Abstract
Description:
This guideline updates the 2017 American College of Physicians (ACP) recommendations on pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults.
Methods:
The ACP Clinical Guidelines Committee based these recommendations on an updated systematic review of evidence and graded them using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) system.
Audience and Patient Population:
The audience for this guideline includes all clinicians. The patient population includes adults with primary osteoporosis or low bone mass.
Recommendation 1a:
ACP recommends that clinicians use bisphosphonates for initial pharmacologic treatment to reduce the risk of fractures in postmenopausal females diagnosed with primary osteoporosis (strong recommendation; high-certainty evidence).
Recommendation 1b:
ACP suggests that clinicians use bisphosphonates for initial pharmacologic treatment to reduce the risk of fractures in males diagnosed with primary osteoporosis (conditional recommendation; low-certainty evidence).
Recommendation 2a:
ACP suggests that clinicians use the RANK ligand inhibitor (denosumab) as a second-line pharmacologic treatment to reduce the risk of fractures in postmenopausal females diagnosed with primary osteoporosis who have contraindications to or experience adverse effects of bisphosphonates (conditional recommendation; moderate-certainty evidence).
Recommendation 2b:
ACP suggests that clinicians use the RANK ligand inhibitor (denosumab) as a second-line pharmacologic treatment to reduce the risk of fractures in males diagnosed with primary osteoporosis who have contraindications to or experience adverse effects of bisphosphonates (conditional recommendation; low-certainty evidence).
Recommendation 3:
ACP suggests that clinicians use the sclerostin inhibitor (romosozumab, moderate-certainty evidence) or recombinant PTH (teriparatide, low-certainty evidence), followed by a bisphosphonate, to reduce the risk of fractures only in females with primary osteoporosis with very high risk of fracture (conditional recommendation).
Recommendation 4:
ACP suggests that clinicians take an individualized approach regarding whether to start pharmacologic treatment with a bisphosphonate in females over the age of 65 with low bone mass (osteopenia) to reduce the risk of fractures (conditional recommendation; low-certainty evidence).
Primary osteoporosis (osteoporosis that is not secondary to a separate condition or medication) is characterized by decreasing bone mass and density and reduced bone strength leading to a higher risk for fracture (Appendix Table 1, available at Annals.org) (1, 2). Fractures can occur in any bone, but hip and spine fractures are most common, accounting for 42% of all osteoporotic fractures. Fractures are associated with serious morbidity and mortality, and people with prevalent fractures are at much higher risk for future fractures (3–5). Overall, an estimated 10.2 million persons aged 50 years or older in the United States have osteoporosis, and about 43.3 million persons (>40% of older U.S. adults) have low bone mass associated with a high risk for progression to osteoporosis (6).
The clinical and economic burden of osteoporotic fractures is increasing over time in certain racial and ethnic groups compared with White Americans, although differences in treatment effects for these populations remain unclear (7). Over the past decade, the prevalence of osteoporosis in the United States increased in females but not males (6, 8). However, males with osteoporotic hip fractures have greater morbidity and mortality than females with hip fractures and receive treatments aimed at fracture prevention less often than females (9–12). There is substantial burden for working patients due to absenteeism and loss of productivity (13–15).
The American College of Physicians (ACP) has previously published clinical recommendations on screening and pharmacologic interventions for osteoporosis (16, 17), with the most recent guideline, published in 2017, aimed at treatment of osteoporosis (18).
Scope And Purpose
The purpose of this ACP guideline is to present a focused update on clinical recommendations for pharmacologic treatments (Table) of osteoporosis and low bone mass to prevent fractures in adults, based on the best available evidence of the benefits and harms of treatments and consideration of patient values, preferences, and costs (Figures 1 to 3). Since publication of the 2017 ACP guideline (18), evidence has emerged on the efficacy of human parathyroid hormone–related peptides (24, 25), sclerostin inhibitors (26, 27), the comparative effectiveness of treatments (28–30), and treatments in males. This update also adds key questions on values and preferences and costs of interventions and incorporates network meta-analysis. The update of the evidence regarding use of estrogen, treatment duration, drug discontinuation (31), and serial bone mineral density monitoring (32) was not addressed in this update but will be reevaluated by the Clinical Guidelines Committee (CGC) during the living review process.
Table.
Medications Licensed in the United States for Treatment of Osteoporosis
| Drug Name (Class) | Route; Frequency | Types of Fractures Examined in Randomized Clinical Trials at Long-Term Follow-up (>36 mo) | Average Annual Medicare Spending Per Beneficiary in 2019 | FDAWarning | |||
|---|---|---|---|---|---|---|---|
| Hip | Clinical Vertebral | Any Clinical | Radiographic Vertebral | ||||
| Antiresorptive drugs | |||||||
| Alendronate (bisphosphonate)*†‡ | By mouth (tablet or solution); once a day (10 mg) or once a week (70 mg)§ | Yes | No | Yes | Yes | $793-$1306 (brand-name); $39 (generic) | Upper gastrointestinal irritation; osteonecrosis of the jaw; atypical femur fractures; severe bone, joint, and muscle pain |
| Risedronate (bisphosphonate)*†‡ | By mouth; once a day, once a week, or 2 dir a row once per month§ | Yes | No | No | Yes | $2036-$2732 (brand-name); $604 (generic) | Upper gastrointestinal irritation; osteonecrosis of the jaw; atypical femur fractures; severe bone, joint, and muscle pain |
| Ibandronate (bisphosphonate)*‡ | By mouth; once a month§ | No | No | No | Yes | $1379 (brand-name); $220 (generic) | Upper gastrointestinal irritation; osteonecrosis of the jaw; atypical femur fractures; severe bone, joint, and muscle pain |
| Zoledronate (bisphosphonate)*†‡ | Intravenous; once s year§ | Yes | Yes | Yes | Yes | $855 (brand-name); $316-$987 (generic) | Osteonecrosisofthe jaw; atypical femur fractures; severe bone, joint, and muscle pain |
| Denosumab (RANK ligand inhibitor)†∥ | By injection (subcutane-ous); every 6 mo¶ | Yes | Yes | Yes | Yes | $1913-$12 241 (brand-name) | Dermatologic reactions and serious infection, including skin infections; suppression of bone turnover contributing to adverse outcomes, such as osteonecrosis ofthe jaw, atypical fractures, and delayed fracture healing |
| Anabolic drugs | |||||||
| Abaloparatide (parathyroid hormone-related protein)∥ | By injection (subcutaneous); once a day | No | No | Yes** | Yes** | $9873 (brand-name) | Hereditary osteosarcoma disorders†† |
| Teriparatide (recombinant human parathyroid hormone)∥‡‡ | By injection (subcutaneous); once a day | Yes** | Yes** | Yes** | Yes** | $22 156 (brand-name) | Hereditary osteosarcoma disorders†† |
| Romosozumab (sclerostin inhibitor)∥ | By injection (subcutaneous); once a month for 12 mo§§ | No | Yes** | Yes** | Yes** | $5574 (brand-name) | Cardiovascular risk Stroke history or risk∥∥ |
| Estrogen agonist on bones | |||||||
| Raloxifene (selective estrogen receptor modulator)*‡ | By mouth; once a day | Yes | Yes | Yes | Yes | $1730 (brand-name); $593 (generic) | Stroke history or risk Thromboembolism history or risk¶¶ |
FDA = U.S. Food and Drug Administration; RANK = receptor activator of nuclear factor κB.
Indicated for treatment of osteoporosis in postmenopausal females.
Indicated for males. Bisphosphonates have been approved for males with primary osteoporosis based on improvement in bone mineral density, and denosumab is approved for males with secondary osteoporosis based on a reduction in risk for vertebral fractures (19).
Indicated for the prevention of osteoporosis in postmenopausal females with low bone mass.
All patients receiving bisphosphonate therapy should have the need for continued therapy reevaluated periodically. Patients at low risk for fracture should be considered for drug discontinuation after 3 to 5 years of use. Patients who discontinue therapy should have their risk for fracture reevaluated periodically.
Indicated for postmenopausal females with osteoporosis who are at high risk for fracture, defined as a history of osteoporotic fracture or multiple risk factors for fracture or patients who have failed or are intolerant to other available osteoporosis therapy.
Denosumab discontinuation is associated with multiple vertebral fractures in some patients (20).
Short-term follow-up (12 to 36 months).
Dose-dependent increase in incidence of osteosarcoma in preclinical studies.
Indicated for males; increase in bone mass in males with primary or hypogonadal osteoporosis who are at high risk for fracture.
Use of romosozumab should be limited to 12 monthly doses because the anabolic effect wanes after 12 monthly doses (21).
The analysis of the FDA Adverse Event Reporting System suggested higher risk for major adverse cardiovascular events associated with romosozumab (22). The current FDA safety warnings recommend avoiding use of romosozumab in patients with high risk for major cardiovascular events (21).
Higher risk for venous thromboembolism and fatal stroke in females who have documented coronary heart disease or are at increased risk for major coronary events (23).
Figure 1.

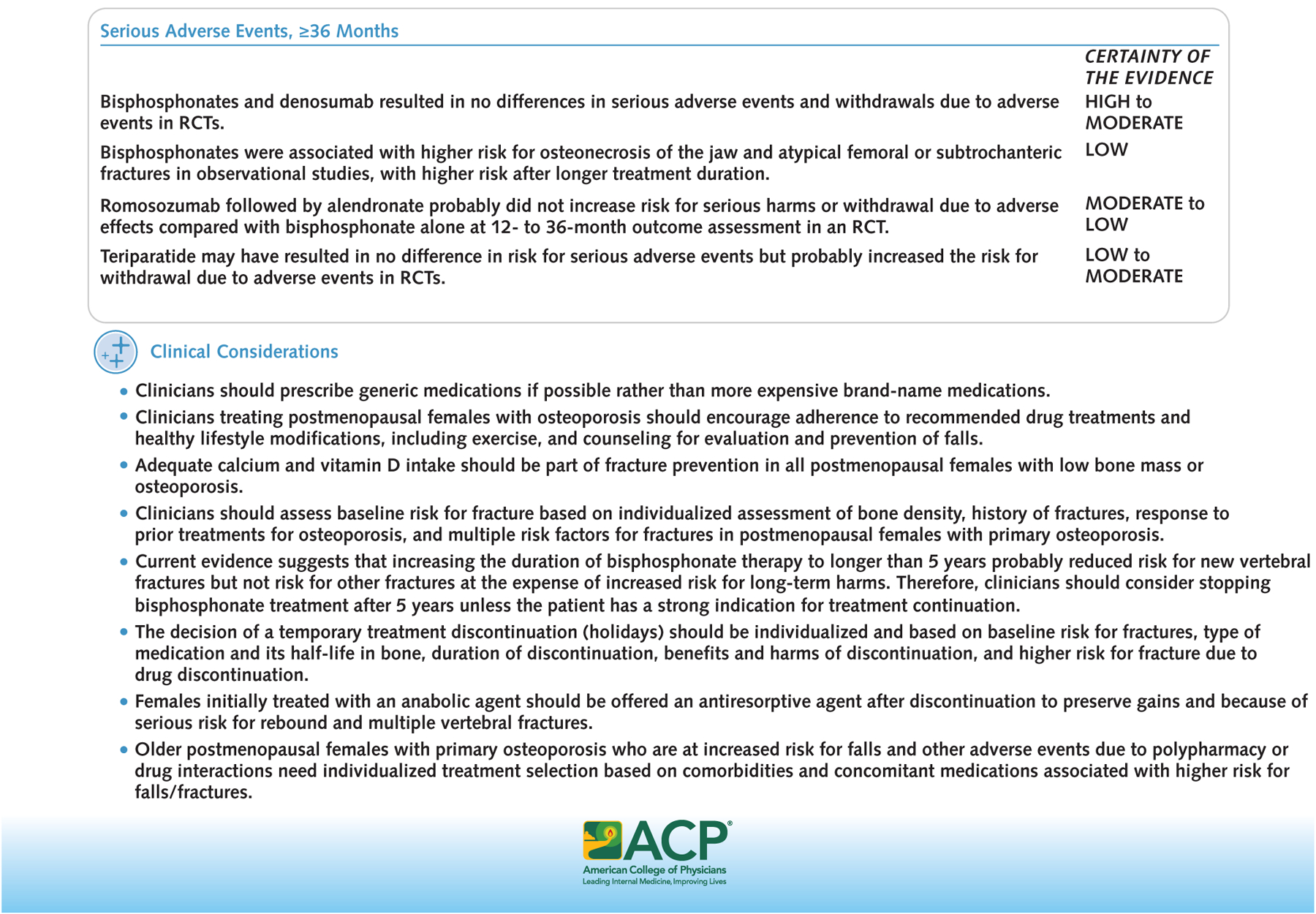
Treatments to reduce fractures in postmenopausal females with primary osteoporosis.
ACP = American College of Physicians; PTH = parathyroid hormone; RANK = receptor activator of nuclear factor κB; RCT = randomized controlled trial.
Figure 3.

Treatments to reduce fractures in postmenopausal females with low bone mass.
ACP = American College of Physicians.
We evaluated the following pharmacologic interventions: an analogue of human parathyroid hormone–related protein (PTHrP) (abaloparatide), bisphosphonates (alendronate, ibandronate, risedronate, zoledronate), a receptor activator of nuclear factor κB (RANK) ligand inhibitor (denosumab), recombinant human parathyroid hormone (recombinant PTH) (teriparatide), a sclerostin inhibitor (romosozumab), and selective estrogen receptor modulators (SERMs) (bazedoxifene, raloxifene). Appendix Table 2 (available at Annals.org) summarizes definitions of fracture outcomes, and the Table provides an overview of medications licensed in the United States for treatment of osteoporosis. We focused on effectiveness and harms of active drugs compared with placebo or bisphosphonates.
Population
The population is adults (premenopausal and postmenopausal females and males) with low bone mass (33) or primary osteoporosis as diagnosed in primary studies (34). In assessing baseline risk for fracture, we consider diagnosis of osteoporosis, history of osteoporotic fractures (clinical or incidental), multiple risk factors for fractures, or failure or intolerability of osteoporosis medications rather than scores from available tools (35–39). The recommendations are based on biological sex assigned at birth because most studies reported sex rather than gender and the majority enrolled only older females.
Intended Audience
The intended audience is all clinicians. The management of secondary osteoporosis in people with cancer (40–43) and other serious illnesses is outside the scope of this guideline.
Guideline Development Process
The CGC developed this guideline according to ACP’s guideline development process (44) and its policy on disclosure of interests and management of conflicts of interest (45). The CGC used Evidence-to-Decision tables when reporting the evidence (Supplement Appendixes 1 to 3, available at Annals.org) and graded the recommendations using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach (Appendix Figure, available at Annals.org) (46). Supplement Appendix 4 (available at Annals.org) presents baseline patient characteristics, and Supplement Appendix 5 (available at Annals.org) lists the key questions for the supporting systematic review and details about the methods for the guideline and systematic review. ACP completes a Guidelines International Network (GIN) standards reporting form for each guideline it publishes, which can be found in GIN’s International Guideline Library or on ACP’s website (www.acponline.org/clinical-information/guidelines/guideline-process).
Because there are many ongoing studies (Table 5c of Supplement Appendix 5), the CGC is planning to maintain this topic as a living guideline with quarterly literature surveillance and periodic updating of the systematic review and the clinical recommendations. The CGC will consider quantitative and qualitative factors, such as the certainty of the evidence, the balance between benefits and harms, and contextual considerations to assess whether the new evidence may lead to changes to the recommendations and the need for an update. The CGC may decide to retire the topic from living status if it is no longer considered a priority for decision making, when there is confidence that conclusions are not likely to change with new evidence, or if it becomes unlikely that new evidence will emerge (47).
Systematic Review and Summary of Findings
This guideline is based on an accompanying systematic review and network meta-analysis completed by the ACP Center for Evidence Reviews at the Portland Veterans Affairs Research Foundation and funded by ACP. The accompanying systematic review and the Supplement Appendixes provide the appraised evidence of benefits and harms of evaluated pharmacologic interventions (34).
Outcomes of Interest
Benefits and Harms
Critical outcomes that were evaluated included patient-oriented clinical outcomes of fractures (Appendix Table 2), patient functional status, quality of life, and serious adverse events, and important outcomes included withdrawals due to adverse events. When evaluating the net benefits of the various treatments, we looked at fracture rates at longer time (≥36 months) and shorter time (12 to <36 months) to outcome assessment (48). The CGC prioritized benefits and harms that lasted at least 36 months over those only assessed at 12 to less than 36 months (34).
Each study contributed to outcomes at 1 time point of fracture assessment (12 to <36 months or ≥36 months). In addition, we prioritized prevention of hip fractures and clinical vertebral fractures followed by prevention of any clinical or radiographic vertebral fractures based on the high risk for disability, institutionalization, morbidity, and mortality in people with clinical fractures (3, 4) and the high risk for future fractures in people with radiographic fractures (49). We also prioritized serious adverse events reported in randomized controlled trials (RCTs) and observational studies as more clinically important than withdrawals due to adverse events, which were usually available only from RCTs. Overall, we contextualized the balance between benefits and harms based on the direction and magnitude of treatment effects across all outcomes and the certainty of evidence.
Public and Patient Values and Preferences
The CGC considered values and preferences of the public and patients when assessing the value of the interventions.
Costs
The CGC considered costs and burden of care when assessing the value of the interventions.
Recommendations
Figures 1 to 3 summarize the recommendations.
Treatments to Reduce Fractures in Adults Diagnosed With Osteoporosis
Recommendation 1a: ACP recommends that clinicians use bisphosphonates for initial pharmacologic treatment to reduce the risk of fractures in postmenopausal females diagnosed with primary osteoporosis (strong recommendation; high-certainty evidence).
Recommendation 1b: ACP suggests that clinicians use bisphosphonates for initial pharmacologic treatment to reduce the risk of fractures in males diagnosed with primary osteoporosis (conditional recommendation; low-certainty evidence).
Recommendation 2a: ACP suggests that clinicians use the RANK ligand inhibitor (denosumab) as a second-line pharmacologic treatment to reduce the risk of fractures in postmenopausal females diagnosed with primary osteoporosis who have contraindications to or experience adverse effects of bisphosphonates (conditional recommendation; moderate-certainty evidence).
Recommendation 2b: ACP suggests that clinicians use the RANK ligand inhibitor (denosumab) as a second-line pharmacologic treatment to reduce the risk of fractures in males diagnosed with primary osteoporosis who have contraindications to or experience adverse effects of bisphosphonates (conditional recommendation; low-certainty evidence).
Rationale
Bisphosphonates should be used as first-line treatment in both females and males with primary osteoporosis. In postmenopausal females and males with osteoporosis, bisphosphonates had the most favorable balance among benefits, harms, patient values and preferences, and cost among the drug classes we evaluated (Tables 1a to 1c of Supplement Appendix 1) (34). However, bisphosphonates were associated with higher risk for osteonecrosis of the jaw and atypical femoral or subtrochanteric fractures in observational studies compared with people with osteoporosis who were not treated with bisphosphonates (low certainty of evidence) (Tables 1b.i to 1b.iii of Supplement Appendix 1) (34). In addition to net clinical benefits, bisphosphonates are much cheaper (Table) than other pharmacologic treatments and are available in generic formulations.
These recommendations are applicable to bisphosphonates studied in the eligible primary RCTs (alendronate, risedronate, or zoledronate), which were evaluated in the accompanying evidence review (34). There is no evidence that ibandronate reduces hip fractures (34). The RANK ligand inhibitor (denosumab) can be used as a second-line treatment in both females and males at high risk for fracture. Evidence from RCTs showed that denosumab had a favorable long-term net benefit in postmenopausal females with primary osteoporosis, a history of osteoporotic fractures, and a history of treatment with bisphosphonates (Table 4a of Supplement Appendix 4) (34). Use of denosumab was not associated with a higher risk for osteonecrosis of the jaw (34); however, events were detected in the extension trials and more data are needed to clarify the risk.
Benefits and Harms of Bisphosphonates
Evidence from the network meta-analysis suggested no greater benefits from other drug classes compared with bisphosphonates (Table 2a of Supplement Appendix 2) (34). High-certainty evidence showed that bisphosphonates reduced risk for hip fractures (absolute risk difference [ARD], 6 fewer events per 1000 patients), clinical vertebral fractures (ARD, 18 fewer events per 1000 patients), any clinical fracture (ARD, 24 fewer events per 1000 patients), and radiographic vertebral fractures (ARD, 56 fewer events per 1000 patients) compared with placebo in RCTs assessing outcomes at least 36 months after treatment initiation (Table 1a of Supplement Appendix 1). High-certainty evidence showed no differences between bisphosphonates and placebo in serious adverse events and withdrawals due to adverse events at least 3 years after initiation of treatment in included RCTs (Table 1a of Supplement Appendix 1) (34). However, evidence from observational studies showed that bisphosphonates were associated with higher risk for atypical femoral fractures and osteonecrosis of the jaw (pooled from 5 observational studies; adjusted risk ratio, 3.4 [95% CI, 1.9 to 5.2]; low certainty) at least 2 to 3 years after treatment initiation compared with people with osteoporosis who were not treated with bisphosphonates (Tables 1b.i to 1b.iii of Supplement Appendix 1), although observed events were uncommon (unadjusted incidence of osteonecrosis of the jaw was 0.01% to 0.3% of bisphosphonate users) (34). Longer treatment duration with bisphosphonates may have been associated with higher risk for osteonecrosis of the jaw (34) and atypical femoral fractures (34). Higher risk for atypical femoral fractures was observed in Asian females compared with non-Hispanic White females (595 vs. 109 per 100 000 person-years) (34).
Compared with other medications, evidence from RCTs suggested that there may be no differences between bisphosphonates and denosumab in fracture risk reduction at 36 months or beyond (low certainty; Table 2a of Supplement Appendix 2). Raloxifene probably reduced radiographic fractures compared with placebo but increased risk for withdrawal due to adverse events in RCTs and was associated with higher risk for venous thromboembolism in RCTs (34). Evidence from studies with shorter follow-up (12 to <36 months) showed no greater net benefit from other drug classes compared with bisphos-phonates (Tables 2a and 2b of Supplement Appendix 2) (34).
Benefits and Harms of the RANK Ligand Inhibitor (Denosumab)
Currently, denosumab is the only available RANK ligand inhibitor. Evidence showed that denosumab reduced clinical vertebral fractures (ARD, 16 fewer events per 1000 patients; high certainty) and probably reduced risk for hip fractures (ARD, 4 fewer events per 1000 patients; moderate certainty), any clinical fracture (ARD, 14 fewer events per 1000 patients; moderate certainty), and radiographic vertebral fractures (ARD, 48 fewer events per 1000 patients; moderate certainty) in RCTs assessing outcomes at least 3 years after treatment initiation (Table 1a of Supplement Appendix 1). Denosumab probably reduced risk for radiographic vertebral fractures at shorter follow-up (12 to <36 months) (ARD, 64 fewer events per 1000 patients; moderate certainty) (Table 1a of Supplement Appendix 1).
Evidence from RCTs showed there are probably no differences in serious adverse effects and withdrawal due to adverse effects at 36 months between denosumab and placebo (moderate certainty; Table 1a of Supplement Appendix 1).
Treatment in Males
There was no evidence suggesting differences in treatment benefits and harms by sex (34). Evidence was limited on the effect of bisphosphonates and fracture prevention in males with primary osteoporosis (Table 1c of Supplement Appendix 1) (34). Therefore, we complemented low-certainty conclusions of the effect of bisphosphonate treatment for males by extrapolating results from trials that included females in order to recommend the same first- and second-line treatments for males and females. We downgraded the overall certainty of evidence from the available data in females to low due to indirectness, and we downgraded the strength of the recommendation to conditional.
The systematic review identified 10 studies (6 RCTs and 4 observational studies) that included only males with osteoporosis or patients stratified by sex (34). Low-certainty evidence showed that bisphosphonates may have reduced radiographic vertebral fractures (ARD, 140 fewer events per 1000 patients) compared with placebo in RCTs assessing outcomes at least 36 months from treatment initiation in males (Table 1c of Supplement Appendix 1). No RCTs evaluated hip fractures. Bisphosphonates probably reduced radiographic vertebral but not any clinical fractures at 12 to less than 36 months (moderate certainty) (34). Evidence from RCTs assessing harms at 12 to less than 36 months showed no differences in the risk for serious adverse events (high certainty) and probably no difference in withdrawals due to adverse events (moderate certainty) and atrial fibrillation (low certainty) in males (Table 1c of Supplement Appendix 1) (34). Longer treatment with bisphosphonates was associated with higher risk for atypical femoral fractures and osteonecrosis of the jaw (34). For other harms, zoledronate increased the likelihood of pyrexia, myalgia, and arthralgia (50–52).
Applicability
Most studies enrolled adults at high risk for fracture, although definitions of baseline risk were heterogeneous due to different scoring scales used in the RCTs and different proportions of adults with prior vertebral fractures at baseline (Table 4a of Supplement Appendix 4) (34). Appendix Table 3 (available at Annals.org) summarizes risk factors for fractures (36–39). Primary studies did not consistently report on prior treatment response, although most allowed previous treatments with bisphosphonates (Table 4a of Supplement Appendix 4) (34). Only bisphosphonates have been tested as first-line treatment in treatment-naive patients (34). Primary studies enrolled adults with osteoporosis who were already taking vitamin D, calcium, or both supplements (Table 4a of Supplement Appendix 4) (34). Most studies included females and a very small number of males with primary osteoporosis (Table 4b of Supplement Appendix 4), but few RCTs assessed the effect of zoledronate in males with osteoporosis (34).
Values and Preferences
Limited evidence on values and preferences related to net benefit from oral or injectable medications (34, 53, 54) showed that females considered the effectiveness and adverse effects of treatments equally, followed by convenience of taking the medication and effect on daily routine (they preferred less frequent dosing, oral route of administration, and injectable route over oral if taken at a lower frequency) (34). Out-of-pocket costs were considered extremely important factors (34). Bisphosphonates can be taken through various routes and at various frequencies, giving patients an opportunity to tailor treatment to their preferences (Table). Views from the CGC Public Panel reported preferences for use of bisphosphonates to treat osteoporosis. Similar to the research evidence, the Public Panel’s preferences were also driven by the profile of benefits and harms.
Costs
We considered national data on resource use and published systematic reviews of economic analyses of lifetime horizon cost applicable to the United States (55). National Medicare data suggested that bisphosphonates are substantially less expensive than the other drug classes (Table 1d and Figures 1b and 1c of Supplement Appendix 1). Medicare data also showed that generic bisphosphonates (oral alendronate or intravenous zoledronate) were the least expensive compared with brand-name formulations (Table 1e of Supplement Appendix 1). The overall treatment cost was probably higher for injectable intravenous formulations because it included reimbursement for clinic visits, infusion costs (intravenous), and potential missed work hours for working patients. Systematic reviews concluded that the most cost-effective initial therapy for postmenopausal osteoporosis was generic zoledronate or oral alendronate (Table 1f of Supplement Appendix 1) (34, 56) and that the maximum net benefit from bisphosphonates is observed in patients with high baseline risk for fractures (Table 1g of Supplement Appendix 1) (34). These analyses did not address poor adherence to oral bisphosphonates or additional costs associated with injectable drugs or brand-name formulations. The absolute cost to use denosumab, romosozumab, or teriparatide is higher because discontinuation should be followed by an alternative sequential treatment to prevent rebound fractures.
Evidence from the published cost-effectiveness analysis (CEA) was insufficient to conclude economic value of drugs for osteoporosis (34). The most recent systematic review of CEAs of osteoporosis drugs included 12 CEAs, but only 1 was from the United States (57). The review suggested that baseline risk for fracture, the magnitude of medication effects on fracture prevention, medication adherence and persistence, and drug cost contributed to cost-effectiveness of available medications (58). A single CEA conducted in the United States (57) concluded that denosumab was cost-effective compared with other osteoporosis treatments in older U.S. males with osteoporosis, based on indirect evidence from a single RCT in postmenopausal females (59).
Recommendation 3: ACP suggests that clinicians use the sclerostin inhibitor (romosozumab, moderate-certainty evidence) or recombinant PTH (teriparatide, low-certainty evidence), followed by a bisphosphonate, to reduce the risk of fractures only in females with primary osteoporosis with very high risk of fracture (conditional recommendation).
Rationale
Evidence showed that the benefits after 24 months of treatment with recombinant PTH (teriparatide) or the sclerostin inhibitor (romosozumab) may have outweighed harms only in a select population of postmenopausal females (mean age, >74 years) with osteoporosis and very high risk for fracture (Table 1a of Supplement Appendix 1 and Table 2a of Supplement Appendix 2) (34, 60–65). We developed our recommendations on the basis of the assessment of very high risk for fracture in primary RCTs (60, 65). Very high risk was based on older age, a recent fracture (for example, within the past 12 months), history of multiple clinical osteoporotic fractures, multiple risk factors for fracture (see Appendix Table 3), or failure of other available osteoporosis therapy (30, 66–68) (Table 4a of Supplement Appendix 4).
Currently, romosozumab is the only available sclerostin inhibitor and teriparatide is the only available recombinant PTH. Discontinuation of romosozumab or teriparatide treatment may result in rapid bone loss and higher fracture risk and should be followed by administration of an antiresorptive agent (69, 70).
Because this is a conditional recommendation for females, we did not make a recommendation for males because any further downgrading due to indirectness was not sufficient to support a clinical recommendation.
Benefits and Harms of Recombinant PTH (Teriparatide)
None of the included studies evaluated the long-term benefits of teriparatide (Table 1a of Supplement Appendix 1). Evidence showed that teriparatide reduced risk for any clinical fractures and radiographic vertebral fractures (ARD, 27 and 69 fewer events per 1000 patients, respectively; high certainty) and may have reduced clinical vertebral fractures (ARD, 45 fewer events per 1000 patients; low certainty) compared with placebo at 24-month outcome assessment (34) but may have resulted in no difference in risk for hip fractures (low certainty). Evidence from RCTs showed that teriparatide may have resulted in no difference in risk for serious adverse effects (low certainty) but probably increased risk for withdrawal due to adverse effects at 36- and 24-month follow-up (ARD, 127 and 17 more events per 1000 patients, respectively; moderate certainty), most commonly due to nausea, dizziness, vomiting, headache, palpitations, and leg cramps (34, 71).
Compared with bisphosphonates at 24-month outcome assessment, evidence showed that teriparatide probably reduced risk for radiographic vertebral fractures (ARD, 66 fewer events per 1000 patients; moderate certainty), may have reduced risk for any clinical fracture (ARD, 46 fewer events per 1000 patients; low certainty), and may have resulted in no differences in serious adverse events (low certainty) or withdrawal due to adverse events (moderate certainty). However, teriparatide increased risk for withdrawal due to adverse events in the longer term (36 months) (risk ratio, 3.1; low certainty) (Table 2a of Supplement Appendix 2) (34). There is not yet sufficient evidence on the benefits and harms of sequential therapy with bisphosphonates after 72 weeks of teriparatide (34, 72).
Benefits and Harms of the Sclerostin Inhibitor (Romosozumab)
None of the included studies evaluated the long-term benefits and harms of romosozumab or reported its effect on risk for hip fractures (34). Moderate-certainty evidence from RCTs assessing outcomes at 12 to 36 months after treatment initiation showed that romosozumab probably reduced clinical vertebral fractures (ARD, 4 fewer events per 1000 patients), radiographic vertebral fractures (ARD, 13 fewer events per 1000 patients), and any clinical fractures (ARD, 9 fewer events per 1000 patients) compared with placebo, but prevention of hip fractures was not reported (Table 1a of Supplement Appendix 1) (34). At 12 months, romosozumab compared with bisphosphonates may have resulted in no differences in clinical or radiographic vertebral fractures (low certainty) with no evidence about its effect in hip fractures (34). Evidence from RCTs showed that romosozumab may have resulted in no differences in serious adverse events (moderate certainty) or withdrawals due to adverse events (low certainty) compared with placebo (34). Romosozumab increased risk for cardiovascular events compared with alendronate (hazard ratio, 1.9 [CI, 1.1 to 3.1]) (21, 34, 60).
Benefits and Harms of Sequential Therapy
Evidence from RCTs that looked explicitly at sequential therapy with bisphosphonates after initial treatment with denosumab, romosozumab, or teriparatide was limited (34, 73). Moderate-certainty evidence from a single large RCT (60) showed that romosozumab followed by alendronate probably reduced all clinical fractures compared with placebo and probably reduced hip fractures (ARD, 12 fewer events per 1000 patients), clinical vertebral fractures (ARD, 13 fewer events per 1000 patients), any clinical fracture (ARD, 33 fewer events per 1000 patients), and radiographic vertebral fractures (ARD, 40 fewer events per 1000 patients) compared with a bisphosphonate alone at 12- to 36-month outcome assessment, without higher risk for serious harms or withdrawal due to adverse effects (Table 1a of Supplement Appendix 1 and Table 2a of Supplement Appendix 2) (34).
Applicability
Primary studies of romosozumab or teriparatide enrolled postmenopausal females (mean age, >74 years) with osteoporosis and very high risk for fracture (Table 1a of Supplement Appendix 1 and Table 2a of Supplement Appendix 2) (34). An estimated 10% of females older than 50 years in the general U.S. population would be characterized as being at very high risk (68) as defined by the level of risk in females enrolled in RCTs of romosozumab (Table 4a of Supplement Appendix 4). Primary studies enrolled postmenopausal females with osteoporosis who were already taking vitamin D, calcium, or both supplements (Table 4a of Supplement Appendix 4) (34). Because this is a conditional recommendation for females, we did not make a recommendation for males because any further downgrading due to indirectness was not sufficient to support a clinical recommendation.
Values and Preferences
The systematic review did not identify any studies of patient values and preferences in adults treated with romosozumab or teriparatide (34).
Costs
Teriparatide is the most expensive of the reviewed treatments, with an average annual cost per Medicare beneficiary of $22 156. Romosozumab is more expensive than bisphosphonates (average annual cost per Medicare beneficiary is $5574 vs. a range of $39 to $2700) but may be less expensive than denosumab (range of $1913 to $12 241) (Figure 1c of Supplement Appendix 1). The systematic review did not identify any CEAs applicable to the United States for either treatment (34). The evidence from the published CEAs was insufficient to conclude economic value of sequential treatments for osteoporosis (34). Indirect evidence from a single RCT extension with high risk of bias was used in CEAs of sequential therapy with abaloparatide or teriparatide followed by alendronate (74–76). Because teriparatide and romosozumab should be followed by bisphosphonates after discontinuation, the absolute cost would be higher than the cost of monotherapy, although the cost-effectiveness of sequential therapy has not been examined (34). Romosozumab and teriparatide are administered by subcutaneous injection, but teriparatide can be administered by self-injection, whereas romosozumab is often injected by clinicians (Table), increasing the overall cost of treatment.
Treatments to Reduce Fractures in Adults With Low Bone Mass
Recommendation 4: ACP suggests that clinicians take an individualized approach regarding whether to start pharmacologic treatment with a bisphosphonate in females over the age of 65 with low bone mass (osteopenia) to reduce the risk of fractures (conditional recommendation; low-certainty evidence).
Rationale
Evidence was limited on treatments in adults with low bone mass and was largely informed by a single trial in older females that showed zoledronate may reduce any clinical or vertebral fractures (34). Fracture prevention in females with low bone mass needs to be balanced with harms and costs of bisphosphonates based on an individualized assessment of baseline risk for fractures. Diagnostic criteria for low bone mass in females varied in the primary studies (Table 4c of Supplement Appendix 4). The effectiveness across different individual bisphosphonates has not been directly evaluated in females with low bone mass.
The systematic review did not identify any studies reporting on fracture outcomes for males with low bone mass or on differences in treatment outcomes by sex (34). Because the certainty of evidence was low in females, further extrapolation downgraded the certainty in males to insufficient due to indirectness (34). Therefore, evidence was very uncertain to make a recommendation for or against treatment in males with low bone mass.
Benefits and Harms of Bisphosphonates
Low-certainty evidence from a long-term (6 years) RCT of older females with higher baseline risk for fracture (2.3%) than older females with low bone mass (34, 77) showed that zoledronate may have reduced any clinical or vertebral fractures, although evidence was very uncertain for the effect on hip fractures, withdrawals due to adverse events, or risk for atrial fibrillation (Table 3a of Supplement Appendix 3) (34, 77, 78). Evidence showed there may have been no differences in serious adverse events (34). The limited evidence on the effects of alendronate or risedronate on fractures was very uncertain (insufficient) (34).
Applicability
The RCT enrolled females aged 65 years or older diagnosed with low bone mass at either the total hip or the femoral neck on either side. Females with osteoporosis at 1 hip site, history of nonvertebral fracture (in 24%), prevalent vertebral fracture (in 13%), and a median 10-year risk for osteoporotic fracture of 12% were also eligible for the trial (77) (Table 4c of Supplement Appendix 4).
Values and Preferences
The systematic review did not identify any studies of patient values and preferences in adults with low bone mass (34).
Costs
The systematic review did not identify any CEAs applicable to the United States in adults with low bone mass, but as previously noted, bisphosphonates are a less expensive option and provide patients with choices for medication route and frequency (34).
Clinical Considerations
Clinicians should prescribe generic medications if possible rather than more expensive brand-name medications.
Clinicians treating adults with osteoporosis should encourage adherence to recommended treatments and healthy lifestyle modifications, including exercise, and counseling for evaluation and prevention of falls.
Adequate calcium and vitamin D intake should be part of fracture prevention in all adults with low bone mass or osteoporosis.
Clinicians should assess baseline risk for fracture based on individualized assessment of bone density, history of fractures, response to prior treatments for osteoporosis, and multiple risk factors for fractures (Appendix Table 3). There are many available risk assessment tools with varying predictive value, which were not evaluated in the systematic review (34) or in this guideline.
Current evidence suggests that increasing the duration of bisphosphonate therapy to longer than 3 to 5 years reduces risk for new vertebral fractures but not risk for other fractures (34, 79–81). However, there is increased risk for long-term harms (34). Therefore, clinicians should consider stopping bisphosphonate treatment after 5 years unless the patient has a strong indication for treatment continuation.
The decision for a temporary bisphosphonate treatment discontinuation (holiday) and its duration should be individualized and should be based on baseline risk for fractures, type of medication and its half-life in bone, benefits, and harms (higher risk for fracture due to drug discontinuation).
Females initially treated with an anabolic agent should be offered an antiresorptive agent after discontinuation to preserve gains and because of serious risk for rebound and multiple vertebral fractures (21, 69, 70, 82).
Older adults (for example, those aged >65 years) with osteoporosis may be at increased risk for falls and other adverse events due to polypharmacy or drug interactions. Individualized treatment selection should address contraindications and cautions for drugs indicated to treat osteoporosis based on comorbidities and concomitant medications (Tables 1j and 1k of Supplement Appendix 1) as well as reassessment of other drugs associated with higher risk for falls and fractures.
There is variable risk for low bone mass in transgender persons based on age at gonadectomy, therapy with sex hormones, distribution of comorbidities, and behavioral risk factors for osteoporosis and fractures. When considering the potential risk for fractures, history of gonadectomy (including age) and sex steroid therapy should be considered in treatment decisions for secondary osteoporosis.
Evidence Gaps and Research Needs
Future RCTs should be designed to shed light on long-term comparative benefits and harms from all available treatments in patients with primary osteoporosis or low bone mass, specifically in less-studied populations, such as premenopausal females, males, intersex persons, transgender persons after any transitioning treatment, residents of long-term care facilities, and people with multimorbidity and polypharmacy. More studies should assess whether fracture outcomes vary depending on baseline risk for fracture and prior response to treatments. Benefits and harms from delayed (83–85) or off-label longer treatment duration with denosumab or anabolic treatments should be examined in well-designed, real-world evidence studies.
Areas With Inconclusive Evidence
Evidence on benefits and harms was inconclusive to recommend for or against PTHrP (abaloparatide) or SERMs (raloxifene, bazedoxifene) (Table 1a of Supplement Appendix 1 and Table 2a of Supplement Appendix 2). Long-term safety of abaloparatide in humans has yet to be determined. The included studies provided sparse data to assess whether treatment benefits and harms varied according to baseline risk for fracture, age (Table 1h of Supplement Appendix 1 and Table 2c of Supplement Appendix 2), race, and ethnicity. Ongoing studies are expected to provide evidence on the benefits and harms of romosozumab and combined therapies in males with osteoporosis (86–91).
Areas With No Evidence
None of the included studies assessed long-term benefits and harms of pharmacologic therapy compared with nonpharmacologic therapy, abaloparatide, romosozumab, or sequential therapy with available drugs for adults with primary osteoporosis. Treatments to mitigate rebound bone loss in patients with contraindications to bisphosphonates or harms after bisphosphonate treatment are unknown. No included studies assessed effects of anabolic drugs on fracture prevention in patients with low bone mass and multiple risk factors for fractures. No included studies specifically examined fracture prevention in transgender persons with osteoporosis or low bone mass.
Clinical guidelines are meant to guide care based on the best available evidence and may not apply to all patients or individual clinical situations. They should not be used as a replacement for a clinician’s judgment. Any reference to a product or process contained in a guideline is not intended as an endorsement of any specific commercial product. All ACP clinical guidelines are considered automatically withdrawn or invalid 5 years after publication or once an update has been issued.
Supplementary Material
Figure 2.

Treatments to reduce fractures in males with primary osteoporosis.
ACP = American College of Physicians; RANK = receptor activator of nuclear factor κB; RCT = randomized controlled trial.
Acknowledgment:
The Clinical Guidelines Committee would like to acknowledge members of the ACP Guidelines Public Panel for their review and comments on the article from a patient perspective: Cynthia Appley, Ray Haeme, Billy Oglesby, James Pantelas, Missy Carson Smith, and Lelis Vernon. The authors also thank Jennifer Yost, RN, PhD, for her methodological review and input on the draft guideline.
Financial Support:
Financial support for the development of this guideline came exclusively from the ACP operating budget.
Disclosures:
All financial and intellectual disclosures of interest were declared, and potential conflicts were discussed and managed. Dr. Mustafa disclosed a high-level conflict (site principal investigator for industry-funded trial) during work and upon identification was recused from further discussion, authorship, and voting. Dr. Williams acquired a high-level conflict (household member receiving industry consulting fees) during work development and upon identification was recused from further discussion, authorship, and voting. Dr. Wilt was recused from authorship and voting due to a moderate-level conflict of interest (household member recently authored relevant publications and served on technical expert panel for supporting systematic review). Drs. Crandall and Owens were recused from authorship and voting due to moderate-level conflicts of interest (recently authored relevant publications). A record of disclosures of interest and management of conflicts is kept for each Clinical Guidelines Committee meeting and conference call and can be viewed at www.acponline.org/clinical_information/guidelines/guidelines/conflicts_cgc.htm. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-1034.
Appendix
Appendix Figure.
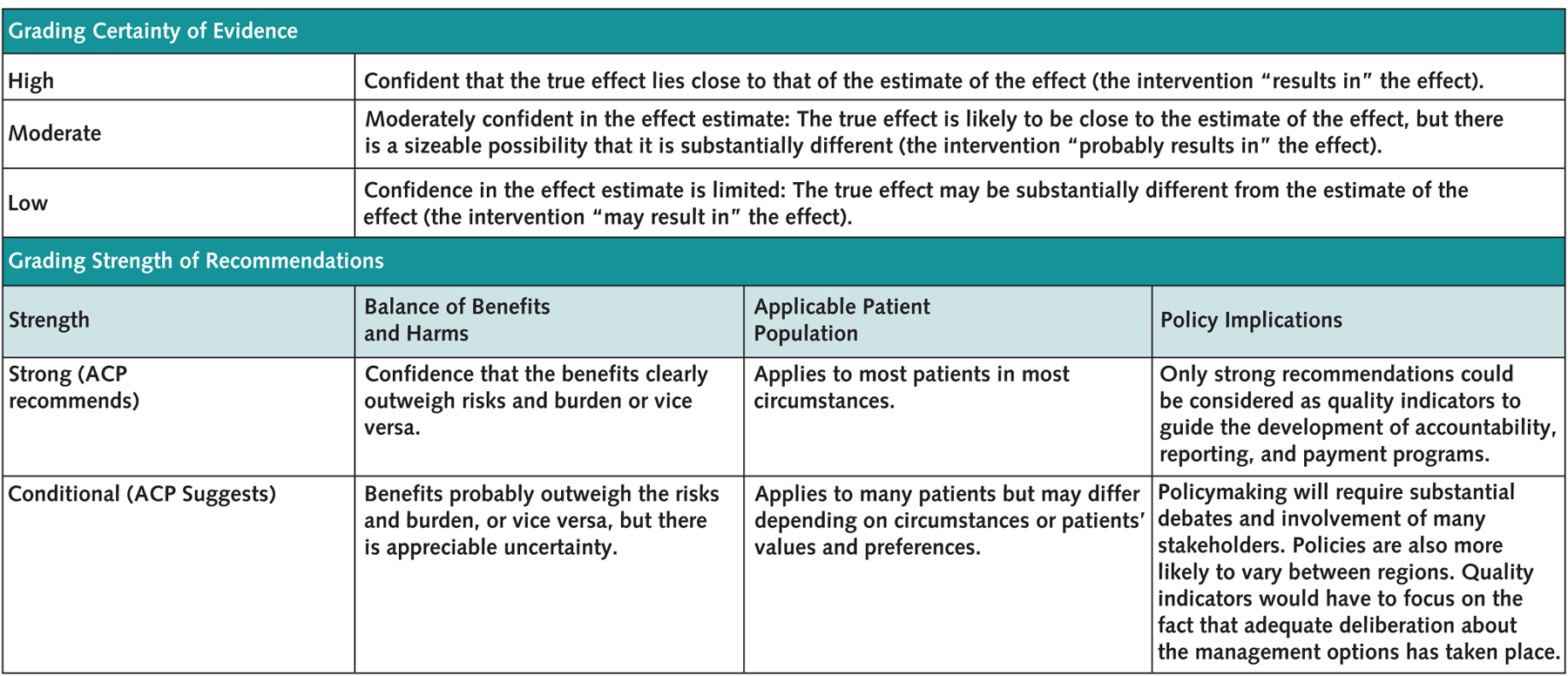
Grading the certainty of evidence and strength of recommendations in ACP clinical guidelines using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach.
ACP = American College of Physicians.
Appendix Table 1.
Definitions of Low Bone Mass and Osteoporosis
| Condition | Definition |
|---|---|
| Low bone mass (osteopenia) | A BMD value at the femoral neck, the lumbar spine, or both that is between 1 and 2.5 SDs below the mean BMD value for a young woman (18, 92, 93) |
| Osteoporosis | A BMD value at the femoral neck, the lumbar spine, or both that is ≥2.5 SDs below the mean BMD value for ayoung woman; osteoporosis may be diagnosed in postmenopausal women and in men aged ≥50 y ifthe T-score forthe lumbar spine, total hip, or femoral neck is −2.5 or less (in certain circumstances, the 33% radius [also called the 1/3 radius] may be used) (18, 92, 93) The reference standard from which the T-score is calculated is the White female population aged 20–29 y in the NHANES III database A uniform White (not adjusted for race) female reference was used for men in all ethnic groups |
BMD = bone mineral density; NHANES III = Third National Health and Nutrition Examination Survey.
Appendix Table 2.
Osteoporotic Fracture Outcomes Reference Guide*
| Fracture Outcome | Fracture Location | Definition | Example |
|---|---|---|---|
| Clinical† | Any fracture | Anyfracture (vertebral or nonvertebral) discovered because the patient is symptommatic; verified by radiograph‡ | A patient seeks care for symptoms that are suggestive of fracture after a fall from a standing height, and the clinician orders radiographs by which the fracture is subsequently confirmed. |
| Clinical vertebral | Spine | A vertebral fracture discovered because the patient is symptomatic; verified by radiograph | After a fall from a standing height, a patient seeks care dueto symptoms highly suggestive of vertebral fracture, and the clinician orders radiographs confirming fracture. |
| Nonvertebral† | All nonspine | Clinical fractures outside the spine§, excludeing fractures not considered to be related to osteoporosis (e.g., in the toes, skull, face, or fingers) | A patient breaks their tibia during a fall from a standing height, and fracture is subsequently confirmed by radiograph. |
| Hip | Hip | Clinical fracture at the top of the femur | A patient falls and cannot get up due to hip pain. Hip fracture is subsequently confirmed by radiograph. |
| Radiographic vertebral | Spine | Anyvertebral fracture appearing on a radiography∥, regardless of whether the patient is symptomatic | A study performs spinal radiographs on all participants entering the study and again after treatment. |
Most studies limited data to fragility fractures (i.e., fractures resulting from a fall from a standing height or lower) and radiographic vertebral fractures; pathologic and high-trauma fractures were generally excluded. A combined outcome of any clinical fracture was created specifically for the network meta-analysis (34).
Not a GRADE (Grading of Recommendations Assessment, Development and Evaluation) outcome, but analyzed as any clinical fracture for network meta-analysis.
Major osteoporotic fractures (fractures of hip, spine [clinical], wrist, or humerus) were included as any clinical fracture.
Several studies limited nonvertebral fractures to predetermined sites. For example, the MOVER (MOnthly intraVenous ibandronatE versus daily oral Risedronate) study defined nonvertebral fractures as those at 6 major sites: the femur, the forearm, the humerus, the clavicle, the tibia/fibula, and the pelvis (94).
Most studies used semiquantitative and/or quantitative morphometry assessment to determine prevalent and incident vertebral fractures.
Appendix Table 3.
Risk Factors for Osteoporotic Fracture*
| Increasing age |
| Female sex |
| Postmenopause (females) |
| Hypogonadism or premature ovarian failure |
| Low body weight |
| History of hip fracture in parent |
| Racial background (White persons are at higher risk than Black persons) |
| Previous clinical or morphometric vertebral fracture |
| Previous fracture due to minimal trauma (i.e., previous osteoporotic fracture) |
| Rheumatoid arthritis |
| Current smoking |
| Current alcohol intake (≥3 drinks daily) |
| Low bone mineral mass |
| Vitamin D deficiency |
| Low calcium intake |
| Hyperkyphosis |
| Falling and immobilization |
| Long-term use of certain medications, the most implicated being gluco-corticoids, anticoagulants, anticonvulsants, aromatase inhibitors, cancer chemotherapeutic drugs, and gonadotrophin-releasing hormone agonists |
Footnotes
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Correction: This article was amended on 16 May 2023 to correct reporting and editorial errors. The Supplement has also been corrected. None of the corrections affect the overall conclusions. A correction has been published (doi:10.7326/L23-0120).
References
- 1.Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–6. doi: 10.1002/jbmr.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curry SJ, Krist AH, Owens DK, et al. ; US Preventive Services Task Force. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319: 2521–31. doi: 10.1001/jama.2018.7498 [DOI] [PubMed] [Google Scholar]
- 3.Lee SB, Park Y, Kim DW, et al. Association between mortality risk and the number, location, and sequence of subsequent fractures in the elderly. Osteoporos Int. 2021;32:233–41. doi: 10.1007/s00198-020-05602-x [DOI] [PubMed] [Google Scholar]
- 4.Rizkallah M, Bachour F, Khoury ME, et al. Comparison of morbidity and mortality of hip and vertebral fragility fractures: which one has the highest burden. Osteoporos Sarcopenia. 2020;6:146–50. doi: 10.1016/j.afos.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crandall CJ, Hunt RP, LaCroix AZ, et al. After the initial fracture in postmenopausal women, where do subsequent fractures occur. EClinicalMedicine. 2021;35:100826. doi: 10.1016/j.eclinm.2021.100826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarafrazi N, Wambogo EA, Shepherd JA. Osteoporosis or low bone mass in older adults: United States, 2017–2018. NCHS Data Brief. 2021:1–8. [PubMed] [Google Scholar]
- 7.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–75. [DOI] [PubMed] [Google Scholar]
- 8.Looker AC, Sarafrazi Isfahani N, Fan B, et al. Trends in osteoporosis and low bone mass in older US adults, 2005–2006 through 2013–2014. Osteoporos Int. 2017;28:1979–88. doi: 10.1007/s00198-017-3996-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roux C, Thomas T, Paccou J, et al. Refracture and mortality following hospitalization for severe osteoporotic fractures: the Fractos Study. JBMR Plus. 2021;5:e10507. doi: 10.1002/jbm4.10507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riska BSL, Forsén L, Omsland TK, et al. Does the association of comorbidity with 1-year mortality after hip fracture differ according to gender? The Norwegian Epidemiologic Osteoporosis Studies (NOREPOS). J Am Geriatr Soc. 2018;66:553–8. doi: 10.1111/jgs.15207 [DOI] [PubMed] [Google Scholar]
- 11.Kannegaard PN, van der Mark S, Eiken P, et al. Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing. 2010;39:203–9. doi: 10.1093/ageing/afp221 [DOI] [PubMed] [Google Scholar]
- 12.Herrera A, Lobo-Escolar A, Mateo J, et al. Male osteoporosis: a review. World J Orthop. 2012;3:223–34. doi: 10.5312/wjo.v3.i12.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran O, Silverman S, Xu X, et al. Long-term direct and indirect economic burden associated with osteoporotic fracture in US postmenopausal women. Osteoporos Int. 2021;32:1195–1205. doi: 10.1007/s00198-020-05769-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen D, Pelizzari PM, Pyenson BS. Medicare cost of osteoporotic fractures: 2021 updated report. Milliman; 30 March 2021. Accessed at www.milliman.com/en/insight/medicare-cost-of-osteoporotic-fractures-2021-updated-report on 2 December 2022. [Google Scholar]
- 15.Hansen D, Bazell C, Pelizzari P, et al. Medicare cost of osteoporotic fractures. Milliman; August 2019. Accessed at https://assets.milliman.com/ektron/Medicare_cost_of_osteoporotic_fractures.pdf on 2 December 2022. [Google Scholar]
- 16.Qaseem A, Snow V, Shekelle P, et al. ; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:404–15. [PubMed] [Google Scholar]
- 17.Qaseem A, Snow V, Shekelle P, et al. ; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680–4. [DOI] [PubMed] [Google Scholar]
- 18.Qaseem A, Forciea MA, McLean RM, et al. ; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818–39. doi: 10.7326/M15-1361 [DOI] [PubMed] [Google Scholar]
- 19.Prolia® (denosumab). Highlights of prescribing information. Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2011/125320s5s6lbl.pdf on 29 November 2022.
- 20.Tsourdi E, Zillikens MC, Meier C, et al. Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. 2020. doi: 10.1210/clinem/dgaa756 [DOI] [PubMed] [Google Scholar]
- 21.EVENITY™ (romosozumab-aqqg) injection, for subcutaneous use. Highlights of prescribing information. Accessed at www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761062Orig1s000Lbl.pdf on 29 November 2022.
- 22.Vestergaard Kvist A, Faruque J, Vallejo-Yagüe E, et al. Cardiovascular safety profile of romosozumab: a pharmacovigilance analysis of the US Food and Drug Administration Adverse Event Reporting System (FAERS). J Clin Med. 2021;10. doi: 10.3390/jcm10081660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EVISTA (raloxifene hydrochloride) Tablet for Oral Use. Highlights of prescribing information. Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2007/022042lbl.pdf on 29 November 2022.
- 24.Miller PD, Hattersley G, Riis BJ, et al. ; ACTIVE Study Investigators. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316:722–33. doi: 10.1001/jama.2016.11136 [DOI] [PubMed] [Google Scholar]
- 25.Greenspan SL, Fitzpatrick LA, Mitlak B, et al. Abaloparatide followed by alendronate in women ≥80 years with osteoporosis: post hoc analysis of ACTIVExtend. Menopause. 2020;27:1137–42. doi: 10.1097/GME.0000000000001593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv F, Cai X, Yang W, et al. Denosumab or romosozumab therapy and risk of cardiovascular events in patients with primary osteoporosis: systematic review and meta-analysis. Bone. 2020;130:115121. doi: 10.1016/j.bone.2019.115121 [DOI] [PubMed] [Google Scholar]
- 27.Kaveh S, Hosseinifard H, Ghadimi N, et al. Efficacy and safety of romosozumab in treatment for low bone mineral density: a systematic review and meta-analysis. Clin Rheumatol. 2020;39:3261–76. doi: 10.1007/s10067-020-04948-1 [DOI] [PubMed] [Google Scholar]
- 28.Migliorini F, Maffulli N, Colarossi G, et al. Effect of drugs on bone mineral density in postmenopausal osteoporosis: a Bayesian network meta-analysis. J Orthop Surg Res. 2021;16:533. doi: 10.1186/s13018-021-02678-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson EL, Martyn-St James M, Hamilton J, et al. Clinical effectiveness of denosumab, raloxifene, romosozumab, and teriparatide for the prevention of osteoporotic fragility fractures: a systematic review and network meta-analysis. Bone. 2020;130:115081. doi: 10.1016/j.bone.2019.115081 [DOI] [PubMed] [Google Scholar]
- 30.Davis S, Simpson E, Hamilton J, et al. Denosumab, raloxifene, romosozumab and teriparatide to prevent osteoporotic fragility fractures: a systematic review and economic evaluation. Health Technol Assess. 2020;24:1–314. doi: 10.3310/hta24290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fink HA, MacDonald R, Forte ML, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171:37–50. doi: 10.7326/M19-0533 [DOI] [PubMed] [Google Scholar]
- 32.Kline GA, Morin SN, Feldman S, et al. Diminishing value from multiple serial bone densitometry in women receiving antiresorptive medication for osteoporosis. J Clin Endocrinol Metab. 2021;106:2718–25. doi: 10.1210/clinem/dgab211 [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Prevention and Management of Osteoporosis: Report of a WHO Scientific Group. WHO Technical Report Series; 921. 2003. Accessed at https://apps.who.int/iris/bitstream/handle/10665/42841/WHO_TRS_921.pdf?sequence=1 on December 6 2022. [PubMed]
- 34.Ayers C, Kansagara D, Lazur B, et al. Effectiveness and safety of treatments to prevent fractures in people with low bone mass or primary osteoporosis: a living systematic review and network meta-analysis for the American College of Physicians. Ann Intern Med. 2023;176:182–195. doi: 10.7326/M22-0684 [DOI] [PubMed] [Google Scholar]
- 35.Kehoe T, Blind E, Janssen H. Regulatory aspects of the development of drugs for metabolic bone diseases - FDA and EMA perspective. Br J Clin Pharmacol. 2019;85:1208–12. doi: 10.1111/bcp.13791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaudoin C, Moore L, Gagné M, et al. Performance of predictive tools to identify individuals at risk of non-traumatic fracture: a systematic review, meta-analysis, and meta-regression. Osteoporos Int. 2019;30:721–40. doi: 10.1007/s00198-019-04919-6 [DOI] [PubMed] [Google Scholar]
- 37.El-Hajj Fuleihan G, Chakhtoura M, Cauley JA, et al. Worldwide fracture prediction. J Clin Densitom. 2017;20:397–424. doi: 10.1016/j.jocd.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 38.Kanis JA, Harvey NC, Johansson H, et al. Overview of fracture prediction tools. J Clin Densitom. 2017;20:444–50. doi: 10.1016/j.jocd.2017.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marques A, Ferreira RJ, Santos E, et al. The accuracy of osteoporotic fracture risk prediction tools: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:1958–67. doi: 10.1136/annrheumdis-2015-207907 [DOI] [PubMed] [Google Scholar]
- 40.Hassett MJ, Somerfield MR, Baker ER, et al. Management of male breast cancer: ASCO guideline. J Clin Oncol. 2020;38:1849–63. doi: 10.1200/JCO.19.03120 [DOI] [PubMed] [Google Scholar]
- 41.Joseph JS, Lam V, Patel MI. Preventing osteoporosis in men taking androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Eur Urol Oncol. 2019;2:551–61. doi: 10.1016/j.euo.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 42.Shapiro CL, Van Poznak C, Lacchetti C, et al. Management of osteoporosis in survivors of adult cancers with nonmetastatic disease: ASCO clinical practice guideline. J Clin Oncol. 2019;37:2916–46. doi: 10.1200/JCO.19.01696 [DOI] [PubMed] [Google Scholar]
- 43.Trémollieres FA, Ceausu I, Depypere H, et al. Osteoporosis management in patients with breast cancer: EMAS position statement. Maturitas. 2017;95:65–71. doi: 10.1016/j.maturitas.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 44.Qaseem A, Kansagara D, Lin JS, et al. ; Clinical Guidelines Committee of the American College of Physicians. The development of clinical guidelines and guidance statements by the Clinical Guidelines Committee of the American College of Physicians: update of methods. Ann Intern Med. 2019;170:863–70. doi: 10.7326/M18-3290 [DOI] [PubMed] [Google Scholar]
- 45.Qaseem A, Wilt TJ, Forciea MA, et al. ; Clinical Guidelines Committee of the American College of Physicians. Disclosure of interests and management of conflicts of interest in clinical guidelines and guidance statements: methods from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2019;171:354–61. doi: 10.7326/M18-3279 [DOI] [PubMed] [Google Scholar]
- 46.Schünemann H, Brożek J, Guyatt G, et al. , eds. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. Accessed at http://gdt.guidelinedevelopment.org/app/handbook/handbook.html on 1 July 2022.
- 47.Akl EA, Meerpohl JJ, Elliott J, et al. ; Living Systematic Review Network. Living systematic reviews: 4. Living guideline recommendations. J Clin Epidemiol. 2017;91:47–53. doi: 10.1016/j.jclinepi.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 48.Willems D, Javaid MK, Pinedo-Villanueva R, et al. Importance of time point-specific indirect treatment comparisons of osteoporosis treatments: a systematic literature review and network meta-analyses. Clin Ther. 2022;44:81–97. doi: 10.1016/j.clinthera.2021.11.015 [DOI] [PubMed] [Google Scholar]
- 49.Nevitt MC, Ettinger B, Black DM, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med. 1998;128:793–800. [DOI] [PubMed] [Google Scholar]
- 50.Boonen S, Orwoll E, Magaziner J, et al. ; HORIZON Recurrent Fracture Trial. Once-yearly zoledronic acid in older men compared with women with recent hip fracture. J Am Geriatr Soc. 2011;59:2084–90. doi: 10.1111/j.1532-5415.2011.03666.x [DOI] [PubMed] [Google Scholar]
- 51.Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012; 367:1714–23. doi: 10.1056/NEJMoa1204061 [DOI] [PubMed] [Google Scholar]
- 52.Nakamura T, Fukunaga M, Nakano T, et al. Efficacy and safety of once-yearly zoledronic acid in Japanese patients with primary osteoporosis: two-year results from a randomized placebo-controlled double-blind study (ZOledroNate treatment in Efficacy to osteoporosis; ZONE study). Osteoporos Int. 2017;28:389–98. doi: 10.1007/s00198-016-3736-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrionuevo P, Gionfriddo MR, Castaneda-Guarderas A, et al. Women’s values and preferences regarding osteoporosis treatments: a systematic review. J Clin Endocrinol Metab. 2019;104:1631–6. doi: 10.1210/jc.2019-00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morizio P, Burkhart JI, Ozawa S. Denosumab: a unique perspective on adherence and cost-effectiveness compared with oral bisphosphonates in osteoporosis patients. Ann Pharmacother. 2018;52:1031–41. doi: 10.1177/1060028018768808 [DOI] [PubMed] [Google Scholar]
- 55.Hiligsmann M, Reginster JY, Tosteson ANA, et al. Recommendations for the conduct of economic evaluations in osteoporosis: outcomes of an experts’ consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the US branch of the International Osteoporosis Foundation. Osteoporos Int. 2019;30:45–57. doi: 10.1007/s00198-018-4744-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Albert SG, Reddy S. Clinical evaluation of cost efficacy of drugs for treatment of osteoporosis: a meta-analysis. Endocr Pract. 2017;23: 841–56. doi: 10.4158/EP161678.RA [DOI] [PubMed] [Google Scholar]
- 57.Silverman S, Agodoa I, Kruse M, et al. Denosumab for elderly men with osteoporosis: a cost-effectiveness analysis from the US payer perspective. J Osteoporos. 2015;2015:627631. doi: 10.1155/2015/627631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li N, Cornelissen D, Silverman S, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39:181–209. doi: 10.1007/s40273-020-00965-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McClung MR, Boonen S, Törring O, et al. Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosis. J Bone Miner Res. 2012;27:211–8. doi: 10.1002/jbmr.536 [DOI] [PubMed] [Google Scholar]
- 60.Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417–27. doi: 10.1056/NEJMoa1708322 [DOI] [PubMed] [Google Scholar]
- 61.Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532–43. [DOI] [PubMed] [Google Scholar]
- 62.Cosman F, Crittenden DB, Ferrari S, et al. Romosozumab FRAME study: a post hoc analysis of the role of regional background fracture risk on nonvertebral fracture outcome. J Bone Miner Res. 2018;33: 1407–16. doi: 10.1002/jbmr.3439 [DOI] [PubMed] [Google Scholar]
- 63.Nakamura T, Sugimoto T, Nakano T, et al. Randomized Teriparatide [human parathyroid hormone (PTH) 1–34] Once-Weekly Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab. 2012;97:3097–3106. doi: 10.1210/jc.2011-3479 [DOI] [PubMed] [Google Scholar]
- 64.Fujita T, Fukunaga M, Itabashi A, et al. Once-weekly injection of low-dose teriparatide (28.2 μg) reduced the risk of vertebral fracture in patients with primary osteoporosis. Calcif Tissue Int. 2014;94:170–5. doi: 10.1007/s00223-013-9777-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904 [DOI] [PubMed] [Google Scholar]
- 66.Al-Saleh Y, Al-Daghri NM, Sabico S, et al. Diagnosis and management of osteoporosis in postmenopausal women in Gulf Cooperation Council (GCC) countries: consensus statement of the GCC countries’ osteoporosis societies under the auspices of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Arch Osteoporos. 2020;15:109. doi: 10.1007/s11657-020-00778-5 [DOI] [PubMed] [Google Scholar]
- 67.Sanchez-Rodriguez D, Bergmann P, Body JJ, et al. The Belgian Bone Club 2020 guidelines for the management of osteoporosis in postmenopausal women. Maturitas. 2020;139:69–89. doi: 10.1016/j.maturitas.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 68.Kanis JA, Johansson H, Harvey NC, et al. An assessment of intervention thresholds for very high fracture risk applied to the NOGG guidelines: a report for the National Osteoporosis Guideline Group (NOGG). Osteoporos Int. 2021;32:1951–60. doi: 10.1007/s00198-021-05942-2 [DOI] [PubMed] [Google Scholar]
- 69.McClung MR, Brown JP, Diez-Perez A, et al. Effects of 24 months of treatment with romosozumab followed by 12 months of denosumab or placebo in postmenopausal women with low bone mineral density: a randomized, double-blind, phase 2, parallel group study. J Bone Miner Res. 2018;33:1397–1406. doi: 10.1002/jbmr.3452 [DOI] [PubMed] [Google Scholar]
- 70.Leder BZ, Tsai JN, Uihlein AV, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015;386:1147–55. doi: 10.1016/S0140-6736(15)61120-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.FORTEO (teriparatide injection) for subcutaneous use. Highlights of prescribing information. Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf on 29 November 2022.
- 72.Hagino H, Sugimoto T, Tanaka S, et al. A randomized, controlled trial of once-weekly teriparatide injection versus alendronate in patients at high risk of osteoporotic fracture: primary results of the Japanese Osteoporosis Intervention Trial-05. Osteoporos Int. 2021;32: 2301–11. doi: 10.1007/s00198-021-05996-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller SA, St Onge EL, Whalen KL. Romosozumab: a novel agent in the treatment for postmenopausal osteoporosis. J Pharm Technol. 2021;37:45–52. doi: 10.1177/8755122520967632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hiligsmann M, Williams SA, Fitzpatrick LA, et al. Cost-effectiveness of sequential treatment with abaloparatide vs. teriparatide for United States women at increased risk of fracture. Semin Arthritis Rheum. 2019;49:184–96. doi: 10.1016/j.semarthrit.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 75.Le QA, Hay JW, Becker R, et al. Cost-effectiveness analysis of sequential treatment of abaloparatide followed by alendronate versus teriparatide followed by alendronate in postmenopausal women with osteoporosis in the United States. Ann Pharmacother. 2019;53:134–43. doi: 10.1177/1060028018798034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mori T, Crandall CJ, Ganz DA. Cost-effectiveness of sequential teriparatide/alendronate versus alendronate-alone strategies in high-risk osteoporotic women in the US: analyzing the impact of generic/biosimilar teriparatide. JBMR Plus. 2019;3:e10233. doi: 10.1002/jbm4.10233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reid IR, Horne AM, Mihov B, et al. Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med. 2018;379: 2407–16. doi: 10.1056/NEJMoa1808082 [DOI] [PubMed] [Google Scholar]
- 78.Reid IR, Horne AM, Mihov B, et al. Anti-fracture efficacy of zoledronate in subgroups of osteopenic postmenopausal women: secondary analysis of a randomized controlled trial. J Intern Med. 2019;286:221–9. doi: 10.1111/joim.12901 [DOI] [PubMed] [Google Scholar]
- 79.Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27:243–54. doi: 10.1002/jbmr.1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Black DM, Schwartz AV, Ensrud KE, et al. ; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–38. [DOI] [PubMed] [Google Scholar]
- 81.Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015;30:934–44. doi: 10.1002/jbmr.2442 [DOI] [PubMed] [Google Scholar]
- 82.Reid IR, Billington EO. Drug therapy for osteoporosis in older adults. Lancet. 2022;399:1080–92. doi: 10.1016/S0140-6736(21)02646-5 [DOI] [PubMed] [Google Scholar]
- 83.Lyu H, Yoshida K, Zhao SS, et al. Delayed denosumab injections and fracture risk among patients with osteoporosis: a population-based cohort study. Ann Intern Med. 2020;173:516–26. doi: 10.7326/M20-0882 [DOI] [PubMed] [Google Scholar]
- 84.Lyu H, Zhao SS, Yoshida K, et al. Delayed denosumab injections and bone mineral density response: an electronic health record-based study. J Clin Endocrinol Metab. 2020;105:1435–44. doi: 10.1210/clinem/dgz321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mazziotti G, Betella N, Lania AG. Letter to the editor: “Delayed denosumab injections and bone mineral density response: an electronic health record-based study” [Letter]. J Clin Endocrinol Metab. 2020;105. doi: 10.1210/clinem/dgaa384 [DOI] [PubMed] [Google Scholar]
- 86.Lewiecki EM, Blicharski T, Goemaere S, et al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab. 2018;103: 3183–93. doi: 10.1210/jc.2017-02163 [DOI] [PubMed] [Google Scholar]
- 87.Walker MD, Cusano NE, Sliney J Jr, et al. Combination therapy with risedronate and teriparatide in male osteoporosis. Endocrine. 2013;44:237–46. doi: 10.1007/s12020-012-9819-4 [DOI] [PubMed] [Google Scholar]
- 88.Efficacy and Safety of Zoledronic Acid for the Treatment of Osteoporosis in Men [clinical trial]. Accessed at https://clinicaltrials.gov/show/NCT00097825 on 29 November 2022.
- 89.Novel Combination Therapy for Osteoporosis in Men (Osteo-Men) [clinical trial]. Accessed at https://clinicaltrials.gov/show/NCT03994172 on 29 November 2022.
- 90.A Study to Compare the Safety and Efficacy of Romosozumab (AMG 785) Versus Placebo in Men With Osteoporosis (BRIDGE) [clinical trial]. Accessed at https://clinicaltrials.gov/show/NCT02186171 on 29 November 2022.
- 91.Combination Risedronate - Parathyroid Hormone Trial in Male Osteoporosis (RPM) [clinical trial]. Accessed at https://clinicaltrials.gov/show/NCT01611571 on 29 November 2022.
- 92.Anderson PA, Freedman BA, Brox WT, et al. Osteoporosis: recent recommendations and positions of the American Society for Bone and Mineral Research and the International Society for Clinical Densitometry. J Bone Joint Surg Am. 2021;103:741–7. doi: 10.2106/JBJS.20.01248 [DOI] [PubMed] [Google Scholar]
- 93.International Society for Clinical Densitometry. Adult Official Positions. 2019. Accessed at https://iscd.org/wp-content/uploads/2021/09/2019-Official-Positions-Adult-1.pdf on 2 December 2022.
- 94.Nakamura T, Nakano T, Ito M, et al. ; MOVER Study Group. Clinical efficacy on fracture risk and safety of 0.5 mg or 1 mg/month intravenous ibandronate versus 2.5 mg/day oral risedronate in patients with primary osteoporosis. Calcif Tissue Int. 2013;93:137–46. doi: 10.1007/s00223-013-9734-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


