Abstract
This study explored the developmental changes in small intestinal barrier function and the potential regulatory roles of intestinal microbiota and metabolites in different breeds of piglets during suckling and weaning periods. Taoyuan black (TB), Xiangcun black (XB), and Duroc (DR) piglets (10 litters per breed; half male and half female) were selected for sampling to evaluate the intestinal barrier-related indexes and intestinal microbiota and metabolites at 1, 10, 21 (weaned), and 24 (3 d after weaning) d old. The results showed that weaning led to severe shedding of small intestinal microvilli and sparse microvilli arrangement. D-lactate level in the ileum of TB and XB piglets during suckling and weaning periods was lower (P < 0.01) than that of DR piglets, as well as the ileal diamine oxidase level at 1 d old. The expression level of mucin 1 was higher (P < 0.05) in the ileum of TB and XB piglets than that of DR piglets, and it was the highest in the ileum of TB piglets at 21 d old. The expression levels of mucin 2 and mucin 13 were higher (P < 0.10) in TB and XB piglets than those of DR piglets at 21 d old, whereas mucin 2 and mucin 13 in the ileum of TB and XB piglets were higher (P < 0.05) than those of DR piglets at 24 d old. TB and XB piglets had a lower relative abundance of Escherichia_Shigella at 21 and 24 d old, but they had higher Streptococcus at 1 and 24 d old than DR piglets (P < 0.01). Differential metabolites between the three breeds of piglets were mainly related to oxidative phosphorylation, steroid biosynthesis, and bile acid synthesis. Collectively, these findings suggest that different pig breeds present differences in the development of the small intestinal barrier function. Compared with DR piglets, TB and XB piglets had higher intestinal permeability during the suckling period and a stronger intestinal mechanical barrier after weaning. Moreover, intestinal microbiota and metabolites are the key factors for developing small intestinal barrier functions in different breeds of piglets.
Keywords: Chinese indigenous piglet, Duroc piglet, Immune function, Intestinal barrier function, Weaning stress
1. Introduction
The integrity of the intestinal barrier is essential for optimal digestion, absorption, and immune response in animals, especially for young ones (Okumura and Takeda, 2017). Meanwhile, intestinal epithelial cell (IEC) are the first line of defense for immunity, which can form the intestinal barrier by secreting mucins and interacting with intestinal microbiota and their metabolites (Turner, 2009). Suckling and weaning are the two key window periods for intestinal barrier development. Despite differences between species, most animals (including pigs) experience a decrease in intestinal permeability during 2 to 3 weeks after birth (De Quelen et al., 2011). Commercial piglets are weaned earlier than their natural weaning time, which poses additional challenges to piglets. Early weaning blocks the acquisition of passive immunity from breast milk, while stressors associated with early weaning disrupt the intestinal barrier function of piglets (Moeser et al., 2017). Due to changes in diets and separation from the maternal environment, weaning not only causes significant changes in the structure and function of the small intestine of piglets but also leads to intestinal inflammation, thereby damaging the villous crypt structure and intestinal barrier function (Montagne et al., 2007). Several factors, including genetic background, microbial colonization, and colostrum and milk composition are associated with the early intestinal barrier development of piglets (Rogier et al., 2014). However, the exact mechanism remains unclear.
Intestinal symbiotic microbiota directly or indirectly contributes to the intestinal health of piglets by enhancing intestinal barrier function (Adak and Khan, 2019). For instance, intestinal microbiota, including Lactobacillus and Bifidobacterium, can directly inhibit the survival of pathogenic bacteria by competing for their ecological niche and nutrient (Bauer et al., 2018). The composition and diversity of the intestinal microbiota in pigs change dynamically (Petri et al., 2010). After weaning, the diversity and abundance of beneficial bacteria decrease while the abundance of pathogenic bacteria increases (Tao et al., 2015). Intestinal microbiota promotes the maturation of adaptive immunity by providing low-level immune stimulation, such as inducing the production of IgA and regulating the baseline level of anti-inflammatory factors that promote the maintenance of epithelial barrier and tight junctions (Hiippala et al., 2018). Moreover, intestinal microbiota could regulate intestinal barrier function by fermenting carbohydrates in the intestine to produce short-chain fatty acids (SCFA), which can transport nutrients for IEC and play a positive role in IEC regeneration and mechanical barrier function (Adak and Khan, 2019; Peng et al., 2009). The changes in intestinal microbial composition are closely related to the health of animals. Therefore, it is of great significance to elucidate the changes in intestinal microbiota and metabolites during the suckling and weaning periods to maintain the health of piglets.
The intestinal barrier is the functional isolation zone of the normal intestine, which separates the intestinal cavity from the internal environment of the body and prevents the invasion of pathogenic antigens, especially for young animals (Pluske et al., 2018). However, the development of the intestinal barrier of Chinese indigenous piglets and their resistance to weaning stress still need to be explored. Most studies on weaned piglets are focused on commercially hybrid pigs, and limited information exists on Chinese indigenous pigs. The Xiangcun black (XB) pig is a cross-breed pig breed of the Taoyuan black (TB) pig as a female parent and the Duroc pig (DR) as a male parent. Our previous study showed that TB and XB piglets could resist the immune stress caused by weaning, but the differences in intestinal barrier function after weaning need to be elucidated (Ding et al., 2022). Therefore, it is necessary to understand the differences in intestinal barrier function establishment in different pig breeds to prevent weaning stress efficiently. Previous research evidence suggests that there are differences in intestinal microbial composition between Chinese indigenous and commercial pigs (Xiao et al., 2018), which might be an important reason for the resistance to weaning stress of indigenous pigs. Therefore, we hypothesized that there might be differences in the intestinal barrier function between Chinese indigenous (TB and XB) and foreign (DR) breeds of piglets, which may be related to the differences in intestinal microbiota and metabolites of different breeds of piglets. Thus, the present study aimed to evaluate the developmental changes in intestinal barrier function and the potential regulatory roles of intestinal microbiota and metabolites in different breeds of piglets during suckling and weaning periods. The findings will provide a theoretical basis for explaining the resistance to weaning stress of Chinese indigenous piglets.
2. Materials and methods
2.1. Animal ethics statement
All experiments complied with the ARRIVE guidelines. The procedures of animal experiments were carried out in accordance with the Animal Care and Use Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (Approval no. 20200018).
2.2. Animals and experimental protocol
A total of 30 litters of healthy newborn piglets, including TB, XB, and DR piglets (10 litters per breed), were selected from their respective sows with similar parities (2 to 3) and litter sizes (9 to 11), respectively. Suckling piglets were not given creep feed. All piglets were weaned at 21 d old and fed creep feed, and the diet composition is shown in Table 1. Feeding management refers to the feeding management model of commercial companies. The experimental piglets were not received any vaccination during the trial.
Table 1.
Ingredients composition and nutrient levels of diets (dry matter basis).
| Item | Content, % |
|---|---|
| Ingredients | |
| Corn (8% CP) | 22.70 |
| Broken rice | 8.50 |
| Wheat flour | 10.00 |
| Soybean meal (46% CP) | 5.50 |
| Fermented soybean meal | 6.00 |
| Extruded soybeans (35.5% CP) | 18.00 |
| Pentapeptide | 6.25 |
| Fish meal (67% CP) | 3.00 |
| Fish solubles (55% CP) | 1.25 |
| Yeast hydrolyzate (45% CP) | 1.25 |
| Fat powder | 1.25 |
| Limestone powder | 0.30 |
| Ca(H2PO4)2 | 0.80 |
| Whey powder (low-protein) | 7.50 |
| Glucose | 6.25 |
| Zinc oxide | 0.20 |
| L-Lysine hydrochloride (78.5%) | 0.50 |
| DL-Methionine (99%) | 0.12 |
| L-Threonine (98.5%) | 0.13 |
| Premix1 | 0.50 |
| Total | 100.00 |
| Nutrient levels2 | |
| Digestive energy, MJ/kg | 14.20 |
| Crude protein | 18.11 |
| Lysine | 1.41 |
| Methionine | 0.45 |
| Threonine | 0.91 |
| Calcium | 0.65 |
| Available phosphorus | 0.45 |
Per kilogram of premix containing 2,500 IU vitamin A, 575 IU vitamin D3, 80 IU vitamin E, 0.8 mg vitamin K, 0.62 mg vitamin B1, 2 mg vitamin B2, 7 mg vitamin B6, 10 mg vitamin B12, 10 mg niacin, 6 mg pantothenic acid, 2.5 mg biotin, 6 mg Cu (CuSO4), 100 mg Fe (Fe2(SO4)3·H2O), 100 mg Zn (ZnSO4·H2O), 4 mg Mn (MnSO4·H2O), 0.20 mg I (Ca(IO3)2), and 0.3 mg Se (Na2SeO3).
Nutrient levels are measured values.
2.3. Sample collection
At 1, 10, 21 (weaned), and 24 (3 d after weaning) d old, 12 h after the last feeding, 10 piglets per breed (one piglet from each litter; half male and half female; male piglets were not castrated) close to the average body weight per litter were selected for sampling. The piglets were euthanized for sampling after intramuscular injection of Zoletil 50 (Beijing Lab Anim Tech Develp Co., Ltd., Beijing, China). Posterior segments of the ileal samples (3 to 5 cm) were collected and stored at −80 °C after snap-freezing in liquid nitrogen until further analysis of barrier function-related indexes. The contents of the ileum were collected in sterile tubes and stored at −80 °C for microbial composition and metabolomics analyses. The excised ileal tissues were rinsed with phosphate-buffered saline (PBS) at 4 °C, cut into 0.50 cm × 0.50 cm small pieces, and fixed with 2.50% glutaraldehyde for microvilli morphology analysis. The body weight and feed intake were recorded to calculate average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR).
2.4. Determination of ileal development and injury-related indexes
Ileal development and injury-related indexes, including transforming growth factor-beta (TGF-β), epidermal growth factor (EGF), epidermal growth factor receptor (EGFR), D-lactate, diamine oxidase (DAO), and 8-hydroxyl-deoxyguanosine (8-OHdG), were analyzed by the colorimetric method with commercially available enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Kexing Trading Co., Ltd., Shanghai, China) according to the protocols provided by the manufacturer. The total protein content of the ileal tissues was determined using the BCA protein assay kit (Beyotime, Shanghai, China), and the final concentration of indexes in the ileal tissues was normalized to unit protein per sample.
2.5. Analysis of ileal barrier function-related gene expression
The total RNA was extracted from ileal tissues using the Trizol reagent (Accurate Biology, Hunan, China). The extracted RNA concentration was measured by NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). The primers used in this study are presented in Supplementary Table S1. Real-time PCR analysis was performed on the LightCycler 480 II Real-Time PCR System (Roche, Basel, Switzerland) with SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Biology). Target gene expression was normalized in comparison with the β-actin, and the 2−ΔΔCt method was used to calculate the relative gene expression level (Rao et al., 2013).
2.6. Observation of ileal microvilli morphology
The fixed ileal segments were washed with 0.01 M PBS solution. The tissue specimens were dehydrated step by step with ethanol and graded alcohol, removed with a gradient of tert-butanol, and then freeze-dried. Subsequently, tissue specimens were attached to an aluminum stub plated with silver colloid, and a thin layer of gold was plated on the specimens by sputtering to make it a conductor. Finally, tissue specimens were observed using a field emission scanning electron microscope (SU8010, Hitachi, Tokyo, Japan) (Xu et al., 2015).
2.7. Analysis of ileal microbial composition by 16S rRNA pyrosequencing
Ileal content samples stored at −80 °C were thawed at 4 °C overnight for microbial composition analysis by the Shanghai Personal Biotechnology Co., Ltd., Shanghai, China. The analysis process from DNA extraction to on-board sequencing mainly included the following steps. Firstly, ileal DNA was extracted for quality inspection and recovery according to the manufacturer's instructions (TransGen Biotech, Beijing, China). Secondly, the sequences of bacterial genes from the V3−V4 region were amplified by PCR with primers 338 F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Finally, PCR amplification products were added to fluorescent reagents and quantified using a microplate reader. Sequencing libraries were prepared using the TruSeq Nano DNA LT Library Prep Kit (Illumina, San Diego, CA, USA). The gene library with a concentration of >2 nm was sequenced by the MiSeq sequencer (Illumina).
The offline sequences were divided into library and sample according to the index and barcode information, and the barcode sequences were removed. Sequence denoising or operational taxonomic unit (OTU) clustering was performed using the QIIME2 DADA2 analysis process. The specific composition of each sample (group) at different taxonomic levels was displayed to understand the overall structure. The alpha diversity (including Chao, Shannon, Simpson, Faith_pd, Observed_species, and Pielou_e index) of each sample was assessed according to the distribution of OTU levels in different samples. At taxonomic composition, numerous unsupervised and supervised classifications combined with corresponding statistical testing methods were used to determine further differences in species abundance composition among different samples (groups) and to find marker species.
2.8. Analysis of ileal metabolite profiles by metabolomics
Untargeted metabolomics analysis was performed using a liquid chromatography-mass spectrometry (LC-MS) entrusted to Suzhou PANOMIX Biomedical Tech Co. Ltd., Suzhou, China. Briefly, approximately 200 mg of ileal contents were mixed with pre-cooled 80% methanol, stood at −20 °C for 20 min, and centrifuged at 4 °C and 10,000 × g for 10 min. All samples were prepared for quality control and testing under the same treatment and centrifugation conditions. The detection was performed in an Agilent 6545 Q-TOF LC/MS system, and the raw data were converted to XML format (v3.0.8789). The R-package XCMS (R-v3.1.3) was used for peak identification, filtering, and alignment. After quality control, the human metabolome database (HMDB, http://www.hmdb.ca), Metlin (http://www.hmdb.ca), mzcloud (https://www.mzcloud.org), and the metabolome databases were used to annotate metabolites of LC-MS data. The metabolite database constructed by BioNovoGene was used to verify LC-MS data to avoid missing important metabolites (BioNovoGene Co., Ltd., Suzhou, China). Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were performed by Soft Independent Modeling of Class Analogy (SIMCA) software (V16.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden). Differential metabolites between groups were identified using the statistically significant VIP ≥ 1 and P < 0.05 values. In addition, pathway enrichment analysis was performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg/) and MetaboAnalyst 5.0 databases (https://www.metaboanalyst.ca/faces/home.xhtml).
2.9. Statistical analysis
All data were expressed as means with standard error of the mean (SEM). The individual piglets were considered the experimental unit. Statistical analysis was performed by a two-way ANOVA for pig breed and day of age using the SPSS 22.0 software package (SPSS Inc., Chicago, IL, USA). Differences between the means of the experimental groups were analyzed using the one-way ANOVA and Tukey's multiple comparison test. Probability values < 0.05 were considered to indicate statistical significance, and P-values between 0.05 and 0.10 were considered a trend.
3. Results
3.1. Changes in growth performance, ileal morphology, and barrier function in different breeds of piglets during suckling and weaning periods
The developmental characteristics of the intestinal morphology and growth performance in different breeds of piglets are presented in Table 2 and Fig. 1. TB and XB piglets had lower (P < 0.05) ADG at 1 to 10 d old than DR piglets, whereas XB piglets had lower (P < 0.01) ADG at 10 to 21 d old than TB and DR piglets. In addition, TB piglets had lower ADFI at 21 to 24 d old than XB and DR piglets, and DR piglets had higher ADFI than TB and XB piglets (P < 0.01; Table 2). The scanning electron microscope analysis showed that the ileal villi became shorter at 24 d old than at 21 d old, and the atrophy of the ileum microvilli was slower in TB and XB piglets compared with DR piglets (Fig. 1A). Compared with DR piglets, the ileal microvilli of DR piglets were remarkably damaged at 24 d old, with more broken villus tops and disordered and shed microvilli. However, TB and XB piglets had no obvious changes in villi disorder compared with DR piglets (Supplementary Fig. S1).
Table 2.
Growth performance of different breeds of piglets.
| Item | TB | XB | DR | SEM | P-values |
|---|---|---|---|---|---|
| Average daily gain, g/d | |||||
| 1 to 10 d old | 129.00B | 136.00B | 225.00A | 25.815 | 0.024 |
| 10 to 21 d old | 219.00A | 74.00B | 195.00A | 31.101 | 0.006 |
| 21 to 24 d old | −230.00 | −69.00 | −87.00 | 74.594 | 0.266 |
| Average daily feed intake, g/d | |||||
| 21 to 24 d old | 57.78C | 76.67B | 117.96A | 5.885 | <0.001 |
| Feed conversion ratio | |||||
| 21 to 24 d old | −0.25 | −1.11 | −1.35 | 0.740 | 0.534 |
DR = Duroc piglet; TB = Taoyuan black piglet; XB = Xiangcun black piglet.
Data are presented as means and their SEM (n = 10).
A, B, C Within a row, different superscript uppercase letters indicate significant differences between piglet breeds (P < 0.05).
Fig. 1.
Changes in ileal morphology, permeability, and barrier function of different breeds of piglets during suckling and weaning periods. (A) Ileal morphology (400× magnification; scale bar = 500 μm), (B) ileal permeability indexes, (C) ileal barrier function-related genes, (D) ileal mucin related genes, and (E) ileal development-related indexes. Data are presented as means with their SEM (n = 10). Bars without a common uppercase letter indicate significant differences between different age stages of the same breed of piglets, and bars without a common lowercase letter indicate significant differences between different breeds of piglets at the same age (P < 0.05). DR = Duroc piglet; TB = Taoyuan black piglet; XB = Xiangcun black piglet.
Regardless of breed, TB piglets had lower (P < 0.01) ileal D-lactate content at 1 and 10 d old than at 21 d old. XB piglets had higher (P < 0.01) ileal D-lactate content at 10 d old than at 1 and 21 d old, and had the lowest D-lactate content at 1 d old. DR piglets had lower (P < 0.05) ileal D-lactate content at 1 d old than at 10 and 21 d old. Moreover, XB piglets had higher (P < 0.01) ileal DAO content at 10 d old than at other three age stages, while they had the highest (P < 0.01) DAO content at 21 and 24 d old than at 1 d old (Fig. 1B). The occludin expression level in the ileum of XB piglets was lower (P < 0.05) at 1 d old than at 10 and 24 d old (Fig. 1C). The mucin 1 expression level in the ileum of DR piglets was higher (P < 0.01) at 10 d old than at other three age stages, while mucin 13 expression level in the ileum of TB piglets was lower (P < 0.01) at 1 and 10 d old than at 21 and 24 d old (Fig. 1D). The TGF-β content in the ileum of TB piglets was lower (P < 0.01) at 1 d old than at other three age stages, while EGFR content was higher (P < 0.01) at 21 and 24 d old than at 1 and 10 d old. Moreover, TGF-β content in the ileum of XB piglets was lower (P < 0.01) at 1 d old than at other three age stages and had the highest (P < 0.01) content at 10 d old, while they had higher (P < 0.05) EGF content at 24 d old than at 10 and 21 d old and higher (P < 0.01) EGFR content at 21 and 24 d old than at 1 d old. The EGFR content in the ileum of DR piglets was higher (P < 0.01) at 21 d old than at other three age stages (Fig. 1E).
Regardless of age, weaning resulted in higher (P < 0.05) D-lactate content in the ileum of three breeds of piglets. TB and XB piglets had lower (P < 0.01) D-lactate content in the ileum at 1, 21, and 24 d old, as well as DAO content at 1 d old than DR piglets. TB piglets had lower D-lactate content and higher 8-OHdG content in the ileum at 10 d old compared with DR piglets (P < 0.05). Moreover, TB piglets had higher (P < 0.01) D-lactate content in the ileum at 21 d old than XB piglets (Fig. 1B). Ileal tight junction-related gene expression levels had no differences between different breeds of piglets (P > 0.05; Fig. 1C).
Compared with DR piglets, TB and XB piglets had higher (P < 0.05) ileal mucin 1 expression level at 21 d old, and TB piglets had higher (P < 0.05) mucin 2 and mucin 13 expression levels at 21 and 24 d old, and they had lower (P < 0.05) ileal EGF content and EGFR content at 21 d old (Fig. 1D and E). Compared with DR piglets, TB piglets had higher (P < 0.05) ileal mucin 2 expression level at 21 d old and lower (P < 0.05) ileal EGFR content at 10 and 24 d old (Fig. 1D and E). Moreover, TB piglets had lower (P < 0.05) ileal TGF-β content at 10 d old than XB and DR piglets.
3.2. Changes in ileal microbial diversity in different breeds of piglets during suckling and weaning periods
The changes in ileal microbial diversity in different breeds of piglets are presented in Fig. 2 and Supplementary Fig. S2. The principal coordinate analysis results revealed that there were clear separations between TB, XB, and DR piglets (Supplementary Fig. S2). TB piglets had higher (P < 0.05) Chao, Shannon, Faith_pd, Observed_species, and Pielou_e indexes at 24 d old than at other three age stages, whereas DR piglets had higher (P < 0.05) Chao, Faith_pd, and Observed_species indexes at 24 d old than at other three age stages (Fig. 2).
Fig. 2.
Changes in ileal microbial diversity of different breeds of piglets during suckling and weaning periods. (A) Chao index, (B) Shannon index, (C) Simpson index, (D) Faith_pd index, (E) Observed_species index, and (F) Pielou_e index. Data are presented as means with their SEM (n = 10). Bars without a common uppercase letter indicate significant differences between different age stages of the same breed of piglets, and bars without a common lowercase letter indicate significant differences between different breeds of piglets at the same age (P < 0.05). DR = Duroc piglet; TB = Taoyuan black piglet; XB = Xiangcun black piglet.
XB and DR piglets had higher (P < 0.05) Chao, Shannon, Simpson, Faith_pd, Observed_species, and Pielou_e indexes at 21 d old than TB piglets (Fig. 2).
3.3. Changes in ileal microbiota composition in different breeds of piglets during suckling and weaning periods
Changes in ileal microbial composition in different breeds of piglets are presented in Fig. 3 (phylum level) and Fig. 4 (genus level). At the phylum level, Firmicutes (56.58% to 99.28%), Actinobacteria (0.10% to 18.13%), and Proteobacteria (0.16% to 35.08%) were the most dominant phyla in the ileum of the three breeds of piglets, accounting for more than 95% of the total abundance (Fig. 3A). TB piglets had a lower relative abundance of Firmicutes, while had a higher relative abundance of Actinobacteria in the ileum at 1 and 24 d old than at 10 and 21 d old, as well as Proteobacteria at 1 d old and Bacteroidetes at 24 d old than at other three age stages (P < 0.01). XB piglets had a lower relative abundance of Firmicutes and a higher relative abundance of Proteobacteria at 1 d old than at other three age stages (P < 0.01), whereas they had a higher relative abundance of Actinobacteria at 1 and 24 d old than at 10 and 21 d old (P < 0.01). DR piglets had a higher (P < 0.05) relative abundance of Bacteroidetes at 24 d old than at other three age stages (Fig. 3B−E).
Fig. 3.
Changes in ileal microbial composition of different breeds of piglets during suckling and weaning periods at the phylum level. (A) Abundances of top ten phyla, and the relative abundances of (B) Firmicutes, (C) Actinobacteria, (D) Proteobacteria, and (E) Bacteroidetes. Data are presented as means with their SEM (n = 10). Bars without a common uppercase letter indicate significant differences between different age stages of the same breed of piglets, and bars without a common lowercase letter indicate significant differences between different breeds of piglets at the same age (P < 0.05). DR = Duroc piglet; TB = Taoyuan black piglet; XB = Xiangcun black piglet.
Fig. 4.
Changes in ileal microbial composition of different breeds of piglets during suckling and weaning periods at the genus level. (A) Relative abundances of top twenty genera, and the relative abundances of (B) Lactobacillus, (C) Escherichia–Shigella, (D) Streptococcus, (E) Fusobacterium, (F) Bacteroides, (G) Muribaculaceae, (H) Veillonella, (I) Rothia, and (J) Romboutsia. Data are presented as means with their SEM (n = 10). Bars without a common uppercase letter indicate significant differences between different age stages of the same breed of piglets, and bars without a common lowercase letter indicate significant differences between different breeds of piglets at the same age (P < 0.05). DR = Duroc piglet; TB = Taoyuan black piglet; XB = Xiangcun black piglet.
At the genus level, Lactobacillus (4.20% to 98.78%), Escherichia–Shigella (0.05% to 32.51%), Streptococcus (0.36% to 60.48%), and Veillonella (0.07% to 0.62%) were the most dominant genera in the ileum of the three breeds of piglets (Fig. 4A). TB piglets had higher (P < 0.01) relative abundances of Streptococcus and Muribaculaceae at 24 d old than at other three age stages, as well as Escherichia–Shigella at 1 d old and Bacteroides at 24 d old than at other three age stages and Lactobacillus at 10 and 21 d old than at 1 and 24 d old. XB piglets had higher relative abundances of Escherichia–Shigella (P < 0.01) at 1 d old and Streptococcus (P < 0.01) and Rothia (P < 0.05) at 24 d old than at other three age stages, as well as Lactobacillus (P < 0.01) at 10 and 21 d old than at 1 and 24 d old. DR piglets had higher relative abundances of Escherichia–Shigella (P < 0.05) at 1 d old and Streptococcus (P < 0.01) at 24 d old than at other three age stages, as well as Veillonella (P < 0.01) at 10 d old than at 1 and 24 d old (Fig. 4B−J).
At the phylum level, TB piglets had higher (P < 0.05) relative abundance of Actinobacteria at 24 d old than XB and DR piglets, and the relative abundance of Firmicutes in TB piglets displayed an increasing trend (P = 0.09) at 10 d old. In addition, XB piglets had higher (P < 0.01) relative abundance of Proteobacteria at 1 d old than TB and DR piglets, whereas TB and XB piglets had lower (P < 0.05) relative abundance of Proteobacteria at 10 d old than DR piglets (Fig. 3B−E). At the genus level, TB piglets had higher (P < 0.01) relative abundance of Lactobacillus at 10 and 21 d old than DR piglets, and they had higher (P < 0.01) relative abundance of Rothia at 1 d old than XB and DR piglets. XB piglets had higher (P < 0.01) relative abundance of Escherichia–Shigella at 1 d old than TB and DR piglets. Moreover, TB and XB piglets had lower (P < 0.01) relative abundance of Lactobacillus at 1 and 24 d old, as well as Escherichia–Shigella (P < 0.05) at 21 and 24 d old but higher (P < 0.01) relative abundance of Streptococcus at 1 and 24 d old, when compared with DR piglets (Fig. 4B−J).
3.4. Changes in ileal microbial biomarkers in different breeds of piglets during suckling and weaning periods
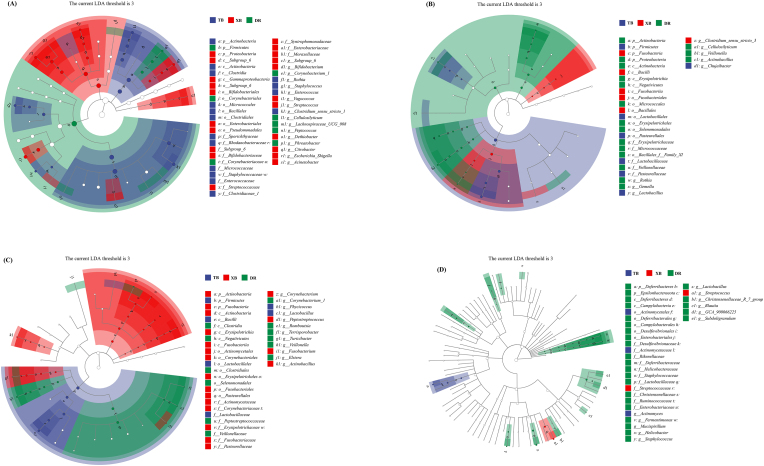
The LEfSe analysis among the three pig breeds is presented in Fig. 5 and Supplementary Fig. S3. At 1 d old, the top five bacterial biomarkers (LDA > 3.0, P < 0.05) in the ileum of TB piglets were Firmicutes, Lachnospiraceae_UGG_008, Peptococus, Corynebacteriales, and Corynebacteriales, and those of XB piglets were Clostridiaceae_1, Clostridium_sensu_stricto_1, Clostridia, Clostridiales, and Actinobacteria, and those of DR piglets were Proteobacteria, Gammaproteria, Enterobacteriaceae, Enterobacteriales, and Escherichia-Shigella (Fig. 5A and Supplementary Fig. S3A). At 10 d old, Veillonellaceae, Selenomonadales, Negativicutes, Veillonella, and Proteobacteria in TB piglets, Lactobacillus, Lactobacillaceae, Lactobacillales, Firmicutes, and Pasteurellales in XB piglets, and Fusobacteria, Fusobacteriia, Fusobacteriales, Bacillales, and Clostridium_sensu_stricto_3 in DR piglets were the top five bacterial biomarkers (LDA > 3.0, P < 0.05; Fig. 5B and Supplementary Fig. S3B) in the ileum. At 21 d old, Clostridiales, Peptostreptococcaceae, Selenomonadales, Negativicutes, and Veillanellaceae in TB piglets; Lactobacillaceae, Lactobacillus, Lactobacillales, Bacilli, and Firmicutes in XB piglets; and Actinobacteria, Actinomycetaceae, Actinomycetales, Peptostreptococcus, and Erysipelotrichales in DR piglets were the top five bacterial biomarkers (LDA > 3.0, P < 0.05; Fig. 5C and Supplementary Fig. S3C) in the ileum. Moreover, Lactobacillaceae, Lactobacillus, Staphylococcaceae, Staphylococcus, and Enterobacteriaceae in TB piglets, Actinomycetales, Actinomycetaceae, and Actinomyces in XB piglets, and Streptococcus and Streptococcaceae in DR piglets were the most dominant bacterial biomarkers at 24 d old (LDA > 3.0, P < 0.05; Fig. 5D and Supplementary Fig. S3D) in the ileum.
Fig. 5.
Linear discriminant analysis combined effect size measurements (LEfSe) analysis of ileal microbiota of different breeds of piglets during suckling and weaning periods. (A−D) 1, 10, 21, and 24 d old. DR = Duroc piglet; TB = Taoyuan black piglet; XB = Xiangcun black piglet.
3.5. Function prediction of ileal microbiota in different breeds of piglets during suckling and weaning periods
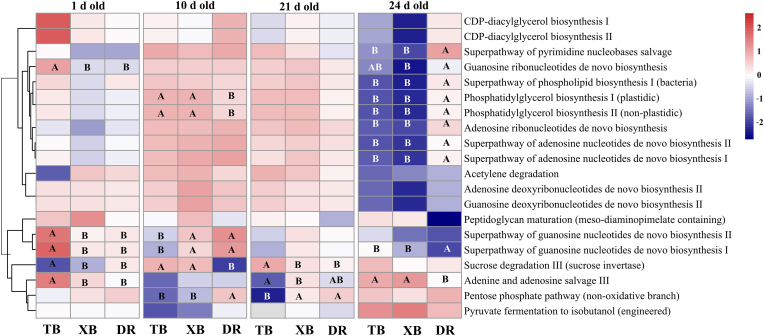
The potential functions of the ileal microbiota were predicted by the PICRUSt2 software package, which is presented in Fig. 6. At 1 d old, the relative abundances of genes involved in guanosine ribonucleotides de novo biosynthesis (P < 0.05), superpathway of guanosine nucleotides de novo biosynthesis I (P < 0.01), superpathway of guanosine nucleotides de novo biosynthesis II (P < 0.01), and adenine and adenosine salvage III (P < 0.05) were enriched, while the sucrose degradation III (P < 0.05) was reduced in TB piglets compared with XB and DR piglets.
Fig. 6.
Abundances of KEGG pathways in the functional prediction of ileal microbiota of different breeds of piglets during suckling and weaning periods. A, B Boxes without a common letter indicate significant differences between piglet breeds at the same age (P < 0.05). DR = Duroc piglet; TB = Taoyuan black piglet; XB = Xiangcun black piglet.
At 10 d old, the relative abundances of genes involved in phosphatidylglycerol biosynthesis I (P < 0.05), phosphatidylglycerol biosynthesis II (P < 0.05), and sucrose degradation III (P < 0.01) were enriched, but the pentose phosphate pathway was reduced (P < 0.01) in TB and XB piglets compared with DR piglets. In addition, TB piglets had reduced (P < 0.01) relative abundances of genes involved in superpathway of guanosine nucleotides de novo biosynthesis Ⅰ and superpathway of guanosine nucleotides de novo biosynthesis II compared with XB and DR piglets.
At 21 d old, the relative abundance of the gene involved in sucrose degradation III was enriched (P < 0.05), whereas the pentose phosphate pathway was reduced (P < 0.01) in TB piglets compared with XB and DR piglets. Moreover, the relative abundance of the gene involved in adenine and adenosine salvage III was reduced (P < 0.05) in TB piglets compared with XB piglets.
At 24 d old, genes involved in the superpathway of pyrimidine nucleobases salvage (P < 0.05), superpathway of phospholipid biosynthesis I (P < 0.05), phosphatidylglycerol biosynthesis I (P < 0.05), phosphatidylglycerol biosynthesis II (P < 0.05), adenosine ribonucleotides de novo biosynthesis (P < 0.01), superpathway of adenosine nucleotides de novo biosynthesis I (P < 0.01), and superpathway of adenosine nucleotides de novo biosynthesis II (P < 0.01) were reduced, while superpathway of guanosine nucleotides de novo biosynthesis I and adenine and adenosine salvage III (P < 0.05) were enriched in TB and XB piglets compared with DR piglets.
3.6. Changes in ileal metabolite profiles in different breeds of piglets during suckling and weaning periods
The PCA results showed that there were no significant separations (Supplementary Fig. S4), but the OPLS-DA score plots showed significant separations between TB, XB, and DR piglets at different age stages (Fig. 7). Six types of differential metabolites, including amino acids (35.19%), lipids (20.37%), carbohydrates (7.41%), cofactors and vitamins (14.81%), xenobiotics (7.41%), and nucleotides (14.81%) were detected in the ileum of all pig breeds (Supplementary Fig. S5). Further metabolomics results of different breeds of piglets are shown in Fig. 8. At 1 d old, XB piglets had higher (P < 0.01) calcitriol, 4,4-dimethyl-5a-cholesta-8,24-dien-3-b-ol, dehydroepiandrosterone, 7-dehydrodesmosterol, 17alpha,21-dihydroxypregnenolone, 2-ketobutyric acid, 20a,22b-dihydroxycholesterol, acetylphosphate, glucosamine, and D-lyxose levels while having a lower (P < 0.05) alpha-dimorphecolic acid level, when compared with TB and DR piglets. TB piglets had higher glycerophosphocholine and arachidonic acid levels but a lower glycochenodeoxycholic acid level compared with XB and DR piglets (P < 0.01), while having the lowest level of glycochenodeoxycholic acid in XB piglets (Fig. 8A).
Fig. 7.
The orthogonal partial least squares-discriminant analysis (OPLS-DA) score plots of ileal microbiota in different breeds of piglets during suckling and weaning periods (n = 10). (A−D) Piglets at 1, 10, 21, and 24 d old in the negative ion mode; (E−H) piglets at 1, 10, 21, and 24 d old in the positive ion mode. DR = Duroc piglet; TB = Taoyuan black piglet; XB = Xiangcun black piglet.
Fig. 8.
Differential metabolites in the ileal contents of different breeds of piglets during suckling and weaning periods. (A−D) Piglets at 1, 10, 21, and 24 d old, respectively. Bars without a common uppercase letter indicate significant differences between piglet breeds at the same age (P < 0.05). DR = Duroc piglet; TB = Taoyuan black piglet; XB = Xiangcun black piglet.
At 10 d old, XB piglets had higher (P < 0.01) calcitriol, 7-dehydrodesmosterol, 25-hydroxycholesterol, dehydroepiandrosterone, oleic acid, 4,4-dimethyl-5a-cholesta-8,24-dien-3-b-ol, sphingosine, sphinganine,12-keto-leukotriene b4, methyl jasmonate, chitobiose, sucrose, and D-mannonate levels compared with TB and DR piglets, and those metabolites were lowest in DR piglets. TB piglets had a lower (P < 0.01) galactitol level compared with XB piglets, while they had a higher (P < 0.01) alpha-dimorphecolic acid level compared with XB and DR piglets (Fig. 8B).
At 21 d old, TB piglets had higher (P < 0.05) 21-hydroxypregnenolone, D-mannose, and palmitoleic acid levels but lower (P < 0.01) levels of taurocholic acid, 14,15-DiHETrE, 20a,22b-dihydroxycholesterol, dodecanoic acid, dehydroepiandrosterone, pregnenolone, methyl jasmonate, 4,4-dimethyl-5a-cholesta-8,24-dien-3-b-ol, 20alpha-hydroxycholesterol, 2-ketobutyric acid, and sucrose, when compared with XB and DR piglets. TB piglets had lower (P < 0.05) levels of choline, 17alpha,21-dihydroxypregnenolone, succinic acid, maleic acid, acetylphosphate, and sorbitol compared with XB and DR piglets, and those metabolites were the highest (P < 0.05) in DR piglets (Fig. 8C).
At 24 d old, TB piglets had higher levels of stearic acid, deoxycholic acid, palmitic acid, 7-dehydrodesmosterol, estrone glucuronide, 12-keto-leukotriene b4, and mannitol but had a lower D-mannose level compared with XB and DR piglets (P < 0.05). XB piglets had higher levels of 13-L-hydroperoxylinoleic acid, maleic acid, sucrose, and sorbitol while had a lower level of glucosamine compared with TB and DR piglets (P < 0.01). XB piglets had a higher level of glycerophosphocholine but a lower level of oxoglutaric acid compared with DR piglets (P < 0.01). In addition, TB piglets had higher levels of sphinganine and succinic acid, and TB and XB piglets had a higher level of chenodeoxycholic acid compared with DR piglets (P < 0.01; Fig. 8D).
Moreover, KEGG enrichment analysis of differential metabolites showed that the differential pathways of metabolites among different pig breeds were mainly enriched in protein digestion and absorption, GABAergic synapse, and amino acid metabolism and synthesis (Supplementary Fig. S6).
3.7. Correlation among differential ileal microbiota, metabolites, and barrier function indexes
Pearson's correlations among intestinal microbiota and differential metabolites, intestinal permeability, and barrier function are shown in Fig. 9. Firmicutes was negatively correlated with 2-ketobutyric acid (P < 0.01), dehydroepiandrosterone (P < 0.01), 4-pyridoxic acid (P < 0.01), 7-dehydrodesmosterol (P < 0.05), and creatinine (P < 0.05) levels, while it was positively (P < 0.05) correlated with quinolinic acid level. Lactobacillus was negatively correlated with 2-ketobutyric acid (P < 0.01), dehydroepiandrosterone (P < 0.01), 7-dehydrodesmosterol (P < 0.01), 4-pyridoxic acid (P < 0.01), creatinine (P < 0.01), and 4,4-dimethyl-5a-cholesta-8,24-dien-3-b-ol (P < 0.05) levels. Escherichia–Shigella and Proteobacteria were positively (P < 0.01) correlated with 2-ketobutyric acid and dehydroepiandrosterone levels, while those were negatively (P < 0.01) correlated with quinolinic acid level. Proteobacteria was positively (P < 0.05) correlated with creatinine level. Streptococcus, Actinobacteria, and Rothia were positively (P < 0.05) correlated with 2-ketobutyric acid, dehydroepiandrosterone, creatinine, 7-dehydrodesmosterol, and 4-pyridoxic acid levels. Streptococcus was positively (P < 0.05) correlated with calcitriol level (Fig. 9A).
Fig. 9.
Correlation analysis among ileal microbiota, differential metabolites, permeability, and barrier function of piglets. (A) Microbiota and metabolites, (B) metabolites and permeability indexes, (C) metabolites and barrier function-related indexes, (D) microbiota and permeability indexes, and (E) microbiota and barrier function-related indexes. 8-OHdG = 8-hydroxyl-deoxyguanosine; DAO = diamine oxidase; EGF = epidermal growth factor; TGF-β = transforming growth factor-beta; EGFR = epidermal growth factor receptor; ZO-1 = zonula occludens-1. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
The correlations between differential ileal metabolites and intestinal permeability are shown in Fig. 9B. DAO content was positively (P < 0.05) correlated with quinolinic acid and calcitriol levels. TGF-β content was positively correlated with calcitriol (P < 0.01), L-isoleucine (P < 0.01), creatinine (P < 0.05), and 4,4-dimethyl-5a-cholesta-8,24-dien-3-b-ol (P < 0.05) levels. D-lactate content was positively (P < 0.05) correlated with calcitriol, L-isoleucine, 4-pyridoxic acid, and 4,4-dimethyl-5a-cholesta-8,24-dien-3-b-ol levels. Moreover, EGFR content was positively correlated with calcitriol (P < 0.01), L-isoleucine (P < 0.01), creatinine (P < 0.01), 4-pyridoxic acid (P < 0.05), and 4,4-dimethyl-5a-cholesta-8,24-dien-3-b-ol (P < 0.05) levels.
The correlations between differential ileal metabolites and barrier-related genes are shown in Fig. 9C. Mucin 13 expression was positively correlated with calcitriol (P < 0.01), 4,4-dimethyl-5a-cholesta-8,24-dien-3-b-ol (P < 0.01), 2-ketobutyric acid (P < 0.01), dehydroepiandrosterone (P < 0.05), 7-dehydrodesmosterol (P < 0.01), and 4-pyridoxic acid (P < 0.01) levels. ZO-1 expression was positively (P < 0.05) correlated with 4,4-dimethyl-5a-cholesta-8,24-dien-3-b-ol level, while occludin was negatively (P < 0.05) correlated with 2-ketobutyric acid level, as well as mucin 1 with creatinine and 4-pyridoxic acid levels.
The correlations between ileal microbiota and permeability indexes are shown in Fig. 9D. Ileal DAO content was positively correlated with Firmicutes (P < 0.01) and Lactobacillus (P < 0.05), while it was negatively correlated with Escherichia–Shigella (P < 0.05), Proteobacteria (P < 0.01), Actinobacteria (P < 0.05), and Rothia (P < 0.05). D-lactate content was negatively (P < 0.05) correlated with Actinobacteria. TGF-β content was positively correlated with Firmicutes, while it was negatively correlated with Proteobacteria (P < 0.05). 8-OHdG content was positively (P < 0.05) correlated with Proteobacteria, as well as EGF content with Streptococcus.
The correlations between ileal microbiota and barrier-related genes are shown in Fig. 9E. The occludin expression was positively (P < 0.05) correlated with Firmicutes and Lactobacillus but negatively correlated with Rothia (P < 0.05), Actinobacteria (P < 0.01), Escherichia–Shigella (P < 0.01), Streptococcus (P < 0.01), and Proteobacteria (P < 0.01).
4. Discussion
Piglets are prone to immune stress and intestinal injury after weaning due to reduced immune capacity, digestive tract underdevelopment, environmental change, and weakened intestinal barrier function, leading to diarrhea and affecting the growth of piglets (Campbell et al., 2013; Chen et al., 2017). Our previous findings revealed that there were discrepancies in the development of immune function among different breeds of pigs and the immune regulation capacity of TB and XB piglets against weaning stress was better than that of DR piglets (Ding et al., 2022). However, studies on the intestinal barrier function of TB and XB pigs need to be further explored. Intestinal microbiota plays an important role in improving intestinal barrier function during the suckling stage, as well as the defense function during the post-weaning stage of piglets (Li et al., 2021). Therefore, the present study explored the influences of intestinal microbiota on the intestinal barrier function of TB, XB, and DR piglets. The results demonstrated that the intestinal mechanical barrier of TB and XB piglets was stronger than that of DR piglets. The intestinal microbiota composition of the three breeds of piglets was different, which may affect the synthesis of steroids and bile acids, leading to the differences in intestinal barrier function of the three pig breeds.
China has abundant resources of indigenous pig breeds. They have higher reproduction rates, and better meat quality and they adapt to extensive feeding and management (Jiang et al., 2011). However, due to the slow growth and low lean meat rates, the breeding of indigenous pigs in large-scale commercial farms is limited (Cesar et al., 2010). The present study showed that the ADG of DR piglets was higher than that of TB and XB piglets from 1 to 10 d old, but there was no significant difference in the ADG among the three pig breeds from 21 to 24 d old. Moreover, TB and XB piglets had lower ADFI than DR piglets. Our previous study showed that TB and XB piglets have lower growth rates than DR piglets, which may be related to the lower lipid metabolic capacity and ileal digestive enzyme activity of Chinese indigenous piglets (Cheng et al., 2023); however, the specific mechanisms still need to be further elucidated.
The intestinal morphological integrity favors the proliferation and specialization of IEC. Specialized IEC forms the barrier surface that separates the host from the environment (Peterson and Artis, 2014). Therefore, IEC maintains the optimal intestinal morphology and influences the development of intestinal barrier function (Peterson and Artis, 2014). Weaning stress leads to impaired intestinal morphology and atrophy of the intestinal villi and then reduces the surface area and nutrient absorption (Zong et al., 2018). In the present study, weaning led to severe shedding of the small intestinal microvilli, which were sparsely and irregularly arranged, but the villi of TB and XB piglets were better than those of DR piglets. The feed source of piglets before weaning is easily digestible liquid milk, and after weaning it is transformed into a solid feed that is not easily digestible. When solid feed enters the gastrointestinal tract of piglets, it will cause certain damage to the intestinal villi, resulting in shorter intestinal villi length and weakened intestinal digestion function, which seriously affects the digestion and absorption of feed, leading to the occurrence of post-weaning diarrhea (Gresse et al., 2017; Montagne et al., 2007). A previous study reported that the most significant changes in the villus of Polish Landrace × Pietland (PP) and Duroc × Hampshire × Wild Boar (DHW) crossbred piglets were the villus morphology and size. Moreover, DHW piglets had a deeper transversal furrow compared with the PP piglets, suggesting that DHW piglets had a greater potential for villus growth (Skrzypek et al., 2005, 2007). Combined with the present study findings, the villi development status of TB and XB piglets was better than that of DR piglets, and XB piglets presented heterosis, which might be because XB piglets had a stronger ability of IEC proliferation.
Intestinal permeability leads to a large outflow of DAO secreted by IEC, while D-lactic acid produced by intestinal microbiota enters the blood circulation through the epithelial barrier and leads to intestinal inflammation (Xiao et al., 2013). In the present study, the plasma D-lactate level of TB and XB piglets was lower after weaning than that of DR piglets, suggesting that the intestinal permeability of TB and XB piglets was lower than DR piglets after weaning. Early weaning resulted in intestinal morphological damage and increased plasma D-lactic acid and DAO contents in Wuzhishan mini-piglets (Xun et al., 2018). Moreover, Bama mini-pigs showed higher intestinal permeability than Landrace pigs during the nursery and growing periods, but there was no difference in the finishing stage (Jiang et al., 2016).
Mucin is the main component of the intestinal epithelium and serves as the first line of defense against invasion by pathogenic microorganisms (Martens et al., 2018). TB and XB piglets had higher mucin 2 and mucin 13 expression levels in the ileum compared with DR piglets after weaning in this study, suggesting that TB and XB piglets have stronger intestinal barriers to resist the damage of weaning stress on intestinal barrier function. Intestinal epithelial cells regulate microbiota to maintain intestinal homeostasis and control inflammation. A previous study reported that weaned piglets have abnormal mucin O-glycosylation profiles in the intestine, dysregulation of intestinal microbiota, and impaired intestinal mucosal barrier function, resulting in impaired intestinal homeostasis (Xia et al., 2022). Additionally, our previous study also demonstrated that TB and XB piglets have a higher ileal IL-6 level than DR piglets after weaning (Ding et al., 2022). Therefore, the disruption of intestinal barrier function ultimately leads to intestinal inflammation, and the integrity of intestinal barrier function is crucial for maintaining intestinal health.
Microbial diversity enhances the resilience of intestinal microecology; that is, when intestinal microecology is disturbed, a species with relatively similar functions is allowed to fill the disturbed niche, which plays a vital role in animal health (Ding et al., 2019; Kim and Isaacson, 2015). A sustainable increase in intestinal microbial diversity of piglets begins with their exposure to the microbiota of the corresponding sows and their surroundings (Slifierz et al., 2015). After weaning, the intestinal microbial diversity and composition of piglets continue to change due to the diet shift, especially to solid feeds containing complex carbohydrates (Bian et al., 2016). The present study found that the microbial diversity of the three breeds of piglets increased continuously during the suckling period, and XB piglets had higher microbial diversity than TB piglets, suggesting that XB piglets exhibited hybridization effects with a better microecosystem. A previous study indicated that fecal microbiota transplantation from Congjiang miniature (CM) piglets to Landrace × Yorkshire (LY) piglets altered the intestinal microbiota diversity and alleviated the diarrhea of early-weaned piglets (Hu et al., 2018a). In agreement with those findings, cross-breed XB piglets may have more potential to resist weaning stress than foreign piglets.
The deterioration of intestinal microbiota, such as Fusobacterium, Bacteroides fragilis, and Escherichia coli, leads to the dysfunction of the intestinal barrier and the infiltration of inflammatory mediators and chemokines (Ahmad Kendong et al., 2021). In the present study, TB and XB piglets had a lower abundance of Escherichia–Shigella at 24 d old while having a higher abundance of Streptococcus in comparison with DR piglets. Previously, it has been found that Duroc × Landrace × Yorkshire piglets weaned at 24 d old had increased relative abundances of Lachnospiraceae, Selenomonadales, E. coli, and Campylobacterales and decreased Lactobacillus and Alloprevotella (Quan et al., 2018). Research evidence suggests that Escherichia–Shigella plays a pathogenic role in the development of nonalcoholic fatty liver disease and is a potential pathogen causing dysentery and diarrhea (Choudhury and Kleerebezem, 2022; Xin et al., 2022). A previous study has also indicated that Escherichia–Shigella may cause diarrhea and dysentery in young animals, while breastfeeding can promote the production of lactic acid in the intestines of young animals, thus inhibiting the growth of Escherichia–Shigella (Konstantinov et al., 2006). At the same time, our previous study showed that TB and XB piglets might obtain more immune protection from the mother than DR piglets (Ding et al., 2022). Combined with the present study results, this may be the reason for the decrease in the relative abundance of Escherichia–Shigella in the ileum of indigenous piglets.
Dysregulation of the host intestinal microbiota and bile acid is detrimental to intestinal barrier function. Microbiota dysregulation leads to impaired bile acid conversion, resulting in increased primary and conjugated bile acids concentrations and decreased secondary bile acids concentrations, whereas secondary bile acids have anti-inflammatory capacity (Sinha et al., 2020; Yang et al., 2021). The present study found that intestinal microbiota could lead to the differences in intestinal barriers in different breeds of piglets via the biosynthesis of steroids, steroid hormone, and primary bile acids. Furthermore, steroids regulate intestinal barrier function. For instance, progesterone and estradiol could reduce the permeability of chloride ions in IEC (Condliffe et al., 2001; Mayol et al., 2002), and estradiol could up-regulate the expression of occludin (Braniste et al., 2009). However, the regulatory mechanism of steroids on intestinal barrier function needs to be further explored.
Intestinal microbial metabolites not only participate in intestinal immune regulation, but also participate in the regulation of intestinal barrier function (Ghosh et al., 2021). The substances produced by intestinal microbiota metabolism are divided into derivative metabolites, including compound K, de novo metabolites such as SCFA, and bile acid metabolites, which affect homeostasis and the integrity of intestinal barrier function (Kho and Lal, 2018). The present study found that TB piglets have higher relative levels of metabolites involved in oxidative phosphorylation, steroid biosynthesis, and bile acid synthesis during the suckling period, while they had lower levels of metabolites involved in the tricarboxylic acid cycle, oxidative phosphorylation, and pentose phosphate pathway during the weaning period compared with XB and DR piglets. Research evidence has shown that the TCA cycle, oxidative phosphorylation, and pentose phosphate are important pathways of oxidative state and active mitochondria in the body (Alvarez et al., 2016; Perl et al., 2011). Lactobacillus gasseri LA39 isolated from the piglet intestine can activate the oxidative phosphorylation pathway of porcine epithelial cells to increase energy production and play an important role in enhancing the intestinal health of piglets (Hu et al., 2018b). Moreover, the oxidative phosphorylation pathway can activate oxidative branches according to the cell demand, mainly producing nicotinamide adenine dinucleotide phosphate that maintains the redox balance in cells or producing nucleotide and amino acid precursors that support cell growth and division (Patra and Hay, 2014; Stincone et al., 2015). The regulation of intestinal microbiota and metabolites on intestinal barrier function is significant and complex, and the specific mechanism of action or interaction needs to be further explored.
Intestinal microbiota affects intestinal barrier function, and metabolites produced by intestinal microbiota (i.e., SCFA, secondary bile acids, and bacteriocins) can interact with several receptors or directly promote mucus secretion and enhance the expression of tight junctions to protect the intestinal epithelial barrier (Kumar et al., 2013). In the present study, Lactobacillus was positively correlated with occludin expression and negatively correlated with 2-ketobutyric acid level. Moreover, Escherichia–Shigella, Streptococcus, and Rothia were positively correlated with 2-ketobutyric acid. These findings suggest that Lactobacillus may enhance intestinal barrier function by inhibiting the production of 2-ketobutyric acid by opportunistic pathogenic bacteria such as Escherichia–Shigella, Streptococcus, and Rothia. A previous study indicated that Lactobacillus fructosus C2 could protect the integrity of Caco-2 cells from E. coli or Salmonella enterica serovar Typhimurium infection (Yu et al., 2015).
5. Conclusion
In summary, there were differences in the development of the small intestine but no differences in the mechanical barrier among different breeds of pigs. During the suckling period, TB and XB piglets' ileal villi atrophy was slower and the permeability at birth was higher compared with DR piglets. After weaning, the chemical barrier function of TB and XB piglets was stronger, the intestinal mucosa secreted more mucin, and the permeability was lower compared with DR piglets to prevent the invasion of harmful substances. The ileal microbial diversity of the three breeds of pigs increased with age and the differences among the three pig breeds were mainly Firmicutes, Proteobacteria, and Actinobacteria. After weaning, TB and XB piglets had a lower ileal abundance of Escherichia–Shigella than DR piglets. Intestinal microbiota may also lead to intestinal barrier differences among different pig breeds by affecting the biosynthesis of steroids and primary bile acids. These findings will provide a physiological and biochemical basis for explaining the stress resistance of Chinese indigenous pigs, and the theoretical basis and technical support for the prevention of weaning stress of piglets.
Author contributions
Sujuan Ding: investigation, funding, writing – original draft, and editing. Md. Abul Kalam Azad: methodology, writing – review and editing. Qian Zhu and Yating Cheng: validation, investigation, and methodology. Pan Huang: investigation and methodology. Xiangfeng Kong: conceptualization, formal analysis, investigation, funding, writing – review and editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was jointly supported by the Key Project of Regional Innovation and Development Joint Fund of National Natural Science Foundation of China (U20A2056), Youth Foundation of Guangxi Natural Science Foundation (2021JJB130431), Open Foundation of the Key Laboratory of Agro-Ecological Processes in Subtropical Region of Chinese Academy of Sciences (ISA2022105), and Special Funds for Construction of Innovative Provinces in Hunan Province (2019RS3022).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2023.09.005.
Appendix supplementary data
The following is the Supplementary data to this article:
References
- Adak A., Khan M.R. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76(3):473–493. doi: 10.1007/s00018-018-2943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Kendong S.M., Raja Ali R.A., Nawawi K.N.M., Ahmad H.F., Mokhtar N.M. Gut dysbiosis and intestinal barrier dysfunction: potential explanation for early-onset colorectal cancer. Front Cell Infect Microbiol. 2021;11:744606. doi: 10.3389/fcimb.2021.744606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez G.M., Casiró S., Gutnisky C., Dalvit G.C., Sutton-McDowall M.L., Thompson J.G., Cetica P.D. Implications of glycolytic and pentose phosphate pathways on the oxidative status and active mitochondria of the porcine oocyte during IVM. Theriogenology. 2016;86(9):2096–2106. doi: 10.1016/j.theriogenology.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Bauer M.A., Kainz K., Carmona-Gutierrez D., Madeo F. Microbial wars: Competition in ecological niches and within the microbiome. Microb Cell. 2018;5(5):215–219. doi: 10.15698/mic2018.05.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian G., Ma S., Zhu Z., Su Y., Zoetendal E.G., Mackie R., et al. Age, introduction of solid feed and weaning are more important determinants of gut bacterial succession in piglets than breed and nursing mother as revealed by a reciprocal cross-fostering model. Environ Microbiol. 2016;18(5):1566–1577. doi: 10.1111/1462-2920.13272. [DOI] [PubMed] [Google Scholar]
- Braniste V., Leveque M., Buisson-Brenac C., Bueno L., Fioramonti J., Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor β-mediated up-regulation of occludin and junctional adhesion molecule – A in epithelial cells. J Physiol. 2009;587:3317–3328. doi: 10.1113/jphysiol.2009.169300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.M., Crenshaw J.D., Polo J. The biological stress of early weaned piglets. J Anim Sci Biotechnol. 2013;4(1):19. doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesar A.S., Silveira A.C., Freitas P.F., Guimarães E.C., Batista D.F., Torido L.C., Meirelles F.V., Antunes R.C. Influence of Chinese breeds on pork quality of commercial pig lines. Genet Mol Res. 2010;9(2):727–733. doi: 10.4238/vol9-2gmr733. [DOI] [PubMed] [Google Scholar]
- Chen H., Hu H., Chen D., Tang J., Yu B., Luo J., et al. Dietary pectic oligosaccharide administration improves growth performance and immunity in weaned pigs infected by rotavirus. J Agric Food Chem. 2017;65(14):2923–2929. doi: 10.1021/acs.jafc.7b00039. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Ding S., Azad M.A.K., Song B., Kong X. Comparison of the pig breeds in the small intestinal morphology and digestive functions at different ages. Metabolites. 2023;13(1):132. doi: 10.3390/metabo13010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury R., Kleerebezem M. Assessing the impact of diet on the mucosa-adhered microbiome in piglets using comparative analysis of rectal swabs and colon content. Front Microbiol. 2022;13:804986. doi: 10.3389/fmicb.2022.804986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condliffe S.B., Doolan C.M., Harvey B.J. 17β-Oestradiol acutely regulates Cl− secretion in rat distal colonic epithelium. J Physiol. 2001;530(1):47–54. doi: 10.1111/j.1469-7793.2001.0047m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Quelen F., Chevalier J., Rolli-Derkinderen M., Mourot J., Neunlist M., Boudry G. N-3 polyunsaturated fatty acids in the maternal diet modify the postnatal development of nervous regulation of intestinal permeability in piglets. J Physiol. 2011;589(17):4341–4352. doi: 10.1113/jphysiol.2011.214056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Cheng Y., Azad M.A.K., Zhu Q., Huang P., Kong X. Developmental changes of immunity and different responses to weaning stress of Chinese indigenous piglets and Duroc piglets during suckling and weaning periods. Int J Mol Sci. 2022;23(24):15781. doi: 10.3390/ijms232415781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Fang J., Liu G., Veeramuthu D., Naif Abdullah A.D., Yin Y. The impact of different levels of cysteine on the plasma metabolomics and intestinal microflora of sows from late pregnancy to lactation. Food Funct. 2019;10(2):691–702. doi: 10.1039/c8fo01838c. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Whitley C.S., Haribabu B., Jala V.R. Regulation of intestinal barrier function by microbial metabolites. Cell Mol Gastroenterol Hepatol. 2021;11(5):1463–1482. doi: 10.1016/j.jcmgh.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresse R., Chaucheyras-Durand F., Fleury M.A., Van de Wiele T., Forano E., Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017;25(10):851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Hiippala K., Jouhten H., Ronkainen A., Hartikainen A., Kainulainen V., Jalanka J., et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients. 2018;10(8):988. doi: 10.3390/nu10080988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Ma L., Nie Y., Chen J., Zheng W., Wang X., et al. A microbiota-derived bacteriocin targets the host to confer diarrhea resistance in early-weaned piglets. Cell Host Microbe. 2018;24(6):817–832. doi: 10.1016/j.chom.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Hu J., Ma L., Zheng W., Nie Y., Yan X. Lactobacillus gasseri LA39 activates the oxidative phosphorylation pathway in porcine intestinal epithelial cells. Front Microbiol. 2018;9:3025. doi: 10.3389/fmicb.2018.03025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G., Liu Y., Oso A., Li F., Kong X., Ying Y. The differences of bacteria and bacteria metabolites in the colon between fatty and lean pigs. J Anim Sci. 2016;94:349–353. [Google Scholar]
- Jiang Y.Z., Zhu L., Li X.W., Si T. Evaluation of the Chinese indigenous pig breed Dahe and crossbred Dawu for growth and carcass characteristics, organ weight, meat quality and intramuscular fatty acid and amino acid composition. Animal. 2011;5(9):1485–1492. doi: 10.1017/S1751731111000425. [DOI] [PubMed] [Google Scholar]
- Kho Z.Y., Lal S.K. The human gut microbiome – a potential controller of wellness and disease. Front Microbiol. 2018;9:1835. doi: 10.3389/fmicb.2018.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.B., Isaacson R.E. The pig gut microbial diversity: Understanding the pig gut microbial ecology through the next generation high throughput sequencing. Vet Microbiol. 2015;177:242–251. doi: 10.1016/j.vetmic.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Konstantinov S.R., Awati A.A., Williams B.A., Miller B.G., Jones P., Stokes C.R., et al. Post-natal development of the porcine microbiota composition and activities. Environ Microbiol. 2006;8(7):1191–1199. doi: 10.1111/j.1462-2920.2006.01009.x. [DOI] [PubMed] [Google Scholar]
- Kumar M., Nagpal R., Verma V., Kumar A., Kaur N., Hemalatha R., et al. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr Rev. 2013;71(1):23–34. doi: 10.1111/j.1753-4887.2012.00542.x. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang Y., Wei K., He J., Ding N., Hua J., et al. Review: Effect of gut microbiota and its metabolite SCFA on radiation-induced intestinal injury. Front Cell Infect Microbiol. 2021;11:577236. doi: 10.3389/fcimb.2021.577236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens E.C., Neumann M., Desai M.S. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. 2018;16(8):457–470. doi: 10.1038/s41579-018-0036-x. [DOI] [PubMed] [Google Scholar]
- Mayol J.M., Arbeo-Escolar A., Alarma-Estrany P., Adame-Navarrete Y., Fernández-Represa J.A. Progesterone inhibits chloride transport in human intestinal epithelial cells. World J Surg. 2002;26(6):652–656. doi: 10.1007/s00268-001-0284-0. [DOI] [PubMed] [Google Scholar]
- Moeser A.J., Pohl C.S., Rajput M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim Nutr. 2017;3(4):313–321. doi: 10.1016/j.aninu.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne L., Boudry G., Favier C., Le Huërou-Luron I., Lallès J.P., Sève B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br J Nutr. 2007;97(1):45–57. doi: 10.1017/S000711450720580X. [DOI] [PubMed] [Google Scholar]
- Okumura R., Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med. 2017;49(5):e338. doi: 10.1038/emm.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra K.C., Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39(8):347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Li Z.R., Green R.S., Holzman I.R., Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139(9):1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl A., Hanczko R., Telarico T., Oaks Z., Landas S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol Med. 2011;17(7):395–403. doi: 10.1016/j.molmed.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L.W., Artis D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- Petri D., Hill J.E., Kessel A.G.V. Microbial succession in the gastrointestinal tract (GIT) of the preweaned pig. Livest Sci. 2010;133:107–109. [Google Scholar]
- Pluske J.R., Turpin D.L., Kim J.C. Gastrointestinal tract (gut) health in the young pig. Anim Nutr. 2018;4(2):187–196. doi: 10.1016/j.aninu.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan J., Cai G., Ye J., Yang M., Ding R., Wang X., et al. A global comparison of the microbiome compositions of three gut locations in commercial pigs with extreme feed conversion ratios. Sci Rep. 2018;8(1):4536. doi: 10.1038/s41598-018-22692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X., Huang X., Zhou Z., Lin X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinform Biomath. 2013;3(3):71–85. [PMC free article] [PubMed] [Google Scholar]
- Rogier E.W., Frantz A.L., Bruno M.E., Wedlund L., Cohen D.A., Stromberg A.J., et al. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci USA. 2014;111(8):3074–3079. doi: 10.1073/pnas.1315792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S.R., Haileselassie Y., Nguyen L.P., Tropini C., Wang M., Becker L.S., et al. Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe. 2020;27(4):659–670. doi: 10.1016/j.chom.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypek T., Piedra J., Skrzypek H., Kazimierczak W., Szymańczyk S., Pawłowska M., et al. Intestinal villi structure during the development of pig and wild boar crossbreed neonates. Livest Sci. 2007;109(1–3):38–41. [Google Scholar]
- Skrzypek T., Valverde Piedra J.L., Skrzypek H., Woliński J., Kazimierczak W., Szymańczyk S., et al. Light and scanning electron microscopy evaluation of the postnatal small intestinal mucosa development in pigs. J Physiol Pharmacol. 2005;56(Suppl. 3):71–87. [PubMed] [Google Scholar]
- Slifierz M.J., Friendship R.M., Weese J.S. Longitudinal study of the early-life fecal and nasal microbiotas of the domestic pig. BMC Microbiol. 2015;15(1):184. doi: 10.1186/s12866-015-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stincone A., Prigione A., Cramer T., Wamelink M.M., Campbell K., Cheung E., et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc. 2015;90(3):927–963. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X., Xu Z., Wan J. Intestinal microbiota diversity and expression of pattern recognition receptors in newly weaned piglets. Anaerobe. 2015;32:51–56. doi: 10.1016/j.anaerobe.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Turner J.R. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Xia B., Zhong R., Wu W., Luo C., Meng Q., Gao Q., et al. Mucin o-glycan-microbiota axis orchestrates gut homeostasis in a diarrheal pig model. Microbiome. 2022;10(1):139. doi: 10.1186/s40168-022-01326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Wu M.M., Tan B.E., Yin Y.L., Li T.J., Xiao D.F., et al. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: I. Growth performance, immune function, and antioxidation capacity. J Anim Sci. 2013;91(10):4772–4780. doi: 10.2527/jas.2013-6426. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Kong F., Xiang Y., Zhou W., Wang J., Yang H., et al. Comparative biogeography of the gut microbiome between Jinhua and Landrace pigs. Sci Rep. 2018;8(1):5985. doi: 10.1038/s41598-018-24289-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin F.Z., Zhao Z.H., Liu X.L., Pan Q., Wang Z.X., Zeng L., et al. Escherichia fergusonii promotes nonobese nonalcoholic fatty liver disease by interfering with host hepatic lipid metabolism through its own msRNA 23487. Cell Mol Gastroenterol Hepatol. 2022;13(3):827–841. doi: 10.1016/j.jcmgh.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Wang D., Zhang P., Lin Y., Fang Z., Che L., et al. Oral administration of Lactococcus lactis-expressed recombinant porcine epidermal growth factor stimulates the development and promotes the health of small intestines in early-weaned piglets. J Appl Microbiol. 2015;119(1):225–235. doi: 10.1111/jam.12833. [DOI] [PubMed] [Google Scholar]
- Xun W., Shi L., Zhou H., Hou G., Cao T. Effect of weaning age on intestinal mucosal morphology, permeability, gene expression of tight junction proteins, cytokines and secretory IgA in Wuzhishan mini piglets. Ital J Anim Sci. 2018;17(4):976–983. [Google Scholar]
- Yang M., Gu Y., Li L., Liu T., Song X., Sun Y., et al. Bile acid-gut microbiota axis in inflammatory bowel disease: From bench to bedside. Nutrients. 2021;13(9):3143. doi: 10.3390/nu13093143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Yuan L., Deng J., Yang Q. Lactobacillus protects the integrity of intestinal epithelial barrier damaged by pathogenic bacteria. Front Cell Infect Microbiol. 2015;5:26. doi: 10.3389/fcimb.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong E., Huang P., Zhang W., Li J., Li Y., Ding X., et al. The effects of dietary sulfur amino acids on growth performance, intestinal morphology, enzyme activity, and nutrient transporters in weaning piglets. J Anim Sci. 2018;96(3):1130–1139. doi: 10.1093/jas/skx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.