Abstract
Background
Only small randomized trials have investigated the efficacy and safety of direct oral anticoagulants (DOACs) compared with vitamin K antagonists (VKAs) in patients with non–valvular atrial fibrillation (NVAF) and end-stage kidney disease.
Objectives
To perform a systematic review and meta-analysis comparing anticoagulation with DOACs to VKAs in patients with NVAF undergoing chronic hemodialysis.
Methods
A systematic search using Medline, Web of Science, and Embase was performed. All randomized trials comparing DOACs with VKAs in patients with NVAF undergoing chronic hemodialysis were included. As primary endpoint, we analyzed all-cause mortality. As secondary endpoints, we investigated total bleeding events, life-threatening or major bleeding events, and thromboembolic events or stroke. We used the odds ratio as outcome measure and fitted a random-effects model due to the expected heterogeneity.
Results
Three studies fulfilled the inclusion criteria comprising 383 patients (218 on apixaban or rivaroxaban, 165 on VKA). No significant difference between DOACs and VKAs regarding death (OR, 0.94; 95% CI, 0.55-1.63; p = .84), total bleedings (OR, 0.99; 95% CI, 0.63-1.54; p = .96) and life-threatening or major bleeding (OR, 0.65; 95% CI, 0.32-1.33, p = .24) was detected. There was a trend toward a reduction of thromboembolic events or stroke in patients receiving DOACs (OR, 0.39; 95% CI, 0.14-1.05; p = .06).
Conclusion
Orally administered activated factor X inhibitors carried a similar risk of bleeding and death when compared with VKAs in patients with NVAF undergoing chronic hemodialysis. Moreover, there was a trend towards a reduction of thromboembolic events or stroke in patients receiving DOACs.
Keywords: anticoagulation, atrial fibrillation, end-stage kidney disease, hemodialysis, meta-analysis
Essentials
-
•
The clinical profile of direct oral anticoagulants (DOACs) in hemodialysis patients is unknown.
-
•
We performed a meta-analysis to determine the clinical profile of DOACs in hemodialysis patients.
-
•
DOACs carried a similar risk of bleeding and death compared with Vitamin K antagonists.
-
•
There was a trend toward a reduction of stroke in patients receiving DOACs.
1. Introduction
Direct oral anticoagulants (DOACs) are predominantly prescribed in patients with non–valvular-atrial fibrillation (NVAF) at an increased risk of stroke or systemic embolization and in patients with venous thromboembolism (VTE) to prevent thromboembolic events [1,2]. Due to their high efficacy, a more predictable mode of action at fixed dosages, and a better safety profile with less intracranial bleedings, DOACs have replaced vitamin K antagonists (VKAs) as preferred oral anticoagulants in most patients with the above-mentioned indications [3,4]. However, all phase III pivotal trials comparing DOACs to VKAs in patients with NVAF and VTE excluded patients with advanced chronic kidney disease (CKD) and a glomerular filtration rate (GFR) <25 mL/min/1.73 m2 [[5], [6], [7], [8], [9], [10], [11], [12], [13]]. Accordingly, data concerning DOACs in patients with severe CKD are scarce.
However, official licensing differs between Europe and the United States (US): in Europe, the direct thrombin inhibitor dabigatran with its high renal excretion rate is contraindicated in patients with a GFR <30 mL/min/1.73 m2, while activated factor X (FXa) inhibitors should not be prescribed in patients with a GFR <15 mL/min/1.73 m2 and in those with end-stage kidney disease (ESKD) requiring hemodialysis [3,14]. Conversely, in the US, apixaban and rivaroxaban are also approved for use in patients with ESKD, and dabigatran is available at a reduced dose of 75 mg for patients with a GFR of 15 to 30 mL/min/1.73 m2 [[15], [16], [17]].
Since the risk of thromboembolic events in atrial fibrillation increases with decreasing GFR and ESKD itself carries a particularly pronounced bleeding risk as well as the possibility of cumulative drug effects, the optimal choice of oral anticoagulation is of utmost importance in these patients [[18], [19], [20]]. Randomized data on DOACs in patients with NVAF undergoing hemodialysis have been lacking until recently. Nevertheless, many patients with NVAF undergoing hemodialysis already received DOACs as an alternative to VKA due to an anticipated superior safety profile [21]. Although some observational data demonstrated a reduction in the number of bleeding events in patients with ESKD receiving DOACs, particularly for apixaban, this approach was not supported by randomized trials [21].
Recently, several randomized clinical trials investigated the use of the FXa inhibitors apixaban and rivaroxaban versus VKAs at an international normalized ratio (INR) target of 2 to 3 in patients with ESKD undergoing chronic hemodialysis with NVAF [19,22,23]. However, these trials only included small patient numbers and were underpowered to draw any definite conclusions. The RENAL-AF (Apixaban for Patients with Atrial Fibrillation on Hemodialysis: A Multicenter Randomized Controlled Trial) study included 154 hemodialysis patients randomly assigned to receive either 5 mg of apixaban twice daily (2.5 mg twice daily for patients ≥80 years of age, weight ≤60 kg, or both) or dose-adjusted warfarin [19]. The AXADIA-AFNET 8 (A Randomized Controlled Trial Comparing Apixaban With the Vitamin K Antagonist Phenprocoumon in Patients on Chronic Hemodialysis) study comprised 97 patients with ESKD and NVAF, who were randomly assigned to receive either 2.5 mg of apixaban twice daily or phenprocoumon [23]. The VALKYRIE (Safety and Efficacy of VKAs versus Rivaroxaban in Hemodialysis Patients with Atrial Fibrillation: A Multicenter Randomized Controlled Trial) study found that a reduced dose of rivaroxaban significantly decreased the primary composite endpoint of fatal and nonfatal cardiovascular events as well as the composite of life-threatening and major bleeding complications compared with VKAs in 132 hemodialysis patients [22].
In order to allow a better estimation of the benefit-risk ratio, we decided to perform a systematic review and meta-analysis of trials comparing DOACs to VKAs in patients with NVAF undergoing chronic hemodialysis.
2. Methods
2.1. Reference search and study selection
For this analysis, we followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement. The meta-analysis was registered in PROSPERO (ID: CRD42023444989). We performed a systematic search on Medline, Web of Science, and Embase until July 2, 2023, using the following terms and keywords: atrial fibrillation AND (apixaban OR edoxaban OR dabigatran OR rivaroxaban) AND (hemodialysis OR hemodialysis) AND (vitamin K antagonist OR vitamin K antagonist OR warfarin OR phenprocoumon). All randomized trials comparing DOACs with VKAs in patients with NVAF undergoing chronic hemodialysis were included. We used the revised Cochrane Collaboration’s tool to assess the bias risk in the eligible trials [24].
Only studies published in English and performed on human subjects were included. Two independent investigators evaluated the studies by screening all retrieved titles and abstracts, and all full-text articles were checked for other relevant studies and trials. Data extraction was performed via a predefined data recording form: author data, year of publication, number of included patients, data on study medication, endpoints, and patient characteristics.
2.2. Endpoints
As the primary endpoint, we analyzed all-cause mortality. As secondary endpoints, we investigated total bleeding events, major or life-threatening bleeding events, and thromboembolic events or stroke. In VALKYRIE, bleeding events were stratified into life-threatening bleedings (fatal bleedings; symptomatic intracranial bleedings; a decrease in hemoglobin ≥5g/dL; need for transfusion of ≥4 blood units, inotropic agents, or surgery), major bleedings (a decrease in hemoglobin ≥2 g/dL; need for transfusion of ≥2 blood units and not fulfilling the criteria for life-threatening bleeding), and minor bleedings, defined as all other bleedings [22]. In RENAL-AF, bleeding events were major bleedings (a decrease in hemoglobin ≥2 g/dL; need for transfusion of ≥2 blood units; bleeding within a critical site), or clinically relevant nonmajor bleedings (bleeding resulting in hospital admission, physician-guided medical or surgical treatment or change in antithrombotic therapy) [19]. In AXADIA-AFNET, 8 bleeding events were characterized as major bleeding events (fatal bleeding; a decrease in hemoglobin ≥2 g/dL; need for transfusion of ≥2 blood units; bleeding within a critical site) or clinically relevant nonmajor bleedings (bleeding resulting in hospital admission, physician-guided medical or surgical treatment, or change in antithrombotic therapy) [23].
2.3. Statistical analysis
The odds ratio was used as the outcome measure, and a random-effects model was applied to the data as heterogeneity was expected. We used the DerSimonian-Laird estimator (ie, Tau2) to assess heterogeneity and report the Q-test for heterogeneity (Chi2) and the I2 statistic. We used the studentized residuals and Cook’s distances to check for outliers and/or influential data in the context of the model. Outliers were defined as a studentized residual larger than the 100×(1-0.05/(2×k))th percentile of a standard normal distribution. Influential studies were defined as a Cook’s distance larger than the median plus 6 times the interquartile range of the Cook’s distances. No indication of outliers was detected for any model by using studentized residuals. Publication bias was not assessed based on the low number (<10) of included studies. For our analyses, we used R (version 4.2.2) (R Core Team, 2020) and the “metafor” package (version 4.0.0) [25].
Results
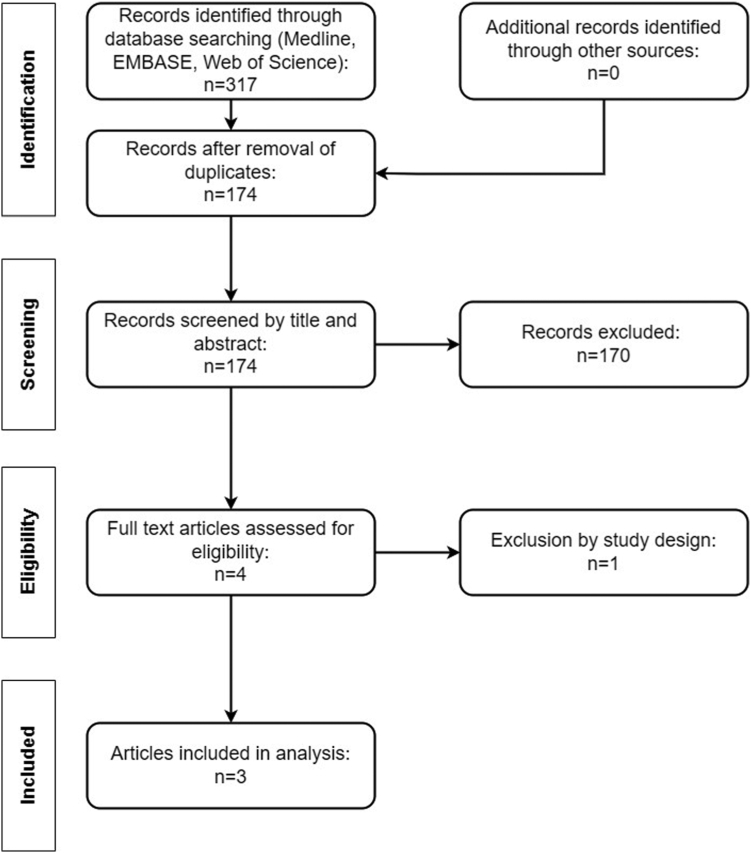
Out of 317 articles, 3 reports fulfilled the inclusion criteria, including 383 patients (218 on DOACs, 165 on VKAs) (Figure 1) [19,22,23]. The preceding publication of the VALKYRIE trial investigating the effect of rivaroxaban on vascular calcification was excluded [26]. Study characteristics are provided in Tables 1, 2, and 3. All studies were considered moderate to high quality (Table 4) [19,22,23].
Figure 1.
Flow diagram.
Table 1.
Characteristics of included trials.
| Characteristics | RENAL-AF [19] | AXADIA AFNET 8 [23] | VALKYRIE [22] |
|---|---|---|---|
| Study design | PROBE | PROBE | PROBE |
| Intervention | Apixaban | Apixaban | Rivaroxaban (±Vitamin K2)a |
| Dose (mg) | 5 or 2.5 mg | 2.5 | 10 |
| Frequency | Twice daily | Twice daily | Once daily |
| Control | Warfarin | Phenprocoumon | VKA (not specified) |
| Target INR | 2.0-3.0 | 2.0-3.0 | 2.0-3.0 |
| Number of patients (intervention) | 82 | 48 | 88 |
| Number of patients (control) | 72 | 49 | 44 |
| Dose reduction criteria | 2.5 mg twice daily for patients ≥80 years of age, weight ≤60 kg, or both | None | None |
| Primary safety endpoint | Major or clinically relevant nonmajor bleeding | Major bleeding, clinically relevant nonmajor bleeding, or all-cause death | Not specified |
| Primary efficacy endpoint | Not specified | Ischemic stroke, all-cause death, myocardial infarction, deep vein thrombosis or pulmonary embolism | Fatal cardiovascular disease and nonfatal stroke, cardiac events, and other vascular events |
| Bleeding definitions | major bleedings: decrease in hemoglobin ≥2 g/dL; need for transfusion of ≥2 blood units; bleeding within a critical site clinically relevant nonmajor bleedings: bleeding resulting in hospital admission, physician-guided medical or surgical treatment or change in antithrombotic therapy |
major bleedings: fatal bleeding; decrease in hemoglobin ≥2 g/dL; need for transfusion of ≥2 blood units; bleeding within a critical site clinically relevant nonmajor bleedings: bleeding resulting in hospital admission, physician-guided medical or surgical treatment or change in antithrombotic therapy |
life-threatening bleedings: fatal bleedings; symptomatic intracranial bleedings; decrease in hemoglobin ≥5 g/dL; need for transfusion of ≥4 blood units, inotropic agents, or surgery major bleedings: decrease in hemoglobin ≥2 g/dL; need for transfusion of ≥2 blood units and not fulfilling the criteria for life-threatening bleeding minor bleedings: all other bleedings |
| Statistical analysis | Cox proportional hazards regression model | Cox proportional hazards regression model | Fine-Gray method (competing risk framework) |
| Follow-up duration, (y) | Intervention group: 0.90 Control group: 0.93 |
Intervention group: 1.17 (0.47-1.92) Control group: 1.38 (0.79-1.92) |
1.88 (1.01-3.38) |
INR, international normalized ratio; PROBE, prospective, randomized, open-label, blinded-outcome evaluation; VKA, vitamin K antagonist.
The VALKYRIE study included 3 treatment arms (VKA, rivaroxaban, and rivaroxaban plus vitamin K2) with a 1:1:1 allocation ratio.
Table 2.
Characteristics of included patients.
| Characteristics | RENAL-AF [19] | AXADIA AFNET 8 [23] | VALKYRIE [22] |
|---|---|---|---|
| Age, (y), (median) | 68 (61-75) | 77 (69-80) | VKA: 80.3 (71.5-84.3) Rivaroxaban: 79.9 (74.4-83.9) Rivaroxaban + Vitamin K2: 79.6 (73.2-83.1) |
| Male sex, No. (%) | 98 (63.6) | 68 (70.1) | 88 (66.7) |
| CHA2DS2-VASc, median (IQR) | 4 (3-5) | 5 (4-6) | 5a |
| HAS-BLED, median (IQR) | NA | 4 (3.5-5) | 5a |
| TTR | 44% | 50.7% | 48% |
| Aspirin, No. (%) | 61 (40.9) | 33 (34.0) | 46 (34.8) |
| Permanent atrial fibrillation, No. (%) | 25 (16.2) | NA | 72 (54.5)= |
| Time on dialysis, median (IQR) | 3.0 (1.5-5.7) y | 962 (363-2147) d | 2.4 ya |
| Black patients, No. (%) | 69 (44.8) | NA | NA |
IQR, interquartile range; NA, not available; No., number; TTR, time in therapeutic range; VKA, vitamin K antagonist.
Interquartile range not available for the overall cohort.
Table 3.
Summary of the endpoints stratified for each trial.
| Endpoints | RENAL-AF [19] |
AXADIA AFNET 8 [23] |
VALKYRIE [22] |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention n = 82 | Control n = 72 | ARR | Intervention n = 48 | Control n = 49 | ARR | Intervention n = 88 | Control n = 44 | ARR | |
| All-cause death, n (%) | 21 (25.6) | 13 (18.1) | 7.5 | 9 (18.7) | 12 (24.5) | -5.7 | 57 (64.7) | 32 (72.7) | -8.0 |
| Total bleedings, n (%) | 21 (25.6) | 16 (22.2) | 3.4 | 15 (31.2) | 15 (30.6) | 0.6 | 43 (48.8) | 24 (54.5) | -5.6 |
| Lift-threatening or major bleedings, n (%) | 9 (10.9) | 7 (9.7) | 1.2 | 5 (10.4) | 6 (12.2) | -1.8 | 17 (19.3) | 17 (38.6) | -19.3 |
| Thromboembolic events or stroke, n (%) | 1 (1.2) | 2 (2.7) | -1.5 | 0 (0) | 1 (2.0) | 2.0 | 6 (6.8) | 7 (15.9) | -9.1 |
ARR, absolute risk reduction.
Table 3 presents potential bias of all in the present meta-analysis included trials as judged by the authors and in accordance with the revised Cochrane’s Collaboration risk of bias assessment tool.
Table 4.
Quality of studies according to the authors’ judgment.
All 3 trials included patients with ESKD on chronic hemodialysis with an indication for long-term anticoagulation due to NVAF. The VALKYRIE trial, including 132 patients, was a three-arm, parallel-group trial, and patients were randomly assigned to receive either warfarin or rivaroxaban (10 mg daily) with or without vitamin K2 for a median treatment duration of 1.88 years [27]. For our analyses, we pooled the patient groups receiving rivaroxaban. RENAL-AF included 154 patients, who were randomly assigned to receive either warfarin or apixaban (5-mg dose twice daily; 2.5-mg dose twice daily for patients ≥80 years or weighing ≤60 kg, or both) for up to 15 months [19]. The AXADIA-AFNET 8 trial enrolled 97 patients and allocated them to either VKA or apixaban (2.5-mg dose twice daily for all patients) with a median duration of 355.5 days [23]. Reported time in therapeutic range (TTR) for VKAs was relatively low in all 3 trials, ranging from 44% to 50.7%.
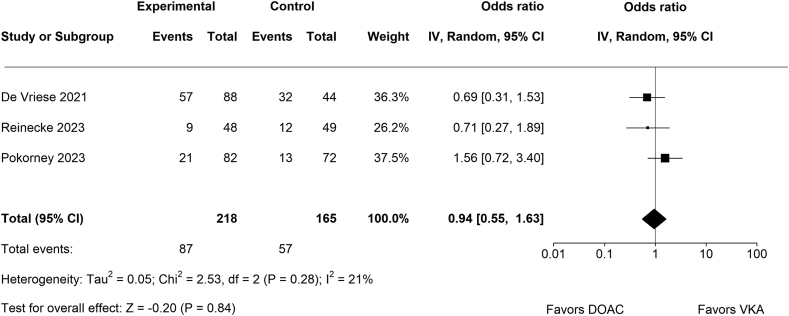
3.1. All-cause mortality
No significant difference between DOACs (n = 87/218) and VKAs (n = 57/165) regarding all-cause death (OR, 0.94; 95% CI, 0.55-1.63; p = .84; Figure 2) was detected. There was no significant amount of heterogeneity in the true outcomes (Chi2 = 2.53, p = .28, Tau2 = 0.05, I2 = 21%). None of the studies could be considered as significantly influential.
Figure 2.
Forest plot for all-cause death.
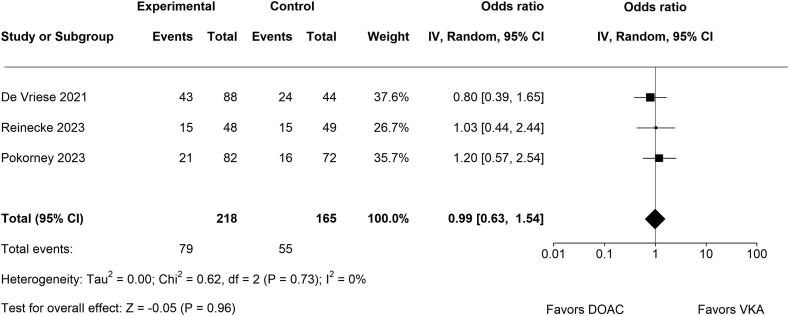
3.2. Total bleeding events
No significant difference between DOACs (n = 79/218) and VKAs (n = 55/165) regarding total bleeding events (OR, 0.99; 95% CI, 0.63-1.54; p = .96; Figure 3) was detected. There was no significant amount of heterogeneity (Chi2 = 0.62, p = .73, Tau2 = 0.00, I2 = 0%). None of the studies could be considered as significantly influential.
Figure 3.
Forest plot for total reported bleeding events.
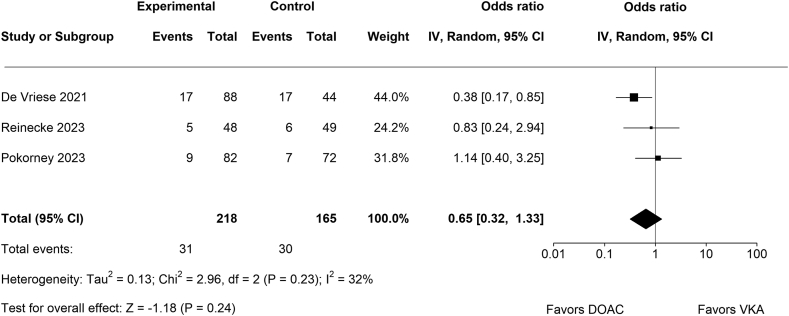
3.3. Life-threatening or major bleeding events
No significant difference between DOACs (n = 31/218) and VKAs (n = 30/165) regarding life-threatening or major bleeding events (OR, 0.65; 95% CI, 0.32-1.33; p = .24; Figure 4) was detected. There was no significant amount of heterogeneity (Chi2 = 2.96, p = .23, Tau2 = 0.13, I2 = 32%). According to the Cook’s distances, none of the studies could be considered as significantly influential.
Figure 4.
Forest plot for life-threatening or major bleeding events.
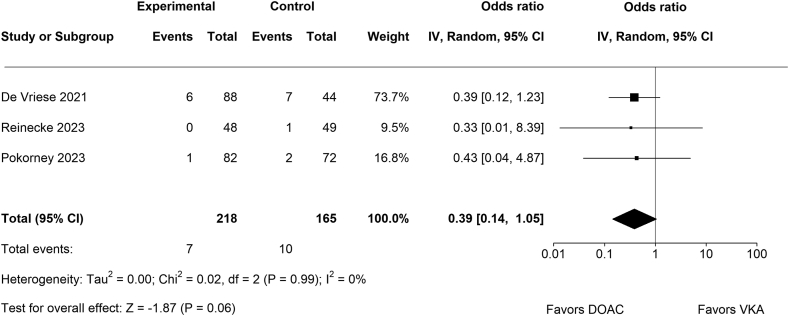
3.4. Thromboembolic events or stroke
There was no significant difference between DOACs (n = 7/218) and VKAs (n = 10/165) regarding the composite of thromboembolic events and stroke (OR, 0.39; 95% CI, 0.14-1.05; p = 0.06; Figure 5). Of note, there was no systemic embolism in all 3 trials, and the presented data are based on rates of ischemic stroke alone. There was no significant amount of heterogeneity (Chi2 = 0.02, p = .99, Tau2 = 0.00, I2 = 0%). None of the studies could be considered as overly influential.
Figure 5.
Forest plot for thromboembolic events or stroke.
4. Discussion
Our meta-analysis systematically compared DOACs to VKAs for oral anticoagulation in patients with NVAF undergoing chronic hemodialysis. In summary, we did not detect a significant difference between patients treated with FXa inhibitors and VKAs regarding death and bleeding. However, there was a trend toward a reduction in stroke rates in patients receiving DOACs.
Chronic kidney disease is an independent risk factor for the development of NVAF [28]. Conversely, NVAF is also a major risk factor for the development of ESKD [29]. These associations underpin the bidirectional relationship between NVAF and CKD as a result of a pro-inflammatory state, the activation of the neurohumoral system, and altered fluid and electrolyte status [30]. Although patients with NVAF undergoing chronic hemodialysis are at high risk of thromboembolic events, this patient group is also at increased risk of bleeding complications and mortality, potentially abating the net clinical benefit of treatment with oral anticoagulation [[30], [31], [32]]. Several observational studies and meta-analyses have questioned the efficacy of oral anticoagulation for the prevention of ischemic stroke and mortality in ESKD, while others have demonstrated some benefit [[33], [34], [35], [36]]. Therefore, the role of oral anticoagulation in patients undergoing chronic hemodialysis remains controversial [30]. The observed differences compared to patients with NVAF and without hemodialysis in whom oral anticoagulation has been proven beneficial may be due to various reasons: patients receive heparin 3 times a week during hemodialysis, potentially mitigating the need for additional oral anticoagulation, especially in the light of high mortality and bleeding rates within this patient population. Also, hemodialysis patients on VKAs commonly have relatively low TTR, a fact that has been confirmed in the 3 current randomized trials comparing DOACs with VKAs [19,22,23]. However, a high TTR is imperative for adequate stroke prevention, as in the general NVAF population, a TTR above 70% has been associated with a relevant reduction in adverse ischemic outcomes and lower bleeding rates [34]. Furthermore, VKAs have been associated with accelerated vascular calcification in patients undergoing hemodialysis, potentially resulting in calciphylaxis, an often life-threatening condition [34].
Nevertheless, if oral anticoagulation is intended, VKAs represent the standard of care in patients with NVAF undergoing chronic hemodialysis [3]. This is mainly due to the lack of data on DOACs in patients with ESKD and the fear of cumulative drug effects potentially resulting in an increased bleeding hazard. Nonetheless, DOACs are already in use in this patient setting, and numerous observational reports have been published with varying efficacy and safety [21,37]. These circumstances underline the importance of randomized controlled trials on oral anticoagulation in patients with NVAF undergoing hemodialysis. Recently, 3 reports have been published on this subject (VALKYRIE, RENAL-AF, and AXADIA-AFNET 8). Although addressing an imperative need for more data, these trials were underpowered for their primary outcome measure (details for each trial are depicted in Tables 1, 2, and 3) [19,22,23]. In our meta-analysis, we could not detect significant differences regarding mortality and bleeding events between VKAs and DOACs. However, we observed a trend toward a benefit for reducing stroke or thromboembolic events in patients treated with DOACs.
Recently, 2 other meta-analyses using the same 3 trials for their calculations have been published [38,39]. Nonetheless, there are significant differences between these analyses and our report: first, Mapili et al. [38] did not report on all-cause mortality but only on cardiovascular mortality. Although cardiovascular mortality constitutes a major proportion of death rates, it neglects other causes of death (ie, bleeding events) potentially associated with anticoagulation therapy. In a population with high overall risk, such as patients undergoing hemodialysis, this poses a major limitation. Second, both meta-analyses failed to include all patients receiving rivaroxaban included in the VALKYRIE trial. VALKYRIE investigated the impact of substituting VKA with rivaroxaban with or without vitamin K2 on vascular calcification in patients with NVAF undergoing hemodialysis. Therefore, the study was designed as a three-arm parallel-group trial with a 1:1:1 allocation ratio (VKA vs rivaroxaban alone vs rivaroxaban and vitamin K2 substitution; Table 1) [26]. Although the study failed to demonstrate the benefit of VKA omission and vitamin K2 substitution on the progression of vascular calcification, the authors identified a potential benefit regarding bleeding complications in patients receiving rivaroxaban. Therefore, follow-up was extended, focusing on the impact of rivaroxaban on bleeding and thromboembolic events. Also, the secondary analysis of VALKYRIE reported the outcomes for all 3 study arms separately. Mapili et al. [38] and Faisaluddin et al. [39] only used the rivaroxaban monotherapy arm (n = 46) for their analyses and excluded the rivaroxaban plus vitamin K2 arm (n = 42). However, vitamin K2 supplementation has no known effect on FXa inhibitors. Therefore, we pooled both rivaroxaban treatment arms for our analyses to increase patient numbers and statistical power further [22]. Finally, Faisaluddin et al. [39] did not include all data on ischemic stroke (AXADIA AFNET 8 was not included in their analysis for stroke).
Interestingly, while mortality and bleeding rates were high in all 3 trials, the rates of stroke were low. Moreover, no systemic embolism was reported in all 3 studies [19,22,23]. Considering the competing risk of mortality and bleeding complications, it remains to be established if the administration of any oral anticoagulation in patients undergoing hemodialysis translates into a net clinical benefit. Unfortunately, current risk scores do not provide sufficient guidance for this vulnerable group of patients, and individualized decision-making remains paramount [40,41]. Additionally, concomitant aspirin was frequently administered in addition to oral anticoagulation in all 3 trials. In an observational trial including patients on hemodialysis, simultaneous use of aspirin abolished the benefits seen with apixaban when compared to VKAs [42].
It needs to be emphasized that all available prospective data on DOACs in hemodialysis patients were generated with the FXa inhibitors apixaban and rivaroxaban. Dabigatran has not been investigated in randomized clinical trials in ESKD, so far, and due to its renal clearance of >80%, it may be more prone to cumulative drug effects in advanced CKD.
The following limitations should be considered when interpreting the current results: we only used study-level data and did not investigate the effects of DOACs versus VKAs at the individual patient level. Therefore, we could not adjust for patient-related factors (ie, therapy duration, age, sex). However, we likely identified all relevant studies using an elaborate search strategy. Moreover, the definition of ischemic and bleeding endpoints varied between the different studies included in the meta-analysis. Also, RENAL-AF and AXADIA-AFNET 8 were terminated early for slow recruitment and, therefore, were underpowered for their primary endpoint [19,23]. Also, ethnicity was not reported in 2 reports thus hindering generalizability [22,23]. Finally, while the control groups received oral anticoagulation with VKAs at a target INR of 2 to 3 in all included studies, different types and/or dosages of FXa inhibitors were administered in the DOAC groups [19,22,23]. Considering the previously published data on FXa inhibitors in NVAF, a class effect seems very likely. However, it remains to be established if the standard dosage or a reduced dosage of the FXa inhibitor or perhaps no oral anticoagulation provides the optimal benefit-risk ratio when treating hemodialysis patients with NVAF. This question can only be resolved by prospective randomized clinical trials.
5. Conclusion
FXa inhibitors carried a similar risk of bleeding and death when compared to VKAs in patients with NVAF undergoing chronic hemodialysis. Moreover, there was a trend toward stroke reduction in patients receiving FXa inhibitors.
Large randomized clinical trials are warranted to confirm our findings and to further investigate the net clinical benefit of oral anticoagulation in patients requiring hemodialysis.
Declaration of generative AI and AI-assisted technologies in the writing process
Not applicable.
Acknowledgments
Author contributions
Conception and design (M.T., D.S., C.A., and T.G.), data collection (M.T. and D.M.), data analysis (M.T. and D.M.), drafting (M.T. and T.G.), revising (D.S., D.M., and C.A.). All authors read and approved the final manuscript.
Relationship disclosure
M.T., D.S., and D.M. have nothing to declare. C.A. received speaker/consulting fees from Bayer, CSL Behring, Novo-Nordisk, Pfizer, Roche, Sobi, and Takeda. T.G. received speaker/consulting fees from AstraZeneca, Amgen, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Novartis, and Pfizer, and grant support from Boehringer-Ingelheim, Bristol Myers Squibb, Medtronic and Abbott.
Funding
None.
Footnotes
Handling Editor: Dr Lana Antoinette Castellucci
Supporting Information
References
- 1.Konstantinides S.V., Meyer G., Becattini C., Bueno H., Geersing G.-J., Harjola V.-P., et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 2.Steffel J., Collins R., Antz M., Cornu P., Desteghe L., Haeusler K.G., et al. 2021 European heart rhythm association practical guide on the use of non-vitamin k antagonist oral anticoagulants in patients with atrial fibrillation. EP Europace. 2021:19. doi: 10.1093/europace/euab157. [DOI] [PubMed] [Google Scholar]
- 3.Gremmel T., Niessner A., Domanovits H., Frossard M., Sengölge G., Steinlechner B., et al. Non-vitamin K antagonist oral anticoagulants in patients with an increased risk of bleeding. Wien Klin Wochenschr. 2018;130:722–734. doi: 10.1007/s00508-018-1381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruff C.T., Giugliano R.P., Braunwald E., Hoffman E.B., Deenadayalu N., Ezekowitz M.D., et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 5.Connolly S.J., Ezekowitz M.D., Yusuf S., Eikelboom J., Oldgren J., Parekh A., et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 6.Patel M.R., Mahaffey K.W., Garg J., Pan G., Singer D.E., Hacke W., et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 7.Granger C.B., Alexander J.H., McMurray J.J.V., Lopes R.D., Hylek E.M., Hanna M., et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 8.Giugliano R.P., Ruff C.T., Braunwald E., Murphy S.A., Wiviott S.D., Halperin J.L., et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 9.Büller H., Décousus H., Grosso M., Mercuri M., Middeldorp S., Prins M., et al. Edoxanban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–1415. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 10.Agnelli G., Buller H.R., Cohen A., Curto M., Gallus A.S., Johnson M., et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 11.EINSTEIN Investigators. Bauersachs R., Berkowitz S.D., Brenner B., Buller H.R., Decousus H., et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 12.Schulman S., Kearon C., Kakkar A.K., Schellong S., Eriksson H., Baanstra D., et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709–718. doi: 10.1056/NEJMoa1113697. [DOI] [PubMed] [Google Scholar]
- 13.Königsbrügge O., Posch F., Antlanger M., Kovarik J., Klauser-Braun R., Kletzmayr J., et al. Prevalence of atrial fibrillation and antithrombotic therapy in hemodialysis patients: cross-sectional results of the Vienna InVestigation of AtriaL Fibrillation and Thromboembolism in Patients on HemoDIalysis (VIVALDI) PLoS One. 2017;12 doi: 10.1371/journal.pone.0169400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kooiman J., van der Hulle T., Maas H., Wiebe S., Formella S., Clemens A., et al. Pharmacokinetics and pharmacodynamics of dabigatran 75 mg b.i.d. in patients with severe chronic kidney disease. J Am Coll Cardiol. 2016;67:2442–2444. doi: 10.1016/j.jacc.2016.03.516. [DOI] [PubMed] [Google Scholar]
- 15.FDA. Drug Approval Package: PRADAXA (dabigatran etexilate mesylate).
- 16.FDA. Drug Approval Package: ELIQUIS (apixaban).
- 17.FDA. Drug Approval Package: XARELTO (rivaroxaban).
- 18.Go A.S., Fang M.C., Udaltsova N., Chang Y., Pomernacki N.K., Borowsky L., et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation. Circulation. 2009;119:1363–1369. doi: 10.1161/CIRCULATIONAHA.108.816082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pokorney S.D., Chertow G.M., Al-Khalidi H.R., Gallup D., Dignacco P., Mussina K., et al. Apixaban for patients with atrial fibrillation on hemodialysis: a multicenter randomized controlled trial. Circulation. 2022;146:1735–1745. doi: 10.1161/CIRCULATIONAHA.121.054990. [DOI] [PubMed] [Google Scholar]
- 20.Königsbrügge O., Ay C. Atrial fibrillation in patients with end-stage renal disease on hemodialysis: Magnitude of the problem and new approach to oral anticoagulation. Res Pract Thromb Haemost. 2019;3:578–588. doi: 10.1002/rth2.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuno T., Takagi H., Ando T., Sugiyama T., Miyashita S., Valentin N., et al. Oral anticoagulation for patients with atrial fibrillation on long-term dialysis. J Am Coll Cardiol. 2020;75:273–285. doi: 10.1016/j.jacc.2019.10.059. [DOI] [PubMed] [Google Scholar]
- 22.De Vriese A.S., Caluwé R., Van Der Meersch H., De Boeck K., De Bacquer D. Safety and efficacy of vitamin K antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: a multicenter randomized controlled trial. J Am Soc Nephrol. 2021;32:1474–1483. doi: 10.1681/ASN.2020111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinecke H., Engelbertz C., Bauersachs R., Breithardt G., Echterhoff H.-H., Gerß J., et al. A randomized controlled trial comparing apixaban with the vitamin K antagonist phenprocoumon in patients on chronic hemodialysis: the AXADIA-AFNET 8 study. Circulation. 2023;147:296–309. doi: 10.1161/CIRCULATIONAHA.122.062779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 25.Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J Stat Softw. 2010;36 [Google Scholar]
- 26.De Vriese A.S., Caluwé R., Pyfferoen L., De Bacquer D., De Boeck K., Delanote J., et al. Multicenter randomized controlled trial of vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: the Valkyrie Study. J Am Soc Nephrol. 2020;31:186–196. doi: 10.1681/ASN.2019060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanifer J.W., Pokorney S.D., Chertow G.M., Hohnloser S.H., Wojdyla D.M., Garonzik S., et al. Apixaban versus warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation. 2020;141:1384–1392. doi: 10.1161/CIRCULATIONAHA.119.044059. [DOI] [PubMed] [Google Scholar]
- 28.Olesen J.B., Lip G.Y.H., Kamper A.-L., Hommel K., Køber L., Lane D.A., et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367:625–635. doi: 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- 29.Bansal N., Fan D., Hsu C., Ordonez J.D., Marcus G.M., Go A.S. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation. 2013;127:569–574. doi: 10.1161/CIRCULATIONAHA.112.123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Vriese A.S., Caluwé R., Raggi P. The atrial fibrillation conundrum in dialysis patients. Am Heart J. 2016;174:111–119. doi: 10.1016/j.ahj.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Murray A.M., Seliger S., Lakshminarayan K., Herzog C.A., Solid C.A. Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol. 2013;24:1166–1173. doi: 10.1681/ASN.2012080841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Findlay M.D., Thomson P.C., Fulton R.L., Solbu M.D., Jardine A.G., Patel R.K., et al. Risk factors of ischemic stroke and subsequent outcome in patients receiving hemodialysis. Stroke. 2015;46:2477–2481. doi: 10.1161/STROKEAHA.115.009095. [DOI] [PubMed] [Google Scholar]
- 33.Königsbrügge O., Meisel H., Beyer A., Schmaldienst S., Klauser-Braun R., Lorenz M., et al. Anticoagulation use and the risk of stroke and major bleeding in patients on hemodialysis: From the VIVALDI, a population-based prospective cohort study. J Thromb Haemost. 2021;19:2984–2996. doi: 10.1111/jth.15508. [DOI] [PubMed] [Google Scholar]
- 34.Van Der Meersch H., De Bacquer D., De Vriese A.S. Vitamin K antagonists for stroke prevention in hemodialysis patients with atrial fibrillation: a systematic review and meta-analysis. Am Heart J. 2017;184:37–46. doi: 10.1016/j.ahj.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Randhawa M.S., Vishwanath R., Rai M.P., Wang L., Randhawa A.K., Abela G., et al. Association between use of warfarin for atrial fibrillation and outcomes among patients with end-stage renal disease. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonde A.N., Lip G.Y.H., Kamper A.-L., Hansen P.R., Lamberts M., Hommel K., et al. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease. J Am Coll Cardiol. 2014;64:2471–2482. doi: 10.1016/j.jacc.2014.09.051. [DOI] [PubMed] [Google Scholar]
- 37.Starr J.A., Pinner N.A., Mannis M., Stuart M.K. A review of direct oral anticoagulants in patients with stage 5 or end-stage kidney disease. Ann Pharmacother. 2022;56:691–703. doi: 10.1177/10600280211040093. [DOI] [PubMed] [Google Scholar]
- 38.Mapili J.A.L., Lim L.C.S., Velando B.M., Aherrera J.A.M. The safety and efficacy of direct oral anticoagulants among chronic kidney disease patients on dialysis with non-valvular atrial fibrillation: a meta-analysis. Front Cardiovasc Med. 2023;10 doi: 10.3389/fcvm.2023.1261183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faisaluddin M., Alwifati N., Naeem N., Balasubramanian S., Narasimhan B., Iqbal U., et al. Safety and efficacy of direct oral anticoagulants versus warfarin for atrial fibrillation in end-stage renal disease on hemodialysis: a meta-analysis of randomized control trials. Am J Cardiol. 2023;206:309–311. doi: 10.1016/j.amjcard.2023.08.116. [DOI] [PubMed] [Google Scholar]
- 40.Nopp S., Spielvogel C.P., Schmaldienst S., Klauser-Braun R., Lorenz M., Bauer B.N., et al. Bleeding risk assessment in end-stage kidney disease: validation of existing risk scores and evaluation of a machine learning-based approach. Thromb Haemost. 2022;122:29–40. doi: 10.1055/a-1754-7551. [DOI] [PubMed] [Google Scholar]
- 41.De Vriese A.S., Heine G. Anticoagulation management in haemodialysis patients with atrial fibrillation: evidence and opinion. Nephrol Dial Transplant. 2022;37:2072. doi: 10.1093/ndt/gfab060. 9. [DOI] [PubMed] [Google Scholar]
- 42.Ionescu F., Cooper C., Petrescu I., George J., Mansuri S. Safety of apixaban compared to warfarin in hemodialysis patients: do antiplatelets make a difference? Eur J Haematol. 2021;106:689–696. doi: 10.1111/ejh.13599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.