Key Teaching Points.
-
•
Rare complications from left bundle branch area pacing include both acute and delayed interventricular septal perforation.
-
•
Septal perforation can manifest as changes in the paced QRS morphology, changes in pacing parameters (threshold, sending, pacing impedance), loss of capture, and ventricular arrhythmias.
-
•
Prompt diagnosis and management of rare complications from left bundle branch area pacing are important to mitigate the risks.
Introduction
Right ventricular pacing with a dual-chamber transvenous pacemaker has historically been used in patients with preserved left ventricular systolic function and atrioventricular block. Pacing from the right ventricle is associated with increased risks of developing arrhythmias and heart failure owing to ventricular dyssynchrony.1 In patients with a reduced left ventricular ejection fraction, cardiac resynchronization therapy (CRT) reduces ventricular dyssynchrony and improves clinical outcomes.2 Given the potential negative impact of right ventricular pacing,2 strategies were developed to directly stimulate the cardiac conduction system. His bundle pacing (HBP) showed promise, as this form of conduction system pacing yielded comparable results to CRT with respect to synchronous ventricular activation and outcomes.3 Limitations of this technique included rising pacing thresholds necessary for His bundle capture during follow-up, poor R-wave amplitudes, and an inability to narrow the paced QRS duration in some patients.3
Left bundle branch area pacing (LBBAP) is an alternative form of conduction system pacing.4 When compared to HBP, LBBAP yields lower pacing thresholds and higher R-wave amplitudes.5 In comparison to CRT, small initial trials of both LBBAP and HBP have demonstrated improved clinical outcomes, including improvements in New York Heart Association class and left ventricular ejection fraction in patients with heart failure with a reduced ejection fraction and left bundle branch block who are referred for CRT.5 LBBAP, which can be performed with both lumenless and stylet-driven leads, involves delivering the lead into the interventricular septum to selectively capture the left bundle and/or fascicular system.6 Complications from this technique include loss of capture (0.3%–11.5%); lead dislodgement (0.3%–10.5%); acute septal perforation, defined as a perforation that occurs during the implant procedure (0%–14.1%); and “delayed” septal perforation, which is defined as a perforation that occurs following the implant procedure (0.08%–0.33%).6,7 Both acute and delayed septal perforation are complications that are specific to LBBAP. Septal perforation, which can be appreciated at the time of implant or during follow-up, is manifested by changes in electrical parameters, including a change in the magnitude and polarity of the current of injury, an increase in the pacing threshold, and a decrease in impedance.8,9 We present a case involving a patient who underwent LBBAP and had a delayed septal lead perforation that was manifested by ventricular tachycardia (VT).
Case report
A 77-year-old man with diastolic heart failure, aortic stenosis with a prior transcatheter valve replacement, chronic kidney disease, obstructive sleep apnea, diabetes mellitus, hypertension, and hyperlipidemia presented for a dual-chamber pacemaker implantation owing to symptomatic Mobitz I and 2:1 atrioventricular block. A dual-chamber permanent pacemaker was implanted using the axillary approach. A 58 cm 2088TC TendrilTM STS lead (Abbott, Abbott Park, IL) was advanced through a number 2 Site Selective Pacing Catheter sheath (Boston Scientific, Marlborough, MA). After the active fixation helix was deployed, serial clockwise lead body rotations were performed. As the lead was advanced into the septum there was a gradual increase in the unipolar pacing impedance. No large decrements in unipolar impedance were noted during lead deployment. Similarly, there was no significant diminution in the R-wave amplitude or abrupt change in the current of injury as the lead was being advanced into the septum. Successful LBBAP with distal left posterior fascicular capture was achieved with a bipolar paced QRS duration of 148 ms and a qR pattern in lead V1 (Figure 1). The R waves were 9.8 mV, the bipolar pacing threshold was 0.5 V at 0.4 ms, and the lead impedance was 836 ohms. Changes in the paced QRS morphology from nonselective LBBAP to septal capture were noted during threshold testing.10 A CapSureFix Novus MRI SureScan 5076 (Medtronic Inc, Minneapolis, MN) lead was implanted in the right atrial appendage. These leads were connected to an Azure XT DR MRI SureScan W1DR01 (Medtronic Inc) dual-chamber pacemaker generator.
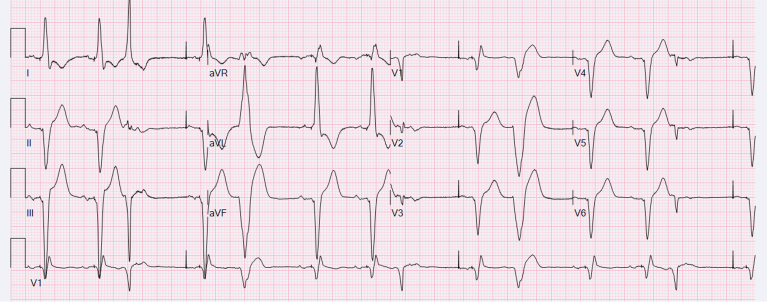
Figure 1.
Twelve-lead electrocardiograms demonstrating left bundle branch area pacing. Twelve-lead electrocardiograms with bipolar pacing at 25 mm/s (left) and with both bipolar and unipolar pacing at 100 mm/s (right). Paced QRS duration (QRSd) 148 ms (bipolar), QRS morphology with a qR pattern in lead V1, and a left axis deviation consistent with distal left posterior fascicular capture.
On the first night following the implant the patient developed sustained VT at a rate of 200 beats per minute on telemetry. A 12-lead electrocardiogram revealed that the VT had a left bundle branch block morphology (qS in lead V1), left superior axis, with a V2 transition (Figure 2). The patient was asymptomatic and the VT was hemodynamically tolerated. The tachycardia was terminated with intravenous lidocaine. The patient was subsequently transferred to the cardiac intensive care unit, where a transthoracic echocardiogram demonstrated evidence that the LBBAP lead had perforated through the interventricular septum and was mobile in the left ventricular cavity (Figure 3). A thrombus was not noted on the lead or septum. A repeat interrogation demonstrated diminution in the R-wave amplitude to 0.9 mV with a bipolar pacing threshold of 0.63 V at 0.4 ms and a lead impedance of 608 ohms. On telemetry, the patient was noted to have premature ventricular contractions (PVCs). A repeat electrocardiogram, performed after the lead had perforated, demonstrated intermittent left ventricular septal capture, which was likely due to anodal capture. Given that the PVCs were not present prior to the septal perforation, it is possible that PVCs may have been due to mechanical stimulation of the left ventricular myocardium (Supplemental Figure 1). PVCs and episodes of nonsustained VT continued to occur until the patient underwent a lead revision the following morning, at which point the ventricular ectopy resolved (Supplemental Figure 2).
Figure 2.
Twelve-lead electrocardiogram of postoperative ventricular tachycardia. Ventricular tachycardia with a left bundle branch block morphology, left superior axis, V2 transition, and a ventricular rate of 200 beats per minute.
Figure 3.
A modified parasternal short-axis view from a transthoracic echocardiogram demonstrating septal perforation of the left bundle branch area pacing lead. LV = left ventricle; RV = right ventricle.
Discussion
With updated guideline recommendations11 supporting the use of LBBAP, it will become increasingly important for providers to be able to promptly recognize postoperative complications that are unique to this form of conduction system pacing. Reports of delayed septal perforation are rare12,13; however, as our case illustrates, this complication can result in significant consequences. In addition to changes in the paced QRS morphology that may result from septal perforation, and the potential risk in pacemaker-dependent patients of the loss of myocardial capture, previous reports of delayed septal perforation have described left ventricular free wall perforation, damage to the lung parenchyma, and laceration of an intercostal artery with hemodynamic compromise.12,13 In our case, it is likely that the delayed septal perforation resulted in the lead’s causing direct mechanical stimulation of the basal inferoseptum. This mechanical stimulation may have induced VT in a fashion similar to mechanical VT induction that can occur owing to catheter ectopy during mapping of the left ventricular endocardium at the time of a catheter ablation procedure. Timely evaluation with a transthoracic echocardiogram allowed for a prompt diagnosis of the perforation. In this instance diagnosing the lead perforation, and the subsequent lead revision, avoided potentially unnecessary treatment strategies for VT, which would not likely have addressed the underlying cause of the arrhythmia.
Delayed septal perforation of LBBAP leads has been previously reported with both the lumenless lead (SelectSecure 3830, Medtronic Inc) and stylet-driven leads including a CapSureFix Novus MRI SureScan 5076.8,9,12, 13, 14 Rates of delayed septal perforation in larger studies range from 0.08% in the 2533 patients in the European MELOS study14 to 0.15% of the 632 patients in the study by Su and colleagues8 and 0.33% of the 612 patients in the report by Chen and colleagues.10 This suggests that the risk of delayed septal perforation may be independent of lead diameter and the composition of outer lead insulation (1.9 mm, polyurethane and silicone copolymer in the case of the 2088TC lead; 2.0 mm, silicone in the case of the CapSureFix Novus MRI SureScan 5076 lead; and 1.4 mm, polyurethane in the case of the SelectSecure 3830 lead). Excess lead slack has been demonstrated to contribute to lead conductor fractures by increasing forces along the lead.15 It is currently unknown if excess lead slack, by the same mechanism, increases the risk of septal perforation when performing LBBAP. Further investigation is needed to determine if any differences exist in the rates of delayed septal perforation between different lead types, as this rare complication is likely due to continued advancement of the lead through the interventricular septum, which may be because of retention of torque that is applied during the implant procedure. The degree of residual torque and subsequent forward migration during the postoperative period may be impacted by factors such as lead diameter, lead composition, number of lead body rotations, and other procedural techniques.
Conclusion
Postoperative complications after LBBAP will likely become better characterized as LBBAP continues to become more widely incorporated into clinical practice. It is critically important that providers be able to recognize and treat rare complications that can arise owing to LBBAP.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
Conflicts of interest for all authors: none.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2023.12.004.
Appendix. Supplementary Data
Supplemental Figure 1.
Premature ventricular contractions
Legend: Electrocardiogram performed following septal perforation with ventricular paced QRS complexes demonstrating a qR morphology in lead V1. Multiple premature ventricular contractions are noted with variable QRS durations. These premature ventricular contractions were not present prior to the septal perforation.
Supplemental Figure 2.
Lead positions
Legend: A comparison of the original lead position of the 2088TC TendrilTM STS lead (left) from the chest xray that was performed immediately following the initial implant procedure with an anteroposterior fluoroscopic image of the final lead position of the SelectSecure 3830 following the lead revision (right).
References
- 1.Kiehl E.L., Makki T., Kumar R., et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016;13:2272–2278. doi: 10.1016/j.hrthm.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 2.Curtis A.B., Worley S.J., Adamson P.B., et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585–1593. doi: 10.1056/NEJMoa1210356. [DOI] [PubMed] [Google Scholar]
- 3.Upadhyay G.A., Vijayaraman P., Nayak H.M., et al. On-treatment comparison between corrective His bundle pacing and biventricular pacing for cardiac resynchronization: a secondary analysis of the His-SYNC Pilot Trial. Heart Rhythm. 2019;16:1797–1807. doi: 10.1016/j.hrthm.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Huang W., Su L., Wu S., et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol. 2017;33:1736 e1–1736 e3. doi: 10.1016/j.cjca.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Wu S., Su L., Vijayaraman P., et al. Left bundle branch pacing for cardiac resynchronization therapy: nonrandomized on-treatment comparison with His bundle pacing and biventricular pacing. Can J Cardiol. 2021;37:319–328. doi: 10.1016/j.cjca.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Cano O., Vijayaraman P. Left bundle branch area pacing: implant technique, definitions, outcomes, and complications. Curr Cardiol Rep. 2021;23:155. doi: 10.1007/s11886-021-01585-1. [DOI] [PubMed] [Google Scholar]
- 7.Burri H., Jastrzebski M., Cano O., et al. EHRA clinical consensus statement on conduction system pacing implantation: endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS) Europace. 2023;25:1208–1236. doi: 10.1093/europace/euad043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su L., Wang S., Wu S., et al. Long-term safety and feasibility of left bundle branch pacing in a large single-center study. Circ Arrhythm Electrophysiol. 2021;14 doi: 10.1161/CIRCEP.120.009261. [DOI] [PubMed] [Google Scholar]
- 9.Chen X., Jin Q., Bai J., et al. The feasibility and safety of left bundle branch pacing vs. right ventricular pacing after mid-long-term follow-up: a single-centre experience. Europace. 2020;22(Suppl_2):ii36–ii44. doi: 10.1093/europace/euaa294. [DOI] [PubMed] [Google Scholar]
- 10.Chen X., Wei L., Bai J., et al. Procedure-related complications of left bundle branch pacing: a single-center experience. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.645947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung M.K., Patton K.K., Lau C.P., et al. 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure. Heart Rhythm. 2023;20:e17–e91. doi: 10.1016/j.hrthm.2023.03.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravi V., Larsen T., Ooms S., Trohman R., Sharma P.S. Late-onset interventricular septal perforation from left bundle branch pacing. HeartRhythm Case Rep. 2020;6:627–631. doi: 10.1016/j.hrcr.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez M., Puerta C., Thareja N., Zhang Y., Pollema T., Thistlethwaite P. Surgical management of left bundle branch pacing lead causing septal and left ventricular perforation. HeartRhythm Case Rep. 2023;9:520–523. doi: 10.1016/j.hrcr.2023.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jastrzebski M., Kielbasa G., Cano O., et al. Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J. 2022;43:4161–4173. doi: 10.1093/eurheartj/ehac445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krahn A.D., Morissette J., Lahm R., et al. Radiographic predictors of lead conductor fracture. Circ Arrhythm Electrophysiol. 2014;7:1070–1077. doi: 10.1161/CIRCEP.114.001612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.