p21Waf1 mRNA expression in mouse embryonal fibroblasts (MEFs) and human colorectal carcinoma (RKO) cells in a p53-dependent manner. The lack of p21Waf1 induction in p53-deficient MEFs and RKO cells correlated with diminished cell survival following UVC irradiation. Unexpectedly, UVC treatment was also found to block the induction of p21Waf1 by various stress-inducing agents such as mimosine in the p53-deficient cells. Additional studies indicated that induction of p21Waf1 by UVC occurs primarily through enhanced mRNA stability rather than increased transcription; in p53−/− MEFs, failure to elevate p21Waf1 after treatment with UVC appears to be due to their inability to stabilize the p21Waf1 transcripts. Treatment of the p53−/− MEFs with the protein tyrosine phosphatase inhibitor vanadate reversed the UVC-induced block on p21Waf1 induction and resulted in their enhanced survival following irradiation. Thus, in cells bearing normal p53, UVC augments p21Waf1 expression by increasing the half-life of p21Waf1 mRNA; without p53, p21Waf1 mRNA remains unstable after UVC, apparently due to a pathway involving tyrosine phosphatase activity.
Exposure of mammalian cells to stressful stimuli triggers a variety of response mechanisms, including alterations in the pattern of gene expression. Ultimately, these modifications determine the global response of the cell, ranging from transformation, growth stimulation, growth inhibition, differentiation, senescence, and cell death. Much of the altered gene expression seen in response to stress occurs through activation of selective gene transcription, and much attention has been focused on delineating the mechanisms regulating these transcriptional events. Central to the stress response is the activation of one or more mitogen-activated protein (MAP) kinase cascades, including those leading to the activation of the extracellular signal-regulated kinases (ERKs) (38, 48, 49), the c-Jun N-terminal kinases (JNKs; also known as stress-activated protein kinases) (3, 29), and a 38-kDa kinase termed CSBP/HOG1 (25, 29, 30). These MAP kinases serve to regulate, via phosphorylation, the activity of critical transcription factors (23). Alterations in posttranscriptional events also contribute to the regulation of gene expression during stress. Enhanced mRNA stability, in particular, has been associated with increased expression of many stress-responsive genes (26), and changes in the rate of translation and/or stability of proteins have likewise been implicated in regulating the expression of particular gene products during the stress response (9, 21). However, the mechanisms regulating these posttranscriptional processes remain largely unknown.
Among the genes that are believed to play an important role in determining cell fate during stress is the cyclin-dependent kinase inhibitor gene p21Waf1. p21Waf1 was originally identified as a gene regulated by the tumor suppressor protein p53 (8), and, indeed, induction of p21Waf1 in response to X irradiation and other DNA-damaging agents relies, to different extents, on its transcriptional upregulation by p53 (19). However, induction of p21Waf1 in response to other stresses, as well as by mitogenic stimulation, occurs via mechanisms that are independent of p53 (1, 8, 16, 27, 31, 35, 40). The role of p21Waf1 during stress remains controversial. Although there is evidence to suggest that p21Waf1 is proapoptotic in certain situations, most studies have provided evidence indicating that it functions as a protective factor during stress, associated with its growth-inhibitory properties. Thus, colorectal carcinoma cells lacking p21Waf1 display enhanced sensitivity to the cytotoxic effects of chemotherapeutic drugs (47). Furthermore, inhibiting p21Waf1 expression results in apoptosis of SH-SY5Y neuroblastoma cells (37) and sensitizes human breast carcinoma MCF-7 cells to killing by prostaglandin A2 (PGA2) (15). Conversely, elevated p21Waf1 expression protects the human colorectal carcinoma RKO line against the cytotoxic effects of PGA2 (15), prevents p53-mediated apoptosis of SK-MEL-110 cells (18), and enhances survival of UVC-treated DLD1 colorectal carcinoma cells (42).
In the present study, we investigated the mechanism(s) contributing to the regulation of p21Waf1 expression in response to UVC treatment and its influence on cell survival. Following exposure of mouse embryonal fibroblasts (MEFs) or RKO cells to UVC, increased p21Waf1 expression was found to depend on the presence of functional p53 and to correlate with enhanced cell survival. Not only did UVC treatment fail to induce p21Waf1 expression in either p53-deficient MEFs (p53−/− MEFs) or p53-deficient RKO cells; it also prevented p21Waf1 induction by mimosine, an agent that induces p21Waf1 expression independently of p53 function. The p53-dependent increase in p21Waf1 expression involves stabilization of p21Waf1 mRNA. However, even in the absence of p53, UVC was found to be capable of augmenting p21Waf1 expression if tyrosine phosphatase activity was blocked by vanadate. These studies implicate p53 in the control of a vanadate-sensitive system, possibly involving regulation of protein tyrosine phosphorylation, in the augmentation of p21Waf1 mRNA stability.
MATERIALS AND METHODS
Cell culture and treatments.
The human colorectal carcinoma cell lines RKO neo (exhibiting wild-type p53 function) and RKO E6 (p53 deficient) (28, 43) were cultured in minimum essential medium (Gibco BRL, Gaithersburg, Md.), and embryonal fibroblasts derived from wild-type, p21Waf1 knockout (p21−/−) (7), and p53 knockout (p53−/−) mice (32) were cultured in Dulbecco’s modified essential medium (Gibco BRL). Both media were supplemented with 10% fetal bovine serum (Hyclone, Logan, Utah) and 50 μg of gentamicin (Gibco BRL) per ml. RKO cells also received 350 μg of neomycin (Gibco BRL) per ml. Suramin, N-acetyl cysteine, rapamycin, sodium orthovanadate, dithiothreitol, H7, staurosporine, wortmannin, genistein, actinomycin D (ActD), and okadaic acid were purchased from Sigma (St. Louis, Mo.). Mimosine was purchased from Aldrich Chemical Co. (St. Louis, Mo.). SB 203580 and PD 098059 were kindly provided by SmithKline Beecham and Parke Davis, respectively. Drugs were added directly to the medium to the final concentrations indicated. For irradiation with UVC, cells were grown to approximately 50% confluence in 100- or 150-mm-diameter plates, and their medium was removed. Cells were then rinsed with phosphate-buffered saline and irradiated, and tissue culture medium was added back. In every experiment, untreated controls were subjected to mock irradiation.
Northern blot analysis.
Total RNA was isolated with STAT-60 (Tel-Test “B,” Friendswood, Tex.), and 20-μg RNA samples were denatured, size fractionated by electrophoresis in 1.2% agarose-formaldehyde gels, and transferred onto GeneScreen Plus nylon membranes (DuPont/NEN, Boston, Mass.). For the detection of p21Waf1 mRNA in RKO cells and gadd153 in MEFs, the p21Waf1 and gadd153 cDNAs were excised from plasmid pCEP-Waf1 (8) or pCMVgadd153, respectively, and labeled by using [α-32P]dCTP with a random primer labeling kit (Boehringer Mannheim, Indianapolis, Ind.). For the detection of p21Waf1 mRNA in MEFs and for normalization of differences in loading and transfer among samples in all Northern blots, an oligomer complementary to the mouse p21Waf1 mRNA (5′-CTCCGTGACGAAGTCAAAGTTCCACCGTTCTCGGGCCTCCTGGAGACAGCC-3′) and an oligomer complementary to the 18S rRNA (5′-ACGGTATCTGATCGTCTTCGAACC-3′ (Integrated DNA Technologies, Coralville, Iowa) were 3′ end labeled with [α-32P]dATP by terminal deoxynucleotidyltransferase (Life Technology Laboratories, Gaithersburg, Md.). Hybridization and washes were performed by the method of Church and Gilbert (4). Incorporation of 32P was visualized by using a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
Transient transfection.
For transfection experiments, 5 × 105 cells were seeded in 100-mm-diameter plates 24 h before transfection. The p21Waf1 promoter-luciferase construct WWT-Luc (5 μg of DNA) was transfected into cells by standard calcium phosphate precipitation methods, and luciferase activity was assayed with a luciferase assay system kit (Promega, Madison, Wis.). Values represent means ± standard errors of the means (SEM) of three independent experiments.
Colony formation assays.
Cell survival was measured by using a standard clonogenic assay. Twenty hours after treatment, 5 × 105 cells were trypsinized and serially diluted according to the expected surviving fraction (from 1:10 to 1:1,000,000). Plates were then returned to the incubator and cultured for an additional 12 to 14 days. The plates were fixed and stained with a crystal violet solution (10% [vol/vol] ethanol, 0.1% [wt/vol] crystal violet), and colonies (defined as greater than 50 cells) were counted. The surviving fraction was measured as the number of colonies divided by the dilution factor. For each UVC dose, plates were seeded at four different dilutions, and routinely, three of these were counted. Each colony formation assay was performed at least three times.
Nuclear run-on assay.
Nuclei were prepared from 5 × 107 MEFs 8 h after treatment with either UVC irradiation (20 J/m2) or mimosine (300 μM). Nascent RNA was labeled essentially as previously described previously (20) except that radiolabeled RNA was isolated by using the STAT-60 reagent and precipitated with 0.7 volume of isopropanol. Five micrograms of denatured β-actin and gadd153 cDNAs, 5 μg of an oligomer complementary to mouse p21Waf1 (5′-CT CCGTGACGAAGTCAAAGTTCCACCGTTCTCGGGCCTCCTGGAGACA GCC-3′), and 5 μg of pBlueScript plasmid (included as a negative control) were dot blotted onto nitrocellulose membranes. After blocking with 100 μg of tRNA per ml, membranes were hybridized with 4 × 106 cpm in 2 ml of hybridization buffer for 72 h at 65°C, washed extensively in 1% sodium dodecyl sulfate–1× SSC (0.15 M NaCl plus 0.015 M sodium citrate) at 65°C, and visualized with a PhosphorImager.
Western blot analysis.
Fifty-microgram samples of total cell lysates were size fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes by using standard techniques. p21Waf1 protein was detected with the ECL system (Amersham, Arlington Heights, Ill.) following incubation with a polyclonal rabbit anti-mouse p21Waf1 antibody (PC55) from Calbiochem (Cambridge, Mass.).
RESULTS
Effect of UVC irradiation on p21Waf1 mRNA expression and survival of MEFs and RKO cells differing in p53 status.
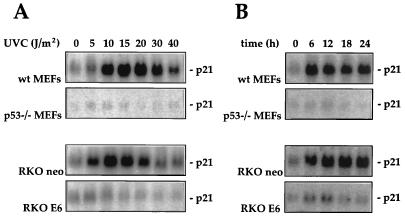
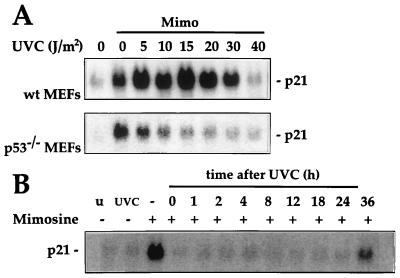
In agreement with earlier reports, exposure of wild-type MEFs (wt MEFs) and parental RKO neo cells to UVC resulted in a dose- and time-dependent elevation in p21Waf1 mRNA. Maximum induction of p21Waf1 mRNA expression (25- to 30-fold) was achieved with doses of UVC between 10 and 20 J/m2 (Fig. 1A) and within 6 to 12 h of treatment. Induction was sustained for up to 24 h but declined thereafter (Fig. 1B and data not shown). To determine whether the enhanced p21Waf1 expression following UVC irradiation was dependent on the presence of functional p53, p53-deficient MEFs and RKO cells were examined. As shown, MEFs derived from a mouse where both p53 alleles had been disrupted (p53−/− MEFs) as well as RKO cells where p53 function was inactivated by constitutive overexpression of the viral oncoprotein E6 (RKO E6 cells) failed to exhibit a substantial induction of p21Waf1 mRNA following UVC exposure, regardless of the UVC doses used or the times examined (Fig. 1).
FIG. 1.
p21Waf1 mRNA expression following UVC irradiation in MEFs or RKO cells differing in p53 status. (A) MEFs and RKO cells with functional p53 (wt MEFs and RKO neo cells, respectively) or nonfunctional p53 (p53−/− MEFs and RKO E6 cells, respectively) were exposed to increasing doses of UVC irradiation. Ten hours later, cells were lysed and p21Waf1 mRNA expression was assessed by Northern blot analysis as described in Materials and Methods. Assessment of loading and transfer of RNA samples, detected by hybridization to an oligomer complementary to 18S rRNA, indicated that all lanes contained equal amounts of RNA (not shown). (B) MEFs and RKO cells differing in p53 status were exposed to UVC at 20 J/m2, and p21Waf1 mRNA expression was monitored at the times indicated.
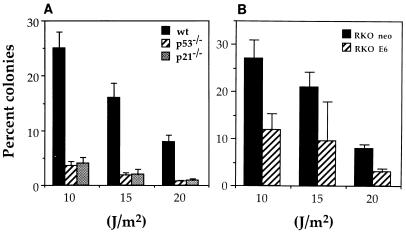
Next, we compared the survival of wild-type versus p53-deficient MEFs and RKO cells following UVC treatment. A colony proliferation assay was used to measure cell survival with several UVC doses, and the results, shown in Fig. 2, are expressed as the percentage of colonies obtained with a given UVC dose relative to untreated controls. p53-deficient cells (p53−/− MEFs and RKO E6 cells) showed significantly lower survival than their wild-type counterparts (wt MEFs and RKO neo cells) at all doses tested. These findings are similar to those recently reported by Sheikh et al. (42), who used a tetracycline-inducible system to modulate the expression exogenous p21Waf1 in p53-deficient cells. Similarly, UVC treatment of p21Waf1-deficient (p21−/−) MEFs was also accompanied by a dramatic reduction in the numbers of colonies recovered. These results support the view that p53 and p21Waf1 are important for cell survival following UVC treatment.
FIG. 2.
Colony survival assay following UVC irradiation of MEFs and RKO cells. (A) Survival of wt, p53−/−, and p21−/− MEFs following UVC irradiation. (B) Survival of RKO neo and RKO E6 cells following UVC irradiation. A total of 5 × 105 cells (in 100-mm-diameter tissue culture dish) were irradiated with the indicated doses of UVC. Twenty-four hours later, cells were trypsinized, serially diluted (10 to 1,000,000 times), replated, and returned to the incubator for an additional 12 to 14 days. Surviving colonies were then fixed with crystal violet and counted. For each UVC dose, plates were seeded at four different dilutions, and routinely, three of these were counted. Experiments were done at least five times. Values represent means ± SEM.
Effect of UVC on p53-independent p21Waf1 induction.
The correlation between p21Waf1 expression and survival of the MEFs and RKO lines was fully consistent with p21Waf1 contributing to the enhanced survival following exposure to UVC. However, we sought to assess the role of p21Waf1 expression in the survival of the p53-deficient lines by inducing an increase in p21Waf1 levels via an alternative, p53-independent mechanism before exposure to UVC. Mimosine was chosen as the inducer, as we had previously reported that mimosine induces p21Waf1 expression in p53-deficient RKO cells independent of p53 function, an effect that was correlated with enhanced survival during subsequent exposure to PGA2 (17).
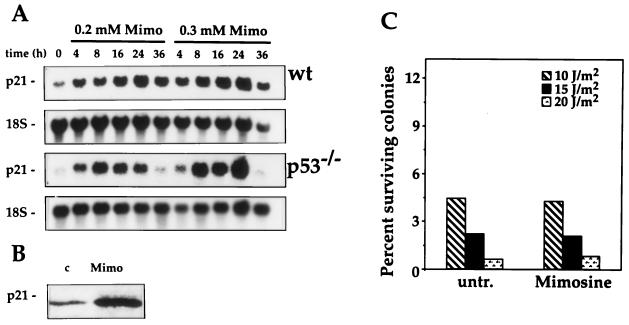
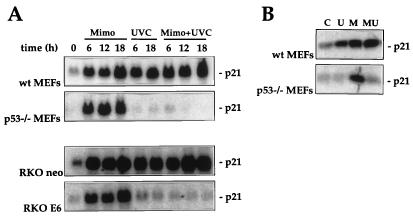
In agreement with our prior observations in RKO cells and those of Alpan and Pardee (2), treatment of MEFs with mimosine led to induction of p21Waf1 mRNA irrespective of p53 activity (Fig. 3A). That the elevated mRNA expression results in increased expression of p21Waf1 protein is shown in Fig. 3B. To determine if such upregulation of p21Waf1 expression could confer enhanced tolerance to UVC in p53−/− MEFs, mimosine was added to the cells immediately following irradiation. This treatment condition was chosen to mimic the time course for p21Waf1 induction seen in wt MEFs treated with UVC (Fig. 1 and 2). To our initial surprise, mimosine treatment did not substantially alter the survival of p53−/− MEFs after UVC treatment; i.e., it did not confer protection as predicted (Fig. 3C). Analysis of p21Waf1 expression in the p53-deficient MEFs subjected to the combined treatments (UVC plus mimosine) revealed that p21Waf1 levels were not increased. UVC blocked the induction of p21Waf1 by mimosine (Fig. 4). This suppressive effect of UVC was also seen in RKO E6 cells but not in the MEFs and RKO lines with normal p53 function (wt MEFs and RKO neo cells, respectively), where p21Waf1 was elevated with either treatment alone as well as with the combination.
FIG. 3.
Effect of mimosine on p21Waf1 expression and UVC-mediated cytotoxicity in p53−/− MEFs. (A) RNA was extracted from wt and p53−/− MEFs treated with 200 or 300 μM mimosine (Mimo) for the indicated times, and p21Waf1 mRNA expression was monitored by Northern blot analysis as described in Materials and Methods. (B) Western blot analysis of p21Waf1 expression in p53−/− MEFs treated with 250 μM mimosine (Mimo) was carried out as described in Materials and Methods. (C) Following irradiation of p53−/− MEFs (5 × 105 cells per 100-mm-diameter tissue culture dish), cells received either normal medium (untr.) or medium containing 200 μM mimosine (Mimosine). Twenty-four hours later, cells were trypsinized, serially diluted (10 to 1,000,000 times), replated, and returned to the incubator for an additional 14 days. Surviving colonies were fixed with crystal violet and counted. For each UVC dose, plates were seeded at four different dilutions, and routinely, three different dilutions were counted. Experiments were done at least three times. Values represent means ± SEM.
FIG. 4.
Dose- and time-dependent effects of UVC on mimosine-induced p21Waf1 expression in MEFs and RKO cells differing in p53 status. (A) MEFs and RKO cells with or without functional p53 status were either treated with 300 μM mimosine (Mimo), exposed to UVC at 20 J/m2 (UVC), or treated with 300 μM mimosine immediately following exposure to UVC at 20 J/m2 (Mimo+UVC). RNA was isolated at the times indicated, and p21Waf1 mRNA expression was analyzed. (B) Western blot analysis of p21Waf1 expression in wt and p53−/− MEFs 16 h after treatment with either UVC at 20 J/m2 (U), 300 μM mimosine (M), or 300 μM mimosine and UVC at 20 J/m2 (MU). C, control.
A more detailed analysis of the time- and dose-dependent relationships for suppression of mimosine-induced p21Waf1 expression by UVC was then undertaken (Fig. 5). With the exception of the highest UVC dose used (40 J/m2), UVC treatment of wild-type MEFs elevated p21Waf1 mRNA levels above that seen with mimosine treatment alone (Fig. 5A). In contrast, in p53−/− MEFs, mimosine-mediated induction of p21Waf1 was inhibited by even the lowest UVC dose tested (5 J/m2). More striking was the length of time over which the inhibitory influence of UVC was sustained (Fig. 5B). Cells that had been exposed to UVC at 20 J/m2 24 h before the mimosine treatment were still totally refractory to induction by mimosine. Lengthening the interval between UVC irradiation and addition of mimosine to 36 h finally led to partial restoration of p21Waf1 induction by mimosine. Thus, the UVC-induced changes that blocked the mimosine-induced upregulation of p21Waf1 lasted nearly 36 h.
FIG. 5.
Dose- and time-dependent effects of UVC on mimosine-induced p21Waf1 mRNA expression. (A) Wild-type and p53−/− MEFs were either left untreated, treated with 300 μM mimosine (Mimo), or UVC irradiated at the doses indicated with 300 μM mimosine. Ten hours after treatment, RNA was extracted and p21Waf1 mRNA expression was analyzed. (B) p53−/− MEFs were irradiated with UVC (20 J/m2) at various time intervals (from 0 to 36 h) prior to treatment with mimosine. p21Waf1 mRNA expression was assessed 10 h following treatment with mimosine. u, untreated control; UVC, cells irradiated 10 h prior to harvesting of RNA.
Posttranscriptional regulation of p21Waf1 expression by UVC.
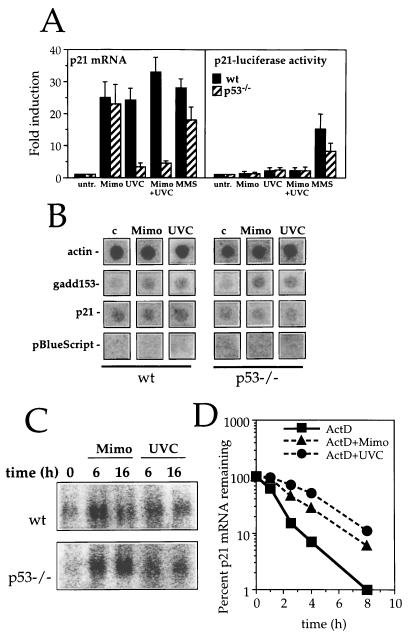
To address the mechanisms whereby UVC inhibits p21Waf1 induction by mimosine, it was first necessary to determine how mimosine acted to induce p21Waf1. Two approaches were used to investigate whether the mimosine effect was due to enhanced transcription of the p21Waf1 gene. First, the activity of the p21Waf1 promoter was measured by using a p21Waf1 promoter-luciferase reporter construct transiently transfected into wt and p53−/− MEFs (Fig. 6A). UVC, mimosine, and combined UVC-plus-mimosine treatments all failed to substantially elevate p21Waf1 promoter activity (Fig. 6A, right), regardless of p53 status. This is in sharp contrast to the effect of these treatments on p21Waf1 mRNA levels (Fig. 6A, left). Treatment with the DNA-alkylating agent methyl methanesulfonate (MMS), included as a positive control, both activated the p21Waf1 promoter and enhanced endogenous levels of p21Waf1 mRNA, although maximal induction in each case required wt p53 function (Fig. 6A). The second approach used nuclear run-on assays to directly determine whether the rate of transcription of the p21Waf1 gene was increased following treatment with either mimosine or UVC. Measurements were performed at 8 h, a time at which p21Waf1 mRNA levels are approaching maximal levels. As shown in Fig. 6B, neither UVC nor mimosine treatment led to any perceptible change in the transcription rates of the p21Waf1 gene, regardless of p53 status. Transcription of the gadd153 gene (included here as a positive control) was modestly increased by the mimosine and UVC treatments. This elevated transcription accounted, at least in part, for the similarly modest elevation in gadd153 mRNA expression in wt and p53−/− MEFs following treatment with either UVC or mimosine (Fig. 6C).
FIG. 6.
Induction of p21Waf1 mRNA expression by UVC and mimosine is not regulated transcriptionally. (A) Right, relative luciferase activity driven by the p21Waf1 promoter in transiently transfected wt or p53−/− MEFs following treatment with 300 μM mimosine (Mimo), UVC at 20 J/m2 (UVC), a combination of 300 μM mimosine plus UVC at 20 J/m2 (Mimo+UVC) or 100 μg of MMS per ml (MMS). Values represent the means of three independent experiments. Left, quantitation of p21Waf1 mRNA levels in identically treated populations of wt and p53−/− MEFs. mRNA expression was analyzed 10 h after addition of 300 μM mimosine (Mimo) and exposure to UVC at 20 J/m2 (UVC), mimosine plus UVC (Mimo+UVC), or 100 μg of MMS per ml (MMS). Values are represented as fold induction relative to the level of expression in untreated control cells (untr.). p21Waf1 mRNA signal was measured with a PhosphorImager and normalized to hybridization signal to 18S rRNA. (B) Nuclear run-on analysis of wt or p53−/− MEFs that were either left untreated (c), treated with mimosine (Mimo), or exposed to UVC at 20 J/m2 (UVC) 8 h prior to collection of cells and nuclear run-on assay, carried out as described in Materials and Methods. (C) Northern blot analysis of gadd153 mRNA expression in wt or p53−/− MEFs following treatment with 300 μM mimosine (Mimo) or exposure to UVC at 20 J/m2 (UVC). (D) The levels of p21Waf1 mRNA in wt MEFs treated with ActD alone or in combination with mimosine (300 μM) or UVC (20 J/m2) were assessed by Northern blot analysis and quantitated with a PhosphorImager.
To determine if the elevation in p21Waf1 mRNA seen in the wt MEFs was associated with enhanced stability of the mRNA, we performed a standard mRNA decay assay. ActD (1 μg/ml) was added to cells to prevent any new gene transcription, and p21Waf1 mRNA levels were monitored over the following 8-h period in cells treated with ActD only and in cells treated with ActD plus either mimosine or UVC. As shown in Fig. 6D, the half-life of p21Waf1 mRNA in ActD-treated cells was about 65 min; mimosine and UVC treatments increased the half-life ∼2.5-fold (to 170 min) and ∼4-fold (240 min), respectively. While a similar study could not be performed in p53−/− MEFs due to their low levels of basal p21Waf1 mRNA expression, our results indicate that both mimosine and UVC induce p21Waf1 mRNA levels primarily by inhibiting its decay.
Vanadate prevents UVC-mediated suppression of p21Waf1 expression through stabilization of p21Waf1 mRNA.
A number of signaling cascades are triggered by UVC and contribute to the cellular changes that characterize the response (22, 45, 46). The evidence presented thus far, indicating that UVC suppresses the induction of p21Waf1 mRNA by other agents, strongly suggests that p53−/− cells do not simply lack a pathway which is otherwise activated by UVC; rather, it is consistent with the existence of an abnormally active mechanism of p21Waf1 mRNA degradation in p53-deficient cells. To begin exploring which, if any, of the established UVC-triggered signaling mechanisms might be important in influencing p21Waf1 mRNA stability, p53−/− MEFs were pretreated with a variety of specific inhibitors of these pathways before treatment with mimosine, UVC, or mimosine plus UVC. p21Waf1 mRNA expression was examined 10 h later to determine if any of the agents tested would alter the UVC-mediated suppression of p21Waf1. No change in the general pattern of p21Waf1 mRNA expression was seen in the presence of any of the agents tested, except for vanadate, an inhibitor of tyrosine phosphatases (Table 1). Vanadate treatment alone did not alter p21Waf1 mRNA levels. However, it effectively allowed for induction of p21Waf1 mRNA by UVC and completely prevented the loss of mimosine-induced p21Waf1 expression by UVC. These findings argue strongly for the involvement of a tyrosine kinase/phosphatase regulatory system in the modulation of p21Waf1 mRNA stability.
TABLE 1.
Effects of various inhibitory agents on p21Waf1 expression in p53−/− MEFs following mimosine treatment, UVC irradiation, or botha
| Inhibitor | Target | Concn | Fold enhancement of p21Waf1 mRNA expressionb
|
||
|---|---|---|---|---|---|
| Mimosine | UVC | Mimosine + UVC | |||
| N-acetyl cysteine | Oxidants | 20 μM | 24 | 2 | 3 |
| Suramin | Growth factor receptor | 0.3 mM | 27 | 2 | 4 |
| SB 203580 | p38/CSBP | 1–10 μM | 25 | 3 | 4 |
| PD 098059 | MEK | 20 μM | 25 | 3 | 4 |
| Wortmannin | Phosphatidylinositol 3-kinase | 100 μM | 26 | 3 | 4 |
| Rapamycin | S6 kinase pathway | 5 ng/ml | 27 | 2 | 4 |
| H7 | PKC | 50 μM | 28 | 2 | 4 |
| Staurosporine | PKC | 50 nM | 25 | 2 | 3 |
| Genistein | Protein Tyr kinases | 20 μM | 28 | 2 | 4 |
| Okadaic acidc | Ser/Thr phosphatases | 30–100 nM | |||
| Vanadate | Protein Tyr phosphatases | 25 μM | 26 | 20 | 24 |
p53−/− MEFs were pretreated for 1 h with the indicated concentrations of the agents listed and then exposed to mimosine (300 μM) and UVC (20 J/m2), separately or in combination. RNA was isolated 10 h later, and the level of p21Waf1 mRNA expression was monitored under each treatment condition.
Calculated relative to the levels of p21Waf1 mRNA seen in cells that were pretreated only with the inhibitor.
Okadaic acid alone induced p21Waf1 mRNA expression, and UVC inhibited this induction.
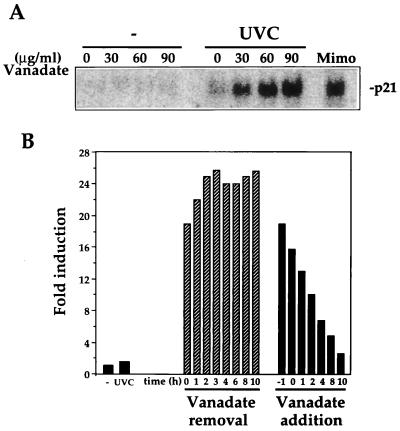
The dose- and time-dependent effects of vanadate are illustrated in Fig. 7. When added alone, vanadate at up to 90 μg/ml (the highest dose used) did not elevate p21Waf1 mRNA expression in the p53−/− MEFs. However, culturing the cells in the presence of increasing concentrations of vanadate allowed for the elevation of p21Waf1 mRNA by UVC (Fig. 7A). The relief from UVC-mediated suppression required only a short period of exposure to vanadate: a 1-h pretreatment period was sufficient to achieve maximum p21Waf1 mRNA induction (greater than 10-fold) even if vanadate was removed at the time of UVC irradiation. As expected, addition of vanadate after UVC irradiation was less effective in relieving the suppression of p21Waf1 mRNA induction (Fig. 7B). Since high doses of vanadate have been reported to inhibit Na-K ATPases, it was important to determine whether the effect of vanadate on p21Waf1 expression might be attributable to the inhibition of these ATPases. Therefore, we examined the effect of ouabain, a specific inhibitor of Na-K ATPases, on p21Waf1 expression following UVC treatment. Ouabain had no effect on p21Waf1 expression in either control or UVC-treated cells (not shown). Therefore, it is unlikely that vanadate influences p21Waf1 expression in UVC-treated cells through inhibition of Na-K ATPases.
FIG. 7.
Dose- and time-dependent effect of vanadate on p21Waf1 expression after UVC irradiation of p53−/− MEFs. (A) Northern blot analysis of p21Waf1 expression in p53−/− MEFs that were treated with increasing doses of vanadate and either left unirradiated (−) or irradiated with UVC at 20 J/m2. Expression of p21Waf1 mRNA was analyzed 10 h after UVC treatment. Mimo, mimosine. (B) Time-dependent effect of 50 μg of vanadate per ml on p21Waf1 mRNA expression. Quantitation of p21Waf1 mRNA levels in p53−/− MEFs subjected to the various treatment regimens. For vanadate removal, cells were treated with vanadate for 1 h before exposure to UVC, and then vanadate was removed at the time of UVC irradiation or at different time intervals thereafter; for vanadate addition, vanadate was added at different times after UVC irradiation. RNA was isolated 10 h after exposure to UVC.
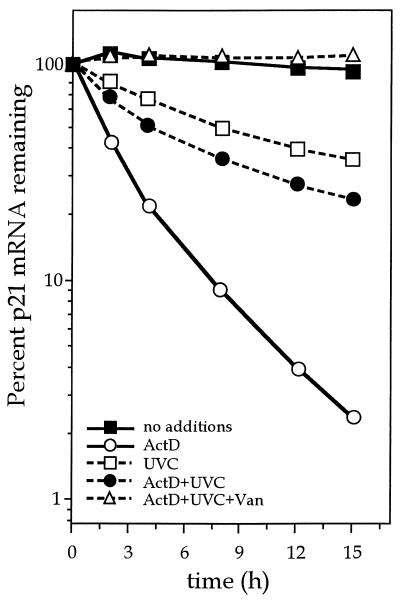
Although we cannot rule out the possibility that the vanadate effects arise from vanadate’s influence on cellular targets other than protein tyrosine phosphatases, the findings described above are consistent with the notion that vanadate relieves UVC-mediated suppression of p21Waf1 expression by inhibiting a tyrosine phosphatase that regulates p21Waf1 mRNA stability. To gain more direct evidence for this view, we examined the decay of p21Waf1 mRNA in p53−/− MEFs following UVC treatment in the absence or presence of vanadate. p53−/− MEFs were treated with mimosine for 12 h prior to the addition of ActD (time zero) to prevent any further transcription. Replicate plates were then either left as such or treated with UVC at (20 J/m2) in the absence or presence of vanadate. p21Waf1 mRNA levels were assessed over the following 15-h period. It is important to note that mimosine remained in the cultures throughout the length of the experiment. As shown in Fig. 8, the addition of ActD led to a loss of p21Waf1 mRNA, similar to that seen in mimosine-ActD-treated wt MEFs (Fig. 6D). UVC-ActD treatment resulted in a similar, though slower, decline in p21Waf1 mRNA. However, vanadate treatment completely blocked the UVC-mediated loss in p21Waf1 mRNA transcripts, suggesting that a tyrosine-phosphorylated protein may be important in regulating mRNA stability (Fig. 8). The fact that p21Waf1 mRNA transcripts are more stable in cells with functional p53 (wt MEFs and RKO cells) relative to p53-negative cells (p53−/− MEFs and RKO E6 cells) following UVC treatment (i.e., UVC treatment does not cause a decline in p21Waf1 mRNA levels in cells with wild-type p53 [Fig. 4 and 5]) suggests that p53 may be somehow involved in regulating the tyrosine kinase/phosphatase activity necessary for stabilization.
FIG. 8.
Influence of vanadate on clearance of p21Waf1 mRNA in p53−/− MEFs. Following exposure to 300 μM mimosine for 14 h, p53−/− MEFs were either pretreated for 1 h with 40 μg of vanadate (Van) per ml or not pretreated and were then exposed to ActD (1 μM), UVC (20 J/m2), or both. At the times indicated, RNA was collected and p21Waf1 mRNA expression was determined by Northern blot analysis. The p21Waf1 mRNA signals were quantitated with a PhosphorImager. Mimosine remained in the culture media throughout the entire treatment period.
Vanadate enhances survival of UVC-treated p53−/− MEFs.
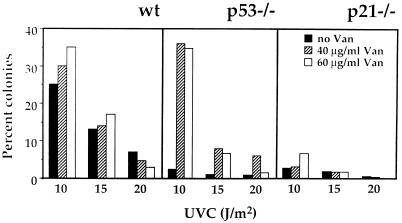
Finally, since p21Waf1 expression could be elevated in p53−/− cells by UVC in the presence of vanadate, it was of interest to compare the survival of p53−/− MEFs following UVC irradiation in the presence or absence of vanadate. p21−/− MEFs were also included in the experiment as a control, since p21Waf1 expression could not be induced in them regardless of vanadate treatment. Wild-type, p53−/−, and p21−/− MEFs were pretreated with either 40 or 60 μg of vanadate per ml for 1 h, after which vanadate was removed and the cells were irradiated with UVC at 10, 15, or 20 J/m2 (Fig. 9). Survival was assessed with a colony proliferation assay 12 to 14 days later. While vanadate pretreatment did not substantially alter the survival of wt MEFs (which already expressed significant amounts of p21Waf1), it markedly enhanced the survival of p53−/− MEFs to levels comparable to those seen in wt cells (Fig. 9). That the enhanced survival was attributed to enhanced p21Waf1 expression rather than some other effect of vanadate is supported by the finding that vanadate did not improve survival of p21−/− MEFs. Taken together, these findings argue strongly that p21Waf1 functions as a survival factor in this stress paradigm.
FIG. 9.
Effect of vanadate treatment on the survival of wt, p53−/−, and p21−/− MEFs following UVC irradiation. MEFs (5 × 105 in 100-mm-diameter tissue culture dishes) were pretreated with 40 or 60 μg of vanadate (Van) per ml for 1 h prior to irradiation with the indicated doses of UVC. Vanadate was removed at the time of irradiation. Twenty-four hours later, cells were trypsinized, serially diluted (10 to 1,000,000 times), replated, and returned to the incubator for an additional 12 to 14 days, whereupon plates were fixed and stained with crystal violet and surviving colonies were counted. For each vanadate and UVC dose, plates were seeded at four different dilutions, and routinely, three of these were counted.
DISCUSSION
Alterations in gene expression in response to external stimuli constitute a key component of the cellular response to stress. Although most studies examining the genetic response to environmental insults have focused on the transcriptional control of the induced genes, it is likely that posttranscriptional mechanisms also contribute significantly to stress-induced changes in gene expression. In support of this view, we have provided evidence that induction of p21Waf1 expression following UVC irradiation is regulated primarily through posttranscriptional events that increase the stability of p21Waf1 mRNA. We provide further evidence indicating that this is mediated by a tyrosine kinase/phosphatase regulatory system and requires the presence of functional p53. In the absence of p53, UVC irradiation not only fails to elevate p21Waf1 expression but potently inhibits p21Waf1 induction by mimosine, and also by other agents such as hydrogen peroxide, okadaic acid, PGA2, or MMS (not shown). This suppressive effect of UVC is likely to reflect a general mechanism important for regulating mRNA stability of stress-responsive genes, as we have found that UVC treatment also inhibits the expression of other stress-inducible genes such as gadd45 and MAP kinase phosphatase 1 genes in cells lacking p53 (not shown).
The regulation of the stability of labile mRNAs is poorly understood. It is thought to employ a number of proteins that control the degradative pathway through interaction with a number of cis elements, most frequently located in the 3′ untranslated region of the mRNA (5). The most common of 3′ untranslated region stability determinants are AU-rich elements (AREs) (with very high incidence of adenosine and uridine residues), notably the pentamer AUUUA (14). AREs have been reported to confer instability to otherwise stable mRNAs (41). Although AREs are typically found in the transcripts encoding cytokines (interleukins and interferons) and oncogenes (c-myc and c-fos), they have also been found in other short-lived stress-inducible mRNAs, including those encoding gadd153, vascular endothelial growth factor, cyclin D1, and p21Waf1. Indeed, both human and mouse p21Waf1 mRNAs contain AREs, including several AUUUA repeats (8, 24). Among the various RNA-binding proteins which have been identified to date are the AU-binding (11, 34, 39) and ELAV families of proteins (12). Members of these families (Hel-N1, Hel-N2, HuR, HuD, Hel-N, and HuC) display high affinity for AREs (10, 33). Several reports have linked protein kinase C (PKC) activation with mRNA stabilization events involving AU-binding proteins following exposure to various agents (13, 14, 34, 44). However, PKC does not appear to contribute to the regulatory events reported here, since PKC inhibitors failed to alter p21Waf1 expression by UVC, mimosine, or combined treatment.
UVC irradiation leads to a rapid increase in tyrosine phosphorylation of cellular proteins, many of which have not been identified. Both receptor-linked and non-receptor-linked tyrosine kinases are believed to play a central role in initiating the UVC response, leading to the activation of MAP kinase signaling cascades, involving ERK, JNK, and p38. The MAP kinase pathways play an important role in activating transcription factors leading to increased gene transcription. Recent reports have provided evidence that both serine/threonine and tyrosine phosphatases play a key role in regulating the activity of the ERK, JNK, and p38 MAP kinases at numerous stages of the signaling cascades. Although a role for MAP kinases in mRNA stabilization has not been explored, neither ERK nor p38 appears to contribute to regulating p21Waf1 mRNA expression, since inhibitors of these pathways did not alter the p21Waf1 mRNA levels. While the involvement of the JNK pathway could not be tested directly in these cells, due to the lack of availability of a suitable inhibitor, other preliminary findings generated in our laboratory suggest that p21Waf1 expression might be modulated by JNK. We have found that transfection of human lung carcinoma A549 cells with a construct that constitutively expresses a dominant-negative isoform of SEK1 (a kinase that specifically phosphorylates, thereby activating, JNK) results in a marked enhancement p21Waf1 mRNA expression by UVC (our unpublished observations). Thus, a JNK-regulated protein might contribute to an enhanced p21Waf1 mRNA degradation following UVC exposure. To the best of our knowledge, ours is the first study to provide evidence that tyrosine phosphorylation contributes to the regulation of mRNA stability. Obviously, the identity of the specific kinases/phosphatases involved will require further investigation. Such activities could function upstream, downstream, or independent of the JNK pathway, but we speculate that the activity of RNA-binding proteins may be subject to regulation either directly by a tyrosine kinase or through a downstream target (e.g., JNK).
The p21Waf1 promoter has been shown to contain multiple p53 binding sites which are important for transcriptional activation of p21Waf1 by ionizing radiation. Given the dependency of p21Waf1 induction by UVC on p53, it was surprising to find that induction by UVC does not involve such transcriptional activation. In addition to its well-established function as a bona fide transcription factor, several other gene regulatory functions have been ascribed to p53. For example, in certain situations, p53 has been shown to repress transcription (36) and translation (9) and to decrease protein stability (21). However, to our knowledge, our studies provide the first evidence that p53 function is involved in the regulation of gene expression by modulating mRNA stability; whether this regulation is direct or indirect remains to be addressed. Although our efforts have focused on the stability of the mRNA encoding p21Waf1, mRNAs encoding other gene products such as MKP-1 and gadd45 also appear to follow a pattern of p53 dependency after UVC irradiation similar to that described here for p21Waf1 mRNA (unpublished observations). Further experiments are required to elucidate the precise role that p53 plays in the expression of these genes and the contribution of transcriptional and posttranscriptional regulatory events.
Finally, our results are consistent with p21Waf1 conferring a survival advantage during the cellular response to UVC irradiation. That is, we observed a strong correlation between expression of p21Waf1 following UVC irradiation and survival, with the p53−/− and p21−/− MEFs exhibiting much greater sensitivity than their wild-type counterparts. These findings contrast with those reported by DeFrank et al. (6), who observed that wt and p53−/− MEFs had comparable survival rates following exposure to UVC irradiation. The cause for this discrepancy remains unclear. Nonetheless, the fact that in our studies vanadate treatment, which restored p21Waf1 expression in p53−/− MEFs, likewise enhanced their survival strongly argues for a protective function of p21Waf1.
In summary, we have provided several novel findings regarding p21Waf1 induction by cellular stress: (i) that p21Waf1 induction in response to UVC occurs largely through posttranscriptional modifications leading to enhanced stability of p21Waf1 mRNA, (ii) that p21Waf1 mRNA stability is regulated via a regulatory system sensitive to vanadate (possibly involving changes in tyrosine phosphorylation), and (iii) that these putative tyrosine kinase/phosphatase activities are subject to modulation by UVC irradiation in a p53-dependent fashion. Future studies will address the identity of the specific tyrosine kinase/phosphatases involved, their downstream targets, and the nature of the p53 dependency of this effect.
ACKNOWLEDGMENTS
We thank M. B. Kastan for the RKO neo and RKO E6 cells, T. Jacks for the wt and p53−/− MEFs, P. Leder for the p21−/− MEFs, and B Vogelstein for plasmids pCEP4Waf1 and WWT-Luc. We are also grateful to S. Shack, A. Passaniti, K. Z. Guyton, and Y. Liu for helpful discussions and D. L. Longo for critical reading of the manuscript.
REFERENCES
- 1.Akashi M, Hachiya M, Osawa Y, Spirin K, Suzuki G, Koeffler H P. Irradiation induces Waf1 expression through a p53-independent pathway in KG-1 cells. J Biol Chem. 1995;270:19181–19187. doi: 10.1074/jbc.270.32.19181. [DOI] [PubMed] [Google Scholar]
- 2.Alpan R S, Pardee A B. p21Waf1/CIP1/SDI1 is elevated through a p53-independent pathway by mimosine. Cell Growth Differ. 1996;7:893–901. [PubMed] [Google Scholar]
- 3.Chen Y R, Wang X, Templeton R J, Davis R J, Tan T H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 4.Church G M, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decker C J, Parker R. Diversity of cytoplasmic functions for the 3′ untranslated region of eukaryotic transcripts. Curr Opin Cell Biol. 1995;7:386–392. doi: 10.1016/0955-0674(95)80094-8. [DOI] [PubMed] [Google Scholar]
- 6.DeFrank J S, Tang W, Powell S N. p53-null cells are more sensitive to ultraviolet light only in the presence of caffeine. Cancer Res. 1996;56:5365–5368. [PubMed] [Google Scholar]
- 7.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21CIP1/Waf1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 8.El-Deiry W S, Tokino T, Velculescu T, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Waf1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 9.Ewen M E, Oliver C J, Sluss H K, Miller S J, Peeper D S. p53-dependent repression of CDK4 translation in TGF-β-induced G1 cell cycle arrest. Genes Dev. 1995;9:204–217. doi: 10.1101/gad.9.2.204. [DOI] [PubMed] [Google Scholar]
- 10.Gao F B, Carson C C, Levine T, Keene J D. Selection of a subset of mRNAs from combinatorial 3′ untranslated region libraries using the neuronal RNA-binding protein Hel-N1. Proc Natl Acad Sci USA. 1994;91:11207–11211. doi: 10.1073/pnas.91.23.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillis P, Malter J S. The adenosine-uridine binding factor recognizes the AU-rich elements of cytokine, lymphokine and oncogene mRNAs. J Biol Chem. 1991;266:3172–3177. [PubMed] [Google Scholar]
- 12.Good P J. A conserved family of elav-like genes in vertebrates. Proc Natl Acad Sci USA. 1995;92:4557–4561. doi: 10.1073/pnas.92.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorospe M, Kumar S, Baglioni C. Tumor necrosis factor increases stability of interleukin-1 mRNA by activating protein kinase C. J Biol Chem. 1993;268:6214–6220. [PubMed] [Google Scholar]
- 14.Gorospe M, Baglioni C. Degradation of unstable interleukin-1 alpha mRNA in a rabbit reticulocyte cell-free system. Localization of an instability determinant to a cluster of AUUUA motifs. J Biol Chem. 1994;269:11845–11851. [PubMed] [Google Scholar]
- 15.Gorospe M, Holbrook N J. Role of p21Waf1 in prostaglandin A2-mediated cellular arrest and death. Cancer Res. 1996;56:475–479. [PubMed] [Google Scholar]
- 16.Gorospe M, Martindale J L, Sheikh M S, Fornace A J, Jr, Holbrook N J. Regulation of p21CIP1/Waf1 expression by cellular stress: p53-dependent and p53-independent mechanisms. Mol Cell Differ. 1996;4:47–65. [Google Scholar]
- 17.Gorospe M, Wang X, Guyton K, Holbrook N J. Protective role of p21Waf1/Cip1 against prostaglandin A2-mediated apoptosis of human colorectal carcinoma cells. Mol Cell Biol. 1996;16:6654–6660. doi: 10.1128/mcb.16.12.6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorospe M, Cirielli C, Wang X, Capogrossi M, Holbrook N J. p21Waf1/Cip1 expression prevents p53-mediated apoptotic cell death of human melanoma cells. Oncogene. 1997;14:929–935. doi: 10.1038/sj.onc.1200897. [DOI] [PubMed] [Google Scholar]
- 19.Gotz C, Montenarh M. p53 and its implications in apoptosis. Int J Oncol. 1995;6:1129–1135. doi: 10.3892/ijo.6.5.1129. [DOI] [PubMed] [Google Scholar]
- 20.Groudine M, Peretz M, Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981;1:281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S, Campbell D, Derijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 22.Holbrook N J, Liu Y, Fornace A J., Jr Signaling events controlling the molecular response to genotoxic stress. EXS. 1996;77:273–288. doi: 10.1007/978-3-0348-9088-5_18. [DOI] [PubMed] [Google Scholar]
- 23.Hunter T, Karin M. The regulation of transcription by phosphorylation. Cell. 1992;70:375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- 24.Huppi K, Siwarski D, Dosik J, Michieli P, Chedid M, Reed S, Mock B, Givol D, Mushinski J F. Molecular cloning, sequencing, chromosomal localization and expression of mouse p21 (Waf1) Oncogene. 1994;9:3017–3020. [PubMed] [Google Scholar]
- 25.Iordanov M, Bender K, Ade T, Schmid W, Sachsenmaier C, Engel K, Gaestel M, Rahmsdorf H J, Herrlich P. CREB is activated by UVC through a p38/HOG1-dependent protein kinase. EMBO J. 1997;16:1009–1022. doi: 10.1093/emboj/16.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackman J, Alamo I J, Fornace A J., Jr Genotoxic stress confers preferential and coordinate messenger RNA stability on the five gadd genes. Cancer Res. 1994;54:5656–5662. [PubMed] [Google Scholar]
- 27.Johnson M, Dimitrov D, Vojta P J, Barret J C, Noda A, Pereira-Smith O M, Smith J R. Evidence for a p53-independent pathway for upregulation of SDI1/CIP1/Waf1/p21 RNA in human cells. Mol Carcinog. 1994;11:59–64. doi: 10.1002/mc.2940110202. [DOI] [PubMed] [Google Scholar]
- 28.Kessis T D, Slebos R J, Nelson W D, Kastan M B, Plunkett B S, Han S M, Lorincz A T, Hedrick L, Cho K R. Human papilloma virus 16 E6 expression disrupts the p53-mediated cellular response to DNA damage. Proc Natl Acad Sci USA. 1993;90:3988–3992. doi: 10.1073/pnas.90.9.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyriakis J M, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1997;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 30.Lee J C, Young P R. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukocyte Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Martindale J L, Gorospe M, Holbrook N J. Regulation of p21Waf1/CIP1 expression through mitogen-activated protein kinase signaling pathway. Cancer Res. 1996;56:1–5. [PubMed] [Google Scholar]
- 32.Lowe S W, Ruley H E, Jacks T, Housman D E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 33.Ma W J, Cheng S, Campbell C, Wright A, Furneaux H. Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J Biol Chem. 1996;271:8144–8151. doi: 10.1074/jbc.271.14.8144. [DOI] [PubMed] [Google Scholar]
- 34.Malter J S, Hong Y. A redox switch and phosphorylation are involved in the post-translational up-regulation of the adenosine-uridine binding factor by phorbol ester and ionophore. J Biol Chem. 1991;266:3167–3171. [PubMed] [Google Scholar]
- 35.Michieli P, Chedid M, Lin D, Pierce J H, Mercer W E, Givol D. Induction of Waf1/CIP1 by a p53-independent pathway. Cancer Res. 1994;54:3391–3395. [PubMed] [Google Scholar]
- 36.Murphy M, Hinman A, Levine A J. Wild-type p53 negatively regulates the expression of a microtubule-associated protein. Genes Dev. 1996;10:2971–2980. doi: 10.1101/gad.10.23.2971. [DOI] [PubMed] [Google Scholar]
- 37.Poluha W, Poluha D K, Chang B, Crosbie N E, Schonhoff C M, Kilpatrick D L, Ross A H. The cyclin-dependent kinase inhibitor p21Waf1 is required for survival of differentiating neuroblastoma cells. Mol Cell Biol. 1996;16:1335–1341. doi: 10.1128/mcb.16.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price M A, Cruzalegui F H, Treisman R. The p38 and ERK MAP kinase pathways cooperate to activate ternary complex factors and c-fos transcription in response to UV light. EMBO J. 1996;15:6552–6563. [PMC free article] [PubMed] [Google Scholar]
- 39.Rondon I J, MacMillan L A, Beckman B S, Goldberg M A, Schneider T, Bunn H F, Malter J S. Hypoxia up-regulates the activity of a novel erythropoietin mRNA binding protein. J Biol Chem. 1991;266:16594–16589. [PubMed] [Google Scholar]
- 40.Russo T, Zambrano N, Esposito F, Ammendola R, Cimino F, Fiscella M, Jackman J, O’Connor P M, Anderson C W, Appella E. A p53-independent pathway for activation of Waf1/CIP1 expression following oxidative stress. J Biol Chem. 1995;270:29386–29391. doi: 10.1074/jbc.270.49.29386. [DOI] [PubMed] [Google Scholar]
- 41.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 42.Sheikh M S, Chen Y Q, Smith M L, Fornace A J., Jr Role of p21Waf1/Cip1/Sdi1 in cell death and DNA repair as studied using a tetracycline-inducible system in p53-deficient cells. Oncogene. 1997;14:1875–1882. doi: 10.1038/sj.onc.1201004. [DOI] [PubMed] [Google Scholar]
- 43.Smith M L, Chen I-T, Zhan Q, O’Connor P M, Fornace A J., Jr Involvement of the p53 tumor suppressor in repair of U.V.-type DNA damage. Oncogene. 1995;9:1053–1059. [PubMed] [Google Scholar]
- 44.Thivierge M, Parent J L, Stankova J, Rola-Pleszczynski M. Modulation of human platelet-activating factor receptor gene expression by protein kinase C activation. J Immunol. 1996;157:4681–4687. [PubMed] [Google Scholar]
- 45.Tyrrell R M. UV activation of mammalian stress proteins. EXS. 1996;77:255–271. [PubMed] [Google Scholar]
- 46.Tyrrell R M. Activation of mammalian gene expression by the UV component of sunlight—from models to reality. Bioessays. 1996;18:139–148. doi: 10.1002/bies.950180210. [DOI] [PubMed] [Google Scholar]
- 47.Waldman T, Lengauer C, Kinzler K W, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 48.Woodgett J R, Avruch J, Kyriakis J. The stress-activated protein kinase pathway. Cancer Surv. 1996;27:127–138. [PubMed] [Google Scholar]
- 49.Zanke B W, Boudreau K, Rubie E, Winnett E, Tibbles L A, Zon L, Kyriakis J, Liu F F, Woodgett J R. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr Biol. 1996;6:606–613. doi: 10.1016/s0960-9822(02)00547-x. [DOI] [PubMed] [Google Scholar]