Abstract
Purpose
We report the results of a phase 1/2 trial of external beam partial breast radiation using proton therapy.
Methods and Materials
Eligible patients included stage 0-IIA breast cancer pTis-T2, N0, and size ≤3 cm. Proton beam radiation was used to deliver 3.85 Gy twice daily to 38.5 Gy. The phase 1 portion determined feasibility based on criteria of successful plan creation, treatment delivery, and acute toxicity grade ≥3 in ≤20% of patients. The phase 2 portion had efficacy goals of acute toxicity grade ≥3 in ≤20% of patients and observing physician-rated cosmesis of excellent or good >85% of patients at 2 years.
Results
From April 2013 to March 2015, there were 12 patients enrolled onto the phase 1 portion, and the preplanned analysis of feasibility was met in all 4 required criteria. From July 2015 through December 2019 there were 28 patients with 29 treated breasts (1 bilateral) enrolled onto the phase 2 portion of the trial out of 45 originally planned. The trial was closed to accrual because of the coronavirus pandemic and not reopened. Thirty-eight breasts were treated with double-scattering and 3 pencil-beam scanning protons. The median follow-up of the 40 patients is 5.4 years (range, 2.3-8.6 years). There was 1 local recurrence. There was no grade ≥3 acute or late toxicity. At baseline all patients had physician-rated cosmesis good or excellent but at 2 years was excellent in 56%, good in 19%, and fair in 25%.
Conclusions
Proton-accelerated partial breast irradiation delivered with a twice-daily fractionation was feasible and associated with very low acute and long-term toxicity. However, the trial did not meet goals for cosmesis outcomes and was closed prematurely. Future study is needed to determine whether pencil-beam scanning protons or different fractionation could improve these outcomes.
Introduction
In 2012, we developed a prospective 1/2 protocol of proton therapy for accelerated partial breast irradiation (APBI). Our rationale for this trial was based on several clinical and physics developments that were occurring simultaneously in the field of radiation therapy (RT) and proton therapy for early-stage breast cancer at that time.
First, there was promising rationale for studying APBI in early-stage breast cancer in the early 2010s. A consensus statement from the American Society for Radiation Oncology in 2009 had recommended that only a small group of patient characteristics were most suitable for treatment outside of a clinical trial.1 Accordingly, clinical studies of APBI published before 2012 were limited primarily to smaller studies, brachytherapy studies, or those with relatively short-term follow-up.2, 3, 4 But based on these early results, APBI was potentially associated with greater convenience for patients, increased utilization of breast conservation, increased utilization of postlumpectomy radiation, and reduced dose to normal structures such as the lungs and heart.
Second, there were limited data on external beam methods for doing APBI. Interstitial brachytherapy and balloon-based brachytherapy methods were the most common forms of APBI with the most data at that time. There were relatively limited and only short-term data on external beam methods of photon radiation.5, 6, 7 Further casting doubt on external beam methods, several studies were concerning for external beam photon APBI because they reported high rates of unacceptable cosmesis and negative normal tissue effects.8,9
Third, proton therapy was under active development at our institution starting in 2009 and seemed particularly well suited to APBI because of the superior physics properties of protons for targeting compared with photons.10,11 Because protons deposit dose at a finite range that depends on the energy of the beam, the exit dose seen with protons is reduced compared with photon therapy. A reduction in exit dose would be expected to significantly reduce the volume of normal breast tissue receiving the prescription dose and to significantly reduce exit dose to the underlying heart and/or lung. By 2012, there were also some favorable early reports from other institutions of good outcomes with proton APBI that were in stark contrast to the other photon external beam APBI reports at that time.12,13
Based on this constellation of developments and trends in research in 2012, we developed a prospective clinical trial that combined all 3 of these elements: (1) APBI as an alternative to whole breast irradiation; (2) external beam radiation as an alternative to brachytherapy methods of APBI; and (3) proton beam radiation as an alternative to existing photon beam radiation. This phase 1/2 trial was intended to study the feasibility and outcomes of proton beam radiation for APBI in early-stage breast cancer.
Methods and Materials
Study design and patient selection
The clinical trial is registered to ClinicalTrials.gov identifier NCT01839838 and was institutional review board–approved under the number UPCC 04113 at the Abramson Cancer Center of the University of Pennsylvania. Inclusion criteria were breast-conserving surgery for histologically confirmed diagnosis of invasive or noninvasive breast carcinoma American Joint Committee on Cancer, 7th edition, Tis, T1, or T2; N0 or N1mic, stage 0-IIA breast cancer. Patient age was ≥50 years with Eastern Cooperative Oncology Group status 0 to 2. Disease was limited to grossly unifocal and microscopic size 3 cm or less. Ductal carcinoma in situ (DCIS) could be clinical N0 and pNX. Receptors needed to be estrogen and/or progesterone positive. A margin at surgery of ≥2 mm was required. Focally close (<2 mm) or positive (tumor cells at the inked edge of the specimen) margins determined to be at an anatomic boundary of resection by the surgeon, such as posterior fascia for posterior margins or skin for anterior margins, were also acceptable without re-excision. In addition, patients presenting with abnormal microcalcifications on a screening mammogram must have had radiographically confirmed excision of the suspicious microcalcifications, either by specimen radiograph or postbiopsy mammograms. All patients underwent a history and physical, bilateral mammogram, chest x-ray or computed tomography (CT) of the chest, and routine complete blood count and comprehensive metabolic panel. Chemotherapy if indicated was permitted >2 weeks after completion of radiation, similar to NSABP B-39. Hormone therapy could be given before, during, or after radiation.
Procedures
CT simulation was done in the supine treatment position for radiation planning. The target lumpectomy cavity needed to be clearly delineated and the target lumpectomy cavity/whole breast reference volume needed to be ≤30% based on the postoperative CT scan. For each patient, NRG Oncology guidelines were used to contour a gross tumor volume (GTV), clinical target volume (CTV), planning target volume (PTV), and evaluation PTV (PTV_Eval). In brief, the GTV in breast cancer contouring guidelines is not gross tumor (because there has been a lumpectomy) but is the volumetric combination of the seroma (radiographic abnormality seen in the breast corresponding with fluid and/or scar tissue in the lumpectomy cavity) and surgical clips. The CTV was the GTV with a margin of 15 mm to account for microscopic extension of disease; however, the CTV was limited to 5 mm from the skin surface and limited posteriorly at the boundary of the breast tissue extent at the pectoral muscle (chest wall and pectoralis muscles were not to be included). The PTV was a 5-mm expansion of the CTV for set-up variability and respiratory motion, and the PTV_Eval excluded areas of dose build-up between air/tissue interfaces including cropping PTV 5 mm from skin and 5 mm from lung.
A total dose of 38.5 Gy (relative biological effectiveness) was prescribed to the PTV. Two fractions, each of 3.85 Gy (relative biological effectiveness) separated by at least 6 hours, were to be administered on 5 treatment days (over a period of 5-10 days) for a total of 10 fractions. For each plan, 2 anterior proton beam fields were used. Proton energy ranged from 100 to 230 MeV with additional range shifter to enable effective coverage at shallow depth. In the early years of the study, treatment was exclusively delivered by passive scattering/double scattering delivery technique; as our facility transitioned to pencil-beam scanning (PBS), several patients late in the study period were treated with the PBS technique. Dose-volume constraints for the treatment planning are shown in Table 1.
Table 1.
Radiation dose-volume treatment results
| DVH parameter | DVH goal | Results (mean) | Results (range) |
|---|---|---|---|
| Protocol-specified goals | |||
| PTV_Eval | V95% ≥ 95% of 38.5 Gy | 97% | 94%-100% |
| Whole breast | D38.5 Gy ≤ 35% | 12% | 1%-22% |
| Whole breast | D19.25 Gy ≤ 60% | 41% | 19%-59% |
| Contralateral breast | Dmax ≤ 1.15 Gy | <0.01 Gy | <0.01 Gy |
| Lung ipsilateral | V11.55 Gy ≤ 15% | 2% | <0.1%-9.3% |
| Lung contralateral | V1.93 Gy ≤ 15% | <0.01 Gy | <0.01 Gy |
| Heart (right-sided) | V1.93 Gy ≤ 5% | <0.01% | <0.01%-0.03% |
| Heart (left-sided) | V1.93 Gy ≤ 40% | <0.01% | <0.01%-0.15% |
| Thyroid | Dmax ≤ 1.15 Gy | <0.01 Gy | <0.01 Gy |
| Additional dose-volume results | |||
| Left-sided patients | |||
| Heart | Mean | 0.03 Gy | <0.01-0.19 Gy |
| Lung | V5 | 0.04% | <0.01%-0.14% |
| Lung | V20 | <0.01% | <0.01%-0.04% |
| Right-sided patients | |||
| Heart | Mean | 0.01 Gy | <0.01-0.15 Gy |
| Lung | V5 | 0.03% | <0.01%-0.12% |
| Lung | V20 | <0.01% | <0.01%-0.05% |
Abbreviations: DVH = dose-volume histogram; PTV_Eval = evaluation planning target volume.
Statistical analysis
For the feasibility phase 1 portion, 12 patients were enrolled. Feasibility was based on multiple radiation planning and treatment parameters: (1) a patient cannot be given treatment because anatomy is such that a dosimetrically satisfactory treatment plan cannot be devised; (2) a patient is unable to tolerate more than 20% of treatments using proton RT (ie, >2 of the 10 fractions); and/or (3) a patient is unable to complete all treatment within 5 days of the estimated date of treatment completion or requires a treatment break of greater than 5 days. A feasibility rate >90% was needed to proceed to the phase 2 portion. In addition to feasibility, no more than 20% of patients experiencing an acute grade 3 or higher toxicity was needed to proceed to the phase 2 portion.
For the phase 2 portion, a total of 57 patients were to be enrolled; 12 patients were included from the feasibility study and 45 additional patients. There were 2 primary objectives. The first was to test whether the rate of grade ≥3 acute toxicity was <20%. With 57 patients, there was 90% power for a χ2 test at a 1-sided 10% significance level to test the null hypothesis that the acute toxicity rate is ≥35% versus the alternative hypothesis that the acute toxicity rate is ≤20%. The second was to test whether the rate of maintained excellent-to-good cosmesis was >85% at 2 years post-RT (only patients with excellent or good cosmesis at baseline contribute to this analysis). With 50 patients, there was 90% power for a χ2 test at a 1-sided 10% significance level, to test the null hypothesis that the cosmesis rate is ≤70% versus the alternative hypothesis that the cosmesis rate is ≥85%.
Toxicity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Radiation acute toxicity was assessed weekly during treatment and 1 month after completion of radiation. Physician-rated cosmesis and patient-reported outcomes were to be obtained at baseline, 1 month after radiation, and every 6 months for 5 years. Physician assessment for the phase 2 endpoint used the Harvard scale that uses the visible sequelae of radiation of the treated breast and symmetry compared with the untreated breast to give an overall score of excellent, good, fair, or poor.14 The patient-reported cosmetic outcome for descriptive analysis (not used for the statistical study endpoints) was collected using the Breast Cancer Treatment Outcome Scale, a 22-item measure that creates 4 subscales of patient-perceived esthetic of the breast, breast-specific pain, breast edema, and arm functional status after breast-conserving surgery and radiation.15 The scoring is based on appearance of the treated breast compared with the opposite breast so that a 1 = no difference, 2 = slight difference, 3 = moderate difference, and 4 = large difference.
Results
From April 2013 to March 2015, there were 12 patients enrolled onto the phase 1 portion of the trial as designed. The protocol treatment was delivered as planned by protons in 11/12 patients, while 1/12 patients had 6 proton treatments and 4 consecutive photon treatments because of proton machine downtime. All patients finished within 5 days of the planned completion day. A preplanned analysis of feasibility of these 12 patients was conducted once there were complete acute toxicity data (within 30 days from end of RT). The treatment met feasibility in 11/12 (92%) patients, and there was no grade ≥3 toxicity. As the feasibility goal of >90% as stipulated in the study requirements was achieved, the study was reopened for phase 2 accrual. From July 2015 through December 2019 there were 28 evaluable patients with 29 treated breasts (1 bilateral) enrolled onto the phase 2 portion of the trial. Two additional patients were enrolled but withdrew before starting radiation, and 1 patient was enrolled in error and removed as ineligible before starting radiation because they refused the bloodwork required to meet eligibility requirements. The study was terminated early during a research pause for the coronavirus (COVID-19) pandemic and was not reopened.
Patient and tumor characteristics are shown in Table 2. The first 38 breasts were treated with a double scattering (DS) delivery system from June 24, 2013 to August 28, 2019, and the last 3 breasts were treated with a PBS delivery system from September 12, 2019 to December 31, 2019, after an upgrade to our proton center (Fig. 1). The median days elapsed for radiation was 7 (range, 5-17). The protocol-specified dose-volume histogram goals and planning results for all patients are shown in Table 1. Only 1 patient did not meet all predefined planning goals because of a PTV_Eval V95% of 94% instead of ≥95%. Additional unplanned exploratory analysis of mean heart dose and V5 and V20 lung dose are also shown in Table 1.
Table 2.
Patient and tumor characteristics
| Total breasts treated (patients) | 41 (40) |
|---|---|
| Age (years) | |
| Median | 60 |
| Range | 53-78 |
| Side | |
| Left | 26 (63%) |
| Right | 15 (37%) |
| Histology | |
| IDC and DCIS/extensive DCIS | 27 (66%)/7 (17%) |
| IDC | 6 (15%) |
| DCIS | 6 (15%) |
| ILC | 1 (2%) |
| ILC and DCIS | 1 (2%) |
| Grade | |
| 1 | 16 (39%) |
| 2 | 20 (49%) |
| 3 | 5 (12%) |
| LVI | |
| Yes | 1 (2%) |
| No | 40 (98%) |
| T size (mm) | |
| Median | 5 |
| Range | 1-25 |
| Quadrant | |
| Upper outer | 25 (61%) |
| Upper inner | 6 (15%) |
| Lower outer | 6 (15%) |
| Lower inner | 3 (7%) |
| Central | 1 (2%) |
| Margins (mm) | |
| >2 | 37 (90%) |
| 1-2 | 3 (7%) |
| > 0, < 1 | 0 (0%) |
| 0 | 1 (2%) |
| Receptors | |
| Invasive ER+/HER2- | 32 (78%) |
| Invasive ER+/HER2+ | 2 (5%) |
| Invasive ER+/HER2 unknown | 1 (2%) |
| DCIS ER+ | 5 (12%) |
| DCIS unknown | 1 (2%) |
| Oncotype (n = 22) | |
| Median | 11 |
| Range | 0-22 |
| Chemotherapy | |
| None | 40 (98%) |
| Docetaxel/cyclophosphamide | 1 (2%) |
| Hormone therapy | |
| AI | 24 (59%) |
| Tamoxifen | 3 (7%) |
| Tamoxifen/AI combination | 1 (2%) |
| None | 13 (32%) |
Abbreviation: AI = aromatase inhibitor; DCIS = ductal carcinoma in situ; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; LVI = lymphovascular space invasion.
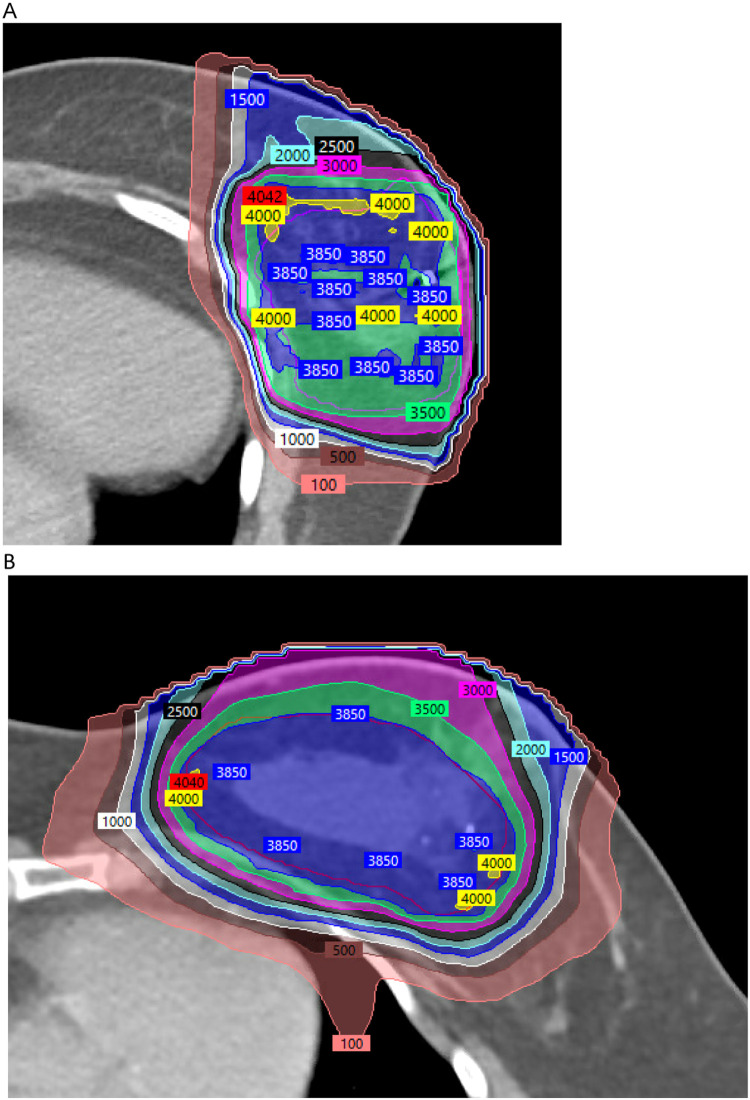
Figure 1.
Isodose plans prescribed to 3850 cGy for 2 left-sided patients. (A) Double scattering isodose plan for patient #13 (first enrolled on the phase 2 trial; mean heart dose 1.2 cGy). (B) Pencil-beam scanning isodose plan for patient #41 (last enrolled on the phase 2 trial; mean heart dose 19.1 cGy). Note the greater ability to optimize (reduce) surface and subcutaneous dose with the pencil-beam scanning delivery technique.
For the 41 breasts treated in the phase 1 and 2 combined study, maximum acute toxicity was grade 0 in 3 cases, grade 1 in 35 cases, and grade 2 in 3 cases. Grade 1 toxicities were dermatitis in 33 cases and pain in 8 cases. Grade 2 toxicities were dermatitis in 3 cases and pain in 1 case. There was grade 1 acute toxicity in 3/3 PBS patients and no late toxicities with 2.3 years’ median follow-up. There was no grade ≥3 acute toxicity, so that the first of the phase 2 endpoints was satisfied (ie, a rate of grade ≥3 acute toxicity <20%).
Median follow-up was 5.4 years (range, 2.3-8.6 years). At last follow-up, 37 patients were alive without disease, 1 was dead of breast cancer, and 2 were dead of other causes. There was 1 local recurrence (of 41 patients, 2.4%) 2.6 years after treatment of a 53-year-old patient with left-sided stage IA IDC, T1a N0, ER+/PR+/HER2- with nonextensive DCIS, grade 1, and margins >2 mm on tamoxifen. There was 1 distant metastasis and subsequent death from breast cancer 4.6 years after treatment in a 62-year-old woman with left-sided stage IA IDC, T1a N0, grade 2, ER +/PR+/HER2-, and oncotype DX recurrence score 22 treated with docetaxel/cyclophosphamide and an aromatase inhibitor.
The maximum late toxicity was grade 0 in 16 cases, grade 1 in 18 cases, grade 2 in 6 cases, and grade 3 in 2 cases. There were no grade ≥4 late toxicities. Grade 1 toxicities that were deemed as likely related to radiation were telangiectasias in 10 cases, fibrosis in 10 cases, hyperpigmentation in 3 cases, pain in 2 cases, and fat necrosis in 2 cases. There were 2 cardiac grade 1 events. A grade 1 case of palpitations was considered as unrelated to radiation, but a grade 1 paroxysmal supraventricular tachycardia 1.3 years after treatment of a patient with left-sided breast cancer was scored as possibly related. Grade 2 toxicities designated as likely related to radiation were telangiectasias in 4 cases and fibrosis in 4 cases. There was 1 case of grade 2 sinus tachycardia after treatment considered not related to radiation. The 2 grade 3 events were both considered likely unrelated to radiation. One grade 3 event was a new diagnosis of coronary artery disease at 6 months and atrial fibrillation at 2 years after radiation in a patient with right-sided treatment. There was 1 case of a stage III left breast cancer 1.5 years after treatment of a patient with right-sided DCIS.
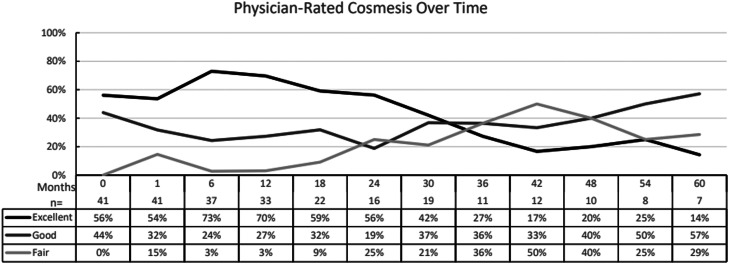
The average physician-reported cosmesis at baseline and subsequent follow-up is shown in Fig. 2. The cosmesis at baseline before radiation was excellent in 56% (23 breasts) and good in 44% (18 breasts). At 2 years, the cosmesis was excellent in 56%, good in 19%, and fair in 25%. This did not satisfy the other phase 2 endpoint goal of whether the rate of excellent or good cosmesis is >85% at 2 years. At the last available patient-reported follow-up for all 41 breasts at various timepoints, the cosmesis was excellent in 56% (23 breasts), good in 29% (12 breasts), and fair in 15% (6 breasts). There were no ratings of poor at any timepoint.
Figure 2.
Physician-rated cosmesis over time. The average scoring for available patient data at each timepoint from best cosmesis to worst cosmesis = excellent, good, fair, poor from baseline after surgery and preradiation to up to 5 years.
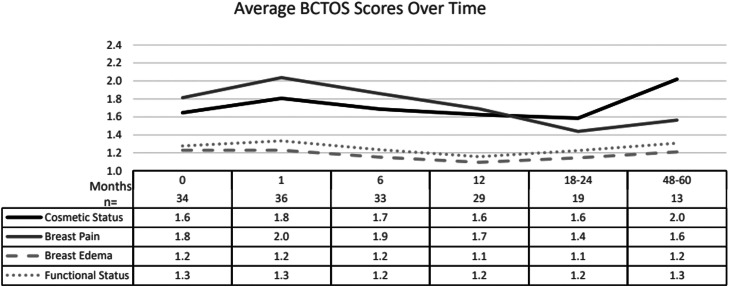
The average patient-reported outcomes from the Breast Cancer Treatment Outcome Scale at baseline and subsequent follow-up are shown in Fig. 3. The average score for overall cosmetic status was 1.6 at baseline, corresponding to a rating of no-to-slight difference between the treated breast and the opposite side. This increased to 1.8 at 1 month after treatment and returned to baseline 1.6 by 1 year. However, for patients with longer follow-up data at 4 to 5 years, this increased to an average of 2.0, corresponding to a rating of a slight difference compared with the opposite side. At baseline, the scores for breast pain were 1.8. This increased to 2.0 at 1 month after treatment and returned to baseline or lower by 1 year. For patients with the longest follow-up at 4 to 5 years, the average score for breast pain was lower than baseline at 1.6. There was minimal-to-no change in the scores over time for breast edema or functional status from baseline to 4 to 5 years.
Figure 3.
Patient-reported cosmetic and quality of life outcomes over time using the Breast Cancer Treatment Outcome Scale. The average scoring for available patient data at each time point from best rating to worst rating = 1 (no difference), 2 (slight difference), 3 (moderate difference), and 4 (large difference) from baseline after surgery and preradiation to up to 5 years.
Discussion
This prospective experience of proton beam APBI met the predetermined phase 1 goals for feasibility that were based on several factors centered around the ability to create a deliverable proton plan, ability to deliver the treatments in a timely fashion, and patients’ tolerability of treatment and acute toxicity (Table 3). For the subsequent phase 2 portion of the study, only 1 of 2 goals was met. The proton treatment well met the criteria for acute toxicity grade ≤3 (0%), but at study closure—which was terminated prematurely because of the COVID-19 pandemic—the trial was not meeting the goal for >85% physician-rated cosmesis ratings of good or excellent at 2 years (75%).
Table 3.
Study endpoints and results
| Phase 1 | Feasibility of treatment delivery >90% | Met |
|---|---|---|
| Grade ≥3 acute toxicity <20% | Met | |
| Phase 2 | Grade ≥3 acute toxicity <20% | Met |
| Good or excellent cosmesis >85% at 2 years | Not met |
In looking at our data on toxicity, there is some evidence for a learning curve with the adoption of this proton APBI technique, as is often the case for any new technology. The 5 grade 2 late toxicities occurred in the first 16 patients with DS protons, but there was no grade ≥2 toxicity in the remaining 24 patients. Further supporting this possible learning curve to the technique was that the 6 breasts with a fair cosmesis occurred in patients treated in the earlier years of the trial; all 6 were in the first 21 patients and 4 were in the first 11 patients. There were no patients with fair cosmesis in the last 20 patients. However, this could also be because of different lengths of follow-up. At trial inception, we had limitations in the available gantry angles that the protons could be delivered, which may have resulted in excessive DS skin doses. In retrospect, the excellent skin cosmesis in 1 earlier trial of proton APBI may have been due in large part to a unique prone position, which was not possible with our gantry and table, so that there was no portion of breast skin in both of the fields in their prone proton technique.12 We altered our technique during the course of the trial to use 2 fields angled 10° to 20° with at least 1 of them optimized to reduce surface dose and telangiectasia risk. Our rate of telangiectasias is comparable to other reports of proton ABI using multiple field DS protons.16 We can only speculate that if the trial were redone today when all patients could be treated with PBS delivery technique, the cosmetic results would have been even better (Fig. 1).
The trial was discontinued before the intended number of patients in the phase 2 portion for many reasons and is a cautionary tale for the successful conduct of any clinical trial in the importance of getting it done in a timely fashion. We had overoptimistically estimated an annual accrual of 10 to 12 patients a year, so that the trial would be completed from April 2013 to April 2018. However, by December 2019 when the last patient was enrolled, the trial was still 16 patients short of the original goal of 57 patients. There were many attempts to address the slow enrollment onto the trial. When the trial first opened, the eligibility was relatively strict and limited to most favorable patients from an early American Society for Radiation Oncology consensus statement on APBI.1 Over the course of the trial enrollment period, however, eligibility was expanded to include characteristics originally at the start considered disqualifying for APBI so that patients with pure DCIS, invasive lobular, and who were aged 40 to 50 were allowed to be enrolled. However, the major factor that ultimately limited enrollment was insurance coverage. Very few plans, such as Medicare or selected commercial plans with special arrangements with our institution, covered protons at all or outside of a national phase 3 trial context, and unfortunately, the trial was not funded to treat patients without insurance or private payment.
There were 2 principal events that led to our decision to discontinue the phase 2 portion of the trial prematurely before accrual goal. The first was the publication of several phase 3 randomized trials comparing whole breast radiation therapy to APBI using photon radiation. When we started the trial in 2013, a reasonable case could be made that external beam APBI using any method had relatively little prospective trial data and long-term follow-up. However, by 2019 to 2020 there were very large phase 3 trials published with 10-year follow-up showing that APBI had either equivalent17,18 or comparable (ie, point estimates within 1%)19 local control efficacy compared with whole breast radiation therapy at 10 years. And although our trial was not meeting 1 of the phase 3 goals of good/excellent cosmesis at 2 years >85%, the Florence trial in particular had 98% good/excellent cosmesis. Given their excellent outcomes, the convenience of their 5-treatment regimen compared with our 10-treatment fraction regimen, and the relative ease of insurance approval for the intensity modulated RT (IMRT) method used in that trial compared with protons, the Florence regimen became our APBI technique of choice in 2020. Given the concerns we had about proton skin dosing, discussed further in the following sections, we would not recommend using with protons the Florence 6 Gy fraction size that was so successful with IMRT. It is not known but would require further study whether PBS protons would be able to be delivered with low enough skin dose and inhomogeneity to use 6 Gy per fraction. So, in summary, we did not feel we should proceed with our proton phase 2 trial given the demonstrated efficacy and cosmesis of the Florence regimen.
The second reason for early termination was the COVID pandemic starting in early 2020. The pandemic shut down clinical trial operations for >1 year, effectively ending any additional enrollment. In addition, most scheduled return patient appointments to collect data at 6-month intervals that were stipulated for already-enrolled patients effectively ended. Return visits were routinely cancelled during the pandemic by patients or were conducted only by telemedicine. This ended our ability to collect required data for physician-rated cosmesis and questionnaires. For all of these reasons, reopening the trial by 2022 was felt to be very unlikely to change any of the ultimate conclusions we were reaching from the data already collected to date.
Given a number of potential options for APBI, what case can be made for proton APBI for a patient with early-stage breast cancer? Our experience with proton APBI was demonstrated to be feasible in plan creation and plan delivery with very low acute and long-term toxicity. The efficacy appears to be comparable to other forms of APBI, with only a single local recurrence at median follow-up over 5 years. Proton APBI has an advantage of noninvasiveness, and we did not observe problems with increased risk for infection, wound complications, or seroma that can occur with brachytherapy APBI options. The greatest advantage of protons is the very minimal exposure to heart and lung (Fig. 1 and Table 1) so that it may be the preferred choice for patients with prior chest radiation as well as those with cardiac or pulmonary disease. However, we did observe a rate of good/excellent cosmesis that did not meet our phase 2 goals of >85%. A rate of 25% or higher fair cosmesis may be inferior to that observed with IMRT but may be comparable to other reports of 3-dimensional APBI using the same twice daily fractionation in the NSABP B-39 and RAPID trials. Our greatest hurdle to enrollment was the higher cost and lack of insurance coverage for protons compared with APBI methods using IMRT or 3-dimensional photons. The higher cost of protons and limited insurance approval may be less a factor in the future if a single payment cost structure for any modality used becomes adopted nationally in the next 5 years.
Conclusion
This prospective clinical trial demonstrates that proton APBI is feasible and safe with excellent local control in early-stage breast cancer. However, the phase 2 portion was unable to be completed and did not meet goals for the good or excellent cosmesis outcomes. It is possible that with future research, use of PBS protons and greater limitation of the superficial dose could be associated with improved cosmetic outcomes. It is also possible that outcomes with protons could be improved by using a daily or every other day fractionation and lower total dose could improve proton cosmetic outcomes. Currently, the role of proton APBI remains investigational outside of a trial and is likely limited outside of a trial to very select patients where standard methods of APBI using 3-dimensional, IMRT, or brachytherapy are not possible or acceptable options.
Disclosures
Taoran Li reports a grant and honorarium from Varian Medical Systems, and a consulting fee from Boston Scientific. Neil K. Taunk reports a grant from Varian Medical Systems, as well as a consulting fee and honorarium from Boston Scientific.
Acknowledgments
The authors thank Rosemarie Mick, MS (retired), Department of Biostatistics, Perelman School of Medicine of the University of Pennsylvania, Philadelphia, Pennsylvania, for the statistical design of the trial.
Footnotes
Sources of support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Oluwadamilola M. Fayanju is supported by the National Institutes of Health (NIH) under award number 7K08CA241390-03 (PI: Fayanju). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The data used to conduct this analysis can be provided upon request to the corresponding author.
References
- 1.Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys. 2009;74:987–1001. doi: 10.1016/j.ijrobp.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 2.Vicini F, Beitsch P, Quiet C, et al. Five-year analysis of treatment efficacy and cosmesis by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in patients treated with accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;79:808–817. doi: 10.1016/j.ijrobp.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 3.Polgar C, Major T, Fodor J, et al. Accelerated partial-breast irradiation using high-dose-rate interstitial brachytherapy: 12-year update of a prospective clinical study. Radiother Oncol. 2010;94:274–279. doi: 10.1016/j.radonc.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Arthur DW, Winter K, Kuske RR, et al. A phase II trial of brachytherapy alone after lumpectomy for select breast cancer: Tumor control and survival outcomes of RTOG 95-17. Int J Radiat Oncol Biol Phys. 2008;72:467–473. doi: 10.1016/j.ijrobp.2007.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vicini F, Winter K, Straube W, et al. A phase I/II trial to evaluate three-dimensional conformal radiation therapy confined to the region of the lumpectomy cavity for stage I/II breast carcinoma: Initial report of feasibility and reproducibility of Radiation Therapy Oncology Group (RTOG) Study 0319. Int J Radiat Oncol Biol Phys. 2005;63:1531–1537. doi: 10.1016/j.ijrobp.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Vicini F, Winter K, Wong J, et al. Initial efficacy results of RTOG 0319: Three-dimensional conformal radiation therapy (3D-CRT) confined to the region of the lumpectomy cavity for stage I/II breast carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:1120–1127. doi: 10.1016/j.ijrobp.2009.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berrang TS, Olivotto I, Kim D-H, et al. Three-year outcomes of a Canadian multicenter study of accelerated partial breast irradiation using conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:1220–1227. doi: 10.1016/j.ijrobp.2010.07.2003. [DOI] [PubMed] [Google Scholar]
- 8.Jagsi R, Ben-David MA, Moran JM, et al. Unacceptable cosmesis in a protocol investigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys. 2010;76:71–78. doi: 10.1016/j.ijrobp.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hepel JT, Tokita M, MacAusland SG, et al. Toxicity of three-dimensional conformal radiotherapy for accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2009;75:1290–1296. doi: 10.1016/j.ijrobp.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Amos RA, Zhang X, et al. External-beam accelerated partial breast irradiation using multiple proton beam configurations. Int J Radiat Oncol Biol Phys. 2011;80:1464–1472. doi: 10.1016/j.ijrobp.2010.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon SH, Shin KH, Kim TH, et al. Dosimetric comparison of four different external beam partial breast irradiation techniques: Three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, helical tomotherapy, and proton beam therapy. Radiother Oncol. 2009;90:66–73. doi: 10.1016/j.radonc.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Bush DA, Slater JD, Garberoglio C, et al. Partial breast irradiation delivered with proton beam: Results of a phase II trial. Clin Breast Cancer. 2011;11:241–245. doi: 10.1016/j.clbc.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Kozak KR, Katz A, Adams J, et al. Dosimetric comparison of proton and photon three-dimensional, conformal, external beam accelerated partial breast irradiation techniques. Int J Radiat Oncol Biol Phys. 2006;65:1572–1578. doi: 10.1016/j.ijrobp.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Beadle GF, Silver B, Botnick L, Hellman S, Harris JR. Cosmetic results following primary radiation therapy for early breast cancer. Cancer. 1984;54:2911–2918. doi: 10.1002/1097-0142(19841215)54:12<2911::aid-cncr2820541216>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Stanton AL, Krishnan K, Collins CA. Form or function? Part 1. Subjective cosmetic and functional correlates of quality of life in women treated with breast-conserving surgical procedures and radiotherapy. Cancer. 2001;91:2273–2281. [PubMed] [Google Scholar]
- 16.Pasalic D, Strom EA, Allen PK, et al. Proton accelerated partial breast irradiation: Clinical outcomes at a planned interim analysis of a prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2021;109:441–448. doi: 10.1016/j.ijrobp.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Whelan TJ, Julian JA, Berrang TS, et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): A randomised controlled trial. Lancet. 2019;394:2165–2172. doi: 10.1016/S0140-6736(19)32515-2. [DOI] [PubMed] [Google Scholar]
- 18.Meattini I, Marrazzo L, Saieva C, et al. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: Long-term results of the randomized phase III APBI-IMRT-Florence trial. J Clin Oncol. 2020;38:4175–4183. doi: 10.1200/JCO.20.00650. [DOI] [PubMed] [Google Scholar]
- 19.Vicini FA, Cecchini RS, White JR, et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: A randomised, phase 3, equivalence trial. Lancet. 2019;394:2155–2164. doi: 10.1016/S0140-6736(19)32514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]